Characteristics of Ground Reaction Force Variability During Walking in Post-Stroke Patients
Highlights
- Paretic-side anterior–posterior ground reaction force variability during pre-swing phase was lower than in age-matched control, independent of speed.
- The reduction in GRF variability during walking was suggested for maintaining gait and avoiding falls in post-stroke patients.
- GRF variability may reflect the adaptive gait control of participants.
- Rehabilitation that permits GRF variability may be beneficial for post-stroke patients with mild deficits.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Gait Assessment
2.4. Shoe Sensor System
2.5. Data Processing
2.6. Ground Reaction Force Variability
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Comparison of GRF Across Stance Sub-Phase Among the Paretic Side, Non-Paretic Side and Control’s Left Side
3.3. Comparison of GRF Variability Across Stance Sub-Phase Among the Paretic Side, Non-Paretic Side, and Control’s Left Side
3.4. Association Between GRF Variability and Balance Ability Across Stance Sub-Phase in Post-Stroke Patients
3.5. Differences in GRF and GRF Variability Between the Faller Group and Non-Faller Group of Post-Stroke Patients
4. Discussion
4.1. GRF Variability During Gait in Post-Stroke Patients
4.2. Association Between GRF Variability During Gait and Balance Ability
4.3. The Difference of GRF Variability Characteristics Between Faller and Non-Faller Groups
4.4. GRF During Gait in Post-Stroke Patients
5. Limitations of the Study and Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Stance Phase (%) | Anterior–Posterior | Vertical |
|---|---|---|
| 10% | 0.604 | 0.491 |
| 20% | 0.596 | 0.544 |
| 30% | 0.569 | 0.557 |
| 40% | 0.642 | 0.620 |
| 50% | 0.720 | 0.675 |
| 60% | 0.727 | 0.689 |
| 70% | 0.710 | 0.716 |
| 80% | 0.699 | 0.705 |
| 90% | 0.735 | 0.714 |
| 100% | 0.752 | 0.872 |
| Paretic Side | Non-Paretic Side | Control | Unadjusted Test Statistics | Unadjusted p-Value | Adjusted F Value | Adjusted p-Value | Adjusted η2 | Adjusted 1 − β | Adjusted 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 16 | 19 | ||||||||
| Stance phase | ||||||||||
| 1–10% | −0.445 (−0.414) | −0.411 (0.387) | −0.284 (0.349) | 0.884 | 0.420 | 0.569 | 0.572 | 0.035 | 0.203 | 0.012–0.053 |
| 11–20% | −1.027 (0.800) | −0.967 (0.654) | −0.487 (0.542) | 3.531 | 0.037 * | 2.277 | 0.121 | 0.128 | 0.658 | 0.045–0.14 |
| 21–30% | −1.222 (0.999) | −1.192 (0.776) | −0.605 (0.704) | 6.316 | 0.043 * | 2.309 | 0.118 | 0.116 | 0.605 | 0.046–0.142 |
| 31–40% | −1.222 (1.131) | −1.226 (0.826) | −0.543 (0.804) | 7.249 | 0.026 * | 1.823 | 0.180 | 0.119 | 0.617 | 0.037–0.119 |
| 41–50% | −1.128 (1.274) | −1.152 (0.836) | −0.324 (0.910) | 8.111 | 0.017 * | 1.638 | 0.213 | 0.138 | 0.697 | 0.033–0.11 |
| 51–60% | −1.100 (1.443) | −1.054 (0.935) | −0.148 (1.124) | 7.668 | 0.022 * | 1.765 | 0.190 | 0.133 | 0.679 | 0.035–0.116 |
| 61–70% | −1.049 (1.701) | −0.895 (1.142) | 0.178 (1.357) | 6.649 | 0.036 * | 2.564 | 0.095 | 0.142 | 0.715 | 0.051–0.153 |
| 71–80% | −0.668 (1.845) | −0.611 (1.322) | 0.815 (1.435) | 5.293 | 0.008 * | 2.998 | 0.066 | 0.181 | 0.837 | 0.059–0.172 |
| 81–90% | 0.324 (1.350) | 0.139 (1.043) | 1.387 (0.989) | 6.343 | 0.004 * | 1.143 | 0.332 | 0.209 | 0.900 | 0.023–0.084 |
| 91–100% | 0.479 (0.551) | 0.463 (0.661) | 1.167 (0.781) | 6.276 | 0.004 * | 1.060 | 0.360 | 0.207 | 0.897 | 0.022–0.080 |
| Paretic Side | Non-Paretic Side | Control | Unadjusted Test Statistics | Unadjusted p-Value | Adjusted F Value | Adjusted p-Value | Adjusted η2 | Adjusted 1 − β | Adjusted 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 16 | 19 | ||||||||
| Stance phase (%) | ||||||||||
| 1–10% | 0.825 (0.228) | 0.759 (0.209) | 0.858 (0.201) | 1.7456 | 0.4178 | 0.311 | 0.735 | 0.038 | 0.218 | 0.006–0.039 |
