Compensation Strategies in Post-Stroke Individuals: Insights from Upper Body Kinematics Analysis Based on Inertial Sensors
Highlights
- The use of inertial measurement units (IMUs) during the Box and Block Test enabled detailed kinematic analysis and the identification of typical compensation strategies in post-stroke individuals.
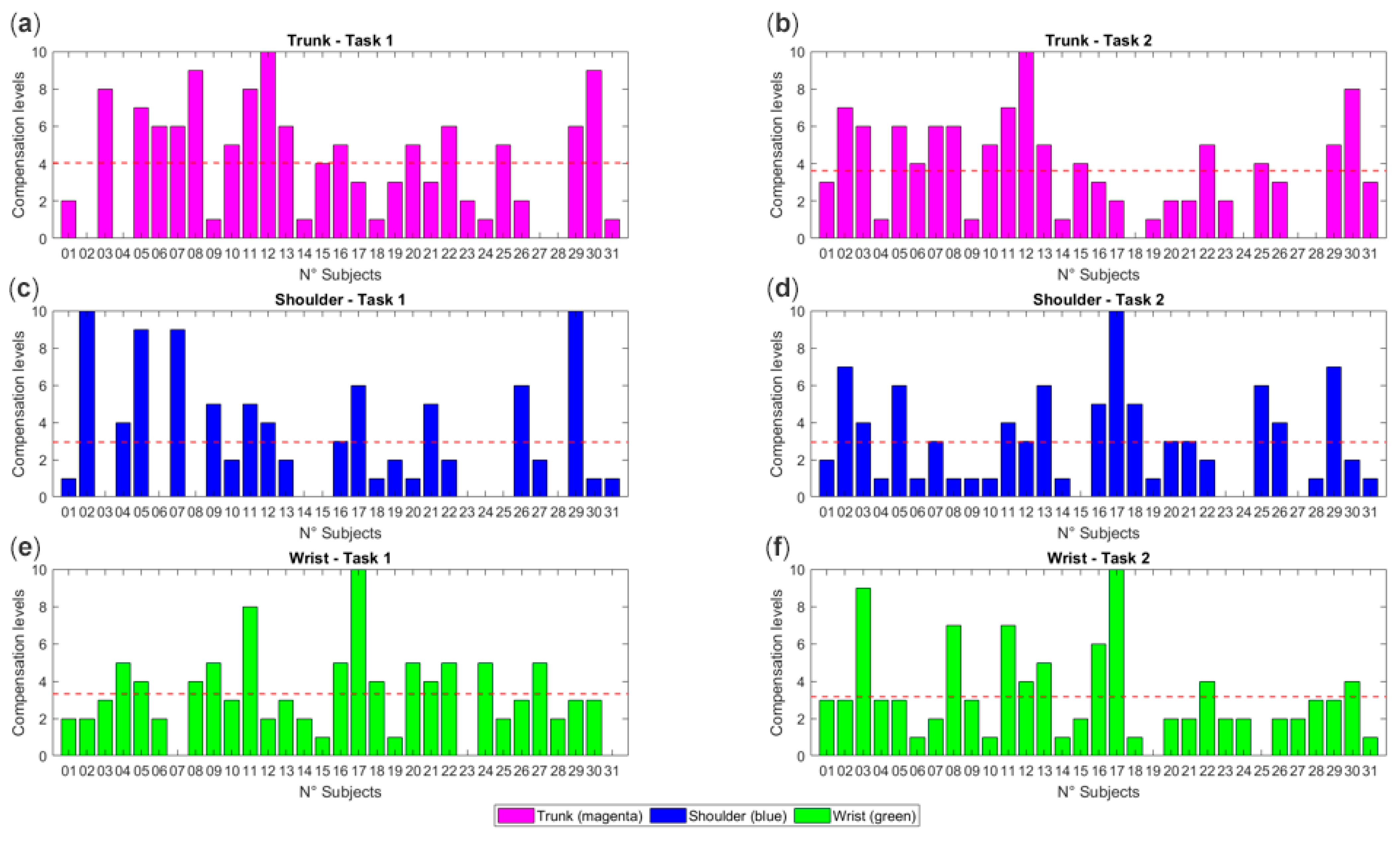
- Overuse of the wrist, shoulder, and trunk was quantified, with 88% of participants showing compensation at the wrist and trunk, and 68% at the shoulder.
- IMUs provide a simple, eco-friendly, and effective tool for objectively assessing movement quality in clinical settings.
