Ratio Fluorescence Determination of Tetracycline with Europium(III)-Doped Boron Nitride
Abstract
1. Introduction
2. Experimental Section
2.1. Chemical and Materials
2.2. Characterization
2.3. Preparation of BN-Eu
2.4. Ratiometric Fluorescence Detection of Tetracycline (TC)
2.5. Anti-Interference Performance of BN-Eu
2.6. Sample Treatment and Detection
2.7. Visual Detection for TC
3. Results and Discussion
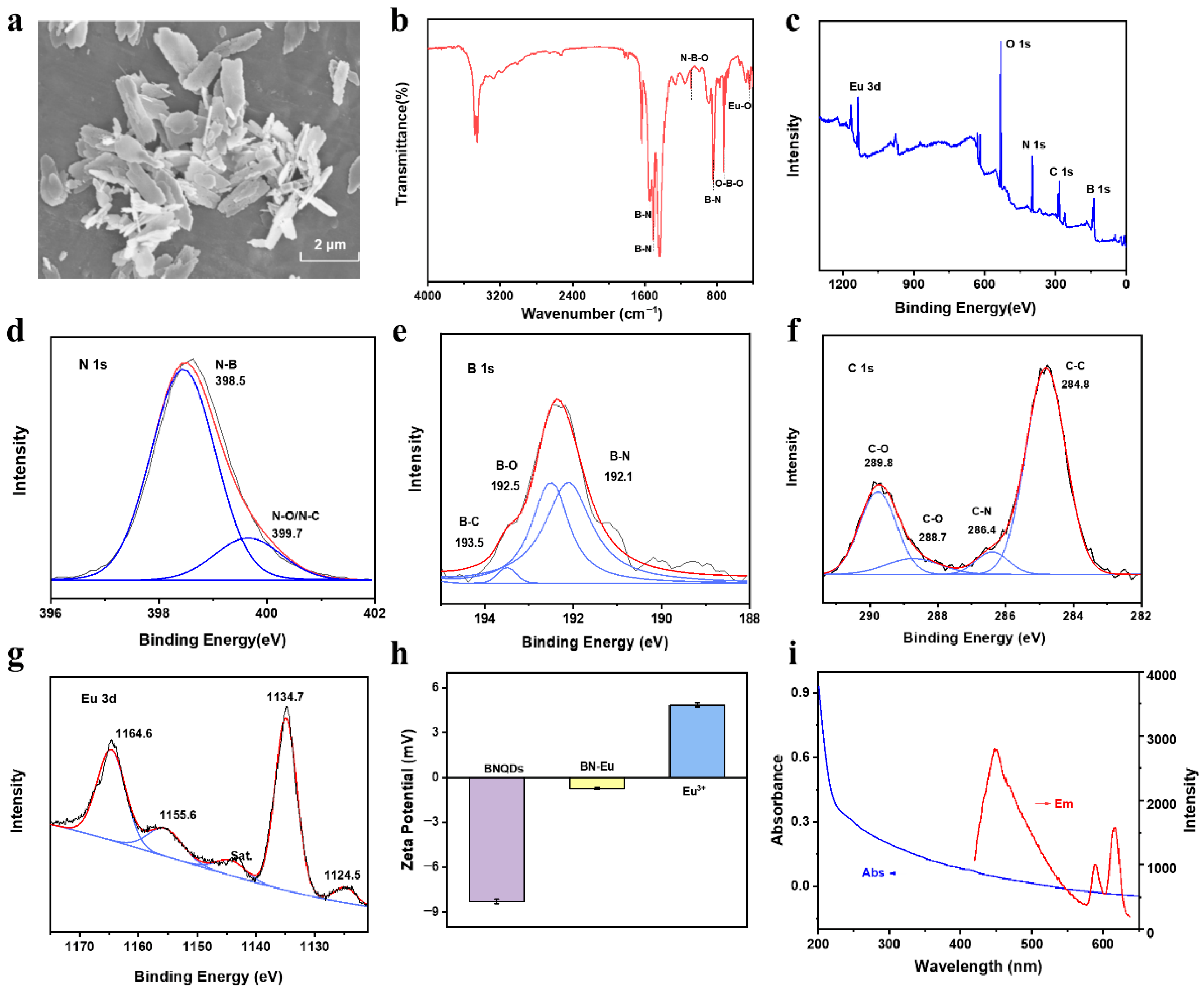
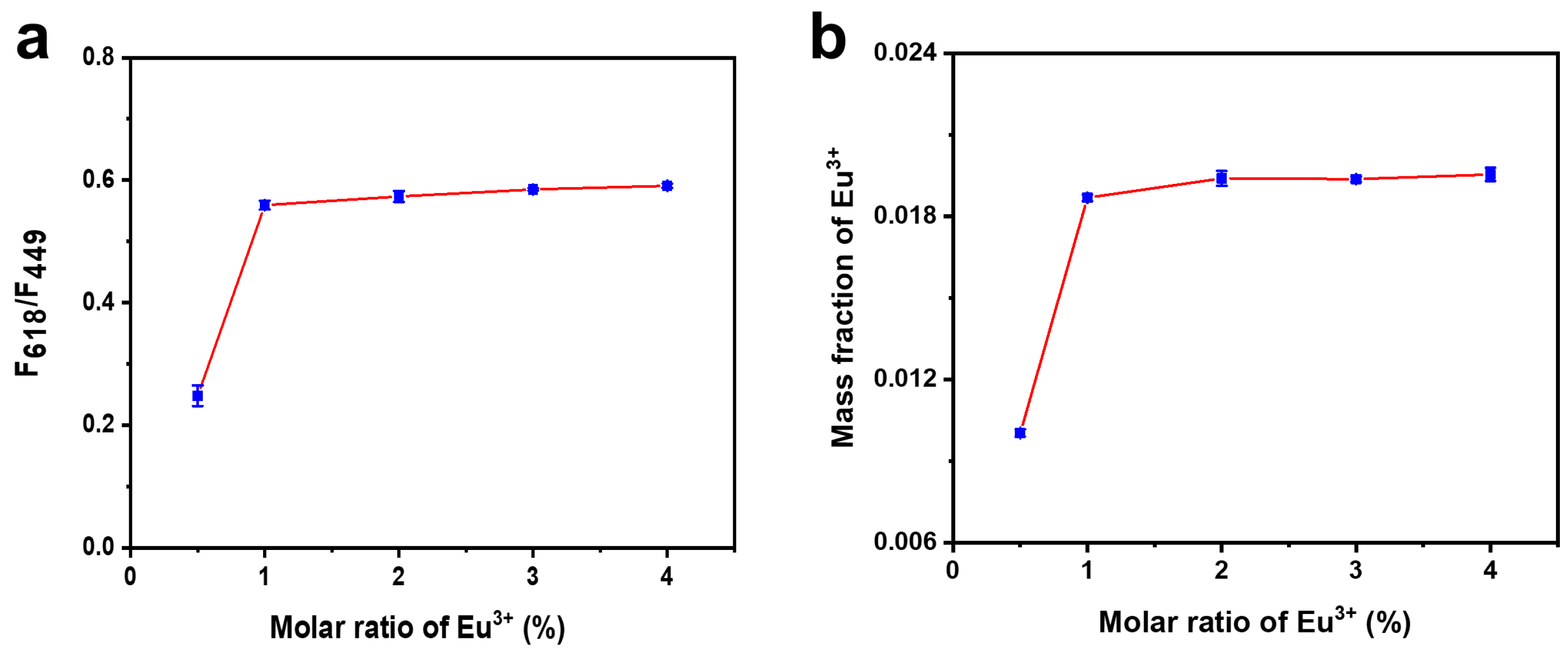
3.1. Characterization of BN-Eu
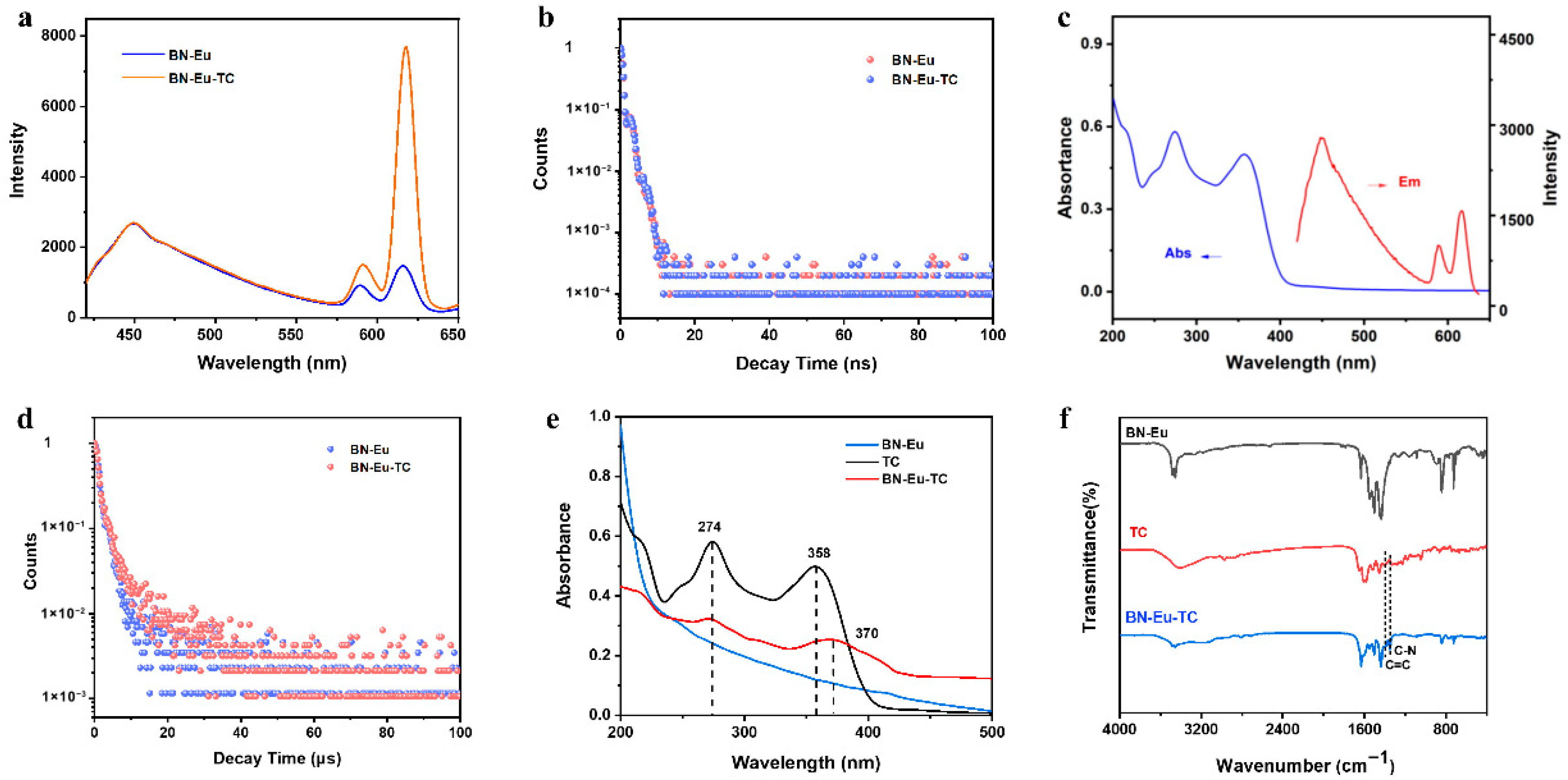
3.2. Sensing Mechanism on Fluorescent Response of BN-Eu to TC
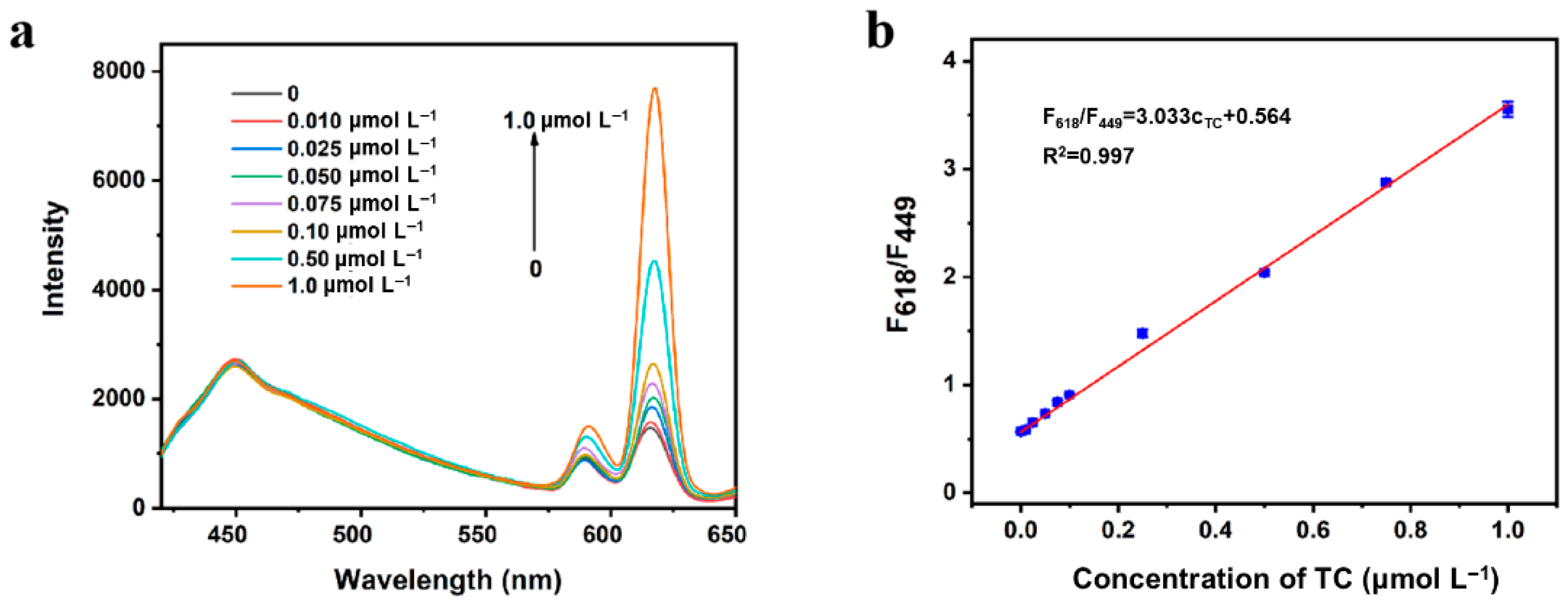
3.3. The Sensing Performance of TC Assay
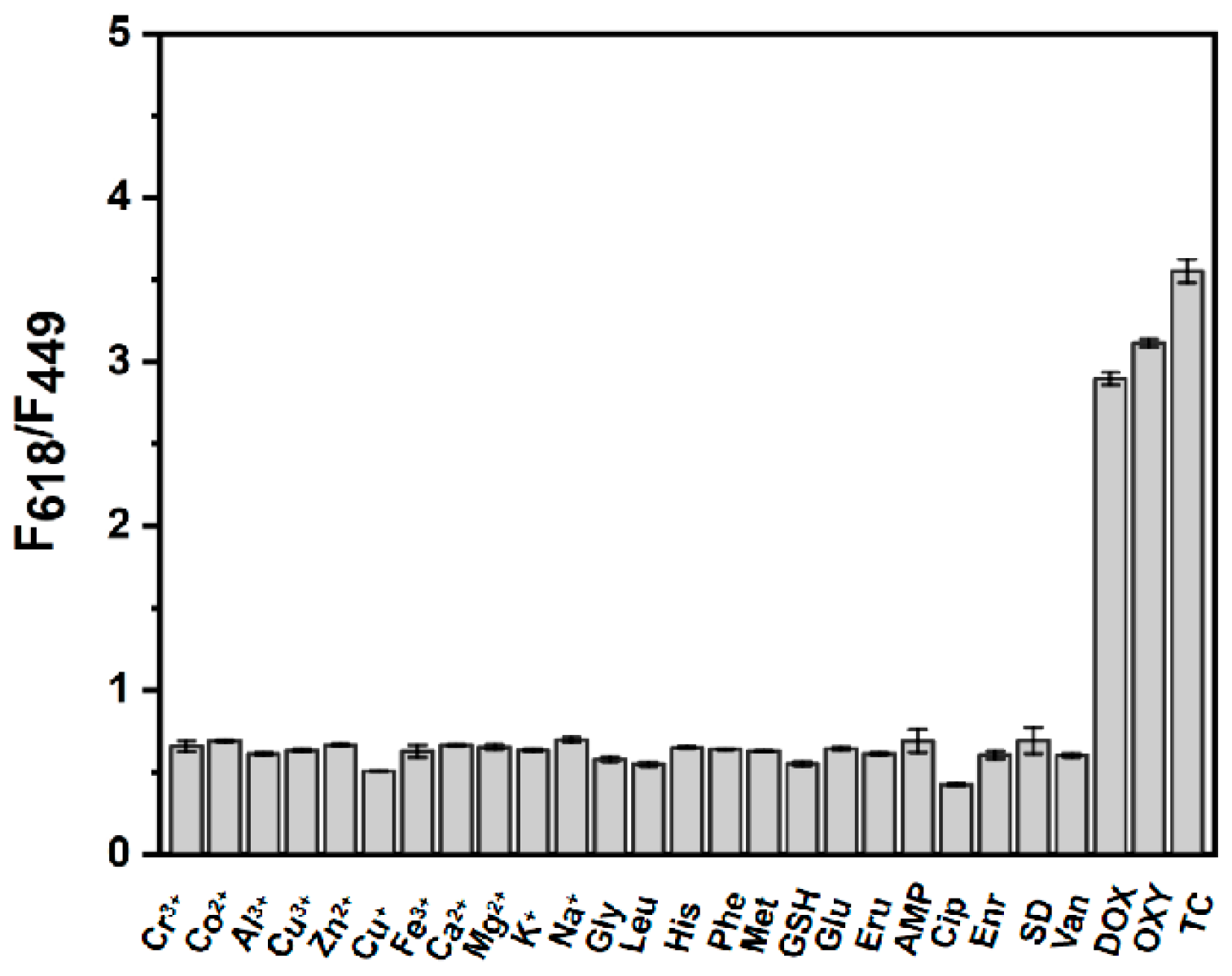
3.4. Selectivity Assay of TC by the BN-Eu Sensing Platform
3.5. Assay of TC in Actual Samples
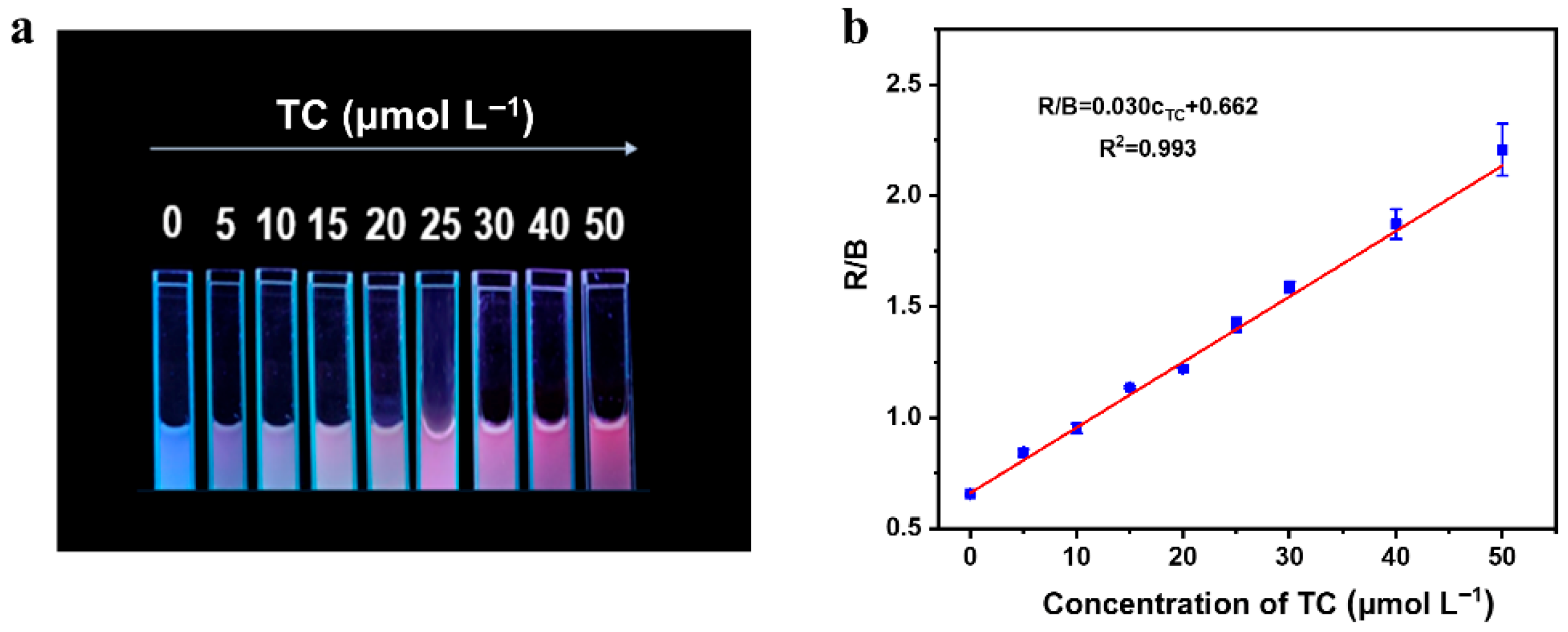
3.6. Visual Detection of TC Based on Smartphone Analysis Device
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Y.J.; Su, M.; Shi, Y.E.; Liu, X.T.; Shen, S.G.; Dong, J.X. A ratiometric fluorescent sensor for tetracyclines detection in meat based on pH-dependence of targets with lanthanum-doped carbon dots as probes. Anal. Bioanal. Chem. 2022, 414, 2597–2606. [Google Scholar] [CrossRef]
- Li, P.; Rao, D.; Wang, Y.; Hu, X. Adsorption characteristics of polythiophene for tetracyclines and determination of tetracyclines in fish and chicken manure by solid phase extraction-HPLC method. Microchem. J. 2022, 173, 106935. [Google Scholar] [CrossRef]
