Quantification of Volatile Compounds in Mixtures Using a Single Thermally Modulated MOS Gas Sensor with PCA–ANN Data Processing
Highlights
- Methodology enhancing the selectivity of MOS gas sensors for measuring ethanol and methanol levels in liquid mixtures.
- Approach to interpreting the output signals of thermally modulated MOS gas sensor by integrating two data processing techniques: PCA and ANN.
- The proposed methodology, integrating measurements from a single thermally modulated MOS gas sensor with a PCA–ANN-based machine learning algorithm, can be applied for the quantitative determination of VOCs in mixtures and demonstrates potential applicability to other types of sensors and volatile compounds.
Abstract
1. Introduction
2. Materials and Methods
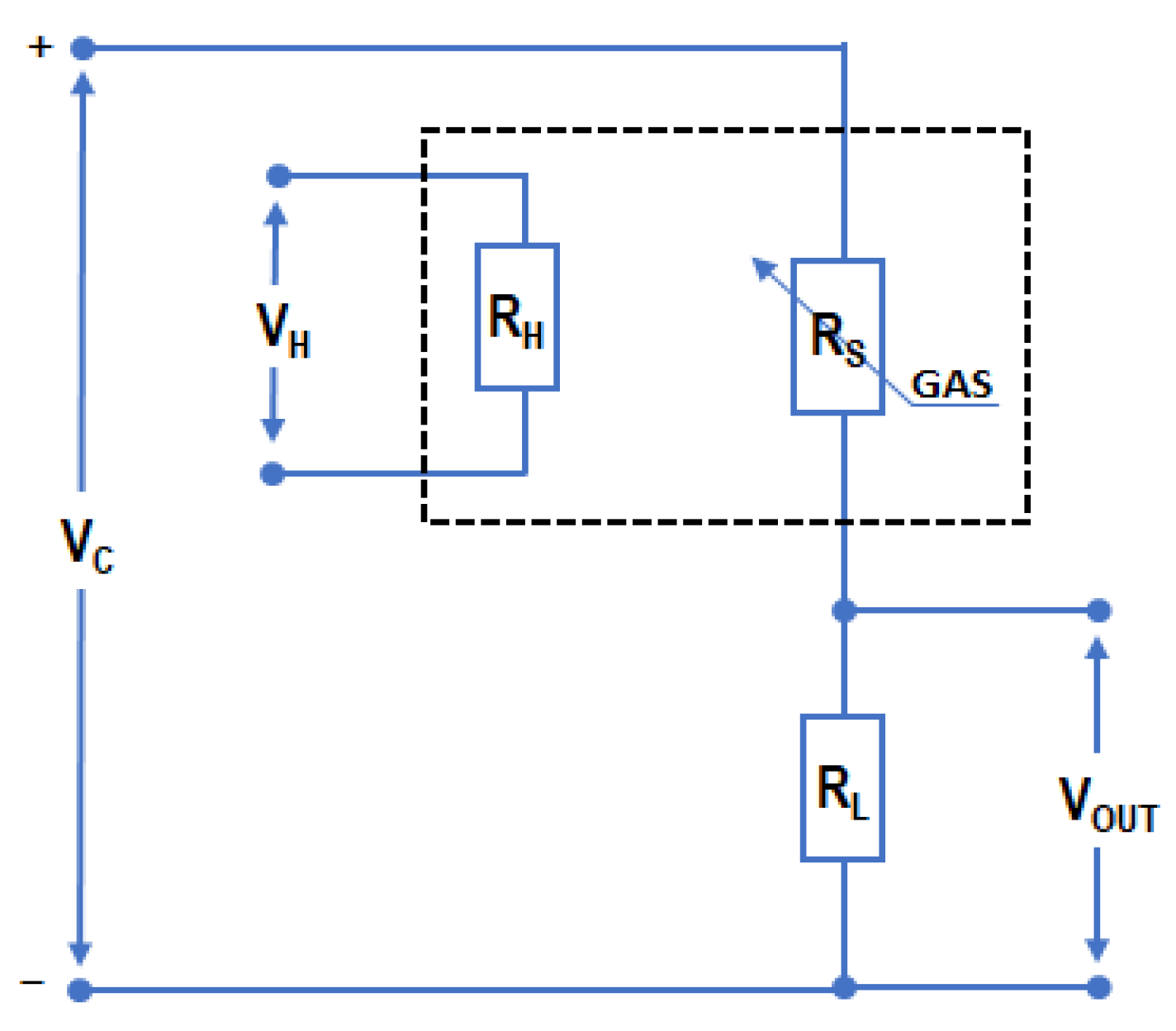
2.1. Measurement System and Methodology
2.2. Preparation of Analyzed Solutions
2.3. MOS Gas Sensor Output Signals Processing
2.3.1. Output Signal Feature Extraction
2.3.2. Development of a Model for Estimating Liquid Mixture Composition
Data Preparation
Artificial Neural Networks in Modeling of Liquid Mixtures Composition
2.4. Statistical Data Analysis
3. Results
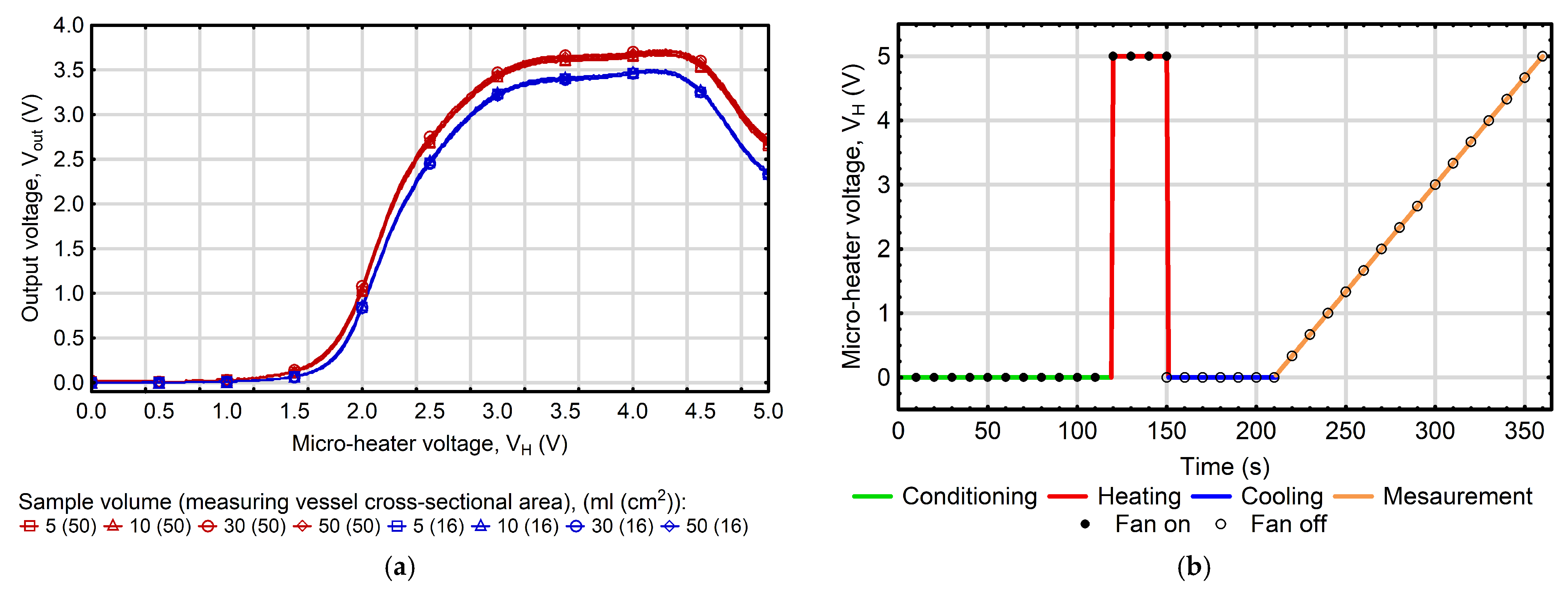
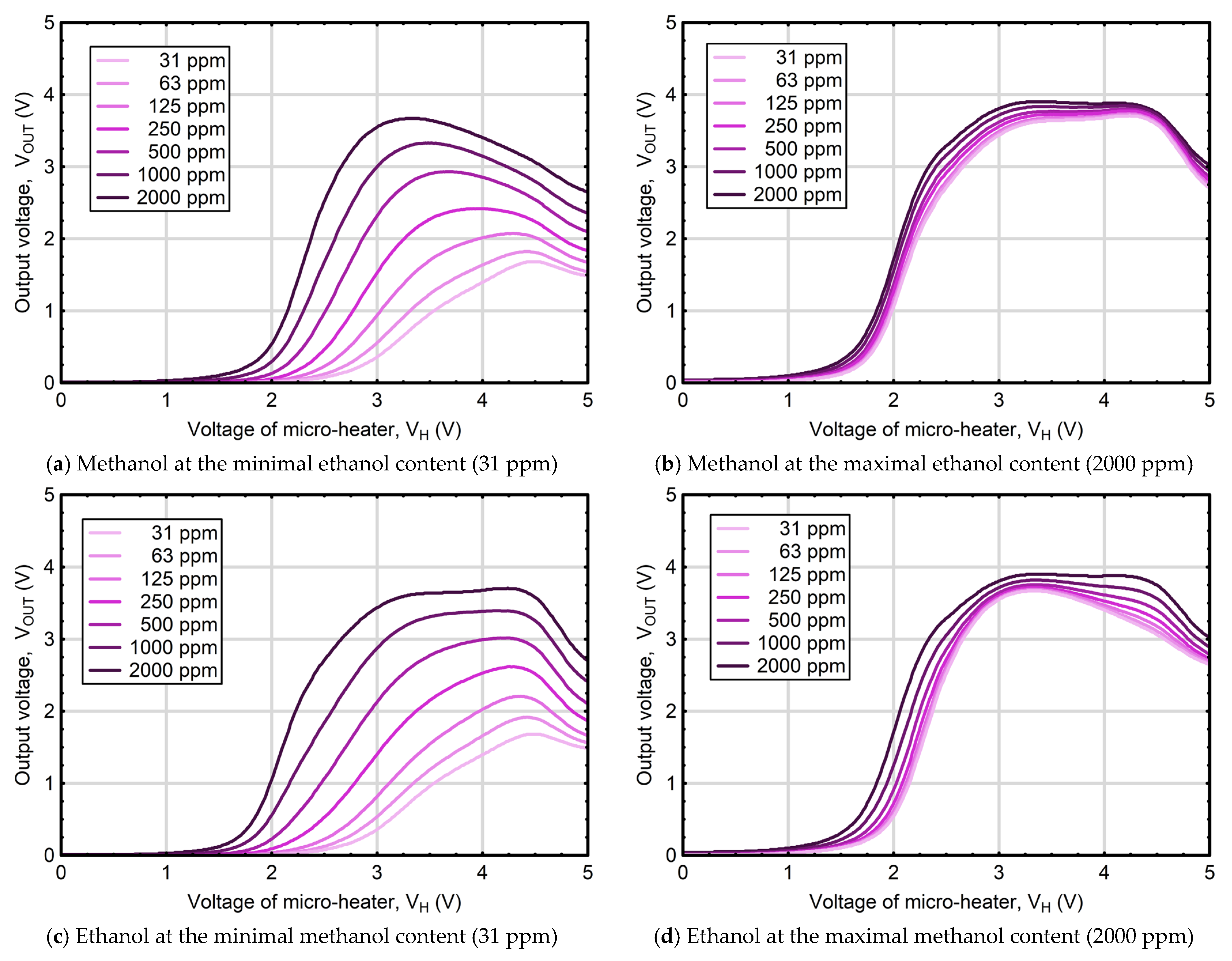
3.1. Dynamics of Thermally Modulated MOS Gas Sensor Responses
3.2. Reduction in Data Redundancy
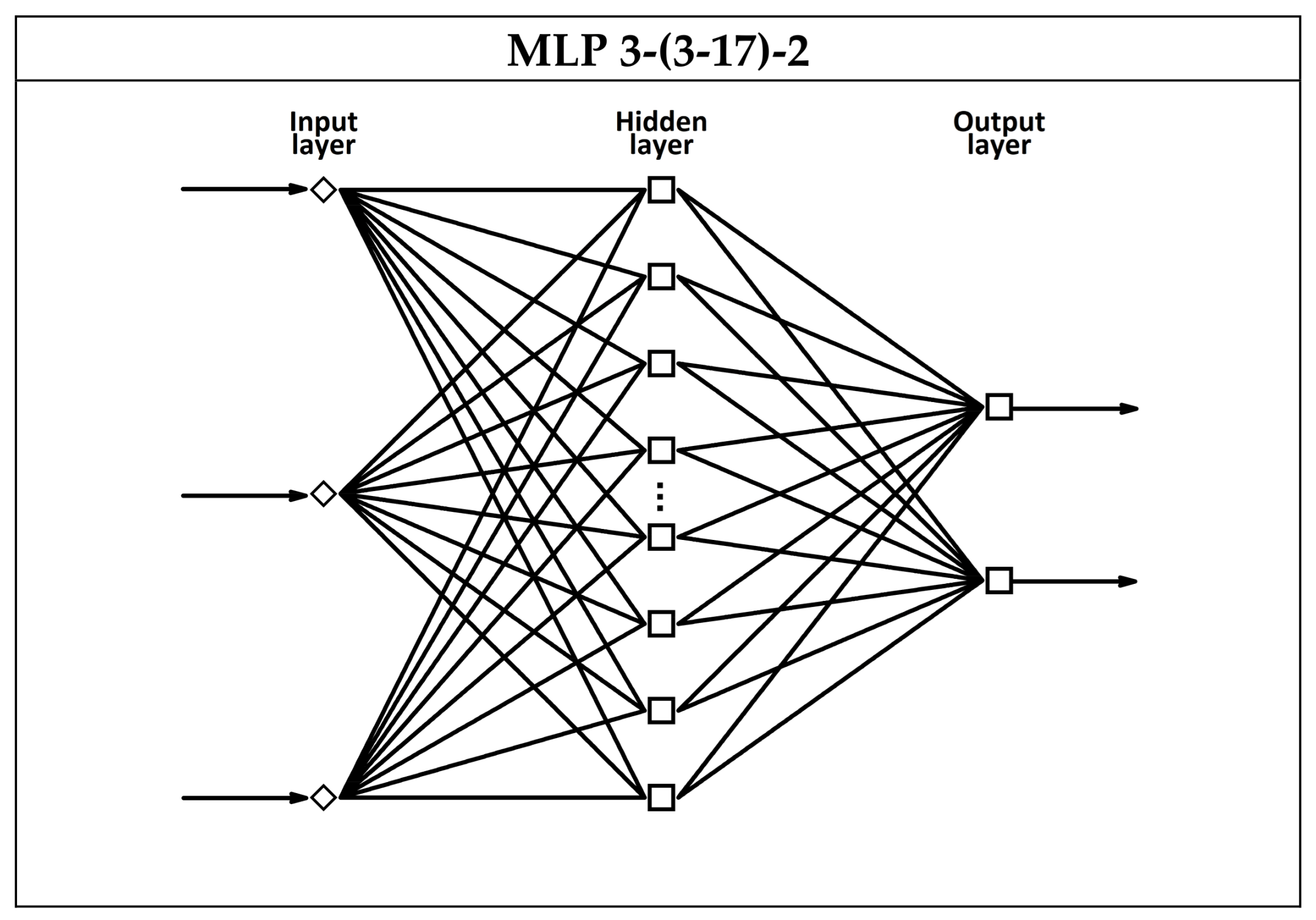
3.3. Artificial Neural Network Models for Estimating Liquid Mixture Composition
Evaluation of Model Performance
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, S. A critical review: Application of methanol as a fuel for internal combustion engines and effects of blending methanol with diesel/biodiesel/ethanol on performance, emission, and combustion characteristics of engines. Heat Transf. 2022, 51, 3334–3352. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol Production and Applications: An Overview; Elsevier Publishing: London, UK, 2018; ISBN 9780444640109. [Google Scholar]
- Ronni, W. Alcohol and the skin. Clin. Dermatol. 1999, 17, 351–352. [Google Scholar] [PubMed]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Randall Bond, G.; Krenzelok, E.P.; Cooper, H.; Allister Vale, J. American Academy of Clinical Toxicology Practice Guidelines on the Treatment of Methanol Poisoning The American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning: * Donald G For personal use only. Clin. Toxicol. 2002, 40, 415–446. [Google Scholar]