- Detecting and quantifying compensation supports the development of personalized rehabilitation approaches and contributes to optimizing functional recovery after stroke.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.2.1. Control Group
2.2.2. Stroke Group
2.3. Clinical Assessments
2.4. Experimental Setup–IMU Setup & Calibration
- First calibration phase: 90° rotations around the three axes (to ensure a global reference and define the initial alignment of the quaternion reference system).
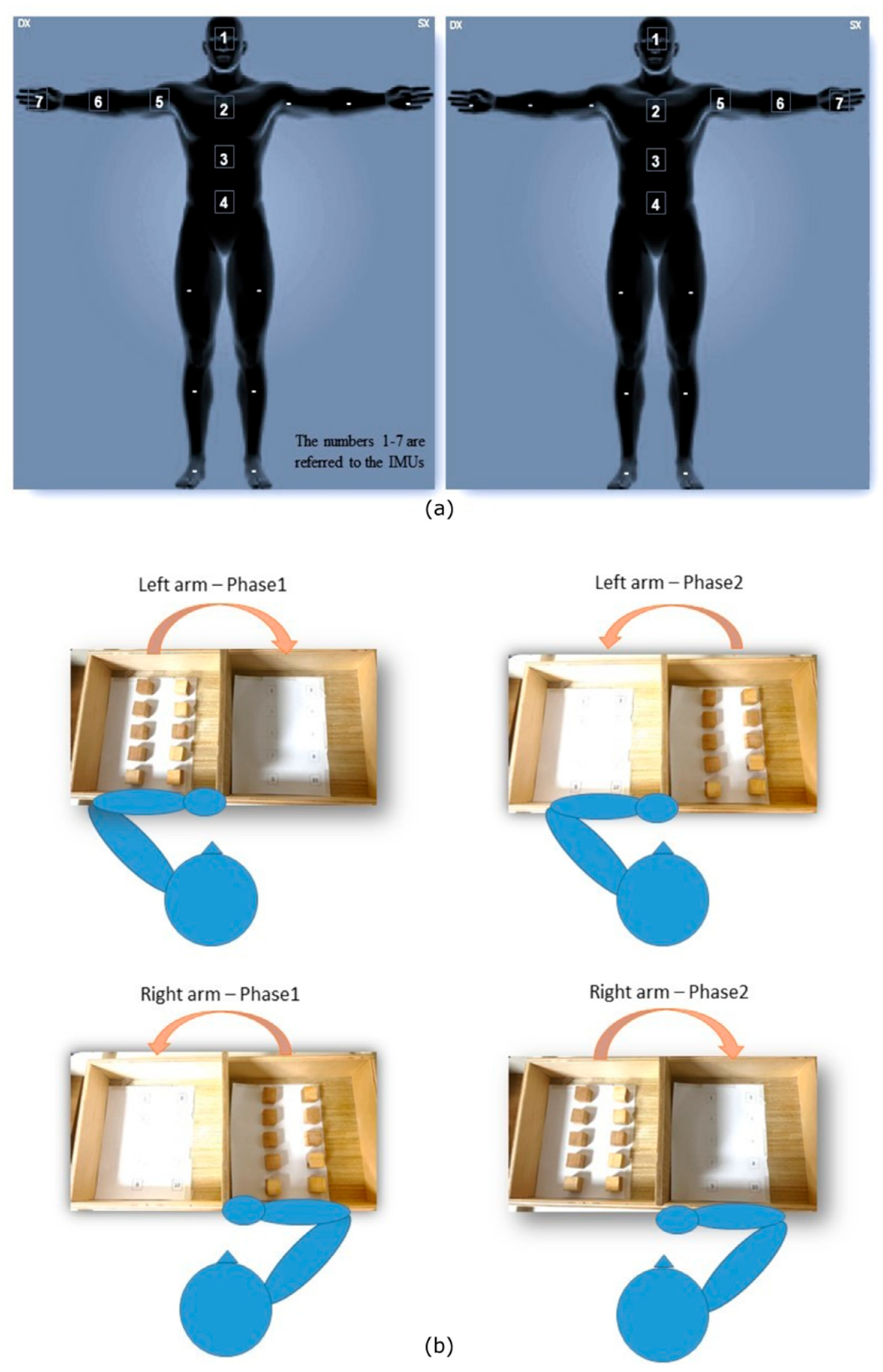
2.5. Task Description
2.6. Data Processing
2.7. Compensation Levels Calculation
2.8. Statistical Analysis
3. Results
4. Discussion
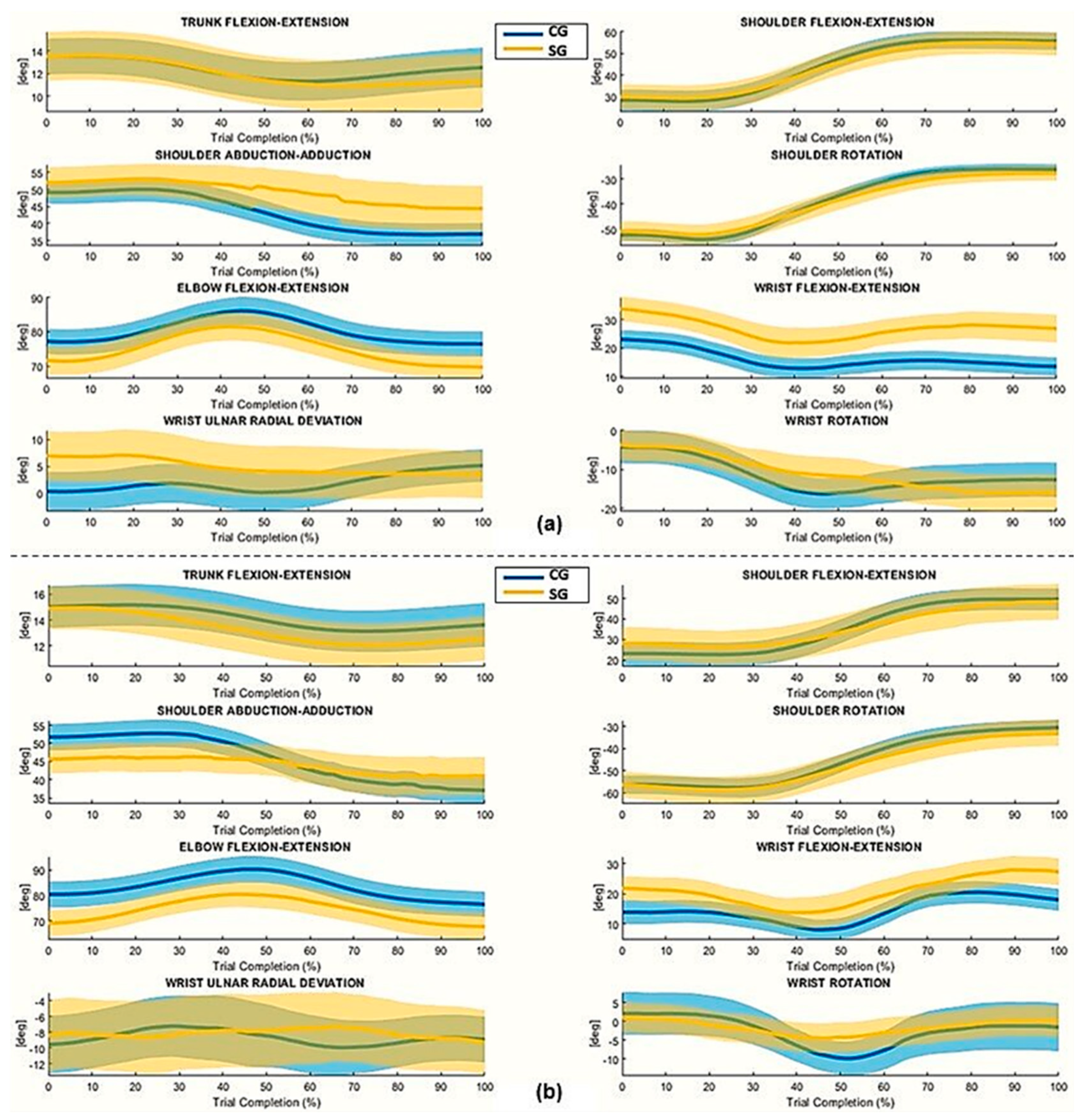
4.1. The Affected Limb
4.2. The Unaffected Limb
4.3. Compensation Levels
4.4. Clinical Implications
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBT | Box and Block Test |
| IMU | Inertial Measurement Unit |
| PD | Parkinson’s Disease |
| CG | Control Group |
| SG | Stroke Group |
| FMA-UL | Fugl-Meyer Assessment-Upper Limb |
| MAS | Modified Ashworth Scale |
| TACI | Total Anterior Circulation Infarct |
| PACI | Partial Anterior Circulation Infarct |
| POCI | Posterior Circulation Infarct |
| LACI | Lacunar Infarct |
| TCT | Trunk Control Test |
| FA | Functional Alignment |
| DLJ | DimensionLess Jerk index |
| LDLJ | Log-DimensionLess Jerk index |
| ROE | Range of Execution |
| F.E. | Flexion–Extension |
| P.S. | Prono–Supination |
| L.B. | Lateral Bending |
| ROT | Rotation |
| A.A. | Ab-/Adduction |
| URD | Ulnar–Radial Deviation |
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, S.M.; Dannenbaum, R.; Levin, M.F. Task-specific training with trunk restraint on arm recovery in stroke: Randomized control trial. Stroke 2006, 37, 186–192. [Google Scholar] [CrossRef]
- Fery-Lemonnier, E. La prévention et la prise en charge des accidents vasculaires cérébraux en France: Rapport à Madame la ministre de la santé et des sports. République Française. Available online: https://www.vie-publique.fr/rapport/30747-la-prevention-et-la-prise-en-charge-des-accidents-vasculaires-cerebraux (accessed on 1 October 2009).
