Application of Wireless EMG Sensors for Assessing Agonist–Antagonist Muscle Activity During 50-m Sprinting in Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
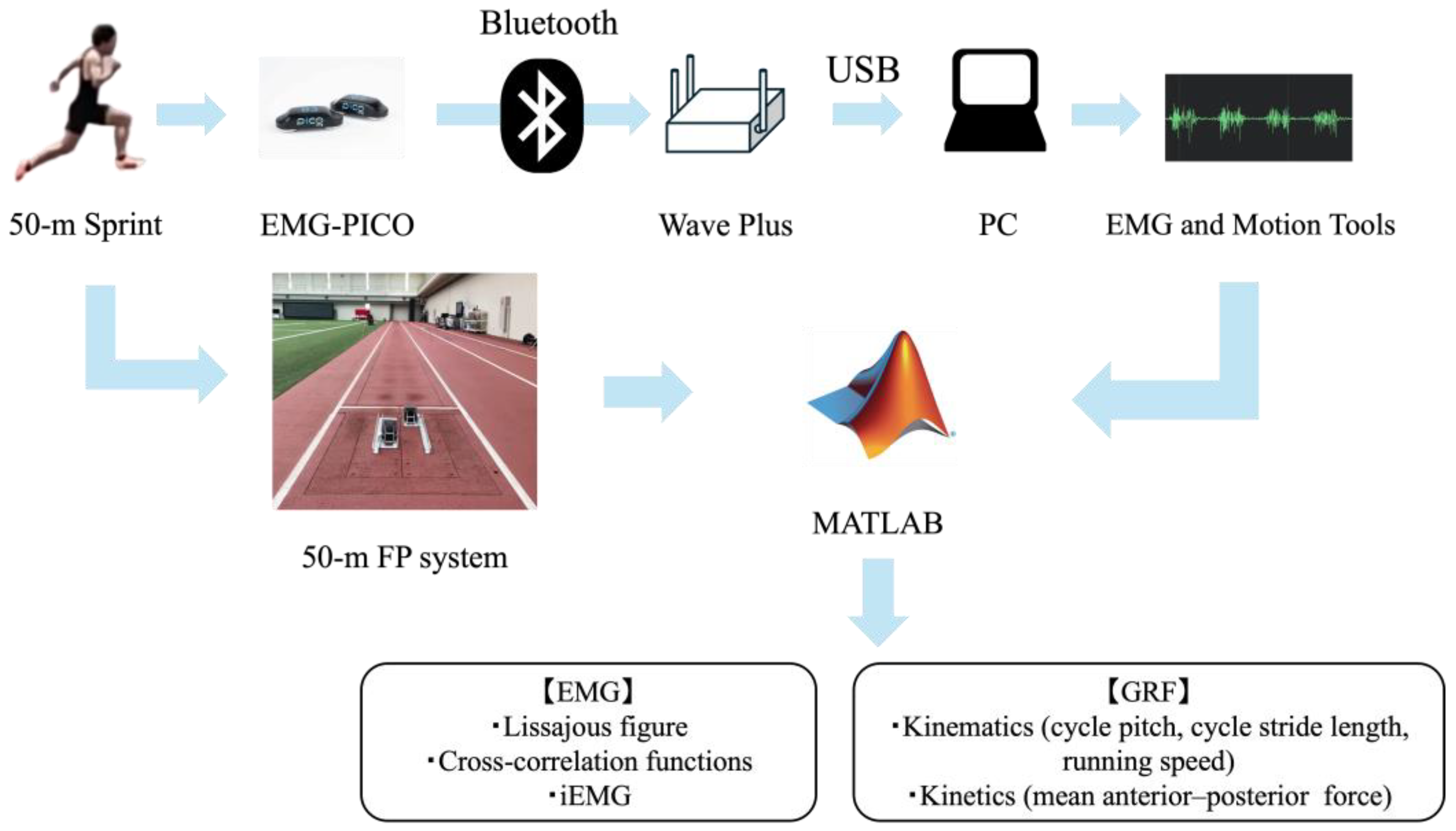
2.3. Instrumentation and Data Collection
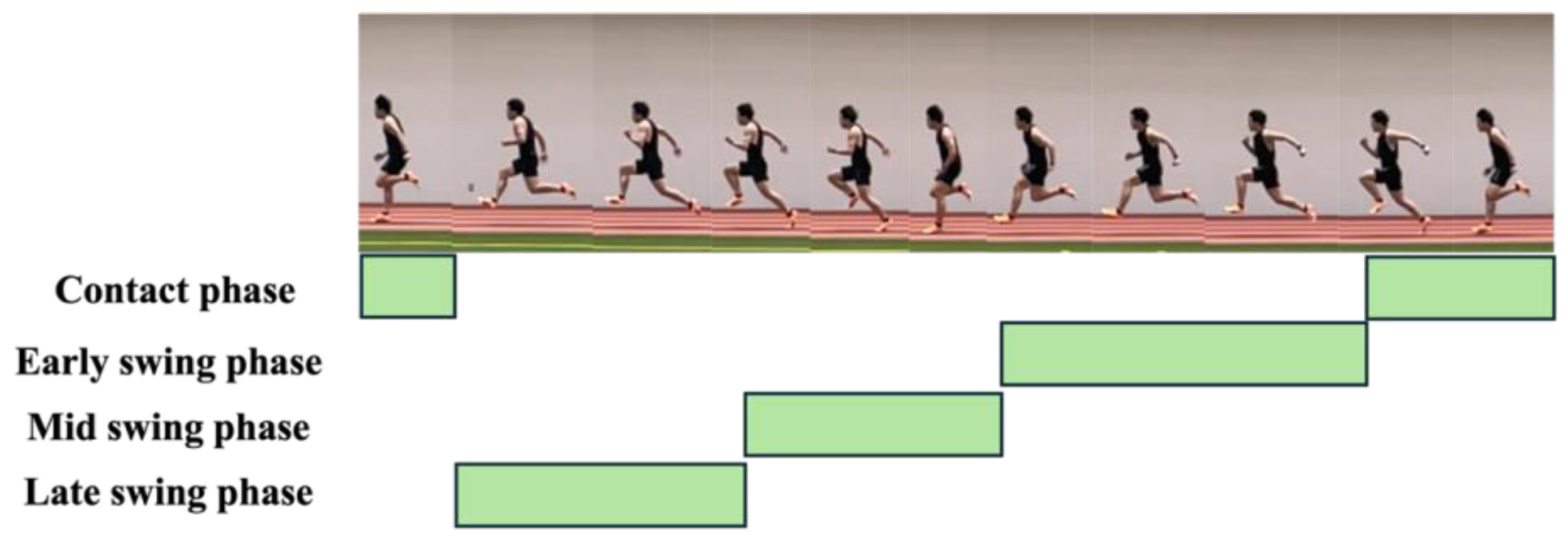