- Deng, B.Y.; Xu, Q.X.; Lu, H.; Ye, L.; Wang, Y. Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electro; phoresis on-line coupled with electrochemiluminescence detection. Food Chem. 2012, 134, 2350–2354. [Google Scholar] [CrossRef]
- Pang, Y.H.; Lv, Z.Y.; Sun, J.C.; Yang, C.; Shen, X.F. Collaborative compounding of metal–organic frameworks for dispersive solid-phase extraction HPLC–MS/MS determination of tetracyclines in honey. Food Chem. 2021, 355, 129411. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Qing, T.; Zhang, P.; Feng, B. Amplified colorimetric detection of tetracycline based on an enzyme-linked aptamer assay with multivalent HRP-mimicking DNAzyme. Analyst 2019, 144, 1948–1954. [Google Scholar] [CrossRef]
- Li, Y.T.; Qu, L.L.; Li, D.W.; Song, Q.X.; Fathi, F.; Long, Y.T. Rapid and sensitive in-situ detection of polar antibiotics in water using a disposable Ag–graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2013, 43, 94–100. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, Q.Z.; Wang, H.H.; Zhangsun, H.; Sun, X.Y.; Bu, T.; Liu, Y.N.; Wang, W.Z.; Xu, Z.H. Europium-based metal-organic framework containing characteristic metal chains: A novel turn-on fluorescence sensor for simultaneous high-performance detection and removal of tetracycline. Sens. Actuators B Chem. 2021, 334, 129610. