Localized Surface Plasmon Resonance-Based Gas Sensor with a Metal–Organic-Framework-Modified Gold Nano-Urchin Substrate for Volatile Organic Compounds Visualization
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Au Nano-Urchins
2.2. Fabrication of the ZIF-8-Modified Au Nano-Urchin Substrates
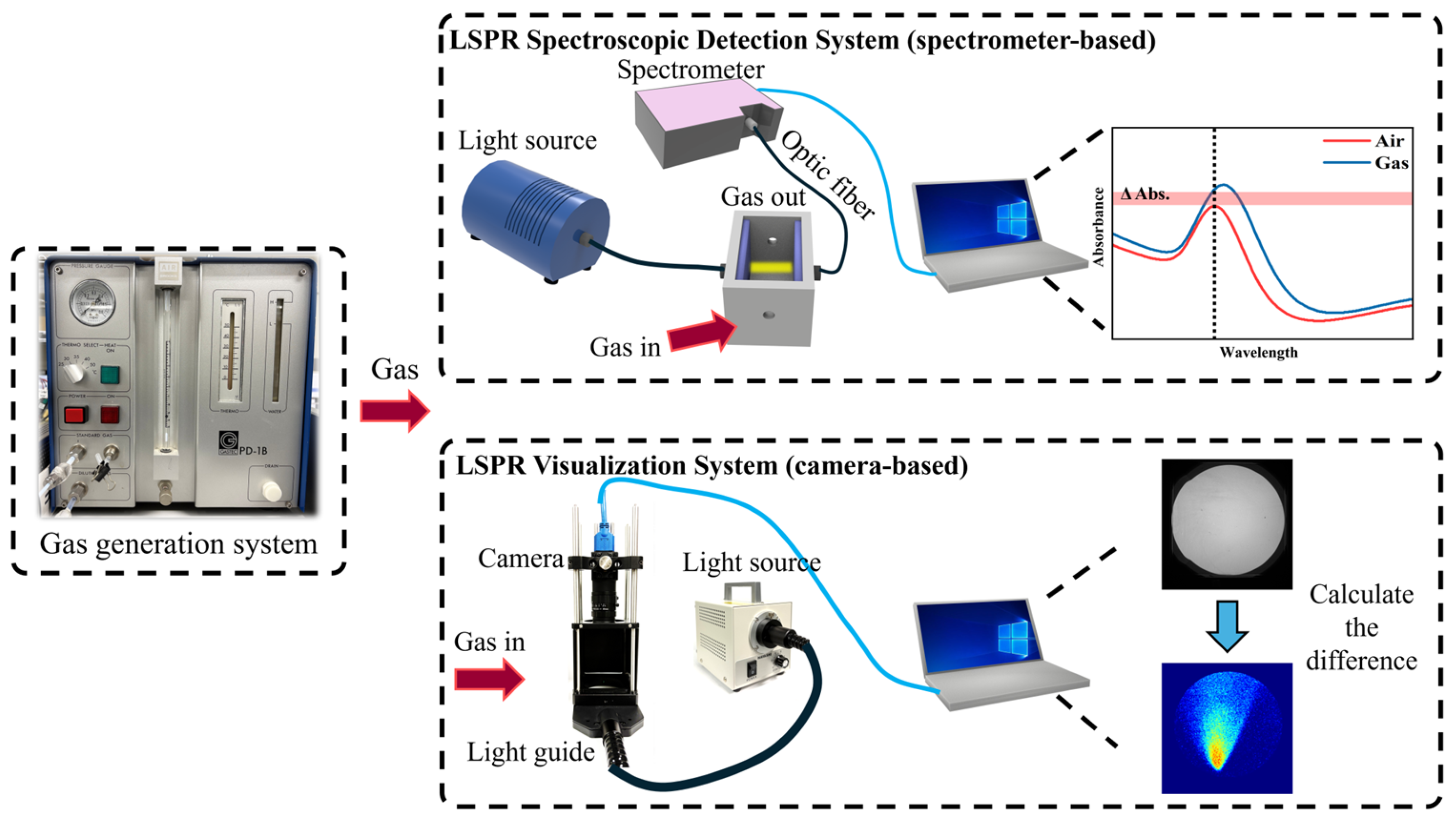
2.3. Gas Generation and Optical Measurement Systems
2.4. Experimental Setup for Gas Visualization
3. Results and Discussion
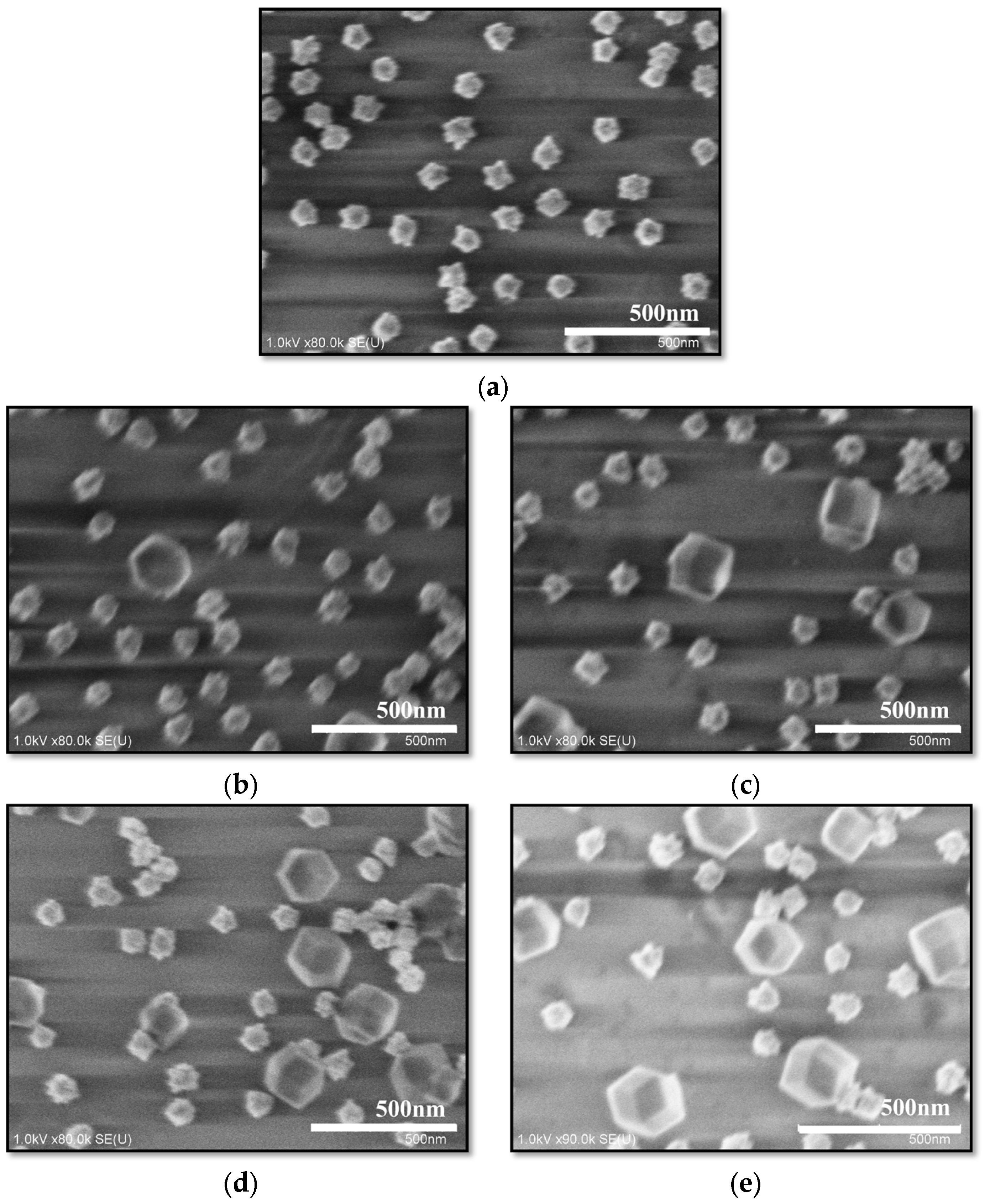
3.1. Morphological Evolution and Optical Properties of the ZIF-8-Modified Au Nano-Urchin Substrates
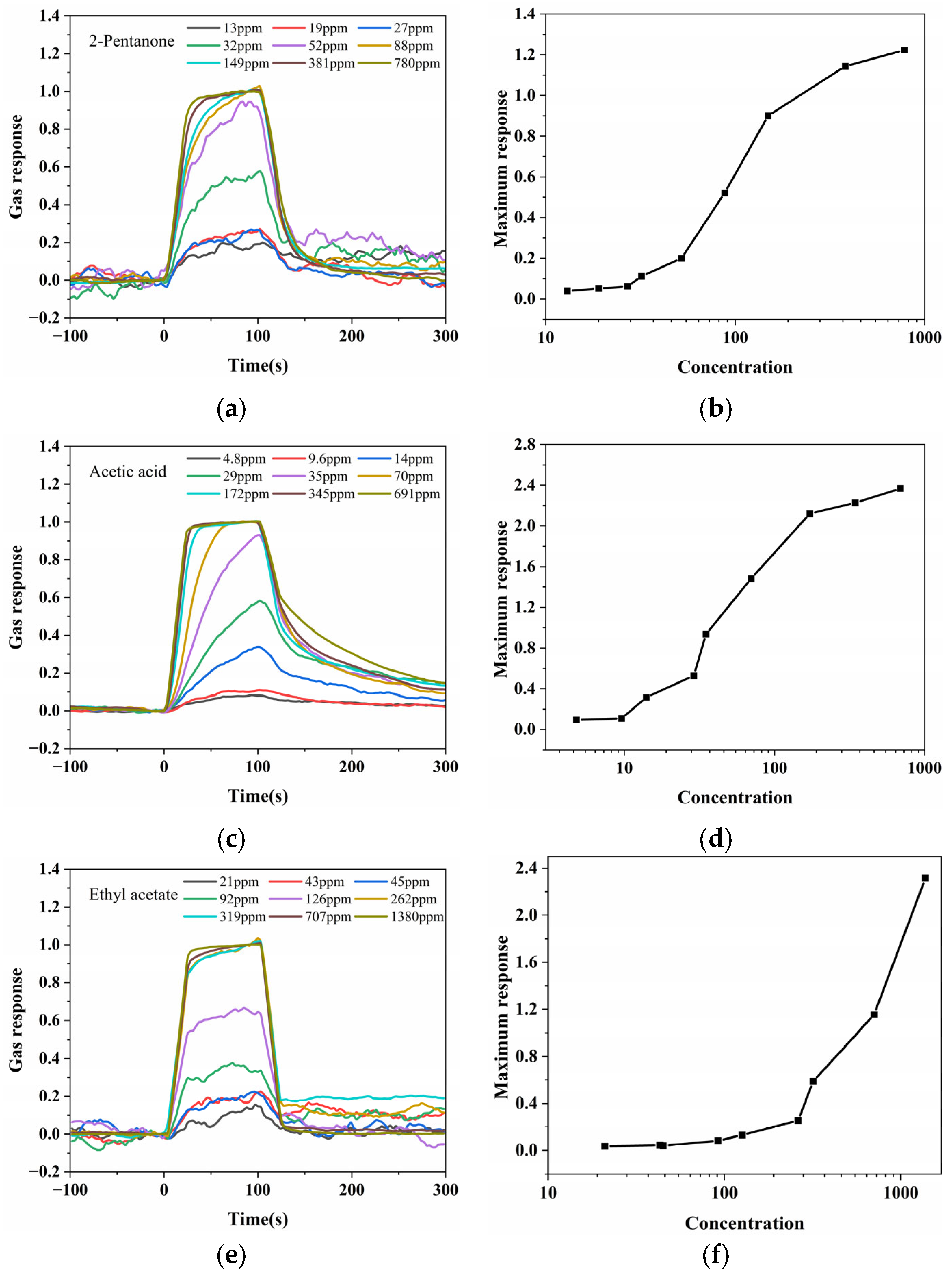
3.2. Gas Sensing Performance of Different VOCs Using the Spectrometer-Based System
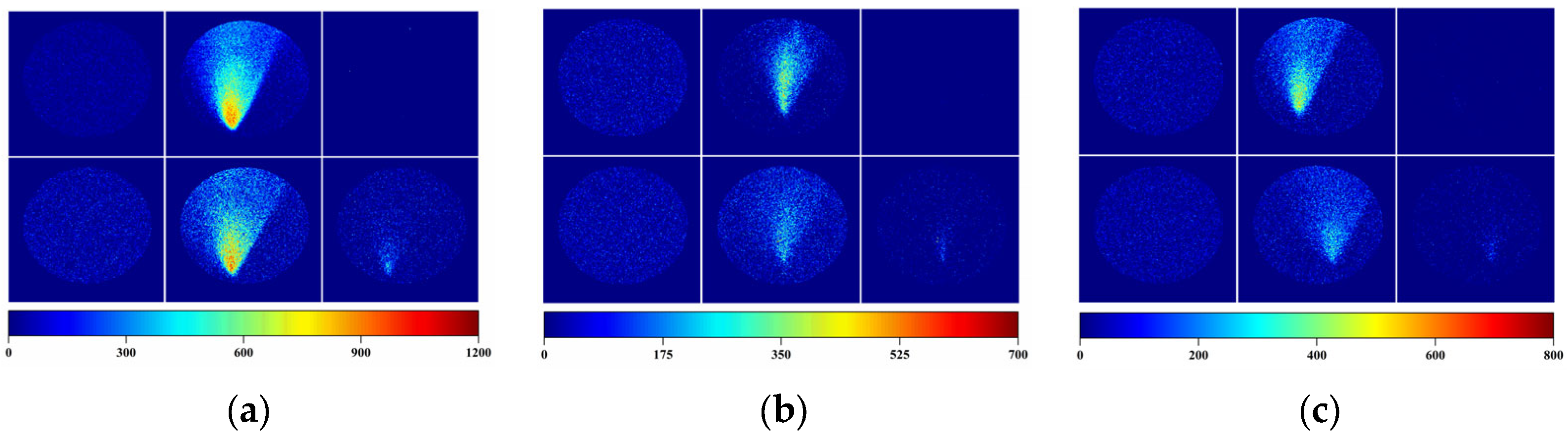
3.3. Visualization of Gas Distributions Using the Camera-Based LSPR System
3.4. Visualization of VOC Evaporation Using the Droplet Evaporation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and Human Health Impacts of Volatile Organic Compounds: A Perspective Review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and Other Secondary Photochemical Pollutants: Chemical Processes Governing Their Formation in the Planetary Boundary Layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Xu, S.; Tang, W.; Chase, D.B.; Sparks, D.L.; Rabolt, J.F. A Highly Sensitive, Selective, and Reproducible SERS Sensor for Detection of Trace Metalloids in the Environment. ACS Appl. Nano Mater. 2018, 1, 1257–1264. [Google Scholar] [CrossRef]
- Gallego, E.; Perales, J.F.; Calaf, J.M. Continuous Monitoring of Volatile Organic Compounds through Sensorization. Automatic Sampling during Pollution/Odour/Nuisance Episodic Events. Atmos. Environ. 2023, 299, 119657. [Google Scholar] [CrossRef]
