The Effect of Knee Extension Limitation on Lumbar Intervertebral Disc Compression Force During Walking: A 3D Musculoskeletal Analysis
Highlights
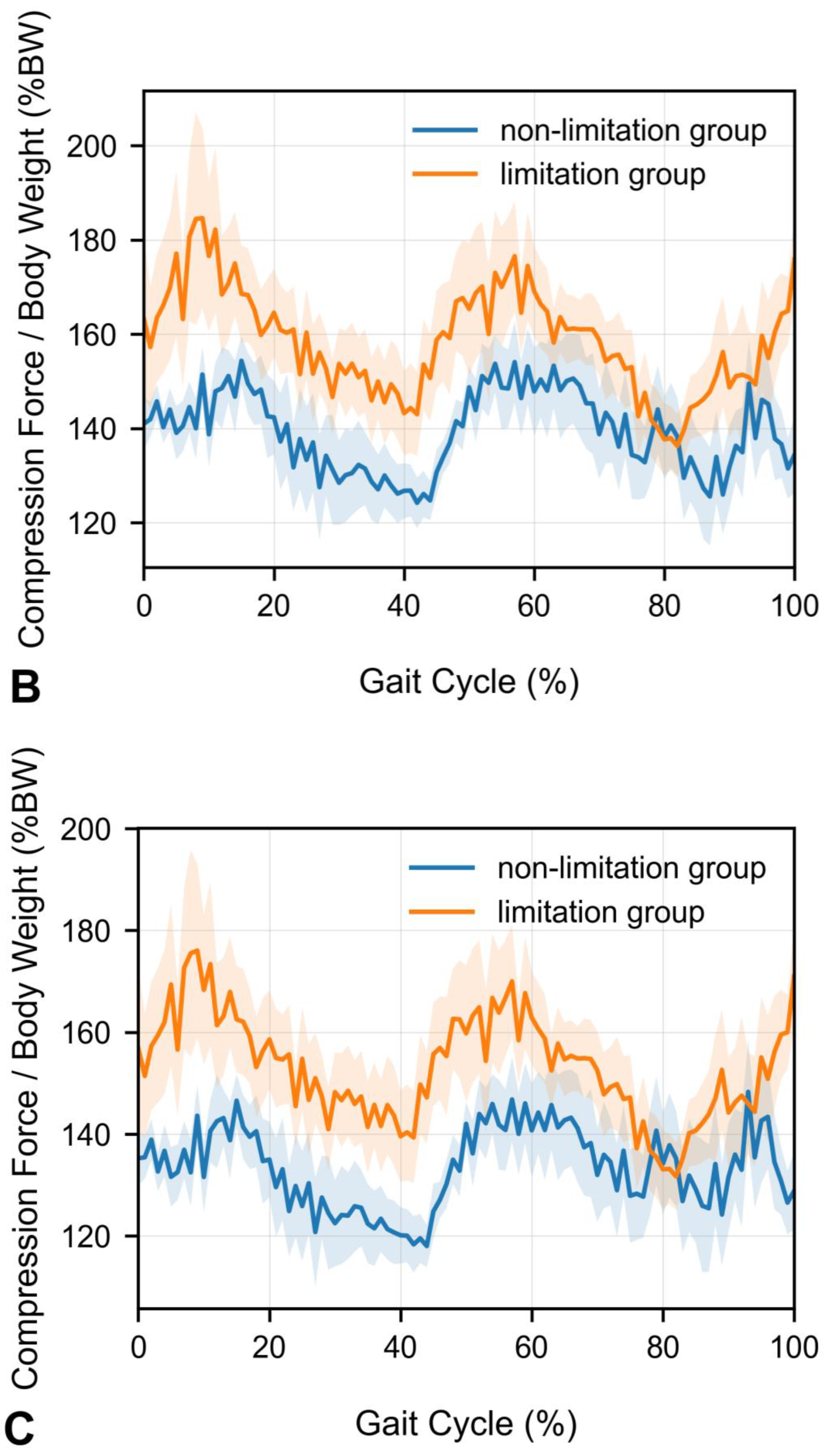
