Integrating an Extended-Gate Field-Effect Transistor in Microfluidic Chips for Potentiometric Detection of Creatinine in Urine
Abstract
1. Introduction
2. Materials and Methods
2.1. Microfluidic Chip
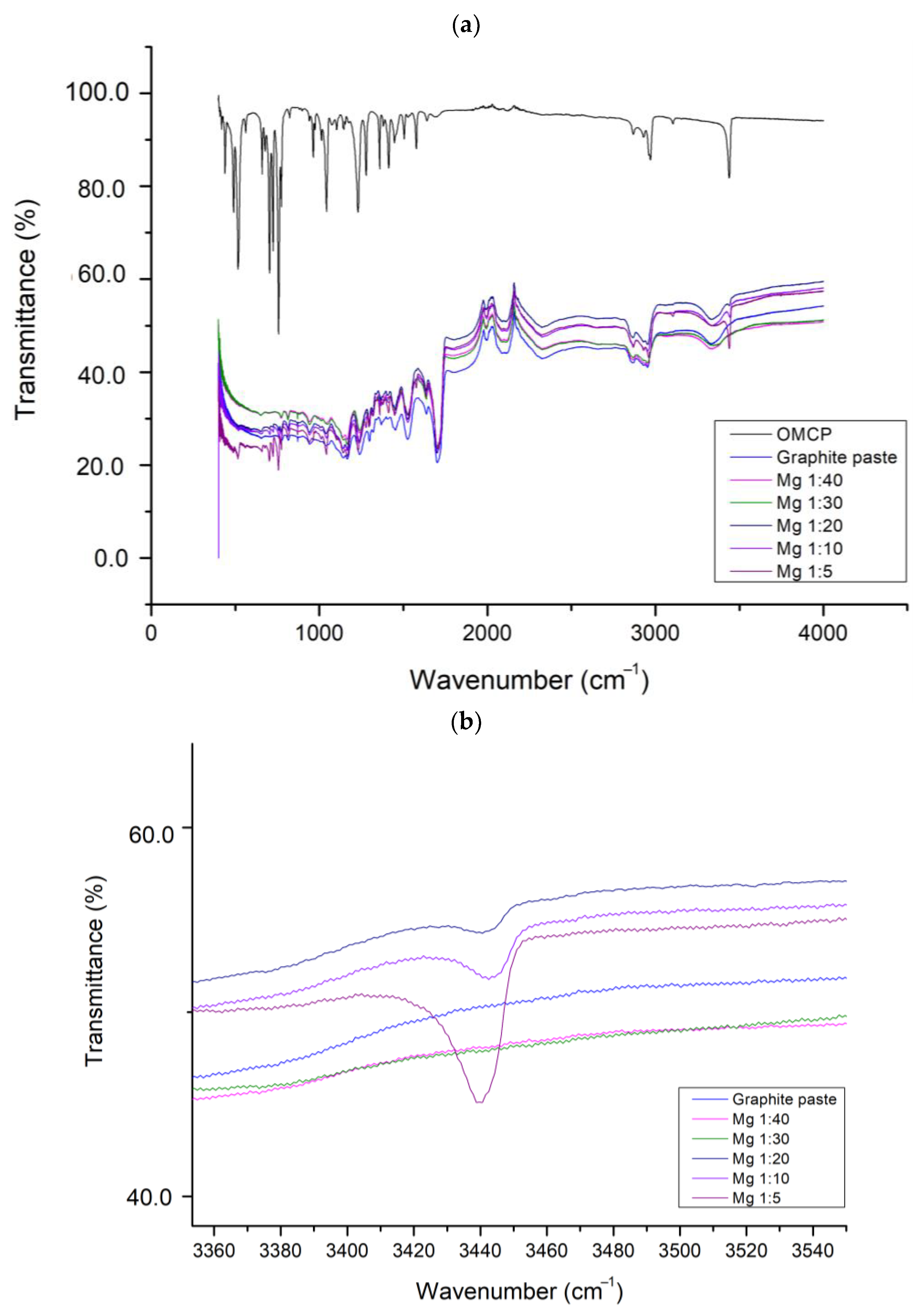
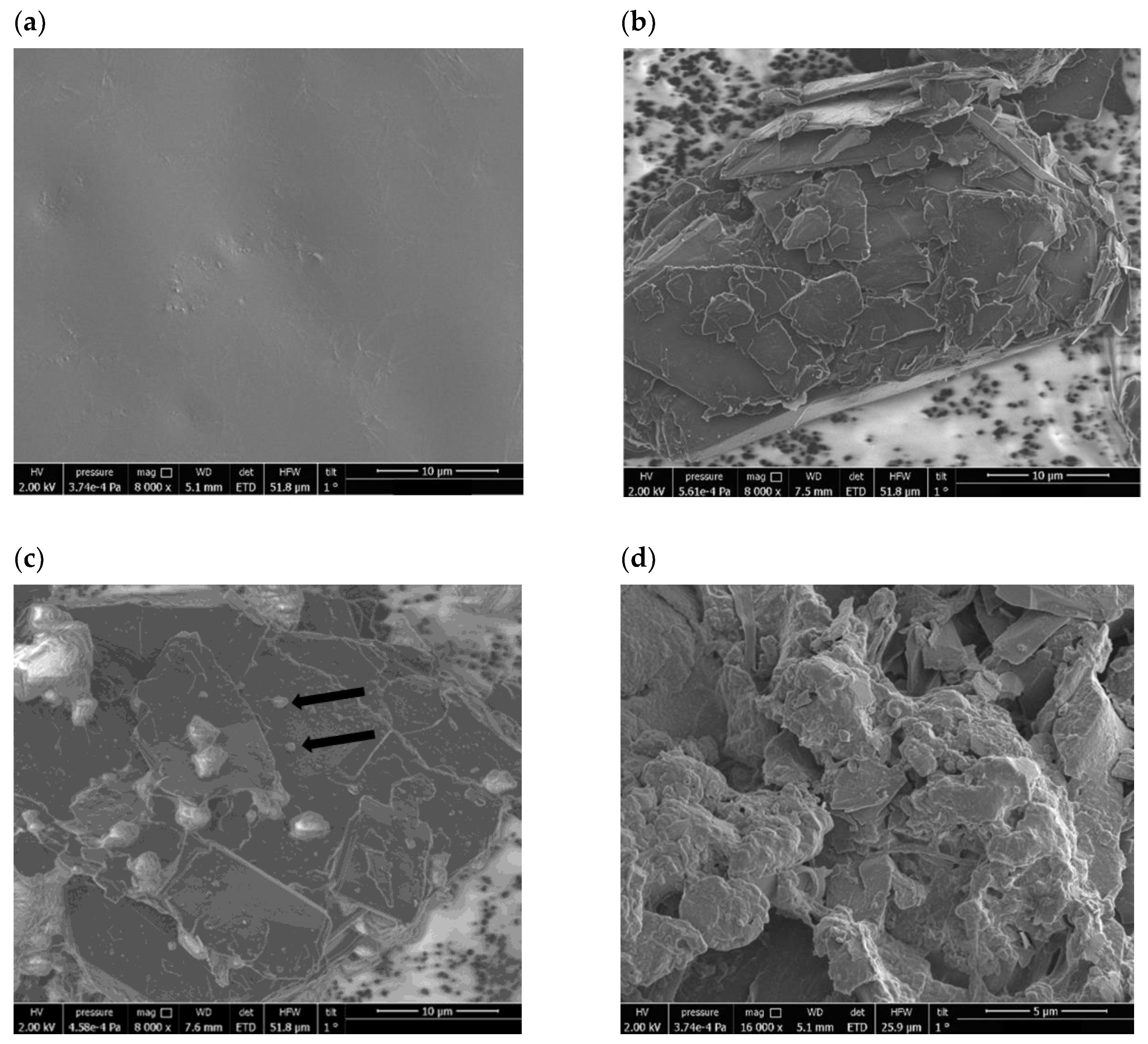
2.2. Creatinine Sensing Membrane: Construction and Characterization
2.3. Commercial MOS Transistor
2.4. Measurements
2.5. Sensor Evaluation Parameters
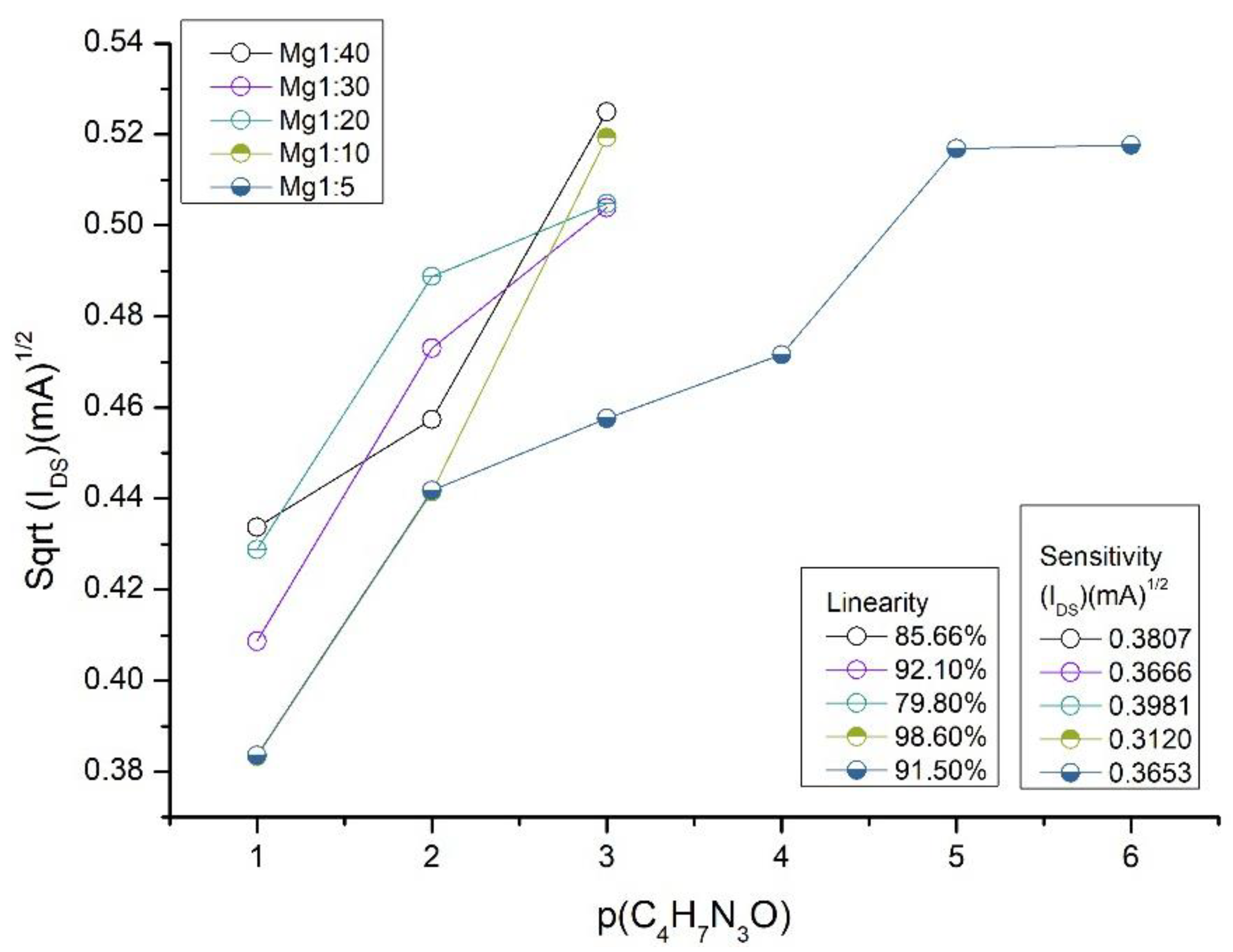
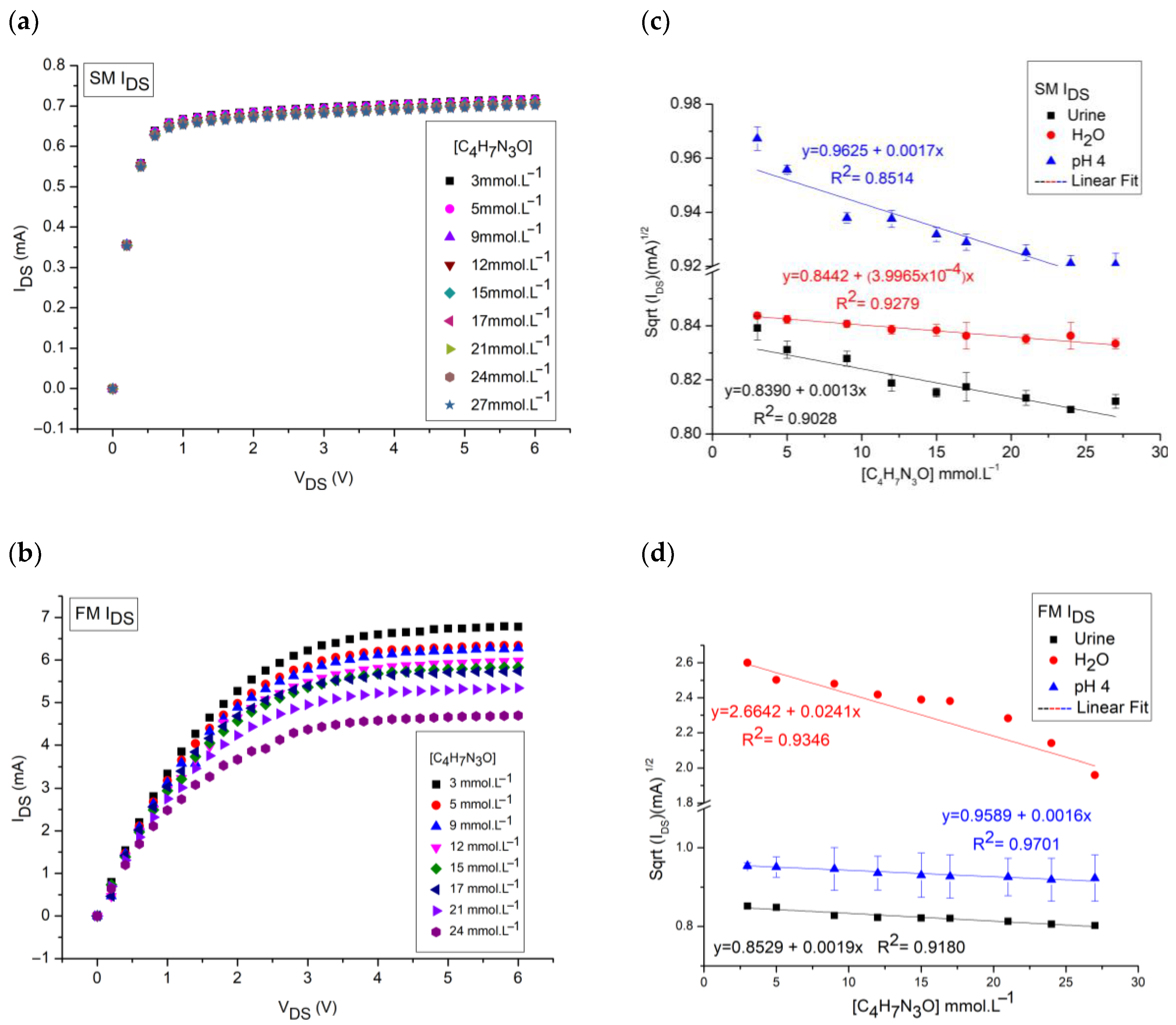
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mello, H.J.N.P.D.; Bueno, P.R.; Mulato, M. Comparing glucose and urea enzymatic electrochemical and optical biosensors based on polyaniline thin films. Anal. Methods 2020, 12, 4199–4210. [Google Scholar] [CrossRef]
- Chiang, J.-L.; Shang, Y.-G.; Yadlapalli, B.K.; Yu, F.-P.; Wuu, D.-S. Ga2O3 nanorod-based extended-gate field-effect transistors for pH sensing. Mater. Sci. Eng. B 2022, 276, 115542. [Google Scholar] [CrossRef]
- Niu, P.; Jiang, J.; Liu, K.; Wang, S.; Jing, J.; Xu, T.; Wang, T.; Liu, Y.; Liu, T. Fiber-integrated WGM optofluidic chip enhanced by microwave photonic analyzer for cardiac biomarker detection with ultra-high resolution. Biosens. Bioelectron. 2022, 208, 114238. [Google Scholar] [CrossRef]
- Sinha, A.; Tai, T.-Y.; Li, K.-H.; Gopinathan, P.; Chung, Y.-D.; Sarangadharan, I.; Ma, H.-P.; Huang, P.-C.; Shiesh, S.-C.; Wang, Y.-L.; et al. An integrated microfluidic system with field-effect-transistor sensor arrays for detecting multiple cardiovascular biomarkers from clinical samples. Biosens. Bioelectron. 2019, 129, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, K.; Scott, P.; Khan, N.I.; Mahmud, M.S.; Song, E. A wearable graphene transistor-based biosensor for monitoring il-6 biomarker. Microelectron. Eng. 2022, 262, 111835. [Google Scholar] [CrossRef]
- Song, P.; Fu, H.; Wang, Y.; Chen, C.; Ou, P.; Rashid, R.T.; Duan, S.; Song, J.; Mi, Z.; Liu, X. A microfluidic field-effect transistor biosensor with rolled-up indium nitride microtubes. Biosens. Bioelectron. 2021, 190, 113264. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Lim, J.-C.; Lai, Y.-H.; Chen, Y.-T.; Lo, Y.-H.; Huang, J.-J. Characterizations of protein-ligand reaction kinetics by transistor-microfluidic integrated sensors. Anal. Chim. Acta 2020, 1110, 1–10. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Yang, T.-H.; Wu, N.-T.; Chen, Y.-T.; Huang, J.-J. Transient analysis of streptavidin-biotin complex detection using an IGZO thin film transistor-based biosensor integrated with a microfluidic channel. Sens. Actuators B Chem. 2017, 244, 642–648. [Google Scholar] [CrossRef]