- Matharage, S.Y.; Liu, Q.; Davenport, E.; Wilson, G.; Walker, D.; Wang, Z.D. Methanol detection in transformer oils using gas chromatography and ion trap mass spectrometer. In Proceedings of the 2014 IEEE 18th International Conference on Dielectric Liquids, ICDL, Bled, Slovenia, 30 June–3 July 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Neto, Á.C.; Oliveira, E.C.S.; Lacerda, V.; Castro, E.V.R.; Romão, W.; Silva, R.C.; Pereira, R.G.; Sten, T.; Filgueiras, P.R.; Poppi, R.J. Quality control of ethanol fuel: Assessment of adulteration with methanol using 1H NMR. Fuel 2014, 135, 387–392. [Google Scholar] [CrossRef]
- Qiu, Q.; Jiang, N.; Ge, L.; Li, X.; Chen, X. The electrochemical sensor for methanol detection based on trimetallic PtAuAg nanotubes. J. Mater. Sci. 2020, 55, 15681–15694. [Google Scholar] [CrossRef]
- Han, T.; Yang, J.; Miao, R.; Liu, K.; Li, J.; Wang, D.; Liu, T.; Fang, Y. Direct Distinguishing of Methanol over Ethanol with a Nanofilm-Based Fluorescent Sensor. Adv. Mater. Technol. 2021, 6, 2000933. [Google Scholar] [CrossRef]
- Li, K.; Wu, Y.; Chen, M.; Rong, Q.; Zhu, Z.; Liu, Q.; Zhang, J. High Methanol Gas-Sensing Performance of Sm2O3/ZnO/SmFeO3 Microspheres Synthesized Via a Hydrothermal Method. Nanoscale Res. Lett. 2019, 14, 57. [Google Scholar] [CrossRef]
- Voss, H.G.J.; Mendes Júnior, J.J.A.; Farinelli, M.E.; Stevan, S.L. A Prototype to Detect the Alcohol Content of Beers Based on an Electronic Nose. Sensors 2019, 19, 2646. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, I.-T.; Yang, H.-C.; Chen, Y.J. An AI-powered Electronic Nose System with Fingerprint Extraction for Aroma Recognition of Coffee Beans. Micromachines 2022, 13, 1313. [Google Scholar] [CrossRef]
- Štefániková, J.; Árvay, J.; Miškeje, M.; Kačániová, M. Determination of Volatile Organic Compounds in Slovak Bryndza Cheese By the Electronic Nose and the Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry. Potravin. Slovak J. Food Sci. 2020, 14, 767–773. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, Y.; Zou, L.; Zhu, H.; Cao, R.; Zhao, G. Combination of machine learning and intelligent sensors in real-time quality control of alcoholic beverages. Food Sci. Technol. 2022, 42, e54622. [Google Scholar] [CrossRef]