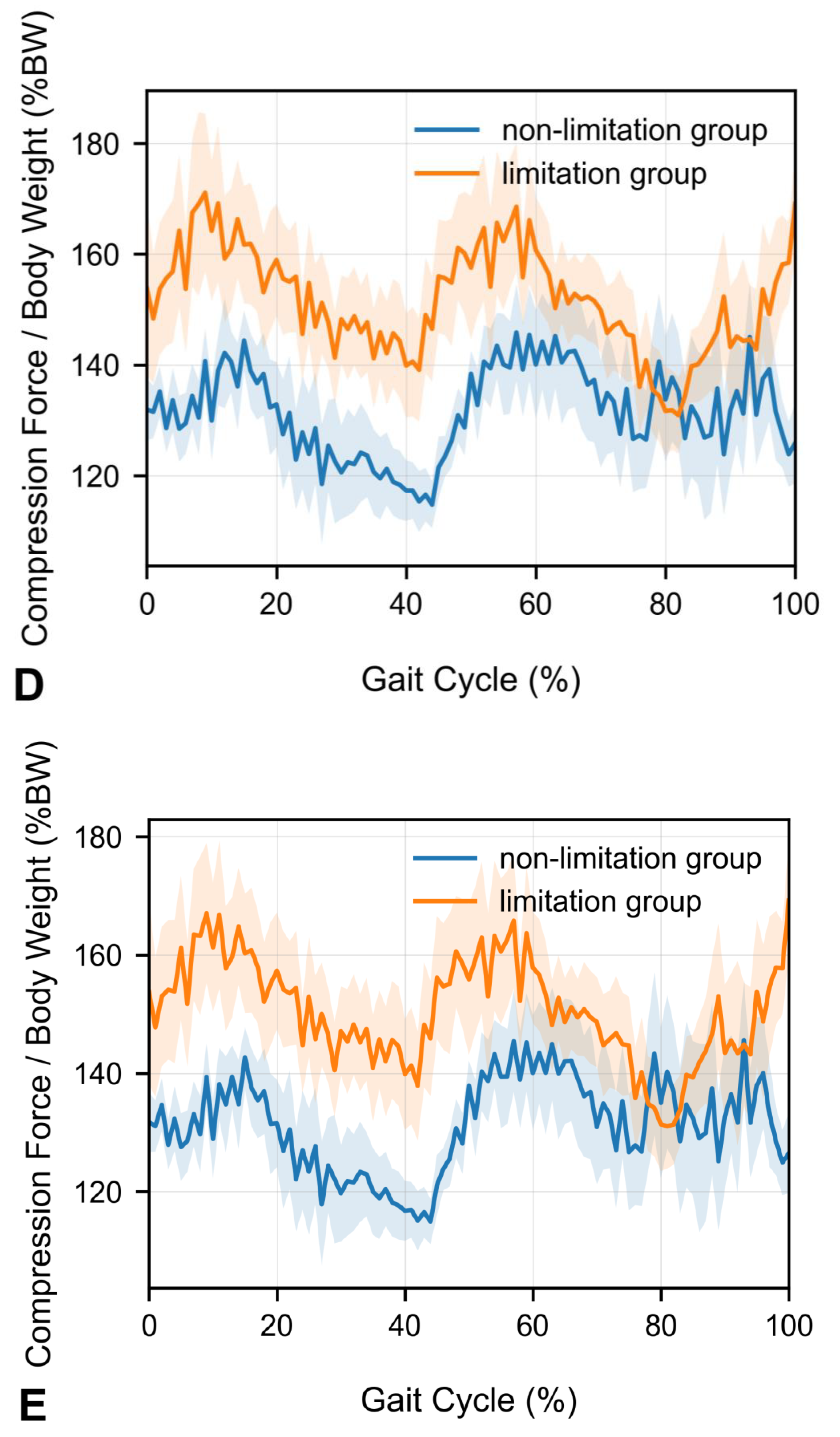
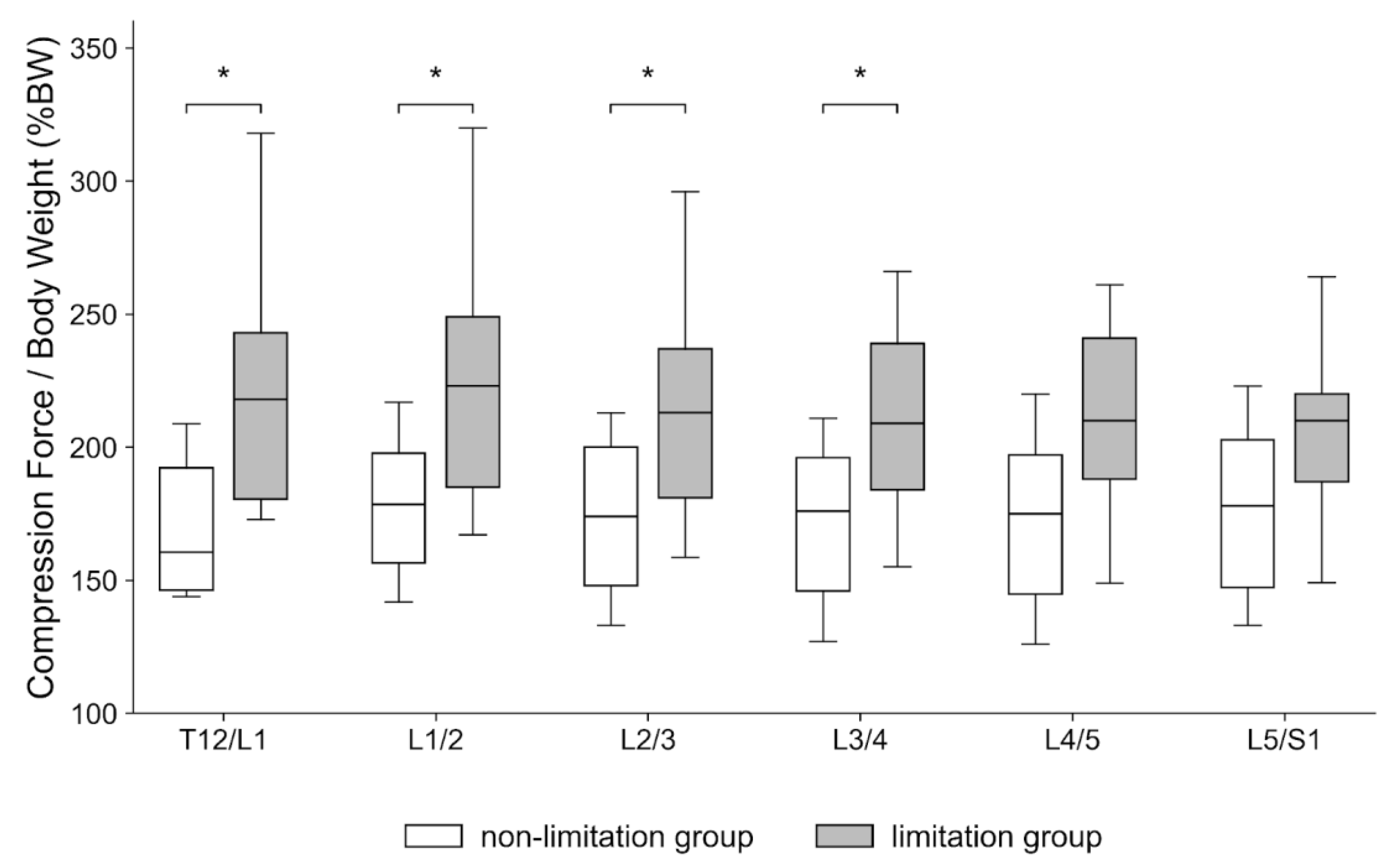
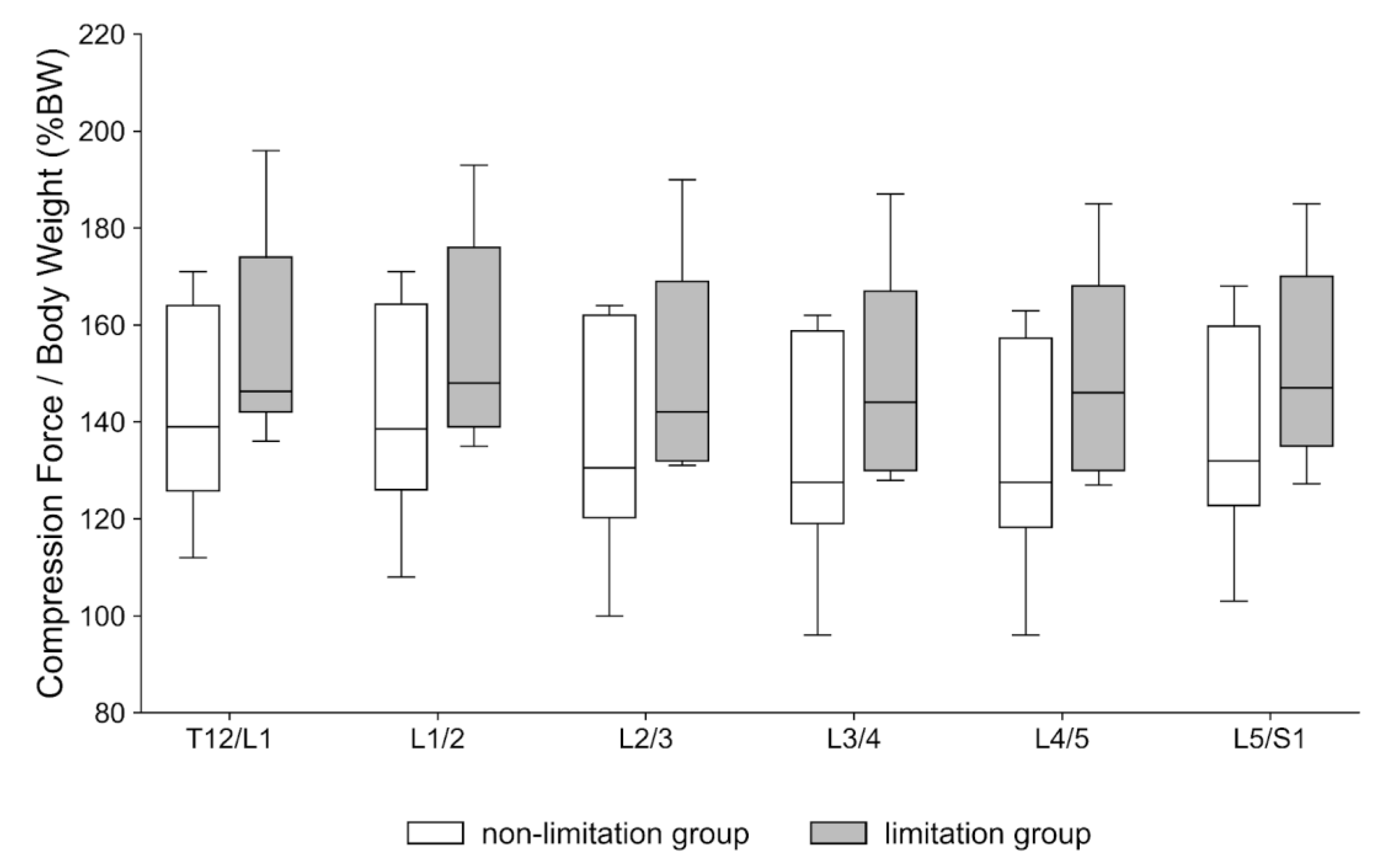
- Peak compression force at the upper lumbar intervertebral discs during walking is significantly increased in patients with knee extension limitations.
- Mean compression forces across the gait cycle remain unchanged in patients with knee extension limitations.
- The findings underscore the heightened risk of disc overload during everyday activities in patients.