- Khizir, H.A.; Abbas, T.A.-H. Hydrothermal synthesis of Tio2 nanorods as sensing membrane for extended-gate field-effect transistor (EGFET) pH sensing applications. Sens. Actuators A Phys. 2022, 333, 113231. [Google Scholar] [CrossRef]
- Manjakkal, L.; Sakthivel, B.; Gopalakrishnan, N.; Dahiya, R. Printed flexible electrochemical pH sensors based on CuO nanorods. Sens. Actuators B Chem. 2018, 263, 50–58. [Google Scholar] [CrossRef]
- Casimero, C.; McConville, A.; Fearon, J.-J.; Lawrence, C.L.C.; Taylor, M.; Smith, R.B.; Davis, J. Sensor systems for bacterial reactors: A new flavin-phenol composite film for the in situ voltammetric measurement of pH. Anal. Chim. Acta 2018, 1027, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-J.; Lim, C.-M. Sensing properties of separative paper-based extended-gate ion-sensitive field-effect transistor for cost effective pH sensor applications. Solid-State Electron. 2018, 140, 96–99. [Google Scholar] [CrossRef]
- Rasheed, H.S.; Ahmed, N.M.; Matjafri, M. Ag metal mid layer based on new sensing multilayers structure extended gate field effect transistor (EGFET) for pH sensor. Mater. Sci. Semicond. Process. 2018, 74, 51–56. [Google Scholar] [CrossRef]
- Slewa, L.H.; Abbas, T.A.; Ahmed, N.M. Synthesis of quantum dot porous silicon as extended gate field effect transistor (EGFET) for a pH sensor application. Mater. Sci. Semicond. Process. 2019, 100, 167–174. [Google Scholar] [CrossRef]
- Kang, J.-W.; Cho, W.-J. Achieving enhanced pH sensitivity using capacitive coupling in extended gate FET sensors with various high-K sensing films. Solid-State Electron. 2019, 152, 29–32. [Google Scholar] [CrossRef]
- Bazilah, A.; Awang, Z.; Shariffudin, S.S.; Hamid, N.H.; Herman, S.H. Sensing and physical properties of ZnO nanostructures membrane. Mater. Today Proc. 2019, 16, 1864–1870. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Sabah, F.A.; Kabaa, E.; Myint, M.T.Z. Single- and double-thread activated carbon fibers for pH sensing. Mater. Chem. Phys. 2019, 221, 288–294. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Silverio, A.A.; Tsai, V.F.; Cheng, C.; Chang, S.-Y.; Zhou, M.-Y.; Kao, C.-Y.; Chen, S.-Y.; Pijanowska, D.G.; Rustia, D.; et al. An implementation of an electronic tongue system based on a multi-sensor potentiometric readout circuit with embedded calibration and temperature compensation. Microelectron. J. 2016, 57, 1–12. [Google Scholar] [CrossRef]
