A ZIF-8-Based High-Performance Glucose Electrochemical Detection Platform Constructed Using a Multi-Layer Interface Optimization Strategy
Highlights
- A high-performance glucose biosensor was fabricated using a multilayered MWCNTs/PB/ZIF-8@GOx/CS composite.
- The sensor achieved rapid response, wide linear range, high sensitivity, ultra-low detection limit, and excellent operational stability.
- The developed sensor shows strong potential for real-time and accurate clinical glucose monitoring.
- This fabrication approach can be extended to construct high-performance biosensing platforms for other biomolecules.
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Equipment
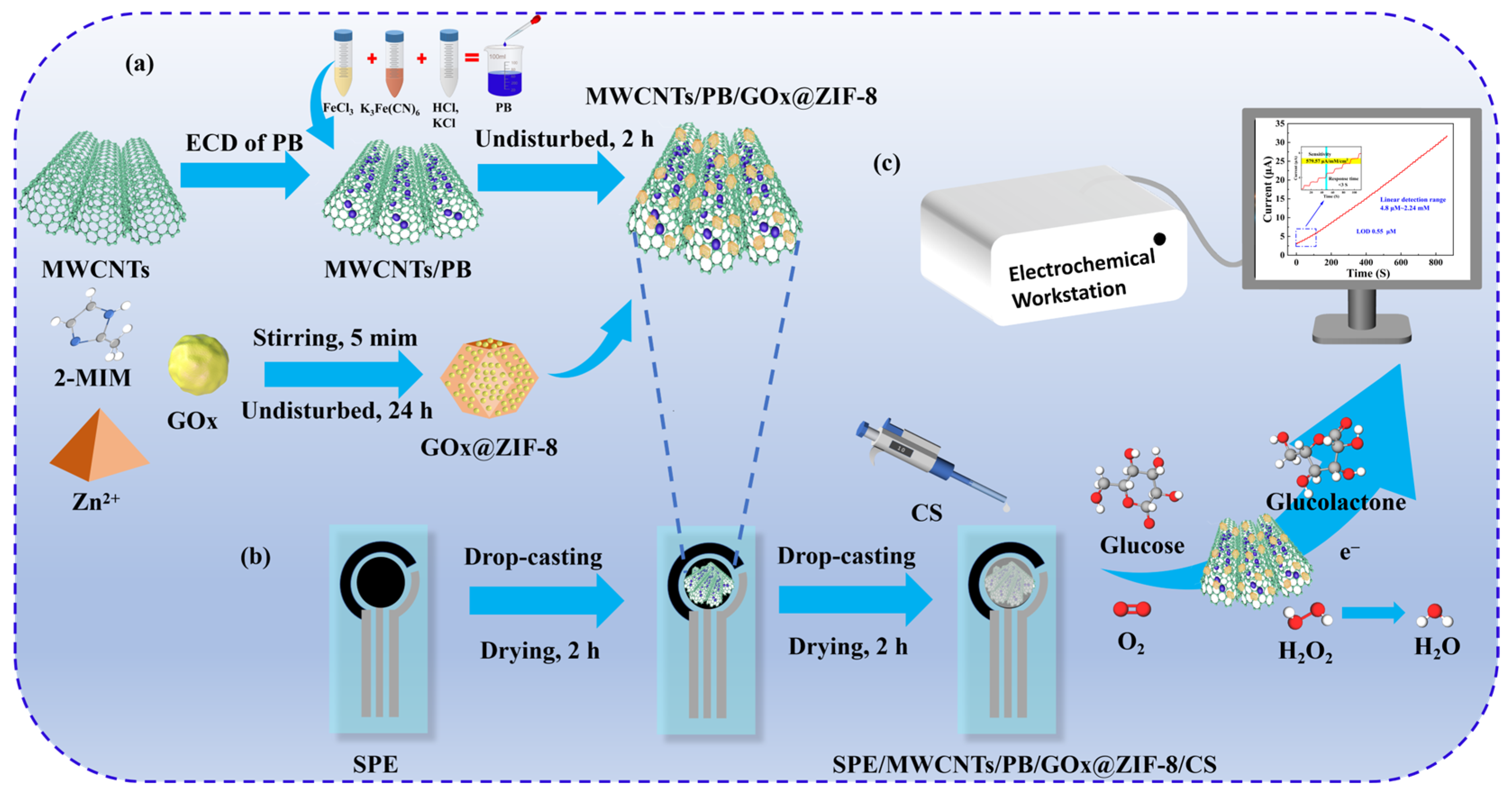
2.3. Preparation of Sensor Electrodes
3. Results and Discussion
3.1. Morphological and Structural Characterization
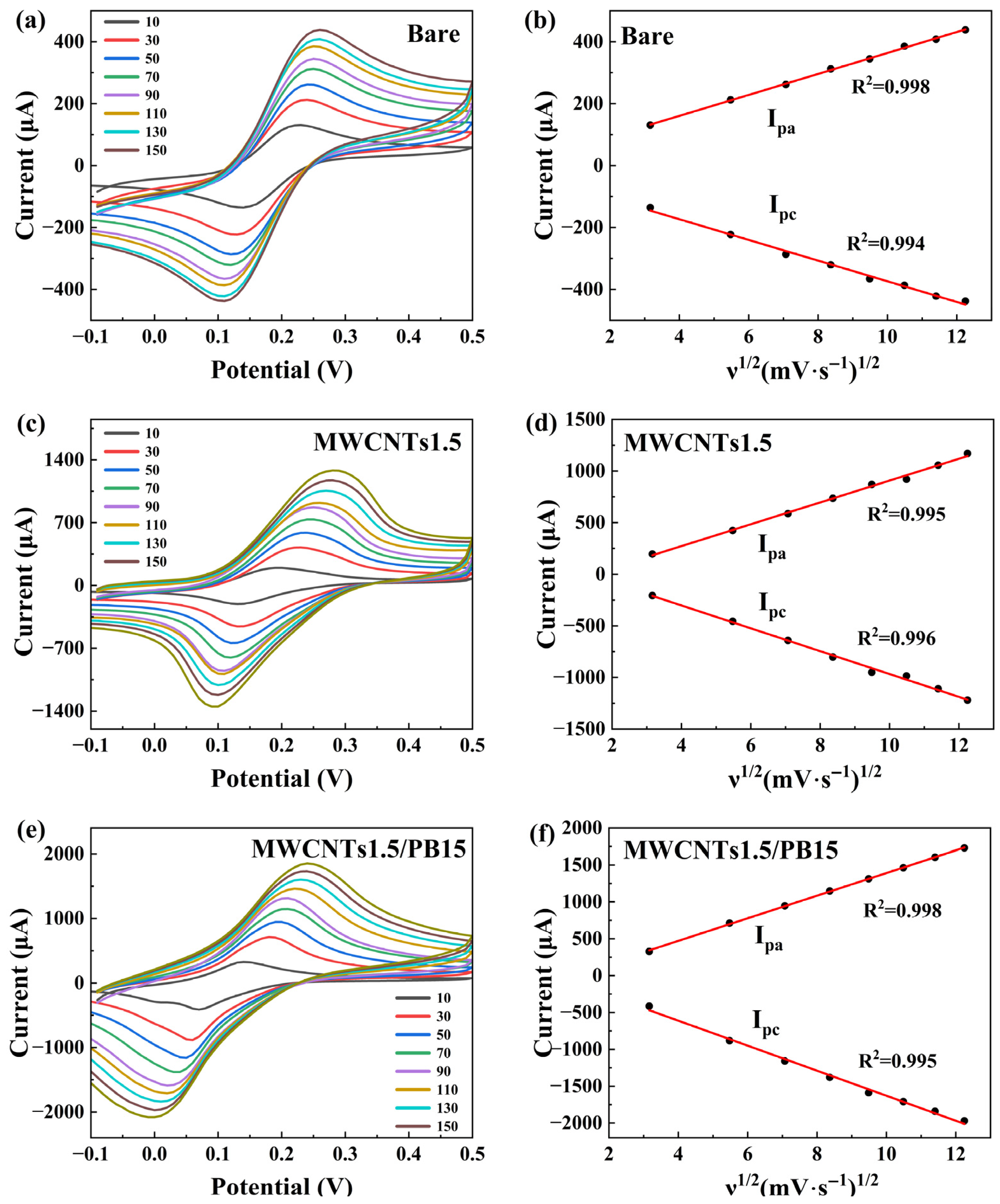
3.2. Interfacial Electron Transfer Properties
3.3. Optimization of Electrode Modification Parameters
3.4. Amperometric Glucose Sensing Performance
3.5. Stability and Selectivity Evaluation
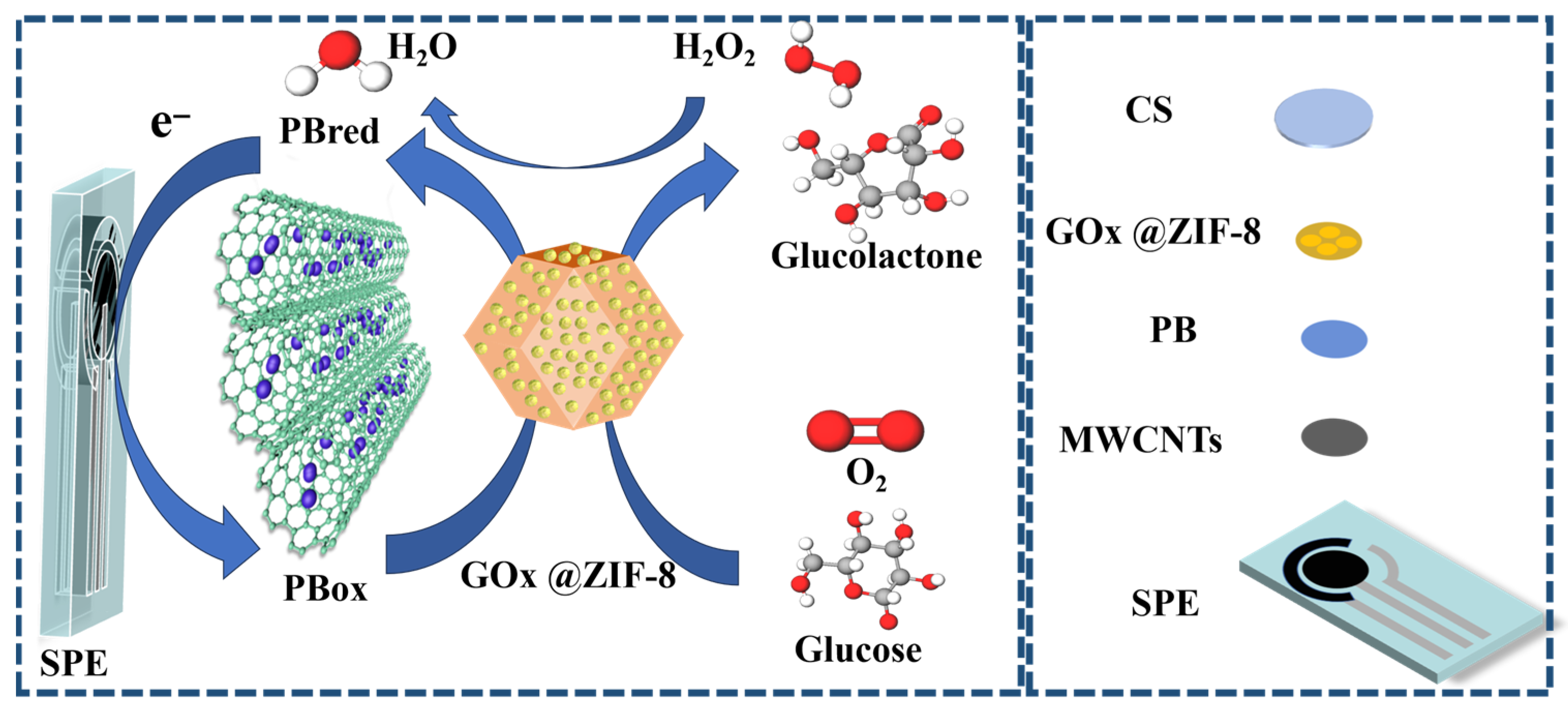
3.6. Comparative Analysis and Catalytic Mechanism
| Electrode | Linear Range (mM) | Sensitivity (μA/mM/cm2) | LOD (μM) | Operational Potential | Ref. |
|---|---|---|---|---|---|
| Cr/Au/PB | 1–10 | 0.23 | 68 | −0.1 v vs. Ag/AgCl | [33] |
| PB-MWCNTs | 0–3.7 | 1.34 | 9.1 | 0.03 v vs. Ag/AgCl | [17] |
| AuNPs/ MWCNTs/CS | 0.001–1 | 27.7 | 0.5 | 0.20 v vs. Ag/AgCl | [19] |
| Ti3C2Tx/ZIF-67 | 0.005–7.5 | 0.379 | 0.66 | 0.35 v vs. Ag/AgCl | [34] |
| Ag@TiO2@ ZIF-67 | 0.048–1 | 788 | 0.99 | 0.4 v vs. Ag/AgCl | [35] |
| AuNPs/ZIF-8-C | 0.01–0.3 | 143 | 4.99 | 0.8 v vs. Ag/AgCl | [36] |
| LIG/CA/PU/ ZIF-8 | 0.62–20 | 1.778 | 160 | 0.45 v vs. Ag/AgCl | [22] |
| Fe3O4/PPy@ ZIF-8 | 0.001–2 | 78 | 0.333 | 0.6 v vs. Ag/AgCl | [37] |
| PMWCNT | 0.2–5.8 | 6.6 | 45 | / | [38] |
| Bi2Ru2O7/MWCNTs | 0.01–8 | 319.88 | 3.5 | / | [39] |
| MWCNTs/PB/ ZIF-8@GOx/CS | 0.0048–2.24 | 579.57 | 0.55 | 0 v vs. Ag/AgCl | This work |