- Clinically assessing and managing knee extension can potentially mitigate adverse upper lumbar loading and reduce the risk of degenerative progression.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Participants and Eligibility
2.3. Radiographic Measurements
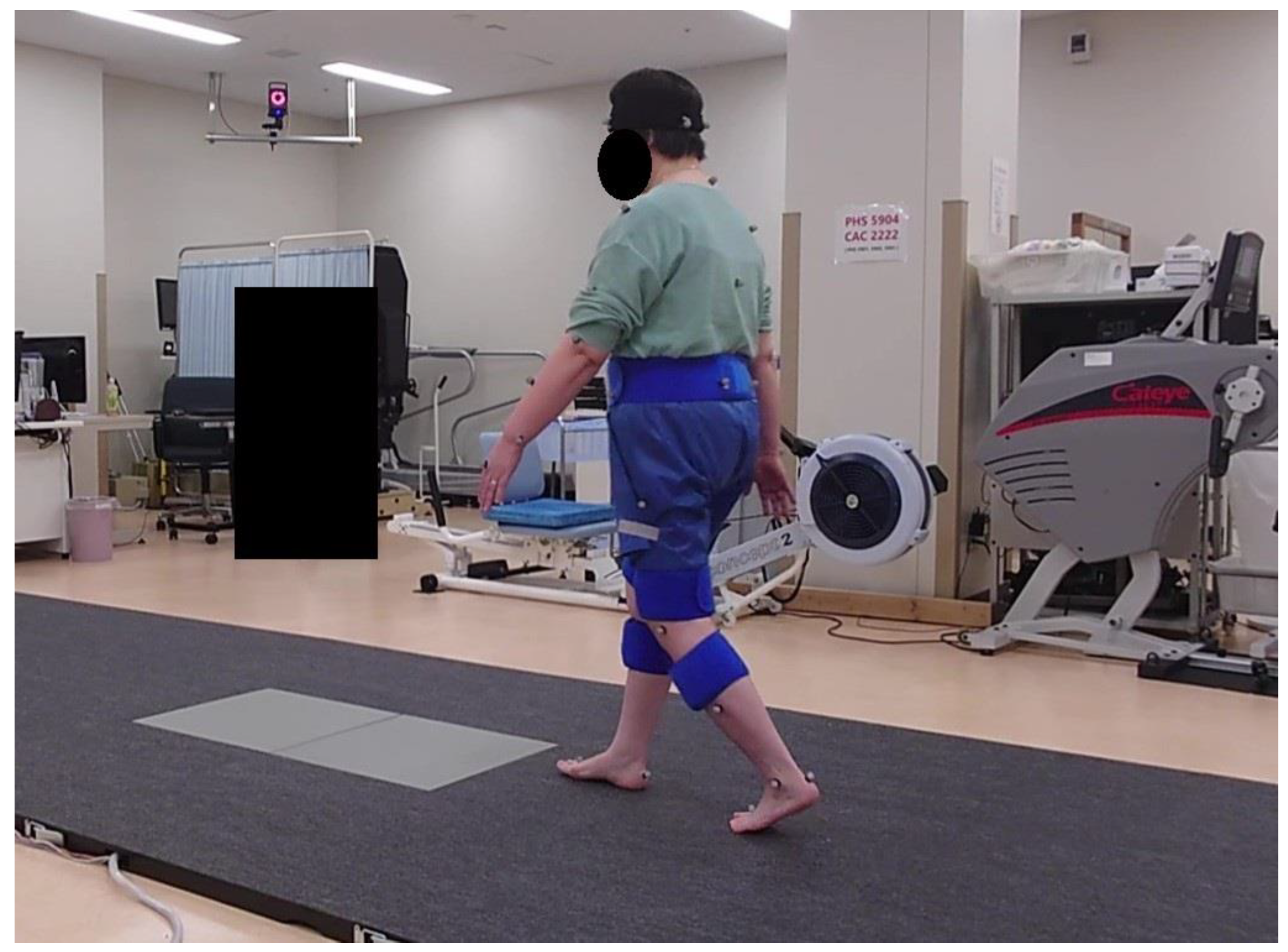
2.4. Gait Acquisition and Pre-Processing
2.5. Musculoskeletal Modeling Workflow
2.6. Outcome Definitions
2.7. Grouping Strategy and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMMR | AnyBody Managed Model Repository |
| ASD | adult spinal deformity |
| BW | body weight |
| DOF | degrees of freedom |
| KEA | knee extension angle |
| LL | lumbar lordosis |
| PI | pelvic incidence |
| PT | pelvic tilt |
| SS | sacral slope |
| SVA | sagittal vertical axis |
| PCF | peak compression force |
References
- Safaee, M.M.; Ames, C.P.; Smith, J.S. Epidemiology and Socioeconomic Trends in Adult Spinal Deformity Care. Neurosurgery 2020, 87, 25–32. [Google Scholar] [CrossRef]
- Takahashi, S.; Hoshino, M.; Ohyama, S.; Hori, Y.; Yabu, A.; Kobayashi, A.; Tsujio, T.; Kotake, S.; Nakamura, H. Relationship of back muscle and knee extensors with the compensatory mechanism of sagittal alignment in a community-dwelling elderly population. Sci. Rep. 2021, 11, 2179. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.M.; Prenovost, K.; Crandall, C. Narrative review: Hyperkyphosis in older persons. Ann. Intern. Med. 2007, 147, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Atsuta, Y.; Matsuno, T.; Takeda, N. A longitudinal study of congruent sagittal spinal alignment in an adult cohort. Spine 2004, 29, 671–676. [Google Scholar] [CrossRef]
- Imagama, S.; Hasegawa, Y.; Matsuyama, Y.; Sakai, Y.; Ito, Z.; Hamajima, N.; Ishiguro, N. Influence of sagittal balance and physical ability associated with exercise on quality of life in middle-aged and elderly people. Arch. Osteoporos. 2011, 6, 13–20. [Google Scholar] [CrossRef]
- Ito, T.; Sakai, Y.; Sugiura, H.; Kawai, K.; Morita, Y.; Yamazaki, K. Association between Trunk Muscle Strength and Fall Risk in Older Men and Women with Lumbar Spondylosis. Healthcare 2021, 9, 521. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Kato, S.; Lenke, L.G.; Nakashima, H.; Nagoshi, N.; Shaffrey, C.I.; Cheung, K.M.C.; Carreon, L.; Dekutoski, M.B.; Schwab, F.J.; et al. Incidence and Risk Factors of Postoperative Neurologic Decline after Complex Adult Spinal Deformity Surgery: Results of the Scoli-RISK-1 Study. Spine J. 2018, 18, 1733–1740. [Google Scholar] [CrossRef]
- Hasegawa, K.; Okamoto, M.; Hatsushikano, S.; Shimoda, H.; Ono, M.; Homma, T.; Watanabe, K. Standing sagittal alignment of the whole axial skeleton with reference to the gravity line in humans. J. Anat. 2017, 230, 619–630. [Google Scholar] [CrossRef]