- Cao, R.; Lu, Z.; Hu, J.; Zhang, Y. Carbon-Based FET-Type Gas Sensor for the Detection of Ppb-Level Benzene at Room Temperature. Chemosensors 2024, 12, 179. [Google Scholar] [CrossRef]
- Duffy, E.; Morrin, A. Endogenous and Microbial Volatile Organic Compounds in Cutaneous Health and Disease. TrAC Trends Anal. Chem. 2019, 111, 163–172. [Google Scholar] [CrossRef]
- Leong, S.X.; Leong, Y.X.; Tan, E.X.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Park, J.-Y.; Choi, J.-W.; Warner, I.M. Coating-Based Quartz Crystal Microbalance Detection Methods of Environmentally Relevant Volatile Organic Compounds. Chemosensors 2021, 9, 153. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An Electronic Nose Based on Carbon Nanotube-Titanium Dioxide Hybrid Nanostructures for Detection and Discrimination of Volatile Organic Compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Jha, S.K.; Liu, C.; Hayashi, K. Molecular Imprinted Polyacrylic Acids Based QCM Sensor Array for Recognition of Organic Acids in Body Odor. Sens. Actuators B Chem. 2014, 204, 74–87. [Google Scholar] [CrossRef]
- Ahmed, L.R.; Lüder, J.; Chuang, C.-H.; EL-Mahdy, A.F.M. Covalent-Organic-Framework-Modified Quartz Crystal Microbalance Sensor for Selective Detection of Hazardous Formic Acid. ACS Appl. Mater. Interfaces 2024, 16, 30408–30420. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.M.; Torad, N.L. Quartz Crystal Microbalance Sensor for Detection of Aliphatic Amines Vapours. Sens. Actuators B Chem. 2010, 147, 481–487. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C. Volatile Organic Compounds Gas Sensor Based on Quartz Crystal Microbalance for Fruit Freshness Detection: A Review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef]