| 11–20% | 2.178 (0.928) | 1.800 (0.659) | 2.153 (0.504) | 0.6928 | 0.7072 | 2.160 | 0.131 | 0.057 | 0.313 | 0.043–0.135 |
| 21–30% | 2.977 (1.033) | 2.664 (0.927) | 2.957 (0.793) | 0.4651 | 0.7925 | 1.750 | 0.191 | 0.024 | 0.151 | 0.035–0.115 |
| 31–40% | 3.379 (0.792) | 3.229 (1.048) | 3.412 (0.898) | 1.5863 | 0.4524 | 0.582 | 0.565 | 0.008 | 0.080 | 0.012–0.054 |
| 41–50% | 3.653 (0.988) | 3.518 (1.176) | 3.789 (0.916) | 2.7982 | 0.2468 | 0.112 | 0.895 | 0.013 | 0.099 | 0.002–0.027 |
| 51–60% | 4.112 (1.289) | 4.204 (1.439) | 4.287 (0.959) | 2.5108 | 0.285 | 0.074 | 0.929 | 0.004 | 0.063 | 0.002–0.025 |
| 61–70% | 4.720 (1.288) | 5.383 (1.282) | 4.691 (1.208) | 1.7153 | 0.4241 | 0.772 | 0.470 | 0.063 | 0.340 | 0.016–0.065 |
| 71–80% | 4.941 (1.193) | 6.198 (1.928) | 4.832 (1.420) | 1.8204 | 0.4024 | 1.640 | 0.208 | 0.144 | 0.722 | 0.033–0.110 |
| 81–90% | 3.621 (0.790) | 4.752 (1.955) | 4.104 (1.053) | 2.0716 | 0.3549 | 1.710 | 0.196 | 0.106 | 0.561 | 0.034–0.113 |
| 91–100% | 1.106 (0.217) | 1.332 (0.999) | 1.504 (0.420) | 2.7742 | 0.2498 | 2.192 | 0.127 | 0.068 | 0.368 | 0.044–0.136 |
| Paretic Side | Non-Paretic Side | Control | Unadjusted Test Statistics | Unadjusted p-Value | Adjusted F Value | Adjusted p-Value | Adjusted η2 | Adjusted 1 − β | Adjusted 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 16 | 19 | ||||||||
| Stance phase (%) | ||||||||||
| 1–10% | 0.825 (0.228) | 0.759 (0.209) | 0.858 (0.201) | 1.746 | 0.4178 | 0.140 | 0.870 | 0.016 | 0.114 | 0.003–0.029 |
| 11–20% | 2.178 (0.928) | 1.800 (0.659) | 2.153 (0.504) | 0.693 | 0.7072 | 0.090 | 0.914 | 0.004 | 0.063 | 0.002–0.026 |
| 21–30% | 2.977 (1.033) | 2.664 (0.927) | 2.957 (0.793) | 0.465 | 0.7925 | 0.027 | 0.974 | 0.003 | 0.063 | 0.001–0.022 |
| 31–40% | 3.379 (0.792) | 3.229 (1.048) | 3.412 (0.898) | 1.586 | 0.4524 | 0.396 | 0.676 | 0.024 | 0.149 | 0.008–0.044 |
| 41–50% | 3.653 (0.988) | 3.518 (1.176) | 3.789 (0.916) | 2.798 | 0.2468 | 1.001 | 0.378 | 0.045 | 0.253 | 0.02–0.077 |
| 51–60% | 4.112 (1.289) | 4.204 (1.439) | 4.287 (0.959) | 2.511 | 0.285 | 0.753 | 0.479 | 0.036 | 0.205 | 0.015–0.064 |
| 61–70% | 4.720 (1.288) | 5.383 (1.282) | 4.691 (1.208) | 1.715 | 0.4241 | 0.453 | 0.640 | 0.030 | 0.175 | 0.009–0.047 |
| 71–80% | 4.941 (1.193) | 6.198 (1.928) | 4.832 (1.420) | 1.820 | 0.4024 | 0.593 | 0.560 | 0.042 | 0.233 | 0.012–0.055 |
| 81–90% | 3.621 (0.790) | 4.752 (1.955) | 4.104 (1.053) | 2.072 | 0.3549 | 0.597 | 0.556 | 0.036 | 0.204 | 0.012–0.055 |
| 91–100% | 1.106 (0.217) | 1.332 (0.999) | 1.504 (0.420) | 2.774 | 0.2498 | 1.341 | 0.275 | 0.055 | 0.302 | 0.027–0.095 |
| Faller | Non-Faller | Unadjusted p | Adjusted W | Adjusted p | Adjusted Effect Size r | Adjusted 95% CI | |
|---|---|---|---|---|---|---|---|
| Number | 4 | 12 | |||||
| Stance phase (%) | |||||||
| 1–10% | −0.723 (0.217) | −0.352 (0.429) | 0.181 | 36 | 0.856 | 0.045 | −0.461–0.529 |
| 11–20% | −1.514 (0.310) | −0.865 (0.855) | 0.249 | 37 | 0.847 | 0.076 | −0.436–0.551 |
| 21–30% | −1.913 (0.594) | −0.992 (1.017) | 0.181 | 28 | 0.721 | 0.167 | −0.359–0.612 |
| 31–40% | −2.277 (0.624) | −0.871 (1.048) | 0.057 | 25 | 0.505 | 0.258 | −0.273–0.668 |
| 41–50% | −2.445 (0.738) | −0.689 (1.107) | 0.043 * | 21 | 0.325 | 0.379 | −0.144–0.736 |
| 51–60% | −2.542 (0.646) | −0.619 (1.310) | 0.045 * | 19 | 0.325 | 0.440 | −0.072–0.768 |
| 61–70% | −2.764 (0.581) | −0.477(1.558) | 0.043 * | 20 | 0.325 | 0.409 | −0.108–0.752 |
| 71–80% | −2.596 (0.547) | −0.025 (1.661) | 0.043 * | 21 | 0.325 | 0.379 | −0.144–0.736 |
| 81–90% | −0.997 (1.078) | 0.764 (1.151) | 0.050 | 22 | 0.326 | 0.349 | −0.178–0.720 |
| 91–100% | 0.063 (0.648) | 0.618 (0.463) | 0.181 | 30 | 0.839 | 0.106 | −0.411–0.572 |
References
- Vieira, E.R.; Palmer, R.C.; Chaves, P.H.M. Prevention of Falls in Older People Living in the Community. BMJ 2016, 353, i1419. [Google Scholar] [CrossRef]
