Moisture Content Detection in Mango (Mangifera indica L., cv. Ataulfo) and Papaya (Carica papaya) Slices During Drying Using an MMI-Based Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Water and Sugar Solution
2.1.2. Mango and Papaya Samples
2.2. Drying Process
2.3. Measurement of the NIR Absorption Spectrum of the Samples
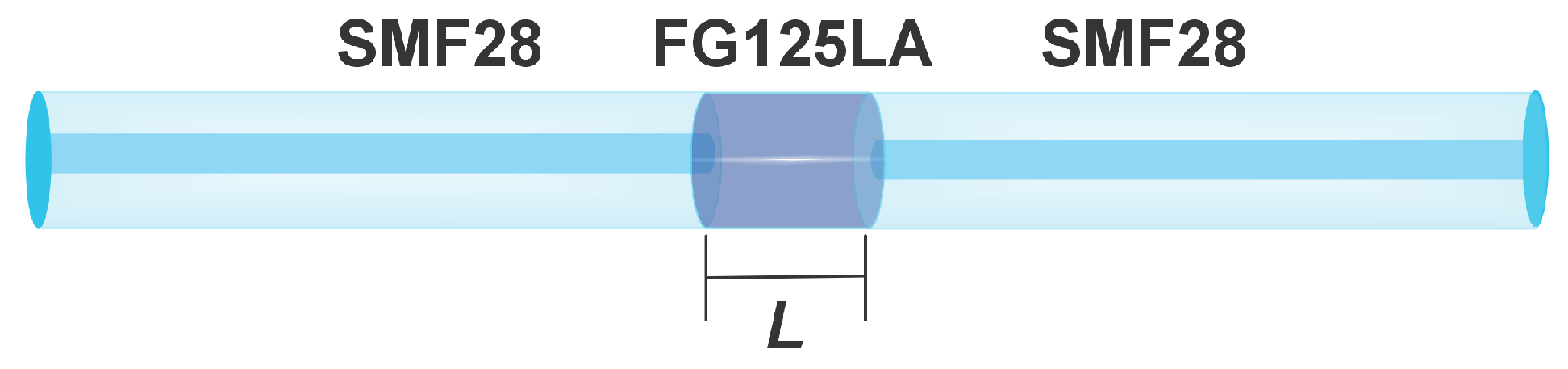
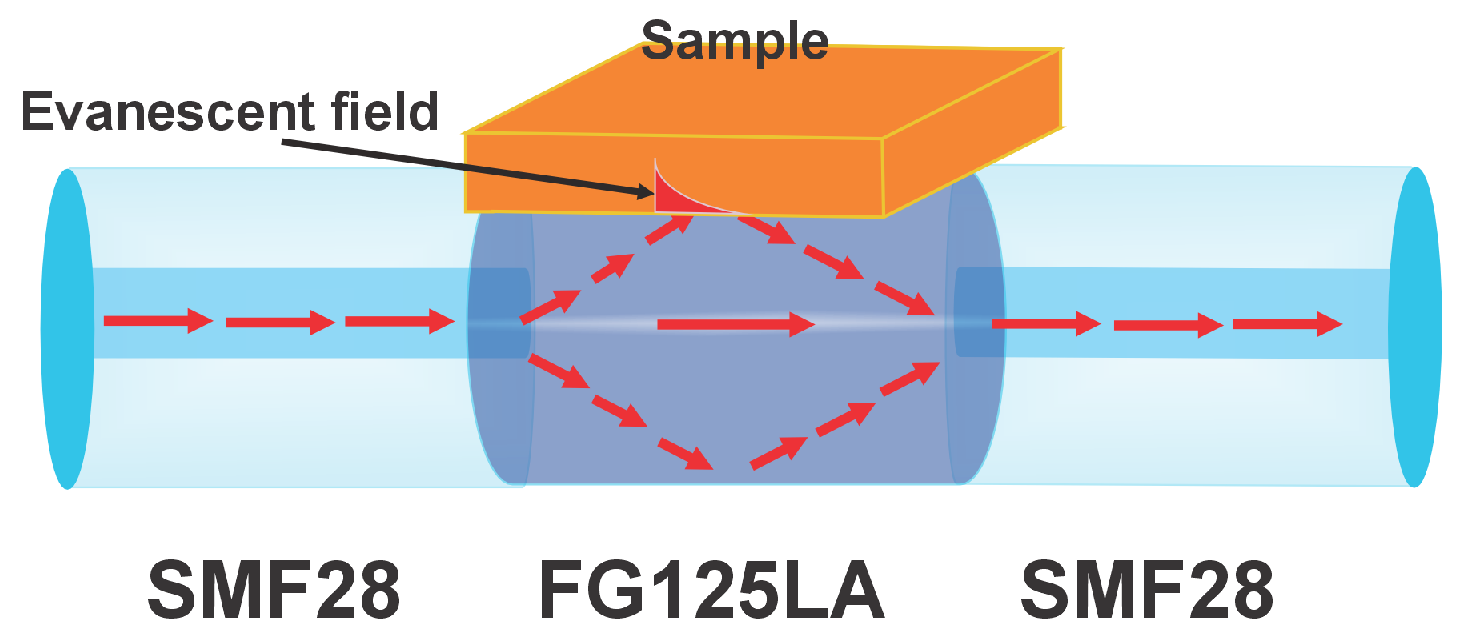
2.4. Sensor Preparation
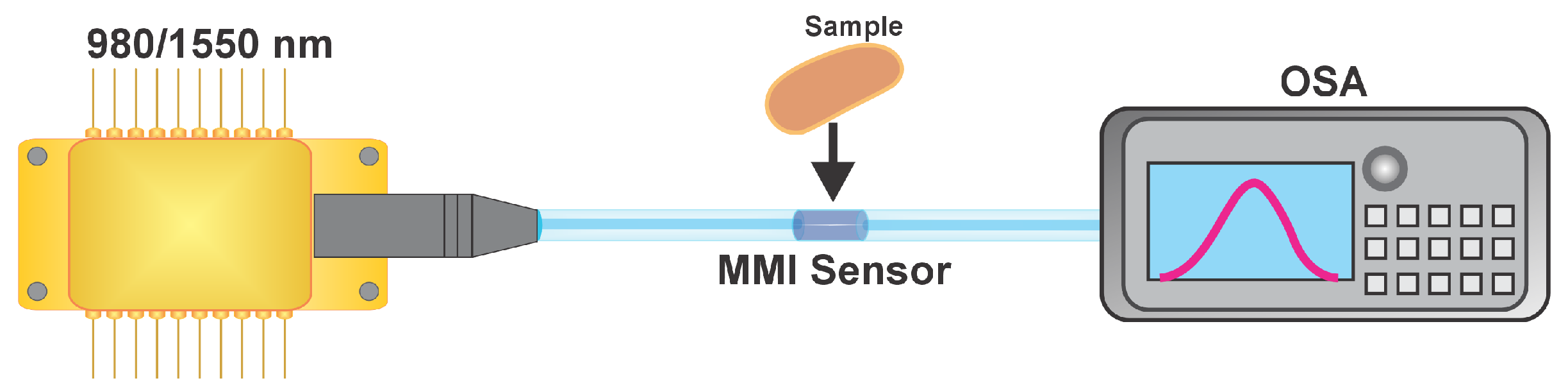
Measurement Procedure
3. Results
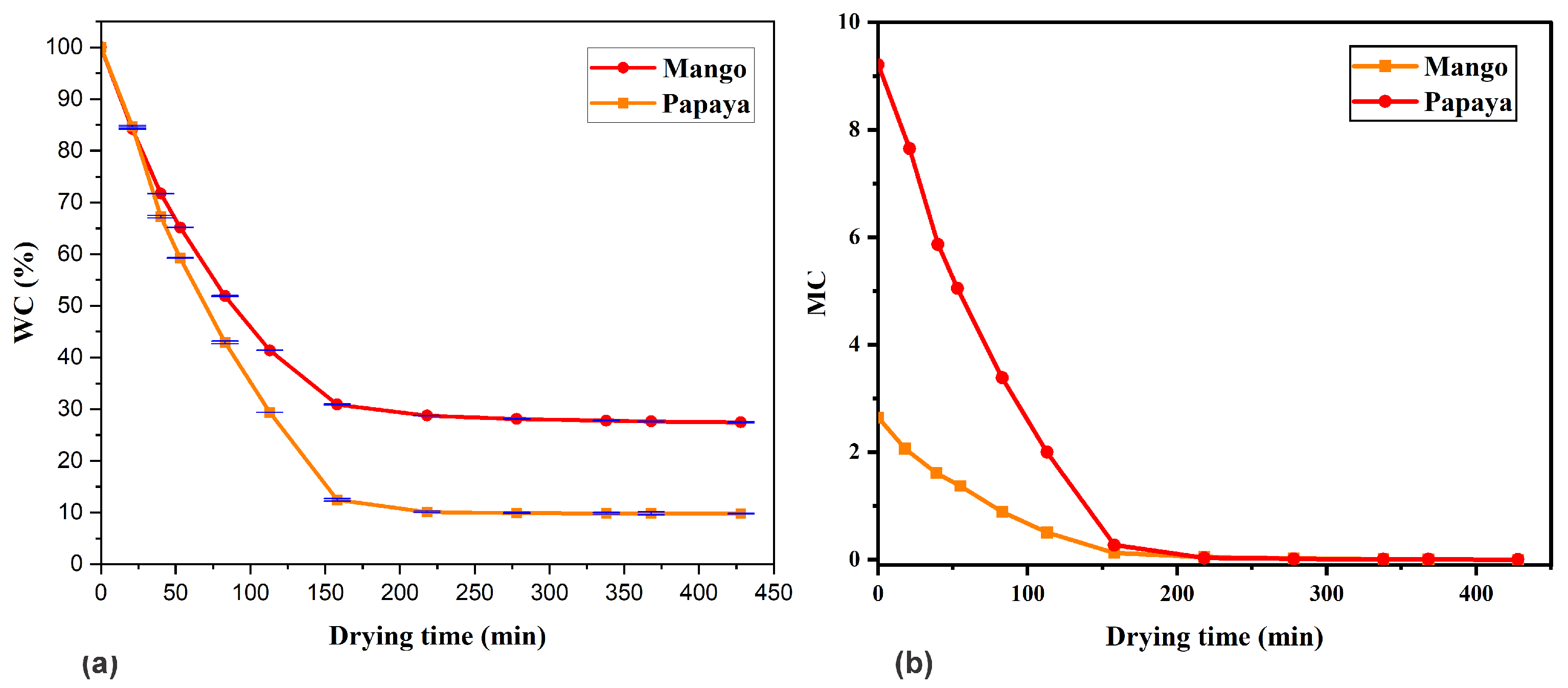
3.1. Drying Curve
3.2. NIR Spectra Response of Papaya Sample
3.3. MMI Sensor Response to Sample