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MWCNTs | Multi-walled carbon nanotubes |
| 2-MIM | 2-Methylimidazole |
| BSA | Bovine serum albumin |
| GOx | glucose oxidase |
| HCl | hydrochloric acid |
| CS | chitosan |
| FeCl3 | Ferric chloride |
| NaCl | sodium chloride |
| KCl | potassium chloride |
| NaH2PO4 | sodium dihydrogen phosphate |
| Na2HPO4 | disodium hydrogen phosphate |
| K3[Fe(CN)6] | potassium ferricyanide |
| UA | uric acid |
| LA | lactic acid |
| AA | ascorbic acid |
| SPCEs | Screen-printed electrodes |
| SEM | scanning electron microscope |
| FT-IR | Fourier transform infrared spectrometer |
| XPS | X-ray photoelectron spectrometer |
| XRD | X-ray diffractometer |
| MOFs | Metal–organic frameworks |
| ZIF-8 | Zeolite imidazolate framework-8 |
| HRP | horseradish peroxidase |
| PBS | phosphate-buffered saline |
Appendix A



References
- Sims, E.K.; Carr, A.L.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 Years of Insulin: Celebrating the Past, Present and Future of Diabetes Therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and Implantable Devices for Drug Delivery: Applications and Challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef]
- Saha, T.; Khan, M.I.; Sandhu, S.S.; Yin, L.; Earney, S.; Zhang, C.; Wang, J. A Passive Perspiration-Inspired Wearable Platform for Continuous Glucose Monitoring. Adv. Sci. 2024, 11, 2405518. [Google Scholar] [CrossRef]
- Kong, X.; Kang, S.; Wang, T.; Liu, B.; Wu, Y.; Lv, B.; Wu, Y. Global, Regional, and National Burden and Trends of Type 2 Diabetes Mellitus among Women of Childbearing Age from 1990 to 2021, with Projections to 2030: A Systematic Analysis for the Global Burden of Disease Study 2021. Endocrine 2025, 90, 122–132. [Google Scholar] [CrossRef]
- Jiao, Y.; Lin, R.; Hua, X.; Churilov, L.; Gaca, M.J.; James, S.; Ekinci, E.I. A Systematic Review: Cost-Effectiveness of Continuous Glucose Monitoring Compared to Self-Monitoring of Blood Glucose in Type 1 Diabetes. Endocrinol. Diabetes Metab. 2022, 5, e369. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose Biosensors in Clinical Practice: Principles, Limits and Perspectives of Currently Used Devices. Theranostics 2022, 12, 493–510. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Dey, K.; Santra, T.S.; Tseng, F.G. Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Appl. Sci. 2025, 15, 2523. [Google Scholar] [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Aktar, M.N.; Preda, V.; Nasiri, N. Advances in Electrochemical Sensors for Real-Time Glucose Monitoring. Sens. Diagn. 2024, 3, 893–913. [Google Scholar] [CrossRef]
- Wang, T.T.; Huang, X.F.; Huang, H.; Luo, P.; Qing, L.S. Nanomaterial-Based Optical and Electrochemical Biosensors for Urine Glucose Detection: A Comprehensive Review. Adv. Sens. Energy Mater. 2022, 1, 100016. [Google Scholar] [CrossRef]
- Hussein, B.A.; Tsegaye, A.A.; Shifera, G.; Taddesse, A.M. A Sensitive Non-Enzymatic Electrochemical Glucose Sensor Based on a ZnO/Co3O4/Reduced Graphene Oxide Nanocomposite. Sens. Diagn. 2023, 2, 347–360. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tai, N.H. Carbon Nanomaterials and Their Composites for Electrochemical Glucose Biosensors: A Review on Fabrication and Sensing Properties. J. Taiwan Inst. Chem. Eng. 2024, 154, 104957. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Liang, J.; Zhang, L.; Zhang, J. Evaluation of a High Sensitivity Glucose Biosensor Based on Carbon Nanotubes and Gold Nanoparticles. IEEE Sens. 2024, 24, 36316–36323. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, T.; Wang, X.; Zhang, T.; Zhang, R.; Xu, Z.; Shi, F. Nanoparticles based on Prussian Blue for biosensor applications: A review. ACS Appl. Nano Mater. 2023, 6, 22568–22593. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Fan, Z.; Sun, Y.; Sun, Y.; Yang, Y.; Zhu, Z. A Dual-Function Wearable Electrochemical Sensor for Uric Acid and Glucose Sensing in Sweat. Biosensors 2023, 13, 105. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Du, G.; Lu, J.; Wang, Q.; Wu, K.; Pang, H. Spiral-Concave Prussian Blue Crystals with Rich Steps: Growth Mechanism and Coordination Regulation. Angew. Chem. Int. Ed. 2025, 64, e202414650. [Google Scholar] [CrossRef]
