Continuous Wireless Vital Sign Sensors for Detecting Severe Deviations at a Transitional Care Facility—An Observational Feasibility Study
Abstract
1. Introduction
2. Methods
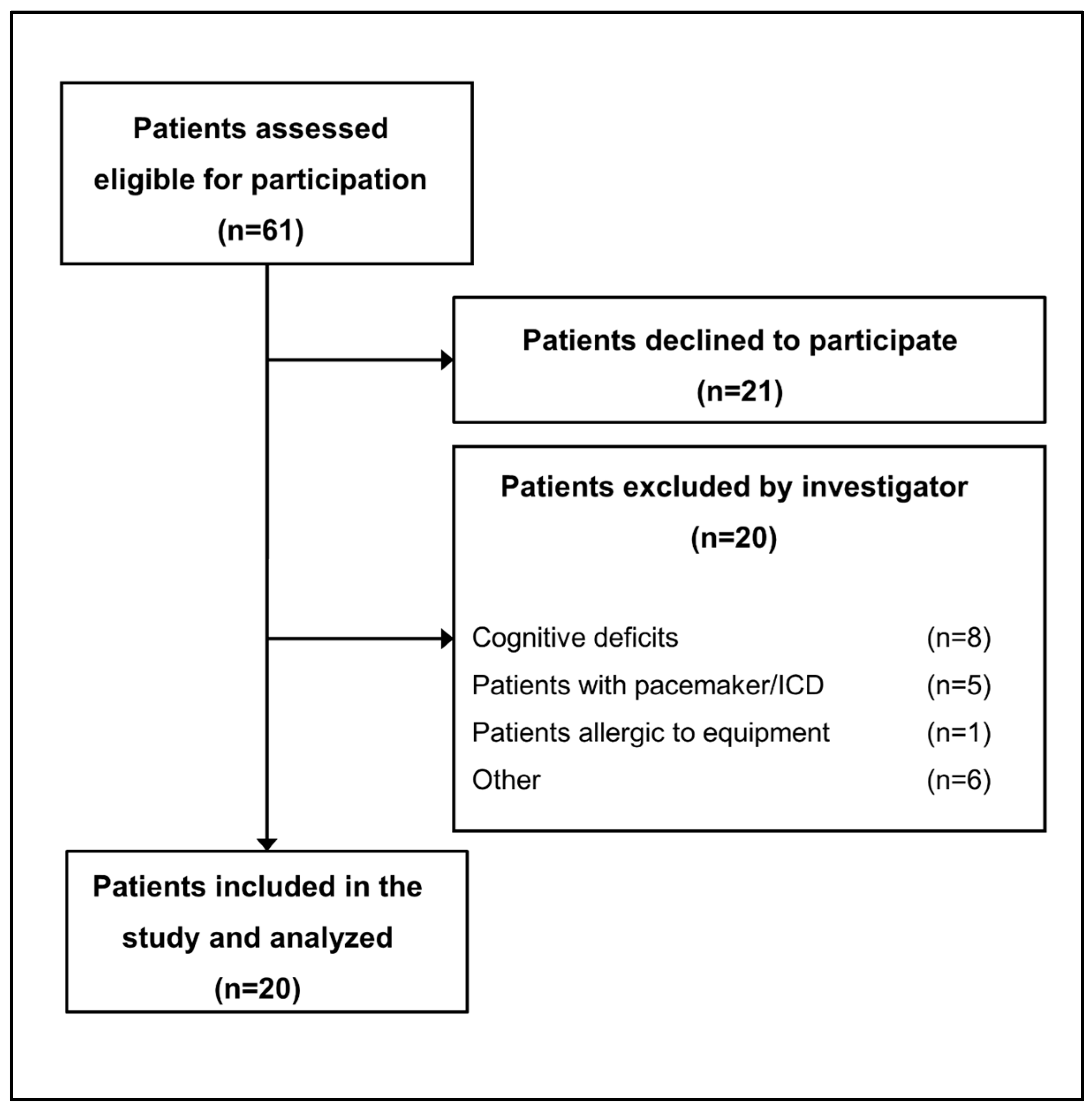
2.1. Study Design and Participants
2.2. Resources and Daily Routine at the Transitional Care Facility
2.3. Description of the Wireless Devices and Abnormal Vital Sign Thresholds
2.4. Abnormal Vital Signs
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Feasibility of Continuous Monitoring
3.3. Abnormal Vital Signs
3.4. Complications and Readmissions
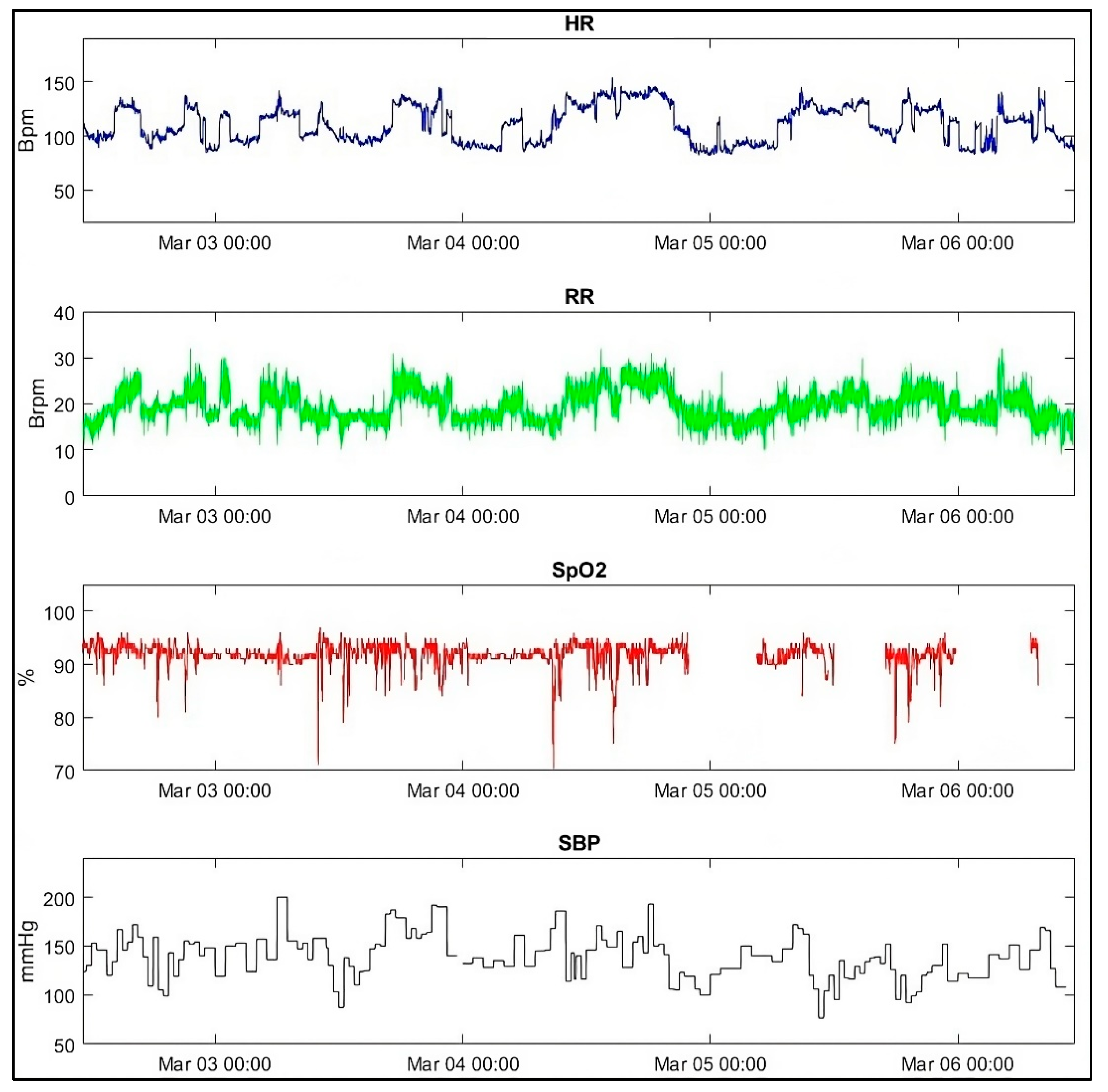
3.5. Patient Case
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists Classification |
| BMI | Body Mass Index |
| BP | Blood pressure |
| Brpm | Breaths per minute |
| EWS | Early warning score |
| HR | Heart rate |
| IQR | Interquartile range |
| mmHg | Millimeter mercury |
| PCI | Percutaneous coronary intervention |
| RR | Respiratory rate |
| SBP | Systolic blood pressure |
| SpO2 | Peripheral oxygen saturation |
References
- Zuckerman, R.B.; Sheingold, S.H.; Orav, E.J.; Ruhter, J.; Epstein, A.M. Readmissions, Observation, and the Hospital Readmissions Reduction Program. New Engl. J. Med. 2016, 374, 1543–1551. [Google Scholar] [CrossRef]
