Review of Hydrogen Sensors in Aerobic and Anaerobic Environments Coupled with Artificial Intelligence Tools
Highlights
- This article provides a comprehensive and recent survey of intelligent and resistive hydrogen sensors according to type of application, in comparison with other sensor technologies, and identifies those coupled with artificial intelligence, detailing the methods and algorithms used, and identifying those in place within sensors in the literature.
- In the literature, very few studies deal jointly with aerobic and anaerobic hydrogen applications.
- Existing hydrogen sensor technologies in the literature are listed and compared according to potential hydrogen applications.
- This review shows the types of hydrogen sensors coupled with a software layer.
- Each field of application is matched to the type of sensor used, with explicit detection ranges based on hydrogen-specific standards and regulations.
- In this paper, the use of Artificial Intelligence within aerobic and anaerobic hydrogen sensors is necessary for performance improvement.
- The main methods and algorithms used in hydrogen sensors, from the simplest to the most complex, are discussed and compared with sensors coupled with AI tools. Their performance is also assessed.
Abstract
1. Introduction
2. Standards and Applications Using Hydrogen Sensors
2.1. Standards
| Oxygen level in case of leak | <19.5% |
| Lower Explosive Limit (LEL) | 4% |
| Upper Explosive Limit (UEL) | 75% |
| Average Exposure Limit (AEL) | 8 h (1000 ppm) |
| Short Term Exposure Limit (STEL) | 15 min (1000 ppm) |
| Sensor Detection Limit (SDL) | 0.1 ppm–100% in volume (environment) |
| Recommended sensitivity range | 0.1 ppm–1% is often sufficient for
|
2.2. Hydrogen Sensors
2.3. Anaerobic Applications
2.3.1. Power to Gas (P2G)
2.3.2. Monitoring Tanks on Future Vehicles
2.4. Aerobie Applications
2.4.1. Leak Detection
2.4.2. Fuel Cells
2.4.3. Bioreactors–Electrolyzers–White Hydrogen Research
2.4.4. Chemical and Metallurgical Process Controls
2.4.5. Wastewater Treatment
2.4.6. Biology–Bacteriology
2.4.7. Healthcare–Pharmaceutical Industry
2.5. Influence of Oxygen on Hydrogen Sensors
2.6. Synthesis
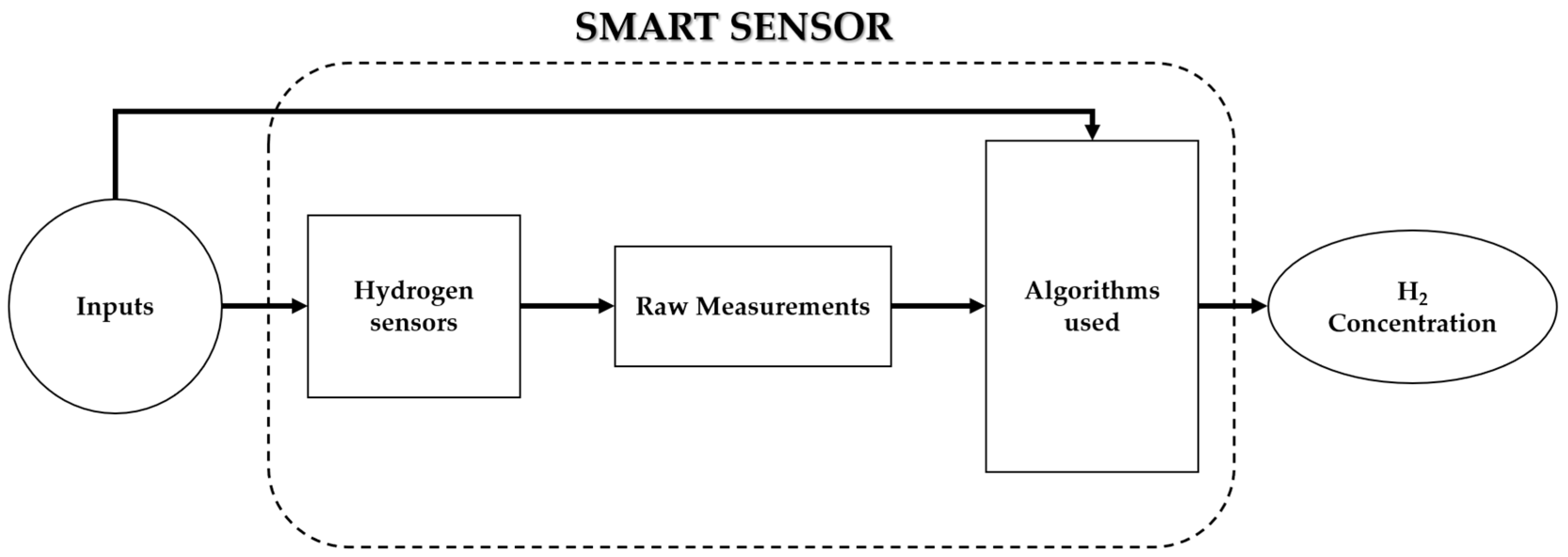
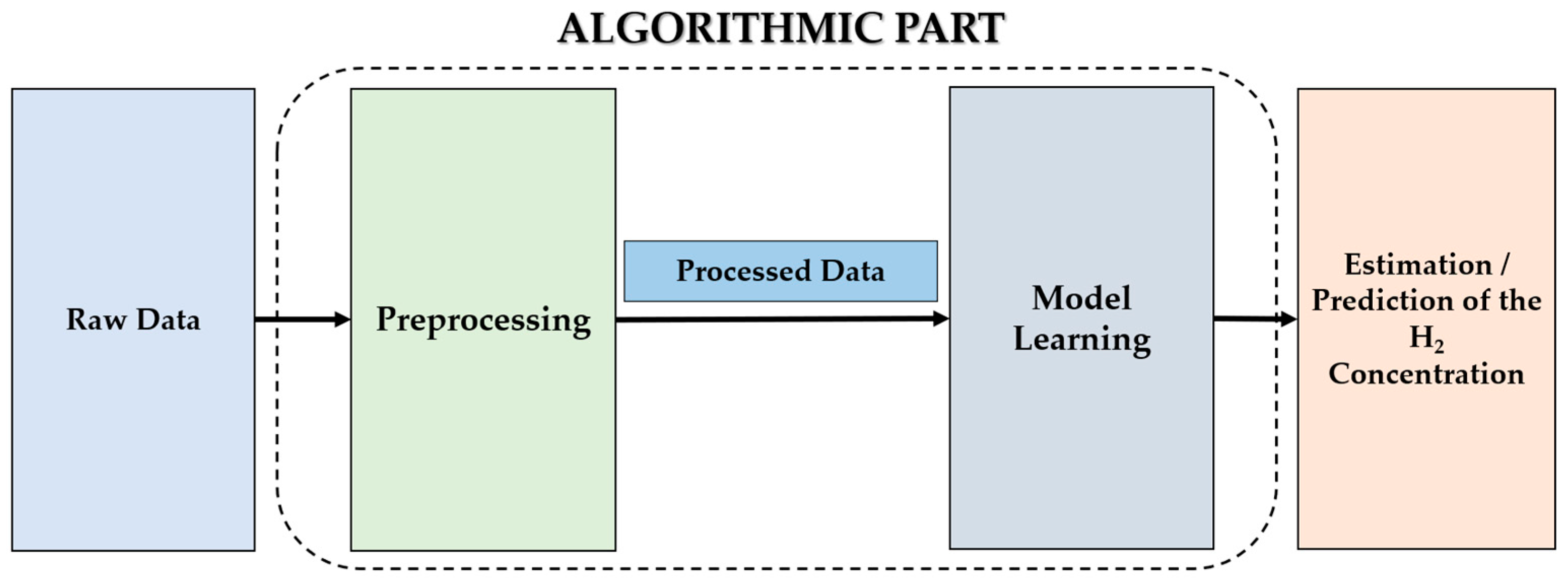
3. Hydrogen Sensors Coupled with Artificial Intelligence
3.1. Hydrogen Sensor Design Improvement
3.2. Improving the Performance of Hydrogen Sensors
- Support Vector Machines (SVM) [164] are based on separation plane calculations [165,166]. They can be used to classify hydrogen concentration levels or perform complex regressions [167]. SVMs are used for classification problems with a nonlinear feature space in [168,169]. In [126,127], SVMs achieve 96% performance for hydrogen selectivity.
- SVMs and RFs are powerful tools for regression and classification. When classes overlap and are not physically separable (by hyperplanes), the RF algorithm is more appropriate as it is based on a probabilistic decision process [171,172]. If the classes are separable, even when the separating hyperplanes are strongly nonlinear, an SVM, thanks to the multitude of kernels and kernel combinations, manages to compute the separating hyperplanes [167,169,173].
- Convolutional Neural Networks (CNNs) [165,166] combines feature extraction and model learning. Inputs are raster images where each pixel corresponds to the normalized resistance obtained by an optical sensor [14,132,133]; CNNs [174,177] are frequently used for classification [178] and lead to optimum performance (almost 100%) for the identification of hydrogen among CO, NH3, or H2S [14]. Although used for image analysis, CNNs can also be applied to time series and sensor signal data, since they can extract important features from resistance signals such as patterns or trends, and thus improve the accuracy of predictions [179].
- Continuous Markov models are stochastic models that can be used to estimate hydrogen concentrations in the presence of uncertainties [188].
- Recent work in the IM2NP laboratory has enabled us to study the prediction of hydrogen content in natural gas pipelines supplying industrial machinery, in the context of power to gas technology, using geometric models associated with a PdAu alloy resistive hydrogen sensor, providing results for hydrogen detection, with promising performance in terms of selectivity, stability, and sensitivity [45].
- Filtering methods: These methods are not based on the classification/regression model. They evaluate each feature independently, usually using statistical measures or correlation coefficients with the target variable. Consequently, model training and performance evaluation take place after the relevant features have been selected. They do not take into account the impact of each feature on the others, but simply evaluate them individually [193,194,195].
- Wrapper methods: These methods select features according to their impact on model performance. The specific machine learning algorithm is used to evaluate the effect of each feature or group of features on model performance. Features are retained or removed according to their ability to improve model performance. This technique is distinguished by its accuracy and its ability to take into account interactions between features [195,196].
- Integrated methods: These methods enable features to be selected directly during model training. Each model or algorithm selects the most important features according to the mechanism used during training (coefficient evaluation, data slicing, etc.). Unlike the Wrapper method, which involves training the model on selected features and then evaluating its performance, the Embedded technique stands out for its speed and high accuracy. However, it may not always achieve the same level of accuracy as the Wrapper method [195,197]. The CNN used in [14,19,132,133] is one of the methods that integrates the extraction/selection step through its convolutional layer.
3.3. Artificial Intelligence in Gas Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| IEA | International Energy Agency |
| OSHA | Occupational Safety and Health Administration |
| INRS | National Institute for Research and Security |
| NIOSH | National Institute for Occupational Safety and Health |
| LEL | Lower Explosive Limit |
| UEL | Upper Explosive Limit |
| AEL | Average Exposure Limit |
| STEL | Short Term Exposure Limit |
| SDL | Sensor Detection Limit |
| HDR | Hydrogen Detection Response |
| ML | Machine Learning |
| DL | Deep Learning |
| MM | Mathematical Modeling |
| GEP | Gene Expression Program |
| LS-SVR | Least Squares Support Vector Regression |
| MLPNN | Multilayer Perceptron Neural Networks |
| SVM | Support Vector Machine |
| PCA | Principal Component Analysis |
| CNN | Convolutional Neural Network |
| ANN | Artificial Neural Network |
| RNN | Recurrent Neural Network |
| MLP | Multi-Layer Perceptron |
| FFT | Fast Fourier Transform |
| RF | Random Forest |
| KNN | K-nearest neighbors |
| LSTM | Long Short-Term Memory |
References
- Hydrogen, IEA. Available online: https://www.iea.org/energy-system/low-emission-fuels/hydrogen (accessed on 10 April 2025).
- International Energy Agency (IEA). World Energy Outlook 2023; International Energy Agency (IEA): Paris, France, 2023; 355p. [Google Scholar]
- European Environment Agency’s Home Page. Available online: https://www.eea.europa.eu/en (accessed on 10 April 2025).
- Accueil|France Stratégie. Available online: https://www.strategie.gouv.fr/ (accessed on 10 April 2025).
- Behrendt, F. The future of industrial hydrogen: Renewable sources and applications for the next 15 years. Clean Energy 2025, 9, 3–8. [Google Scholar] [CrossRef]
- France Hydrogène. Mémento de l’Hydrogène; France Hydrogène: Paris, France, 2019. [Google Scholar]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Patil, R.R.; Calay, R.K.; Mustafa, M.Y.; Thakur, S. Artificial Intelligence—Driven Innovations in Hydrogen Safety. Hydrogen 2024, 5, 312–326. [Google Scholar] [CrossRef]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic Noses: From Advanced Materials to Sensors Aided with Data Processing. Adv. Mater. Technol. 2019, 4, 1800488. [Google Scholar] [CrossRef]
- Galstyan, V.E.; Martirosyan, K.S.; Aroutiounian, V.M.; Arakelyan, V.M.; Arakelyan, A.H.; Soukiassian, P.G. Investigations of hydrogen sensors made of porous silicon. Thin Solid Film. 2008, 517, 239–241. [Google Scholar] [CrossRef]
- Shi, C.; Pei, W.; Jin, C.; Alizadeh, A.; Ghanbari, A. Prediction of the SnO2-based sensor response for hydrogen detection by artificial intelligence techniques. Int. J. Hydrogen Energy 2023, 48, 19834–19845. [Google Scholar] [CrossRef]
- Van, K.D.; Hieu, N.V.; Yang, T.C.-K.; Manh, T.L. Rapid Detection of Ultralow H2S Concentration with on-chip Fabrication of SnO2-based Gas Sensors by Direct Electrodeposition from Non-Aqueous Solvents. J. Electrochem. Soc. 2024, 171, 097506. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Luo, C.; Wang, X.; Yang, M.; Xiong, Z.; Gu, J.; Gong, Z.; Wei, Z.; Qian, F. SnO2-based resistive hydrogen gas sensor: A comprehensive review from performance to function optimization. Mater. Sci. Semicond. Process. 2025, 188, 109209. [Google Scholar] [CrossRef]
- Yan, S.; Cao, Y.; Su, Y.; Huang, B.; Chen, C.; Yu, X.; Xu, A.; Wu, T. Hydrogen Sensors Based on Pd-Based Materials: A Review. Sensors 2025, 25, 3402. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. ppb-Level Selective Hydrogen Gas Detection of Pd-Functionalized In2O3-Loaded ZnO Nanofiber Gas Sensors. Sensors 2019, 19, 4276. [Google Scholar] [CrossRef] [PubMed]
- Ilnicka, A.; Lukaszewicz, J.P. Graphene-Based Hydrogen Gas Sensors: A Review. Processes 2020, 8, 633. [Google Scholar] [CrossRef]
- Patel, P.; Garaniya, V.; Baalisampang, T.; Arzaghi, E.; Abbassi, R.; Salehi, F. A technical review on quantitative risk analysis for hydrogen infrastructure. J. Loss Prev. Process Ind. 2024, 91, 105403. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, H.; Fan, Y.; Yang, X.; Zhang, H.; Wu, W.; Zhou, Y.; Hu, H. Recent advances in gas and environmental sensing: From micro/nano to the era of self-powered and artificial intelligent (AI)-enabled device. Microchem. J. 2022, 181, 107833. [Google Scholar] [CrossRef]
- Salimian, A. Quantitative hydrogen and methane gas sensing via implementing AI based spectral analysis of plasma discharge. Int. J. Hydrogen Energy 2024, 50, 1157–1173. [Google Scholar] [CrossRef]
- Hu, D.; Gao, P.; Cheng, Z.; Shen, Y.; He, R.; Yi, F.; Lu, M.; Wang, J.; Liu, S. Comprehensive Review of Hydrogen Leakage in Relation to Fuel Cell Vehicles and Hydrogen Refueling Stations: Status, Challenges, and Future Prospects. Energy Fuels 2024, 38, 4803–4835. [Google Scholar] [CrossRef]
- An, Y.; Loh, T.Y.; Bhaskara, P.S.; Sin, S.M.I. Review of safety regulations, codes, and standards (RCS) for hydrogen distribution and application. Int. J. Green Energy 2024, 21, 1757–1765. [Google Scholar] [CrossRef]
- OSHA. Occupational Safety and Health Administration. Available online: https://www.osha.gov/ (accessed on 10 April 2025).