- Skvortsov, D.V.; Kaurkin, S.N.; Grebenkina, N.V.; Ivanova, G.E. Typical Changes in Gait Biomechanics in Patients with Subacute Ischemic Stroke. Diagnostics 2025, 15, 511. [Google Scholar] [CrossRef]
- Schmid, A.A.; Yaggi, H.K.; Burrus, N.; McClain, V.; Austin, C.; Ferguson, J.; Fragoso, C.; Sico, J.J.; Miech, E.J.; Matthias, M.S.; et al. Circumstances and Consequences of Falls among People with Chronic Stroke. J. Rehabil. Res. Dev. 2013, 50, 1277–1286. [Google Scholar] [CrossRef]
- Nagano, H.; Said, C.M.; James, L.; Sparrow, W.A.; Begg, R. Biomechanical Correlates of Falls Risk in Gait Impaired Stroke Survivors. Front. Physiol. 2022, 13, 833417. [Google Scholar] [CrossRef] [PubMed]
- Tasseel-Ponche, S.; Delafontaine, A.; Godefroy, O.; Yelnik, A.P.; Doutrellot, P.L.; Duchossoy, C.; Hyra, M.; Sader, T.; Diouf, M. Walking Speed at the Acute and Subacute Stroke Stage: A Descriptive Meta-Analysis. Front. Neurol. 2022, 13, 989622. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Taylor-Piliae, R.E.; Yang, C.; Peng, X.; Yang, Q.; Zhang, Q. Risk Factors for Falls and Recurrent Falls in Older Stroke Survivors: A Systematic Review and Meta-Analysis of Prospective Studies. Int. J. Older People Nurs. 2025, 20, e70050. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, F.A.; Mackintosh, S.F.; Said, C.M.; Hill, K.D. Falls after Stroke. Int. J. Stroke 2012, 7, 482–490. [Google Scholar] [CrossRef]
- Goto, Y.; Otaka, Y.; Suzuki, K.; Inoue, S.; Kondo, K.; Shimizu, E. Incidence and Circumstances of Falls among Community-Dwelling Ambulatory Stroke Survivors: A Prospective Study. Geriatr. Gerontol. Int. 2019, 19, 240–244. [Google Scholar] [CrossRef]
- Lee, D.; Cho, I.Y.; Chang, W.H.; Yoo, J.E.; Choi, H.L.; Park, J.; Shin, D.W.; Han, K. Fracture Risk among Stroke Survivors According to Poststroke Disability Status and Stroke Type. Stroke 2024, 55, 1498–1506. [Google Scholar] [CrossRef]
- Karisik, A.; Dejakum, B.; Moelgg, K.; Granna, J.; Felicetti, S.; Pechlaner, R.; Mayer-Suess, L.; Toell, T.; Buergi, L.; Scherer, L.; et al. Incidence, Characteristics, and Consequences of Fractures after Acute Ischemic Stroke and TIA—A Prospective Cohort Study. Int. J. Stroke 2025, 20, 1141–1149. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, J.; Shen, H.; Hu, H.; Cao, L. Predictors of Prognosis for Elderly Patients with Poststroke Hemiplegia Experiencing Hip Fractures. Clin. Orthop. Relat. Res. 2009, 467, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Brough, L.G.; Klute, G.K.; Neptune, R.R. Biomechanical Response to Mediolateral Foot-Placement Perturbations during Walking. J. Biomech. 2021, 116, 110213. [Google Scholar] [CrossRef]
- Debelle, H.; Maganaris, C.N.; O’Brien, T.D. Biomechanical Mechanisms of Improved Balance Recovery to Repeated Backward Slips Simulated by Treadmill Belt Accelerations in Young and Older Adults. Front. Sports Act. Living 2021, 3, 708929. [Google Scholar] [CrossRef]
- Williams, S.E.; Gibbs, S.; Meadows, C.B.; Abboud, R.J. Classification of the Reduced Vertical Component of the Ground Reaction Force in Late Stance in Cerebral Palsy Gait. Gait Posture 2011, 34, 370–373. [Google Scholar] [CrossRef]
- Cikajlo, I.; Matjačić, Z. Directionally Specific Objective Postural Response Assessment Tool for Treatment Evaluation in Stroke Patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 91–100. [Google Scholar] [CrossRef]
- Kwon, M.S.; Kwon, Y.R.; Park, Y.S.; Kim, J.W. Comparison of Gait Patterns in Elderly Fallers and Non-Fallers. Technol. Health Care 2018, 26, S427–S436. [Google Scholar] [CrossRef]
- Pan, J.W.; Sidarta, A.; Wu, T.L.; Kwong, W.H.P.; Ong, P.L.; Tay, M.R.J.; Phua, M.W.; Chong, W.B.; Ang, W.T.; Chua, K.S.G. Unraveling Stroke Gait Deviations with Movement Analytics, More than Meets the Eye: A Case Control Study. Front. Neurosci. 2024, 18, 1425183. [Google Scholar] [CrossRef]