- Glance, L.G.; Kellermann, A.L.; Osler, T.M.; Li, Y.; Mukamel, D.B.; Lustik, S.J.; Eaton, M.P.; Dick, A.W. Hospital Readmission after Noncardiac Surgery: The Role of Major Complications. JAMA Surg. 2014, 149, 439–445. [Google Scholar] [CrossRef]
- Spence, J.; LeManach, Y.; Chan, M.T.; Wang, C.Y.; Sigamani, A.; Xavier, D.; Pearse, R.; Alonso-Coello, P.; Garutti, I.; Srinathan, S.K.; et al. Association between Complications and Death within 30 Days after Noncardiac Surgery. CMAJ 2019, 191, E830–E837. [Google Scholar] [CrossRef]
- Jacobs, B.L.; He, C.; Li, B.Y.; Helfand, A.; Krishnan, N.; Borza, T.; Ghaferi, A.A.; Hollenbeck, B.K.; Helm, J.E.; Lavieri, M.S.; et al. Variation in Readmission Expenditures after High-Risk Surgery. J. Surg. Res. 2017, 213, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kum Ghabowen, I.; Epane, J.P.; Shen, J.J.; Goodman, X.; Ramamonjiarivelo, Z.; Zengul, F.D. Systematic Review and Meta-Analysis of the Financial Impact of 30-Day Readmissions for Selected Medical Conditions: A Focus on Hospital Quality Performance. Healthcare 2024, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Joshi, G.P.; Kehlet, H. Enhanced Recovery Pathways: Looking Into the Future. Anesth. Analg. 2019, 128, 5–7. [Google Scholar] [CrossRef]
- Kehlet, H.; Wilmore, D.W. Evidence-Based Surgical Care and the Evolution of Fast-Track Surgery. Ann. Surg. 2008, 248, 189–198. [Google Scholar] [CrossRef]
- Braet, A.; Weltens, C.; Sermeus, W. Effectiveness of Discharge Interventions from Hospital to Home on Hospital Readmissions: A Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 106–173. [Google Scholar] [CrossRef]
- Varadhan, K.K.; Neal, K.R.; Dejong, C.H.C.; Fearon, K.C.H.; Ljungqvist, O.; Lobo, D.N. The Enhanced Recovery after Surgery (ERAS) Pathway for Patients Undergoing Major Elective Open Colorectal Surgery: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2010, 29, 434–440. [Google Scholar] [CrossRef]
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Illness Severity in the NHS. Updated Report of a Working Party. London: RCP, 2017; Royal College of Physicians. Available online: https://www.rcp.ac.uk/media/a4ibkkbf/news2-final-report_0_0.pdf (accessed on 30 October 2025).