- INRS. Valeurs Limites D’exposition Pour la Prévention des Risques Chimique; INRS: Paris, France, 2016. [Google Scholar]
- INRS. Détecteurs Portables de Gaz et de Vapeurs; INRS: Paris, France, 2022. [Google Scholar]
- ISO/TR 15916:2015; Basic Considerations for the Safety of Hydrogen Systems. ISO: Geneve, Switzerland, 2015.
- INRS. Hydrogène—Fiche Toxicologique; INRS: Paris, France, 2021. [Google Scholar]
- Ministères Aménagement du Territoire Transition Écologique. Risques Technologiques: La Directive SEVESO et la loi Risques. Available online: https://www.ecologie.gouv.fr/politiques-publiques/risques-technologiques-directive-seveso-loi-risques (accessed on 2 November 2025).
- CDC. National Institute for Occupational Safety and Health; National Institute for Occupational Safety and Health (NIOSH): Washington, DC, USA, 2025. Available online: https://www.cdc.gov/niosh/index.html (accessed on 10 April 2025).
- INRS. Valeurs Limites D’exposition Professionnelle aux Agents Chimiques en France; INRS: Paris, France, 2016. [Google Scholar]
- Comment Fonctionnent les Capteurs des Détecteurs de gaz?—ANATECS. Available online: https://safetylife.fr/content/20-comment-fonctionnent-capteurs-detecteurs-gaz?srsltid=AfmBOopdv58L3uuFIawsRzbkqaNQYSDcodWa3EkpQ9KUSJe1sNeq-GL8 (accessed on 19 June 2025).
- Ivanov, I.I.; Baranov, A.M.; Talipov, V.A.; Mironov, S.M.; Akbari, S.; Kolesnik, I.V.; Orlova, E.D.; Napolskii, K.S. Investigation of catalytic hydrogen sensors with platinum group catalysts. Sens. Actuators B Chem. 2021, 346, 130515. [Google Scholar] [CrossRef]
- Panama, G.; Lee, H.-O.; Bae, J.; Lee, S.S. Hydrogen Sensor with a Thick Catalyst Layer Anchored on Polyimide Film. Adv. Mater. Technol. 2024, 9, 2400445. [Google Scholar] [CrossRef]
- Menon, S.K.; Kumar, A.; Mondal, S. Advancements in hydrogen gas leakage detection sensor technologies and safety measures. Clean Energy 2025, 9, 263–277. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef]
- Li, W.; Sokolovskij, R.; Jiang, Y.; Wen, K.; Hu, Q.; Deng, C.; Wang, Q.; Yu, H. Hydrogen Detection Performance of a Pt-AlGaN/GaN HEMT Sensor at High Temperatures in Air Ambient. J. Electrochem. Soc. 2024, 171, 127513. [Google Scholar] [CrossRef]
- Bas, S.Z.; Cummins, C.; Borah, D.; Ozmen, M.; Morris, M.A. Electrochemical Sensing of Hydrogen Peroxide Using Block Copolymer Templated Iron Oxide Nanopatterns. Anal. Chem. 2018, 90, 1122–1128. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrogen Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Djeziri, M.; Benmoussa, S.; Bendahan, M.; Seguin, J.L. Review on data-driven approaches for improving the selectivity of MOXsensors. Microsyst. Technol. 2024, 7, 791–807. [Google Scholar] [CrossRef]
- Bendimerad, M. Memoire Online—Réalisation d’un capteur de gaz MOX. Available online: https://www.memoireonline.com/11/10/4074/m_Realisation-dun-capteur-de-gaz-MOX5.html (accessed on 3 October 2024).
- Kafil, V.; Sreenan, B.; Hadj-Nacer, M.; Wang, Y.; Yoon, J.; Greiner, M.; Chu, P.; Wang, X.; Fadali, M.S.; Zhu, X. Review of noble metal and metal-oxide-semiconductor based chemiresistive hydrogen sensors. Sens. Actuators Phys. 2024, 373, 115440. [Google Scholar] [CrossRef]
- Tang, Y.; Zhoa, Y.; Liu, H. Room-Temperature Semiconductor Gas Sensors: Challenges and Opportunities. ACS Sens. 2022, 7, 3582–3597. [Google Scholar] [CrossRef]
- Occelli, C.; Fiorido, T.; Perrin-Pellegrino, C.; Seguin, J.-L. Sensors for anaerobic hydrogen measurement: A comparative study between a resistive PdAu based sensor and a commercial thermal conductivity sensor. Int. J. Hydrogen Energy 2023, 48, 17729–17741. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.; Choi, S.; Eom, T.H.; Cho, S.H.; Park, S.; Park, S.H.; Kim, J.Y.; Kim, J.; Nam, G.B.; et al. Highly Durable Chemoresistive Micropatterned PdAu Hydrogen Sensors: Performance and Mechanism. ACS Sens. 2024, 9, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, I.; Khairunnisa, S.Z.; Tomeček, D.; Langhammer, C. Optimization of the Composition of PdAuCu Ternary Alloy Nanoparticles for Plasmonic Hydrogen Sensing. ACS Appl. Nano Mater. 2021, 4, 8716–8722. [Google Scholar] [CrossRef]
- Djeziri, M.; Benmoussa, S.; Occelli, C.; Fiorido, T.; Seguin, J.-L.; Bendahan, M. Hydrogen rate prediction in natural-gas pipes supplying industrial machines in the frame of power-to-gas technology. IFAC-Paper 2024, 58, 479–483. [Google Scholar] [CrossRef]
- Sowmya, N.; Ponnusamy, V. Development of Spectroscopic Sensor System for an IoT Application of Adulteration Identification on Milk Using Machine Learning. IEEE Access 2021, 9, 53979–53995. [Google Scholar] [CrossRef]
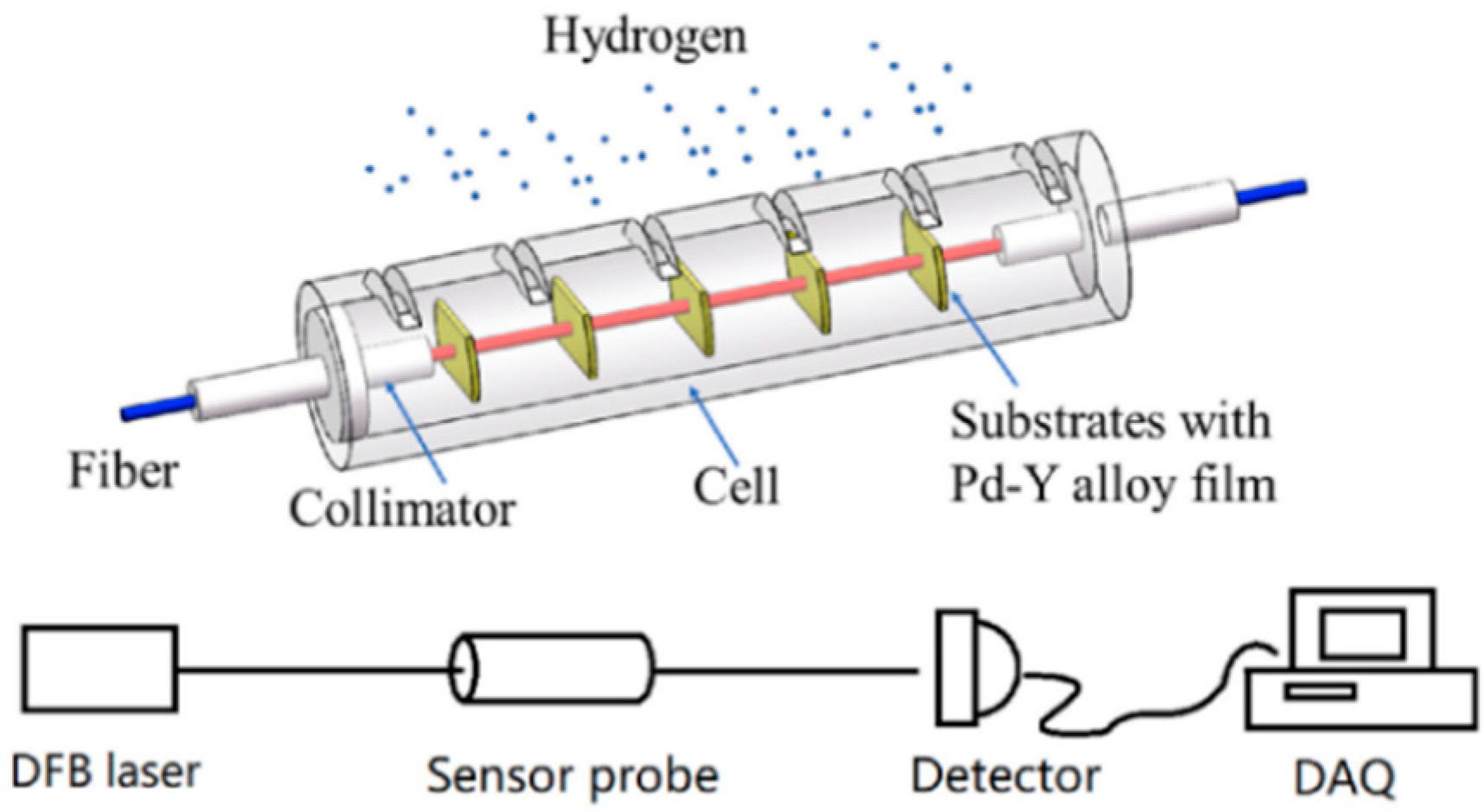
- Liu, Y.; Li, Y. Signal analysis and processing method of transmission optical fiber hydrogen sensors with multi-layer Pd–Y alloy films. Int. J. Hydrogen Energy 2019, 44, 27151–27158. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Darmadi, I.; Zhdanov, V.P.; Langhammer, C. Universal Scaling and Design Rules of Hydrogen-Induced Optical Properties in Pd and Pd-Alloy Nanoparticles. ACS Nano 2018, 12, 9903–9912. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, L.; Wang, G.; Xiang, F.; Qin, Y.; Wang, M.; Yang, M. Optical Fiber Grating Hydrogen Sensors: A Review. Sensors 2017, 17, 577. [Google Scholar] [CrossRef]
- Shen, C.; Xie, Z.; Huang, Z.; Yan, S.; Sui, W.; Zhou, J.; Wang, Z.; Han, W.; Zeng, X. Review of the Status and Prospects of Fiber Optic Hydrogen Sensing Technology. Chemosensors 2023, 11, 473. [Google Scholar] [CrossRef]
- Jupiter 1000—Power-to-Gas—Le Projet. Available online: https://www.jupiter1000.eu/projet (accessed on 10 April 2025).
- Occelli, C. Micro-Capteurs Pour la Mesure de L’hydrogène Injecté dans les Réseaux de gaz Nature, dans le Contexte de la Technologie “Power to Gas”. Ph.D. Thesis, Aix-Marseille University, Marseille, France, 2023. [Google Scholar]
- Korotcenkov, G.; Liu, Y.; Stetter, J.R.; Tang, Y.; Zeng, X. Electrochemical gas sensors: Fundamentals, fabrication and parameters. In Chemical Sensors: Comprehensive Sensor Technologies. Vol. 5. Electrochemical and Optical Sensors; Momentum Press: New York, NY, USA, 2011; Volume 5, pp. 1–89. Available online: https://www.researchgate.net/publication/288761637_Electrochemical_gas_sensors_Fundamentals_fabrication_and_parameters (accessed on 13 June 2025).
- Wang, J.; Liu, Y.; Zhou, H.; Wang, Y.; Wu, M.; Huang, G.; Li, T. Thermal Conductivity Gas Sensor with Enhanced Flow-Rate Independence. Sensors 2022, 22, 1308. [Google Scholar] [CrossRef] [PubMed]
- Aroutiounian, V. Metal oxide hydrogen, oxygen, and carbon monoxide sensors for hydrogen setups and cells. Int. J. Hydrogen Energy 2007, 32, 1145–1158. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, C.; Yang, L.; Zhang, Z.; Han, Z. Étude des propriétés de détection de H 2 d’ un film mince multicouche mésoporeux pur et dopé au Pd de SnO2. Sens. Actuators B Chem. 2019, 283, 399–406. [Google Scholar] [CrossRef]
- Westerwaal, R.J.; Rooijmans, J.S.A.; Leclercq, L.; Gheorghe, D.G.; Radeva, T.; Mooij, L.; Mak, T.; Polak, L.; Slaman, M.; Dam, B.; et al. Nanostructured Pd–Au based fiber optic sensors for probing hydrogen concentrations in gas mixtures. Int. J. Hydrogen Energy 2013, 38, 4201–4212. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Kadkhodazadeh, S.; Wagner, J.B.; Langhammer, C. Rationally Designed PdAuCu Ternary Alloy Nanoparticles for Intrinsically Deactivation-Resistant Ultrafast Plasmonic Hydrogen Sensing. ACS Sens. 2019, 4, 1424–1432. [Google Scholar] [CrossRef]
- ResearchGate. (PDF) Hydrogen Sensing and Detection. Available online: https://www.researchgate.net/publication/265541692_Hydrogen_Sensing_and_Detection (accessed on 5 August 2025).