- Romani, S.; Rodriguez-Estrada, M.T. Chapter 5-Bakery Products and Electronic Nose. In Electronic Noses and Tongues in Food Science; Rodríguez Méndez, M.L., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 39–47. ISBN 978-0-12-800243-8. [Google Scholar]
- Wu, G.; Du, H.; Cha, Y.L.; Lee, D.; Kim, W.; Feyzbar-Khalkhali-Nejad, F.; Oh, T.S.; Zhang, X.; Kim, D.J. A wearable mask sensor based on polyaniline/CNT nanocomposites for monitoring ammonia gas and human breathing. Sens. Actuators B Chem. 2023, 375, 132858. [Google Scholar] [CrossRef]
- Kim, W.; Lee, D.; Wu, G.; Cha, Y.L.; Moazzem, M.S.; Cho, S.; Kim, D.-J. Molecularly Imprinted Chemiresistive Sensor for Specific Recognition of Furaneol as a Biomarker of Strawberry Flavor Conditions. ACS Sens. 2023, 8, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.M.; Ahmed, F.; Saber, O.; Kumar, S. Gases in Food Production and Monitoring: Recent Advances in Target Chemiresistive Gas Sensors. Chemosensors 2022, 10, 338. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, J. Methodology for Quantifying Volatile Compounds in a Liquid Mixture Using an Algorithm Combining B-Splines and Artificial Neural Networks to Process Responses of a Thermally Modulated Metal-Oxide Semiconductor Gas Sensor. Sensors 2022, 22, 8959. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart Gas Sensors: Recent Developments and Future Prospective; Springer Nature: Singapore, 2025; Volume 17, ISBN 0123456789. [Google Scholar]
- Lun, D.; Xu, K. Recent Progress in Gas Sensor Based on Nanomaterials. Micromachines 2022, 13, 919. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors—A Review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Advancements in Improving Selectivity of Metal Oxide Semiconductor Gas Sensors Opening New Perspectives for Their Application in Food Industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Ravindranath, V.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Optimization of extraction solvent and fast blue BB assay for comparative analysis of antioxidant phenolics from Cucumis melo L. Plants 2021, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Ferreira, S.H.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave synthesized ZnO nanorod arrays for UV sensors: A seed layer annealing temperature study. Materials 2016, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Ultrahigh Selective Room-Temperature Ammonia Gas Sensor Based on Tin-Titanium Dioxide/reduced Graphene/Carbon Nanotube Nanocomposites by the Solvothermal Method. ACS Omega 2019, 4, 16916–16924. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-fast microwave synthesis of ZnO nanorods on cellulose substrates for UV sensor applications. Materials 2017, 10, 4–10. [Google Scholar] [CrossRef]
- Singh, S.A.; More, P.S.; Khollam, Y.B.; Kondawar, S.B. Enhanced hydrogen gas sensing characteristics of graphene modified with rubidium (Rb). Mater. Chem. Phys. 2021, 260, 124105. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S. Temperature dependent selective detection of ethanol and methanol using MoS2/TiO2 composite. Sens. Actuators B Chem. 2022, 350, 130798. [Google Scholar] [CrossRef]
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Gas sensor array and classifiers as a means of varroosis detection. Sensors 2020, 20, 117. [Google Scholar] [CrossRef]
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Detecting varroosis using a gas sensor system as a way to face the environmental threat. Sci. Total Environ. 2020, 722, 137866. [Google Scholar] [CrossRef] [PubMed]
- Štefániková, J.; Nagyová, V.; Hynšt, M.; Vietoris, V.; Martišová, P.; Nagyová, Ľ. Application of electronic nose for determination of Slovak cheese authentication based on aroma profile. Potravin. Slovak J. Food Sci. 2019, 13, 262–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakata, S.; Takahara, N. Distinction of gaseous mixtures based on different cyclic temperature modulations. Sens. Actuators B Chem. 2022, 359, 131615. [Google Scholar] [CrossRef]
- Morati, N.; Contaret, T.; Seguin, J.; Bendahan, M.; Morati, N.; Contaret, T.; Seguin, J.; Bendahan, M.; Djedidi, O.; Morati, N.; et al. Data Analysis-Based Gas Identification with a Single Metal Oxide Sensor Operating in Dynamic Temperature Regime. In Proceedings of the ALLSENSORS 2020, The Fifth International Conference on Advances in Sensors, Actuators, Metering and Sensing, Valencia, Spain, 21–25 November 2019. [Google Scholar]
- Wawrzyniak, J. Quantitative determination of volatile compounds in a mixture using a single thermally modulated metal oxide semiconductor gas sensor and convolutional neural networks. Microchem. J. 2025, 211, 113083. [Google Scholar] [CrossRef]
