A Review of Graphene-Integrated Biosensors for Non-Invasive Biochemical Monitoring in Health Applications
Abstract
1. Introduction
1.1. Comparative Mechanistic Attributes of Gr, GrO, and rGrO in Wearable Biosensing
1.2. Complementary 2D Nanomaterials: MXenes, Silicene, and Biochar for Advanced Biosensing
- Consolidates current knowledge and critically assesses the latest developments in the field of graphene-based, wearable biochemical sensors.
- Highlights the innovative potential of continuous, non-invasive biochemical monitoring for healthcare applications.
- Guides future research by addressing key technical and practical challenges.
2. Advancements in Biochemical Sensors
2.1. Biofluid-Based Sensing
2.1.1. Sweat Biomarker Detection
Glucose, Lactate, and Urea Detection
Cortisol and Cytokines Detection
Electrolytes Detection
L-Cysteine Detection
- Sensitivity: A signal change of ≥10% across the detection range to ensure the signal is clearly distinguishable from noise.
- Detection Range: Coverage of clinically relevant concentrations for the target analyte in sweat (e.g., 0.01–25 mM for metabolites—lactate/glucose, 10–100 mM for electrolytes).
- Limit of Detection (LOD): A threshold of <10 M, necessary for detecting baseline levels of key metabolites like glucose.
- Response Time: A rapid response of <60 s, which is important for tracking dynamic physiological changes during exertion.
- Durability: Demonstrated stability for >8 h of continuous use or >100 deformation/use cycles, reflecting the need for robustness during a typical activity session.
2.1.2. Saliva Biomarker Detection
Papillomavirus Detection
Influenza Virus Detection
Lysozyme Detection
Pseudomonas Aeruginosa Detection
Drug Detection
Cardiovascular Diseases Detection
Cortisol Detection
Serotonin Detection
L-Tryptophan Detection
Glucose Detection
Nitrite and Uric Acid(UA) Detection
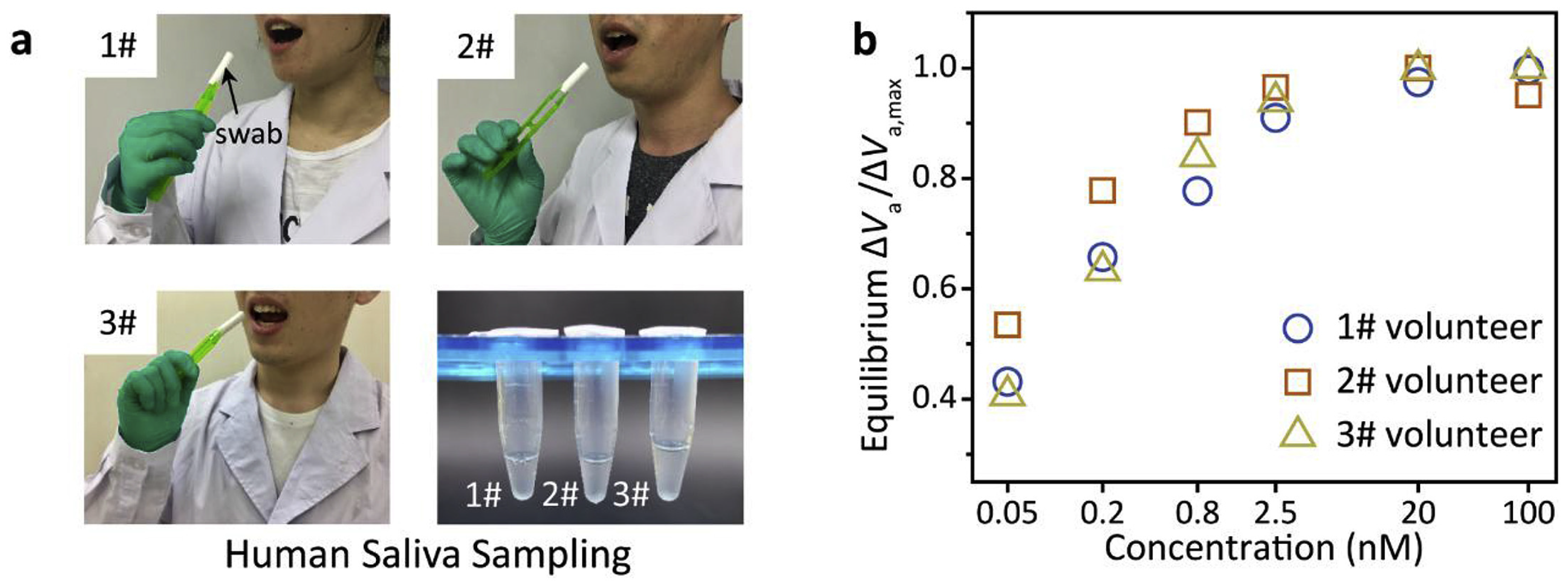



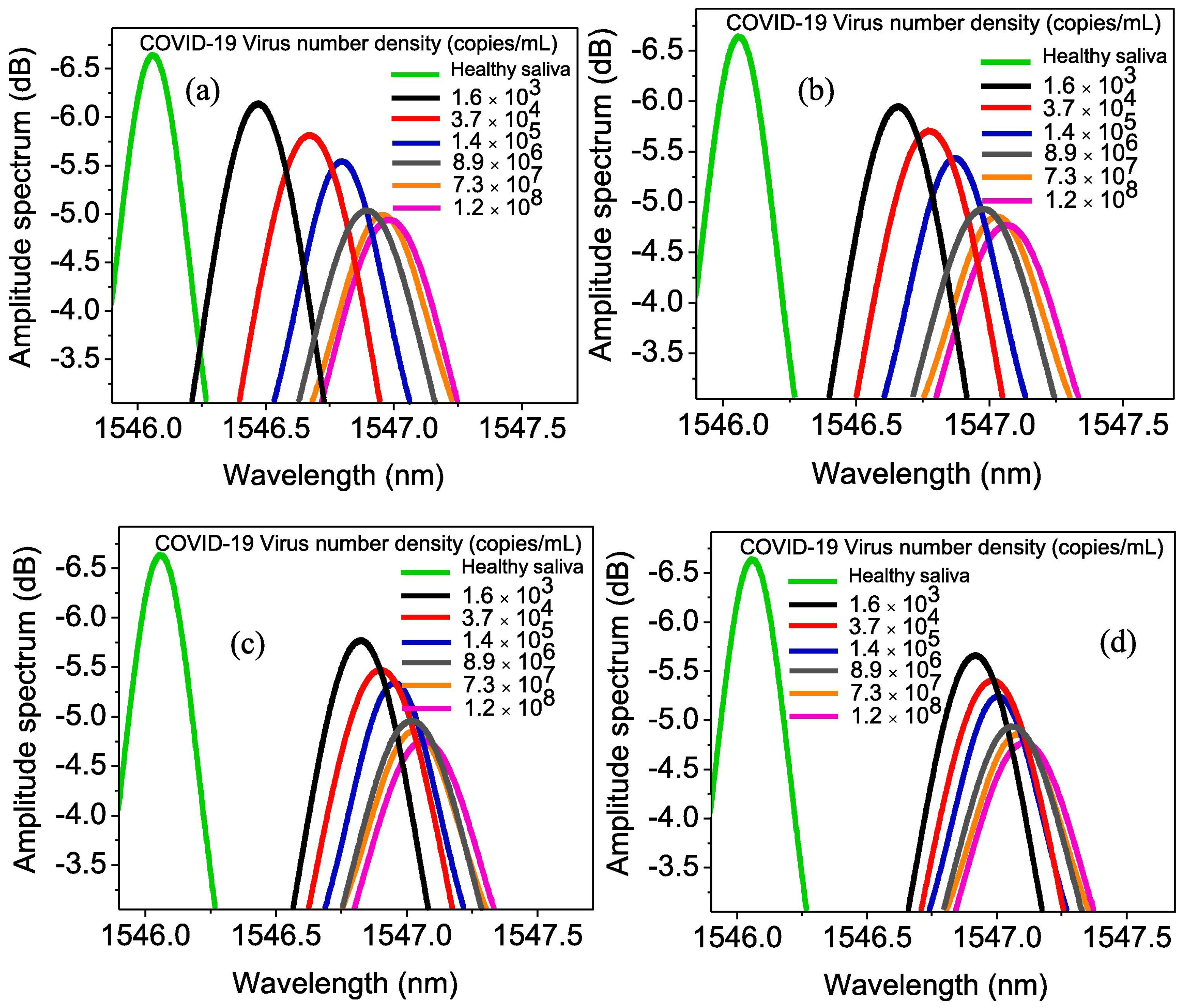
Carbonic Anhydrase 1 Detection
Tumour Detection
Cancer Detection
- Lung Cancer Detection
- Oral Cancer Detection
- Prostate Cancer Detection
- Sensitivity: A signal change of ≥10% across the pM–nM range for clear signal-to-noise in dilute samples.
- Detection Range: The ability to span the clinically relevant picomolar (pM) to nanomolar (nM) concentration range.
- Limit of Detection (LOD): A stringent threshold of <100 , essential for detecting trace analytes for early-stage disease diagnosis (<100 for proteins/viruses or <1 M for metabolites).
- Response Time: A rapid result time of <5 min (300 s) to enable point-of-care use.
- Durability: A minimum storage stability of >30 days, which is a critical parameter for the commercial viability of disposable test kits. This addresses shelf stability and requisite operational lifespan for disposable or semi-disposable systems.
- “U” values are conservatively deemed non-compliant to mitigate overestimation risks
2.1.3. Tear Biomarker Detection
Glucose Detection
Cytokines Detection
Ocular Detection
Myopia Detection
L-Cysteine Detection
- Sensitivity: ≥ signal change.
- Detection Range: Must cover relevant physiological ranges (e.g., 0.1–0.9 mM for glucose, pM cytokines).
- Limit of Detection (LOD): A threshold of <15 M for glucose (critical for hypoglycemia detection) and <1 nM for other biomarkers.
- Response Time: A very rapid response of <30 s is required for real-time physiological feedback.
- Durability: A minimum of >12 h of continuous operational stability to ensure reliability for a full day of wear.
2.2. Breath Sensing Devices
Volatile Organic Compounds (VOCs) Detection
- Sensitivity: ≥ signal change.
- Detection Range: Spanning the relevant parts-per-billion (ppb) to low parts-per-million (ppm) range.
- Limit of Detection (LOD): A highly sensitive threshold of <100 ppb is necessary to distinguish pathological VOC levels from healthy baselines.
- Response Time: A rapid response of <60 s to capture transient breath components.
- Durability: Demonstrated stability in the face of the primary interferent, high humidity, defined here as stable operation at ≥ Relative Humidity (RH).