- Y a-t-il une Place Pour L’hydrogène dans la Transition Énergétique. Available online: https://www.actu-environnement.com/media/pdf/news-22533-hydrogene-france-strategie.pdf (accessed on 11 April 2025).
- Lee, J.-S.; An, J.W.; Bae, S.; Lee, S.-K. Review of Hydrogen Gas Sensors for Future Hydrogen Mobility Infrastructure. Appl. Sci. Converg. Technol. 2022, 31, 79–84. [Google Scholar] [CrossRef]
- Amid, A.; Mignard, D.; Wilkinson, M. Seasonal storage of hydrogen in a depleted natural gas reservoir. Int. J. Hydrogen Energy 2016, 41, 5549–5558. [Google Scholar] [CrossRef]
- Chi, G.; Xu, S.; Yu, D.; Wang, Z.; He, Z.; Wang, K.; Zhou, Q. A brief review of structural health monitoring based on flexible sensing technology for hydrogen storage tank. Int. J. Hydrogen Energy 2024, 80, 980–998. [Google Scholar] [CrossRef]
- Li, X. Status and development of hydrogen preparation, storage and transportation. Chin. Sci. Bull. 2022, 67, 425–436. [Google Scholar] [CrossRef]
- Qanbar, M.W.; Hong, Z. A Review of Hydrogen Leak Detection Regulations and Technologies. Energies 2024, 17, 4059. [Google Scholar] [CrossRef]
- A Leakage Detection Method for Hydrogen-Blended Natural Gas Pipelines in Utility Tunnels Based on Multi-Task LSTM and CFD Simulation-Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:001375529400001 (accessed on 11 June 2025).
- Javahiraly, N.; Perrotton, C.; Meyrueis, P.; Dam, B. Study of a fiber optic sensor for hydrogen leak detection. In Photonic Applications for Aerospace, Commercial, and Harsh Environments IV, Proceedings of the SPIE, Baltimore, MD, USA, 29 April–1 May 2013; Kazemi, A.A., Kress, B.C., Thibault, S., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2013; Volume 8720, p. 872004. [Google Scholar]
- A Robust Organic Hydrogen Sensor for Distributed Monitoring Applications-Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:001438316100001 (accessed on 11 April 2025).
- Collina, G.; Bucelli, M.; Paltrinieri, N. Multi-stage monitoring of hydrogen systems for improved maintenance approaches: An extensive review. Int. J. Hydrogen Energy 2025, 105, 458–480. [Google Scholar] [CrossRef]
- Preti, D.; Squarcialupi, S.; Fachinetti, G. Aerobic, Copper-Mediated Oxidation of Alkaline Formaldehyde to Fuel-Cell Grade Hydrogen and Formate: Mechanism and Applications. Angew. Chem. Int. Ed. 2009, 48, 4763–4766. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, K.; Roger, M.; Gutierrez-Sanchez, C.; Ilbert, M.; Nitsche, S.; Byrne-Kodjabachian, D.; Marchi, V.; Lojou, E. Hydrogen bioelectrooxidation on gold nanoparticle-based electrodes modified by Aquifex aeolicus hydrogenase: Application to hydrogen/oxygen enzymatic biofuel cells. Bioelectrochemistry 2015, 106, 47. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Ezugwu, C.I.; Ghosh, P.C. Microbial Fuel Cell-Based Biological Oxygen Demand Sensors for Monitoring Wastewater: State-of-the-Art and Practical Applications. ACS Sens. 2020, 5, 2297–2316. [Google Scholar] [CrossRef]
- Markov, S.A. Bioreactors for hydrogen production. In Biohydrogen; Zaborsky, O.R., Ed.; Plenum Press Div Plenum Publishing Corp: New York, NY, USA, 1998; pp. 383–390. [Google Scholar]
- Markov, S.A. Hydrogen production in bioreactors: Current trends. In Proceedings of the WHEC 2012 Conference Proceedings—19th World Hydrogen Energy Conference, Toronto, ON, Canada, 3–7 June 2012; Elsevier Science Bv: Amsterdam, The Netherlands, 2012; Volume 29, pp. 394–400. [Google Scholar]
- Qyyum, M.A.; Ihsanullah, I.; Ahmad, R.; Ismail, S.; Khan, A.; Nizami, A.-S.; Tawfik, A. Biohydrogen production from real industrial wastewater: Potential bioreactors, challenges in commercialization and future directions. Int. J. Hydrogen Energy 2022, 47, 37154–37170. [Google Scholar] [CrossRef]
- Shandarr, R.Y.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Losiewicz, B. Technology for Green Hydrogen Production: Desk Analysis. Energies 2024, 17, 4514. [Google Scholar] [CrossRef]
- Akyuz, S.; Telli, E.; Farsak, M. Hydrogen generation electrolyzers: Paving the way for sustainable energy. Int. J. Hydrogen Energy 2024, 81, 1338–1362. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Xiao, A.; Zhu, L.; Yang, Z. Hydrogen sensing towards palladium-based nanocomposites: A review. Int. J. Hydrogen Energy 2024, 136, 1282–1305. [Google Scholar] [CrossRef]
- Fetter, K.L.; Munera, L.; Watts, M.A.; Pineda, D.I. Interband cascade laser absorption sensor for sensitive measurement of hydrogen chloride in smoke-laden gases using wavelength modulation spectroscopy. Appl. Opt. 2024, 63, 8517–8525. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. An overview of solar decarbonization processes, reacting oxide materials, and thermochemical reactors for hydrogen and syngas production. Int. J. Hydrogen Energy 2020, 45, 25783–25810. [Google Scholar] [CrossRef]
- Qiu, Z.; Yue, Q.; Yan, T.; Wang, Q.; Sun, J.; Yuan, Y.; Che, Z.; Wang, Y.; Du, T. Gas utilization optimization and exergy analysis of hydrogen metallurgical shaft furnace. Energy 2023, 263, 125847. [Google Scholar] [CrossRef]
- Protasova, L.; Snijkers, F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75–93. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Adeniran, J.A.; Lototskyy, M.; Asadi, A. Simultaneous brewery wastewater treatment and hydrogen generation via hydrolysis using Mg waste scraps. J. Clean. Prod. 2020, 276, 123198. [Google Scholar] [CrossRef]
- Salem, R.M.M.; Saraya, M.S.; Ali-Eldin, A.M.T. An Industrial Cloud-Based IoT System for Real-Time Monitoring and Controlling of Wastewater. IEEE Access 2022, 10, 6528–6540. [Google Scholar] [CrossRef]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef]
- Mccurry, M.D.; D’Agostino, G.D.; Walsh, J.T.; Bisanz, J.E.; Zalosnik, I.; Dong, X.; Morris, D.J.; Korzenik, J.R.; Edlow, A.G.; Balskus, E.P.; et al. Gut bacteria convert glucocorticoids into progestins in the presence of hydrogen gas. Cell 2024, 187, 2952–2968. [Google Scholar] [CrossRef] [PubMed]
- Gullino, A.; Grassini, S.; Gugliandolo, G.; Moulaee, K.; Donato, N.; Parvis, M.; Lombardo, L. Hydrogen chemoresistive sensor for the analysis of gut health. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications (IEEE MeMeA 2021), Lausanne, Switzerland, 23–25 June 2021; IEEE: New York, NY, USA, 2021. [Google Scholar]
- Barbu, A.; Jansson, L.; Sandberg, M.; Quach, M.; Palm, F. The use of hydrogen gas clearance for blood flow measurements in single endogenous and transplanted pancreatic islets. Microvasc. Res. 2015, 97, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Logue, F.N.; Ramaswamy, K.; Hersh, J.H. Investigation of illness associated with exposure to hydrogen sulfide among Pennsylvania school students. J. Environ. Health 2001, 63, 9–13. [Google Scholar]
- Hutchins, K.M. Functional materials based on molecules with hydrogen-bonding ability: Applications to drug co-crystals and polymer complexes. R. Soc. Open Sci. 2018, 5, 180564. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kiwa, T.; Tsukada, K.; Yokosawa, K. Oxygen interference mechanism of platinum-FET hydrogen gas sensor. Sens. Actuators Phys. 2007, 136, 244–248. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, Z.; Liu, X.; Chen, Z.; Sun, Y.; Dai, N.; Ge, M. Humidity-tolerant and highly sensitive gas sensor for hydrogen sulfide based on WO 3 nanocubes modified with CeO2. RSC Adv. 2024, 14, 15039–15047. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ye, J.; Zheng, L.; Zou, K. The hydrogen sensing properties of Pt–Pd/reduced graphene oxide based sensor under different operating conditions. RSC Adv. 2016, 6, 24880–24888. [Google Scholar] [CrossRef]
- Antony, C.E.; Jayakumar, A.; Yadav, A.; Sivakumar, N.S.; Kamath, N.; Kamble, V.B.; Jaiswal-Nagar, D. Metal-polymer hybrid chemiresistive sensor for low concentration fast hydrogen detection. arXiv 2020, arXiv:2011.07599. [Google Scholar] [CrossRef]
- Luong, H.M.; Pham, M.T.; Guin, T.; Madhogaria, R.P.; Phan, M.-H.; Larsen, G.K.; Nguyen, T.D. Sub-second and ppm-level Optical Sensing of Hydrogen Using Templated Control of Nano-hydride Geometry and Composition. Nat. Commun. 2021, 12, 2414. [Google Scholar] [CrossRef]
- Kumar, V.; Gautam, Y.K.; Gautam, D.; Kumar, A.; Adalati, R.; Singh, B.P. Highly Sensitive and Selective Hydrogen Gas Sensor with Humidity Tolerance Using Pd-Capped SnO2 Thin Films of Various Thicknesses. Fuels 2023, 4, 279–294. [Google Scholar] [CrossRef]
- Melios, C.; Winters, M.; Strupinski, W.; Panchal, V.; Giusca, C.E.; Jayawardena, K.D.G.I.; Rorsman, N.; Silva, S.R.P.; Kazakova, O. Tuning epitaxial graphene sensitivity to water by hydrogen intercalation. Nanoscale 2017, 9, 3440–3448. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-Performance Nanostructured Palladium-Based Hydrogen Sensors—Current Limitations and Strategies for Their Mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Lawaniya, S.D.; Kumar, S.; Dwivedi, P.K.; Awasthi, K. A highly selective, efficient hydrogen gas sensor based on bimetallic (Pd–Au) alloy nanoparticle (NP)-decorated SnO2 nanorods. J. Mater. Chem. A 2023, 11, 26687–26697. [Google Scholar] [CrossRef]
- Meillaud, L. La Guyane se Lance dans L’hydrogène Pour la Fusée Ariane 6. H2Today. Available online: https://hydrogentoday.info/guyane-hydrogene-fusee-ariane-6/ (accessed on 29 October 2025).
- IMOCA OceansLab: Le Bateau de Course à Hydrogène sur le Point D’achever sa Construction, Bateau-Electrique.com. Available online: https://www.bateau-electrique.com/actualites/imoca-oceanslab-bateau-course-hydrogene-sur-point-achever-construction/ (accessed on 29 October 2025).
- Cet Avion Hypersonique à Hydrogène Pourrait être le Plus Rapide au Monde. Available online: https://www.h2-mobile.fr/actus/avion-hypersonique-hydrogene-plus-rapide-monde/ (accessed on 29 October 2025).