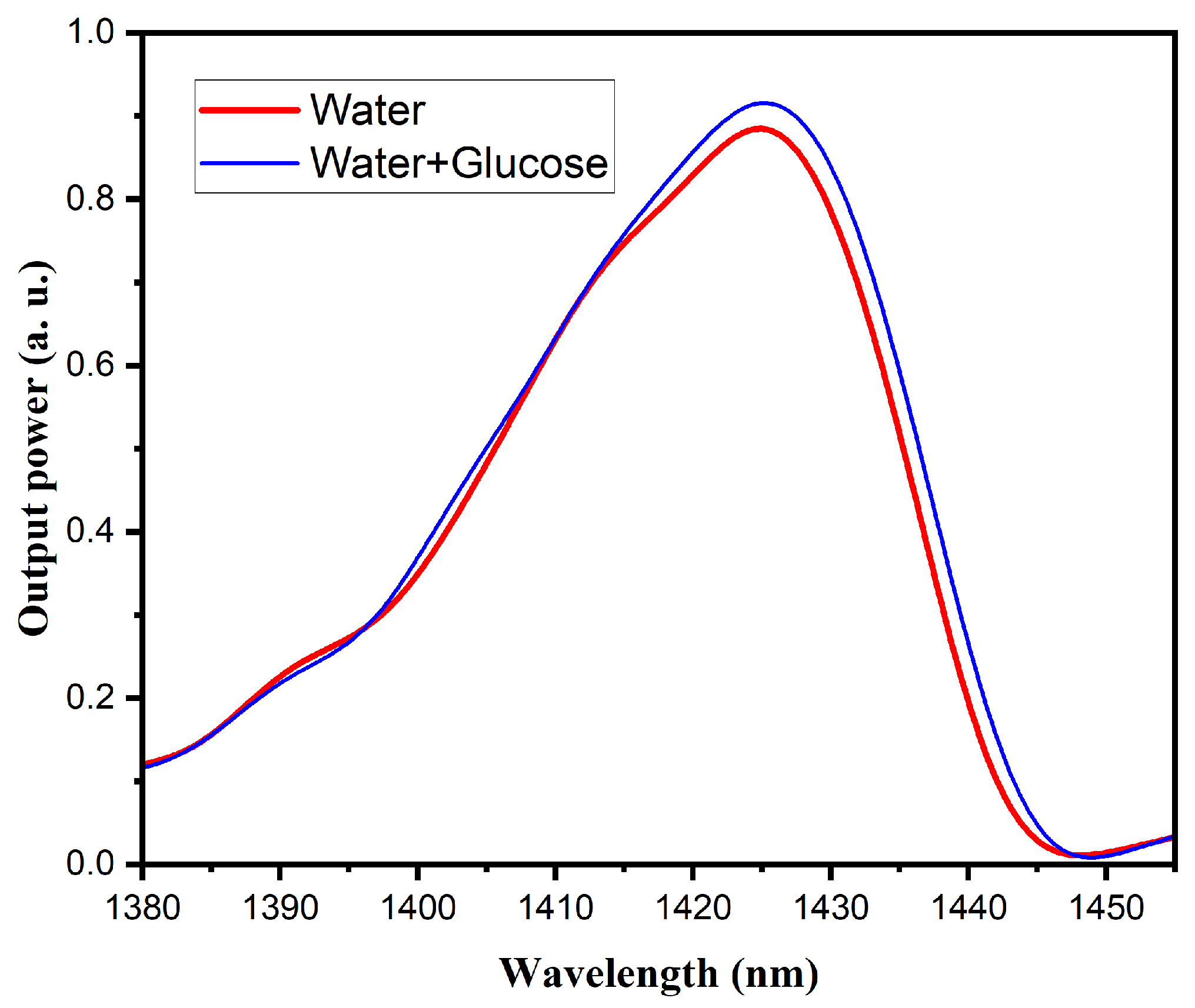
3.3.1. Water and Glucose Mixture
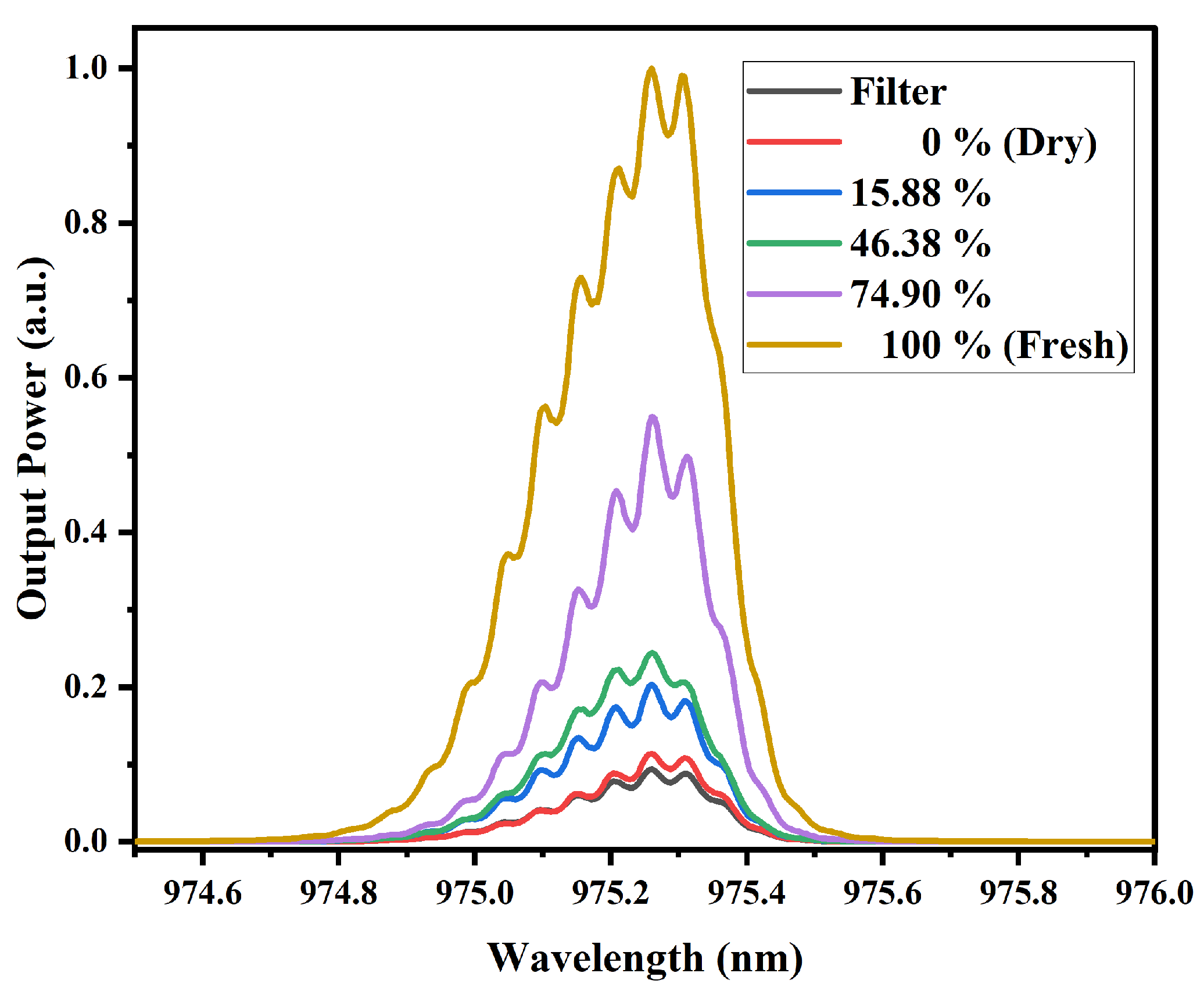
3.3.2. MMI Sensor for the 980 nm Laser Diode
3.3.3. MMI Sensor for the Superluminescent Laser Diode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMF | Single Mode Fiber |
| MMF | Multimode Fiber |
| NCF | No-Core Fiber |
| MMI | Multimodal Interference |
References
- Sagar, V.; Suresh Kumar, P. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Srivastav, P.P.; Karizaki, V.M.; Suleria, H.A.R. Effects of drying technology on physiochemical and nutritional quality of fruits and vegetables. In Emerging Thermal and Nonthermal Technologies in Food Processing; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 69–116. [Google Scholar]
- de Información Agroalimentaria y Pesquera, S. Producción Nacional Agrícola. 2025. Available online: https://nube.agricultura.gob.mx/avance_agricola/ (accessed on 18 June 2025).
