Method for Bioimpedance Assessment of Superficial Head Tissue Microcirculation
Abstract
1. Introduction
2. Materials and Methods
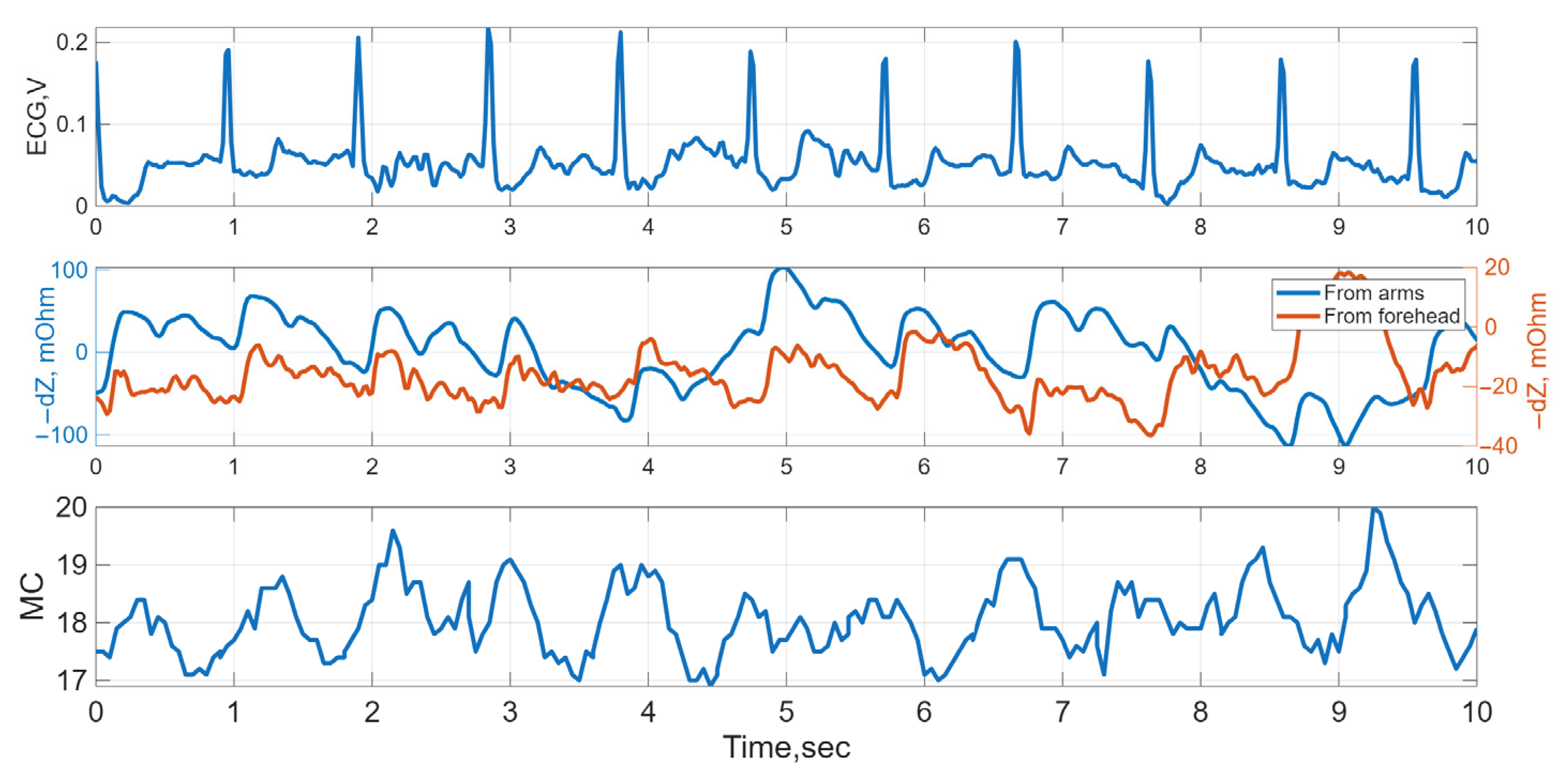
2.1. Recorded Biosignals
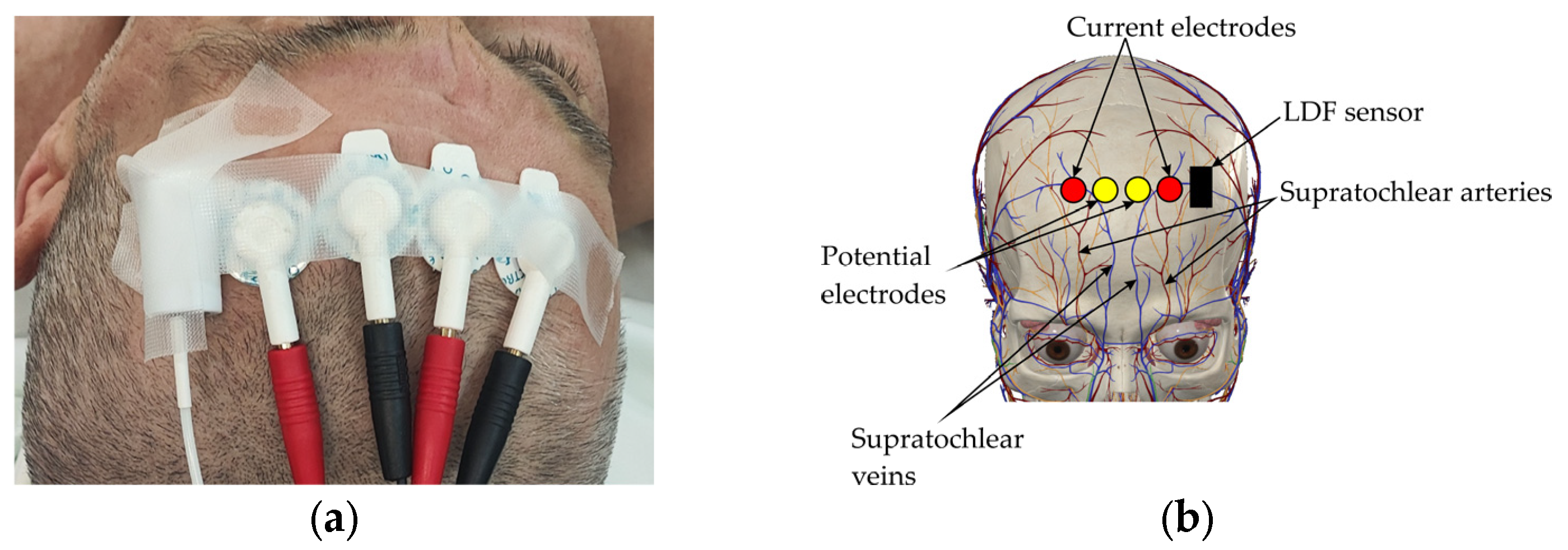
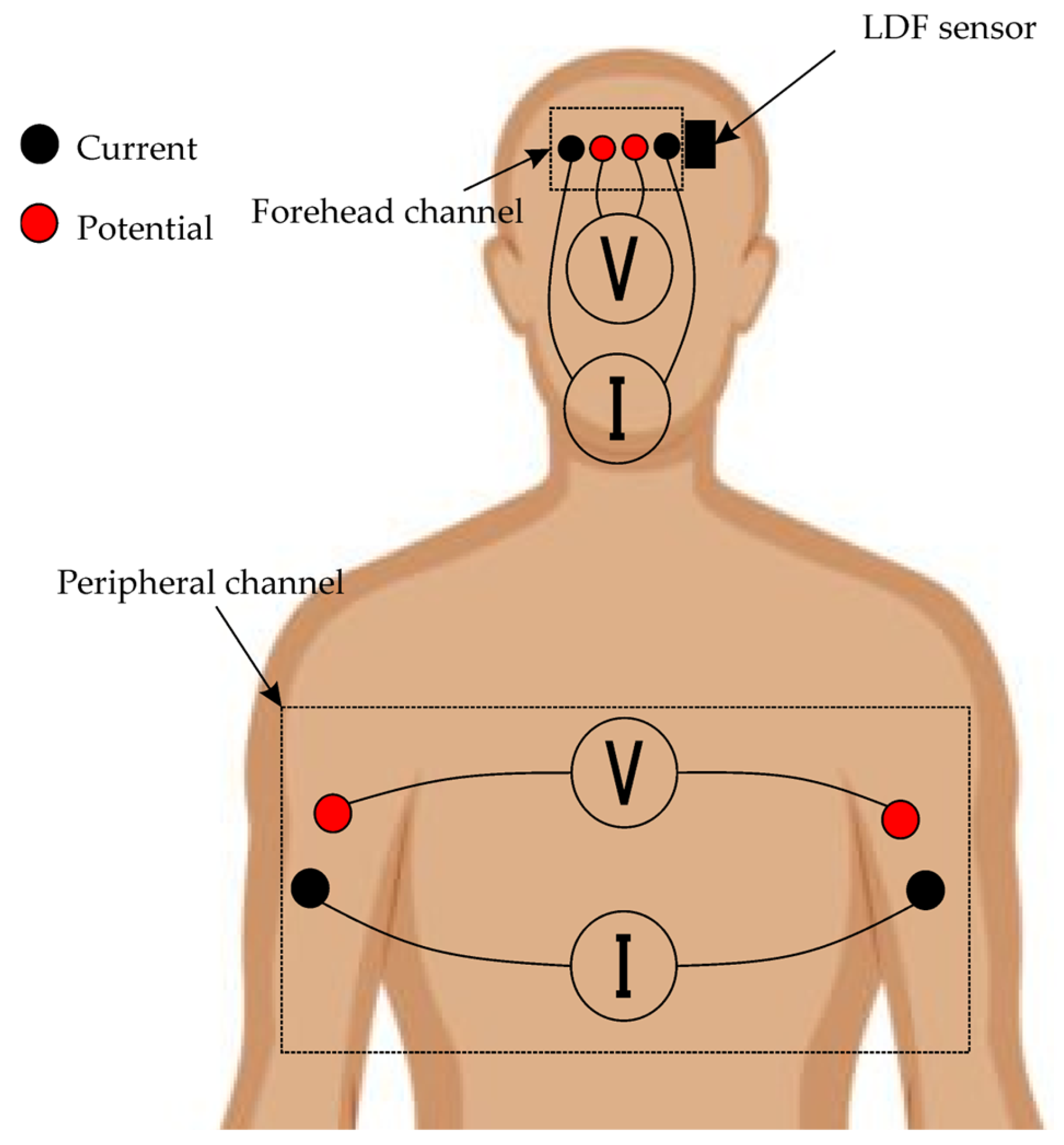