- Li, H.-W.; Onoue, K. Compressed Hydrogen: High-Pressure Hydrogen Tanks. In Hydrogen Energy Engineering: A Japanese Perspective; Sasaki, K., Li, H.-W., Hayashi, A., Yamabe, J., Ogura, T., Lyth, S.M., Eds.; Springer: Tokyo, Japan, 2016; pp. 273–278. ISBN 978-4-431-56042-5. [Google Scholar]
- Dai, J.; Li, Y.; Ruan, H.; Ye, Z.; Chai, N.; Wang, X.; Qiu, S.; Bai, W.; Yang, M. Fiber Optical Hydrogen Sensor Based on WO3-Pd2Pt-Pt Nanocomposite Films. Nanomaterials 2021, 11, 128. [Google Scholar] [CrossRef]
- Pathak, A.K.; Verma, S.; Sakda, N.; Viphavakit, C.; Chitaree, R.; Rahman, B.M.A. Recent Advances in Optical Hydrogen Sensor including Use of Metal and Metal Alloys: A Review. Photonics 2023, 10, 122. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Li, J.; Luo, C.; Xu, X.; Qian, F. Solid-state electrochemical hydrogen sensors: A review. Int. J. Hydrogen Energy 2023, 48, 31377–31391. [Google Scholar] [CrossRef]
- Hoffmann, M.; Wienecke, M.; Ciudin, R. MEMS-Based Hydrogen Sensors: A State of the Art Review | Request PDF. In Proceedings of the 2023 International Interdisciplinary PhD Workshop, Wismar, Germany, 3–5 May 2023. [Google Scholar] [CrossRef]
- Lee, B.; Cho, S.; Jeong, B.J.; Lee, S.H.; Kim, D.; Kim, S.H.; Park, J.-H.; Yu, H.K.; Choi, J.-Y. Highly responsive hydrogen sensor based on Pd nanoparticle-decorated transfer-free 3D graphene. Sens. Actuators B Chem. 2024, 401, 134913. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Guo, Y.; Ye, S.; Wang, T.; Fu, Y.; Zhang, X. Hydrogen-Induced Aggregation of Au@Pd Nanoparticles for Eye-Readable Plasmonic Hydrogen Sensors. ACS Sens. 2022, 7, 2778–2787. [Google Scholar] [CrossRef]
- Xing, Q.; Chen, X.; Cai, Y.; Zhang, M. Composites homogènes à base de SnO2 riches en défauts d’oxygène pour capteur d’hydrogène à réponse et récupération rapides. Sens. Actuators B Chem. 2024, 419, 136407. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Tang, M.; Wang, Z.; Zhang, D. Capteur d’hydrogène à réponse rapide basé sur l’hétérojonction de nanofeuilles de MXène et de SnO2 pour la détection des défaillances des batteries lithium-ion. Sens. Actuators B Chem. 2024, 405, 135229. [Google Scholar] [CrossRef]
- Xing, Q.; Cai, Y.; Zhang, M. A sub-second response/recovery hydrogen sensor based on multifunctional palladium oxide modified heterojunctions. Sens. Actuators B Chem. 2024, 401, 134956. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, Z.; Jiang, C.; Zhang, X.; Qiao, Z.; Zhang, J.; Liang, J.; Wang, S.; Duan, Z.; Wu, Y.; et al. Ultrafast Hydrogen Detection System Using Vertical Thermal Conduction Structure and Neural Network Prediction Algorithm Based on Sensor Response Process. ACS Sens. 2025, 10, 2181–2190. [Google Scholar] [CrossRef]
- Morsi, I. A microcontroller based on multi sensors data fusion and artificial intelligent technique for gas identification. In IECON 2007: 33rd Annual Conference of the IEEE Industrial Electronics Society, Vols 1–3, Conference Proceedings; Institute of Electrical and Electronics Engineers, Industrial Electronics Society: Piscataway, NJ, USA, 2007; pp. 2203–2208. [Google Scholar]
- Abiola, A.; Manzano, F.S.; Andujar, J.M. A Novel Deep Reinforcement Learning (DRL) Algorithm to Apply Artificial Intelligence-Based Maintenance in Electrolysers. Algorithms 2023, 16, 541. [Google Scholar] [CrossRef]
- Shemyakina, A.A.; Levina, A.I.; Korablev, V.V.; Lepekhin, A.A. Architecture of the management system for hydrogen production at hydropplications. Int. J. Hydrogen Energy 2024, 69, 1227–1235. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Phanichphant, S. H2 Sensing Response of Flame-spray-made Ru/SnO2 Thick Films Fabricated from Spin-Coated Nanoparticles. Sensors 2009, 9, 8996–9010. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, Q.; Xu, L.; Gui, Y.; Zhao, Z.; Tang, C.; Chen, W. Synthesis and Characterization of Highly Sensitive Hydrogen (H2) Sensing Device Based on Ag Doped SnO2 Nanospheres. Materials 2018, 11, 492. [Google Scholar] [CrossRef]
- Liu, L.; Guo, C.; Li, S.; Wang, L.; Dong, Q.; Li, W. Improved H2 sensing properties of Co-doped SnO2 nanofibers. Sens. Actuators B Chem. 2010, 150, 806–810. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Tuantranont, A.; Wisitsoraat, A.; Phanichphant, S. The effect of Pt nanoparticles loading on H2 sensing properties of flame-spray-made SnO2 sensing films. Mater. Chem. Phys. 2014, 147, 661–672. [Google Scholar] [CrossRef]
- Nazari, A. Prediction performance of PEM fuel cells by gene expression programming. Int. J. Hydrogen Energy 2012, 37, 18972–18980. [Google Scholar] [CrossRef]
- Nabipour, N.; Qasem, S.N.; Salwana, E.; Baghban, A. Evolving LSSVM and ELM models to predict solubility of non-hydrocarbon gases in aqueous electrolyte systems. Measurement 2020, 164, 107999. [Google Scholar] [CrossRef]
- Moosavi, S.R.; Vaferi, B.; Wood, D.A. Auto-detection interpretation model for horizontal oil wells using pressure transient responses. Adv. Geo-Energy Res. 2020, 4, 305–316. [Google Scholar] [CrossRef]
- Ghate, V.N.; Dudul, S.V. Cascade Neural-Network-Based Fault Classifier for Three-Phase Induction Motor. IEEE Trans. Ind. Electron. 2011, 58, 1555–1563. [Google Scholar] [CrossRef]
- Kumar, M.; Singh Bhati, V.; Ranwa, S.; Singh, J.; Kumar, M. Pd/ZnO nanorods based sensor for highly selective detection of extremely low concentration hydrogen. Sci. Rep. 2017, 7, 236. [Google Scholar] [CrossRef]
- Zhu, J.; Zhan, Y.; Ni, X.; Gao, Y. Artificial Intelligence of Things in Hydrogen Sensing: Toward Optic and Intelligent System. Research 2025, 8, 0750. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Son, Y.; Lee, D.H.; Lim, M.H.; Han, J.K.; Jang, M.; Park, S.; Kang, S.; Yim, S.; Myung, S.; et al. Enhancing selectivity and sensitivity in gas sensors through noble metal-decorated ZnO and machine learning. Appl. Surf. Sci. 2025, 693, 162750. [Google Scholar] [CrossRef]
- Durand, B. Conception et Réalisation D’une Nouvelle Génération de Nano-Capteurs de gaz à base de Nanofils Semiconducteurs. Ph.D. Thesis, UPS Toulouse-Université Toulouse 3 Paul Sabatier, Toulouse, France, 2016. [Google Scholar]
- Tang, X.-Y.; Yang, W.-W.; Liu, Z.; Li, J.-C.; Ma, X. Deep learning performance prediction for solar-thermal-driven hydrogen production membrane reactor via bayesian optimized LSTM. Int. J. Hydrogen Energy 2024, 82, 1402–1412. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.-H.; Seo, H. Pd on MoO3 nanoplates as small-polaron-resonant eye-readable gasochromic and electrical hydrogen sensor. Sens. Actuators B Chem. 2017, 247, 357–365. [Google Scholar] [CrossRef]
- Diao, S.; Li, H.; Wang, J.; Wei, C.; Yao, Y.; Yu, M. Hydrogen leakage location prediction for fuel cell vehicles in parking lots: A combined study of CFD simulation and CNN-BiLSTM modeling. Int. J. Hydrogen Energy 2025, 109, 115–128. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhao, T.; Zou, Z.; Shen, B.; Yang, L. A New Convolutional Neural Network With Random Forest Method for Hydrogen Sensor Fault Diagnosis. IEEE Access 2020, 8, 85421–85430. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, Y.; Li, C.; Wang, D.; Wu, H. Electronic Noses: From Gas-Sensitive Components and Practical Applications to Data Processing. Sensors 2024, 24, 4806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-S.; Du, Y.; Guo, X.-M. Gas-sensing performance of Au loading Sn0.97Cu0.03O2 and its use on quanti-fying CO and H2 concentration by BP-temperature modulation method. Mater. Sci. Semicond. Process. 2023, 156, 107291. [Google Scholar] [CrossRef]
- Zeng, X.; Shahzeb, M.; Cheng, X.; Shen, Q.; Xiao, H.; Xia, C.; Xia, Y.; Huang, Y.; Xu, J.; Wang, Z. An Enhanced Gas Sensor Data Classification Method Using Principal Component Analysis and Synthetic Minority Over-Sampling Technique Algorithms. Micromachines 2024, 15, 1501. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, X.; Li, C.; Zhou, M.; Liu, T.; Liu, J.; Zhang, L. A Review of Machine Learning-Assisted Gas Sensor Arrays in Medical Diagnosis. Biosensors 2025, 15, 548. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Gao, W. Design of hydrogen sensor relying on Pd-MWCNT/WO3 sensing materials for selective and rapid hydrogen detection. Sens. Actuators B Chem. 2025, 422, 136648. [Google Scholar] [CrossRef]
- Mirzaei, H.; Ramezankhani, M.; Earl, E.; Tasnim, N.; Milani, A.S.; Hoorfar, M. Investigation of a Sparse Autoencoder-Based Feature Transfer Learning Framework for Hydrogen Monitoring Using Microfluidic Olfaction Detectors. Sensors 2022, 22, 7696. [Google Scholar] [CrossRef]
- Seo, J.; Noh, Y.; Kang, Y.-J.; Lim, J.; Ahn, S.; Song, I.; Kim, K.C. Graph neural networks for anomaly detection and diagnosis in hydrogen extraction systems. Eng. Appl. Artif. Intell. 2024, 135, 108846. [Google Scholar] [CrossRef]
- Machine Learning Based Approach to Selective Measurements of Hydrogen for Catalytic Gas Sensors. Available online: https://xplorestaging.ieee.org/document/10036195 (accessed on 12 June 2025).
- Selective Low-Temperature Hydrogen Catalytic Sensor. Available online: https://xplorestaging.ieee.org/document/9759978 (accessed on 12 June 2025).
- Gao, D.; Gao, S.; Deng, H.; Liu, H.; Hou, D.; Lu, Q.; He, X.; Huang, S. Modulation de la structure électronique de surface de PdO/SnO2 par chargement de Pd pour des performances de détection d’hydrogène supérieures. Chem. Eng. J. 2025, 515, 163694. [Google Scholar] [CrossRef]
- Franić, N.; Pivac, I.; Barbir, F. A review of machine learning applications in hydrogen electrochemical devices. Int. J. Hydrogen Energy 2025, 102, 523–544. [Google Scholar] [CrossRef]
- Wang, L.; Song, J. Review-Recent Progress in the Design of Chemical Hydrogen Sensors. J. Electrochem. Soc. 2024, 171, 017510. [Google Scholar] [CrossRef]
- Thanh, H.V.; Rahimi, M.; Tangparitkul, S.; Promsuk, N. Modeling the thermal transport properties of hydrogen and its mixtures with greenhouse gas impurities: A data-driven machine learning approach. Int. J. Hydrogen Energy 2024, 83, 1–12. [Google Scholar] [CrossRef]
- Gardner, E.L.W.; Gardner, J.W.; Udrea, F. Micromachined Thermal Gas Sensors—A Review. Sensors 2023, 23, 681. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhang, X.; Peng, W. Fiber Optics-Mechanics Coupling Sensor for High-Performance Hydrogen Detection. Photonic Sens. 2025, 15, 250314. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Peng, W. Status and development of fiber optic hydrogen sensing technology (invited). Infrared Laser Eng. 2025, 54, 20250072. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwata, C.; Nakada, M.; Kurita, S.; Kakiuchi, H. Chromatographic Analysis of Molecular Hydrogen (H2) in the Atmosphere for Understanding Atmospheric Tritiated Hydrogen (HT)). Plasma Fusion Res. 2023, 18, 2405038. [Google Scholar] [CrossRef]
- Sutarya, D.; Mahendra, A. Virtual Sensor for Time Series Prediction of Hydrogen Safety Parameter in Degussa Sintering Furnace. In Proceedings of the 2015 2nd International Conference on Information Technology, Computer, and Electrical Engineering (ICITACEE), Semarang, Indonesia, 16–18 October 2015; Isnanto, R., Facta, M., Widianto, E., Eridani, D., Eds.; Diponegoro University: Semarang, Indonesia, 2015; pp. 81–86. [Google Scholar]
- Kawano, T.; Tsuboi, N.; Tsujii, H.; Sugiyama, T.; Asakura, Y.; Uda, T. Infinitesimal concentration hydrogen Analyzer using trace reduction detector (TRD). Jpn. J. Appl. Phys. Part 2-Lett. 2003, 42, L549–L551. [Google Scholar] [CrossRef]
- Ohira, S.-I.; Toda, K. Micro gas analyzers for environmental and medical applications. Anal. Chim. Acta 2008, 619, 143–156. [Google Scholar] [CrossRef]
- Arrhenius, K.; Buker, O.; Fischer, A.; Persijn, S.; Moore, N.D. Development and evaluation of a novel analyser for ISO14687 hydrogen purity analysis. Meas. Sci. Technol. 2020, 31, 075010. [Google Scholar] [CrossRef]
- Higuchi, K.; Yamamoto, K.; Kajioka, H.; Toiyama, K.; Honda, M.; Orimo, S.; Fujii, H. Remarkable hydrogen storage properties in three-layered Pd/Mg/Pd thin films. J. Alloys Compd. 2002, 330, 526–530. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- The Role of Palladium in a Hydrogen Economy. Scilit. Available online: https://www.scilit.com/publications/bb5261e34ecb7df566a58e03bf0c442a (accessed on 11 June 2025).
- Lin, B.; Wu, X.; Xie, L.; Kang, Y.; Du, H.; Kang, F.; Li, J.; Gan, L. Atomic Imaging of Subsurface Interstitial Hydrogen and Insights into Surface Reactivity of Palladium Hydrides. Angew. Chem. Int. Ed. 2020, 59, 20348–20352. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Innovations in WO3 gas sensors: Nanostructure engineering, functionalization, and future perspectives. Heliyon 2024, 10, e27740. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.-H.; Liu, Y.-C.; Yuan, S.-H.; Chiang, Z.-X.; Zhang, S.; Wu, R.-J. Mesoporous WO3-TiO2 heterojunction for a hydrogen gas sensor. Sens. Actuators B Chem. 2021, 341, 130035. [Google Scholar] [CrossRef]
- Yamada, T.; Yamaguchi, T.; Hara, K. WO3-based Hydrogen Gas Sensors Using Stacked Thin Films with Interspace. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; IEEE: New York, NY, USA, 2019. [Google Scholar]
- Boudiba, A.; Zhang, C.; Umek, P.; Bittencourt, C.; Snyders, R.; Olivier, M.-G.; Debliquy, M. Sensitive and rapid hydrogen sensors based on Pd-WO3 thick films with different morphologies. Int. J. Hydrogen Energy 2013, 38, 2565–2577. [Google Scholar] [CrossRef]
- Kishnani, V.; Yadav, A.; Mondal, K.; Gupta, A. Palladium-Functionalized Graphene for Hydrogen Sensing Performance: Theoretical Studies. Energies 2021, 14, 5738. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Mirzaei, S.; Nasiri, N. Nanomaterial Innovations and Machine Learning in Gas Sensing Technologies for Real-Time Health Diagnostics. ACS Sens. 2025, 10, 1620–1640. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cirgue, G. Apprendre le ML en une Semaine. Machinelearnia. 2019. Available online: https://machinelearnia.com (accessed on 20 February 2025).
- MATLAB for Machine Learning, 2nd edition. Available online: https://fr.mathworks.com/academia/books/matlab-for-machine-learning-ciaburro.html (accessed on 22 April 2025).
- Djeziri, M.; Djedidi, O.; Morati, N.; Seguin, J.-L.; Bendahan, M.; Contaret, T. A temporal-based SVM approach for the detection and identification of pollutant gases in a gas mixture. Appl. Intell. 2021, 52, 6065–6078. [Google Scholar] [CrossRef]
- Dreyfus, G. Apprentissage Statistique, 3rd ed.; Eyrolles: Paris, France, 2008; ISBN 978-2-212-12229-9. [Google Scholar]
- Ding, X.; Liu, J.; Yang, F.; Cao, J. Random radial basis function kernel-based support vector machine. J. Frankl. Inst. 2021, 358, 10121–10140. [Google Scholar] [CrossRef]
- Biernat, É. Data Science: Fondamentaux et Études de Cas. Available online: https://elmoukrie.com/wp-content/uploads/2022/05/eric-biernat-michel-lutz-yann-lecun-data-science-_-fondamentaux-et-etudes-de-cas-_-machine-learning-avec-python-et-r-eyrolles-2015.pdf (accessed on 22 April 2025).