- Nakra, S.; Tripathy, S.; Srivastav, P.P. Drying as a preservation strategy for medicinal plants: Physicochemical and functional outcomes for food and human health. Phytomedicine Plus 2025, 5, 100762. [Google Scholar] [CrossRef]
- Moutia, I.; Lakatos, E.; Kovács, A.J. Impact of Dehydration Techniques on the Nutritional and Microbial Profiles of Dried Mushrooms. Foods 2024, 13, 3245. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- Vera Zambrano, M.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.D. Water content of natural cyclodextrins and their essential oil complexes: A comparative study between Karl Fischer titration and thermal methods. Food Chem. 2012, 132, 1741–1748. [Google Scholar] [CrossRef]
- López-Morales, G.; López-Páez, M.F.; López, P.; Carriles, R.; Vilchis, H. Detection of moisture ratio and carotenoid compounds in mamey (Pouteria sapota) fruit during dehydration process using spectroscopic techniques. J. Food Sci. Technol. 2023, 60, 1952–1959. [Google Scholar] [CrossRef]
- Javanbakht, N.; Xiao, G.; Amaya, R.E. A Comprehensive Review of Portable Microwave Sensors for Grains and Mineral Materials Moisture Content Monitoring. IEEE Access 2021, 9, 120176–120184. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Q.; Gao, K.; Xia, Z.; Wang, H. Electrical Impedance Sensors for Multi-Phase Flow Measurement: A Review. IEEE Sens. J. 2021, 21, 27252–27267. [Google Scholar] [CrossRef]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of near-infrared (NIR) spectroscopy and hyperspectral imaging for quality and safety assessment of fruits: An overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Saidane, A. Optical Sensors and Microsystems: New Concepts, Materials, Technologies-S. Martellucci, AN Chester, AG Migrani (Eds.); Kluwer Academic/Plenum Press, New York, 2000, 315 pages, hardbound, ISBN 0-306-46380-6. Microelectron. J. 2001, 32, 621–622. [Google Scholar]
- Joe, H.E.; Yun, H.; Jo, S.H.; Jun, M.B.; Min, B.K. A review on optical fiber sensors for environmental monitoring. Int. J. Precis. Eng. Manuf.-Green Technol. 2018, 5, 173–191. [Google Scholar] [CrossRef]
- Soldano, L.B.; Pennings, E.C. Optical multi-mode interference devices based on self-imaging: Principles and applications. J. Light. Technol. 1995, 13, 615–627. [Google Scholar] [CrossRef]
- Nazemosadat, E.; Mafi, A. Nonlinear multimodal interference and saturable absorption using a short graded-index multimode optical fiber. J. Opt. Soc. Am. B 2013, 30, 1357–1367. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Li, C.; Zhang, J.; Xu, S. Mode-locked Tm fiber laser using SMF-SIMF-GIMF-SMF fiber structure as a saturable absorber. Opt. Express 2017, 25, 26546–26553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, Y.; Xiao, X.; Yang, C. Nonlinear multimode interference-based dual-color mode-locked fiber laser. Opt. Lett. 2020, 45, 1655–1658. [Google Scholar] [CrossRef]
- Silva, S.; Pachon, E.G.; Franco, M.A.; Hayashi, J.G.; Malcata, F.X.; Frazão, O.; Jorge, P.; Cordeiro, C.M. Ultrahigh-sensitivity temperature fiber sensor based on multimode interference. Appl. Opt. 2012, 51, 3236–3242. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, D.P.; Wei, L.; Liu, W.K.; Lit, J.W. Temperature-and phase-independent lateral force sensor based on a core-offset multi-mode fiber interferometer. Opt. Express 2008, 16, 19291–19296. [Google Scholar] [CrossRef]
- Ascorbe, J.; Corres, J.M.; Arregui, F.J.; Matias, I.R. Recent developments in fiber optics humidity sensors. Sensors 2017, 17, 893. [Google Scholar] [CrossRef]
- Tian, K.; Farrell, G.; Wang, X.; Yang, W.; Xin, Y.; Liang, H.; Lewis, E.; Wang, P. Strain sensor based on gourd-shaped single-mode-multimode-single-mode hybrid optical fibre structure. Opt. Express 2017, 25, 18885–18896. [Google Scholar] [CrossRef]