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Zhang, C.; Yi, H.; Li, B.; Deng, R.; Liu, S.; et al. Recent advances in sensors for tetracycline antibiotics and their applications. Trends Anal. Chem. 2018, 109, 260–274. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Yang, G.; Zhang, S.; Yang, L.; Dai, W.; Wang, H. Zeolitic imidazolate framework-8 for ratiometric fluorescence sensing tetracyclines in environmental water based on AIE effects. Anal. Chim. Acta 2022, 1199, 339576. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.S.; Sher Mohammed, N.M.; Omer, K.M. Ultra-small highly fluorescent zinc-based metal organic framework nanodots for ratiometric visual sensing of tetracycline based on aggregation induced emission. Talanta 2023, 254, 124178. [Google Scholar] [CrossRef]
- Sun, X.; Xin, X.; He, W.; Cao, X.; Shen, J. Tandem Förster resonance energy transfer induced visual ratiometric fluorescence sensing of tetracyclines based on zeolitic imidazolate framework-8 incorporated with carbon dots and safranine T. Analyst 2022, 147, 1152–1158. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, C.; Yan, X.; Cheng, S.; Zhang, Y. The synthesis of N-doped carbon dots for visual differentiating and detection of tetracyclines. Luminescence 2023, 38, 188–195. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Z.; Qian, J.; Zhou, H.; Zhang, K. Copper doped zinc sulfide quantum dots as ratiometric fluorescent probes for rapid and specific detection of tetracycline residues in milk. Anal. Chim. Acta 2022, 1216, 339991. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J. Zhuang. Y.; Huang. S.; Chen. J.; Han. Z. Dual-mode turn-on ratiometric fluorescence sensor based on carbon dots and CuInS2/ZnS quantum dots for detection of chlorotetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120851. [Google Scholar]
- Miao, J.; Ji, W.; Yu, J.; Cheng, J.; Huang, Y.; Arabi, M.; Zhou, N.; Li, B.; Zhang, Z.; Chen, L.; et al. A triple-emission ratiometric fluorescence sensor based on carbon dots-Au nanoclusters nanocomposite for detection of tetracycline. Sens. Actuators B Chem. 2023, 384, 054477. [Google Scholar]
- Han, L.; Fan, Y.Z.; Qing, M.; Liu, S.G.; Yang, Y.Z.; Li, N.B.; Luo, H.Q. Smartphones and Test Paper-Assisted Ratiometric Fluorescent Sensors for Semi-Quantitative and Visual Assay of Tetracycline Based on the Target-Induced Synergistic Effect of Antenna Effect and Inner Filter Effect. ACS Appl. Mater. Interfaces 2020, 12, 47099–47107. [Google Scholar] [CrossRef]
- Li, X.; Fan, K.; Yang, R.; Du, X.; Qu, B.; Miao, X.; Lu, L. A long lifetime ratiometrically luminescent tetracycline nanoprobe based on Ir(III) complex-doped and Eu3+-functionalized silicon nanoparticles. J. Hazard. Mater. 2020, 386, 121929. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, X.; Jia, L.; Zhou, T.; Ma, T.; Xu, Z.; Cao, J.; Ge, Z.; Bi, N.; Zhu, T.; et al. A novel visual ratiometric fluorescent sensing platform for highly-sensitive visual detection of tetracyclines by a lanthanide- functionalized palygorskite nanomaterial. J. Hazard. Mater. 2018, 342, 158–165. [Google Scholar] [CrossRef]
- Kainthola, A.; Bijalwan, K.; Negi, S.; Sharma, H.; Dwivedi, H. Hydrothermal synthesis of highly stable boron nitride nanoparticles. Mater. Today Proc. 2020, 28, 138–140. [Google Scholar] [CrossRef]
- Hirsch, L.M.; Van Geel, T.F.; Winefordner, J.D.; Kelly, R.N.; Schulman, S.G. Characteristics of the binding of europium(III) to tetracycline. Anal. Chim. Acta 1985, 166, 207–219. [Google Scholar] [CrossRef]
- Zhao, C.X.; Zhang, X.P.; Shu, Y.; Wang, J.H. Europium−pyridinedicarboxylate−adenine light-up fluorescence nanoprobes for selective detection of phosphate in biological fluids. ACS Appl. Mater. Interfaces 2020, 12, 22593–22600. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-J.; Oh, S.-J.; Suga, S.; Suzuki, T.; Kasuya, T. Electronic structure study of Eu intermetallic compounds by photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1996, 77, 173–181. [Google Scholar] [CrossRef]
- Wu, W.J.; Zhao, Q.; Zhou, R.; Liang, Y.C.; Zhao, W.B.; Shan, C.X. Ratiometric fluorescence sensor based on europium-grafted ZnO quantum dots for visual and colorimetric detection of tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119901. [Google Scholar]