2.3. Breath Comparative Insights
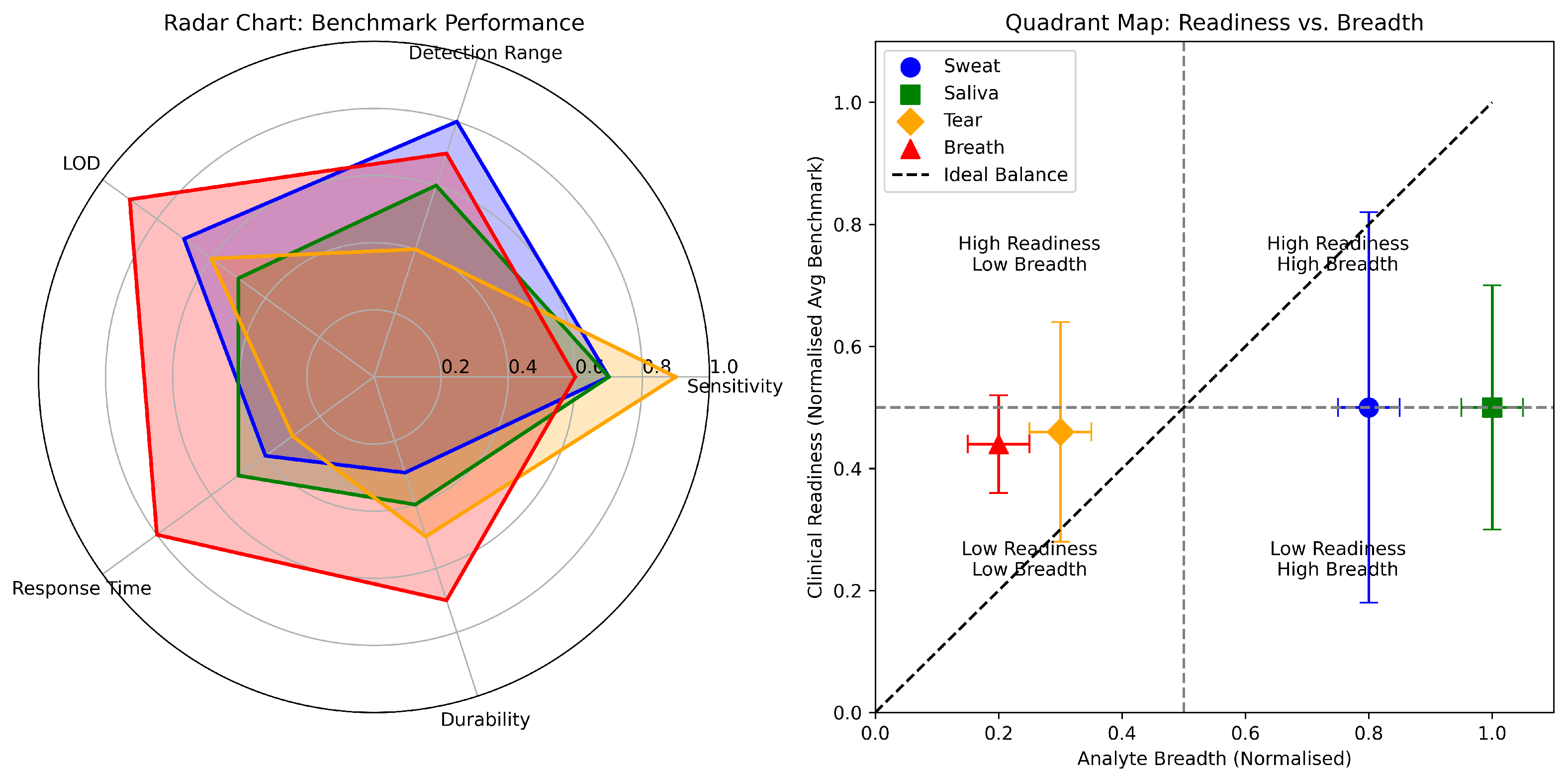
3. Unified Benchmarking of Biofluid Platforms
4. Challenges and Limitations in Graphene-Based Biosensor Development
4.1. Technical Limitations
4.2. Biological and Physiological Constraints
4.3. Sensitivity, Selectivity and Matrix Interference
4.4. Practical and Commercial Challenges
4.5. Ethical and Societal Considerations
5. Conclusions and Future Perspectives
- Translation Challenges: Most devices remain at the proof-of-concept stage, with limited evaluation under physiologically relevant conditions. Variations in biofluid composition, motion artefacts, and environmental interference impede reproducibility. Bridging this gap requires systematic in vivo validation, standardised biofluid sampling, and long-term stability assessment under real-world wear conditions.
- Clinical Benchmarks: For clinical adoption, graphene-based sensors must satisfy regulatory standards for accuracy, sensitivity, and specificity equivalent to gold-standard invasive assays. Achieving clinically relevant detection limits for glucose, lactate, cytokines, and tumour markers, while maintaining robustness across diverse populations, will require benchmark datasets and multi-site validation frameworks.
- Design Trade-offs: The same properties that make graphene appealing also introduce constraints. Pristine graphene offers high mobility but limited functionalisation, while graphene oxide improves bioreceptor immobilisation at the cost of conductivity. Increasing sensitivity often compromises mechanical integrity or biocompatibility. Future designs should explicitly balance these trade-offs through optimised hybrid materials and biofluid-specific architectures.
- Research Priorities: Key research directions include (i) scalable, reproducible synthesis of graphene derivatives; (ii) integration with wireless, low-power electronics for continuous data transmission; (iii) multiplexed platforms capable of detecting biochemical and biophysical cues; and (iv) longitudinal clinical studies to establish predictive value for disease monitoring.
- Standardisation: Community-wide protocols for fabrication, functionalisation, and validation under physiologically relevant conditions.
- Multimodal Integration: Combining signals from multiple biofluids (e.g., sweat lactate and salivary cortisol) to deliver comprehensive physiological insights.
- Intelligent Systems: Machine learning-driven calibration, drift correction, and multimodal data fusion to address variability and temporal lag.
- Durability and Power Management: Extending operational lifespans (>1 month for disposables, >1 year for wearables) and developing self-sustaining or energy-efficient systems for autonomous operation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNWs | Silver Nanowires |
| APTES | 3-Aminopropyl triethoxy silane |
| AuPt NPs | Gold and Platinum Alloy Nanoparticles |
| BSA | Bovine Serum Albumin |
| CA1 | Carbonic Anhydrase 1 |
| CAT | Catalase |
| CEA | Carcinoembryonic Antigen |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CO | Carbon Monoxide |
| CR | Chemiresistive |
| CS | Chitosan |
| CV | Cyclic Voltammetry |
| CVD | Chemical Vapour deposition |
| cTnI | Cardiac Troponin I |
| CYFRA-21-1 | Cytokeratin 19 Fragment |
| DA | Dopamine |
| DNA | Deoxyribonucleic Acid |
| DPV | Differential Pulse Voltammetry |
| EC | Electrochemical |
| ECD | Electrochemical Deposition |
| ECG | Electrocardiograms |
| EEG | Electroencephalograms |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMG | Electromyograms |
| EPD | Electrophoretic Deposition |
| EtOH | Ethanol |
| GCE | Glassy Carbon Electrode |
| GFET | Graphene Field-Effect Transistor |
| GNFET | Graphene-Nafion Field-Effect Transistor |
| GOx | Glucose Oxidase |
| Gr | Graphene |
| GrO | Graphene Oxide |
| GrP | Graphene on Paper |
| HIS-rGrO | L-Histidine-Modified Reduced Graphene Oxide |
| HWE | Hybrid Working Electrode |
| IDO | Indium Tin Oxide |
| IFN- | Interferon Gamma |
| IgG | Immunoglobulin G |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IoT | Internet of Things |
| ITO | Indium Tin Oxide |
| LBGr | Laser-Burned Graphene |
| LDH | Lactate Dehydrogenase |
| LDT | Low Detection Threshold |
| LIG | Laser-Induced Graphene |
| LOD | Limit of Detection |
| LOQ | Quantification Limit |
| LOx | Lactate Oxidase |
| MeOH | Methanol |
| MMP-9 | Matrix Metalloproteinase-9 |
| MOF | Metal–Organic Framework |
| MOFs | Metal–Organic Frameworks |
| MWCNT | Multiwalled Carbon Nanotube |
| Na+ | Sodium Ion |
| NH+ | Ammonium Ion |
| NH3 | Ammonia |
| NMO | Nanostructured Metal Oxide |
| nHfO2 | Nanostructured Hafnium Oxide |
| NO | Nitric Oxide |
| NTA | Nanoparticle Tracking Analysis |
| OA | Octylamine |
| OS | Oxidative Stress |
| OSI | Ocular Scatter Index |
| PAH | Polycyclic Aromatic Hydrocarbon |
| PANI | Polyaniline |
| PASE | 1-Pyrenebutanoic Acid Succinimidyl Ester |
| PBS | Phosphate Buffer Saline |
| PDMS | Polydimethylsiloxane |
| PEDOT-Gr | Poly(3,4-ethylene dioxythiophene)-Graphene |
| PI | Polyimide |
| PMMA | Polymethyl Methacrylate |
| POC | Point-of-Care |
| ppb | Parts Per Billion |
| ppm | Parts Per Million |
| PGr | Porous Graphene |
| PGr-Cu BTC | Pristine Graphene-Copper Benzene-1,3,5-tricarboxylate |
| PGr-SnO2 | Pristine Graphene-doped Tin Oxide |
| PGr-UiO 66 | Pristine Graphene-Zirconium 1,4-dicarboxybenzene |
| PGr-ZIF 8 | Pristine Graphene-2-methylimidazole Zinc Salt |
| PS67-b-PAA27 | Polystyrene-block-poly(acrylic acid) |
| PSA | Prostate-Specific Antigen |
| PTFE | Polytetrafluoroethylene |
| PVA | Polyvinyl Alcohol |
| rGrO | Reduced Graphene Oxide |
| RNA | Ribonucleic Acid |
| RLC | Resistor-Inductor-Capacitor |
| RSD | Relative Standard Deviation |
| SNR | Signal-to-Noise Ratio |
| SWCNTs | Single Walled Carbon Nanotubes |
| TEGrO | Thermally Exfoliated Reduced Graphene Oxide |
| THF | Tetrahydrofuran |
| TNF- | Tumor Necrosis Factor-alpha |
| UiO 66 | University of Oslo Framework 66 |
| VOC | Volatile Organic Compound |
| VPP | Vapour Phase Polymerisation |
| WO3/Au/WO3 | Tungsten Trioxide/Gold/Tungsten Trioxide |
| WSAS | Wireless Self-powered Acetone Sensor |
| ZIF 8 | Zeolitic Imidazolate Framework 8 |
| ZnO | Zinc Oxide |
References
- Chu, Z.; Zhang, W.; You, Q.; Yao, X.; Liu, T.; Liu, G.; Zhang, G.; Gu, X.; Ma, Z.; Jin, W. A Separation-Sensing Membrane Performing Precise Real-Time Serum Analysis During Blood Drawing. Angew. Chem. 2020, 132, 18860–18867. [Google Scholar] [CrossRef]
- Wang, Y.; Haick, H.; Guo, S.; Wang, C.; Lee, S.; Yokota, T.; Someya, T. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 2022, 51, 3759–3793. [Google Scholar] [CrossRef]
- Ahmad Tarar, A.; Mohammad, U.; K. Srivastava, S. Wearable skin sensors and their challenges: A review of transdermal, optical, and mechanical sensors. Biosensors 2020, 10, 56. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Chen, Y.; Ling, H.; Zhao, L.; Luo, G.; Wang, X.; Hartel, M.C.; Liu, H.; Xue, Y.; et al. Gelatin methacryloyl-based tactile sensors for medical wearables. Adv. Funct. Mater. 2020, 30, 2003601. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Cao, Z.; Pan, L.; Shi, Y. Advanced wearable microfluidic sensors for healthcare monitoring. Small 2020, 16, 1903822. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Jang, B.; Kim, S.; Sharma, B.K.; Kim, J.H.; Ahn, J.H. Graphene-based stretchable/wearable self-powered touch sensor. Nano Energy 2019, 62, 259–267. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of nanomaterials in wearables: A review on wearable actuators and sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef]
- Sreenilayam, S.P.; Ahad, I.U.; Nicolosi, V.; Garzon, V.A.; Brabazon, D. Advanced materials of printed wearables for physiological parameter monitoring. Mater. Today 2020, 32, 147–177. [Google Scholar] [CrossRef]
- Ho, D.H.; Hong, P.; Han, J.T.; Kim, S.Y.; Kwon, S.J.; Cho, J.H. 3D-printed sugar scaffold for high-precision and highly sensitive active and passive wearable sensors. Adv. Sci. 2020, 7, 1902521. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Tai, H.; Liu, B.; Duan, Z.; Yuan, Z.; Pan, H.; Xie, G.; Du, X.; Su, Y. An integrated flexible self-powered wearable respiration sensor. Nano Energy 2019, 63, 103829. [Google Scholar] [CrossRef]