2.2. Biosensor System and Measurement Setup
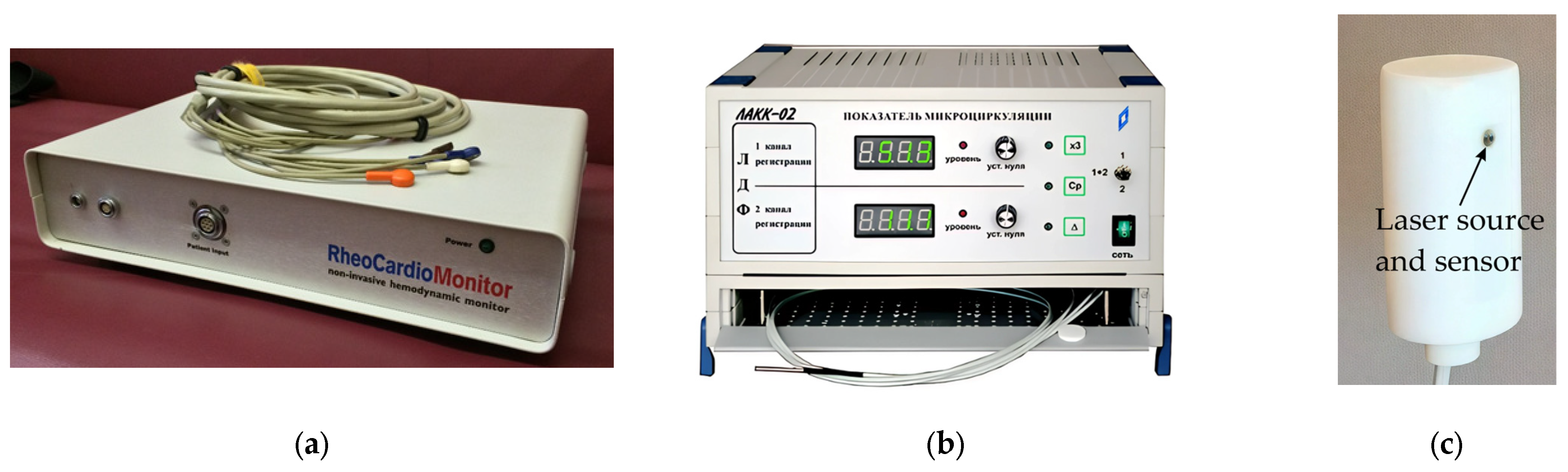
2.3. Equipment
2.4. Patients
2.5. Research Methodology
3. Data Processing
3.1. Software
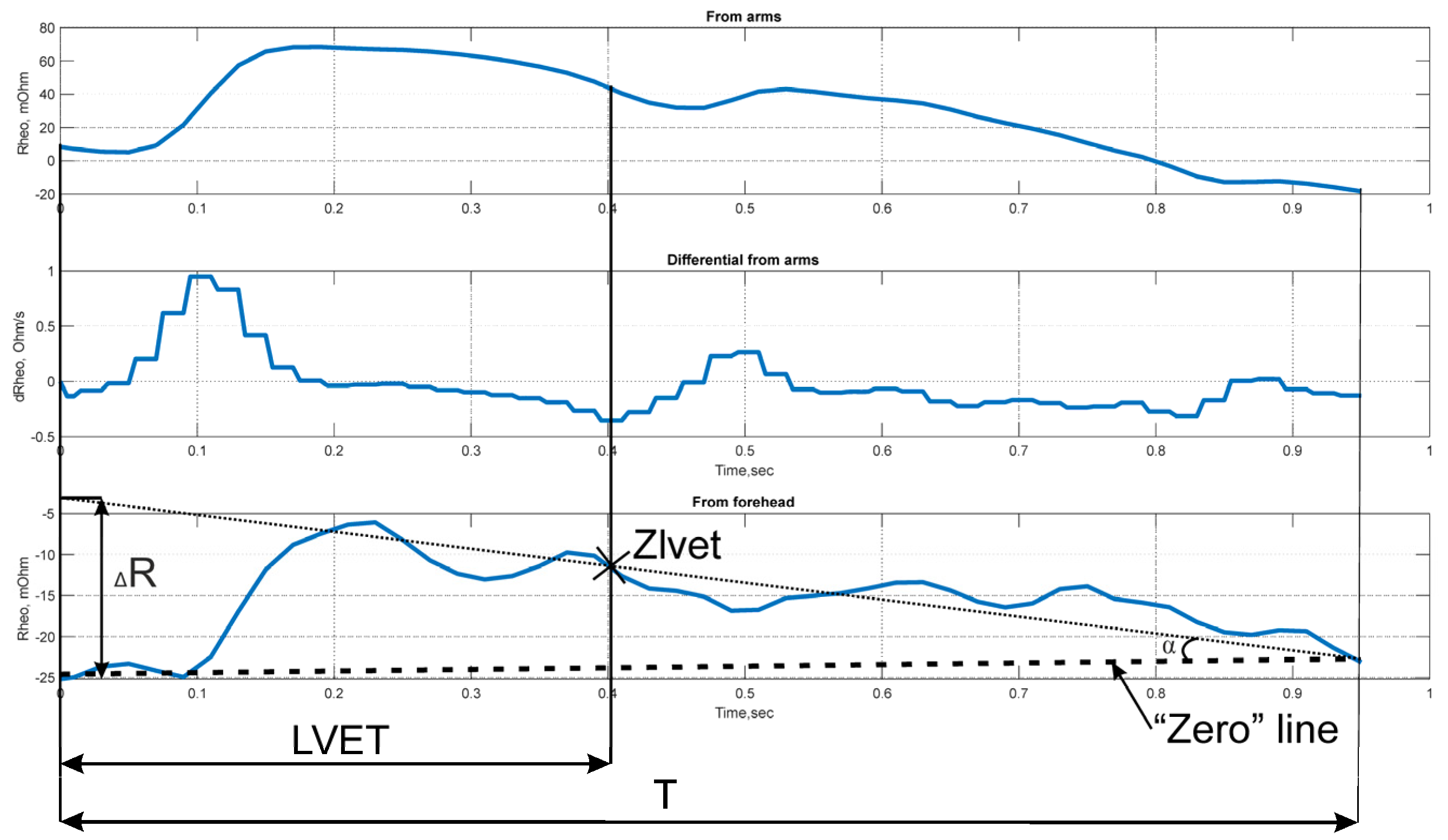
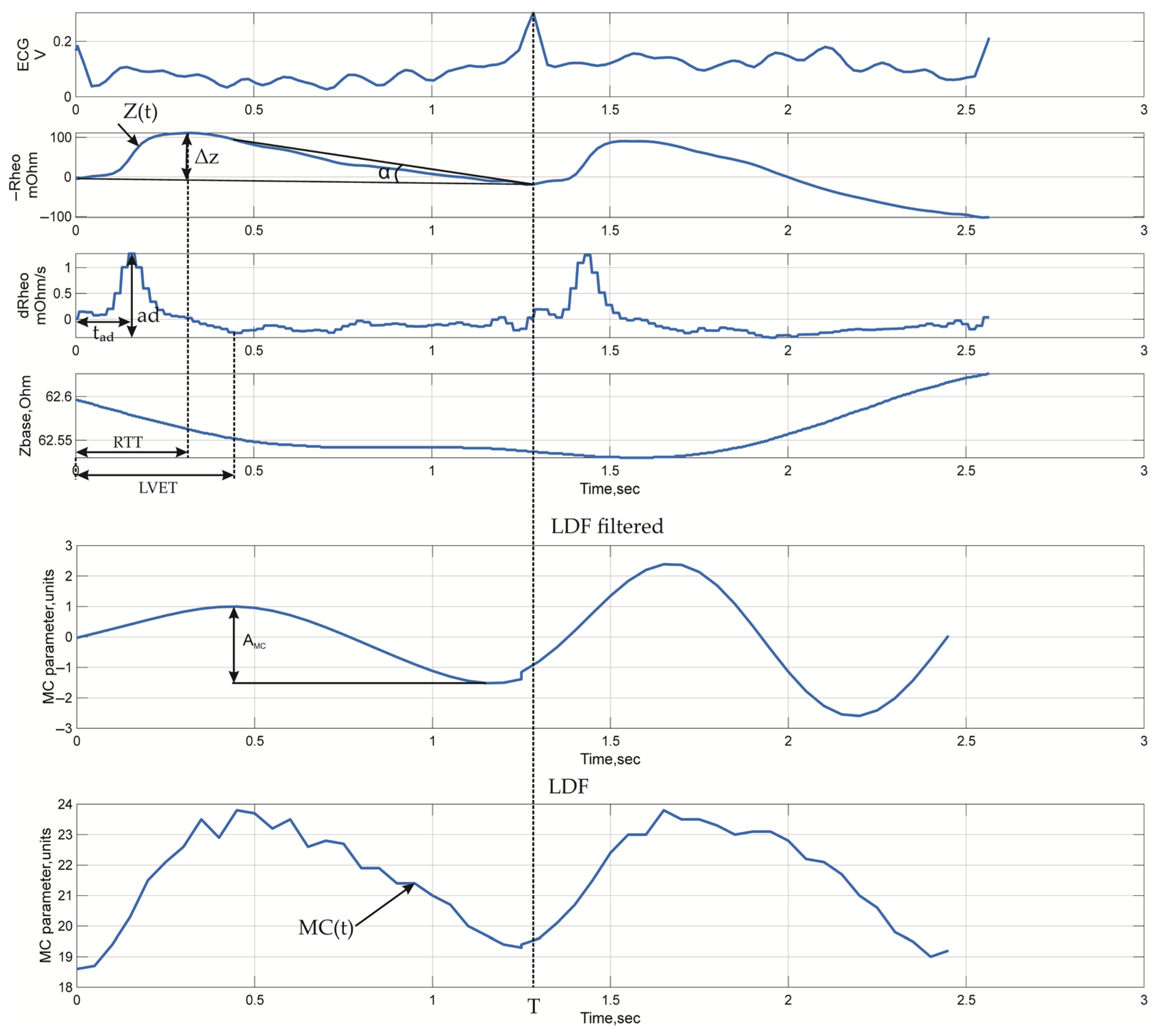
3.2. Signal Metrics
- (1)
- Impedance signal parameters recorded from the forehead surface;
- (2)
- Impedance signal parameters recorded from the surface of the arms;
- (3)
- Impedance signal parameters utilizing values from both recording channels;
- (4)
- LDF signal parameters.