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Ferko, N.; Djeziri, M.A.; Al Sheikh, H.; Moubayed, N.; Bendahan, M.; Seguin, J.-L. Detection and Estimation of Ethanol Concentration in a Disturbed Environment Utilizing Random Forest. In Proceedings of the 2024 IEEE International Conference on Design, Test and Technology of Integrated Systems (DTTIS), Aix-En-Provence, France, 14–16 October 2024; pp. 1–5. Available online: https://ieeexplore.ieee.org/abstract/document/10780114/ (accessed on 30 October 2025).
- Shomope, I.; Al-Othman, A.; Tawalbeh, M.; Alshraideh, H.; Almomani, F. Machine learning in PEM water electrolysis: A study of hydrogen production and operating parameters. Comput. Chem. Eng. 2025, 194, 108954. [Google Scholar] [CrossRef]
- Zhu, X.; Goldberg, A.B. Introduction to Statistical Machine Learning. In Introduction to Semi-Supervised Learning; Zhu, X., Goldberg, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2009; pp. 1–8. ISBN 978-3-031-01548-9. [Google Scholar]
- Song, W.; Gao, C.; Zhao, Y.; Zhao, Y. A Time Series Data Filling Method Based on LSTM—Taking the Stem Moisture as an Example. Sensors 2020, 20, 5045. [Google Scholar] [CrossRef]
- Katterbauer, K.; Al Shehri, A.; Qasim, A.; Yousef, A. Analyzing Hydrogen Flow Behavior Based on Deep Learning Sensor Selection Optimization Framework. J. Fluids Eng.-Trans. ASME 2024, 146, 071112. [Google Scholar] [CrossRef]
- Rapid Forecasting of Hydrogen Concentration Based on a Multilayer CNN-LSTM Network|Request PDF. ResearchGate. Available online: https://www.researchgate.net/publication/368705758_Rapid_forecasting_of_hydrogen_concentration_based_on_a_multilayer_CNN-LSTM_network (accessed on 10 June 2025).
- Hassan, A.; Refaat, M.; Hemeida, A.M. Image classification based deep learning: A Review. Aswan Univ. J. Sci. Technol. 2022, 2, 11–35. [Google Scholar] [CrossRef]
- Li, A.; Lang, Z.; Ni, C.; Tian, H.; Wang, B.; Cao, C.; Du, W.; Qian, F. Deep learning-based source term estimation of hydrogen leakages from a hydrogen fueled gas turbine. Int. J. Hydrogen Energy 2024, 86, 875–889. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yin, Y.; Zhang, J.; Grzegorzek, M. Applications of artificial neural networks in microorganism image analysis: A comprehensive review from conventional multilayer perceptron to popular convolutional neural network and potential visual transformer. Artif. Intell. Rev. 2023, 56, 1013–1070. [Google Scholar] [CrossRef]
- Raheli, B.; Aalami, M.T.; El-Shafie, A.; Ghorbani, M.A.; Deo, R.C. Uncertainty assessment of the multilayer perceptron (MLP) neural network model with implementation of the novel hybrid MLP-FFA method for prediction of biochemical oxygen demand and dissolved oxygen: A case study of Langat River. Environ. Earth Sci. 2017, 76, 503. [Google Scholar] [CrossRef]
- IBM SPSS Neural Networks 31. Available online: https://www.ibm.com/docs/en/SSLVMB_31.0.0/nl/fr/pdf/IBM_SPSS_Neural_Network.pdf (accessed on 17 June 2025).
- Karri, V.; Ho, T.; Madsen, O. Artificial neural networks and neuro-fuzzy inference systems as virtual sensors for hydrogen safety prediction. Int. J. Hydrogen Energy 2008, 33, 2857–2867. [Google Scholar] [CrossRef]
- Mendizabal, J.; Vernon, D.; Martin, B.; Bajon-Fernandez, Y.; Soares, A. Short-term memory artificial neural network modelling to predict concrete corrosion in wastewater treatment plant inlet chambers using sulphide sensors. J. Water Process Eng. 2025, 69, 106821. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; Jung, E.S.; Kwon, S. Implantable hybrid chrome silicide temperature sensor for power MEMS devices. Micro Nano Lett. 2011, 6, 895–899. [Google Scholar] [CrossRef]
- Banerjee, T.; Chowdhury, K.; Agrawal, D.P. Tree based data aggregation in sensor networks using polynomial regression. In Proceedings of the 2005 7th International Conference on Information Fusion (Fusion), Vols 1 and 2, Philadelphia, PA, USA, 25–28 July 2005; IEEE: New York, NY, USA, 2005; pp. 1146–1153. [Google Scholar]
- Lee, G.; Jung, M.; Song, M.; Choo, J. Unsupervised anomaly detection of the gas turbine operation via convolutional auto-encoder. In Proceedings of the 2020 IEEE International Conference on Prognostics and Health Management (ICPHM), Detroit, MI, USA, 8–10 June 2020; pp. 1–6. [Google Scholar]
- Xing, Y.; Wang, B.; Gong, Z.; Hou, Z.; Xi, F.; Mou, G.; Du, Q.; Gao, F.; Jiao, K. Data-Driven Fault Diagnosis for PEM Fuel Cell System Using Sensor Pre-Selection Method and Artificial Neural Network Model. IEEE Trans. Energy Convers. 2022, 37, 1589–1599. [Google Scholar] [CrossRef]
- Arroyo, P.; Meléndez, F.; Suárez, J.I.; Herrero, J.L.; Rodríguez, S.; Lozano, J. Electronic Nose with Digital Gas Sensors Connected via Bluetooth to a Smartphone for Air Quality Measurements. Sensors 2020, 20, 786. [Google Scholar] [CrossRef]
- Gwiżdż, P.; Brudnik, A.; Zakrzewska, K. Hydrogen Detection with a Gas Sensor Array—Processing and Recognition of Dynamic Responses Using Neural Networks. Metrol. Meas. Syst. 2015, 22, 3–12. [Google Scholar] [CrossRef]
- Yan, J.; Guo, X.; Duan, S.; Jia, P.; Wang, L.; Peng, C.; Zhang, S. Electronic Nose Feature Extraction Methods: A Review. Sensors 2015, 15, 27804–27831. [Google Scholar] [CrossRef]
- Cai, J.; Luo, J.; Wang, S.; Yang, S. Feature selection in machine learning: A new perspective. Neurocomputing 2018, 300, 70–79. [Google Scholar] [CrossRef]
- Attouri, K.; Mansouri, M.; Hajji, M.; Kouadri, A.; Bouzrara, K.; Nounou, H. Enhanced Neural Network Method-Based Multiscale PCA for Fault Diagnosis: Application to Grid-Connected PV Systems. Signals 2023, 4, 381–400. [Google Scholar] [CrossRef]
- Velliangiri, D.S. A Review of Dimensionality Reduction Techniques for Efficient Computation. Procedia Comput. Sci. 2019, 165, 104–111. [Google Scholar] [CrossRef]
- Visalakshi, S.; Radha, V. A literature review of feature selection techniques and applications: Review of feature selection in data mining. In Proceedings of the 2014 IEEE International Conference on Computational Intelligence and Computing Research, Coimbatore, India, 18–20 December 2014; pp. 1–6. [Google Scholar]
- Feature Selection Methods: Case of Filter and Wrapper Approaches for Maximising Classification Accuracy. ResearchGate. Available online: https://www.researchgate.net/publication/322920304_Feature_selection_methods_Case_of_filter_and_wrapper_approaches_for_maximising_classification_accuracy (accessed on 30 June 2025).