- Jeon, H.M.; Chung, E.H.; Bak, S.Y.; Kim, H.; Shin, S.; Baek, H.; Kim, M.Y. Comparison of Biomechanical Parameters in Lower Limb Joints of Stroke Patients According to Conventional Evaluation Scores during Level Walking. Front. Bioeng. Biotechnol. 2024, 12, 1320337. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hong, P.W.; Chen, C.L.; Chou, S.W.; Wu, C.Y.; Cheng, P.T.; Tang, F.T.; Chen, H.C. Ground Reaction Force Patterns in Stroke Patients with Various Degrees of Motor Recovery Determined by Plantar Dynamic Analysis. Chang Gung Med. J. 2007, 30, 62–72. [Google Scholar] [PubMed]
- Dean, J.C.; Bowden, M.G.; Kelly, A.L.; Kautz, S.A. Altered Post-Stroke Propulsion Is Related to Paretic Swing Phase Kinematics. Clin. Biomech. 2020, 72, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Tanabe, S.; Tamari, M.; Katsuhira, J. Patterns of Change in Propulsion Force and Late Braking Force in Patients with Stroke Walking at Comfortable and Fast Speeds. Sci. Rep. 2024, 14, 22316. [Google Scholar] [CrossRef]
- Ohta, M.; Tanabe, S.; Katsuhira, J.; Tamari, M. Kinetic and Kinematic Parameters Associated with Late Braking Force and Effects on Gait Performance of Stroke Patients. Sci. Rep. 2023, 13, 7729. [Google Scholar] [CrossRef]
- Hida, N.; Kokue, T.; Sugawara, K. Propulsive and Braking Mechanisms during Acceleration and Deceleration in Human Gait. Hum. Mov. Sci. 2025, 104, 103420. [Google Scholar] [CrossRef]
- Rabuffetti, M.; Bovi, G.; Quadri, P.L.; Cattaneo, D.; Benvenuti, F.; Ferrarin, M. An Experimental Paradigm to Assess Postural Stabilization: No More Movement and Not yet Posture. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 420–426. [Google Scholar] [CrossRef]
- La Scaleia, V.; Ivanenko, Y.P.; Zelik, K.E.; Lacquaniti, F. Spinal Motor Outputs during Step-to-Step Transitions of Diverse Human Gaits. Front. Hum. Neurosci. 2014, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Bizovska, L.; Svoboda, Z.; Kutilek, P.; Janura, M.; Gaba, A.; Kovacikova, Z. Variability of Centre of Pressure Movement during Gait in Young and Middle-Aged Women. Gait Posture 2014, 40, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, D.; Singh, N.B.; Van Dieën, J.H.; Heller, M.O.; Taylor, W.R. Kinematic Measures for Assessing Gait Stability in Elderly Individuals: A Systematic Review. J. R. Soc. Interface 2011, 8, 1682–1698. [Google Scholar] [CrossRef]
- Chau, T.; Young, S.; Redekop, S. Managing Variability in the Summary and Comparison of Gait Data. J. Neuroeng. Rehabil. 2005, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Isho, T.; Tashiro, H.; Usuda, S. Accelerometry-Based Gait Characteristics Evaluated Using a Smartphone and Their Association with Fall Risk in People with Chronic Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 1305–1311. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Arai, T.; Takaishi, S.; Takamura, H.; Maruki, H. Increased Stride Time Variability Is Associated with a Higher Risk of Falls in Patients with Ataxia after Stroke. Physiother. Theory Pract. 2024, 40, 2916–2924. [Google Scholar] [CrossRef]
- Lord, S.; Howe, T.; Greenland, J.; Simpson, L.; Rochester, L. Gait Variability in Older Adults: A Structured Review of Testing Protocol and Clinimetric Properties. Gait Posture 2011, 34, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, E.; Burger, L.M.; Mergen, D.F.; Orth, M.; Pohlemann, T.; Ganse, B. The Walking Surface Influences Vertical Ground Reaction Force and Centre of Pressure Data Obtained with Pressure-Sensing Insoles. Front. Digit. Health 2024, 6, 1476335. [Google Scholar] [CrossRef]