- Puspita, I.; Rahmah, F.; Hatta, A.M.; Koentjoro, S. Load effect on an SMS fiber structure embedded in a high-density polyethylene. In Proceedings of the International Seminar on Photonics, Optics, and Its Applications (ISPhOA 2014), Sanur, Indonesia, 14–15 October 2014; SPIE: Bellingham, WA, USA, 2015; Volume 9444, pp. 174–178. [Google Scholar]
- Álvarez-Tamayo, R.I.; Aguilar-Soto, J.G.; Durán-Sánchez, M.; Antonio-López, J.E.; Ibarra-Escamilla, B.; Kuzin, E.A. MMI filters configuration for dual-wavelength generation in a ring cavity erbium-doped fibre laser. J. Eur. Opt. Soc.-Rapid Publ. 2016, 12, 20. [Google Scholar] [CrossRef]
- Guzmán-Sepúlveda, J.R.; Guzmán-Cabrera, R.; Castillo-Guzmán, A.A. Optical sensing using fiber-optic multimode interference devices: A review of nonconventional sensing schemes. Sensors 2021, 21, 1862. [Google Scholar] [CrossRef]
- Quiñones-Flores, A.A.; Guzman-Sepulveda, J.R.; Castillo-Guzman, A.A. Relative Humidity Measurement Based on a Tapered, PVA-Coated Fiber Optics Multimode Interference Sensor. Optics 2023, 4, 473–481. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Jian, S. Multimode interference refractive index sensor based on coreless fiber. Photonic Sens. 2014, 4, 21–27. [Google Scholar] [CrossRef]
- Ran, Y.; Xia, L.; Han, Y.; Li, W.; Rohollahnejad, J.; Wen, Y.; Liu, D. Vibration fiber sensors based on SM-NC-SM fiber structure. IEEE Photonics J. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Wang, K.; Dong, X.; Köhler, M.H.; Kienle, P.; Bian, Q.; Jakobi, M.; Koch, A.W. Advances in optical fiber sensors based on multimode interference (MMI): A review. IEEE Sens. J. 2020, 21, 132–142. [Google Scholar] [CrossRef]
- Ertekin, C.; Firat, M.Z. A comprehensive review of thin-layer drying models used in agricultural products. Crit. Rev. Food Sci. Nutr. 2017, 57, 701–717. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Manzoor, S.; Yusof, Y.A.; Tawakkal, I.S.M.A.; Fikry, M.; Sin, C.L. Thin-layer Drying Characteristics of Papaya (Carica papaya) Peel using Convection Oven and Microwave Drying. Pertanika J. Sci. Technol. 2019, 27, 1207–1226. [Google Scholar]
- Goyal, R.; Kingsly, A.; Manikantan, M.; Ilyas, S. Thin-layer drying kinetics of raw mango slices. Biosyst. Eng. 2006, 95, 43–49. [Google Scholar] [CrossRef]
- Huang, L.S.; Lin, G.R.; Fu, M.Y.; Sheng, H.J.; Sun, H.T.; Liu, W.F. A refractive-index fiber sensor by using no-core fibers. In Proceedings of the 2013 International Symposium on Next-Generation Electronics, Eindhoven, The Netherlands, 25–26 November 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 100–102. [Google Scholar]
- Costantini, I.; Cicchi, R.; Silvestri, L.; Vanzi, F.; Pavone, F.S. In-vivo and ex vivo optical clearing methods for biological tissues. Biomed. Opt. Express 2019, 10, 5251–5267. [Google Scholar] [CrossRef]
- Cairós, C.; Oliva-García, R.; Siverio, G.; Trujillo-Sevilla, J.M.; Rodríguez-Ramos, J.M.; Acebes, Á. Refractive index estimation in biological tissues by quantitative phase imaging. Opt. Mater. 2023, 142, 114087. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Zhao, Q. Review of no-core optical fiber sensor and applications. Sens. Actuators A Phys. 2020, 313, 112160. [Google Scholar] [CrossRef]
- Wang, K.; Mizuno, Y.; Dong, X.; Kurz, W.; Fink, M.; Lee, H.; Jakobi, M.; Koch, A.W. Fiber-optic multimode interference sensing: Comprehensive characterization and its potential for strain-insensitive temperature sensing. arXiv 2022, arXiv:2204.11044. [Google Scholar]
- Das Majumdar, A.; Kamboj, U.; Munjal, N. Characterization of Mango with Physicochemical Analysis and Infrared Spectroscopy. 2025. Available online: https://ssrn.com/abstract=4998092 (accessed on 15 August 2025).