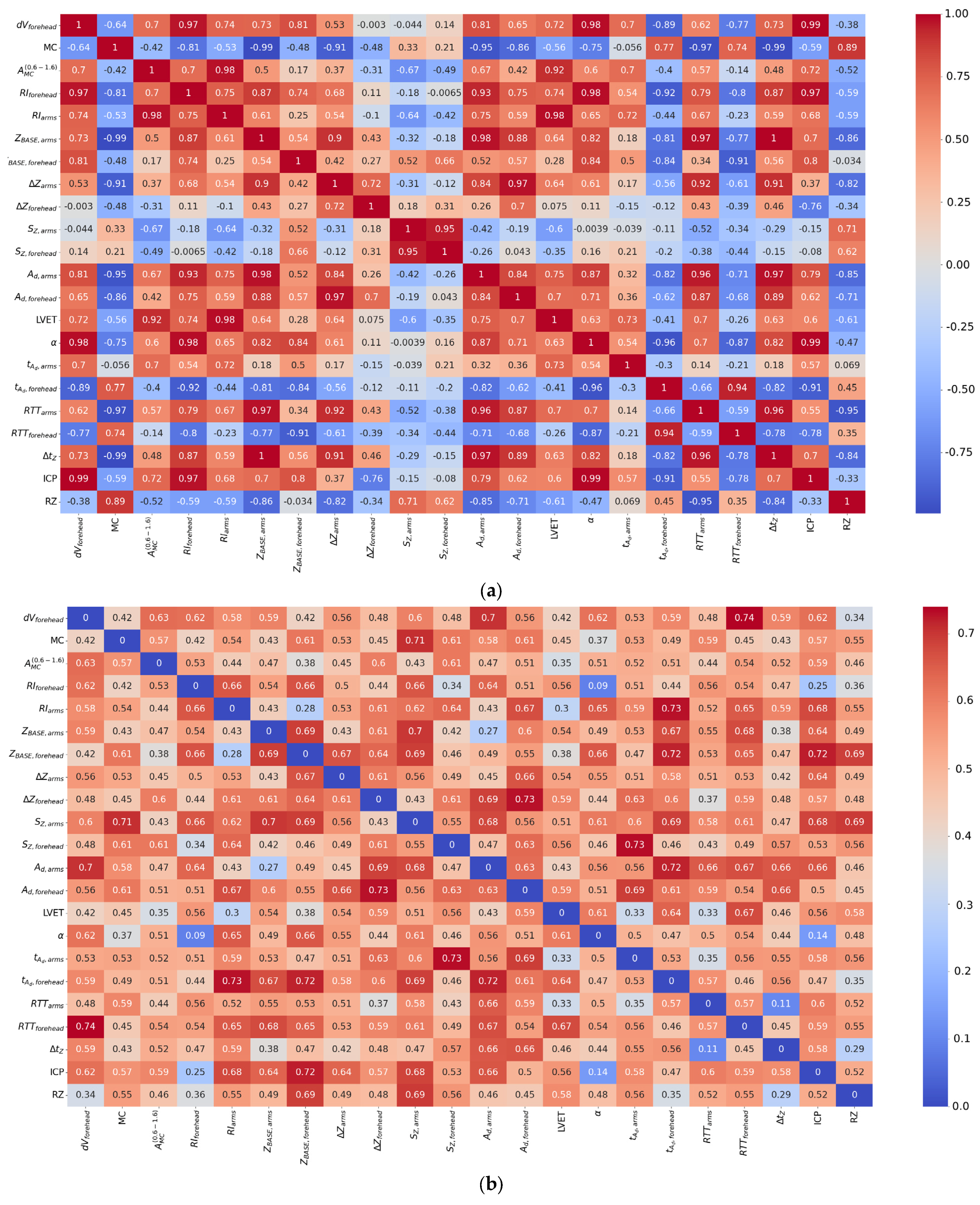
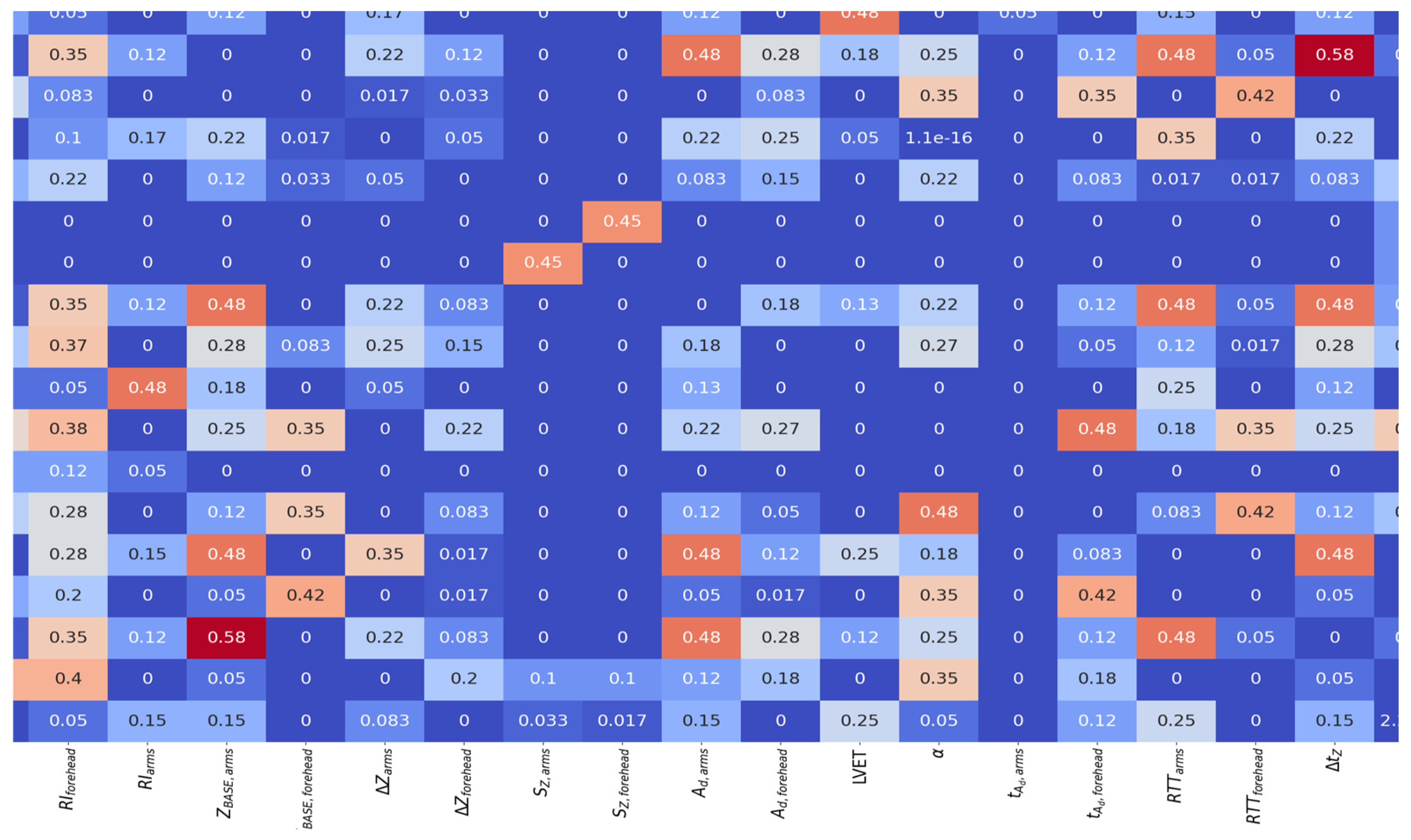
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Formulas for Calculating Volumetric Blood Filling of Forehead Tissues
References
- Shakhnovich, P.G. Clinical Significance of Microcirculation Assessment in Patients with Acute Coronary Syndrome. Ph.D. Thesis, S.M. Kirov Military Medical Academy, Saint Petersburg, Russia, 2016. (In Russian). [Google Scholar]
- Spies, C.; Haude, V.; Fitzner, R.; Schröder, K.; Overbeck, M.; Runkel, N.; Schaffartzik, W. Serum Cardiac Troponin T as a Prognostic Marker in Early Sepsis. Chest 1998, 113, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Kosovskikh, A.A.; Churlyaev, Y.A.; Kan, S.L.; Lyzlov, A.N.; Kirsanov, T.V.; Martyanov, A.R. Central Hemodynamics and Microcirculation in Critical Conditions. Gen. Reanimatol. 2013, 9, 18–22. (In Russian) [Google Scholar] [CrossRef]
- Østergaard, L.; Granfeldt, A.; Secher, N.; Tietze, A.; Iversen, N.K.; Jensen, M.S.; Andersen, K.K.; Nagenthiraja, K.; Gutiérrez-Lizardi, P.; Mouridsen, K.; et al. Microcirculatory Dysfunction and Tissue Oxygenation in Critical Illness. Acta Anaesthesiol. Scand. 2015, 59, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Gruartmoner, G.; Mesquida, J.; Ince, C. Microcirculatory Monitoring in Septic Patients: Where Do We Stand? Med. Intensiv. 2017, 41, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.-L.; Shen, M.; Zhang, G. Intestinal Microcirculation Dysfunction in Sepsis: Pathophysiology, Clinical Monitoring, and Therapeutic Interventions. World J. Emerg. Med. 2022, 13, 343–348. [Google Scholar] [CrossRef]
- Fu, S.-J.; Xu, M.-T.; Wang, B.; Li, B.-W.; Ling, H.; Li, Y.; Wang, Q.; Liu, X.-T.; Zhang, X.-Y.; Li, A.-L. Global Trend and Future Landscape of Intestinal Microcirculation Research from 2000 to 2021: A Scientometric Study. World J. Gastroenterol. 2023, 29, 1523–1535. [Google Scholar] [CrossRef]
- Lewis, C.V.; Taylor, W.R. Intestinal Barrier Dysfunction as a Therapeutic Target for Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1227–H1233. [Google Scholar] [CrossRef]
- Flick, M.; Hilty, M.P.; Duranteau, J.; Saugel, B. The Microcirculation in Perioperative Medicine: A Narrative Review. Br. J. Anaesth. 2024, 132, 25–34. [Google Scholar] [CrossRef]
- Man, Y.; Maji, D.; An, R.; Ahuja, S.P.; Little, J.A.; Suster, M.A.; Mohseni, P.; Gurkan, U.A. Microfluidic Electrical Impedance Assessment of Red Blood Cell-Mediated Microvascular Occlusion. Lab Chip 2021, 21, 1036–1048. [Google Scholar] [CrossRef]
- Duranteau, J.; De Backer, D.; Donadello, K.; Shapiro, N.I.; Hutchings, S.D.; Rovas, A.; Legrand, M.; Harrois, A.; Ince, C. The Future of Intensive Care: The Study of the Microcirculation Will Help to Guide Our Therapies. Crit. Care 2023, 27, 190. [Google Scholar] [CrossRef]
- De Backer, D.; Donadello, K.; Cortes, D.O. Monitoring the microcirculation. J. Clin. Monit. Comput. 2012, 26, 361–366. [Google Scholar] [CrossRef]
- De Backer, D.; Ospina-Tascon, G.; Salgado, D.; Favory, R.; Creteur, J.; Vincent, J.-L. Monitoring the Microcirculation in the Critically Ill Patient: Current Methods and Future Approaches. Intens. Care Med. 2010, 36, 1813–1825. [Google Scholar] [CrossRef]
- Cortelazzo, G.M. Evaluation of Coronary Blood Flow in Exercise-Induced Acute Myocardial Infarction by Biomicroscopy. Int J. Health Policy Manag. 2020, 1, 36–50. [Google Scholar] [CrossRef]