- Diebo, B.G.; Shah, N.V.; Boachie-Adjei, O.; Zhu, F.; Rothenfluh, D.A.; Paulino, C.B.; Schwab, F.J.; Lafage, V. Adult spinal deformity. Lancet 2019, 394, 160–172. [Google Scholar] [CrossRef]
- Imagama, S.; Ito, Z.; Wakao, N.; Seki, T.; Hirano, K.; Muramoto, A.; Sakai, Y.; Matsuyama, Y.; Hamajima, N.; Ishiguro, N.; et al. Influence of spinal sagittal alignment, body balance, muscle strength, and physical ability on falling of middle-aged and elderly males. Eur. Spine J. 2013, 22, 1346–1353. [Google Scholar] [CrossRef]
- Miura, T.; Miyakoshi, N.; Saito, K.; Kijima, H.; Iida, J.; Hatakeyama, K.; Suzuki, K.; Komatsu, A.; Iwami, T.; Matsunaga, T.; et al. Association between global sagittal malalignment and increasing hip joint contact force, analyzed by a novel musculoskeletal modeling system. PLoS ONE 2021, 16, e0259049. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Takahashi, K.; Yamagata, M.; Hanaoka, E.; Moriya, H. The knee–spine syndrome. Association between lumbar lordosis and extension of the knee. J. Bone Jt. Surg. Br. 2003, 85, 95–99. [Google Scholar] [CrossRef]
- Harato, K.; Nagura, T.; Matsumoto, H.; Otani, T.; Toyama, Y.; Suda, Y. A gait analysis of simulated knee flexion contracture to elucidate knee–spine syndrome. Gait Posture 2008, 28, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, S.; Kawada, M.; Takeshita, Y.; Matsuzawa, Y.; Hata, K.; Araki, S.; Kiyama, R. Effect of Unilateral Knee Extension Restriction on the Lumbar Region during Gait. J. Healthc. Eng. 2022, 2022, 1151753. [Google Scholar] [CrossRef]
- Rohlmann, A.; Graichen, F.; Kayser, R.; Bender, A.; Bergmann, G. Loads on a telemeterized vertebral body replacement measured in two patients. Spine 2008, 33, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Damsgaard, M.; Rasmussen, J.; Christensen, S.T.; Surma, E.; de Zee, M. Analysis of musculoskeletal systems in the AnyBody Modeling System. Simul. Modell. Pract. Theor. 2006, 14, 1100–1111. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D.; DeWald, C.; Mehdian, H.; Shaffrey, C.; Tribus, C.; et al. Scoliosis research society–Schwab adult spinal deformity classification: A validation study. Spine 2012, 37, 1077–1082. [Google Scholar] [CrossRef]
- Yamada, K.; Aota, Y.; Higashi, T.; Ishida, K.; Nimura, T.; Saito, T. Accuracies in measuring spinopelvic parameters in full-spine lateral standing radiograph. Spine 2015, 40, E640–E646. [Google Scholar] [CrossRef]