- Pundir, C.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef]
- Dong, Y.; Luo, X.; Liu, Y.; Yan, C.; Li, H.; Lv, J.; Yang, L.; Cui, Y. A disposable printed amperometric biosensor for clinical evaluation of creatinine in renal function detection. Talanta 2022, 248, 123592. [Google Scholar] [CrossRef]
- Humphries, T.L.; Vesey, D.A.; Galloway, G.J.; Gobe, G.C.; Francis, R.S. Identifying disease progression in chronic kidney disease using proton magnetic resonance spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 134, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Abitbol, C.L. Standardized urine biomarkers in assessing neonatal kidney function: Are we there yet? J. Pediatr. 2021, 97, 476–477. [Google Scholar] [CrossRef]
- Balhara, N.; Devi, M.; Balda, A.; Phour, M.; Giri, A. Urine; a new promising biological fluid to act as a non-invasive biomarker for different human diseases. URINE 2023, 5, 40–52. [Google Scholar] [CrossRef]
- Lorente, D.G.; Lorente, J.G.; Usach, T.S. Repeticion de la medicion de creatinina serica en atencion primaria: No todos tienen insuficiencia renal crônica. Nefrologia 2015, 35, 395–402. [Google Scholar] [CrossRef]
- Nkuipou-Kenfack, E.; Latosinska, A.; Yang, W.Y.; Fournier, M.C.; Blet, A.; Mujaj, B.; Thijs, L.; Feliot, E.; Gayat, E.; Mischak, H.; et al. A novel urinary biomarker predicts 1-year mortality after discharge from intensive care. Cr. Care 2020, 24, 10. [Google Scholar] [CrossRef]
- Askenazi, D.J. No matter the hemisphere or language, neonatal acute kidney injury is common and is associated with poor outcomes. J. Pediatr. 2023, 99, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Stasyuk, N.; Zakalskiy, A.; Nogala, W.; Gawinkowski, S.; Ratajczyk, T.; Bonarowska, M.; Demkiv, O.; Zakalska, O.; Gonchar, M. A reagentless amperometric biosensor for creatinine assay based on recombinant creatinine deiminase and n-methylhydantoin-sensitive CoCu nanocomposite. Sens. Actuators B Chem. 2023, 393, 134276. [Google Scholar] [CrossRef]