- Zebari, R.; Abdulazeez, A.; Zeebaree, D.; Zebari, D.; Saeed, J. A Comprehensive Review of Dimensionality Reduction Techniques for Feature Selection and Feature Extraction. J. Appl. Sci. Technol. Trends 2020, 1, 56–70. [Google Scholar] [CrossRef]
- Zhu, X.; Goldberg, A.B. Semi-Supervised Support Vector Machines. In Introduction to Semi-Supervised Learning; Zhu, X., Goldberg, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2009; pp. 57–67. ISBN 978-3-031-01548-9. [Google Scholar]
- Zhu, X.; Goldberg, A.B. Overview of Semi-Supervised Learning. In Introduction to Semi-Supervised Learning; Zhu, X., Goldberg, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2009; pp. 9–19. ISBN 978-3-031-01548-9. [Google Scholar]
- Nkulikiyinka, P.; Yan, Y.; Gulec, F.; Manovic, V.; Clough, P.T. Prediction of sorption enhanced steam methane reforming products from machine learning based soft-sensor models. Energy AI 2020, 2, 100037. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Ba Hashwan, S.S.; Junaid, M.M. A Review of Actuation and Sensing Mechanisms in MEMS-Based Sensor Devices. Nanoscale Res. Lett. 2021, 16, 16. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Z.; Tang, Y.; Sun, J.; Wei, X.; Zhang, Q.; Tian, G.; Wang, H. MEMS-based resistive hydrogen sensor with high performance using a palladium-gold alloy thin film. J. Alloys Compd. 2023, 930, 167398. [Google Scholar] [CrossRef]
- Bévenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clément, M. Hydrogen leak detection using an optical fibre sensor for aerospace applications. Sens. Actuators B Chem. 2000, 67, 57–67. [Google Scholar] [CrossRef]
- Samsudin, M.R.; Shee, Y.G.; Adikan, F.R.M.; Razak, B.B.A.; Dahari, M. Fiber Bragg Gratings (FBG) Hydrogen Sensor for Transformer Oil Degradation Monitoring | Request PDF. IEEE Sens. J. 2016, 16, 2993–2999. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, S.; Song, J.; Zhao, F.; Yan, Z.; Hong, X.; Zhang, T.; Zhang, W.; Zhou, K.; Li, C.; et al. High temperature resistant ultra-short DBR Yb-doped fiber laser. Appl. Opt. 2019, 58, 4474–4478. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, Y.; Guo, J.-Y.; Li, X.-L.; Tao, L.; Hu, J.-Y.; Cao, J.-X.; Tang, P.-H.; Zhang, Y. Gas sensor array based on carbon-based thin-film transistor for selective detection of indoor harmful gases. RARE Met. 2024, 43, 4401–4411. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Rivai, M.; Aulia, D. Use of Electronic Nose to Identify Levels of Cooking Cookies. IEEE Access 2024, 12, 97235–97247. [Google Scholar] [CrossRef]
- Ferko, N.; Djeziri, M.A.; Sheikh, H.A.; Moubayed, N.; Bendahan, M.; El Rafei, M.; Seguin, J.-L. Methodology for estimating ethanol concentration with artificial intelligence in the presence of interfering gases and measurement delay. Sens. Actuators B Chem. 2024, 421, 136502. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. MetaArXiv 2020. [Google Scholar] [CrossRef]
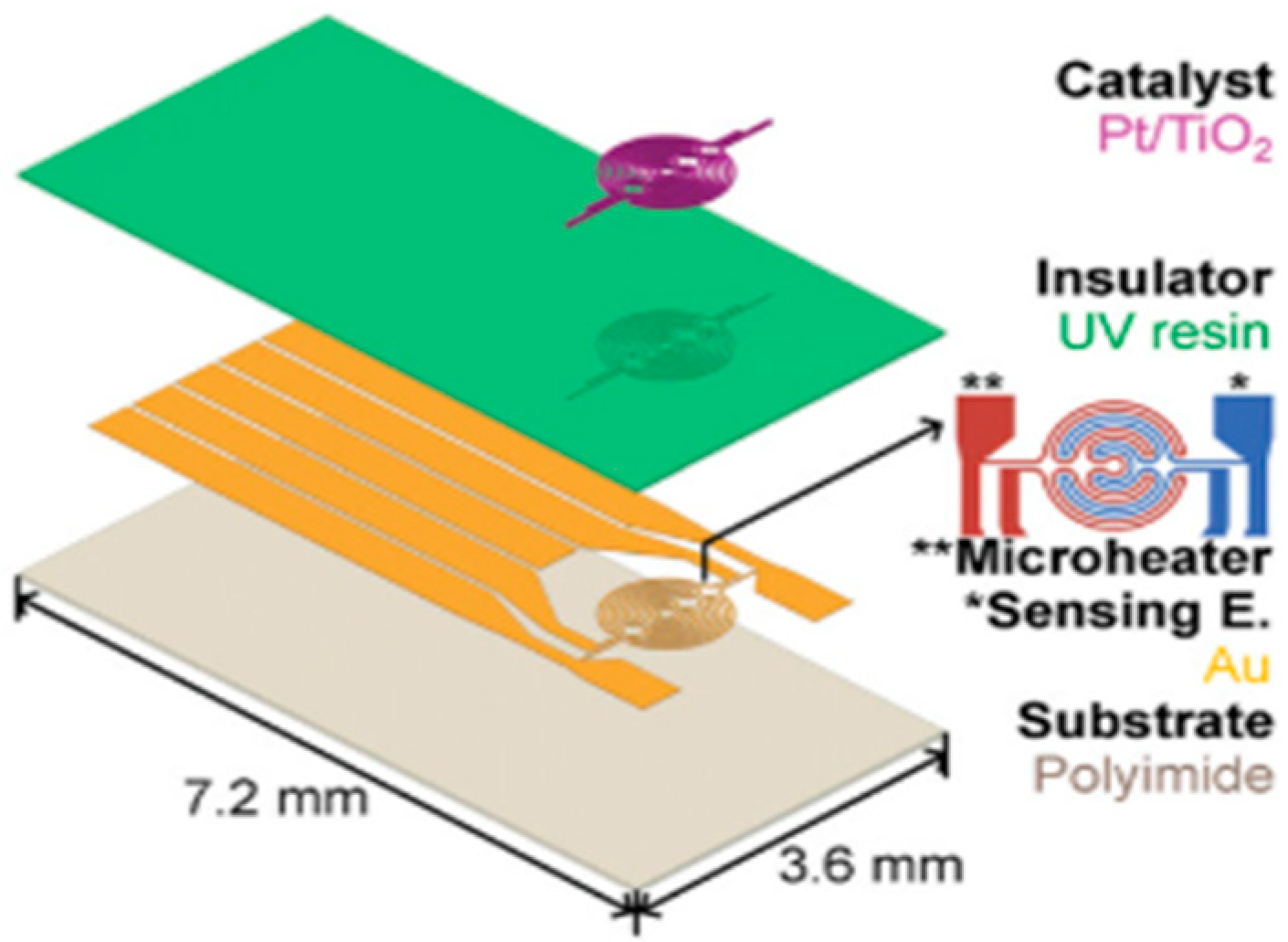
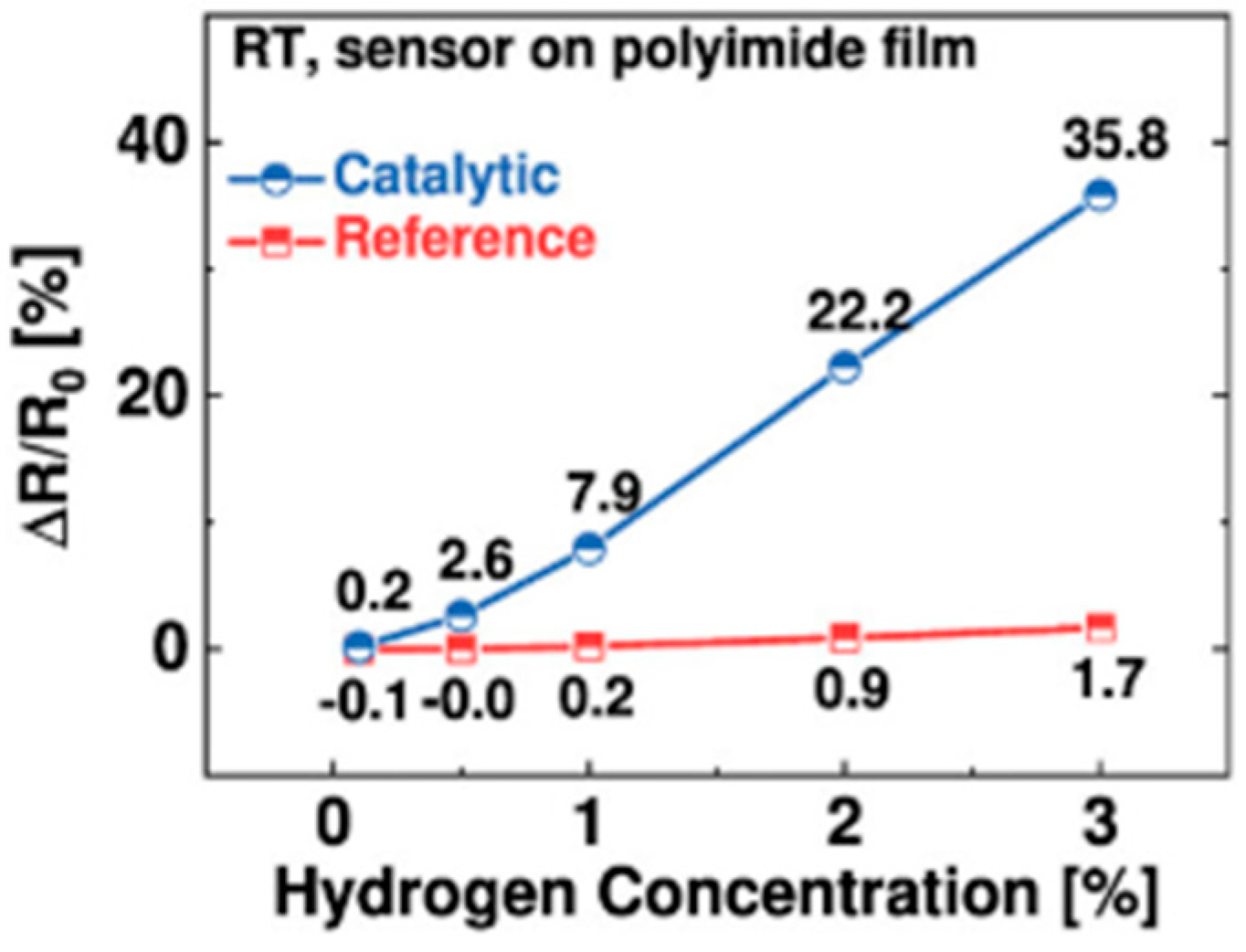
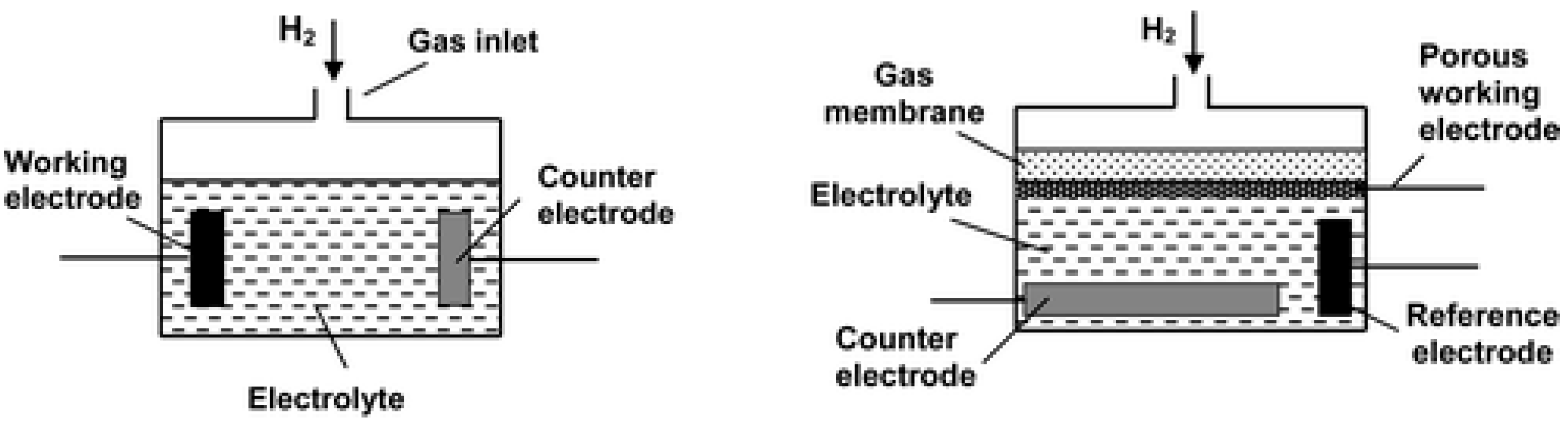
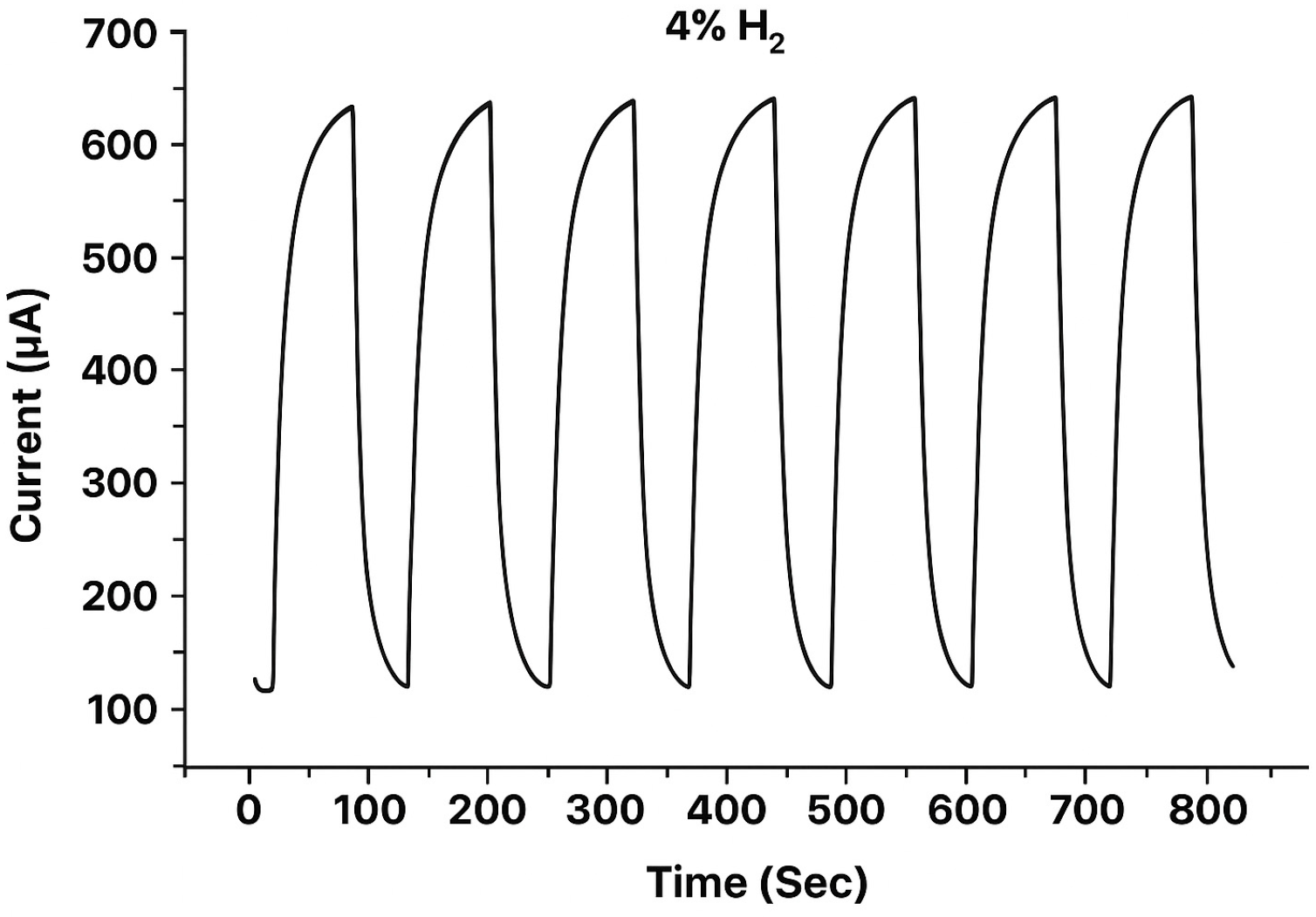
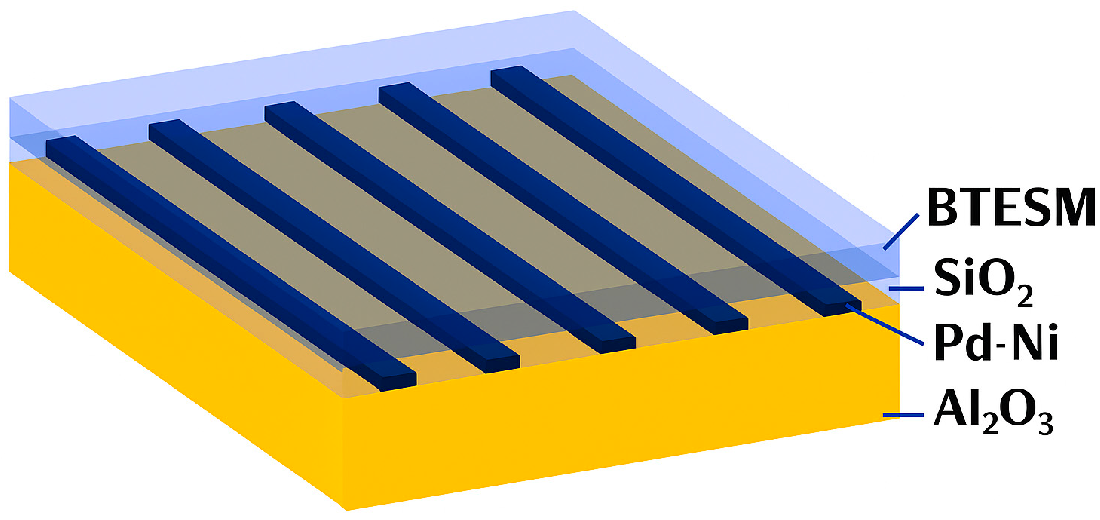
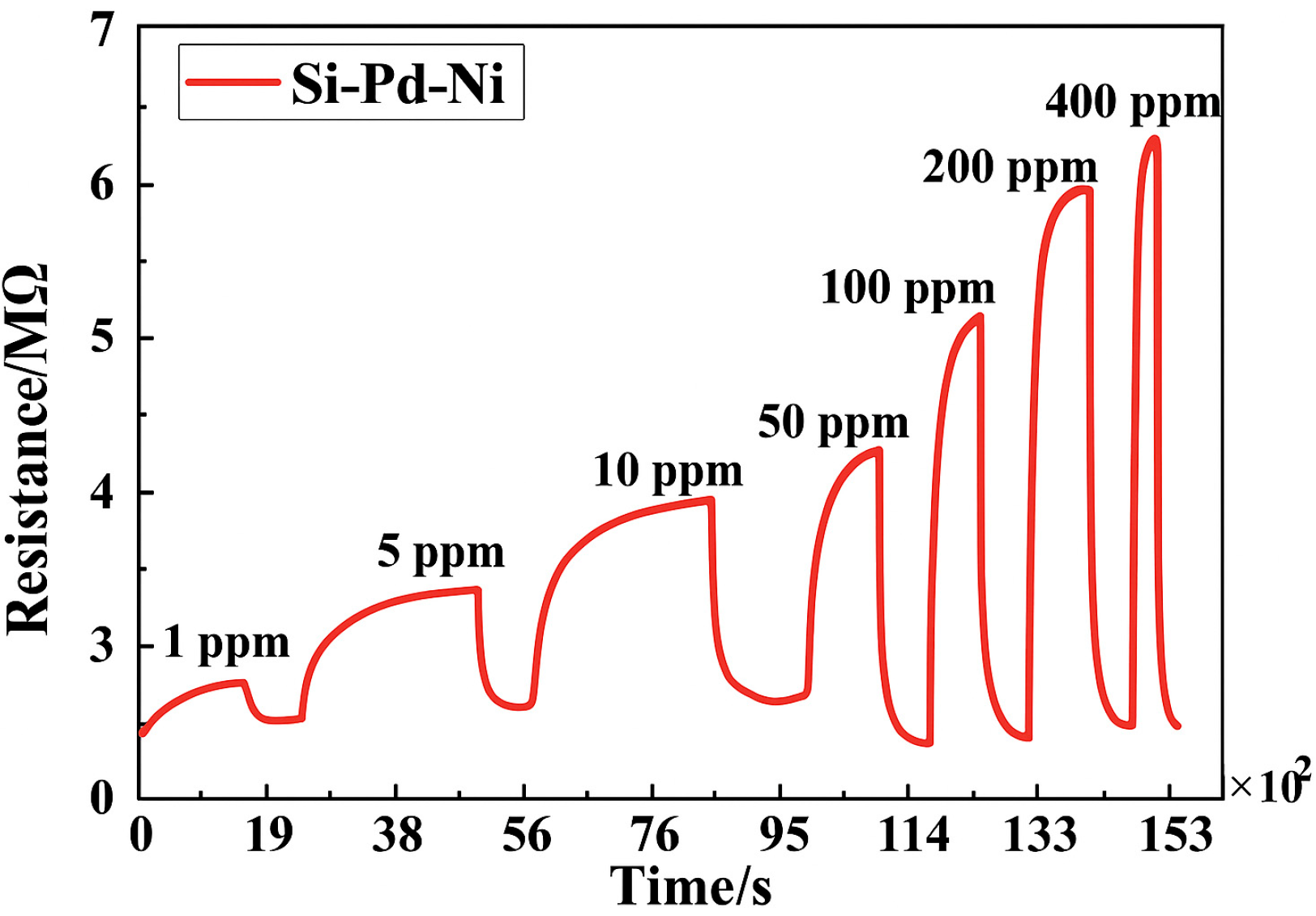

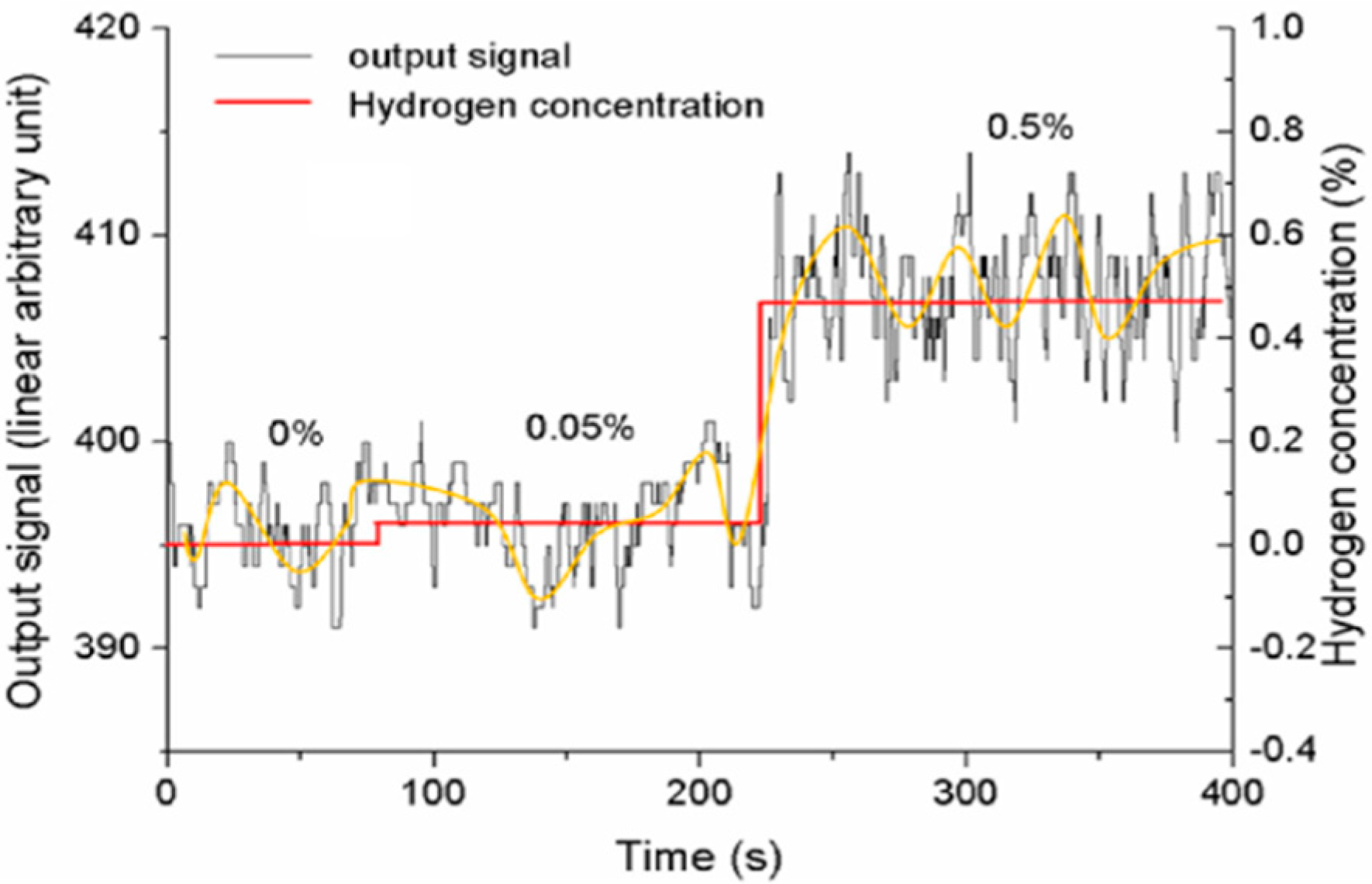



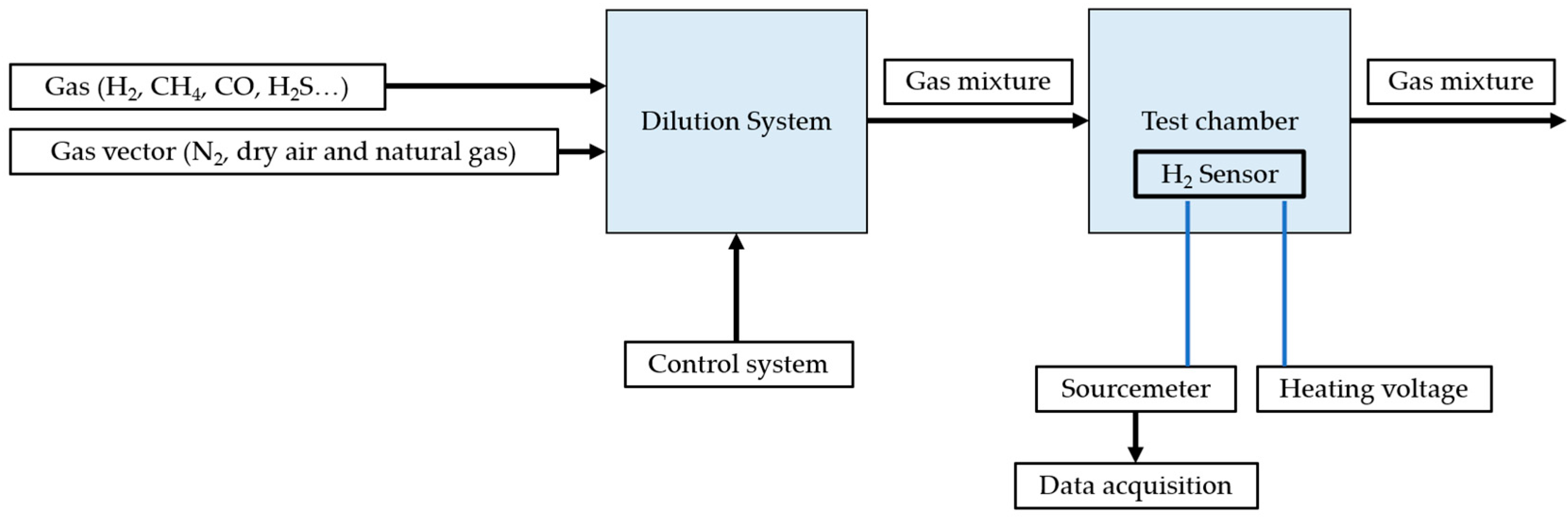
| Specifications Requirements | Catalytic | Electrochemical | MOX | Thermal Conductivity | Metal Films |
|---|---|---|---|---|---|
| Anaerobic | ✗ | ✓ | ✗ | ✓ | ✓ |
| Dry environment | ✓ | ✗ | ✓ | ✓ | ✓ |
| Temperature < 100 °C | ✗ | ✓ | ✗ | ✓ | ✓ |
| Measurement 0–100% H2 | ✗ | ✓ | ✗ | ✓ | ✓ |
| Selective to H2 | ✗ | ✓ | ✗ | ✗ | ✓ |
| T90/T10 < 1 min | ✓ | ✓ | ✓ | ✓ | ✓ |
| Vehicle Types | Sensitivity | Advantages |
|---|---|---|
| Planes | 10 ppm to 4% | Priority given to rapid detection to avoid any risk in flight |
| Trains and buses | 100 ppm to 4% | Robustness against vibrations and interference |
| Vehicles | 100 ppm to 4% | Compact and low cost |
| High pressure tanks | 10 ppm to 100% | Low leakage than hydrogen-saturated environments |
| Sensors | Measurement Ranges | Advantages |
|---|---|---|
| Semiconductors (SnO2, ZnO) | 1 ppm to 1% | Sensitive to low concentrations |
| Electrochemical (Pt, Pd, Au, Ni or Co) | 10 ppm to 10% | Accurate and reliable for critical applications |
| A palladium | 1 ppm to 100% | Very sensitive and specific to hydrogen |
| Optics (Pd) | 10 ppm to 100% | Demanding environments (airplanes, trains) |
| Types of Leaks | Nature | Concentration Range H2 |
|---|---|---|
| Primary | Trace–Background Leak | <10 ppm |
| Early | Beginning of leak | 10–100 ppm |
| Secondary | High risk | 100 ppm–1% |
| Tertiary | Immediate danger | 1–100% |
| Settings | Concentration (in % by Volume) |
|---|---|
| Short-term exposure threshold | 1% for 15 min |
| Average exposure threshold | 1% over an 8 h period |
| Minimum flammability limit | 4% |
| Alarm threshold in natural cavities | 1% to 2% |
| Average concentration monitored for safety in the natural environment | 0.1% to 2% |
| Technology | Detection Limit Value (in % by Volume) of H2 |
|---|---|
| Electrochemical | 0.01% |
| Semiconductors | 0.1% |
| Thermals | 0.1% to 0.5% |
| Palladium effect | 0.001% to 0.01% |
| Spectroscopiques | 0.001% to 0.01% |
| Nature of the Leak | Recommended Sensors | Advantages |
|---|---|---|
| Slow leak at very low H2 concentration | Electrochemical or MOX sensor | High sensitivity (ppm); low cost; use for environmental monitoring |
| Rapid leak at moderate H2 concentration | Catalytic sensor or thermal sensor | Detection around 4% H2–Fast response |
| Continuous leak in controlled atmosphere | Optical sensor or Pd-based metal film sensor | Stable; reliable; little sensitive to external interference |
| Significant leak with high risk of explosion (ATEX zones) | Catalytic sensor | Robust and suitable for complex environments |
| Leaks in environments with multiple gases | Fiber-optic sensor or infrared spectroscopy sensor | High selectivity to H2; little cross-interference |
| Sensors Technologies | Detection Limit Value (in % by Volume) |
|---|---|
| Electrochemical | 0.01% |
| Semiconductors | 0.1% to 0.5% |
| Thermals | 0.1% |
| Palladium effect | 0.001% to 0.01% |
| Spectroscopiques | 0.001% to 0.01% |