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants Characteristics
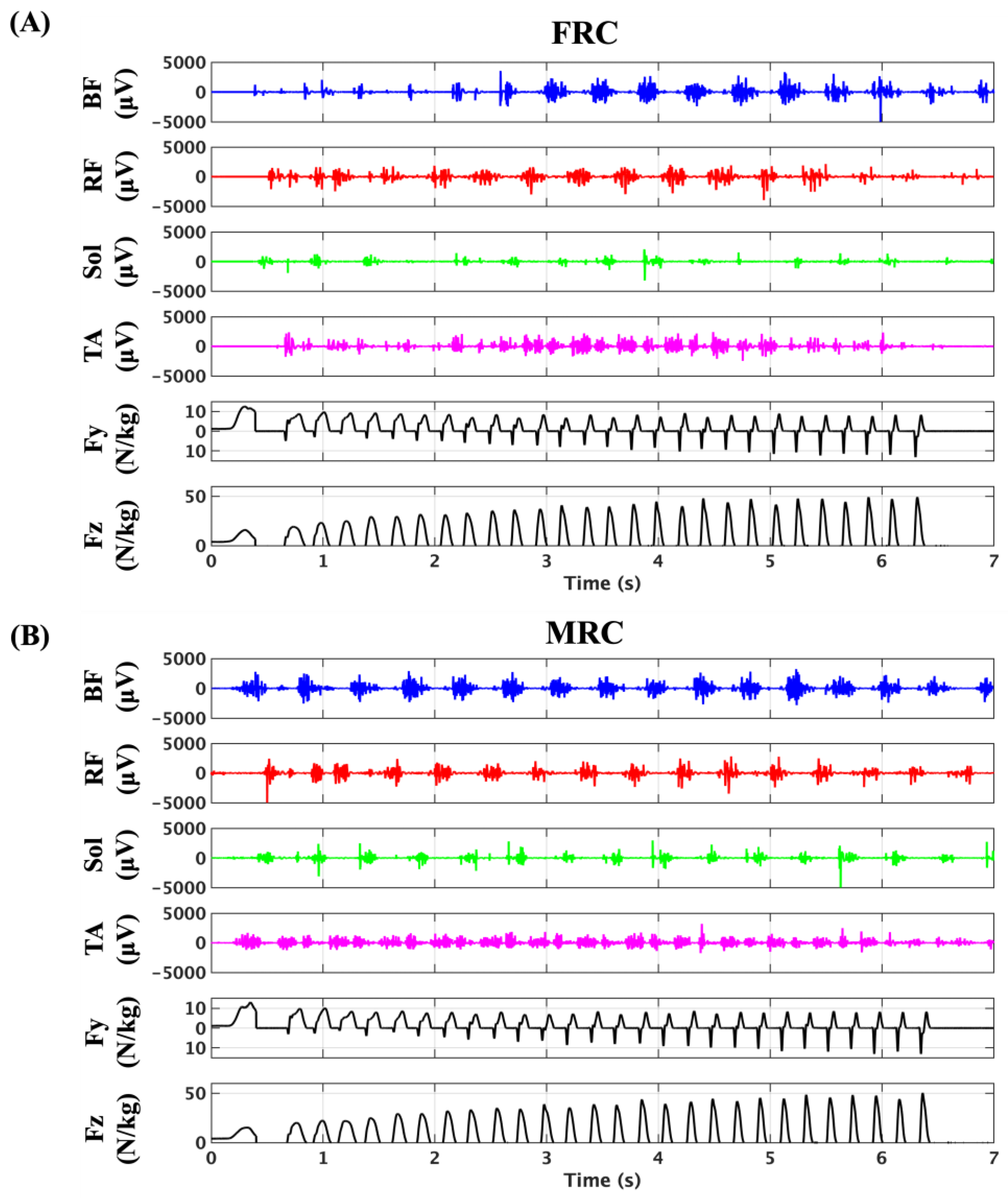