- De Pouvourville, G. Coût de la prise en charge des accidents vasculaires cérébraux en France. Arch. Cardiovasc. Dis. Suppl. 2016, 8, 161–168. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Vive-Larsen, J.; Støier, M.; Olsen, T.S. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 399–405. [Google Scholar] [CrossRef]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef]
- Levin, M.F.; Kleim, J.A.; Wolf, S.L. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil. Neural Repair 2009, 23, 313–319. [Google Scholar] [CrossRef]
- Alaverdashvili, M.; Foroud, A.; Lim, D.H.; Whishaw, I.Q. “Learned baduse” limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: A new analysis of the effect of gestures on success. Behav. Brain Res. 2008, 188, 281–290. [Google Scholar] [CrossRef]
- Allred, R.P.; Cappellini, C.H.; Jones, T.A. The “good” limb makes the “bad” limb worse: Experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav. Neurosci. 2010, 124, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Melendez-Calderon, A.; Roby-Brami, A.; Burdet, E. On the analysis of movement smoothness. J. Neuroeng. Rehabil. 2015, 12, 112. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Melendez-Calderon, A.; Burdet, E. A robust and sensitive metric for quantifying movement smoothness. IEEE Trans. Biomed. Eng. 2012, 59, 2126–2136. [Google Scholar] [CrossRef]
- Hogan, N.; Sternad, D. Sensitivity of smoothness measures to movement duration, amplitude, and arrests. J. Mot. Behav. 2009, 41, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, S.M.; Gates, D.H. Reliability of upper limb movement quality metrics during everyday tasks. Gait Posture 2019, 71, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Alouche, S.; Molad, R.; Demers, M.; Levin, M.F. Development of a comprehensive outcome measure for motor coordination; Step 1: Three-phase content validity process. Neurorehabil. Neural Repair 2020, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Wang, S.; Hsu, C.J.; Trent, L.; Ryan, T.; Kearns, N.T.; Civillico, E.F.; Kontson, K.L. Evaluation of Performance-Based Outcome Measures for the Upper Limb: A Comprehensive Narrative Review. PM R 2018, 10, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; Goffredo, M.; Pournajaf, S.; Paravati, S.; Agosti, M.; De Pisi, F.; Galafate, D.; Posteraro, F. Predictors of activities of daily living outcomes after upper limb robot-assisted therapy in subacute stroke patients. PLoS ONE 2018, 13, e0193235. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Mazzoleni, S.; Gison, A.; Infarinato, F.; Pournajaf, S.; Galafate, D.; Agosti, M.; Posteraro, F.; Franceschini, M. Kinematic Parameters for Tracking Patient Progress during Upper Limb Robot-Assisted Rehabilitation: An Observational Study on Subacute Stroke Subjects. Appl. Bionics Biomech. 2019, 2019, 4251089. [Google Scholar] [CrossRef]
- Collins, K.C.; Kennedy, N.C.; Clark, A.; Pomeroy, V.M. Getting a kinematic handle on reach-to-grasp: A meta-analysis. Physiotherapy 2018, 104, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.C.; Kennedy, N.C.; Clark, A.; Pomeroy, V.M. Kinematic Components of the Reach-to-Target Movement After Stroke for Focused Rehabilitation Interventions: Systematic Review and Meta-Analysis. Front. Neurol. 2018, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Y.; Ye, B.; Babineau, J.; Ding, Y.; Mihailidis, A. Technology-Based Compensation Assessment and Detection of Upper Extremity Activities of Stroke Survivors: Systematic Review. J. Med. Internet Res. 2022, 24, e34307. [Google Scholar] [CrossRef]