- Mo, X.; Zhou, H.; Li, W.; Xu, Z.; Duan, J.; Huang, L.; Hu, B.; Zhou, J. Piezoelectrets for wearable energy harvesters and sensors. Nano Energy 2019, 65, 104033. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, H.; Wang, H.; Wang, F. Immune tolerance induced by immune-homeostatic particles. Eng. Regen. 2021, 2, 133–136. [Google Scholar] [CrossRef]
- Zhang, H.; He, R.; Niu, Y.; Han, F.; Li, J.; Zhang, X.; Xu, F. Graphene-enabled wearable sensors for healthcare monitoring. Biosens. Bioelectron. 2022, 197, 113777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, stretchable sensors for wearable health monitoring: Sensing mechanisms, materials, fabrication strategies and features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced carbon for flexible and wearable electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-based sensors for human health monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Kayser, L.V.; Lipomi, D.J. Stretchable conductive polymers and composites based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Koo, J.; Xie, Z.; Avila, R.; Yu, X.; Ning, X.; Zhang, H.; Liang, X.; Kim, S.B.; Yan, Y.; et al. A bioresorbable magnetically coupled system for low-frequency wireless power transfer. Adv. Funct. Mater. 2019, 29, 1905451. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Liao, M.; Sun, H.; Zhang, J.; Wu, J.; Xie, S.; Fu, X.; Sun, X.; Wang, B.; Peng, H. Multicolor, fluorescent supercapacitor fiber. Small 2018, 14, 1702052. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, B.; Lee, H.; Cha, G.D.; Lee, H.S.; Cho, Y.; Kim, S.Y.; Seo, H.; Lee, W.; Son, D.; et al. Ultra-wideband multi-Dye-sensitized upconverting nanoparticles for information security application. Adv. Mater. 2017, 29, 1603169. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-enabled wearable sensors for healthcare. Adv. Healthc. Mater. 2018, 7, 1700889. [Google Scholar] [CrossRef]
- Das, K.K.; Basu, B.; Maiti, P.; Dubey, A.K. Piezoelectric nanogenerators for self-powered wearable and implantable bioelectronic devices. Acta Biomater. 2023, 171, 85–113. [Google Scholar] [CrossRef]
- Huang, T.; Yang, S.; He, P.; Sun, J.; Zhang, S.; Li, D.; Meng, Y.; Zhou, J.; Tang, H.; Liang, J.; et al. Phase-separation-induced PVDF/graphene coating on fabrics toward flexible piezoelectric sensors. ACS Appl. Mater. Interfaces 2018, 10, 30732–30740. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, W. One-dimensional nanomaterials for soft electronics. Adv. Electron. Mater. 2017, 3, 1600314. [Google Scholar] [CrossRef]
- Qin, J.; Yin, L.J.; Hao, Y.N.; Zhong, S.L.; Zhang, D.L.; Bi, K.; Zhang, Y.X.; Zhao, Y.; Dang, Z.M. Flexible and stretchable capacitive sensors with different microstructures. Adv. Mater. 2021, 33, 2008267. [Google Scholar] [CrossRef]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable electrical conductivity of individual graphene oxide sheets reduced at “low” temperatures. Nano Lett. 2008, 8, 4283–4287. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, X.; Huang, M.; Zhen, Z.; Zhong, Y.; Chen, Q.; Zhao, X.; He, Y.; Hu, R.; Yang, T.; et al. The physics and chemistry of graphene-on-surfaces. Chem. Soc. Rev. 2017, 46, 4417–4449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, G. An introduction to the chemistry of graphene. Phys. Chem. Chem. Phys. 2015, 17, 28484–28504. [Google Scholar] [CrossRef]
- Umar, E.; Ikram, M.; Haider, J.; Nabgan, W.; Imran, M.; Nazir, G. 3D graphene-based material: Overview, perspective, advancement, energy storage, biomedical engineering and environmental applications a bibliometric analysis. J. Environ. Chem. Eng. 2023, 11, 110339. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, Y.; Feng, W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mater. Sci. Eng. R Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Ning, G.; Fan, Z.; Wang, G.; Gao, J.; Qian, W.; Wei, F. Gram-scale synthesis of nanomesh graphene with high surface area and its application in supercapacitor electrodes. Chem. Commun. 2011, 47, 5976–5978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, Z.; Yang, Z.; Ke, L.; Kitipornchai, S.; Yang, J. Functionally graded graphene reinforced composite structures: A review. Eng. Struct. 2020, 210, 110339. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Kuzmenko, A.B.; Van Heumen, E.; Carbone, F.; Van Der Marel, D. Universal optical conductance of graphite. Phys. Rev. Lett. 2008, 100, 117401. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Graphene-based biosensors. Interface Focus 2018, 8, 20160132. [Google Scholar] [CrossRef]
- Wan, S.; Zhu, Z.; Yin, K.; Su, S.; Bi, H.; Xu, T.; Zhang, H.; Shi, Z.; He, L.; Sun, L. A highly skin-conformal and biodegradable graphene-based strain sensor. Small Methods 2018, 2, 1700374. [Google Scholar] [CrossRef]
- Miao, P.; Wang, J.; Zhang, C.; Sun, M.; Cheng, S.; Liu, H. Graphene nanostructure-based tactile sensors for electronic skin applications. Nano-Micro Lett. 2019, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Lee, M.S.; Kim, K.; Ji, S.; Kim, Y.T.; Park, J.; Na, K.; Bae, K.H.; Kyun Kim, H.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, L.; Chen, Y.; Zhang, T.; Wang, W.; Liu, Z.; Fu, L. Human-like sensing and reflexes of graphene-based films. Adv. Sci. 2016, 3, 1600130. [Google Scholar] [CrossRef]
- Choi, C.; Lee, Y.; Cho, K.W.; Koo, J.H.; Kim, D.H. Wearable and implantable soft bioelectronics using two-dimensional materials. Accounts Chem. Res. 2018, 52, 73–81. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and its derivatives: Synthesis and application in the electrochemical detection of analytes in sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, H.; Li, S.; Liang, X.; Zhang, M.; Dai, X.; Zhang, Y. Flexible electrodes for in vivo and in vitro electrophysiological signal recording. Adv. Healthc. Mater. 2021, 10, 2100646. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, C.; Liu, F.; Du, S.; Li, G.; Wang, X. Eliminating heat injury of zeolite in hemostasis via thermal conductivity of graphene sponge. ACS Appl. Mater. Interfaces 2019, 11, 23848–23857. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Le, H.S.; Dang, T.M.L.; Ju, S.; Park, S.Y.; Lee, N.E. Freestanding, fiber-based, wearable temperature sensor with tunable thermal index for healthcare monitoring. Adv. Healthc. Mater. 2018, 7, 1800074. [Google Scholar] [CrossRef]
- Yun, Y.J.; Ju, J.; Lee, J.H.; Moon, S.H.; Park, S.J.; Kim, Y.H.; Hong, W.G.; Ha, D.H.; Jang, H.; Lee, G.H.; et al. Highly elastic graphene-based electronics toward electronic skin. Adv. Funct. Mater. 2017, 27, 1701513. [Google Scholar] [CrossRef]
- Xu, G.; Li, Y.; Zhang, H.; Chen, Y. Recent Advancements in MXene-Based Biosensors for Health and Environmental Applications. Biosensors 2024, 14, 497. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Liu, Z.; Zhao, Q. Recent Progress in MXene Hydrogel for Wearable Electronics. Biosensors 2023, 13, 495. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, L.; Chen, X. Two-Dimensional Silicene/Silicon and Its Derivatives: Properties and Utilisations in Sensors. Mater. Today 2023, 63, 34–48. [Google Scholar] [CrossRef]
- Akash, R.; Sakthi Balaji, A.; Janani Sivasankar, K.; Rajalakshmi Mohanraj, H.; Thiruvadigal, D.J. N-Doped Armchair Silicene Nanoribbon as a Promising Biosensor for Detection of VOCs in Healthcare Monitoring: A First Principles Study. Mech. Syst. Signal Process. 2025, 189, 109302. [Google Scholar] [CrossRef]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Contini, G.; Micheli, L.; Signori, E. Cost-Effective and Disposable Label-Free Voltammetric Immunosensor for Sensitive Detection of Interleukin-6. Biosens. Bioelectron. 2022, 213, 114467. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease detection with molecular biomarkers: From chemistry of body fluids to nature-inspired chemical sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Kęsy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef]
- Singh, S.; Chatterjee, S.; Lone, S.; Ho, H.H.; Kaswan, K.; Peringeth, K.; Khan, A.; Chiang, Y.W.; Lee, S.; Lin, Z.H. Advanced wearable biosensors for the detection of body fluids and exhaled breath by graphene. Microchim. Acta 2022, 189, 236. [Google Scholar] [CrossRef]
- Swann, G. The skin is the body’s largest organ. J. Vis. Commun. Med. 2010, 33, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Shiomi, M.; Fujita, Y.; Arie, T.; Akita, S.; Takei, K. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat. Electron. 2018, 1, 596–603. [Google Scholar] [CrossRef]