- Xu, X.; Cang, H.; Li, C.; Zhao, Z.K.; Li, H. Quartz Crystal Microbalance Sensor Array for the Detection of Volatile Organic Compounds. Talanta 2009, 78, 711–716. [Google Scholar] [CrossRef]
- Vaughan, S.R.; Pérez, R.L.; Chhotaray, P.; Warner, I.M. Quartz Crystal Microbalance Based Sensor Arrays for Detection and Discrimination of VOCs Using Phosphonium Ionic Liquid Composites. Sensors 2020, 20, 615. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Yao, H.; Wang, P.; Zhu, M.; Shi, X.; Xu, S. Recent Advances in Metal Oxide Semiconductor Heterojunctions for the Detection of Volatile Organic Compounds. Chemosensors 2024, 12, 244. [Google Scholar] [CrossRef]
- Long, H.; Li, Y.; Chai, K.; Zeng, W. Metal Oxide Semiconductor-Based Core-Shell Nanostructures for Chemiresistive Gas Sensing: A Review. Sens. Actuators B Chem. 2024, 417, 136183. [Google Scholar] [CrossRef]
- Kau, N.; Jindal, G.; Kaur, R.; Rana, S. Progress in Development of Metal Organic Frameworks for Electrochemical Sensing of Volatile Organic Compounds. Results Chem. 2022, 4, 100678. [Google Scholar] [CrossRef]
- Tian, R.; Ding, Y.; Wang, Q.; Song, P. Advanced Triethylamine Sensor Utilizing 3D Microspheres of La-Doped MoO3: Performance Enhancement and Mechanism Insights. Sens. Actuators B Chem. 2024, 412, 135817. [Google Scholar] [CrossRef]
- Tamersit, K. WS2 Nanosheet-Based Ultrascaled Field-Effect Transistor for Hydrogen Gas Sensing: Addressing the Sensitivity-Downscaling Trade-Off. Sensors 2024, 24, 6730. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Wu, G.; Cao, J.; Zhang, Z.; Zhang, Y. Carbon Nanotube-Based Field-Effect Transistor-Type Sensor with a Sensing Gate for Ppb-Level Formaldehyde Detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319. [Google Scholar] [CrossRef]