- Weenk, M.; van Goor, H.; Frietman, B.; Engelen, L.J.; van Laarhoven, C.J.; Smit, J.; Bredie, S.J.; van de Belt, T.H. Continuous Monitoring of Vital Signs Using Wearable Devices on the General Ward: Pilot Study. JMIR Mhealth Uhealth 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Elvekjaer, M.; Aasvang, E.K.; Olsen, R.M.; Sørensen, H.B.D.; Porsbjerg, C.M.; Jensen, J.U.; Haahr-Raunkjær, C.; Meyhoff, C.S. Physiological Abnormalities in Patients Admitted with Acute Exacerbation of COPD: An Observational Study with Continuous Monitoring. J. Clin. Monit. Comput. 2020, 34, 1051–1060. [Google Scholar] [CrossRef]
- Weenk, M.; Koeneman, M.; van de Belt, T.H.; Engelen, L.J.L.P.G.; van Goor, H.; Bredie, S.J.H. Wireless and Continuous Monitoring of Vital Signs in Patients at the General Ward. Resuscitation 2019, 136, 47–53. [Google Scholar] [CrossRef]
- Granholm, A.; Pedersen, N.E.; Lippert, A.; Petersen, L.F.; Rasmussen, L.S. Respiratory Rates Measured by a Standardised Clinical Approach, Ward Staff, and a Wireless Device. Acta Anaesthesiol. Scand. 2016, 60, 1444–1452. [Google Scholar] [CrossRef]
- Haahr-Raunkjaer, C.; Mølgaard, J.; Elvekjaer, M.; Rasmussen, S.M.; Achiam, M.P.; Jorgensen, L.N.; Søgaard, M.I.V.; Grønbæk, K.K.; Oxbøll, A.B.; Sørensen, H.B.D.; et al. Continuous Monitoring of Vital Sign Abnormalities; Association to Clinical Complications in 500 Postoperative Patients. Acta Anaesthesiol. Scand. 2022, 66, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Bignami, E.G.; Fornaciari, A.; Fedele, S.; Madeo, M.; Panizzi, M.; Marconi, F.; Cerdelli, E.; Bellini, V. Wearable Devices in Healthcare Beyond the One-Size-Fits All Paradigm. Sensors 2025, 25, 6472. [Google Scholar] [CrossRef]
- Rowland, B.A.; Motamedi, V.; Michard, F.; Saha, A.K.; Khanna, A.K. Impact of Continuous and Wireless Monitoring of Vital Signs on Clinical Outcomes: A Propensity-Matched Observational Study of Surgical Ward Patients. Br. J. Anaesth. 2024, 132, 519–527. [Google Scholar] [CrossRef]
- Mølgaard, J.; Grønbæk, K.K.; Rasmussen, S.S.; Eiberg, J.P.; Jørgensen, L.N.; Achiam, M.P.; Rohrsted, M.; Singh, U.M.; Hoang, T.-H.; Søgaard, M.; et al. Continuous Vital Sign Monitoring at the Surgical Ward for Improved Outcomes After Major Noncardiac Surgery: A Randomized Clinical Trial. Anesth. Analg. 2025, 141, 807–817. [Google Scholar] [CrossRef]