- Kangkamano, T.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan Cryogel with Embedded Gold Nanoparticles Decorated Multiwalled Carbon Nanotubes Modified Electrode for Highly Sensitive Flow-Based Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2017, 246, 854–863. [Google Scholar] [CrossRef]
- Kuang, G.; Wang, Z.; Bilal, M.; Wang, Z.; Feng, Y.; Du, Y.; Cui, J. Metal–Organic Frameworks: A Potential Platform from Enzyme Immobilization to Mimetic Enzyme. Aggregate 2025, 6, e724. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Wu, J.; Peng, Y.; Pang, H. Pyridine-Regulated Lamellar Nickel-Based Metal–Organic Framework (Ni-MOF) for Nonenzymatic Electrochemical Glucose Sensor. Adv. Sci. 2023, 10, 2304102. [Google Scholar]
- Kuang, X.; Zhao, G.; Guo, J.; Tang, Q.; Yu, J.; Wang, F. Development of a ZIF-8/LIG-Based Electrochemical Biosensor for Accurate and Reliable Glucose Monitoring in Blood. Microchim. Acta 2025, 192, 436. [Google Scholar]
- Weng, Y.; Chen, R.; Hui, Y.; Chen, D.; Zhao, C.X. Boosting Enzyme Activity in Enzyme Metal–Organic Framework Composites. Chem. Bio Eng. 2024, 1, 99–112. [Google Scholar] [CrossRef]
- Rogacka, J.; Labus, K. Metal–Organic Frameworks as Highly Effective Platforms for Enzyme Immobilization—Current Developments and Future Perspectives. Braz. J. Chem. Eng. 2024, 42, 1273–1301. [Google Scholar] [CrossRef]
- Liang, W.; Zheng, S.; Shu, Y.; Huang, J. Machine Learning Optimizing Enzyme/ZIF Biocomposites for Enhanced Encapsulation Efficiency and Bioactivity. JACS Au 2024, 4, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, W.; Wang, Y.; Su, R.; He, Z. A Facile Strategy for Enzyme Immobilization with Highly Stable Hierarchically Porous Metal–Organic Frameworks. Nanoscale 2017, 9, 17561–17570. [Google Scholar] [CrossRef] [PubMed]