- Zhang, L.; Wang, Y.; Jia, L.; Bi, N.; Bie, H.; Chen, X.; Zhang, C.; Xu, J. Ultrasensitive and visual detection of tetracycline based on dual-recognition units constructed multicolor fluorescent nano-probe. J. Hazard. Mater. 2021, 409, 124935. [Google Scholar] [CrossRef]
- Yang, K.R.; Jia, P.; Hou, J.J.; Bu, T.; Sun, X.; Liu, Y.; Wang, L. Innovative dual-emitting ratiometric fluorescence sensor for tetracyclines detection based on boron nitride quantum dots and europium ions. ACS Sustain. Chem. Eng. 2020, 8, 17185–17193. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Wei, Y.L.; Chen, H.H.; Wu, Z.; Ye, Y.; Han, D. Liposome-encapsulated aggregation-induced emission fluorogen assisted with portable smartphone for dynamically on-site imaging of residual tetracycline. Sens. Actuators B Chem. 2022, 350, 130871. [Google Scholar] [CrossRef]
- Yao, R.H.; Li, Z.J.; Liu, G.; Fan, C.B.; Pu, S.Z. Luminol-Eu-based ratiometric fluorescence probe for highly selective and visual determination of tetracycline. Talanta 2021, 234, 122612. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Sun, Q.X.; Liu, X.; Li, H.Y.; Wang, J.H.; Chen, M.L. Ratiometric fluorescence detection of tetracycline for tetracycline adjuvant screening in bacteria. Sens. Actuators B Chem. 2022, 372, 132687. [Google Scholar] [CrossRef]