- Liu, J.; Kim, H.B.; Wolf, S.L.; Kesar, T.M. Comparison of the Immediate Effects of Audio, Visual, or Audiovisual Gait Biofeedback on Propulsive Force Generation in Able-Bodied and Post-Stroke Individuals. Appl. Psychophysiol. Biofeedback 2020, 45, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, E.; Catelli, D.S.; Lamontagne, M. A Waveform Test for Variance Inequality, with a Comparison of Ground Reaction Force during Walking in Younger vs. Older Adults. J. Biomech. 2021, 127, 110657. [Google Scholar] [CrossRef]
- Alam, Z.; Rendos, N.K.; Vargas, A.M.; Makanjuola, J.; Kesar, T.M. Timing of Propulsion-Related Biomechanical Variables Is Impaired in Individuals with Post-Stroke Hemiparesis. Gait Posture 2022, 96, 275–278. [Google Scholar] [CrossRef]
- Hsiang, S.M.; Chang, C. The Effect of Gait Speed and Load Carrying on the Reliability of Ground Reaction Forces. Saf. Sci. 2002, 40, 639–657. [Google Scholar] [CrossRef]
- Tomoda, Y.; Nagao, T.; Uchida, T.; Sato, H.; Oonishi, M.; Okamoto, T.; Tajitsu, M.; Alt Murphy, M. Cross-Cultural Adaptation and Validation of the Japanese Translation of the Fugl-Meyer Assessment for Upper and Lower Extremity Sensorimotor Function After Stroke. J. Rehabil. Med. 2025, 57, jrm43350. [Google Scholar] [CrossRef]
- Chino, N.; Sonoda, S.; Domen, K.; Saitoh, E.; Kimura, A. Stroke Impairment Assessment Set (SIAS)—A New Evaluation Instrument for Stroke Patients. In Functional Evaluation of Stroke Patients; Springer: Tokyo, Japan, 1996. [Google Scholar]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Hauer, K.A.; Kempen, G.I.J.M.; Schwenk, M.; Yardley, L.; Beyer, N.; Todd, C.; Oster, P.; Zijlstra, G.A.R. Validity and Sensitivity to Change of the Falls Efficacy Scales International to Assess Fear of Falling in Older Adults with and without Cognitive Impairment. Gerontology 2011, 57, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Kamide, N.; Shiba, Y.; Sakamoto, M.; Sato, H.; Kawamura, A. Fall-Related Efficacy Is a Useful and Independent Index to Detect Fall Risk in Japanese Community-Dwelling Older People: A 1-Year Longitudinal Study. BMC Geriatr. 2019, 19, 293. [Google Scholar] [CrossRef]
- Cho, H.Y.; Heijnen, M.J.H.; Craig, B.A.; Rietdyk, S. Falls in Young Adults: The Effect of Sex, Physical Activity, and Prescription Medications. PLoS ONE 2021, 16, e0250360. [Google Scholar] [CrossRef]
- Nagano, H.; Sparrow, W.A.; Mizukami, K.; Sarashina, E.; Begg, R. A Cross-Sectional Study of Foot-Ground Clearance in Healthy Community Dwelling Japanese Cohorts Aged 50, 60 and 70 Years. BMC Geriatr. 2021, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Bisi, M.C.; Stagni, R. Gait Variability and Stability Measures: Minimum Number of Strides and within-Session Reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Takahashi, Y.; Sasaki, Y. Prediction of Three-Directional Ground Reaction Forces during Walking Using a Shoe Sole Sensor System and Machine Learning. Sensors 2023, 23, 8985. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tomosada, M.; Nishi, T.; Sasaki, Y.; Sakurai, R.; Yamaguchi, T. Comparing the Ground Reaction Forces, Toe Clearances, and Stride Lengths of Young and Older Adults Using a Novel Shoe Sensor System. Sensors 2024, 24, 6871. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Seeley, M.K. An Investigation of Lower-Extremity Functional Asymmetry for Non-Preferred Able-Bodied Walking Speeds. Int. J. Exerc. Sci. 2010, 3, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Shorter, A.L.; Richardson, J.K.; Finucane, S.B.; Joshi, V.; Gordon, K.; Rouse, E.J. Characterization and Clinical Implications of Ankle Impedance during Walking in Chronic Stroke. Sci. Rep. 2021, 11, 16726. [Google Scholar] [CrossRef]
- Covarrubias-Escudero, F.; Appelgren-Gonzalez, J.P.; Nuñez-Saavedra, G.; Urrea-Baeza, D.; Varas-Diaz, G. Enhancing Gait Biomechanics in Persons with Stroke: The Role of Functional Electrical Stimulation on Step-To-Step Transition. Physiother. Res. Int. 2025, 30, e70080. [Google Scholar] [CrossRef]