- Roussouly, P.; Gollogly, S.; Berthonnaud, E.; Dimnet, J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine 2005, 30, 346–353. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- de Zee, M.; Hansen, L.; Wong, C.; Rasmussen, J.; Simonsen, E.B. A generic detailed rigid-body lumbar spine model. J. Biomech. 2007, 40, 1219–1227. [Google Scholar] [CrossRef]
- Lund, M.E.; Tørholm, S.; Dzialo, C.M.; Jensen, B.K. The AnyBody Managed Model Repository (AMMR). Zenodo 2019. [CrossRef]
- Shiba, Y.; Taneichi, H.; Inami, S.; Moridaira, H.; Takeuchi, D.; Nohara, Y. Dynamic global sagittal alignment evaluated by three-dimensional gait analysis in patients with degenerative lumbar kyphoscoliosis. Eur. Spine J. 2016, 25, 2572–2579. [Google Scholar] [CrossRef]
- Miura, T.; Hongo, M.; Kasukawa, Y.; Kijima, H.; Kudo, D.; Saito, K.; Kimura, R.; Iwami, T.; Miyakoshi, N. Relationship between intervertebral disc compression force and sagittal spinopelvic lower limb alignment in elderly women in standing position with a patient-specific whole body musculoskeletal model. Int. J. Environ. Res. Public Health 2022, 19, 16452. [Google Scholar] [CrossRef]
- Alkhatib, B.; Rosenzweig, D.H.; Krock, E.; Roughley, P.J.; Beckman, L.; Steffen, T.; Weber, M.H.; Ouellet, J.A.; Haglund, L. Acute mechanical injury of the human intervertebral disc: Link to degeneration and pain. Eur. Cell Mater. 2014, 28, 98–110. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.H.; Shin, S.H.; Seo, J.S.; Kim, K.H.; Jang, J.S. Radiological analysis of upper lumbar disc herniation and spinopelvic sagittal alignment. Eur. Spine J. 2016, 25, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, J.S.; Choi, H.G.; Kim, T.W.; Lee, Y.S. Spinal flexibility is an important factor for improvement in spinal and knee alignment after total knee arthroplasty: Evaluation using a whole body EOS system. J. Clin. Med. 2020, 9, 3498. [Google Scholar] [CrossRef]
- Han, H.S.; Yun, K.R.; Cho, K.; Kim, T.W.; Kang, S.B. Relationships between the changes in flexion contracture and standing flexion angle of the knee and sagittal spinal alignment after total knee arthroplasty. Knee 2021, 29, 374–380. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Hsu, L.-C.; Su, C.-C.; Li, C.-Y.; Chao, C.-T.; Chang, Y.-T.; Chang, C.-M.; Wang, W.-F.; Lien, W.-C. Gait abnormalities and longitudinal fall risk in older patients with end-stage kidney disease and sarcopenia. BMC Geriatr. 2024, 24, 937. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Kawamura, K.; Wada, T.; Ishihara, N.; Yokota, A.; Suginoshita, T.; Yokoyama, S. Gait cycle time variability in patients with knee osteoarthritis and its possible associating factors. J. Phys. Ther. Sci. 2022, 34, 140–145. [Google Scholar] [CrossRef] [PubMed]