- Rumpel, J.; Spray, B.J.; Chock, V.Y.; Kirkley, M.J.; Slagle, C.L.; Frymoyer, A.; Cho, S.-H.; Gist, K.M.; Blaszak, R.; Poindexter, B.; et al. Urine biomarkers for the assessment of acute kidney injury in neonates with hypoxic ischemic encephalopathy receiving therapeutic hypothermia. J. Pediatr. 2022, 241, 133–140.e3. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.H.A.; Krebs, V.L.J.; Koch, V.H.K.; Magalhaes, N.A.M.; Carvalho, W.B. Risk factors of acute kidney injury in very low birth weight infants in a tertiary neonatal intensive care unit. J. Pediatr. 2023, 99, 235–240. [Google Scholar] [CrossRef]
- Barrio, R.C.; Agustin, J.A.; Manzano, M.C.; Garcıa-Rubira, J.C.; Fernandez-Ortiz, A.; Vilacosta, I.; Macaya, C. In-hospital prognostic value of glomerular filtration rate in patients with acute coronary syndrome and a normal creatinine level. Rev. Esp. Cardiol. (Engl. Ed.) 2007, 60, 714–719. [Google Scholar] [CrossRef]
- Ashakirin, S.N.; Zaid, M.H.M.; Haniff, M.A.S.M.; Masood, A.; Wee, M.F.M.R. Sensitive electrochemical detection of creatinine based on electrodeposited molecular imprinting polymer modified screen printed carbon electrode. Measurement 2023, 210, 112502. [Google Scholar] [CrossRef]
- Gonzalez-Gallardo, C.L.; Arjona, N.; Álvarez-Contreras, L.; Guerra-Balcázar, M. Electrochemical creatinine detection for advanced point-of-care sensing devices: A review. RSC Adv. 2022, 12, 30785–30802. [Google Scholar] [CrossRef]
- Jadhav, R.B.; Patil, T.; Tiwari, A.P. Trends in sensing of creatinine by electrochemical and optical biosensors. Appl. Surf. Sci. Adv. 2024, 19, 100567. [Google Scholar] [CrossRef]
- Guinovart, T.; Hernandez-Alonso, D.; Adriaenssens, L.; Blondeau, P.; Rius, F.X.; Ballester, P.; Andrade, F.J. Characterization of a new ionophore-based ion-selective electrode for the potentiometric determination of creatinine in urine. Biosens. Bioelectron. 2017, 87, 587–592. [Google Scholar] [CrossRef]
- Kukkar, D.; Zhang, D.; Jeon, B.; Kim, K.-H. Recent advances in wearable biosensors for non-invasive monitoring of specific metabolites and electrolytes associated with chronic kidney disease: Performance evaluation and future challenges. TrAC Trends Anal. Chem. 2022, 150, 116570. [Google Scholar] [CrossRef]
- Gohiya, P.; Nadkarni, J.; Mishira, M. Study of neonatal acute kidney injury based on KDIGO criteria. Pediatr. Neonatol. 2022, 63, 66–70. [Google Scholar] [CrossRef]