- Alizadeh, A.; Burns, A.; Lenigk, R.; Gettings, R.; Ashe, J.; Porter, A.; McCaul, M.; Barrett, R.; Diamond, D.; White, P.; et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip 2018, 18, 2632–2641. [Google Scholar] [CrossRef]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, eaaw0285. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Khan, A.; Winder, M.; Hossain, G. Modified graphene-based nanocomposite material for smart textile biosensor to detect lactate from human sweat. Biosens. Bioelectron. X 2022, 10, 100103. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Recent advances in noninvasive flexible and wearable wireless biosensors. Biosens. Bioelectron. 2019, 141, 111422. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P. Lactate in human sweat: A critical review of research to the present day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef]
- Onor, M.; Gufoni, S.; Lomonaco, T.; Ghimenti, S.; Salvo, P.; Sorrentino, F. Potentiometric sensor for non-invasive lactate determination in human sweat. Anal. Chim. Acta 2017, 989, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Yoo, J.H.; Woo, B.W.; Kim, S.S.; Cha, G.S.; Nam, H. Disposable amperometric glucose sensor electrode with enzyme-immobilized nitrocellulose strip. Talanta 2001, 54, 1105–1111. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Lai, Z.; Wang, R.; Guo, X.; Wu, X.; Zhang, G.; Zhang, Z.; Wang, Y.; Chen, Z. Planar amperometric glucose sensor based on glucose oxidase immobilized by chitosan film on Prussian blue layer. Sensors 2002, 2, 127–136. [Google Scholar] [CrossRef]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef]
- Garg, V.; Gupta, T.; Rani, S.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Qiao, L.; Liu, G. A hierarchically designed nanocomposite hydrogel with multisensory capabilities towards wearable devices for human-body motion and glucose concentration detection. Compos. Sci. Technol. 2021, 213, 108894. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Kim, J.Y.; Hui, X.; Das, P.S.; Yoon, H.S.; Park, J.Y. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018, 120, 160–167. [Google Scholar] [CrossRef]
- Zaryanov, N.V.; Nikitina, V.N.; Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Nonenzymatic sensor for lactate detection in human sweat. Anal. Chem. 2017, 89, 11198–11202. [Google Scholar] [CrossRef]
- Wang, Z.; Gui, M.; Asif, M.; Yu, Y.; Dong, S.; Wang, H.; Wang, W.; Wang, F.; Xiao, F.; Liu, H. A facile modular approach to the 2D oriented assembly MOF electrode for non-enzymatic sweat biosensors. Nanoscale 2018, 10, 6629–6638. [Google Scholar] [CrossRef] [PubMed]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Zúñiga, M.E.; Estremadoyro, L.O.; León, C.P.; Huapaya, J.A.; Cieza, J.A. Validation of the salivary urea test as a method to diagnose chronic kidney disease. J. Nephrol. 2012, 25, 431. [Google Scholar] [CrossRef]
- Rosenberg, E.; Kellner, R. Measuring glucose and urea by flow injection analysis with FTIR detection. J. Mol. Struct. 1993, 294, 9–12. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Prim. 2015, 1, 15018. [Google Scholar] [CrossRef]
- Promphet, N.; Hinestroza, J.P.; Rattanawaleedirojn, P.; Soatthiyanon, N.; Siralertmukul, K.; Potiyaraj, P.; Rodthongkum, N. Cotton thread-based wearable sensor for non-invasive simultaneous diagnosis of diabetes and kidney failure. Sens. Actuators B Chem. 2020, 321, 128549. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Aljfary, M.A.; Aldossary, H.; Alshammari, T.; AL-Qaaneh, A.; Aldahhan, R.; Alkhalifah, Z. Mechanism of hormones secretion and action. In Emerging Concepts in Endocrine Structure and Functions; Springer: Cham, Switzerland, 2022; pp. 47–71. [Google Scholar]
- Premalatha, S.J. Hormones and their Endocrine Nature; Shineeks Publishers: Las Vegas, NV, USA, 2022. [Google Scholar]
- Crafa, A.; Calogero, A.E.; Cannarella, R.; Mongioi’, L.M.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. The burden of hormonal disorders: A worldwide overview with a particular look in Italy. Front. Endocrinol. 2021, 12, 694325. [Google Scholar] [CrossRef]
- Zamkah, A.; Hui, T.; Andrews, S.; Dey, N.; Shi, F.; Sherratt, R.S. Identification of suitable biomarkers for stress and emotion detection for future personal affective wearable sensors. Biosensors 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Gupta, N.; Malhotra, B.D. Recent developments in wearable & non-wearable point-of-care biosensors for cortisol detection. Expert Rev. Mol. Diagn. 2023, 23, 217–230. [Google Scholar] [PubMed]
- Diez-Martin, E.; Hernandez-Suarez, L.; Muñoz-Villafranca, C.; Martin-Souto, L.; Astigarraga, E.; Ramirez-Garcia, A.; Barreda-Gómez, G. Inflammatory bowel disease: A comprehensive analysis of molecular bases, predictive biomarkers, diagnostic methods, and therapeutic options. Int. J. Mol. Sci. 2024, 25, 7062. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Electrochemical ELISA protein biosensing in undiluted serum using a polypyrrole-based platform. Sensors 2020, 20, 2857. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- San Nah, J.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J. Cytokines: Past, present, and future. Int. J. Hematol. 2001, 74, 3–8. [Google Scholar] [CrossRef]
- Morán, G.A.G.; Parra-Medina, R.; Cardona, A.G.; Quintero-Ronderos, P.; Rodríguez, É.G. Cytokines, chemokines and growth factors. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Wang, Z.; Hao, Z.; Wang, X.; Huang, C.; Lin, Q.; Zhao, X.; Pan, Y. A flexible and regenerative aptameric graphene–nafion biosensor for cytokine storm biomarker monitoring in undiluted biofluids toward wearable applications. Adv. Funct. Mater. 2021, 31, 2005958. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Lee, R.J.; Foskett, J.K. Ca2+ signaling and fluid secretion by secretory cells of the airway epithelium. Cell Calcium 2014, 55, 325–336. [Google Scholar] [CrossRef]
- Reddy, M.; Quinton, P. Cytosolic potassium controls CFTR deactivation in human sweat duct. Am. J.-Physiol.-Cell Physiol. 2006, 291, C122–C129. [Google Scholar] [CrossRef]
- Brothers, M.C.; DeBrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Accounts Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Nadeem, M.F.; Butt, A.M.; Ashraf, W.; Matti, N.; Farooq, M.A.; Nasim, M.b.; Siddique, M.I.; Khan, T.M. The impact of fluid and electrolyte imbalance on the severities of diseases and their management in developing countries. In Handbook of Medical and Health Sciences in Developing Countries: Education, Practice, and Research; Springer: Cham, Switzerland, 2024; pp. 1–20. [Google Scholar]
- Merrill, A.E.; Chambliss, A.B. Water and electrolyte balance. In Contemporary Practice in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 651–663. [Google Scholar]
- Park, H.J.; Jeong, J.M.; Son, S.G.; Kim, S.J.; Lee, M.; Kim, H.J.; Jeong, J.; Hwang, S.Y.; Park, J.; Eom, Y.; et al. Fluid-dynamics-processed highly stretchable, conductive, and printable graphene inks for real-time monitoring sweat during stretching exercise. Adv. Funct. Mater. 2021, 31, 2011059. [Google Scholar] [CrossRef]
- Hua, Y.; Kwok, S.C.; Wang, X.; Lee, Y.K.; Yuen, M.M. Flexible Sweat monitoring based on all-solid-state metal-organic frameworks/graphene composite sensors. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Yeung, K.K.; Li, J.; Huang, T.; Hosseini, I.I.; Al Mahdi, R.; Alam, M.M.; Sun, H.; Mahshid, S.; Yang, J.; Ye, T.T.; et al. Utilizing gradient porous graphene substrate as the solid-contact layer to enhance wearable electrochemical sweat sensor sensitivity. Nano Lett. 2022, 22, 6647–6654. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Wang, Z.; Wang, H.; Zhao, X.; Pan, Y. An ultraflexible and transparent graphene-based wearable sensor for biofluid biomarkers detection. Adv. Mater. Technol. 2022, 7, 2101131. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Batchelor-McAuley, C.; Cowen, P.J.; Williams, C.; Gonçalves, L.M.; Compton, R.G. Can saliva testing replace blood measurements for health monitoring? Insights from a correlation study of salivary and whole blood glutathione in humans. Analyst 2016, 141, 4707–4712. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef]
- Rathnayake, N.; Gieselmann, D.R.; Heikkinen, A.M.; Tervahartiala, T.; Sorsa, T. Salivary Diagnostics—Point-of-Care diagnostics of MMP-8 in dentistry and medicine. Diagnostics 2017, 7, 7. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.H. Recent advances in graphene-based nanobiosensors for salivary biomarker detection. Biosens. Bioelectron. 2021, 171, 112723. [Google Scholar] [CrossRef]