- Romano, P.; Pournajaf, S.; Ottaviani, M.; Gison, A.; Infarinato, F.; Mantoni, C.; De Pandis, M.F.; Franceschini, M.; Goffredo, M. Sensor Network for Analyzing Upper Body Strategies in Parkinson’s Disease versus Normative Kinematic Patterns. Sensors 2021, 21, 3823. [Google Scholar] [CrossRef]
- Gates, D.H.; Walters, L.S.; Cowley, J.; Wilken, J.M.; Resnik, L. Range of Motion Requirements for Upper-Limb Activities of Daily Living. Am. J. Occup. Ther. 2016, 70, 7001350010p1–7001350010p10. [Google Scholar] [CrossRef]
- Valevicius, A.M.; Jun, P.Y.; Hebert, J.S.; Vette, A.H. Use of optical motion capture for the analysis of normative upper body kinematics during functional upper limb tasks: A systematic review. J. Electromyogr. Kinesiol. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Hebert, J.S.; Lewicke, J.; Williams, T.R.; Vette, A.H. Normative data for modified Box and Blocks test measuring upper-limb function via motion capture. J. Rehabil. Res. Dev. 2014, 51, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Kontson, K.; Marcus, I.; Myklebust, B.; Civillico, E. Targeted box and blocks test: Normative data and comparison to standard tests. PLoS ONE 2017, 12, e0177965. [Google Scholar] [CrossRef] [PubMed]
- Filippeschi, A.; Schmitz, N.; Miezal, M.; Bleser, G.; Ruffaldi, E.; Stricker, D. Survey of Motion Tracking Methods Based on Inertial Sensors: A Focus on Upper Limb Human Motion. Sensors 2017, 17, 1257. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Cattaneo, D.; Ferrarin, M. Quantitative assessment of upper limb motor function in Multiple Sclerosis using an instrumented Action Research Arm Test. J. Neuroeng. Rehabil. 2014, 11, 67. [Google Scholar] [CrossRef]
- Patel, S.; Hughes, R.; Hester, T.; Stein, J.; Akay, M.; Dy, J.; Bonato, P. Tracking motor recovery in stroke survivors undergoing rehabilitation using wearable technology. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6858–6861. [Google Scholar]
- Goffredo, M.; Carli, M.; Conforto, S.; Bibbo, D.; Neri, A.; D’Alessio, T. Evaluation of skin and muscular deformations in a non-rigid motion analysis. In Medical Imaging 2005: Physiology, Function, and Structure from Medical Images; SPIE: Bellingham, WA, USA, 2005; Volume 5746, pp. 535–541. [Google Scholar]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. Neurorehabil. Neural Repair 2017, 31, 793–799. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; Van de Winckel, A.; De Weerdt, W. Clinical tools to measure trunk performance after stroke: A systematic review of the literature. Clin. Rehabil. 2007, 21, 387–394. [Google Scholar] [CrossRef]
- Sainburg, R.; Kalakanis, D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J. Neurophysiol. 2000, 83, 2661–2675. [Google Scholar] [CrossRef]
- Saggio, G.; Tombolini, F.; Ruggiero, A. Technology-Based Complex Motor Tasks Assessment: A 6-DOF Inertial-Based System Versus a Gold-Standard Optoelectronic-Based One. IEEE Sens. J. 2021, 21, 1616–1624. [Google Scholar] [CrossRef]
- Vitali, R.V.; Perkins, N.C. Determining anatomical frames via inertial motion capture: A survey of methods. J. Biomech. 2020, 106, 109832. [Google Scholar] [CrossRef]