- Park, S.; Yoon, S. Validity Evaluation of an Inertial Measurement Unit (IMU) in Gait Analysis Using Statistical Parametric Mapping (SPM). Sensors 2021, 21, 3667. [Google Scholar] [CrossRef]
- Keller, T.S.; Weisberger, A.M.; Ray, J.L.; Hasan, S.S.; Shiavi, R.G.; Spengler, D.M. Relationship between Vertical Ground Reaction Force and Speed during Walking, Slow Jogging, and Running. Clin. Biomech. 1996, 11, 253–259. [Google Scholar] [CrossRef]
- Jordan, K.; Challis, J.H.; Newell, K.M. Walking Speed Influences on Gait Cycle Variability. Gait Posture 2007, 26, 128–134. [Google Scholar] [CrossRef]
- Cohen, J. Quantitative Methods in Psychology—A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Knorr, S.; Brouwer, B.; Garland, S.J. Validity of the Community Balance and Mobility Scale in Community-Dwelling Persons After Stroke. Arch. Phys. Med. Rehabil. 2010, 91, 890–896. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Kuramatsu, Y.; Yamamoto, Y.; Izumi, S.I. Sensorimotor Strategies in Individuals with Poststroke Hemiparesis When Standing up without Vision. Motor Control 2020, 24, 150–167. [Google Scholar] [CrossRef]
- Fickey, S.N.; Browne, M.G.; Franz, J.R. Biomechanical Effects of Augmented Ankle Power Output during Human Walking. J. Exp. Biol. 2018, 221, jeb182113. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Kautz, S.A.; Zajac, F.E. Contributions of the Individual Ankle Plantar Flexors to Support, Forward Progression and Swing Initiation during Walking. J. Biomech. 2001, 34, 1387–1398. [Google Scholar] [CrossRef]
- Winter, D.A. The Biomechanics and Motor Control of Human Gait: Normal, Elderly, and Pathological. J. Biomech. 1992, 25, 949. [Google Scholar]
- Jonkers, I.; Delp, S.; Patten, C. Capacity to Increase Walking Speed Is Limited by Impaired Hip and Ankle Power Generation in Lower Functioning Persons Post-Stroke. Gait Posture 2009, 29, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pardasaney, P.K.; Latham, N.K.; Jette, A.M.; Wagenaar, R.C.; Ni, P.; Slavin, M.D.; Bean, J.F. Sensitivity to Change and Responsiveness of Four Balance Measures for Community-Dwelling Older Adults. Phys. Ther. 2012, 92, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Beschorner, K.E.; Albert, D.L.; Redfern, M.S. Required Coefficient of Friction during Level Walking Is Predictive of Slipping. Gait Posture 2016, 48, 256–260. [Google Scholar] [CrossRef]
- Rozin Kleiner, A.F. Effects of Flooring and Hemi Body on Ground Reaction Forces and Coefficient of Friction in Stroke Gait. Int. J. Neurorehabil. 2014, 1, 1000122. [Google Scholar] [CrossRef]
- Redfern, M.S.; Dipasquale, J. Biomechanics of Descending Ramps. Gait Posture 1997, 6, 119–125. [Google Scholar] [CrossRef]
- Bower, K.; Thilarajah, S.; Pua, Y.H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic Balance and Instrumented Gait Variables Are Independent Predictors of Falls Following Stroke. J. Neuroeng. Rehabil. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Decker, L.M. Human Movement Variability, Nonlinear Dynamics, and Pathology: Is There a Connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Elshehabi, M.; Del Din, S.; Hobert, M.A.; Warmerdam, E.; Sünkel, U.; Schmitz-Hübsch, T.; Behncke, L.M.; Heinzel, S.; Brockmann, K.; Metzger, F.G.; et al. Walking Parameters of Older Adults from a Lower Back Inertial Measurement Unit, a 6-Year Longitudinal Observational Study. Front. Aging Neurosci. 2022, 14, 789220. [Google Scholar] [CrossRef]
- Tsuchiyama, K.; Mukaino, M.; Ohtsuka, K.; Matsuda, F.; Tanikawa, H.; Yamada, J.; Pongpipatpaiboon, K.; Kanada, Y.; Saitoh, E.; Otaka, Y. Effects of Ankle-Foot Orthoses on the Stability of Post-Stroke Hemiparetic Gait. Eur. J. Phys. Rehabil. Med. 2022, 58, 352–362. [Google Scholar] [CrossRef]
- Deffeyes, J.E.; Peters, D.M. Time-Integrated Propulsive and Braking Impulses Do Not Depend on Walking Speed. Gait Posture 2021, 88, 258–263. [Google Scholar] [CrossRef]