- Samavati, A.; Samavati, Z.; Velashjerdi, M.; Ismail, A.F.; Othman, M.; Abdullah, M.S.; Bolurian, M.; Bolurian, M. Sustainable and fast saliva-based COVID-19 virus diagnosis kit using a novel GO-decorated Au/FBG sensor. Chem. Eng. J. 2021, 420, 127655. [Google Scholar] [CrossRef]
- Ban, D.K.; Bodily, T.; Karkisaval, A.G.; Dong, Y.; Natani, S.; Ramanathan, A.; Ramil, A.; Srivastava, S.; Bandaru, P.; Glinsky, G.; et al. Rapid self-test of unprocessed viruses of SARS-CoV-2 and its variants in saliva by portable wireless graphene biosensor. Proc. Natl. Acad. Sci. USA 2022, 119, e2206521119. [Google Scholar] [CrossRef]
- Hao, Z.; Luo, Y.; Huang, C.; Wang, Z.; Song, G.; Pan, Y.; Zhao, X.; Liu, S. An intelligent graphene-based biosensing device for cytokine storm syndrome biomarkers detection in human biofluids. Small 2021, 17, 2101508. [Google Scholar] [CrossRef]
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 26, pp. 13–21. [Google Scholar]
- Abreu, A.L.; Souza, R.P.; Gimenes, F.; Consolaro, M.E. A review of methods for detect human Papillomavirus infection. Virol. J. 2012, 9, 262. [Google Scholar] [CrossRef]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide–based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2021, 413, 779–787. [Google Scholar] [CrossRef]
- Chekin, F.; Bagga, K.; Subramanian, P.; Jijie, R.; Singh, S.K.; Kurungot, S.; Boukherroub, R.; Szunerits, S. Nucleic aptamer modified porous reduced graphene oxide/MoS2 based electrodes for viral detection: Application to human papillomavirus (HPV). Sens. Actuators B Chem. 2018, 262, 991–1000. [Google Scholar] [CrossRef]
- Joshi, S.R.; Sharma, A.; Kim, G.H.; Jang, J. Low cost synthesis of reduced graphene oxide using biopolymer for influenza virus sensor. Mater. Sci. Eng. C 2020, 108, 110465. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Wu, M.; Ma, X.; Yang, X. Graphene-based lysozyme binding aptamer nanocomposite for label-free and sensitive lysozyme sensing. J. Electroanal. Chem. 2013, 702, 49–55. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Gu, P.; Su, S.; Huang, Y.; Feng, X.; Fan, Q.; Huang, W. Highly sensitive fluorometric turn-on detection of lysozyme based on a graphene oxide/ssDNA assembly. IEEE Sens. J. 2017, 17, 5431–5436. [Google Scholar] [CrossRef]
- Kang, D.; Kirienko, D.R.; Webster, P.; Fisher, A.L.; Kirienko, N.V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence 2018, 9, 804–817. [Google Scholar] [CrossRef]
- Peek, M.E.; Bhatnagar, A.; McCarty, N.A.; Zughaier, S.M. Pyoverdine, the major siderophore in Pseudomonas aeruginosa, evades NGAL recognition. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 843509. [Google Scholar] [CrossRef]
- Cernat, A.; Tertis, M.; Gandouzi, I.; Bakhrouf, A.; Suciu, M.; Cristea, C. Electrochemical sensor for the rapid detection of Pseudomonas aeruginosa siderophore based on a nanocomposite platform. Electrochem. Commun. 2018, 88, 5–9. [Google Scholar] [CrossRef]
- Gandouzi, I.; Tertis, M.; Cernat, A.; Bakhrouf, A.; Coros, M.; Pruneanu, S.; Cristea, C. Sensitive detection of pyoverdine with an electrochemical sensor based on electrochemically generated graphene functionalized with gold nanoparticles. Bioelectrochemistry 2018, 120, 94–103. [Google Scholar] [CrossRef]
- Hutchinson, L.; Sinclair, M.; Reid, B.; Burnett, K.; Callan, B. A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br. J. Clin. Pharmacol. 2018, 84, 1089–1108. [Google Scholar] [CrossRef]
- Parate, K.; Karunakaran, C.; Claussen, J.C. Electrochemical cotinine sensing with a molecularly imprinted polymer on a graphene-platinum nanoparticle modified carbon electrode towards cigarette smoke exposure monitoring. Sens. Actuators B Chem. 2019, 287, 165–172. [Google Scholar] [CrossRef]
- Rajendran, J.; Sundramoorthy, A.K.; Ganapathy, D.; Atchudan, R.; Habila, M.A.; Nallaswamy, D. 2D MXene/graphene nanocomposite preparation and its electrochemical performance towards the identification of nicotine level in human saliva. J. Hazard. Mater. 2022, 440, 129705. [Google Scholar] [CrossRef]
- Carrillo, L.R.; Garcia, K.A.; Yalcin, N.; Shah, M. Ketamine and its emergence in the field of neurology. Cureus 2022, 14, e27389. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, R.; He, J.; Bai, H.; Zhang, G. Sensitive detection of ketamine with an electrochemical sensor based on UV-induced polymerized molecularly imprinted membranes at graphene and MOFs modified electrode. Biosens. Bioelectron. 2019, 143, 111636. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Atty, S.A.; Merey, H.A.; Fattah, T.A.; Foster, C.W.; Banks, C.E. Titanium nanoparticles (TiO2)/graphene oxide nanosheets (GO): An electrochemical sensing platform for the sensitive and simultaneous determination of benzocaine in the presence of antipyrine. Analyst 2017, 142, 3674–3679. [Google Scholar] [CrossRef]
- Mishra, V.; Patil, R.; Khanna, V.; Tripathi, A.; Singh, V.; Pandey, S.; Chaurasia, A. Evaluation of Salivary Cardiac Troponin-I as Potential Marker for Detection of Acute Myocardial Infarction. J. Clin. Diagn. Res. 2018, 12, ZC44–ZC47. [Google Scholar] [CrossRef]
- Chekin, F.; Vasilescu, A.; Jijie, R.; Singh, S.K.; Kurungot, S.; Iancu, M.; Badea, G.; Boukherroub, R.; Szunerits, S. Sensitive electrochemical detection of cardiac troponin I in serum and saliva by nitrogen-doped porous reduced graphene oxide electrode. Sens. Actuators B Chem. 2018, 262, 180–187. [Google Scholar] [CrossRef]
- Yoo, S.S.; Kim, S.Y.; Kim, K.S.; Hong, S.; Oh, M.J.; Nam, M.G.; Kim, W.J.; Park, J.; Chung, C.H.; Choe, W.S.; et al. Controlling inter-sheet-distance in reduced graphene oxide electrodes for highly sensitive electrochemical impedimetric sensing of myoglobin. Sens. Actuators B Chem. 2020, 305, 127477. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, Y. A disposable printed liquid gate graphene field effect transistor for a salivary cortisol test. ACS Sens. 2021, 6, 3024–3031. [Google Scholar] [CrossRef]
- Zubarev, A.; Cuzminschi, M.; Iordache, A.M.; Iordache, S.M.; Rizea, C.; Grigorescu, C.E.; Giuglea, C. Graphene-based sensor for the detection of cortisol for stress level monitoring and diagnostics. Diagnostics 2022, 12, 2593. [Google Scholar] [CrossRef]
- Khan, M.S.; Misra, S.K.; Wang, Z.; Daza, E.; Schwartz-Duval, A.S.; Kus, J.M.; Pan, D.; Pan, D. Paper-based analytical biosensor chip designed from graphene-nanoplatelet-amphiphilic-diblock-co-polymer composite for cortisol detection in human saliva. Anal. Chem. 2017, 89, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz-Duval, A.S.; Misra, S.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2019, 144, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, K.; Jung, H.; Kang, H.K.; Jo, J.; Park, I.K.; Lee, H.H. Direct immune-detection of cortisol by chemiresistor graphene oxide sensor. Biosens. Bioelectron. 2017, 98, 473–477. [Google Scholar] [CrossRef]
- Kim, K.S.; Lim, S.R.; Kim, S.E.; Lee, J.Y.; Chung, C.H.; Choe, W.S.; Yoo, P.J. Highly sensitive and selective electrochemical cortisol sensor using bifunctional protein interlayer-modified graphene electrodes. Sens. Actuators B Chem. 2017, 242, 1121–1128. [Google Scholar] [CrossRef]
- Adumitrăchioaie, A.; Tertiș, M.; Suciu, M.; Graur, F.; Cristea, C. A novel immunosensing platform for serotonin detection in complex real samples based on graphene oxide and chitosan. Electrochim. Acta 2019, 311, 50–61. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A. 3D-Printed graphene/polylactic acid electrode for bioanalysis: Biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Nugba, B.E.; El-Moneim, A.; Mousa, N.O.; Osman, A. A nonenzymatic laser-induced flexible amperometric graphene electrode for glucose detection in saliva. Carbon Lett. 2023, 33, 1767–1780. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, H.; Li, X.; Sun, D.; Hu, T.; Xiang, N.; Ni, Z. Paper-based graphene oxide biosensor coupled with smartphone for the quantification of glucose in oral fluid. Biomed. Microdevices 2018, 20, 89. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, X.; Heinig, N.F.; Thomas, J.P.; Zhang, L.; Leung, K.T. Nonenzymatic saliva-range glucose sensing using electrodeposited cuprous oxide nanocubes on a graphene strip. ACS Appl. Nano Mater. 2021, 4, 4790–4799. [Google Scholar] [CrossRef]
- Shang, L.J.; Yu, S.Q.; Shang, X.W.; Wei, X.Y.; Wang, H.Y.; Jiang, W.S.; Ren, Q.Q. A non-invasive glucose sensor based on 3D reduced graphene oxide-MXene and AuNPs composite electrode for the detection of saliva glucose. J. Appl. Electrochem. 2024, 54, 1807–1817. [Google Scholar] [CrossRef]