- Tafner, P.F.D.A.; Chen, F.K.; Rabello Filho, R.; Corrêa, T.D.; Chaves, R.C.D.F.; Serpa Neto, A. Recent Advances in Bedside Microcirculation Assessment in Critically Ill Patients. Rev. Bras. Ter. Intensiv. 2017, 29, 2. [Google Scholar] [CrossRef] [PubMed]
- Zamparini, G.; Butin, G.; Fischer, M.-O.; Gérard, J.-L.; Hanouz, J.-L.; Fellahi, J.-L. Noninvasive Assessment of Peripheral Microcirculation by Near-Infrared Spectroscopy: A Comparative Study in Healthy Smoking and Nonsmoking Volunteers. J. Clin. Monit. Comput. 2015, 29, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Feke, G. Laser Doppler Instrumentation for the Measurement of Retinal Blood Flow: Theory and Practice. Bull. Soc. Belge. Ophtalmol. 2006, 302, 171–184. [Google Scholar]
- Leahy, M.J.; Nilsson, G.E. Laser Doppler Flowmetry for Assessment of Tissue Microcirculation: 30 Years to Clinical Acceptance. In Proceedings of the Dynamics and Fluctuations in Biomedical Photonics VII, San Francisco, CA, USA, 23–25 January 2010; SPIE: Bellingham, WA, USA, 2010; Volume 7563, pp. 68–72. [Google Scholar]
- Blinov, A.A.; Shchukin, S.I.; Volkov, A.K.; Nikolaev, A.P. A Technique for Diagnosis of Intervertebral Disc Pathologies Based on Multichannel Electrical Impedance Mapping: Pilot Research. Biomed. Eng. 2019, 52, 379–382. [Google Scholar] [CrossRef]
- Kapravchuk, V.; Briko, A.; Kobelev, A.; Hammoud, A.; Shchukin, S. An Approach to Using Electrical Impedance Myography Signal Sensors to Assess Morphofunctional Changes in Tissue during Muscle Contraction. Biosensors 2024, 14, 76. [Google Scholar] [CrossRef]
- Ryazantsev, P.G.; Ustinova, A.Y.; Briko, A.N.; Shchukin, S.I.; Akselrod, B.A. Approach to Assessing Electrical Impedance of Microcirculation: A Case Study. In Proceedings of the 2025 7th International Youth Conference on Radio Electronics, Electrical and Power Engineering (REEPE), Moscow, Russia, 27–28 February 2025. [Google Scholar]
- Hammoud, A.; Tikhomirov, A.; Briko, A.; Volkov, A.; Karapetyan, A.; Shchukin, S. Evaluation of the Information Content for Determining the Vascular Tone Type of the Lower Extremities in Varicose Veins: A Case Study. Biosensors 2023, 13, 96. [Google Scholar] [CrossRef]
- Hammoud, A.; Tikhomirov, A.N.; Shaheen, Z. Automatic Bio-Impedance Signal Analysis: Smoothing Processes Efficacy Evaluation in Determining the Vascular Tone Type. In Proceedings of the 2021 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 13–14 May 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 113–116. [Google Scholar]
- Tikhomirov, A.N.; Malakhov, A.I.; Shchukin, S.I. Evaluation of Three Dimensional Motion of Mass Center of the Heart by Electrical Impedance Mapping. In Proceedings of the 2019 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 25–26 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 178–181. [Google Scholar]
- Briko, A.N.; Kobelev, A.V.; Ryazantsev, P.G.; Ustinova, A.Y.; Shchukin, S.I.; Akselrod, B.A. Assessment of the Applicability of Electrical Impedance Methods for the Study of Microcirculation in the LBA Region. Meditsinskaya Tekhnika 2025, 2, 10–13. (In Russian) [Google Scholar]