- Costa, B.M.d.C.; Griveau, S.; Bedioui, F.; d’Orlye, F.; Silva, J.A.F.; Varenne, A. Stereolithography based 3d-printed microfluidic device with integrated electrochemical detection. Electrochim. Acta 2022, 407, 139888. [Google Scholar] [CrossRef]
- Yörük, Ö.; Uysal, D.; Doğan, Ö.M. Carbon-assisted hydrogen production via electrolysis at intermediate temperatures: Impact of mineral composition, functional groups, and membrane effects on current density. Fuel 2025, 380, 133268. [Google Scholar] [CrossRef]
- Sacko, A.; Nure, J.F.; Motsa, M.M.; Nyoni, H.; Mamba, B.; Nkambule, T.; Msagati, T.A.M. Graphitic carbon nitride embedded in polymeric membrane from polyethylene terephthalate microplastic for water treatment. J. Water Process Eng. 2024, 68, 106458. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Jiao, Y.; Pan, C.; Fan, G.; Long, Y.; Yang, H. Multifunctional flexible composite membrane based on nanocellulose-modified expanded graphite/electrostatically spun fiber network structure for solar thermal energy conversion. Energy Rep. 2024, 11, 4564–4571. [Google Scholar] [CrossRef]
- Liu, H.; Duan, P.; Wu, Z.; Liu, Y.; Yan, Z.; Zhong, Y.; Wang, Y.; Wang, X. Silicon/graphite/amorphous carbon composites as anode materials for lithium-ion battery with enhanced electrochemical performances. Mater. Res. Bull. 2025, 181, 113082. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, D.; Yang, G.; Hu, S.; Wang, S.; Pei, P.; Hao, J.; Xu, X. N-doped porous graphite with multilevel pore defects and ultra-high conductivity anchoring Pt nanoparticles for proton exchange membrane water electrolyzers. J. Energy Chem. 2024, 102, 290–301. [Google Scholar] [CrossRef]
- Song, C.; Liu, M.; Du, L.; Chen, J.; Meng, L.; Li, H.; Hu, J. Effect of grain size of graphite powder in carbon paper on the performance of proton exchange membrane fuel cell. J. Power Sources 2022, 548, 232012. [Google Scholar] [CrossRef]
- Corba, A.; Sierra, A.F.; Blondeau, P.; Giussani, B.; Riu, J.; Ballester, P.; Andrade, F.J. Potentiometric detection of creatinine in the presence of nicotine: Molecular recognition, sensing and quantification through multivariate regression. Talanta 2022, 246, 123473. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, T.; Hernández-Alonso, D.; Adriaenssens, L.; Blondeau, P.; Martínez-Belmonte, M.; Rius, F.X.; Andrade, F.J.; Ballester, P. Recognition and Sensing of Creatinine. Angew. Chem. Int. Ed. 2016, 55, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.; Bhatt, K.; Parikh, J.; Patel, N. Multifunctional Calix[4]Pyrrole Derivatives: Optimized Synthesis and a Dual-Action Study Targeting Tuberculosis and Oxidative Stress. Polycycl. Aromat. Compd. 2024, 1–11. [Google Scholar] [CrossRef]