- Cocco, E.S.; Thouant, C.L.; Pietrosanti, L.; Infarinato, F.; Manzia, C.M.; Romano, P.; Torcisi, R.S.M.; Franceschini, M.; Verrelli, C.M.; Pournajaf, S. New gait performance indices and cognitive functions: A pilot study on correlation in people with Parkinson’s disease. Front. Hum. Neurosci. 2025, 19, 1636813. [Google Scholar] [CrossRef]
- Cocco, E.S.; Pournajaf, S.; Romano, P.; Morone, G.; Thouant, C.L.; Buscarini, L.; Manzia, C.M.; Cioeta, M.; Felzani, G.; Infarinato, F.; et al. Comparative analysis of upper body kinematics in stroke, Parkinson’s disease, and healthy subjects: An observational study using IMU-based targeted box and block test. Gait Posture 2024, 114, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sainburg, R. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 2002, 142, 241–258. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Schaefer, S.Y. Interlimb differences in control of movement extent. J. Neurophysiol. 2004, 92, 1374–1383. [Google Scholar] [CrossRef]
- Scano, A.; Molteni, F.; Tosatti, L.M. Low-Cost Tracking Systems Allow Fine Biomechanical Evaluation of Upper-Limb Daily-Life Gestures in Healthy People and Post-Stroke Patients. Sensors 2019, 19, 1224. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, M.C.; Levin, M.F. Compensatory strategies for reaching in stroke. Brain 2000, 123, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Térémetz, M.; Garcia Alvarez, A.; Hanneton, S.; Roby-Brami, A.; Roche, N.; Bensmail, D.; Lindberg, P.; Robertson, J.V.G. Improving upper-limb and trunk kinematics by interactive gaming in individuals with chronic stroke: A single-blinded RCT. Ann. Phys. Rehabil. Med. 2022, 65, 101622. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Geed, S.; Mitchell, A.; Lum, P.S.; Edwards, D.F.; Dromerick, A.W. Characterizing upper extremity motor behavior in the first week after stroke. PLoS ONE 2020, 15, e0221668. [Google Scholar] [CrossRef] [PubMed]
- Mark, V.W.; Taub, E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor. Neurol. Neurosci. 2004, 22, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Desrosiers, J.; Beauchemin, D.; Bergeron, N.; Rochette, A. Development and validation of a scale for rating motor compensations used for reaching in patients with hemiparesis: The reaching performance scale. Phys. Ther. 2004, 84, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Silva, A.; Sousa, A.; Pinheiro, A.R.; Bourlinova, C.; Silva, A.; Salazar, A.; Borges, C.; Crasto, C.; Correia, M.V.; et al. Co-activation of upper limb muscles during reaching in post-stroke subjects: An analysis of the contralesional and ipsilesional limbs. J. Electromyogr. Kinesiol. 2014, 24, 731–738. [Google Scholar] [CrossRef]

| Demographic and Clinical Characteristics (N = 62) | ||
|---|---|---|
| CG (N = 31) | SG (N = 31) | |
| Age | 62.87 10.22 | 67.10 11.06 |
| Gender Male, n° (%) | 16 (52%) | 16 (52%) |
| Arm (n° right limb), n (%) | 31 (100%) | 31 (100%) |
| Affected Side (n° right limb), n (%) | - | 10 (32%) |
| Weight (kg) | 66.32 13.99 | 74.38 13.49 |
| Height (cm) | 167.12 7.74 | 165.29 9.69 |
| Stroke onset (days) | - | 51.30 29.00 |
| sBBT–Dominant or Unaffected Side (n° of cubes) | 68.06 9.19 * | 52.45 9.67 * |
| sBBT–Non-Dominant or Affected Side (n° of cubes) | 65.25 7.35 * | 37.12 12.77 * |
| FMA Upper Extremity | - | 30 (11–36) |
| FMA Wrist | - | 6 (0–10) |
| FMA Hand | - | 9 (7–14) |
| FMA Coordination/Speed | - | 4 (1–5) |
| FMA TOT | - | 50 (23–65) |
| FMA Sensation | - | 12 (6–12) |