- Mugeb, A.; Chernikov, E.S.; Shchukin, S.I.; Gries, T.; Leonhardt, S. Renal Blood Monitoring System Using Bio-Impedance Measurement: Pilot Study. In Proceedings of the 2020 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 16–17 May 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 199–202. [Google Scholar]
- Kralj, L.; Lenasi, H. Wavelet Analysis of Laser Doppler Microcirculatory Signals: Current Applications and Limitations. Front. Physiol. 2023, 13, 1076445. [Google Scholar] [CrossRef]
- Shcherbachev, A.; Kudashov, I.; Itkin, G.; Bychkov, E.; Pavlov, A.; Govorin, A. Approximation Model for Electroimpedance Measurement Finite Diastolic Volume AHV. In Proceedings of the 2022 Ural-Siberian Conference on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 19–21 September 2022; pp. 55–58. [Google Scholar]
- Li, C.; Guan, G.; Reif, R.; Huang, Z.; Wang, R.K. Determining Elastic Properties of Skin by Measuring Surface Waves from an Impulse Mechanical Stimulus Using Phase-Sensitive Optical Coherence Tomography. J. R. Soc. Interface 2012, 9, 831–841. [Google Scholar] [CrossRef]
| Organ | Relative Perfusion Parameters |
|---|---|
| Heart | 0.08–0.10 mL/min/g |
| Brain | 3.5 mL/100 g/min or 0.035 mL/min/g |
| Kidneys | 4–5 mL/100 g/min or 0.04–0.05 mL/min/g |
| Intestines | 1–1.5 mL/100 g/min or 0.01–0.015 mL/min/g |
| Method Name | Description | Signal Parameters | Advantages | Disadvantages |
|---|---|---|---|---|
| Biomicroscopy | A method for non-invasive diagnostics of blood vessels by obtaining images of the superficial layers of the region of interest containing microvessels [13,14] | Assessment of capillary geometry and red blood cell velocity in capillaries | Direct real-time visualization of the capillary state | Limited field of view, high technical requirements for the recording device, and consequently high equipment costs |
| Near-Infrared Spectroscopy (NIRS) | A method for determining oxyhemoglobin and deoxyhemoglobin contents based on the difference in their absorption capacities relative to the absorption capacity of soft tissues [15,16] | Concentration of oxy- and deoxyhemoglobin in the area under study is determined | Easy to implement, non-invasive | Measurement in relative units; influence of central hemodynamics due to greater penetration depth of radiation (compared to LDF) |
| Laser Doppler Flowmetry (LDF) | A method for measuring red blood cell velocity in the area under study [17,18] | Microcirculation index, proportional to the root mean square velocity of red blood cells in the area under study | Non-invasive, shallow penetration depth of radiation, ability to individually assess respiratory, myogenic, neurogenic, and endothelial frequency ranges of vascular tone regulation, no complex equipment required | Measurement in relative units, variability of results, lack of standardization, limited field of view, highly specialized equipment is required |
| Thermometry | Recording infrared radiation from the body surface | Temperature of the tissue area under examination | Ease of use, simultaneous signal acquisition from a large area, compatibility with other techniques, non-invasive, contactless | The method is more suitable for assessing changes, is highly dependent on environmental conditions, and requires highly specialized equipment |