- Dhumale, B.; Bhatt, K.D.; Patel, N.; Modi, K. Reconnoitering the dynamics-calix[4]pyrrole: A heights in research and technology. Results Chem. 2023, 5, 100881. [Google Scholar] [CrossRef]
- Zhang, S.; Bie, W.; Duan, X.; Wu, Z.; Zhang, L.; Li, H.; Wang, Z.; Wei, M.; Kong, F.; Wang, W. Porous calix[4]pyrrole-based polymers with high surface area for efficient removal of polar organic micropollutants from water. Chemosphere 2024, 366, 143548. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; He, S.; Huang, Q.; Zhao, C.; Yu, S.; Jiang, W.; Yao, H.; Wang, L.; Yang, L. An endo-functionalized molecular cage for selective potentiometric determination of creatinine. Chem. Sci. 2024, 15, 14791–14797. [Google Scholar] [CrossRef] [PubMed]
- Sedra, A.S.; Smith, K.C. Microelectronic Circuits, 5th ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Ko, C.; Tseng, C.; Lu, S.; Lee, C.; Kim, S.; Fu, L. Handheld microfluidic multiple detection device for concurrent blood urea nitrogen and creatinine ratio determination using colorimetric approach. Sens. Actuators B Chem. 2025, 422, 136585. [Google Scholar] [CrossRef]
- Parra, L.M.H.; Laucirica, G.; Toimil-Molares, M.E.; Marmisollé, W.; Azzaroni, O. Sensing creatinine in urine via the iontronic response of enzymatic single solid-state nanochannels. Biosens. Bioelectron. 2025, 268, 116893. [Google Scholar] [CrossRef]
- Das, C.; Raveendran, J.; Bayry, J.; Rasheed, P.A. Selective and naked eye colorimetric detection of creatinine through aptamer-based target-induced passivation of gold nanoparticles. RSC Adv. 2024, 14, 33784–33793. [Google Scholar] [CrossRef] [PubMed]
- Chandhana, J.P.; Roshith, M.; Vasu, S.P.; Kumar, D.V.R.; Satheesh, B.T.G. High aspect ratio copper nanowires modified screen-printed carbon electrode for interference-free non-enzymatic detection of serum creatinine in neutral medium. J. Electroanal. Chem. 2024, 971, 118605. [Google Scholar] [CrossRef]
- Peng, S.; Yan, L.; You, R.; Lu, Y.; Liu, Y.; Li, L. Cationic cellulose dispersed Ag NCs/C-CNF paper-based SERS substrate with high homogeneity for creatinine and uric acid detection. Int. J. Biol. Macromol. 2024, 282, 136724. [Google Scholar] [CrossRef] [PubMed]