- Huang, S.; Ibarlucea, B.; Cuniberti, G. Discrimination of Methanol from Ethanol Using Graphene-based Smart Gas Sensors. In Proceedings of the ISOEN 2024-International Symposium on Olfaction and Electronic Nose, Grapevine, TX, USA, 12–15 May 2024; pp. 1–3. [Google Scholar] [CrossRef]
- Durán, C.; Benjumea, J.; Carrillo, J. Response optimization of a chemical gas sensor array using temperature modulation. Electronics 2018, 7, 54. [Google Scholar] [CrossRef]
- Krivetskiy, V.V.; Andreev, M.D.; Efitorov, A.O.; Gaskov, A.M. Statistical shape analysis pre-processing of temperature modulated metal oxide gas sensor response for machine learning improved selectivity of gases detection in real atmospheric conditions. Sens. Actuators B Chem. 2021, 329, 129187. [Google Scholar] [CrossRef]
- Chutia, R.; Bhuyan, M. Best frequency for temperature modulation of tin oxide gas sensor for chemical vapor identification. Int. J. Eng. Technol. 2014, 6, 1158–1166. [Google Scholar]
- Shi, X.; Zhang, H.; Ji, H.; Meng, F. Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor. Chemosensors 2022, 10, 226. [Google Scholar] [CrossRef]
- Mu, F.; Gu, Y.; Zhang, J.; Zhang, L. Milk source identification and milk quality estimation using an electronic nose and machine learning techniques. Sensors 2020, 20, 4238. [Google Scholar] [CrossRef]
- Abdelghani, R.M.; Kashyout, A.E.B.; Morsi, I.; Taha, T.E.; Soliman, N.F.; El-shafai, W. Synthesis and characterization of hematite nanomaterials imprinted with acetone, ethanol and methanol for AI-Based IoT gas sensor arrays. Sci. Rep. 2025, 15, 29821. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.; Chandra, S.K. A Temperature Modulation Circuit for Metal Oxide Semiconductor Gas Sensor. Indian J. Sci. Technol. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Rescalli, A.; Marzorati, D.; Gelosa, S.; Cellesi, F.; Cerveri, P. Temperature Modulation of MOS Sensors for Enhanced Detection of Volatile Organic Compounds. Chemosensors 2023, 11, 501. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics, 67th ed.; CRC Press Inc.: Boca Raton, FL, USA, 1986. [Google Scholar]
- Yoshizawa, T.; Kamijo, Y.; Fujita, Y.; Suzuki, Y.; Hanazawa, T.; Usui, K.; Kishino, T. Mild manifestation of methanol poisoning half a day after massive ingestion of a fuel alcohol product containing 70% ethanol and 30% methanol: A case report. Acute Med. Surg. 2018, 5, 289–291. [Google Scholar] [CrossRef]