- Hong, S.; Wu, M.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Kim, D.; Jang, D.; Lee, J.-H. FET-Type Gas Sensors: A Review. Sens. Actuators B Chem. 2021, 330, 129240. [Google Scholar] [CrossRef]
- Burgmair, M.; Frerichs, H.-P.; Zimmer, M.; Lehmann, M.; Eisele, I. Field Effect Transducers for Work Function Gas Measurements: Device Improvements and Comparison of Performance. Sens. Actuators B Chem. 2003, 95, 183–188. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, G.; Zhang, T.; Shen, F.; Meng, L.; Wang, L.; Li, F.; Zhu, Y.; Zheng, Y.; He, N.; et al. VOC detections with optical spectroscopy. Prog. Electromagn. Res. 2022, 173, 71–92. [Google Scholar] [CrossRef]
- D’Arco, A.; Mancini, T.; Paolozzi, M.C.; Macis, S.; Mosesso, L.; Marcelli, A.; Petrarca, M.; Radica, F.; Tranfo, G.; Lupi, S.; et al. High Sensitivity Monitoring of VOCs in Air through FTIR Spectroscopy Using a Multipass Gas Cell Setup. Sensors 2022, 22, 5624. [Google Scholar] [CrossRef]
- Rubio, R.; Santander, J.; Fonseca, L.; Sabate, N.; Gracia, I.; Cane, C.; Udina, S.; Marco, S. Non-Selective NDIR Array for Gas Detection. Sens. Actuators B Chem. 2007, 127, 69–73. [Google Scholar] [CrossRef]
- Sun, L.; Rotaru, A.; Robeyns, K.; Garcia, Y. A Colorimetric Sensor for the Highly Selective, Ultra-Sensitive, and Rapid Detection of Volatile Organic Compounds and Hazardous Gases. Ind. Eng. Chem. Res. 2021, 60, 8788–8798. [Google Scholar] [CrossRef]
- Duffy, E.; Cauven, E.; Morrin, A. Colorimetric Sensing of Volatile Organic Compounds Produced from Heated Cooking Oils. ACS Omega 2021, 6, 7394–7401. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Umadevi, D.; González-Barcia, L.M.; Delente, J.M.; Byrne, K.; Schmitt, W.; Watson, G.W.; Gunnlaugsson, T. “Turn-on” Fluorescence Sensing of Volatile Organic Compounds Using a 4-Amino-1,8-Naphthalimide Tröger’s Base Functionalised Triazine Organic Polymer. Chem. Commun. 