| Application | Range H2 (%) | Pressure (Bar) | Humidity (%) | Interfering Gases | References |
|---|---|---|---|---|---|
| Anaerobic | |||||
| Power to Gas (P2G) | 1–20 | 30 | CO–H2S | [42,45,48,51,52,53,54,55,56,57,58] | |
| Monitoring of tanks of future vehicles (Train/Bus/Cars) | 0.01–4 | 300–700 | CO–CO2–O2–S | [59,60,61,62,63,64] | |
| Monitoring of H2 aircraft tanks | 0.001–4 | 350–700 | CO–CO2–O2–S | ||
| High pressure tank monitoring | 0.001–100 | 200–700 | CO–CO2–O2–S | ||
| Aerobic | |||||
| Leak detection (safety) | 0–0.1 | 1 | 20–80 | CO–H2S–H2O–O2 | [33,63,65,66,68,69] |
| Fuel cells | 90–100 | 30 | 80–100 | CO–H2S | [39,70,71,72] |
| Bioreactors–Electrolyzers | <1 | 30 | 80 | CO–H2S–S | [40,43,44,59,73,74,75,76,77,79,80] |
| White H2 research or leak detection from storage in natural cavities | 0–1 | 1 | 20–80 | CO–H2S–H2O–O2 | [59,68,69] |
| Chemical and metallurgical process controls | Variable depending on the process | 30 | Possible | CO–H2S | [46,81,82,83] |
| Pharmaceuticals | 0–0.1 | 1 | 20–80 | CO–H2S–H2O–O2 | [88,90,91] |
| Wastewater treatment–Biology–Bacteriology | 0–0.01 | 1 | 20–100 | CO–H2S–H2O–O2 | [6,72,75,84,85,86,87]. |
| Health | 0–0.01 | 1 | 20–100 | CO–H2S–H2O–O2 | [63,88] |
| Anaerobic | Applications | Detection Ranges According to Sensor Types | |||||||||||||||
| Catalytic | Electrochemical | MOX semiconductor | Thermal conductivity | Metallic films | Transistor | Optical fibers | Ultrasound | Laser Spectroscopy | Diode Laser | Infrared | Hall effect | Thermals | Spectroscopiques | Other Optics (reflectivity, transmission) | Medical diagnosis | ||
| Power to Gas | 0.1–100% | 0.5 ppm–1% | 1 ppm–4% | 0.5–100% | 1 ppm–4% | NI | NI | NI | NI | 0.01–100% | NI | NI | NI | 10 ppb–100% | 10 ppb–100% | NI | |
| Monitoring the tanks of future vehicles | NI | 0.5 ppm–1% | 1 ppm–4% | NI | 10 ppb–10% | NI | NI | NI | NI | NI | K-NI | NI | NI | NI | NI | ||
| H2 Aircraft Tank Monitoring | NI | 0.5 ppm–1% | 1 ppm–4% | NI | 10 ppb–10% | NI | NI | NI | NI | 0.01–100% | NI | NI | NI | NI | NI | NI | |
| Monitoring of H2 train tanks | 0.1–100% | 0.5 ppm–1% | 1 ppm–4% | 0.5–100% | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | |
| High pressure tank monitoring | 0.1–100% | 0.5 ppm–1% | 1 ppm–4% | 0.5–100% | 10 ppb–10% | NI | NI | NI | NI | 0.01–100% | NI | NI | NI | 10 ppb–100% | 10 ppb–100% | NI | |
| Aerobic | Applications | Detection ranges according to sensor types | |||||||||||||||
| Catalytic | Electrochemical | MOX semiconductor | Thermal conductivity | Metallic films | Transistor | Spectroscopiques | Ultrasound | Laser Spectroscopy | Diode Laser | Infrared | Hall effect | Thermals | MEMS | Other sensors | Medical diagnosis | ||
| Leak detection | 0.1–100% | 0.5 ppm–1% | 1 ppm–4% | 0.5–100% | 10 ppb–10% | NI | 10 ppb–100% | 0.1–100% | 0.01–100% | NI | 100 ppm–100% | 1 ppm–10% | 0.5–100% | NI | NI | NI | |
| Fuel cells | 0.1–100% | 0.5 ppm–1% | 1 ppm–4% | NI | 10 ppb–10% | NI | NI | NI | NI | 0.01–100% | 100 ppm–100% | NI | NI | 10 ppb–100% | NI | NI | |
| Bioreactors-Electrolyzers | 0.1–100% | 0.01% | 0.1% | NI | 10 ppm–0.01% | NI | 10 ppm–0.01% | NI | 0.01–100% | NI | NI | NI | 0.1–0.5% | NI | NI | NI | |
| White H2 research–Detection of leaks from storage in natural cavities | 0.1–100% | 10 ppm–0.01% | 0.01–0.1% | NI | 1 ppm–0.01% | NI | 0.1 ppm–0.01% | NI | 0.01–100% | NI | NI | NI | 0.01–0.1% | NI | NI | NI | |
| Chemical and metallurgical process controls | NI | 0.01% | 0.1% | NI | 10 ppm–0.01% | NI | 10 ppm–0.01% | NI | NI | NI | NI | NI | 0.1–0.5% | NI | NI | NI | |
| Pharmaceuticals | NI | 0.01–0.1 ppm | 0.1 ppm | NI | 0.01–0.1 ppm | NI | 0.01 ppm | NI | NI | NI | NI | NI | 0.05 ppm–0.1 ppm | NI | NI | NI | |
| Wastewater treatment | NI | 0.01–0.5 ppm | 0.1–0.5 ppm | NI | 0.01–0.1 ppm | NI | 0.01 ppm | NI | NI | NI | NI | NI | Roughly 0.1 ppm | NI | NI | NI | |
| Biology-Bacteriology | NI | 0.01–0.1 ppm | 0.1–0.5 ppm | NI | 0.01–0.1 ppm | NI | <0.01 ppm | NI | NI | NI | NI | NI | Roughly 0.1 ppm | NI | NI | NI | |
| Health | NI | 0.01–0.1 ppm | 0.1–0.5 ppm | NI | 0.01–0.1 ppm | NI | <0.01 ppm | NI | NI | NI | NI | NI | Roughly 0.1 ppm | NI | NI | <0.1 ppm | |
| Sensor Type | Detection Limit | Response Time | Stability-Lifetime | References |
|---|---|---|---|---|
| Pd-based metallic film | 0.1–100 ppm | 1–10 s | Maximum 1 year | [99] |
| Semiconductor | 1–100 ppm | 1 min | More than 1 year | [100] |
| Optical fiber | 10 ppb–1 ppm | 1–30 s | Maximum 2 years | [105,106] |
| Electrochemical | 0.5–10 ppm | 1 min | 1–3 years | [107] |
| MEMS | 100 ppb–1 ppm | Less than 1 s | Maximum 6 months | [108] |
| Graphene-based | 1 ppb–10 ppm | Less than 5 s | Maximum 1 year | [109] |
| PdAu-based plasmonic | 0.1–10 ppm | Less than 2 s | Maximum 1 year | [110] |
| Inputs | Outputs | ||||||
|---|---|---|---|---|---|---|---|
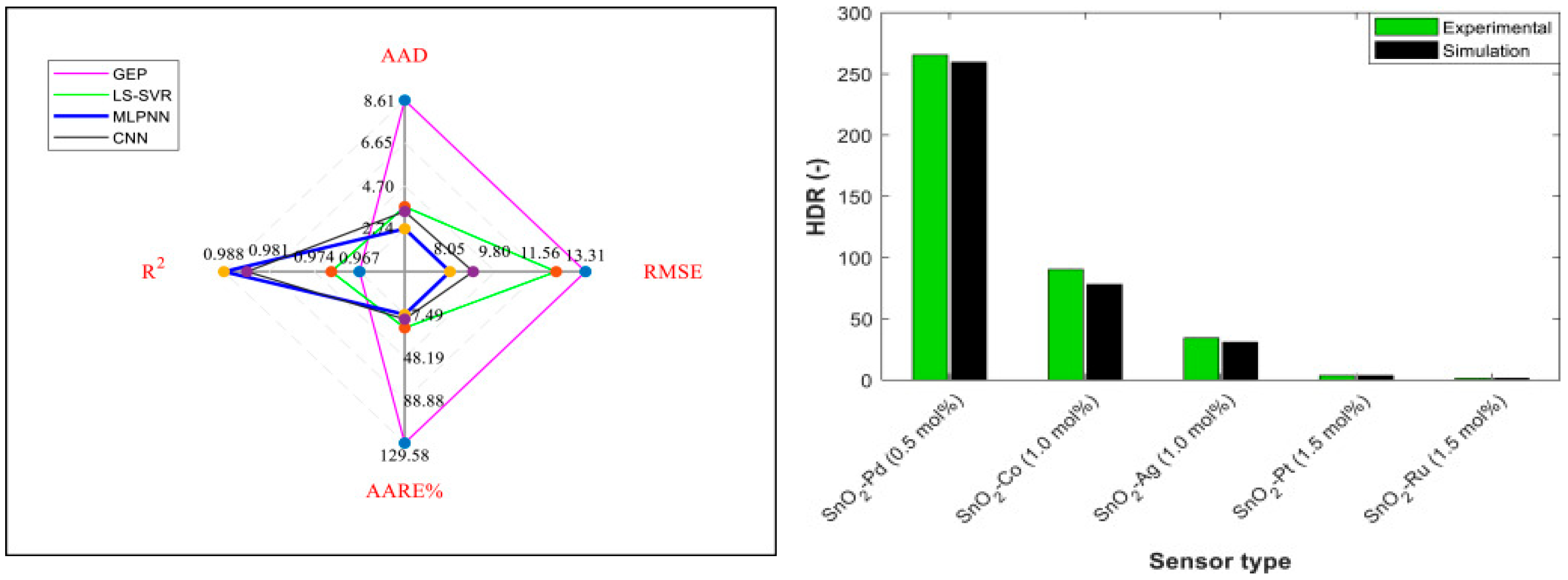
| Sensor | Molecular Weight of the Dopant (g/mol) | Doping Dosage (mol%) | Temperature (°C) | Concentration H2 (ppm) | HDR (−) | Number of Data | References |
| SnO2–Ag | 107.87 | 0–5 | 150–480 | 1.07–2000 | 0.6–80.1 | 137 | [119] |
| SnO2–Co | 58.93 | 0.196–1.195 | 260–400 | 100–35,000 | 1.1–291.4 | 51 | [120] |
| SnO2–Pd | 106.42 | 0.5 | 175–225 | 50–1000 | 105.7–265.5 | 4 | [56] |
| SnO2–Pt | 195.08 | 0.155–1.552 | 150–350 | 500–10,000 | 1.0–148.8 | 46 | [121] |
| SnO2–Ru | 101.7 | 0.298–4.408 | 200–350 | 500–10,000 | 1.2–26.7 | 45 | [118] |
| Type of Hydrogen Sensors | Inputs | Outputs | Objectives | Performance Before | Methods and Algorithms Used | Performance After | References |
|---|---|---|---|---|---|---|---|
| Pd-doped ZnO nanorods | Series of resistors with derivatives and peaks | H2 concentration (5 ppm–500 ppm)–150–200 °C Binary classification marked by the presence or absence of H2 | Evaluating hydrogen selectivity | 75% precision | Support Vector Machines (SVM) | 96% precision | M. Kumar [126], J. Zhu [127] |
| ZnO decorated with noble metals | Resistors Current Voltage | H2 concentration | Improving selectivity and sensitivity | Reliable detection of hydrogen; low selectivity among CO and H2S | Principal Component Analysis (PCA) + Random Forest (RF) Using a series of classifiers to evaluate results | Reduces classification errors by 40% | Yeong Min Kwon [128] |