- Ahumada, L.A.C.; Cruz, A.F.S.; Fox, M.A.G.; Sandoval, O.L.H. Useful Piezoelectric Sensor to Detect False Liquor in Samples with Different Degrees of Adulteration. J. Sens. 2018, 2018, 924094. [Google Scholar] [CrossRef]
- Kraut, J.A. Approach to the Treatment of Methanol Intoxication. Am. J. Kidney Dis. 2016, 68, 161–167. [Google Scholar] [CrossRef]
- Prakasha, B.S.; Shukla, G.; Subramanian, A. Discriminative analysis of volatile organic compounds using machine-learning assisted Au loaded ZnO and TiO2-based thin film sensors. Sens. Actuators A. Phys. 2024, 373, 115385. [Google Scholar] [CrossRef]
- Chen, C.; Liaw, H.; Shu, C.; Hsieh, Y. Autoignition Temperature Data for Methanol, Ethanol, Propanol, 2-Butanol, 1-Butanol, and 2-Methyl-2, 4-pentanediol. J. Chem. Eng. Data 2010, 55, 5059–5064. [Google Scholar] [CrossRef]
- Whan, Y.; Yang, B.; Thi, D.; To, H.; Resendiz, E.; Hyoung, K.; Vincent, N.; Liu, Y. Improved selectivity toward methanol detection via Pd-functionalized tungsten trioxide nanofiber gas sensors. Chem. Eng. J. 2025, 523, 168814. [Google Scholar] [CrossRef]
- Moalaghi, M.; Gharesi, M.; Ranjkesh, A. Topic: Chemical Sensors, P1GS.22-Tin Oxide Gas Sensor on Tin Oxide Microheater for Methane. Sensing 2018, 9, 560–561. [Google Scholar] [CrossRef]
- Tsuruta, A.; Itoh, T.; Mikami, M.; Terasaki, I.; Murayama, N.; Shin, W.S. Trial of an All-Ceramic SnO2 Gas Sensor Equipped with CaCu3Ru4O12 Heater and Electrode. Materials 2018, 11, 981. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Babaei, F.; Amini, A. A breakthrough in gas diagnosis with a temperature-modulated generic metal oxide gas sensor. Sens. Actuators B Chem. 2012, 166–167, 419–425. [Google Scholar] [CrossRef]
- Guo, Q.; Li, T.; Qu, Y.; Wang, X.; Liu, L.; Liu, H.; Wang, Q. Action of phytosterols on thermally induced trans fatty acids in peanut oil. Food Chem. 2021, 344, 128637. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Sun, Q.; Zhou, W.; Zhu, Z. Principal Component Analysis for Big Data. Wiley StatsRef Stat. Ref. Online 2018, 1–13. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Lombardi, G.; Ranaldi, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the carvacrol and thymol chemotypes. Plants 2023, 12, 1344. [Google Scholar] [CrossRef]