2019, 55, 12140–12143. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Yang, Z.; Sassa, F.; Hayashi, K. A Robot Equipped with a High-Speed LSPR Gas Sensor Module for Collecting Spatial Odor Information from On-Ground Invisible Odor Sources. ACS Sens. 2018, 3, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Guo, H.; Ge, L.; Sassa, F.; Hayashi, K. Inkjet-Printed Localized Surface Plasmon Resonance Subpixel Gas Sensor Array for Enhanced Identification and Visualization of Gas Spatial Distributions from Multiple Odor Sources. Sensors 2024, 24, 6731. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, C.; Ge, L.; Shang, L.; Guo, H.; Hayashi, K. AuNU Dimers on ITO Substrate with the Highest Refractive Index Sensitivity as Chemical Sensor. IEEE Sens. J. 2022, 22, 7580–7589. [Google Scholar] [CrossRef]
- Chen, B.; Mou, Q.; Liu, C.; Hayashi, K. Au NUs@SiO2 Sensors for Sensitive and Selective Optical Sensing of Volatile Compounds. Talanta 2025, 294, 128207. [Google Scholar] [CrossRef]
- Estany-Macià, A.; Fort-Grandas, I.; Joshi, N.; Svendsen, W.E.; Dimaki, M.; Romano-Rodríguez, A.; Moreno-Sereno, M. ZIF-8-Based Surface Plasmon Resonance and Fabry–Pérot Sensors for Volatile Organic Compounds. Sensors 2024, 24, 4381. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Chen, L.; Guo, H.; Wang, C.; Chen, B.; Sassa, F.; Hayashi, K. Two-Dimensional SERS Sensor Array for Identifying and Visualizing the Gas Spatial Distributions of Two Distinct Odor Sources. Sensors 2024, 24, 790. [Google Scholar] [CrossRef]
- Iitani, K.; Toma, K.; Arakawa, T.; Mitsubayashi, K. Transcutaneous Blood VOC Imaging System (Skin-Gas Cam) with Real-Time Bio-Fluorometric Device on Rounded Skin Surface. ACS Sens. 2020, 5, 338–345. [Google Scholar] [CrossRef]