- Huang, X.; Shi, W.; Li, J.; Bao, N.; Yu, C.; Gu, H. Determination of salivary uric acid by using poly (3, 4-ethylenedioxythipohene) and graphene oxide in a disposable paper-based analytical device. Anal. Chim. Acta 2020, 1103, 75–83. [Google Scholar] [CrossRef]
- Hao, Z.; Pan, Y.; Shao, W.; Lin, Q.; Zhao, X. Graphene-based fully integrated portable nanosensing system for on-line detection of cytokine biomarkers in saliva. Biosens. Bioelectron. 2019, 134, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gray, M.; Ortiz-Marquez, J.C.; Weber, A.; Desmond, C.R.; Argun, A.; van Opijnen, T.; Burch, K.S. Detection of a multi-disease biomarker in saliva with graphene field effect transistors. Med. Devices Sens. 2020, 3, e10121. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Augustine, S.; Yadav, S.; Yadav, B.K.; Chauhan, R.P.; Dewan, A.K.; Malhotra, B.D. Effect of Brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosens. Bioelectron. 2018, 102, 247–255. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.P. Non-invasive oral cancer detection from saliva using zinc oxide–reduced graphene oxide nanocomposite based bioelectrode. Mrs Commun. 2019, 9, 1227–1234. [Google Scholar] [CrossRef]
- Khan, M.; Dighe, K.; Wang, Z.; Srivastava, I.; Daza, E.; Schwartz-Dual, A.; Ghannam, J.; Misra, S.; Pan, D. Detection of prostate specific antigen (PSA) in human saliva using an ultra-sensitive nanocomposite of graphene nanoplatelets with diblock-co-polymers and Au electrodes. Analyst 2018, 143, 1094–1103. [Google Scholar]
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S. An immunosensing device based on inhibition of mediator’s faradaic process for early diagnosis of prostate cancer using bifunctional nanoplatform reinforced by carbon nanotube. J. Pharm. Biomed. Anal. 2019, 172, 259–267. [Google Scholar] [PubMed]
- Pankratov, D.; González-Arribas, E.; Blum, Z.; Shleev, S. Tear based bioelectronics. Electroanalysis 2016, 28, 1250–1266. [Google Scholar] [CrossRef]
- Ponzini, E.; Santambrogio, C.; De Palma, A.; Mauri, P.; Tavazzi, S.; Grandori, R. Mass spectrometry-based tear proteomics for noninvasive biomarker discovery. Mass Spectrom. Rev. 2022, 41, 842–860. [Google Scholar] [CrossRef]
- Jacob, J.T.; Ham, B. Compositional profiling and biomarker identification of the tear film. Ocul. Surf. 2008, 6, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Bachhuber, F.; Huss, A.; Senel, M.; Tumani, H. Diagnostic biomarkers in tear fluid: From sampling to preanalytical processing. Sci. Rep. 2021, 11, 10064. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Micera, A.; De Piano, M.; Cortes, M.; Bonini, S. Tears and ocular surface disorders: Usefulness of biomarkers. J. Cell. Physiol. 2019, 234, 9982–9993. [Google Scholar] [CrossRef]
- Jalbert, I. Diet, nutraceuticals and the tear film. Exp. Eye Res. 2013, 117, 138–146. [Google Scholar] [CrossRef]
- Aihara, M.; Kubota, N.; Minami, T.; Shirakawa, R.; Sakurai, Y.; Hayashi, T.; Iwamoto, M.; Takamoto, I.; Kubota, T.; Suzuki, R.; et al. Association between tear and blood glucose concentrations: Random intercept model adjusted with confounders in tear samples negative for occult blood. J. Diabetes Investig. 2021, 12, 266–276. [Google Scholar] [CrossRef]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Di Ilio, C.; Sacchetta, P.; Del Boccio, P. Unraveling the molecular repertoire of tears as a source of biomarkers: Beyond ocular diseases. Proteom.–Clin. Appl. 2015, 9, 169–186. [Google Scholar] [CrossRef]
- Alotaibi, S.; Markoulli, M.; Ozkan, J.; Papas, E. Bio-chemical markers of chronic, non-infectious disease in the human tear film. Clin. Exp. Optom. 2022, 105, 166–176. [Google Scholar] [CrossRef]
- Komkova, M.A.; Eliseev, A.A.; Poyarkov, A.A.; Daboss, E.V.; Evdokimov, P.V.; Eliseev, A.A.; Karyakin, A.A. Simultaneous monitoring of sweat lactate content and sweat secretion rate by wearable remote biosensors. Biosens. Bioelectron. 2022, 202, 113970. [Google Scholar] [CrossRef]
- Rentka, A.; Koroskenyi, K.; Harsfalvi, J.; Szekanecz, Z.; Szucs, G.; Szodoray, P.; Kemeny-Beke, A. Evaluation of commonly used tear sampling methods and their relevance in subsequent biochemical analysis. Ann. Clin. Biochem. 2017, 54, 521–529. [Google Scholar] [CrossRef]
- Vijay, A.K.; Fadli, Z.; Lakkis, C.; Coles-Brennan, C.; Willcox, M.D. In vitro compatibility of contact lenses with corneal epithelial cells. Eye Contact Lens 2018, 44, S283–S290. [Google Scholar] [CrossRef] [PubMed]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Kim, S.Y.; Cheong, W.H.; Jang, J.; Park, Y.G.; Na, K.; Kim, Y.T.; Heo, J.H.; Lee, C.Y.; et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, Z.; Yu, S.; Huang, C.; Pan, Y.; Zhao, X. A wearable and deformable graphene-based affinity nanosensor for monitoring of cytokines in biofluids. Nanomaterials 2020, 10, 1503. [Google Scholar] [CrossRef]
- Jang, J.; Kim, J.; Shin, H.; Park, Y.G.; Joo, B.J.; Seo, H.; Won, J.e.; Kim, D.W.; Lee, C.Y.; Kim, H.K.; et al. Smart contact lens and transparent heat patch for remote monitoring and therapy of chronic ocular surface inflammation using mobiles. Sci. Adv. 2021, 7, eabf7194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, G.; Feng, H.; Shan, S.; Huang, L.; Yuan, F.; Bao, B.; Yan, L.; Xia, Z.; Lawson, T.; et al. Wearable corneal biosensors fabricated from PEDOT functionalized sulfur-doped graphene for use in the early detection of myopia. Adv. Mater. Technol. 2020, 5, 2000682. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef]
- Siegel, A.P.; Daneshkhah, A.; Mather, K.J.; Agarwal, M. 968-P: Volatile organic compounds in breath predict hypoglycemia well before plasma glucose levels fall. Diabetes 2019, 68, 968-P. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Xu, H.; Xiang, J.X.; Lu, Y.F.; Zhang, M.K.; Li, J.J.; Gao, B.B.; Zhao, Y.J.; Gu, Z.Z. Multifunctional wearable sensing devices based on functionalized graphene films for simultaneous monitoring of physiological signals and volatile organic compound biomarkers. ACS Appl. Mater. Interfaces 2018, 10, 11785–11793. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Kam, K.W.; Cheung, W.F.; Zhao, N.; Zheng, B. Functionalized graphene-based chemiresistive electronic nose for discrimination of disease-related volatile organic compounds. Biosens. Bioelectron. X 2019, 1, 100016. [Google Scholar] [CrossRef]
- Su, Y.; Yang, T.; Zhao, X.; Cai, Z.; Chen, G.; Yao, M.; Chen, K.; Bick, M.; Wang, J.; Li, S.; et al. A wireless energy transmission enabled wearable active acetone biosensor for non-invasive prediabetes diagnosis. Nano Energy 2020, 74, 104941. [Google Scholar] [CrossRef]
- Sánchez-Vicente, C.; Santos, J.P.; Lozano, J.; Sayago, I.; Sanjurjo, J.L.; Azabal, A.; Ruiz-Valdepeñas, S. Graphene-doped tin oxide nanofibers and nanoribbons as gas sensors to detect biomarkers of different diseases through the breath. Sensors 2020, 20, 7223. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Tran, M.T.; Feller, J.F.; Castro, M.; Van Ngo, T.; Hassan, K.; Nine, M.J.; Losic, D. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon 2020, 159, 333–344. [Google Scholar] [CrossRef]
- Abdelfattah, M.A.; Jamali, S.S.; Kashaninejad, N.; Nguyen, N.T. Wearable biosensors for health monitoring: Advances in graphene-based technologies. Nanoscale Horizons 2025, 10, 1542–1574. [Google Scholar] [CrossRef]
- Heo, J.S.; Hossain, M.F.; Kim, I. Challenges in design and fabrication of flexible/stretchable carbon-and textile-based wearable sensors for health monitoring: A critical review. Sensors 2020, 20, 3927. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Liu, Y. Current development in wearable glucose meters. Chin. Chem. Lett. 2021, 32, 3705–3717. [Google Scholar] [CrossRef]
- Xiao, Y.; Hou, L.; Wang, M.; Liu, R.; Han, L.; Nikolai, M.; Zhang, S.; Cheng, C.; Hu, K. Noninvasive glucose monitoring using portable GOx-Based biosensing system. Anal. Chim. Acta 2024, 1287, 342068. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable electrochemical glucose sensors in diabetes management: A comprehensive review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef] [PubMed]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef]
- Gupta, R.; Mehta, S.; Patel, G. Electronic Nose Based on Graphene Oxide. In Nanostructured Materials for Electronic Nose; Springer: Singapore, 2024; pp. 227–271. [Google Scholar]