- Lima, C.M.G.; Silveira, P.G.; Santana, R.F.; da Piedade Edmundo Sitoe, E.; Bonomo, R.C.F.; Coutinho, H.D.M.; Wawrzyniak, J.; de Carvalho dos Anjos, V.; Bell, M.J.V.; Contado, J.L.; et al. Leveraging infrared spectroscopy for cocoa content prediction: A dual approach with Kohonen neural network and multivariate modeling. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2025, 335, 125975. [Google Scholar] [CrossRef]
- Koraqi, H.; Wawrzyniak, J.; Aydar, A.Y.; Pandiselvam, R.; Khalide, W.; Petkoska, A.T.; Karabagias, I.K.; Ramniwas, S.; Rustagi, S. Application of multivariate analysis and Kohonen Neural Network to discriminate bioactive components and chemical composition of kosovan honey. Food Control 2025, 172, 111072. [Google Scholar] [CrossRef]
- Sitoe, E.; Gonçalves Lima, C.M.; Wawrzyniak, J.; Mourão, M. Integration of PCA, HCA, and KNN to Evaluate Packaging and Storage Conditions for Red Bell Peppers. J. Food Sci. 2025, 90, e70367. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Karamizadeh, S.; Abdullah, S.M.; Manaf, A.A.; Zamani, M.; Hooman, A. An Overview of Principal Component Analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Shrestha, N. Factor Analysis as a Tool for Survey Analysis. Am. J. Appl. Math. Stat. 2021, 9, 4–11. [Google Scholar] [CrossRef]
- Cybenko, G. Approximation by superpositions of a sigmoidal function. Math. Control Signals Syst. 1989, 2, 303–314. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Kodogiannis, V.S. Application of neural networks as a non-linear modelling technique in food mycology. Expert Syst. Appl. 2009, 36, 121–131. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Safari Sinegani, A.A.; Sarikhani, M.R.; Mohammadi, S.A. Comparison of artificial neural network and multivariate regression models for prediction of Azotobacteria population in soil under different land uses. Comput. Electron. Agric. 2017, 140, 409–421. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Application of artificial neural networks to assess the mycological state of bulk stored rapeseeds. Agriculture 2020, 10, 567. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Prediction of fungal infestation in stored barley ecosystems using artificial neural networks. LWT 2021, 137, 110367. [Google Scholar] [CrossRef]
- Huang, Y. Advances in artificial neural networks-Methodological development and application. Algorithms 2009, 2, 973–1007. [Google Scholar] [CrossRef]