- Man, T.; Xu, C.; Liu, X.Y.; Li, D.; Tsung, C.K.; Pei, H.; Li, L. Hierarchically Encapsulating Enzymes with Multi-Shelled Metal–Organic Frameworks for Tandem Biocatalytic Reactions. Nat. Commun. 2022, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fang, Z.; Kan, X. ZIF-8 Encapsulated-Enzymes Integrated Nanozyme Cascade Biocatalysis Platform for the Colorimetric Sensing of Glucose and Lactose in Milk. Food Chem. 2024, 438, 138025. [Google Scholar] [CrossRef]
- Yan, X.; Chai, J.; Yuan, L.; Yin, H. Construction of Pt-GOx@H-ZIF-8 Core–Shell Structure for the Detection of Glutathione. Microchem. J. 2024, 200, 110384. [Google Scholar] [CrossRef]
- Lin, M.; Wang, Q.; Dai, Y.; Chen, J.; Lin, Y. Enzyme-Encapsulated Metal–Organic Framework ZIF-8-Mediated Biosensor for Ultrasensitive Detection of Urinary Prostatic Exosomal Protein Using a Glucose Meter. RSC Adv. 2024, 14, 34848–34854. [Google Scholar] [CrossRef]
- Su, L.; Wu, H.; Zhou, S.; Qian, R.; Cui, C.; Zhang, S.; Pang, H. Narrowing the Kinetic Gap Between Alkaline and Acidic Hydrogen Oxidation Reactions through Intermediate Behaviors Regulated on D–p Hybridized Pd-Based Catalysts. Adv. Sci. 2025, e13616. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Shi, F.; Zhang, R.; Wang, X.; Sun, X.; Li, Y.; Wei, Q. Long-Lasting Chemiluminescence Bioassays for Glucose Enabled by a MOFs-in-Hydrogel Hybrid Platform. Sens. Diagn. 2022, 1, 1044–1051. [Google Scholar] [CrossRef]
- Nguyen, T.N.H.; Jin, X.; Nolan, J.K.; Xu, J.; Le, K.V.H.; Lam, S.; Lee, H. Printable Nonenzymatic Glucose Biosensors Using Carbon Nanotube–PtNP Nanocomposites Modified with AuRu for Improved Selectivity. ACS Biomater. Sci. Eng. 2020, 6, 5315–5325. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cao, K.; Yao, Y.; Zhao, J.; Chai, C.; Dai, P. A Novel Electrochemical Sensor for Glucose Detection Based on a Ti3C2Tx/ZIF-67 Nanocomposite. RSC Adv. 2022, 12, 20138–20146. [Google Scholar] [CrossRef]
- Arif, D.; Hussain, Z.; Sohail, M.; Liaqat, M.A.; Kha, M.A.; Noor, T. A Non-Enzymatic Electrochemical Sensor for Glucose Detection Based on Ag@TiO2@Metal–Organic Framework (ZIF-67) Nanocomposite. Front. Chem. 2020, 8, 573510. [Google Scholar] [CrossRef]