- Syama, S.; Mohanan, P. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar] [CrossRef]
- Debnath, S.; Cheng, Q.; Hedderman, T.G.; Byrne, H.J. A Raman spectroscopy study of the solubilisation of SWCNTs by polycyclic aromatic hydrocarbons. Carbon 2010, 48, 1489–1497. [Google Scholar] [CrossRef]
- Ershova, O.V.; Lillestolen, T.C.; Bichoutskaia, E. Study of polycyclic aromatic hydrocarbons adsorbed on graphene using density functional theory with empirical dispersion correction. Phys. Chem. Chem. Phys. 2010, 12, 6483–6491. [Google Scholar] [CrossRef]
- Debnath, S.; Cheng, Q.; Hedderman, T.G.; Byrne, H.J. An experimental study of the interaction between single walled carbon nanotubes and polycyclic aromatic hydrocarbons. Phys. Status Solidi B 2008, 245, 1961–1963. [Google Scholar] [CrossRef]
- Song, J.; Luo, Y.; Hao, Z.; Qu, M.; Huang, C.; Wang, Z.; Yang, J.; Liang, Q.; Jia, Y.; Song, Q.; et al. Graphene-based wearable biosensors for point-of-care diagnostics: From surface functionalization to biomarker detection. Mater. Today Bio 2025, 32, 101667. [Google Scholar] [CrossRef]
- Hatta, F.F.; Mohammad Haniff, M.A.S.; Mohamed, M.A. A review on applications of graphene in triboelectric nanogenerators. Int. J. Energy Res. 2022, 46, 544–576. [Google Scholar] [CrossRef]
- Gao, J.; He, S.; Nag, A. Electrochemical detection of glucose molecules using laser-induced graphene sensors: A review. Sensors 2021, 21, 2818. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, S.; Wang, X. Applications of graphene-based materials in sensors: A review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef]
- Haq, M.A.U.; Rehman, S.U.; Alhulayyil, H.A.; Alzahrani, T.A.; Alsagri, H.S.; Faheem, M. Wireless antenna sensors for biosimilar monitoring toward cyber-physical systems: A review of current trends and future prospects. IEEE Access 2023, 11, 132037–132054. [Google Scholar] [CrossRef]
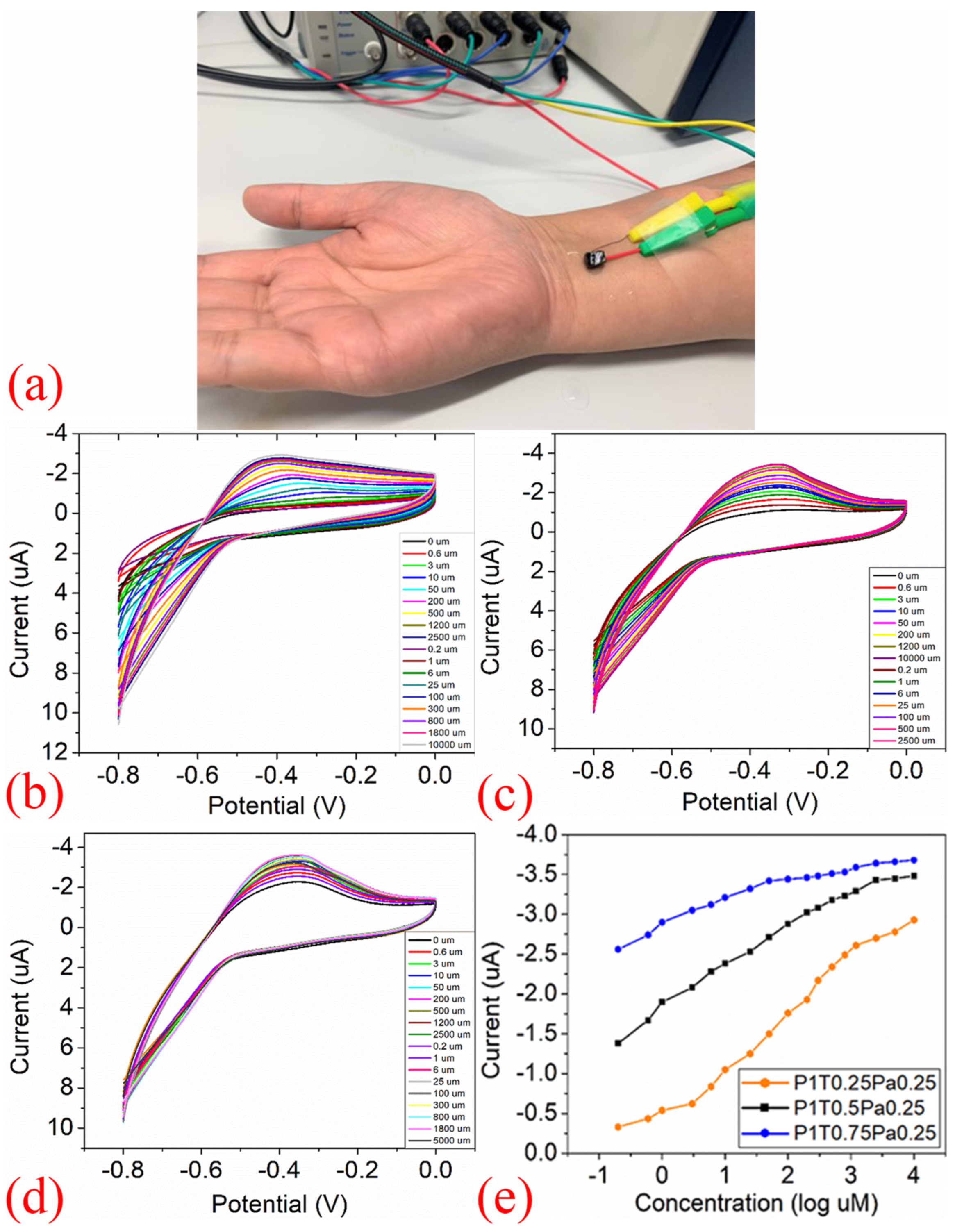
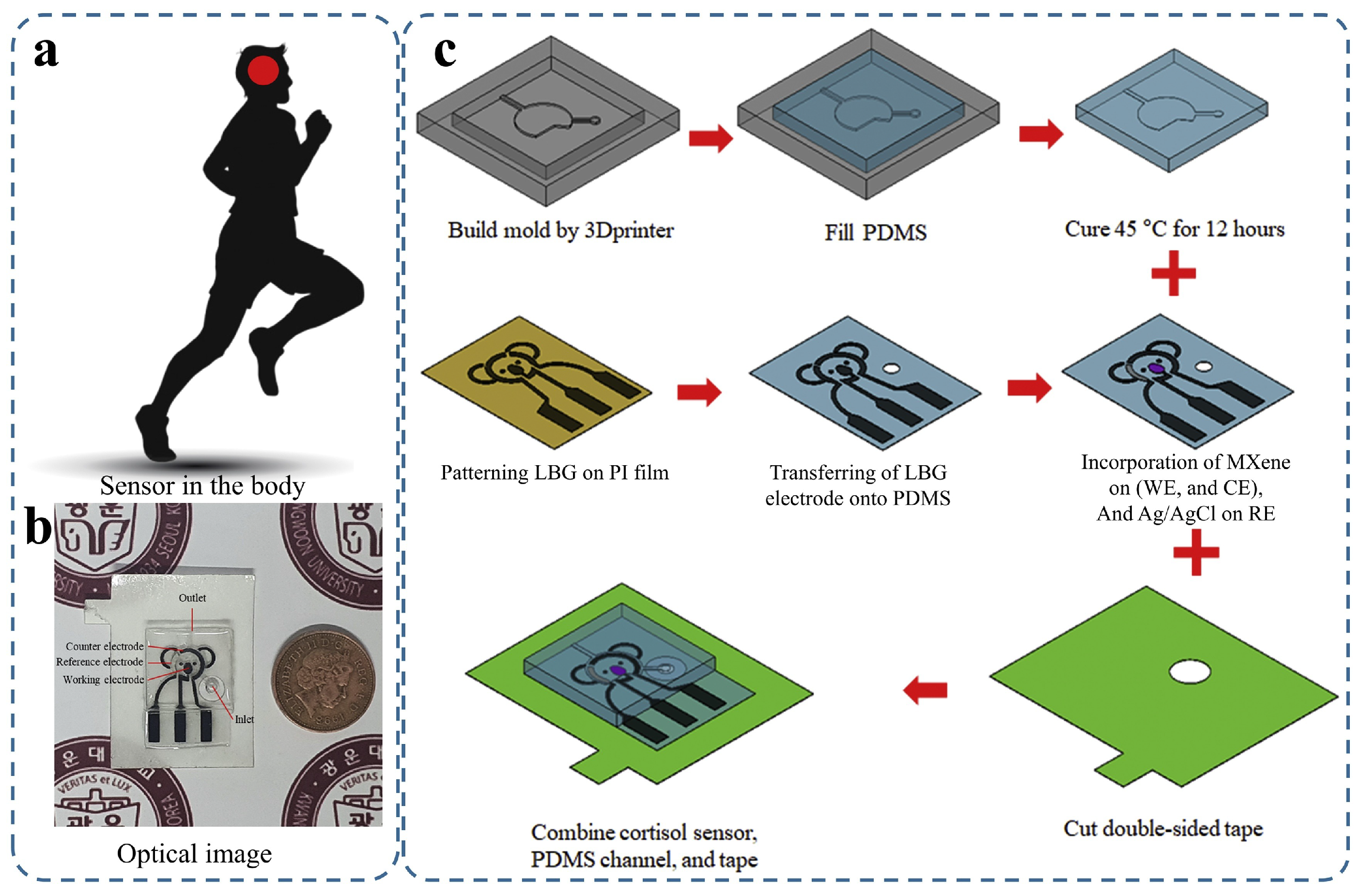







| Property | Gr | GrO | rGrO |
|---|---|---|---|
| Conductivity | Extremely high mobility; ideal for chemiresistive/FET detection (VOCs). | Very low; unsuitable for high-speed sensing without modification. | Intermediate; good for electrochemical biosensors. |
| Functionalisation potential | Limited covalent reactivity; relies on – stacking. | Very high; abundant surface functional groups enable dense enzyme/aptamer attachment. | Moderate; residual groups allow functionalisation and conductivity balance. |
| Mechanical properties | Exceptional (Young’s modulus ∼1 TPa, tensile strength ∼130 GPa). | Reduced strength; brittle in isolation. | Improved over GrO but not as strong as Gr. |
| Biocompatibility | Generally favourable; low cytotoxicity. | Hydrophilic and dispersible; may induce oxidative stress at high dose. | Balanced biocompatibility; less oxidative potential. |
| Application niches | Breath sensing; electrophysiology. | Tear/saliva sensing (protein/aptamer detection). | Sweat/saliva (electrochemical biosensing); flexible sensors. |
| Property/Application | Graphene | MXenes | Silicene | Biochar |
|---|---|---|---|---|
| Structure | Planar honeycomb lattice of carbon atoms | Layered transition metal carbides/nitrides with surface terminations (–O, –OH, –F) | Buckled honeycomb lattice of silicon atoms | Amorphous/graphitic porous carbon from biomass pyrolysis |
| Electronic Properties | Zero bandgap, high conductivity, Dirac fermions | Metallic conductivity, tunable surface chemistry | Small tunable bandgap (∼1.5 meV), high carrier mobility | Moderate conductivity; variable with precursor and processing |
| Surface Chemistry | High surface area, receptor immobilisation | Abundant functional groups, hydrophilic, excellent charge transfer | Reactive surface, analyte binding, functionalisation under development | Rich in O/N groups; enzyme immobilisation possible, but heterogeneous |
| Mechanical Properties | Flexible, strong, lightweight | Flexible in hydrogel form, stability issues | Predicted flexibility, less stable ambient | Robust and porous, limited flexibility in thin films |
| Biocompatibility | Generally good, widely studied | Good with polymers/hydrogels | Under investigation; stability/toxicity concerns | Variable; promising for processed forms, requires further validation |
| Applications in Biosensing | Glucose, lactate, cortisol, DNA/protein sensors | Electrochemical, wearable, enzymatic sensors | Early-stage; sensitive doped nanoribbons | Low-cost biosensors for glucose, dopamine, uric acid, antioxidants |
| Challenges | No intrinsic bandgap; synthesis challenges | Stability in aqueous/biological media; reproducibility | Ambient oxidation; immature synthesis | Lower conductivity, heterogeneity, batch variability |
| Sensing Material | Analyte Sample | Sensing Mechanism | Sensitivity (A mM−1 cm−2) | Detection Range (mM) | LOD (mM) | Response Time (s) | Durability | Meets Benchmarks |
|---|---|---|---|---|---|---|---|---|
| Gr-PU-rGrO-PB/ [67] | Lactate | EC/patch | U | 0.01–10.0 | 0.4 | U | 5 cycles | 1/5 |
| AuPt NPs/rGrO/chitosan- [73] | Glc | AMP | 48 | 0–2.4 | 0.005 | 20 | 192 h | 5/5 |
| PANI/TEGO/ PVA [74] | Glc | EC/patch | U | 0.0002–10 | 0.0002 | U | 125 cycles | 3/5 |
| PBl/Au-doped Gr hybrid/ [75] | Glc | CV/patch | U | 0.01–0.7 | 0.01 | 900 | 6 h | 1/5 |
| AgNC/3D LIGr/PtAuNP [76] | Glc | EC/patch | 6.4 | 0–1.1 | 0.005 | tens of seconds | 408 h | 5/5 |
(BTC)2 [78] | Glc, Lac | EC/patch | Glc: 5360; Lac: 29 | 0.05–1.78; 0.05–22.6 | 0.00003; 0.005 | 5; 3 | 1200 h (for both) | 5/5 |
| SPE/PB/GrO-Ch/GOx [79] | Glc, Lac | FIA | Glc: 8.2; Lac: 0.39 | 0.02–3.8; 1–50 | 6.7; 28 | U U | 35 m;
25 m | 2/5 |
| CNFs/CS-GrO [83] | Glc Urea | COL COL | U U | 0.1–3; 30–180 | 0.1; 30 | U | U | 1/5 |
| MXene /LBGr/PDMS [92] | CORT | IIS | U | U | U | 1/5 | ||
| Graphene/ PI [93] | CORT | EC/patch | U | 0.43–50.2 | 0.08 | 60 | 168 h | 2/5 |
| GNFET [96] | Cytokine | FE | U | 360 | 80 regenerative and 100 crumpling cycles | 1/5 | ||
| EGrFs/TPU, NMP [103] | POT | 58.3 mV/dec | 0.1–100 | 0.0025 | 9.6 | 10,000 cycles | 5/5 | |
| MOF/Gr [104] | POT | 59.23 mV/log | 0.001–100 | 0.001 | U | 168 h | 3/5 | |
| 3D CVD Gr [105] | POT | 65.1 mV/dec | 0.01–100 | U | U | 125 h | 1/5 | |
| Paper-based ISE (rGrO/ FAS) [106] | pH | POT | 57.0 mV/dec 56.7 mV/dec 56 mV/dec 55.7 mV/dec | 6.5 61.4 6.91 49.5 | U | 12 h | 1/5 | |
| GFET [107] | L-Cys | FET | U | 0–4.8 | 0.00022 | U | 100 cycles | 3/5 |
| Sensing Material | Analyte Sample | Sensing Mechanism | Sensitivity (A mM−1 cm−2) | Detection Range | LOD (Lowest Tested) | Response Time (min) | Durability (Day) | Meets Benchmarks |
|---|---|---|---|---|---|---|---|---|
| GrO-Au/FBG [115] | COVID-19 virus | SPR | 0.4250 × 10−8 nm/virus number | 1.6 × 103–1.2 × 108 copiesmL−1 | 1.6 × 103 copiesmL−1 | 0.17 | U | 3/5 |
| DNA-apt-GFET [116] | SARS-CoV-2 virus | EC | U | 0–30 nM (S protein); 0–15 nM (N protein) | 1.28 PFUmL−1; 1.45 PFUmL−1 | 20 | U | 1/5 |
| DGTFET [117] | IFN-; IL-6; TNF- | EC | U | 1 nM–10 pM; 10–200 pM; 10–200 pM | 476 fM; 611 fM; 608 fM | 7 | U | 2/5 |
| rGrO-FET [120] | HPV-16 E7 | EC | U | 30–1000 nM | 1.75 nM | U | 30 | 2/5 |
| rGrO/MoS2 /GCE [121] | HPV-16 L1 | DPV | U | 0.2–2 ngmL−1 (3.5 pM–35.3 pM) | 0.1 ngmL−1 (1.75 pM) | 40 | 30 | 2/5 |
| TrGrO [122] | H1N1 | EC | U | 0–10,000 PFUmL−1 | 33.11 PFUmL−1 | U | 14 | 2/5 |
| LBA-Gr-GCE [123] | LYS | EC | U | 0.01–0.5 pML−1 | 6 fML−1 | 23 | 200 cycles | 2/5 |
| GrO/ssDNA [124] | LYS | FL | U | – | 20 | U | 1/5 | |
| AuNPs/Ppy-COOH/Gr-SPE [127] | PVD | EC/DPV | U | 1–100 | 0.33 | U | 8 tests | 2/5 |
| PtNP@Gr /SPCE [130] | COT | EC/CV | decade−1 | 1–100 | 0.33 | 12 | U | 3/5 |
| MX/Gr [131] | NIC | EC/DPV; EC/AMP | 3.5; 0.527 | 1–55 M; 30–600 nM | 290 nM; 0.28 nM | U | 40 | 4/5 |
| KT-MIM/MOFs @Gr/SPE [133] | KET | DPV | U | 1 × 10−10–4 × 10−5 ML−1 | 4.0 × 10−11 ML−1 | 5 | 60 uses or 60 days | 4/5 |
| TiO2-GrO/CPE [134] | BEN; ANT | EC/SWV | U | 1M–1.0 mM; 12 nM–80 M | 0.25 M; 3 nM | U | 30 | 2/5 |
| N-prGrO-(py-PEG/ PyCOOH)/GCE [136] | cTnI | EC/DPV | 41 A cm−2decade−1 | 0.001–100 ngmL−1 | 1 pgmL−1 | 30 | 10 cycles; 30 days | 4/5 |
| anti-Mb-Ab/d-BSA/rGrO [137] | Mb | EC/EIS | U | 5 pM–10 nM | 2.37 pM | 30 | U | 2/5 |
| Lg-GFET [138] | CORT | EC | U | 0.08–800 nM | U | 30 | U | 1/5 |
| Gr/PPy [139] | CORT | EC/patch | U | 0.5–5 ngmL−1 | 0.5 ngmL−1 | U | U | 1/5 |
| GrP/PS67-b-PAA27 [140] | CORT | EC | 50 (pg mL−1)−1 | 3 pgmL−1–10 gmL−1 | 3 pgmL−1 | 12 | 28 | 3/5 |