- Tan, H.L.; Ma, C.J.; Song, Y.H.; Xu, F.G.; Chen, S.H.; Wang, L. Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. Biosens. Bioelectron. 2013, 50, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, J.Y.; Huang, Z.J.; Qian, C.; Tian, Y.H.; Duan, Y.X. An Eu-doped Zr-metal-organic framework for simultaneous detection and removal of antibiotic tetracycline. J. Environ. Chem. Eng. 2021, 9, 106012. [Google Scholar] [CrossRef]
- Xu, J.; Guo, S.L.; Jia, L.; Zhu, T.H.; Chen, X.Z.; Zhao, T.Q. A smartphone-integrated method for visual detection of tetracycline. Chem. Eng. J. 2021, 416, 127741. [Google Scholar] [CrossRef]
- Liu, Y.S.; Guo, H.; Wu, N.; Peng, L.P.; Wang, M.Y.; Tian, J.Y.; Xu, J.X.; Yang, W. Eu3+-Functionalized nanoporous covalent organic frameworks for fluorescence detection and removal of tetracycline. ACS Appl. Nano Mater. 2023, 6, 6627–6636. [Google Scholar] [CrossRef]


| Materials | Mode | Detection | Linear Range (μM) | LOD (nM) | Ref. |
|---|---|---|---|---|---|
| BNQDs-Eu3+ | ratiometric | tetracycline | 0–50 | 19 | [23] |
| Luminol-Eu-CitNa | ratiometric | tetracycline | 0.5–80 | 39 | [25] |
| ZnO/Eu | ratiometric | tetracycline | 0.005–3 | 4 | [21] |
| g-C3N4/Eu3+ | ratiometric | tetracycline | 0.25–80 | 6.5 | [16] |
| CDs-CuInS2/ZnS | off-on | chlortetracycline | 1–50 | 36 | [13] |
| N-CDs | ratiometric | tetracycline | 0.05–324 | 22.7 | [11] |
| Ir(III)@SiNPs-Eu3+ | ratiometric | tetracycline | 0.01–20 | 4.9 | [17] |