- Elvekjaer, M.; Carlsson, C.J.; Rasmussen, S.M.; Porsbjerg, C.M.; Grønbæk, K.K.; Haahr-Raunkjær, C.; Sørensen, H.B.D.; Aasvang, E.K.; Meyhoff, C.S. Agreement between Wireless and Standard Measurements of Vital Signs in Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Clinical Validation Study. Physiol. Meas. 2021, 42, 055006. [Google Scholar] [CrossRef] [PubMed]
- Haahr-Raunkjaer, C.; Skovbye, M.; Rasmussen, S.M.; Elvekjaer, M.; Sørensen, H.B.D.; Meyhoff, C.S.; Aasvang, E.K. Agreement between Standard and Continuous Wireless Vital Sign Measurements after Major Abdominal Surgery: A Clinical Comparison Study. Physiol. Meas. 2022, 43, 115007. [Google Scholar] [CrossRef]
- Duus, C.L.; Aasvang, E.K.; Olsen, R.M.; Sørensen, H.B.D.; Jørgensen, L.N.; Achiam, M.P.; Meyhoff, C.S. Continuous Vital Sign Monitoring after Major Abdominal Surgery—Quantification of Micro Events. Acta Anaesth. Scand. 2018, 62, 1200–1208. [Google Scholar] [CrossRef]
- Breteler, M.J.M.; KleinJan, E.J.; Dohmen, D.A.J.; Leenen, L.P.H.; van Hillegersberg, R.; Ruurda, J.P.; van Loon, K.; Blokhuis, T.J.; Kalkman, C.J. Vital Signs Monitoring with Wearable Sensors in High-Risk Surgical Patients: A Clinical Validation Study. Anesthesiology 2020, 132, 424–439. [Google Scholar] [CrossRef]
- Sun, Z.; Sessler, D.I.; Dalton, J.E.; Devereaux, P.J.; Shahinyan, A.; Naylor, A.J.; Hutcherson, M.T.; Finnegan, P.S.; Tandon, V.; Darvish-Kazem, S.; et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth. Analg. 2015, 121, 709–715. [Google Scholar] [CrossRef]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Churpek, M.M.; Coca Perraillon, M.; Konetzka, R.T. Understanding Why Patients with COPD Get Readmitted: A Large National Study to Delineate the Medicare Population for the Readmissions Penalty Expansion. Chest 2015, 147, 1219–1226. [Google Scholar] [CrossRef]
- Klinge, M.; Aasbrenn, M.; Öztürk, B.; Christiansen, C.F.; Suetta, C.; Pressel, E.; Nielsen, F.E. Readmission of Older Acutely Admitted Medical Patients after Short-Term Admissions in Denmark: A Nationwide Cohort Study. BMC Geriatr. 2020, 20, 203. [Google Scholar] [CrossRef]
- Legner, V.J.; Massarweh, N.N.; Symons, R.G.; McCormick, W.C.; Flum, D.R. The Significance of Discharge to Skilled Care after Abdominopelvic Surgery in Older Adults. Ann. Surg. 2009, 249, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.V.; Eriksen, V.R.; Rasmussen, S.S.; Meyhoff, C.S.; Aasvang, E.K. Time to Detection of Serious Adverse Events by Continuous Vital Sign Monitoring versus Clinical Practice. Acta Anaesthesiol. Scand. 