| Sensing Material | Analyte Sample | Sensing Mechanism | Sensitivity (A mM−1 cm−2) | Detection Range | LOD | Response Time (min) | Durability (Day) | Benchmarks |
|---|---|---|---|---|---|---|---|---|
| Gr/PS67-b-PAA27 [141] | CORT | EC | U | 0.001– 10 ng mL−1 | 0.87 pg mL−1 | 12 | 42 | 3/5 |
| c-Mab-rGrO/ITO/glass [142] | CORT | EC/CR | U | 1–10 ng mL−1 | 27.6 pM | U | several months | 3/5 |
| Ab/d-BSA/rGrO/Qz [143] | CORT | EC/EIS | U | 10–10,000 pM | 10 pM | 30 | U | 2/5 |
| GrO-CS/GSPE [144] | 5-HT | EC/DPV | 0.05 A | 0.01–100 M | 3.2 nM | 30 | 28 | 2/5 |
| AuNP/rGrO /SPE [145] | Trp | EC/DPV | U | 0.5–500 ML−1 | 0.39 ML−1 | U | U | 1/5 |
| Gr-PLA [146] | UA | EC/BIA-MPA | 0.1332 ALM−1 | 0.5–250 ML−1 | 0.02 ML−1 | |||
| EC/DPV | 0.1723 ALM−1 | 10–70 ML−1 | 0.5 M.L−1 | |||||
| NO2− | EC/BIA-MPA | 0.0922 ALM−1 | 0.5–250 ML−1 | 0.03 ML−1 | U | 15 measurements | 1/5 | |
| EC/DPV | 0.0031 ALM−1 | 50–1300 ML−1 | 30 ML−1 | |||||
| Glc | AMP | U | 0.50– ∼6.30 mML−1 | 15 ML−1 | ||||
| CuNPs/LIGr [147] | Glc | EC | 2665 | 0.03–4.5 mM | 0.023 µM | 0.083 | 35 | 4/5 |
| GrO-PAD [148] | Glc | COL | U | 0–∼1 mM | 0.02 mM | 1 | U | 2/5 |
| Cu2O NC/Gr [149] | Glc | EC | 36.4 | 0.002–17.1 mM | 0.23 M | 1 | 180 | 4/5 |
| Au/rGrO-Ti3C2 [150] | Glc | EC | 355 | 10 µM–21 mM | 3.1 µM | 0.083 | 10 | 2/5 |
| PEDOT-GrO/ITO [151] | UA | EC/CV | U | 2–1000 M | 0.75 M | 1 | 10 | 2/5 |
| GFET [152] | IL-6 | POT | U | 0.05–0.84 nM | 12.2 pM | 6.67 | U | 2/5 |
| GFET [153] | CA1 | EC | 65.4 mVdecade−1 | 330 fM– 3 nM | 330 fM | 60 | U | 3/5 |
| rGrO/ITO [155] | CYFRA-21-1 | EC/DPV | 0.756 mA mLng−1 | 2–22 ngmL−1 | 0.122 ngmL−1 | 16 | 56 | 3/5 |
| BSA/anti-CYFRA21/ APTES/nHfO2 @rGrO/ITO [156] | CYFRA-21-1 | EC/DPV | 18.24 µA mLng−1 | 0–30 ngmL−1 | 0.16 ngmL−1 | 15 | 40 | 3/5 |
| AuNPs-rGrO [157] | IL8 | EC | U | 0.0005–4 ng mL−1 | 72.73 pgmL−1 | 9 | 84 | 3/5 |
| ZnO-rGrO [158] | IL8 | EC | 12.46 µAmLng−1 | 100 fgmL−1– 5 ngmL−1 | 51.53 pgmL−1 | 10 | 70 | 4/5 |
| GrP-PS67-b-PAA27-Au [159] | PSA | EC/CR | 0.875 | 0.0001–100 ng mL−1 | 40 fgmL−1 | 4 | 56 | 5/5 |
| MWCNT/His-rGrO [160] | PSA | EC/DPV | U | 0.01–20,000 pg mL−1 | 2.8 fgmL−1 | 25 | 28 | 2/5 |
| Sensing Material | Analyte Sample | Sensing Mechanism | Detection Sensitivity | Detection Range | LOD | Response Time (s) | Durability | Meets Benchmarks |
|---|---|---|---|---|---|---|---|---|
| Gr-AgNW [46] | Glc | EC | U | 0.001–10 mM | 0.4 M | U | Stable after 5000 cycles; enzyme activity retained 24 h in solution | 3/5 |
| Gr/CAT / [174] | Glc | EC | 22.72% | 0.1–0.9 mM | 12.57 M | ∼1.3 | Stable for 48 h in artificial tears; negligible degradation after 5000 cycles of stretching | 4/5 |
| GFET/ PMMA /PASE [175] | Cytokines TNF- | FE | U | 0.03–500 nM | 2.75 pM | ∼420 | Consistent response after 100% tensile strain | 2/5 |
| Cytokines IFN- | FE | U | 0.03–500 nM | 2.89 pM | U | (Same as TNF-) | 2/5 | |
| GFET/IgG /AgNWs [176] | MMP-9 | FE | 11.1 ng per 1% | 1–500 ng | 0.74 ng | ∼2.5 | Stable after 16 days of accelerated aging (≈1 year storage period) | 3/5 |
| PEDOT-Gr [177] | DA | AMP | 12.9 A | 0–70 M | 101 nM | U | High long-term stability; ∼15% sensitivity loss after 1 month of storage at 4 °C | 1/5 |
| GFET [107] | L-Cys | FE | U | 0–4800 M | 0.02 M in undiluted human sweat; 0.043 M in artificial tears | U | Consistent electrical and mechanical properties after 100 bending/folding/shrinking cycles | 2/5 |
| Sensing Material | Analyte Sample | Sensing Mechanism | Detection Sensitivity | Detection Range (ppm) | LOD (Lowest Tested, ppm) | Response Time (s) | Durability | Meets Benchmarks |
|---|---|---|---|---|---|---|---|---|
| Porphyrin-rGO [182] | Acetone, | EC | Unique VOC patterns | 25–100 | 25 | ∼seconds | U | 2/5 |
| rGrO/OA [183] | EtOH, 2-ethylhexanol, nonanal | CR | High sensitivity at 25 ppm | 25–125 | 25 | 61–200 | Stable response | 2/5 |
| CS-rGrO [184] | Acetone | CR | 27.89% response at 10 ppm | 0–10 | U | U | 5 weeks | 2/5 |
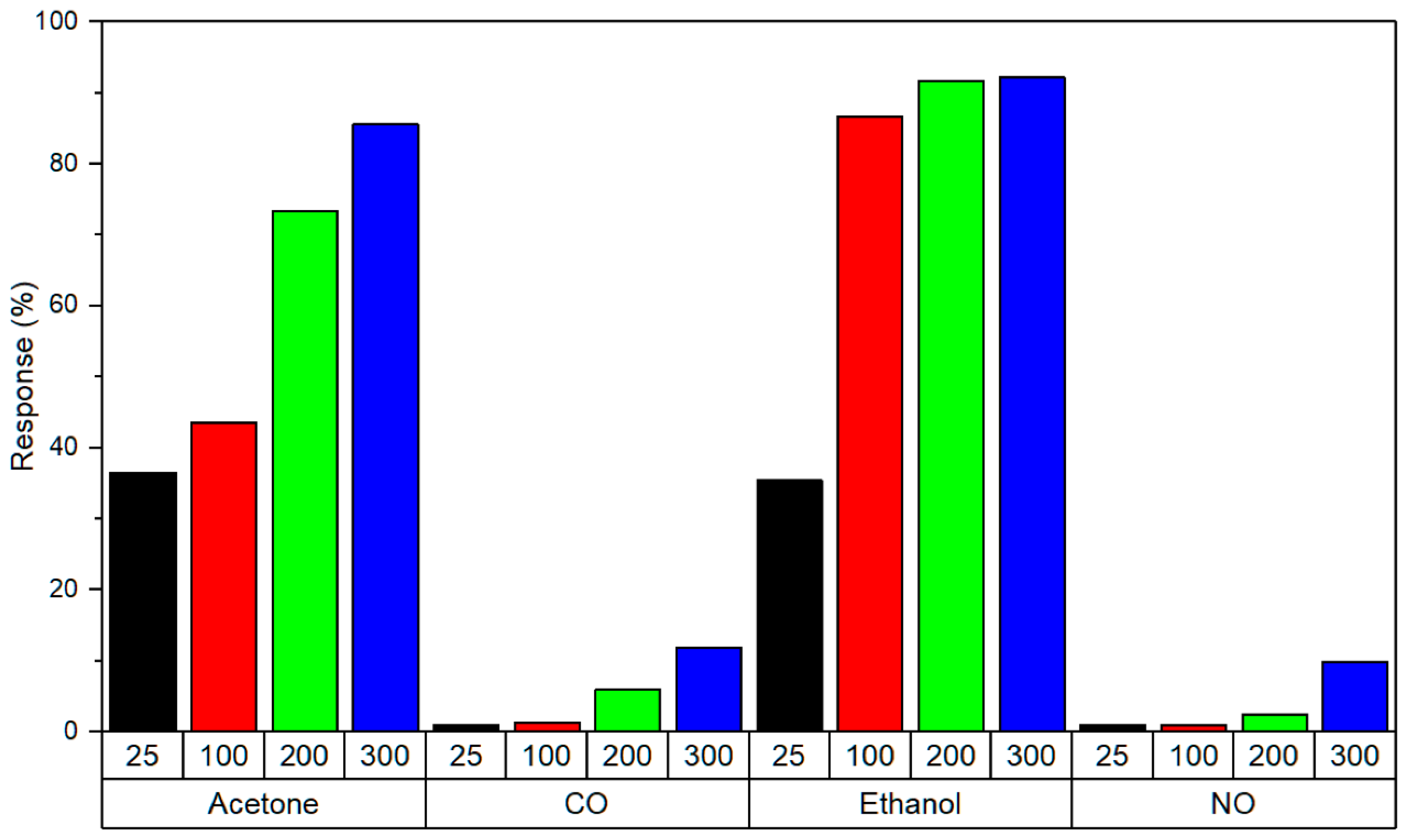
| PGr- [185] | Ethanol | CR | >35% response | 0.5–2 | 0.5 | ∼50 | U | |
| Acetone | >35% response | 0.5–4 | 0.5 | ∼50 | U | |||
| NO | >5% response | 0.01–0.1 | 0.01 | ∼50 | U | 3/5 | ||
| CO | >5% response | 1–5 | 1 | ∼50 | U | |||
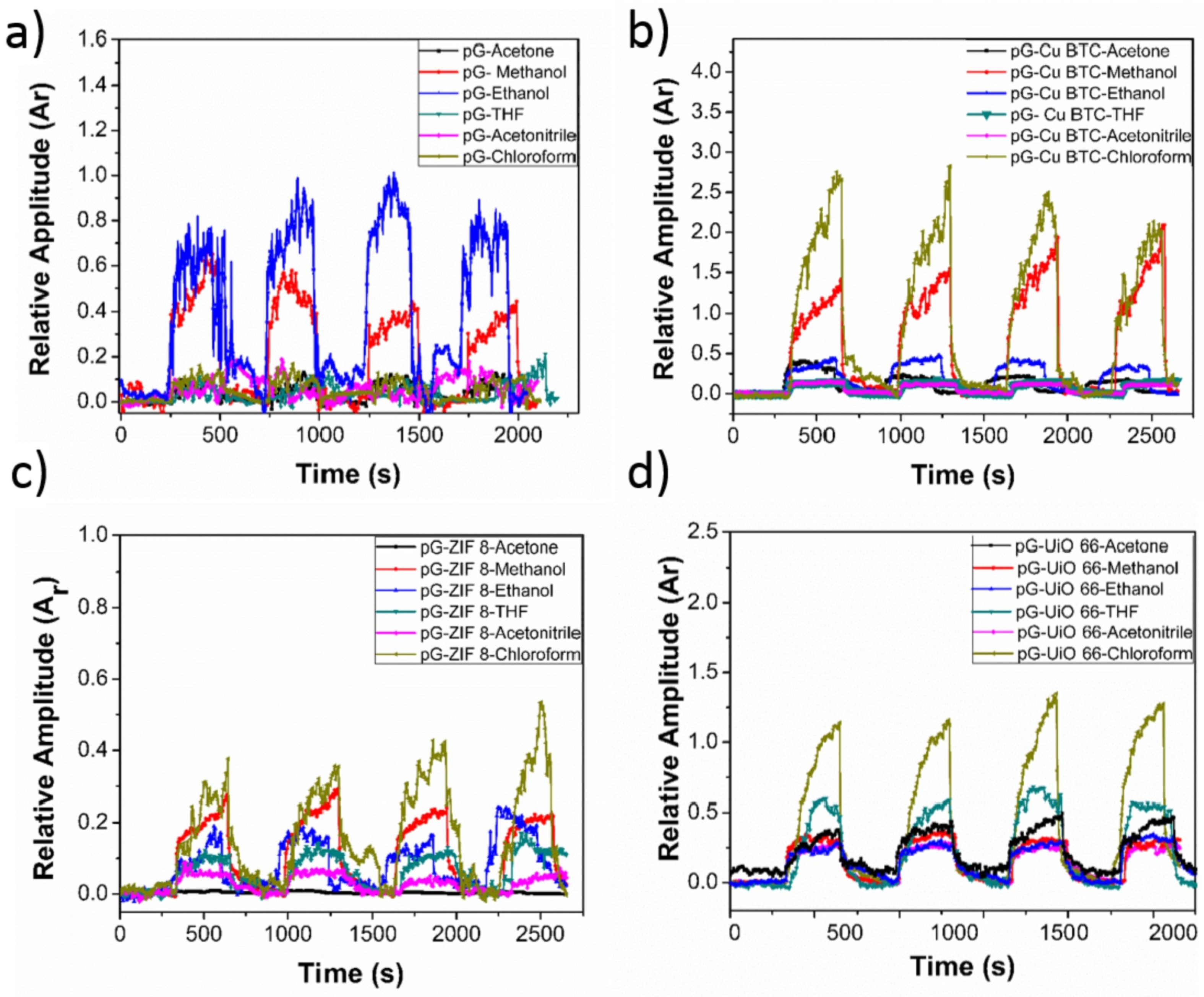
| PGr-Cu BTC [186] | MeOH, Chloroform | CR | Highest sensitivity for Chloroform (value not provided) | 2.82–22.6 | 2.82 | ∼seconds | U | 2/5 |
| Sensors | 1/5 | 2/5 | 3/5 | 4/5 | 5/5 | Avg. ± SD | Strengths | Limitations |
|---|---|---|---|---|---|---|---|---|
| Sweat (16) | 7 (44%) | 2 (13%) | 3 (19%) | 0 (0%) | 4 (25%) | 2.5 ± 1.6 | High durability; rapid response; clinically relevant ranges; multiple perfect-score devices; excellent sensitivity; scalable wearable designs; broad analyte compatibility | Inconsistent LOD; high performance variance; many devices at low maturity level; inconsistent response time reporting; trade-offs: sensitivity vs. responsiveness; durability issues under real sweat conditions; |
| Saliva (37) | 6 (16%) | 15 (41%) | 9 (24%) | 6 (16%) | 1 (3%) | 2.5 ± 1.0 | Exceptional LOD; broad analyte diversity; many devices meet high benchmarks; hybrid nanocomposites enable balanced sensitivity and stability; large device pool with miniaturised designs | Slow response; inconsistent sensitivity reporting; limited real-world stability data; sensitivity–speed trade-off; durability gaps; variable performance metrics; heterogeneous reporting; missing real-matrix validation |
| Tear (6) | 1 (17%) | 3 (50%) | 1 (17%) | 1 (17%) | 0 (0%) | 2.3 ± 0.9 | Ultra-low LOD; rapid response; good durability; suitable for contact-lens integration; good mechanical stability with direct corneal access | Small evidence base; sensitivity–speed–durability trade-offs; few devices fully validated; limited ocular biocompatibility data; incomplete long-term stability testing; limited multiplexing exploration |
| Breath (5) | 0 (0%) | 4 (80%) | 1 (20%) | 0 (0%) | 0 (0%) | 2.2 ± 0.8 | Fast response; clinically relevant VOC detection; high sensitivity; rapid chemiresistive sensor responses; real-time VOC monitoring; unique exhaled breath profiles; strong diagnostic potential | Poor LOD profile and durability in high humidity; low performance variance; material challenges for humidity-resistant selectivity; sparse real-breath validation data; translational immaturity |
| Failure Mode | Underlying Mechanism | Impact on Performance | Representative Mitigation Strategies |
|---|---|---|---|
| Biofouling (molecular) | Non-specific adsorption of proteins, cells, and macromolecules from complex biofluids onto the graphene surface | Reduced sensitivity and specificity; baseline signal drift | Surface functionalisation with anti-fouling coatings (e.g., PEG, zwitterionic polymers); active electrochemical cleaning; microfluidic sample conditioning |
| Mechanical degradation (interfacial) | Microcracking, delamination, or strain-induced disruption of conductive pathways and receptor anchoring sites | Loss of conductivity; reduced binding efficiency; device failure under repeated deformation | Use of stretchable substrates and serpentine interconnects; encapsulation layers; strain-accommodating receptor immobilisation chemistries |
| Signal drift (systems-level) | Instability of recognition elements; environmental fluctuations; compounded effects of biofouling and mechanical degradation | Gradual baseline shift; reduced accuracy and reliability over time | Incorporation of stable synthetic receptors (e.g., aptamers, MIPs); real-time calibration algorithms; reference electrode integration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, S.; Debnath, T.; Paul, M. A Review of Graphene-Integrated Biosensors for Non-Invasive Biochemical Monitoring in Health Applications. Sensors 2025, 25, 6553. https://doi.org/10.3390/s25216553
Debnath S, Debnath T, Paul M. A Review of Graphene-Integrated Biosensors for Non-Invasive Biochemical Monitoring in Health Applications. Sensors. 2025; 25(21):6553. https://doi.org/10.3390/s25216553
Chicago/Turabian StyleDebnath, Sourabhi, Tanmoy Debnath, and Manoranjan Paul. 2025. "A Review of Graphene-Integrated Biosensors for Non-Invasive Biochemical Monitoring in Health Applications" Sensors 25, no. 21: 6553. https://doi.org/10.3390/s25216553
APA StyleDebnath, S., Debnath, T., & Paul, M. (2025). A Review of Graphene-Integrated Biosensors for Non-Invasive Biochemical Monitoring in Health Applications. Sensors, 25(21), 6553. https://doi.org/10.3390/s25216553