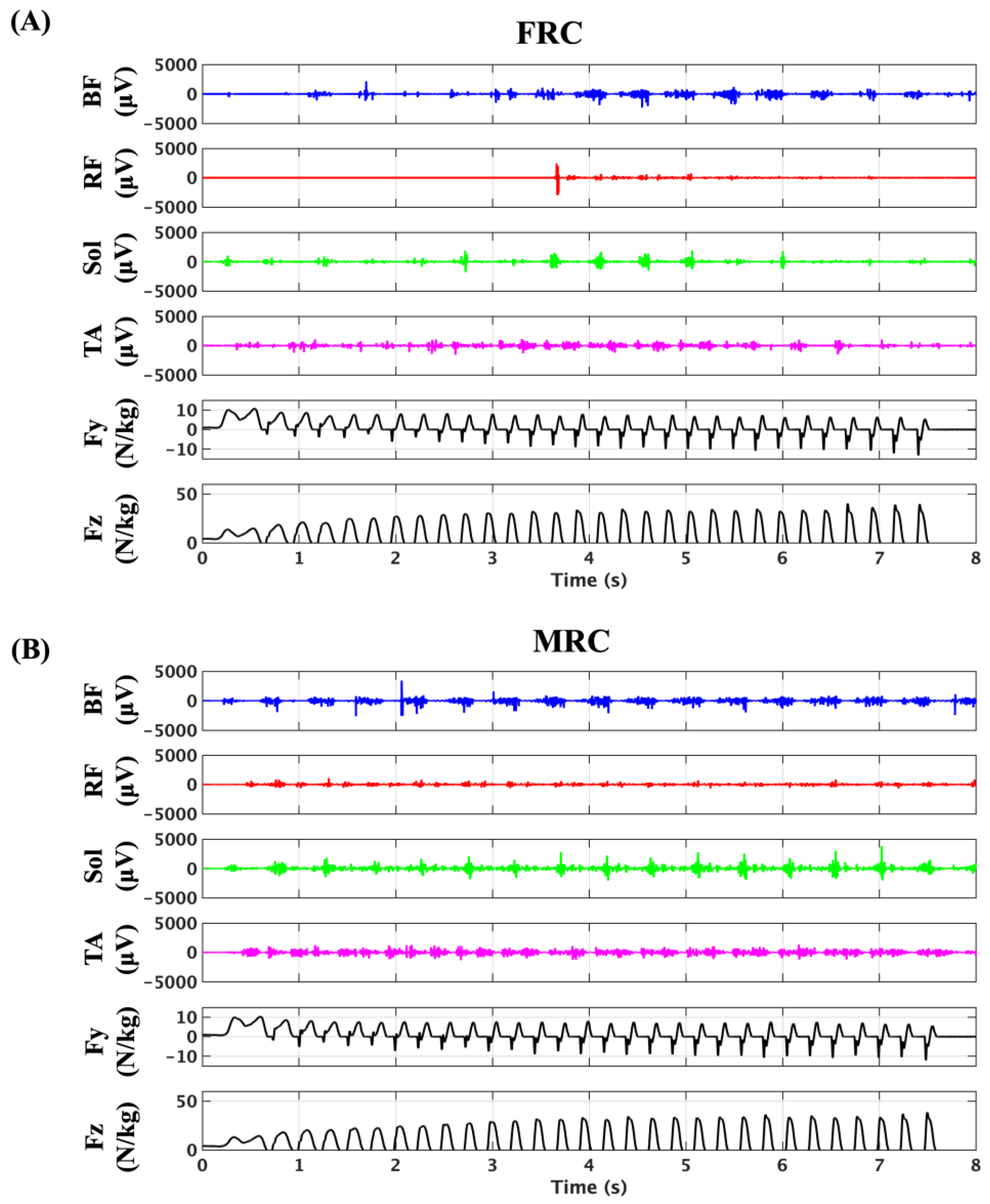
3.2. Feasibility of Continuous sEMG Signal Acquisition in Sprinting
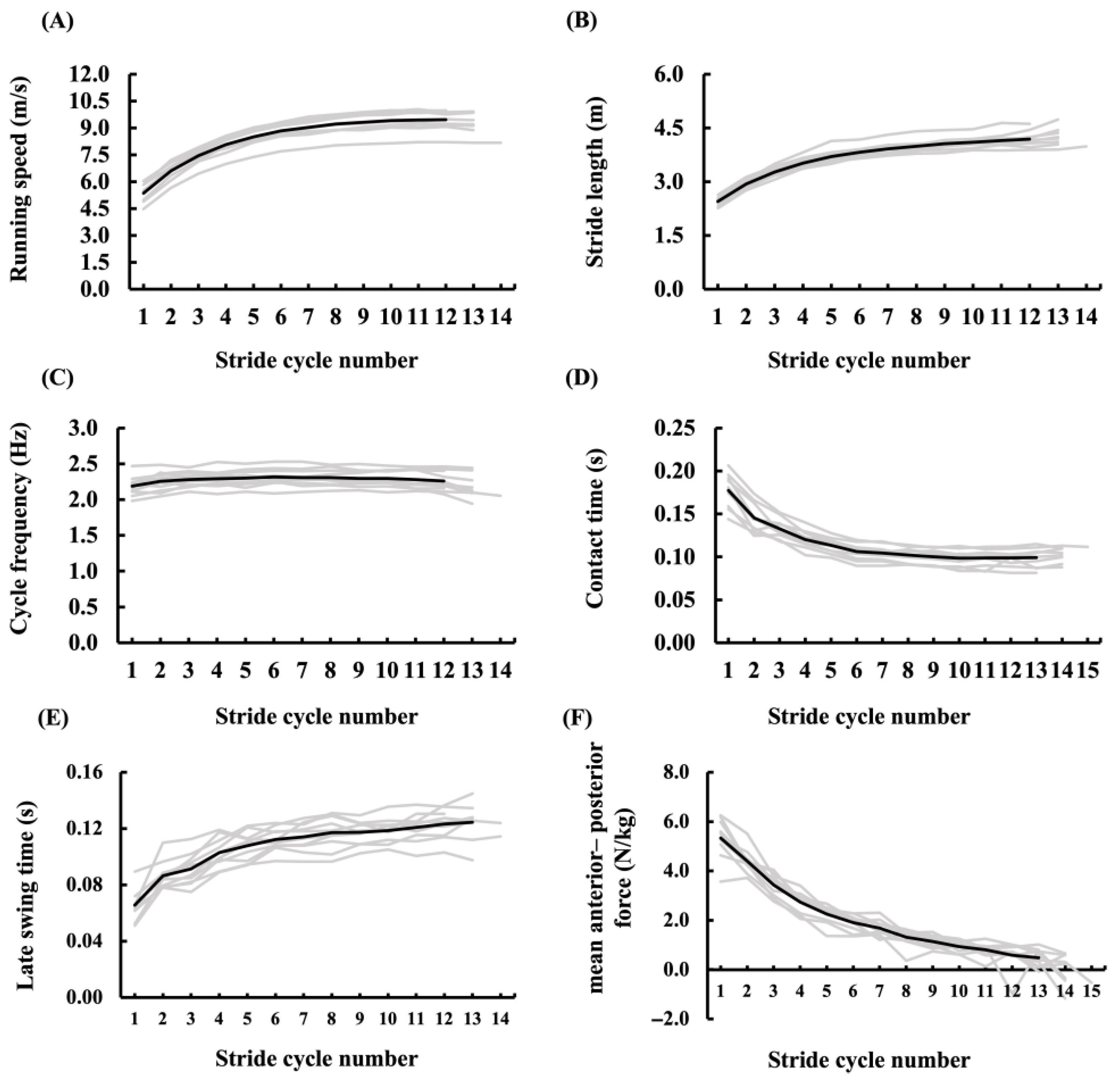