- Vistamehr, A.; Kautz, S.A.; Bowden, M.G.; Neptune, R.R. The Influence of Locomotor Training on Dynamic Balance during Steady-State Walking Post-Stroke. J. Biomech. 2019, 89, 21–27. [Google Scholar] [CrossRef]
- Bowden, M.G.; Balasubramanian, C.K.; Neptune, R.R.; Kautz, S.A. Anterior-Posterior Ground Reaction Forces as a Measure of Paretic Leg Contribution in Hemiparetic Walking. Stroke 2006, 37, 872–876. [Google Scholar] [CrossRef]
- Nascimento, L.R.; Ada, L.; Teixeira-Salmela, L.F. The Provision of a Cane Provides Greater Benefit to Community-Dwelling People after Stroke with a Baseline Walking Speed between 0.4 and 0.8 Metres/Second: An Experimental Study. Physiotherapy 2016, 102, 351–356. [Google Scholar] [CrossRef]
- Huijben, B.; van Schooten, K.S.; van Dieën, J.H.; Pijnappels, M. The Effect of Walking Speed on Quality of Gait in Older Adults. Gait Posture 2018, 65, 112–116. [Google Scholar] [CrossRef] [PubMed]
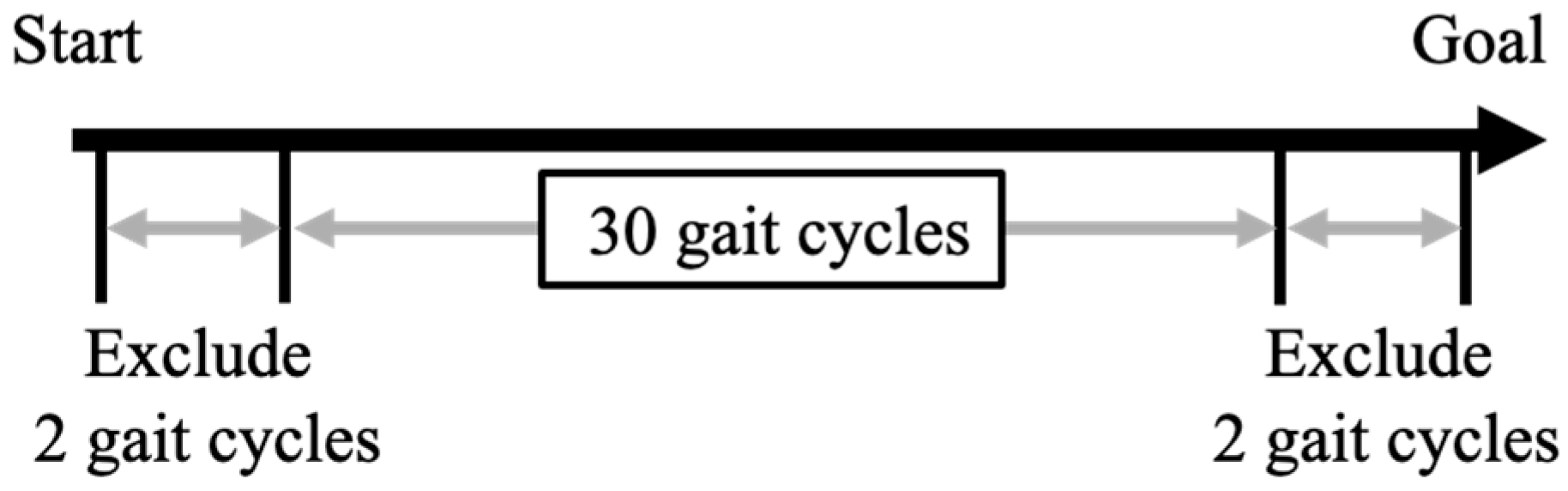
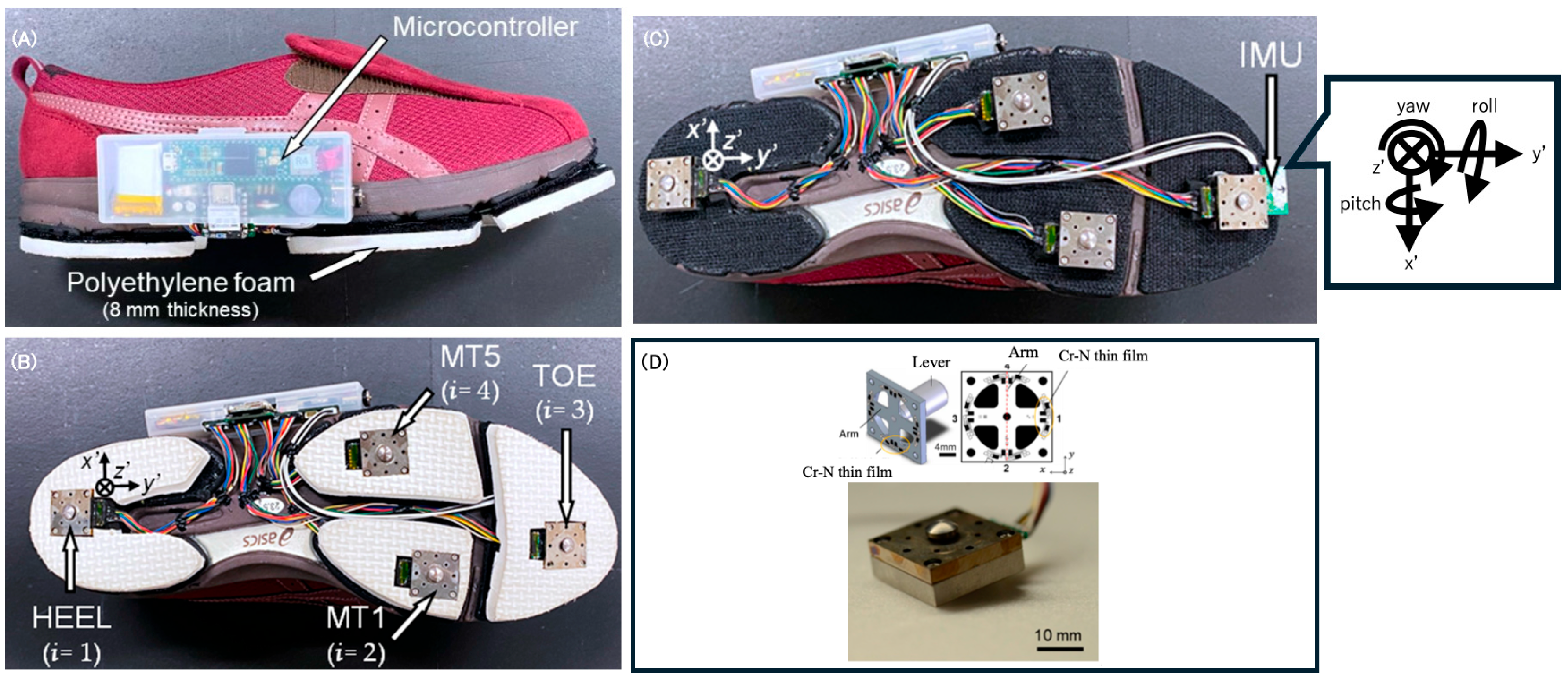


| Post-Stroke Patients | Controls | Test Statistics | p-Value | |
|---|---|---|---|---|
| Number | 16 | 19 | ||
| Age (years) a,b | 72.19 (8.54) | 68.63 (5.73) | 1.467 | 0.151 |
| Sex (male/female) c | 10/6 | 10/9 | 0.347 | 0.556 |
| Height (cm) a,b | 157.14 (12.34) | 161.33 (8.82) | −1.168 | 0.251 |
| Weight (kg) a,b | 55.68 (8.60) | 56.65 (10.57) | −0.294 | 0.77 |
| Cane (T-cane/none) c | 5/11 | 0/19 | 8.883 | 0.003 * |
| Gait speed (m/sec) a,b | 0.81 (0.32) | 1.25 (0.11) | −5.577 | <0.001 * |
| Diagnosis | ||||
| Infarction d | 12 (75%) | - | - | |
| Hemorrhage d | 4 (25%) | - | - | |
| Paretic side | ||||
| Right d | 9 (56%) | - | - | |
| Left d | 7 (44%) | - | - | |
| Time post-stroke (days) a | 91.56 (36.13) | - | - | |
| Fugl–Meyer Assessment a | 30.94 (3.00) | - | - | |
| Berg Balance scale a | 51.69 (4.13) | - | - | |
| Stroke Impairment Assessment Set (1/2/3/4/5) | - | - | ||
| Hip flexion test | 0/0/1/4/11 | - | - | |
| Knee extension test | 0/0/2/5/9 | - | - | |
| Ankle dorsiflexion test | 0/0/0/5/11 | - | - |
| Paretic Side | Non-Paretic Side | Control | Unadjusted Test Statistics | Unadjusted p-Value | Adjusted F Value | Adjusted p-Value | Adjusted η2 | Adjusted 1 – β | Adjusted 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 16 | 19 | ||||||||
| Stance phase (%) | ||||||||||
| 1–10% | −0.445 (−0.414) | −0.411 (0.387) | −0.284 (0.349) | 1.435 | 0.488 | 0.971 | 0.389 | 0.042 | 0.227 | 0.020–0.075 |
| 11–20% | −1.027 (0.800) | −0.967 (0.654) | −0.487 (0.542) | 0.569 | 0.752 | 1.068 | 0.355 | 0.027 | 0.163 | 0.022–0.081 |
| 21–30% | −1.222 (0.999) | −1.192 (0.776) | −0.605 (0.704) | 1.458 | 0.482 | 1.111 | 0.341 | 0.029 | 0.169 | 0.023–0.083 |
| 31–40% | −1.222 (1.131) | −1.226 (0.826) | −0.543 (0.804) | 1.232 | 0.540 | 1.324 | 0.280 | 0.041 | 0.227 | 0.027–0.094 |
| 41–50% | −1.128 (1.274) | −1.152 (0.836) | −0.324 (0.910) | 1.249 | 0.536 | 1.798 | 0.181 | 0.062 | 0.336 | 0.036–0.118 |
| 51–60% | −1.100 (1.443) | −1.054 (0.935) | −0.148 (1.124) | 1.444 | 0.486 | 1.171 | 0.322 | 0.039 | 0.215 | 0.024–0.086 |
| 61–70% | −1.049 (1.701) | −0.895 (1.142) | 0.178 (1.357) | 1.392 | 0.499 | 0.473 | 0.627 | 0.017 | 0.113 | 0.010–0.048 |
| 71–80% | −0.668 (1.845) | −0.611 (1.322) | 0.815 (1.435) | 0.084 | 0.959 | 0.240 | 0.789 | 0.001 | 0.054 | 0.005–0.035 |
| 81–90% | 0.324 (1.350) | 0.139 (1.043) | 1.387 (0.989) | 1.151 | 0.563 | 1.327 | 0.283 | 0.021 | 0.135 | 0.027–0.094 |
| 91–100% | 0.479 (0.551) | 0.463 (0.661) | 1.167 (0.781) | 11.404 | 0.003 * | 3.721 | 0.038 * | 0.232 | 0.937 | 0.072–0.202 |
| Partial Correlation | p-Value | |
|---|---|---|
| BBS total score | 0.08 | 0.78 |
| Stand eye closed | 0.36 | 0.191 |
| Arm reaching | −0.08 | 0.782 |
| Object pick up | 0.23 | 0.403 |