- Saputra, E. A colorimetric detection of creatinine based-on EDTA capped-gold nanoparticles (EDTA-AuNPs): Digital image colorimetry. Sens. Int. 2024, 5, 100286. [Google Scholar] [CrossRef]
- Manikandan, R.; Yoon, J.; Lee, J.; Chang, S. Non-enzymatic disposable paper sensor for electrochemical detection of creatinine. Microchem. J. 2024, 204, 111114. [Google Scholar] [CrossRef]
- Huang, J.; Sokolikova, M.; Ruiz-Gonzalez, A.; Kong, Y.; Wang, Y.; Liu, Y.; Xu, L.; Wang, M.; Mattevi, C.; Davenport, A.; et al. Ultrasensitive colorimetric detection of creatinine via its dual binding affinity for silver nanoparticles and silver ions. RSC Adv. 2024, 14, 9114–9121. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, T.; Shanmugaraj, K.; Nandhini, K.; Kim, J.T.; Ilanchelian, M. Red-emitting copper nanoclusters for ultrasensitive and selective detection of creatinine and its application in portable smartphone-based paper strips and polymer thin film. Surf. Interfaces 2024, 53, 105014. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, R.; Liu, G.; Jiang, S.; Wang, J.; Lin, J.; Wang, T.; Wang, J.; Huang, Z. Smartphone-based low-cost and rapid quantitative detection of urinary creatinine with the Tyndall effect. Methods 2024, 221, 12–17. [Google Scholar] [CrossRef]
- Bajpai, S.; Akien, G.R.; Toghill, K.E. An alkaline ferrocyanide non-enzymatic electrochemical sensor for creatinine detection. Electrochem. Commun. 2024, 158, 107624. [Google Scholar] [CrossRef]
- Tirkey, A.; Babu, P.J. Synthesis and characterization of citrate-capped gold nanoparticles and their application in selective detection of creatinine (A kidney biomarker). Sens. Int. 2024, 5, 100252. [Google Scholar] [CrossRef]
- Chhillar, M.; Kukkar, D.; Yadav, A.K.; Kim, K. Nitrogen doped carbon dots and gold nanoparticles mediated FRET for the detection of creatinine in human urine samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 321, 124752. [Google Scholar] [CrossRef] [PubMed]
- Nazar, H.R.S.; Ahmadi, V.; Nazar, A.R.S. Mach-Zehnder interferometer fiber optic sensor coated with UiO-66 metal organic framework for creatinine detection. Measurement 2024, 225, 114015. [Google Scholar] [CrossRef]