- Rapado-Rincón, D.; van Henten, E.J.; Kootstra, G. Development and evaluation of automated localisation and reconstruction of all fruits on tomato plants in a greenhouse based on multi-view perception and 3D multi-object tracking. Biosyst. Eng. 2023, 231, 78–91. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Guindo, M.L.; Bakhshipour, A.; Fazayeli, H.; Li, X.; Yang, C. Advancing precision agriculture: The potential of deep learning for cereal plant head detection. Comput. Electron. Agric. 2023, 209, 107875. [Google Scholar] [CrossRef]
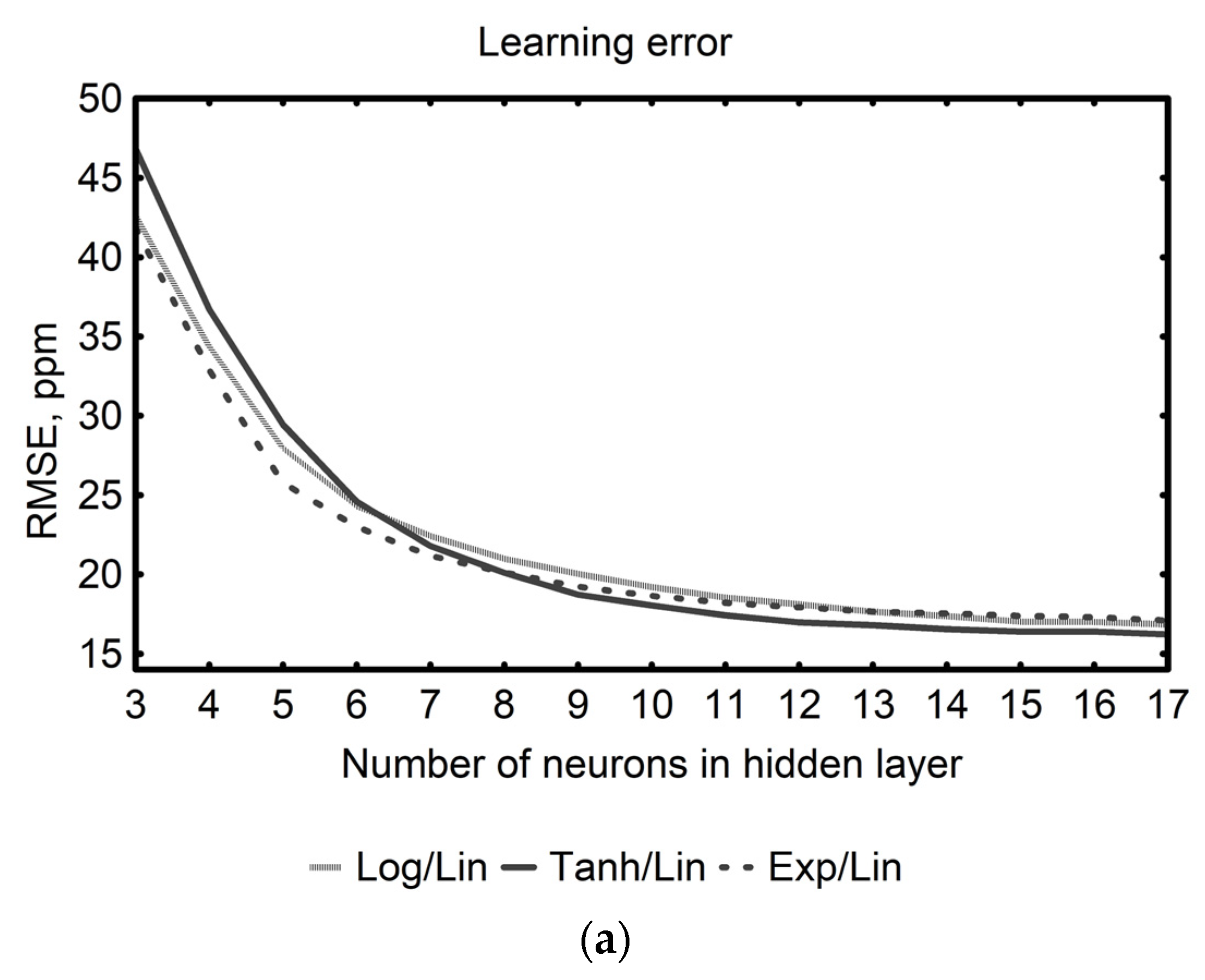
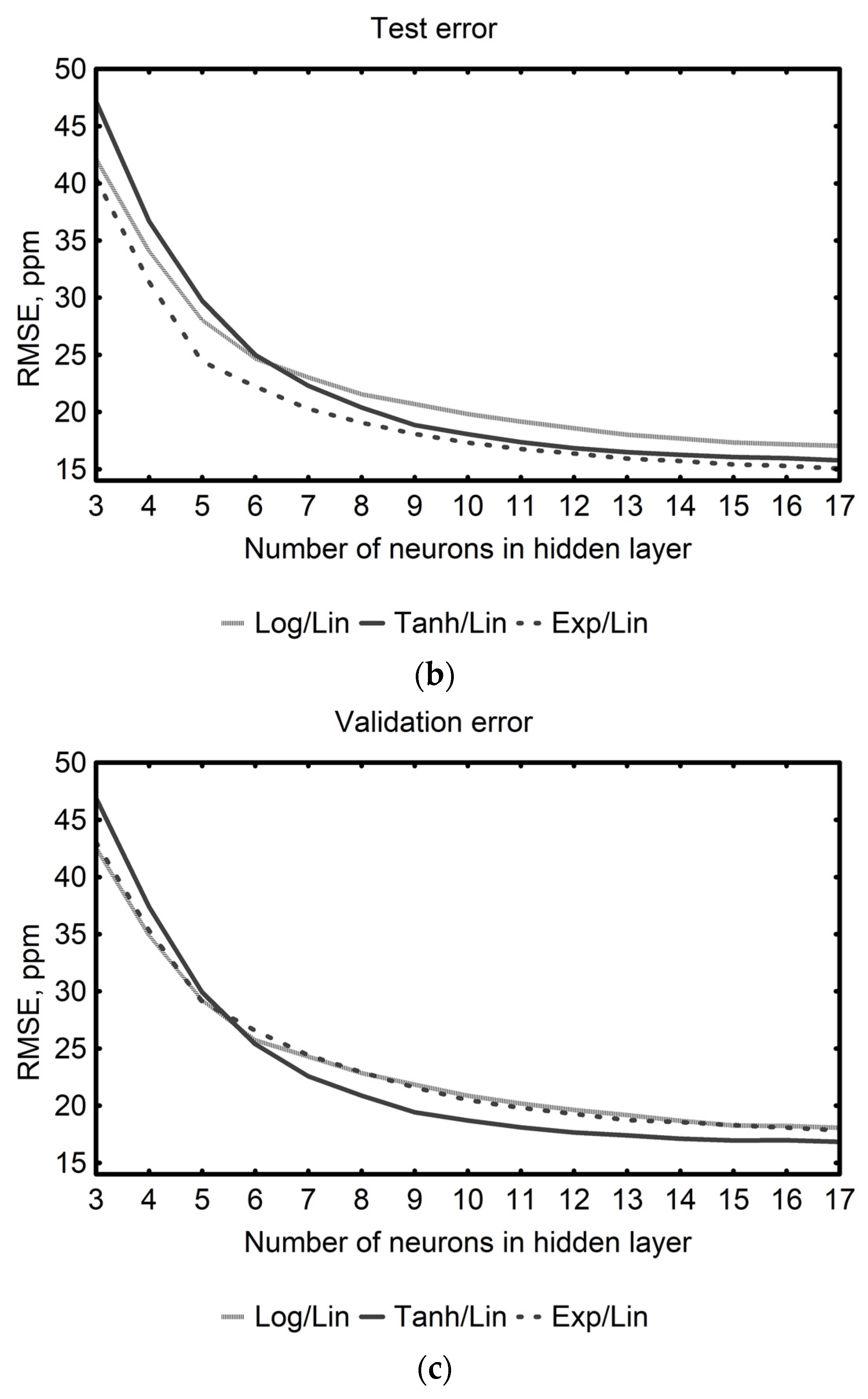
| Neural Networks Topology | Activation Functions Hidden/Output Layer | Network Group/ Error Metric | Errors (ppm) | |||
|---|---|---|---|---|---|---|
| El | Et | Ev | ||||
| MLP 3-12-2 | Tanh/Lin | Best model | 13.27 | 12.01 | 12.57 | |
| Group of 1000 networks | mean | 16.90 | 16.68 | 17.48 | ||
| Group of 1000 networks | SD | 1.72 | 2.32 | 2.52 | ||
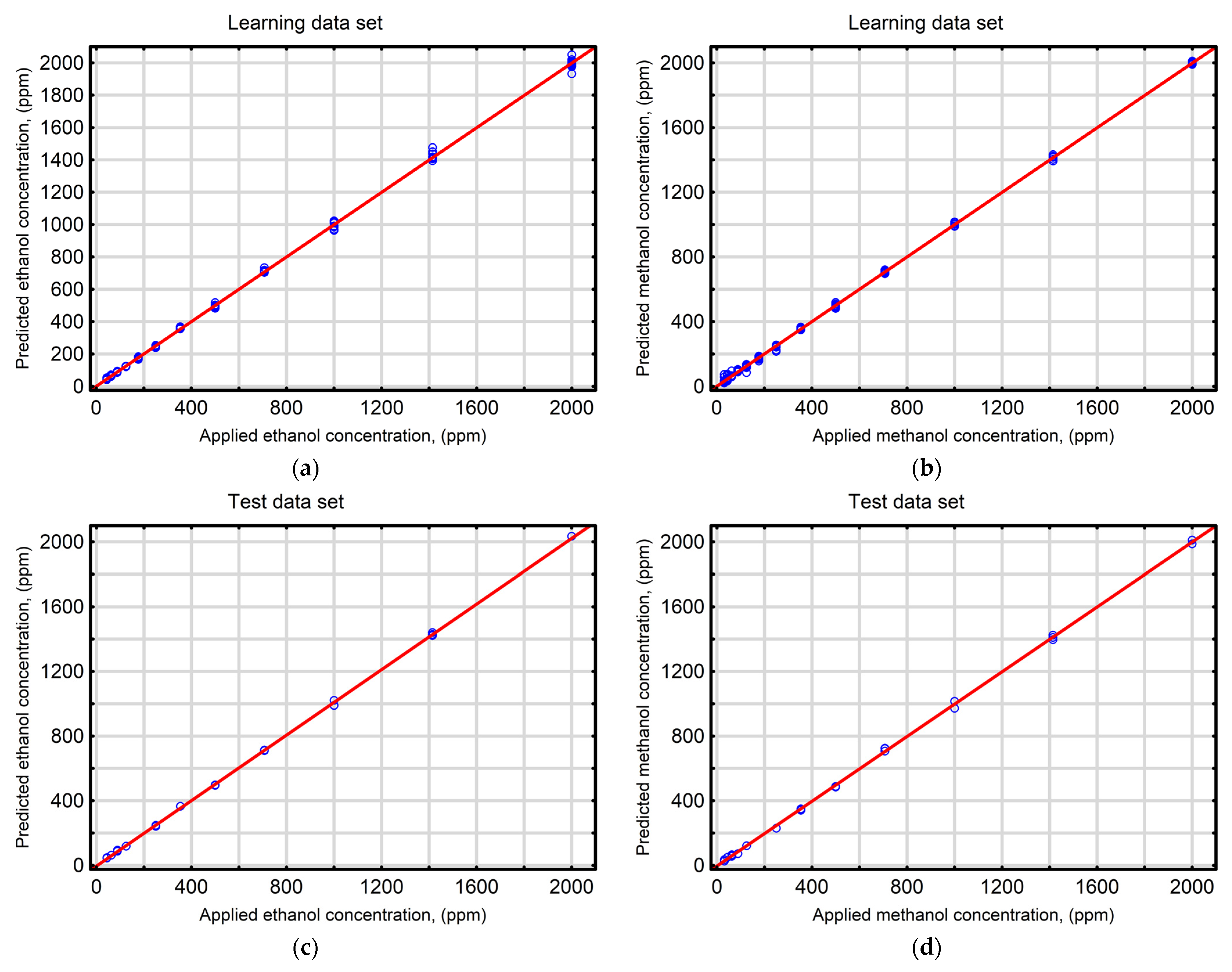
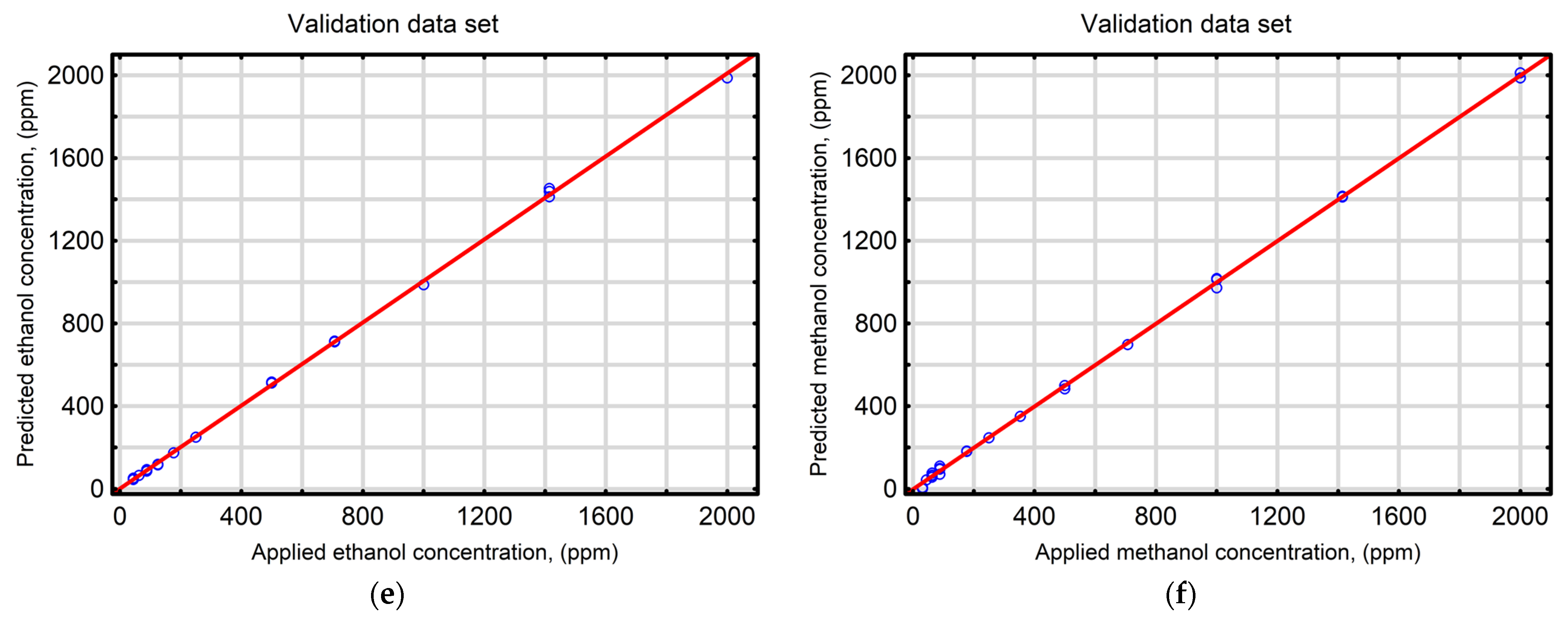
| Statistical Index | Ethanol | Methanol | ||||||
|---|---|---|---|---|---|---|---|---|
| Dataset | ||||||||
| L | T | V | F | L | T | V | F | |
| Coefficient of determination (R2) | 0.9994 | 0.9997 | 0.9996 | 0.9995 | 0.9996 | 0.9996 | 0.9995 | 0.9998 |
| Root mean square error (RMSE) | 14.37 | 11.71 | 11.28 | 13.67 | 11.60 | 12.30 | 13.72 | 12.25 |
| Mean absolute error (MAE) | 9.02 | 8.36 | 7.38 | 8.67 | 8.31 | 10.02 | 10.65 | 8.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, J. Quantification of Volatile Compounds in Mixtures Using a Single Thermally Modulated MOS Gas Sensor with PCA–ANN Data Processing. Sensors 2025, 25, 6913. https://doi.org/10.3390/s25226913
Wawrzyniak J. Quantification of Volatile Compounds in Mixtures Using a Single Thermally Modulated MOS Gas Sensor with PCA–ANN Data Processing. Sensors. 2025; 25(22):6913. https://doi.org/10.3390/s25226913
Chicago/Turabian StyleWawrzyniak, Jolanta. 2025. "Quantification of Volatile Compounds in Mixtures Using a Single Thermally Modulated MOS Gas Sensor with PCA–ANN Data Processing" Sensors 25, no. 22: 6913. https://doi.org/10.3390/s25226913
APA StyleWawrzyniak, J. (2025). Quantification of Volatile Compounds in Mixtures Using a Single Thermally Modulated MOS Gas Sensor with PCA–ANN Data Processing. Sensors, 25(22), 6913. https://doi.org/10.3390/s25226913