2025, 69, e14541. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, K.; Mølgaard, J.; Rasmussen, S.M.; Meyhoff, C.S.; Aasvang, E.K. The Effect of Technical Filtering and Clinical Criteria on Alert Rates from Continuous Vital Sign Monitoring in the General Ward. Hosp. Pract. 2023, 51, 295–302. [Google Scholar] [CrossRef]
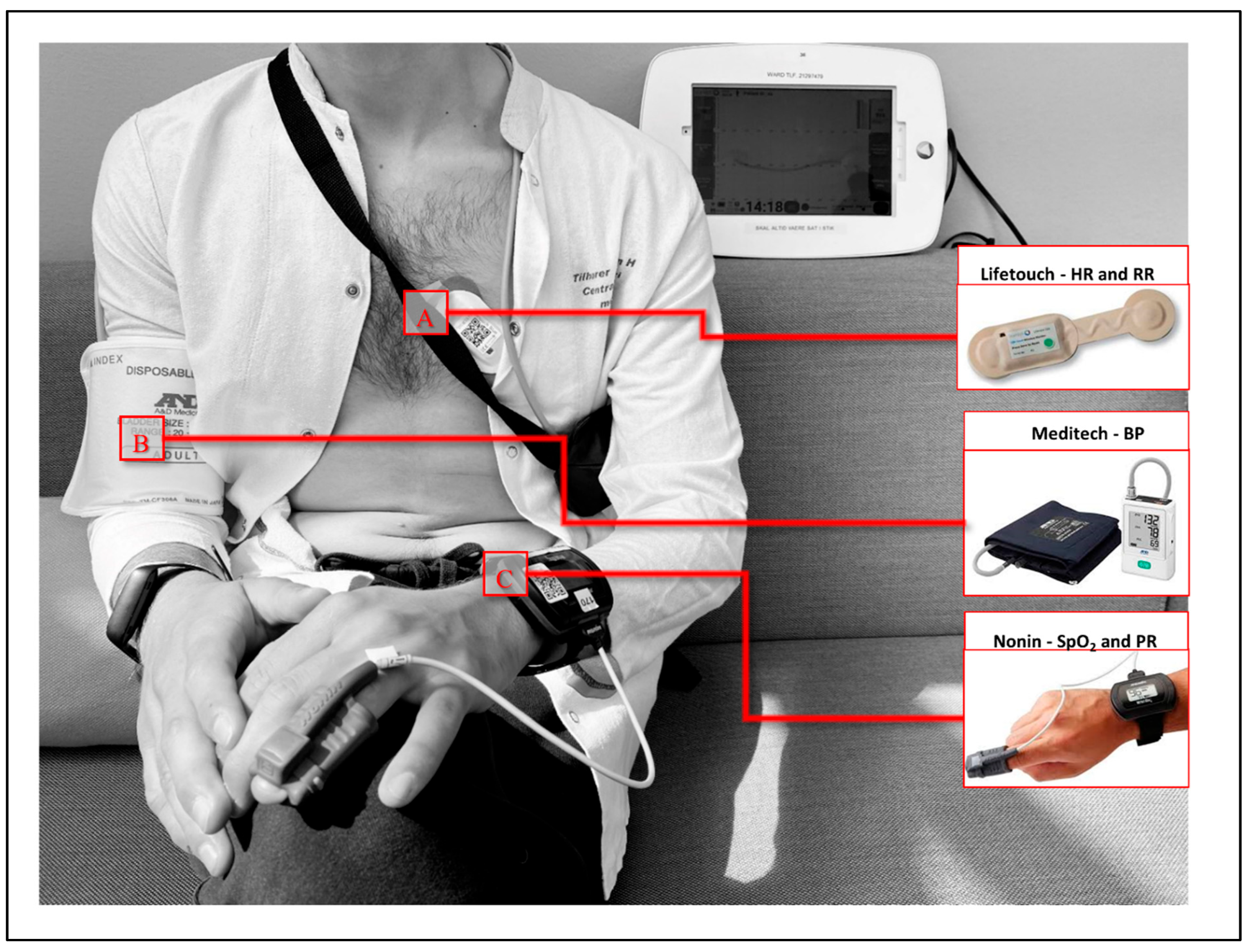
| Parameter | n = 20 |
|---|---|
| Gender, male, female | 8 (40%), 12 (60%), |
| Age, years | 83 [74–89] |
| BMI kg/m2 | 25 [20–29] |
| Smoking history (never/previously/current) | 9/9/2 |
| Excessive alcohol intake | 6 (30%) |
| Reason for hospital admission, surgery/medical | 9 (45%)/11 (55%) |
| Medical history | |
| One comorbidity | 5 (25%) |
| ≥2 comorbidity | 15 (75%) |
| Stroke or transitory ischemic attack | 4 (20%) |
| Epilepsy | 1 (5%) |
| Chronic obstructive pulmonary disease | 5 (25%) |
| Myocardial infarction | 1 (5%) |
| PCI | 1 (5%) |
| Atrial fibrillation | 5 (25%) |
| Hypertensio arterialis | 5 (25%) |
| Congestive heart failure | 4 (20%) |
| Hypercholesterolemia | 2 (10%) |
| Diabetes mellitus | 3 (15%) |
| Chronic kidney disease | 1 (5%) |
| Other disease | 13 (65%) |
| HR, bpm | 77 [69–86] |
| RR, brpm | 16 [14–18] |
| SpO2, % | 97% [96–98] |
| Systolic blood pressure, mmHg | 132 [115–147] |
| Diastolic blood pressure, mmHg | 71 [61–78] |
| Median h [IQR] | % of Theoretical Maximum Monitoring Time (96h) | |
|---|---|---|
| Any device | 80 [50–93] | 83% [52–97%] |
| Lifetouch (HR and RR) | 77 [46–92] | 80% [48–96%] |
| Nonin (SpO2 and PR) | 32 [12–63] | 33% [13–66%] |
| Lifetouch + Nonin (HR, RR, SpO2, and PR) | 20 [8–56] | 21% [21–58%] |
| BP (planned 156 times per 96 h) | 41 times [14–99] | 26% [9–63%] |
| Monitoring by Continuous Wearable Devices in the Entire Monitoring Period | Manual Monitoring by Transitional Care Facility (n = 11) | ||||
|---|---|---|---|---|---|
| Minutes of deviations | Sustained deviations | ||||
| Median duration (min/24 h of monitoring) | Sustained deviation | Number of patients (%) | During monitoring period | 30 days following inclusion | |
| Respiratory vital sign abnormalities | |||||
| SpO2 < 92% | 313.0 [149.0–494.0] | SpO2 < 92% ≥ 60 min | 5 (25) | 0 (0) | 0 (0) |
| SpO2 < 88% | 77.0 [32.0–123.0] | SpO2 < 88% ≥ 10 min | 9 (45) | 0 (0) | 1 (5) |
| SpO2 < 85% | 25.0 [11.0–56.0] | SpO2 < 85% ≥ 5 min | 11 (55) | 0 (0) | 1 (5) |