- Yang, X.; Wu, F.; Huang, H.; Zheng, G.; Zhang, H.; Cai, W.; Kong, Y. Au Nanoparticles Anchored Carbonized ZIF-8 for Enabling Real-Time and Noninvasive Glucose Monitoring in Sweat. Biosens. Bioelectron. 2025, 272, 117138. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, D.; Wang, Y.; Zhang, S.; Li, S. Preparation of Magnetic Fe3O4/PPy@ZIF-8 Nanocomposite for Glucose Oxidase Immobilization and Used as Glucose Electrochemical Biosensor. J. Electroanal. Chem. 2018, 822, 50–56. [Google Scholar] [CrossRef]
- Dinesh, B.; Shalini Devi, K.S.; Krishnan, U.M. Achieving a Stable High Surface Excess of Glucose Oxidase on Pristine Multiwalled Carbon Nanotubes for Glucose Quantification. ACS Appl. Bio Mater. 2019, 2, 1740–1750. [Google Scholar] [CrossRef]
- Isailović, J.; Dapčević, A.; Žunić, M.; Finšgar, M.; Vidović, K.; Tasić, N.; Hočevar, S.B. Study of a Sensitive and Selective Electrochemical Biosensor for Glucose Based on Bi2Ru2O7 Pyrochlore Clusters Combined with MWCNTs. Chemosensors 2025, 13, 109. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Qi, P.; Liu, L.; Chen, Y.; Tong, J. A ZIF-8-Based High-Performance Glucose Electrochemical Detection Platform Constructed Using a Multi-Layer Interface Optimization Strategy. Sensors 2025, 25, 7064. https://doi.org/10.3390/s25227064
Hu C, Qi P, Liu L, Chen Y, Tong J. A ZIF-8-Based High-Performance Glucose Electrochemical Detection Platform Constructed Using a Multi-Layer Interface Optimization Strategy. Sensors. 2025; 25(22):7064. https://doi.org/10.3390/s25227064
Chicago/Turabian StyleHu, Canjie, Pengjia Qi, Lichao Liu, Yang Chen, and Jijun Tong. 2025. "A ZIF-8-Based High-Performance Glucose Electrochemical Detection Platform Constructed Using a Multi-Layer Interface Optimization Strategy" Sensors 25, no. 22: 7064. https://doi.org/10.3390/s25227064
APA StyleHu, C., Qi, P., Liu, L., Chen, Y., & Tong, J. (2025). A ZIF-8-Based High-Performance Glucose Electrochemical Detection Platform Constructed Using a Multi-Layer Interface Optimization Strategy. Sensors, 25(22), 7064. https://doi.org/10.3390/s25227064