- Wang, C.; Shingo, K.; Guo, H.; Wang, Y.; Sassa, F.; Kenshi, H. Integrating LSPR and SERS for Rapid and Effective Visualization and Identification of Volatile Organic Compounds Distribution. In Proceedings of the 2024 IEEE SENSORS, Kobe, Japan, 20 October 2024; pp. 1–4. [Google Scholar]
- Chen, L.; Huang, Y.; Xing, T.T.; Ge, L.; Yang, T.; Chen, B.; Huang, C.Z. A Portable Multi-Channel Sensing Device Using Au Nano-Urchins as Probes for Melamine Detection in Milk. J. Mater. Chem. C 2017, 5, 7806–7812. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Guo, H.; Chen, B.; Yan, J.; Sassa, F.; Hayashi, K. Localized Surface Plasmon Resonance-Based Gas Sensor with a Metal–Organic-Framework-Modified Gold Nano-Urchin Substrate for Volatile Organic Compounds Visualization. Sensors 2025, 25, 6522. https://doi.org/10.3390/s25216522
Wang C, Guo H, Chen B, Yan J, Sassa F, Hayashi K. Localized Surface Plasmon Resonance-Based Gas Sensor with a Metal–Organic-Framework-Modified Gold Nano-Urchin Substrate for Volatile Organic Compounds Visualization. Sensors. 2025; 25(21):6522. https://doi.org/10.3390/s25216522
Chicago/Turabian StyleWang, Cong, Hao Guo, Bin Chen, Jia Yan, Fumihiro Sassa, and Kenshi Hayashi. 2025. "Localized Surface Plasmon Resonance-Based Gas Sensor with a Metal–Organic-Framework-Modified Gold Nano-Urchin Substrate for Volatile Organic Compounds Visualization" Sensors 25, no. 21: 6522. https://doi.org/10.3390/s25216522
APA StyleWang, C., Guo, H., Chen, B., Yan, J., Sassa, F., & Hayashi, K. (2025). Localized Surface Plasmon Resonance-Based Gas Sensor with a Metal–Organic-Framework-Modified Gold Nano-Urchin Substrate for Volatile Organic Compounds Visualization. Sensors, 25(21), 6522. https://doi.org/10.3390/s25216522