| FMA Passive Joint Motion | - | 24 (12–24) |
| FMA Joint Pain | - | 24 (12–24) |
| MAS Wrist | - | 0 (0–2) |
| MAS Elbow | - | 0 (0–3) |
| MAS Shoulder | - | 0 (0–3) |
| Hand Trajectory Parameters | |||||
|---|---|---|---|---|---|
| DA/UA | NDA/AA | ||||
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | ||
| Parameter | Group | Mean SD | Mean SD | Mean SD | Mean SD |
| DLJ | CG | −3.75 1.00 | −4.57 2.83 * | −3.98 1.80 * | −4.99 3.96 * |
| SG | −4.22 1.28 | −5.62 2.68 * | −5.33 2.11 * | −6.45 4.62 * | |
| LDLJ | CG | −1.32 0.01 | −1.52 1.04 * | −1.38 0.58 * | −1.61 1.38 * |
| SG | −1.44 0.25 | −1.73 0.98 * | −1.67 0.75 * | −1.86 1.53 * | |
| CG | 31.18 5.13 * | 26.92 4.56 * | 30.30 4.51 * | 27.16 4.42 * | |
| SG | 23.23 7.29 * | 20.42 6.50 * | 20.52 9.46 * | 20.97 13.55 * | |
| Joint Angles | ||||||
|---|---|---|---|---|---|---|
| DA/UA | NDA/AA | |||||
| Phase 1 | Phase 1 | |||||
| Joint Angles | Group | Mean Angle | ROE | Mean Angle | ROE | |
| Elbow | F.E. | CG | 81.80 21.48 * | 14.87 5.42 | 80.95 17.90 * | 16.93 6.24 |
| SG | 74.17 18.25 * | 18.85 7.02 | 73.04 18.69 * | 17.83 6.70 | ||
| P.S. | CG | 71.01 17.05 * | 17.85 9.42 * | 68.43 15.67 * | 17.66 9.17 * | |
| SG | 66.35 22.38 * | 28.90 10.08 * | 59.59 26.40 * | 25.56 16.75 * | ||
| Shoulder | A.A. | CG | 51.19 22.62 * | 19.15 9.54 | 51.43 24.05 * | 20.06 10.06 |
| SG | 55.25 29.37 * | 20.84 11.20 | 55.15 23.80 * | 20.22 7.98 | ||
| F.E. | CG | 42.22 26.94 | 28.92 13.28 | 44.66 22.99 * | 34.32 12.56 * | |
| SG | 43.34 25.09 | 31.86 15.59 | 39.99 27.38 * | 27.02 12.09 * | ||
| ROT | CG | −42.58 16.20 | 32.48 8.71 | −37.33 11.95 * | 34.42 8.63 * | |
| SG | −39.16 15.99 | 30.87 10.55 | −45.29 26.04 * | 29.32 9.41 * | ||
| Wrist | F.E. | CG | 19.19 16.91 * | 22.32 13.51 | 16.72 17.26 * | 20.53 6.74 * |
| SG | 27.01 19.52 * | 23.18 21.55 | 19.73 20.79 * | 26.44 13.91 * | ||
| URD | CG | 1.57 7.62 * | 8.55 6.43 | −0.52 9.45 * | 8.55 5.13 | |
| SG | 4.98 12.07 * | 8.59 10.08 | −4.06 7.42 * | 7.13 7.06 | ||
| ROT | CG | −11.97 22.95 | 24.85 12.79 | −11.49 18.23 * | 22.15 7.28 | |
| SG | −11.05 20.91 | 25.99 21.45 | −7.18 19.54 * | 24.82 14.77 | ||
| Joint Angles | ||||||
|---|---|---|---|---|---|---|
| DA/UA | NDA/AA | |||||
| Phase 1 | Phase 1 | |||||
| Joint Angles | Group | Mean Angle | ROE | Mean Angle | ROE | |
| Pelvis | L.B. | CG | 3.62 4.58 * | 3.95 1.97 | 4.29 4.15 * | 3.59 1.75 |
| SG | 6.62 0.57 * | 3.95 2.26 | 6.73 1.29 * | 4.80 2.62 | ||
| ROT | CG | 4.73 1.47 | 2.96 1.20 | 4.77 1.37 | 2.79 1.40 | |
| SG | 4.69 12.05 | 3.05 1.35 | 4.21 11.36 | 3.87 1.82 | ||
| TILT | CG | 1.39 2.68 | 2.07 1.16 | 0.06 3.53 * | 2.11 1.53 * | |
| SG | 1.98 8.70 | 2.88 2.07 | 2.11 7.76 * | 4.15 3.02 * | ||
| Trunk | F.E. | CG | 13.25 6.39 | 3.24 1.46 | 11.50 7.57 | 3.38 1.32 * |
| SG | 11.80 10.39 | 4.08 2.02 | 12.25 8.61 | 5.73 3.16 * | ||
| L.B. | CG | 5.45 5.55 * | 5.18 3.03 | −6.20 3.13 * | 6.16 3.31 | |
| SG | 8.74 0.78 * | 6.93 3.21 | −9.85 1.36 * | 7.62 3.40 | ||
| ROT | CG | 9.73 2.98 * | 8.39 1.35 | −5.66 1.52 * | 7.88 2.03 | |
| SG | 18.79 1.33 * | 8.44 2.48 | −20.35 0.66 * | 9.88 2.78 | ||
| Joint Angles | ||||||
|---|---|---|---|---|---|---|
| DA/UA | NDA/AA | |||||
| Phase 2 | Phase 2 | |||||
| Joint Angles | Group | Mean Angle | ROE | Mean Angle | ROE | |
Elbow | F.E. | CG | 84.17 22.40 * | 17.50 4.83 * | 85.06 18.93 * | 18.86 7.07 |
| SG | 78.57 18.23 * | 22.97 7.77 * | 75.89 21.36 * | 21.23 8.32 | ||