| SpO2 < 80% | 6.0 [2.0–15.0] | SpO2 < 80% ≥ 1 min | 17 (85) | 0 (0) | 0 (0) |
| RR < 5min−1 | 0.0 [0.0–0.0] | RR < 5min−1 ≥ 1 min | 7 (35) | 0 (0) | 0 (0) |
| RR < 11 min−1 | 28.0 [4.0–121.0] | RR < 11 min−1 ≥ 5 min | 7 (35) | 0 (0) | 0 (0) |
| RR > 24 min−1 | 10.0 [2.0–46.0] | RR > 24min−1 ≥ 5 min | 9 (45) | 0 (0) | 5 (25) |
| RR > 30 min−1 | 0.0 [0.0–2.0] | RR > 30 min−1 ≥ 1 min | 11 (55) | 0 (0) | 2 (10) |
| Circulatory vital sign abnormalities | |||||
| HR < 30 min−1 | 0.0 [0.0–0.0] | HR < 30/min ≥ 5 min | 0 (0) | 0 (0) | 0 (0) |
| HR < 40 min−1 | 0.0 [0.0–0.0] | HR < 40/min ≥ 5 min | 0 (0) | 0 (0) | 0 (0) |
| HR > 110 min−1 | 20.0 [3.0–43.0] | HR > 110/min ≥ 60 min | 1 (5) | 0 (0) | 2 (10) |
| HR > 130 min−1 | 0.0 [0.0–3.0] | HR > 130/min ≥ 30 min | 1 (5) | 0 (0) | 0 (0) |
| SBP < 70 mmHg | 0.0 [0.0–0.0] | SBP < 70 mmHg ≥ two times | 0 (0) | 0 (0) | 0 (0) |
| SBP < 90 mmHg | 0.0 [0.0–0.0] | SBP < 90 mmHg ≥ two times | 2 (10) | 0 (0) | 1 (5) |
| SBP > 180 mmHg | 0.0 [0.0–10.0] | SBP > 180 mmHg ≥ two times | 4 (20) | 0 (0) | 0 (0) |
| SBP > 220 mmHg | 0.0 [0.0–0.0] | SBP > 220 mmHg ≥ two times | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mølgaard, J.; Haahr-Raunkjaer, C.; Rasmussen, S.S.; Andersen, L.V.; Meyhoff, C.S.; Aasvang, E.K. Continuous Wireless Vital Sign Sensors for Detecting Severe Deviations at a Transitional Care Facility—An Observational Feasibility Study. Sensors 2025, 25, 6970. https://doi.org/10.3390/s25226970
Mølgaard J, Haahr-Raunkjaer C, Rasmussen SS, Andersen LV, Meyhoff CS, Aasvang EK. Continuous Wireless Vital Sign Sensors for Detecting Severe Deviations at a Transitional Care Facility—An Observational Feasibility Study. Sensors. 2025; 25(22):6970. https://doi.org/10.3390/s25226970
Chicago/Turabian StyleMølgaard, Jesper, Camilla Haahr-Raunkjaer, Søren Straarup Rasmussen, Loraine Villacorte Andersen, Christian S. Meyhoff, and Eske K. Aasvang. 2025. "Continuous Wireless Vital Sign Sensors for Detecting Severe Deviations at a Transitional Care Facility—An Observational Feasibility Study" Sensors 25, no. 22: 6970. https://doi.org/10.3390/s25226970
APA StyleMølgaard, J., Haahr-Raunkjaer, C., Rasmussen, S. S., Andersen, L. V., Meyhoff, C. S., & Aasvang, E. K. (2025). Continuous Wireless Vital Sign Sensors for Detecting Severe Deviations at a Transitional Care Facility—An Observational Feasibility Study. Sensors, 25(22), 6970. https://doi.org/10.3390/s25226970