- Purwanto, Y.A.; Budiastra, I.W.; Darmawati, E.; Arifiya, N. Measurement of starch and soluble solid content in papaya using near infrared spectroscopy. J. Chem. Pharm. Res. 2015, 7, 112–116. [Google Scholar]
- Cavaco, A.M.; Passos, D.; Pires, R.M.; Antunes, M.D.; Guerra, R. Nondestructive Assessment of Citrus Fruit Quality and Ripening by Visible–Near Infrared Reflectance Spectroscopy; IntechOpen: London, UK, 2021. [Google Scholar]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Niskanen, I.; Lauri, J.; Ojala, S.; Kolli, T.; Yokota, M.; Heikkilä, R.; Fabritius, T. Refractive index shift from liquid to solid in the drying process. Opt. Rev. 2025, 32, 497–503. [Google Scholar] [CrossRef]
- Katsumata, T.; Aizawa, H.; Komuro, S.; Ito, S.; Matsumoto, T. Non-destructive evaluation of orange juice based on optical scattering intensities. Optik 2019, 182, 1064–1073. [Google Scholar] [CrossRef]
- Gryczynski, Z.; Gryczynski, I.; Lakowicz, J.R. 2-Basics of Fluorescence and FRET. In Molecular Imaging; Periasamy, A., Day, R.N., Eds.; American Physiological Society: San Diego, CA, USA, 2005; pp. 21–56. [Google Scholar] [CrossRef]






| Mango | Papaya | |||||
|---|---|---|---|---|---|---|
| Model | Coef. | RMSE | R2 | Coefficients | RMSE | R2 |
| Lewis | k = 0.0137 | 0.0294 | 0.993 | k = 0.0128 | 0.0459 | 0.984 |
| Page | k = 0.00697 | 0.0219 | 0.996 | k = 0.00324 | 0.0217 | 0.996 |
| n = 1.1562 | n = 1.3166 | |||||
| HP | k = 0.0140 | 0.0299 | 0.993 | k = 0.0136 | 0.0437 | 0.986 |
| a = 1.0184 | a = 1.0524 | |||||
| Log | k = 0.0133 | 0.0285 | 0.993 | k = 0.0126 | 0.0408 | 0.988 |
| a = 1.0336 | a = 1.0774 | |||||
| c = −0.0206 | c = −0.0331 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Morales, G.; Espinosa-Sánchez, Y.M.; Flores-Rosas, A.; Vilchis, H. Moisture Content Detection in Mango (Mangifera indica L., cv. Ataulfo) and Papaya (Carica papaya) Slices During Drying Using an MMI-Based Sensor. Sensors 2025, 25, 6902. https://doi.org/10.3390/s25226902
López-Morales G, Espinosa-Sánchez YM, Flores-Rosas A, Vilchis H. Moisture Content Detection in Mango (Mangifera indica L., cv. Ataulfo) and Papaya (Carica papaya) Slices During Drying Using an MMI-Based Sensor. Sensors. 2025; 25(22):6902. https://doi.org/10.3390/s25226902
Chicago/Turabian StyleLópez-Morales, Guadalupe, Yuliana M. Espinosa-Sánchez, Ariel Flores-Rosas, and Héber Vilchis. 2025. "Moisture Content Detection in Mango (Mangifera indica L., cv. Ataulfo) and Papaya (Carica papaya) Slices During Drying Using an MMI-Based Sensor" Sensors 25, no. 22: 6902. https://doi.org/10.3390/s25226902
APA StyleLópez-Morales, G., Espinosa-Sánchez, Y. M., Flores-Rosas, A., & Vilchis, H. (2025). Moisture Content Detection in Mango (Mangifera indica L., cv. Ataulfo) and Papaya (Carica papaya) Slices During Drying Using an MMI-Based Sensor. Sensors, 25(22), 6902. https://doi.org/10.3390/s25226902