| Electrical impedance | A method for assessing the hemodynamic characteristics of the vascular bed by measuring the complex impedance of tissues [19] | Baseline and pulsatile impedance | Capability to determine volumetric characteristics of blood filling, as well as parameters of vascular wall tone | The need to develop mathematical models for signal interpretation, dependence of measurement results on the quality of “electrode-skin” contact |
| Characteristic | Value |
|---|---|
| Number of impedance recording channels | 2 |
| Number of ECG channels | 1 |
| Channel sampling rate, Hz | 500 |
| Impedance measurement method | Tetrapolar |
| Probing current amplitude, mA | 2.8 |
| Probing current frequency, kHz | 100 |
| Baseline impedance measurement range, Ohm | 1–240 |
| Pulsatile impedance measurement range, mOhm | 10–500 |
| Input-referred electrical impedance noise value, mOhm | <0.5 |
| Input-referred ECG noise value, μV | <5 |
| Characteristic | Value |
|---|---|
| Laser type | Single-mode semiconductor laser diode |
| Laser wavelength, nm | 1064 |
| Laser power at the fiber output, mW | <3.0 |
| Doppler shift frequency recording bandwidth, Hz | 20–24,000 |
| Range of recorded red blood cell velocities, mm/s | 0.3–6 |
| Range of microcirculation index values, perf. units | 0–99 |
| Patient ID | Head Circumference, cm | Thickness of the Superficial Soft Tissue Layer, mm | Age, Years | Sex | Height, cm | Weight, kg |
|---|---|---|---|---|---|---|
| 1 | 60 | 4 | 57 | male | 179 | 94 |
| 2 | 57 | 4 | 60 | male | 175 | 83 |
| 3 | 59 | 4 | 46 | male | 169 | 71 |
| 4 | 56 | 4 | 62 | male | 180 | 85 |
| 5 | 58 | 4 | 41 | male | 170 | 85 |
| 6 | 54 | 3 | 66 | female | 163 | 60 |
| 7 | 58 | 4 | 54 | female | 167 | 74 |
| 8 | 61 | 3 | 43 | male | 195 | 129 |
| 9 | 57 | 4 | 63 | male | 182 | 112 |
| 10 | 61 | 3 | 74 | male | 165 | 95 |
| Stage Number | Description | Stage Duration Time Me (min; max) hh:mm:ss | Physiological Conditions |
|---|---|---|---|
| 1 | Patient’s condition before induction of general anesthesia | 0:25:00 (0:10:00; 0:34:00) | Stable hemodynamics, spontaneous respiration |
| 2 | Maximum hypnotic effect | 0:59:30 (0:43:00; 1:22:00) | Artificial lung ventilation, stabilization of systemic hemodynamics |
| 3 | Patient’s condition before initiation of cardiopulmonary bypass (CPB) | 0:09:00 (0:02:00; 0:18:00) | Maintained anesthesia after thoracotomy, decreased body temperature |
| 4 | Patient’s condition after CPB | 1:20:36 (0:51:00; 1:49:00) | Restoration of spontaneous circulation, normothermia |
| 5 | Patient’s condition after the end of surgery prior to transfer to intensive care unit | 0:20:48 (0:16:30; 0:31:30) | Stable hemodynamics |
| № | Name | Formula | Calculation Group | Description |
|---|---|---|---|---|
| 1 | Baseline impedance | (1), (2) | Reflects non-cardiac tissue perfusion | |
| 2 | Pulse impedance amplitude | (1), (2) | Reflects pulse wave tissue perfusion | |
| 3 | Surface under pulse impedance curve | (1), (2) | Is an indicator of stroke volume | |
| 4 | Rheographic index | (1), (2) | Reflects the volumetric blood flow | |
| 5 | Differential rheogram amplitude | (1), (2) | Reflects the vessels’ activity | |
| 6 | Left ventricular ejection time | LVET | (2) | Reflects cardiac activity |
| 7 | Pulse impedance curve fall angle | α | (1) | Reflects venous vessels’ activity |
| 8 | Time to peak of differential rheogram | (1), (2) | Reflects capillary activity | |