| MoO3 | Resistance time series over 60 sliding seconds | H2 concentration | Predicting H2 concentration levels over time | RMSE around 15 ppm | LSTM | RMSE reduced to 3 ppm | [66,129,130] Other studies suggest an improvement from a materials point of view [131] |
| Pd-doped SnO2 | 5 × 5 images where each pixel corresponds to the normalized resistance | H2 concentration | Evaluate the selectivity of H2 among CO, NH3 and CH4 | 55–65% correct identification of H2 | Classification CNN | 97% identification of H2 | Y. Shubin [14] S. Diao [132] |
| Pd-doped SnO2 | 2D raster images | Binary classification marked by the presence or absence of hydrogen | Diagnosis of sensor faults | 66–75% in normal and noisy environments | Method CNN with RF | 100% in normal and noisy environments | Y.Sun [133] |
| Au-doped SnO2 | Voltage Resistance Heating temperature | Low H2 concentration (1–100 ppm) | Identify low hydrogen concentrations at high noise levels | 65% detection | PCA + KNN | 92% detection | [134,135] This method can be complemented by the work of Zeng [136] |
| Wo3 doped Cu, Fe and Pt | Voltage Current Resistance variations with different gases Slope Area | Gas classification and concentration | Evaluate the selectivity of hydrogen among CO or NH3 | 60% precision | RF | 93% precision | Yu [137] Wang [138] |
| Graphene/SnO2 | Resistance values versus time Voltage Current | H2 concentration Reconstruction errors in order to identify the presence of an anomaly by assessing threshold exceedance | Detect potential leaks or drifts in normal sensor behavior | 58% anomaly detection | Autoencoder method | 98% fault detection | H. Mirzaei [139] J. Seo [140] |
| Catalytic | Temperature Resistance and response Voltage | H2 concentration | Improving hydrogen selectivity and detection | NI | Linear regression Neural network | Constant error level for all gases | D. Spirjakin [141] Ivanov [31,142] |
| Catalytic based PdO/SnO2 | Relative humidity Resistance Voltage Current Heating temperature | H2 concentration | Identify hydrogen concentrations in a very humid environment (80% RH) Evaluate hydrogen selectivity | 65–70% identification but many errors | Classification: PCA + RF (feature extraction and selection) MLP to differentiate hydrogen concentrations | 91% recognition of H2 concentrations | Gao et al. [143] |
| Electrochemical-Thermal | Current Self-biasing voltage Operating temperature Relative humidity | H2 concentration | Improve performance in terms of selectivity and drift | 10–100 ppm Not very selective Time less than 30 s Service life 1 year | MLP to recognize hydrogen among potential interferents + SVM–RF for multi-gas classification | 10 ppm 5–20 s H2 detection with CO and CH4 Service life up to 2 years | N. Franic, I. Pivac, F. Barbir [34,53,144,145] |
| Thermal conductivity (MEMS) | Resistor Heating temperature Pressure | H2 concentration | Detection of hydrogen concentrations from 500 ppm to 3% | 100 ppm for a 5 s response time | Polynomial regression calibration; MLP to compensate for temperature effect | 100 ppm for a 5 s response time | H. Thanh [146] E. Gardner [147] |
| Fiber-optic hydrogen sensors with multilayer Pd-Y films | Wavelength of light source Absorption spectrum | H2 concentration | Improve sensitivity and response speed to hydrogen | Wavelength drift introducing measurement errors and influencing detection | Fast Fourier transform (FFT) filtering methods; moving average algorithm for spectral data analysis | Optimal design enabling a 97% reduction in measurement error Lower wavelength drift error | Y. Liu [47] |
| Pd-based optical fibers-Spectroscopic | Reflected or transmitted wavelength (nm) Temperature Absorption or interference spectrum sometimes | H2 concentration | Study drift and obtain a spatio-temporal profile when using a sensor network | NI | NI Sensors designed for industrial use by international manufacturers. Little information available on the internal software layer. | Measurements are repeatable and temperature effects are compensated from −10 to 80 °C for a detection threshold of 50 ppm | Zhang [148,149] [67,150] |
| Sensor for estimating and predicting hydrogen safety parameters | Heater voltage and current Heating temperature Inlet pressure and flow rate | Hydrogen outlet temperature Hydrogen outlet volumetric flow rate | Estimate hydrogen safety parameters such as pressure, temperature, and flow rates from the outlet of a Degussa sintering furnace. | NI | RNA–ANN Adaptative Neuro-Fuzzy Inference Systems (ANFIS) to solve a prediction problem | ANFIS >> ANN Average RMSE of 0.0387, 0.0283, 0.1301, and MAE of 0.0241, 0.0115, 0.0355 sequentially for temperature, pressure, and hydrogen flow rate | D. Sutarya [151] |
| Hydrogen detection using AI-based plasma discharge spectral analysis | Experimental bench with spectrometer Matrix 50 × 90 (images) | H2 concentration and methane | Detecting hydrogen and methane in plasma and their respective concentrations | Quantitative detection but no estimation of hydrogen content in the presence of methane | Complex CNN model not appropriate Residual model with predictions made after training | Not very precise (improve in terms of materials or involve more in-depth image processing) | A. Salimian [19] |
| Analyzers-Chromatographs | NI | NI | NI | NI | Industry-standard software such as LabVIEW | NI | [150,152,153,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbeck-Tazibt, J.; Djeziri, M.A.; Fiorido, T.; Seguin, J.-L. Review of Hydrogen Sensors in Aerobic and Anaerobic Environments Coupled with Artificial Intelligence Tools. Sensors 2025, 25, 6936. https://doi.org/10.3390/s25226936
Herbeck-Tazibt J, Djeziri MA, Fiorido T, Seguin J-L. Review of Hydrogen Sensors in Aerobic and Anaerobic Environments Coupled with Artificial Intelligence Tools. Sensors. 2025; 25(22):6936. https://doi.org/10.3390/s25226936
Chicago/Turabian StyleHerbeck-Tazibt, Jordan, Mohand A. Djeziri, Tomas Fiorido, and Jean-Luc Seguin. 2025. "Review of Hydrogen Sensors in Aerobic and Anaerobic Environments Coupled with Artificial Intelligence Tools" Sensors 25, no. 22: 6936. https://doi.org/10.3390/s25226936
APA StyleHerbeck-Tazibt, J., Djeziri, M. A., Fiorido, T., & Seguin, J.-L. (2025). Review of Hydrogen Sensors in Aerobic and Anaerobic Environments Coupled with Artificial Intelligence Tools. Sensors, 25(22), 6936. https://doi.org/10.3390/s25226936