- Patel, M.R.; Park, T.J.; Kailasa, S.K. Eu3+ ion-doped strontium vanadate perovskite quantum dots-based novel fluorescent nanosensor for selective detection of creatinine in biological samples. J. Photochem. Photobiol. A Chem. 2024, 449, 115376. [Google Scholar] [CrossRef]
- Afshary, H.; Amiri, M. A fast and highly selective ECL creatinine sensor for diagnosis of chronic kidney disease. Sens. Diagn. 2024, 3, 1562–1570. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, P.; Singh, B.P.; Kedia, R.; Shrivastava, S.; Patra, A.; Hwang, S.; Agrawal, V.V. Advancing paper microfluidics: A strategic approach for rapid fabrication of microfluidic paper-based analytical devices (µPADs) enabling in-vitro sensing of creatinine. J. Mol. Liq. 2024, 411, 125707. [Google Scholar] [CrossRef]
- Das, M.; Chakraborty, T.; Kao, C.H. Sol-gel synthesized RexBi1-x-O thin films for electrochemical creatinine sensing: A facile fabrication approach. Mater. Chem. Phys. 2024, 315, 128889. [Google Scholar] [CrossRef]
- Grochocki, W.; Markuszewski, M.J.; Quirino, J.P. Simultaneous determination of creatinine and acetate by capillary electrophoresis with contactless conductivity detector as a feasible approach for urinary tract infection diagnosis. J. Pharm. Biomed. Anal. 2017, 137, 178–181. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Brito Oliveira, D.C.; Costa, F.H.M.; Beraldo, R.M.; da Silva, J.A.F.; Diniz, J.A. Integrating an Extended-Gate Field-Effect Transistor in Microfluidic Chips for Potentiometric Detection of Creatinine in Urine. Sensors 2025, 25, 779. https://doi.org/10.3390/s25030779
De Brito Oliveira DC, Costa FHM, Beraldo RM, da Silva JAF, Diniz JA. Integrating an Extended-Gate Field-Effect Transistor in Microfluidic Chips for Potentiometric Detection of Creatinine in Urine. Sensors. 2025; 25(3):779. https://doi.org/10.3390/s25030779
Chicago/Turabian StyleDe Brito Oliveira, Dhaniella Cristhina, Fernando Henrique Marques Costa, Renato Massaroto Beraldo, José Alberto Fracassi da Silva, and José Alexandre Diniz. 2025. "Integrating an Extended-Gate Field-Effect Transistor in Microfluidic Chips for Potentiometric Detection of Creatinine in Urine" Sensors 25, no. 3: 779. https://doi.org/10.3390/s25030779
APA StyleDe Brito Oliveira, D. C., Costa, F. H. M., Beraldo, R. M., da Silva, J. A. F., & Diniz, J. A. (2025). Integrating an Extended-Gate Field-Effect Transistor in Microfluidic Chips for Potentiometric Detection of Creatinine in Urine. Sensors, 25(3), 779. https://doi.org/10.3390/s25030779