| Twist turn | −0.17 | 0.545 |
| Turn 360° | 0.23 | 0.408 |
| Step on stool | 0.28 | 0.316 |
| Tandem standing | 0.05 | 0.855 |
| One leg standing | −0.13 | 0.651 |
| Faller | Non-Faller | Test Statistics | p-Value | |
|---|---|---|---|---|
| Number | 4 | 12 | ||
| Age (years) a | 71.75 (11.32) | 72.33 (8.03) | 29 | 0.624 |
| Sex (male/female) b | 4/0 | 6/6 | 4.534 | 0.0332 * |
| Height (cm) ac | 161.50 (11.32) | 155.69 (12.79) | 40 | 0.467 |
| Weight (kg) ac | 55.35 (8.94) | 55.79 (8.88) | 32 | 0.856 |
| Cane (T-cane/none) b | 2/2 | 3/9 | 0.834 | 0.361 |
| Gait speed (m/sec) ac | 0.80 (0.50) | 0.81 (0.26) | 33 | 0.951 |
| Diagnosis bd | 1.636 | 0.201 | ||
| Infarction | 2 (50%) | 10 (83%) | ||
| Hemorrhage | 2 (50%) | 2 (17%) | ||
| Time post-stroke (days) ac | 94.2 (39.2) | 90.7 (36.8) | 35 | 0.870 |
| Affected side b | 0.796 | 0.372 | ||
| Right | 3 (75%) | 6 (50%) | ||
| Left | 1 (25%) | 6 (50%) | ||
| Orthosis (AFO/None) bd | 1/3 | 0/12 | 2.983 | 0.084 |
| Fugl–Meyer Assessment ac | 29.2 (1.5) | 31.5 (3.2) | 24 | 0.287 |
| Berg Balance scale ac | 52.0 (3.7) | 51.6 (4.4) | 32 | 1.000 |
| Short Falls Efficacy Scale -International ac | 11.0 (4.3) | 11.6 (4.6) | 32 | 1.000 |
| Stroke Impairment Assessment Set (1/2/3/4/5) | ||||
| Hip flexion test b | 0/0/0/2/2 | 0/0/1/2/9 | 2.018 | 0.365 |
| Knee extension test b | 0/0/0/3/1 | 0/0/2/2/8 | 4.986 | 0.083 |
| Ankle dorsiflexion test b | 0/0/0/3/1 | 0/0/0/2/10 | 4.563 | 0.033 * |
| Faller | Non-Faller | Unadjusted p | Adjusted W | Adjusted p | Adjusted Effect Size r | Adjusted 95% CI | |
|---|---|---|---|---|---|---|---|
| Number | 4 | 12 | |||||
| Stance phase (%) | |||||||
| 1–10% | 0.154 (0.047) | 0.217 (0.122) | 0.403 | 26 | 0.403 | 0.227 | −0.302–0.650 |
| 11–20% | 0.199 (0.024) | 0.373 (0.242) | 0.431 | 17 | 0.113 | 0.500 | 0.006–0.798 |
| 21–30% | 0.208 (0.052) | 0.413 (0.298) | 0.312 | 18 | 0.120 | 0.470 | −0.034–0.783 |
| 31–40% | 0.198 (0.052) | 0.428 (0.272) | 0.091 | 19 | 0.132 | 0.440 | −0.072–0.768 |
| 41–50% | 0.208 (0.051) | 0.431 (0.236) | 0.045 * | 17 | 0.113 | 0.500 | 0.006–0.798 |
| 51–60% | 0.212 (0.053) | 0.457 (0.253) | 0.045 * | 16 | 0.113 | 0.531 | 0.047–0.813 |
| 61–70% | 0.187 (0.040) | 0.514 (0.270) | 0.045 * | 16 | 0.113 | 0.531 | 0.047–0.813 |
| 71–80% | 0.300 (0.242) | 0.547 (0.186) | 0.169 | 22 | 0.233 | 0.349 | −0.178–0.720 |
| 81–90% | 0.467 (0.431) | 0.595 (0.271) | 0.312 | 28 | 0.505 | 0.167 | −0.359–0.612 |
| 91–100% | 0.158 (0.077) | 0.336 (0.122) | 0.045 * | 23 | 0.254 | 0.318 | −0.211–0.703 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, D.; Sekiguchi, Y.; Honda, K.; Miyagi, M.; Yamaguchi, T.; Nishi, T.; Matsumoto, H.; Nakai, Y.; Sasaki, Y.; Hayasaka, J.-I.; et al. Characteristics of Ground Reaction Force Variability During Walking in Post-Stroke Patients. Sensors 2025, 25, 6940. https://doi.org/10.3390/s25226940
Naito D, Sekiguchi Y, Honda K, Miyagi M, Yamaguchi T, Nishi T, Matsumoto H, Nakai Y, Sasaki Y, Hayasaka J-I, et al. Characteristics of Ground Reaction Force Variability During Walking in Post-Stroke Patients. Sensors. 2025; 25(22):6940. https://doi.org/10.3390/s25226940
Chicago/Turabian StyleNaito, Daiki, Yusuke Sekiguchi, Keita Honda, Midori Miyagi, Takeshi Yamaguchi, Toshiaki Nishi, Hide Matsumoto, Yuzuki Nakai, Yoshihiro Sasaki, Jun-Ichi Hayasaka, and et al. 2025. "Characteristics of Ground Reaction Force Variability During Walking in Post-Stroke Patients" Sensors 25, no. 22: 6940. https://doi.org/10.3390/s25226940
APA StyleNaito, D., Sekiguchi, Y., Honda, K., Miyagi, M., Yamaguchi, T., Nishi, T., Matsumoto, H., Nakai, Y., Sasaki, Y., Hayasaka, J.-I., Haruyama, D., Watanabe, K., & Ebihara, S. (2025). Characteristics of Ground Reaction Force Variability During Walking in Post-Stroke Patients. Sensors, 25(22), 6940. https://doi.org/10.3390/s25226940