| Variable | Median | Range |
|---|---|---|
| Age (years) | 72.0 | 64.0–76.0 |
| Height (cm) | 157.0 | 152.0–158.1 |
| Weight (kg) | 64.0 | 55.0–72.6 |
| BMI (kg/m2) | 25.5 | 21.9–29.9 |
| SVA (mm) | 32.3 | −14.0–50.0 |
| LL (°) | 44.0 | 39.0–49.0 |
| PT (°) | 26.0 | 16.0–27.0 |
| SS (°) | 33.0 | 28.0–36.0 |
| PI (°) | 55.0 | 50.0–63.0 |
| PI–LL (°) | 14.0 | 6.0–21.0 |
| Variable | Limitation (n = 9) | Non-Limitation (n = 8) | p Value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Age (years) | 74.0 | 64.0–80.0 | 70.5 | 63.3–75.0 | 0.766 |
| Height (cm) | 156.1 | 147.0–158.0 | 157.0 | 153.8–158.6 | 0.262 |
| Weight (kg) | 55.1 | 51.6–66.1 | 67.7 | 63.5–73.2 | 0.076 |
| BMI (kg/m2) | 22.5 | 20.5–30.2 | 28.4 | 25.1–29.6 | 0.191 |
| Right KEA (°) | −12.0 | −15 to −10 | −5.0 | −5.5 to −5.0 | <0.001 |
| Left KEA (°) | −12.0 | −15 to −10 | −5.0 | −5.5 to −5.0 | 0.015 |
| Variable | Limitation (n = 9) | Non-Limitation (n = 8) | p Value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| LL (°) | 41.0 | 34.0–45.0 | 45.5 | 41.0–52.8 | 0.180 |
| PT (°) | 20.0 | 14.0–27.0 | 27.0 | 25.5–27.5 | 0.083 |
| SS (°) | 28.0 | 23.0–34.0 | 34.5 | 32.8–36.8 | 0.265 |
| PI (°) | 53.0 | 45.0–55.0 | 62.5 | 57.5–65.3 | 0.006 |
| PI–LL (°) | 14.0 | 3.0–15.0 | 12.0 | 7.5–22.3 | 0.332 |
| SVA (mm) | 50.1 | 32.9–56.0 | 17.8 | 9.45–26.2 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotaki, Y.; Kudo, D.; Kimura, R.; Kasukawa, Y.; Kijima, H.; Saito, H.; Hongo, M.; Iwami, T.; Miyakoshi, N. The Effect of Knee Extension Limitation on Lumbar Intervertebral Disc Compression Force During Walking: A 3D Musculoskeletal Analysis. Sensors 2025, 25, 6605. https://doi.org/10.3390/s25216605
Kotaki Y, Kudo D, Kimura R, Kasukawa Y, Kijima H, Saito H, Hongo M, Iwami T, Miyakoshi N. The Effect of Knee Extension Limitation on Lumbar Intervertebral Disc Compression Force During Walking: A 3D Musculoskeletal Analysis. Sensors. 2025; 25(21):6605. https://doi.org/10.3390/s25216605
Chicago/Turabian StyleKotaki, Yuhei, Daisuke Kudo, Ryota Kimura, Yuji Kasukawa, Hiroaki Kijima, Hidetomo Saito, Michio Hongo, Takehiro Iwami, and Naohisa Miyakoshi. 2025. "The Effect of Knee Extension Limitation on Lumbar Intervertebral Disc Compression Force During Walking: A 3D Musculoskeletal Analysis" Sensors 25, no. 21: 6605. https://doi.org/10.3390/s25216605
APA StyleKotaki, Y., Kudo, D., Kimura, R., Kasukawa, Y., Kijima, H., Saito, H., Hongo, M., Iwami, T., & Miyakoshi, N. (2025). The Effect of Knee Extension Limitation on Lumbar Intervertebral Disc Compression Force During Walking: A 3D Musculoskeletal Analysis. Sensors, 25(21), 6605. https://doi.org/10.3390/s25216605