| 9 | Volumetric blood filling (Appendix A) | (3) | - | |
| Complex parameters | ||||
| 1 | Pulse wave transit time for each channel | (1), (2) | Reflects vessels’ activity | |
| 2 | Pulse wave transit time between channels | (3) | Reflects the difference in macro- and microvessels’ activity | |
| 3 | Central–peripheral ratio index | (3) | Reflects the difference in pulse wave blood perfusion | |
| 4 | Baseline impedance ratio | (3) | Reflects the difference in non-cardiac perfusion | |
| LDF parameters | ||||
| 1 | Microcirculation index | (4) | Is a value proportional to the multiplication of the amount of the blood cells and theirs root mean square velocity | |
| 2 | Microcirculation index amplitude | (4) | Reflects the changes in MC value due to the pulse wave blood perfusion | |
| Stage Number According to Table 6 | , mL/min by 100 g. of the Tissue | , *103 Units | , perf. Units | |||
|---|---|---|---|---|---|---|
| 1 | 5.94 ± 7.28, 5.77 (3.30; 8.38) | 0.86 ± 0.00, 0.88 (0.47; 1.18) | 1.13 ± 0.91, 0.81 (0.54; 1.40) | −0.98 ± 0.02, −0.98 (−0.99; −0.97) | 0.12 ± 0.16, 0.12 (0.01;0.16) | −0.12 ± 0.16, −0.11 (−0.17; 0.011) |
| 2 | 7.35 ± 9.54, 5.83 (2.59; 11.89) * | 0.89 ± 0.00, 0.80 (0.38; 1.40) | 2.07 ± 2.08, 1.68 (0.87; 3.12) * | −0.97 ± 0.03, −0.98 (−0.99; −0.97) | 0.03 ± 0.14, 0.03 (−0.09; 0.11) | −0.01 ± 0.15, −0.03 (−0.12; 0.11) |
| 3 | 7.75 ± 10.89, 6.66 (2.85; 9.56) | 0.75 ± 0.00, 0.84 (0.42; 1.23) | 1.53 ± 1.05, 1.12 (0.74; 2.30) * | −0.98 ± 0.02, −0.98 (−0.99; −0.96) | 0.05 ± 0.10, 0.04 (−0.01; 0.1) | −0.03 ± 0.11, −0.04 (−0.12; 0.04) |
| 4 | 4.57 ± 21.77, 5.13 (0.21; 9.24) * | 0.26 ± 0.00, 0.56 (−0.02; 1.06) * | 1.29 ± 1.02, 1.07 (0.69; 1.65) * | −0.98 ± 0.02, −0.99 (−0.99; −0.97) | 0.18 ± 0.15, 0.20 (0.07; 0.26) | −0.17 ± 0.16, −0.17 (−0.27; −0.07) |
| 5 | 5.08 ± 12.92, 3.22 (1.71; 6.79) * | 0.28 ± 0.00, 0.29 (−0.17; 0.58) * | 0.88 ± 0.64, 0.75 (0.47; 1.09) * | −0.99 ± 0.03, −1 (−1; −1) | −0.07 ± 0.41, 0.06 (−0.12, 0.15) | 0.07 ± 0.41, −0.06 (−0.12; 0.11) |
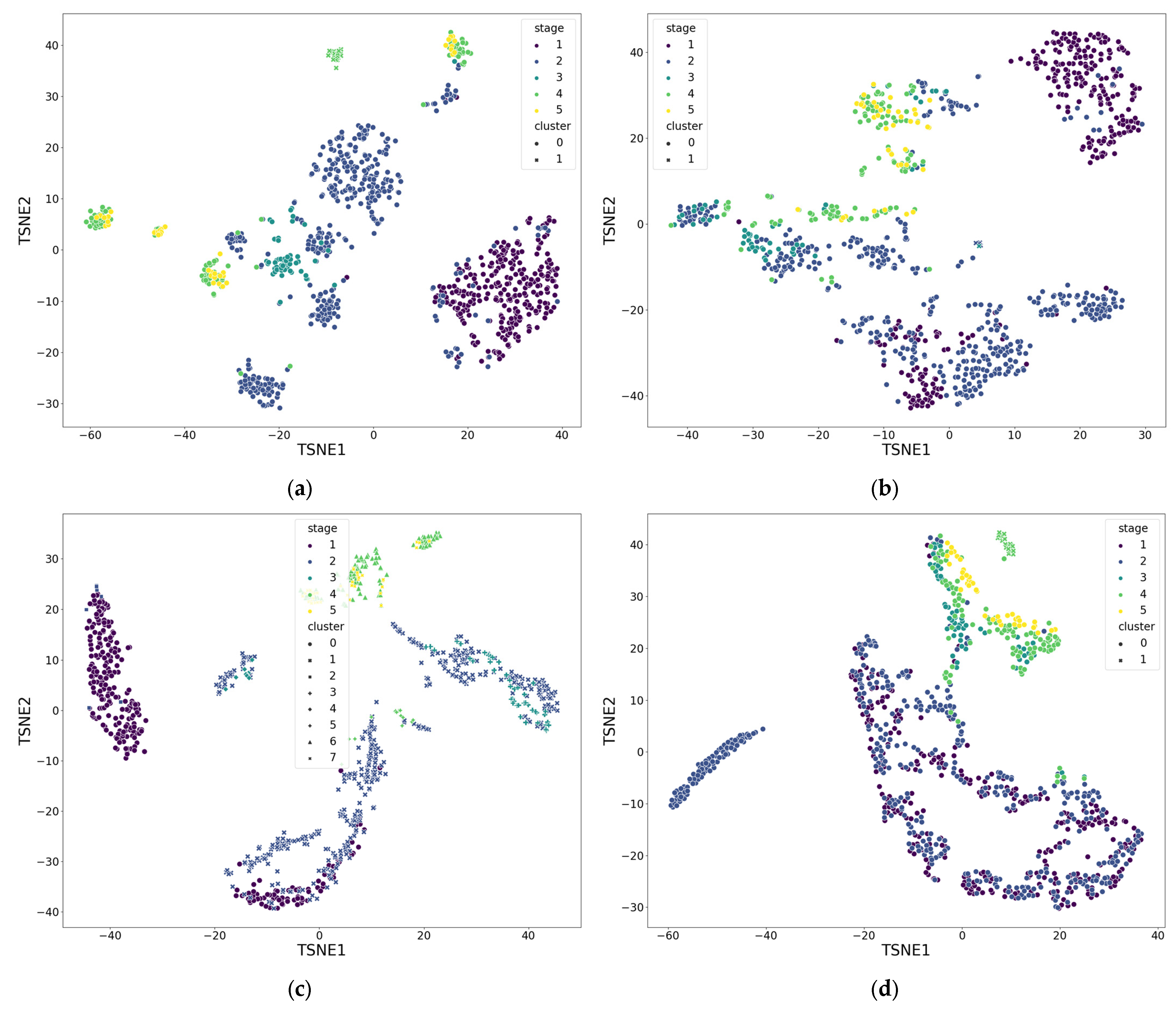
| Functional Groups | Range of Number of Clusters Me (min; max) | Silhouette Coefficient Values M, std (min; max) |
|---|---|---|
| 1 | 2 (2; 3) | 0.468, 0.137 (0.148; 0.586) |
| 2 | 2 (2; 4) | 0.517, 0.142 (0.222; 0.798) |
| 3 | 4 (2; 13) | 0.340, 0.164 (0.195; 0.678) |
| 4 | 4 (2; 6) | 0.452, 0.229 (0.149; 0.730) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briko, A.; Ryazantsev, P.; Gubko, A.; Kapravchuk, V.; Shchukin, S.; Akselrod, B. Method for Bioimpedance Assessment of Superficial Head Tissue Microcirculation. Sensors 2025, 25, 7190. https://doi.org/10.3390/s25237190
Briko A, Ryazantsev P, Gubko A, Kapravchuk V, Shchukin S, Akselrod B. Method for Bioimpedance Assessment of Superficial Head Tissue Microcirculation. Sensors. 2025; 25(23):7190. https://doi.org/10.3390/s25237190
Chicago/Turabian StyleBriko, Andrey, Pavel Ryazantsev, Artem Gubko, Vladislava Kapravchuk, Sergey Shchukin, and Boris Akselrod. 2025. "Method for Bioimpedance Assessment of Superficial Head Tissue Microcirculation" Sensors 25, no. 23: 7190. https://doi.org/10.3390/s25237190
APA StyleBriko, A., Ryazantsev, P., Gubko, A., Kapravchuk, V., Shchukin, S., & Akselrod, B. (2025). Method for Bioimpedance Assessment of Superficial Head Tissue Microcirculation. Sensors, 25(23), 7190. https://doi.org/10.3390/s25237190