| P.S. | CG | 61.72 19.90 | 15.91 6.63 | 58.82 13.51 * | 18.69 10.68 | |
| SG | 59.53 24.21 | 19.79 8.65 | 51.60 29.67 * | 17.17 6.85 | ||
| Shoulder | A.A. | CG | 53.87 22.25 | 21.26 9.51 | 51.64 19.50 | 23.59 10.87 |
| SG | 57.82 28.26 | 20.98 10.19 | 53.82 25.22 | 21.11 11.18 | ||
| F.E. | CG | 39.40 27.36 | 32.18 13.21 | 44.38 24.47 * | 35.97 12.49 * | |
| SG | 37.57 25.80 | 33.50 15.85 | 39.33 33.08 * | 31.49 14.21 * | ||
| ROT | CG | −45.54 17.26 | 34.39 8.41 | −40.77 12.61 * | 37.17 9.07 * | |
| SG | −44.44 17.10 | 33.63 8.91 | −49.44 28.86 * | 31.58 10.18 * | ||
| Wrist | F.E. | CG | 16.77 16.73 * | 26.29 16.53 | 13.38 18.05 * | 26.29 11.20 * |
| SG | 23.14 19.47 * | 28.69 19.22 | 16.97 23.00 * | 30.26 13.92 * | ||
| URD | CG | −6.22 1.65 * | 7.00 3.34 | −10.16 1.63 | 6.79 5.11 | |
| SG | −12.41 2.18 * | 9.63 1.31 | −8.14 8.78 | 6.98 8.14 | ||
| ROT | CG | −5.90 13.08 | 14.27 2.52 * | −4.11 7.88 * | 13.02 2.42 * | |
| SG | −3.91 19.82 | 26.87 18.01 * | −0.76 18.10 * | 26.56 12.63 * | ||
| Joint Angles | ||||||
|---|---|---|---|---|---|---|
| DA/UA | NDA/AA | |||||
| Phase 2 | Phase 2 | |||||
| Joint Angles | Group | Mean Angle | ROE | Mean Angle | ROE | |
| Pelvis | L.B. | CG | 2.75 4.28 * | 3.19 1.89 | 3.95 4.06 * | 2.87 1.70 |
| SG | 6.73 0.33 * | 3.50 2.31 | 6.61 1.68 * | 4.79 2.93 | ||
| ROT | CG | 5.18 1.53 * | 2.88 1.16 | 6.37 1.39 * | 2.61 1.22 | |
| SG | 12.57 5.75 * | 3.14 1.40 | 12.09 4.65 * | 4.08 2.02 | ||
| TILT | CG | 0.44 2.86 * | 1.94 1.13 | 0.24 1.08 | 1.84 1.40 | |
| SG | 2.67 8.79 * | 2.70 1.26 | 1.65 8.13 | 4.09 2.95 | ||
| Trunk | F.E. | CG | 12.06 6.64 | 3.21 1.34 | 13.01 7.36 | 3.25 1.47 * |
| SG | 12.77 10.54 | 4.02 1.79 | 12.49 9.06 | 5.75 4.26 * | ||
| L.B. | CG | 3.87 4.86 * | 5.98 2.99 | 4.29 4.46 * | 5.28 3.28 | |
| SG | 8.36 0.79 * | 5.90 2.52 | 9.01 1.28 * | 7.44 3.77 | ||
| ROT | CG | 4.47 9.83 * | 8.49 2.23 | −4.95 5.55 * | 8.37 2.65 * | |
| SG | 8.42 1.39 * | 9.41 3.52 | −21.50 0.20 * | 11.16 3.50 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thouant, C.-L.; Cocco, E.S.; Morone, G.; Manzia, C.M.; Infarinato, F.; Romano, P.; Cioeta, M.; Goffredo, M.; Franceschini, M.; Pournajaf, S. Compensation Strategies in Post-Stroke Individuals: Insights from Upper Body Kinematics Analysis Based on Inertial Sensors. Sensors 2025, 25, 7609. https://doi.org/10.3390/s25247609
Thouant C-L, Cocco ES, Morone G, Manzia CM, Infarinato F, Romano P, Cioeta M, Goffredo M, Franceschini M, Pournajaf S. Compensation Strategies in Post-Stroke Individuals: Insights from Upper Body Kinematics Analysis Based on Inertial Sensors. Sensors. 2025; 25(24):7609. https://doi.org/10.3390/s25247609
Chicago/Turabian StyleThouant, Carrie-Louise, Elena Sofia Cocco, Giovanni Morone, Carlotta Maria Manzia, Francesco Infarinato, Paola Romano, Matteo Cioeta, Michela Goffredo, Marco Franceschini, and Sanaz Pournajaf. 2025. "Compensation Strategies in Post-Stroke Individuals: Insights from Upper Body Kinematics Analysis Based on Inertial Sensors" Sensors 25, no. 24: 7609. https://doi.org/10.3390/s25247609
APA StyleThouant, C.-L., Cocco, E. S., Morone, G., Manzia, C. M., Infarinato, F., Romano, P., Cioeta, M., Goffredo, M., Franceschini, M., & Pournajaf, S. (2025). Compensation Strategies in Post-Stroke Individuals: Insights from Upper Body Kinematics Analysis Based on Inertial Sensors. Sensors, 25(24), 7609. https://doi.org/10.3390/s25247609