| BN-Eu | ratiometric | tetracycline | 0.01–1.00 | 4.0 | This work |
| Detection Method | Spiked (μM) | TC Concentration (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Ratiometric fluorescence | — | 0.076 ± 0.005 | — | — |
| 0.25 | 0.321 ± 0.019 | 98.0 | 5.9 | |
| 0.50 | 0.575 ± 0.024 | 99.8 | 4.2 | |
| 0.75 | 0.750 ± 0.016 | 89.9 | 2.1 | |
| HPLC | — | 0.085 ± 0.008 | — | — |
| 0.25 | 0.338 ± 0.014 | 101.2 | 4.1 | |
| 0.50 | 0.608 ± 0.020 | 104.6 | 3.3 | |
| 0.75 | 0.828 ± 0.027 | 99.1 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-Q.; Sun, X.-Y.; Liu, K.-X.; Chen, M.-L. Ratio Fluorescence Determination of Tetracycline with Europium(III)-Doped Boron Nitride. Sensors 2025, 25, 7056. https://doi.org/10.3390/s25227056
Zhang S-Q, Sun X-Y, Liu K-X, Chen M-L. Ratio Fluorescence Determination of Tetracycline with Europium(III)-Doped Boron Nitride. Sensors. 2025; 25(22):7056. https://doi.org/10.3390/s25227056
Chicago/Turabian StyleZhang, Shang-Qing, Xiao-Yan Sun, Kai-Xin Liu, and Ming-Li Chen. 2025. "Ratio Fluorescence Determination of Tetracycline with Europium(III)-Doped Boron Nitride" Sensors 25, no. 22: 7056. https://doi.org/10.3390/s25227056
APA StyleZhang, S.-Q., Sun, X.-Y., Liu, K.-X., & Chen, M.-L. (2025). Ratio Fluorescence Determination of Tetracycline with Europium(III)-Doped Boron Nitride. Sensors, 25(22), 7056. https://doi.org/10.3390/s25227056