3.3. Kinetics and Kinematics Data
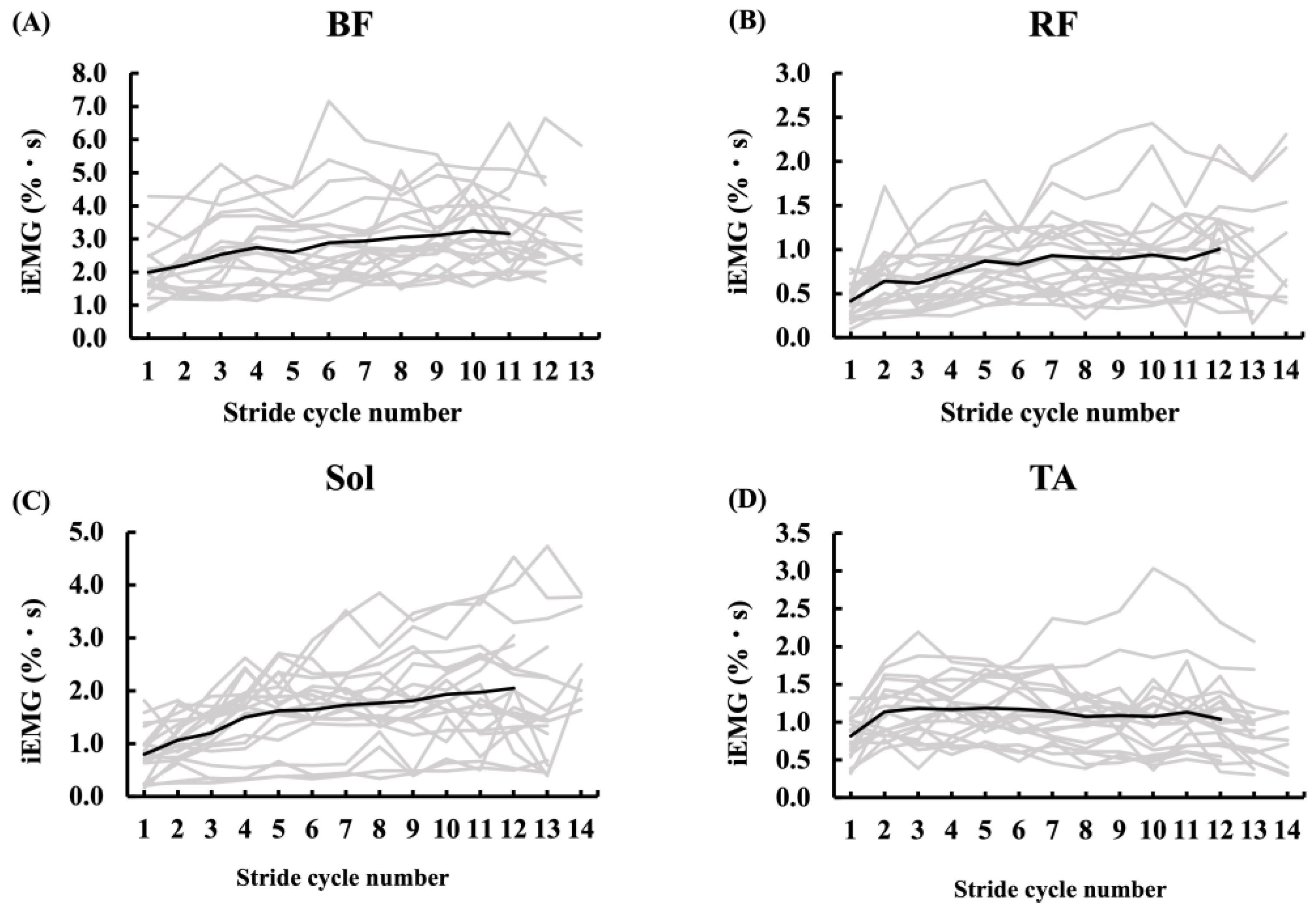
3.4. Muscle Activities During Sprinting
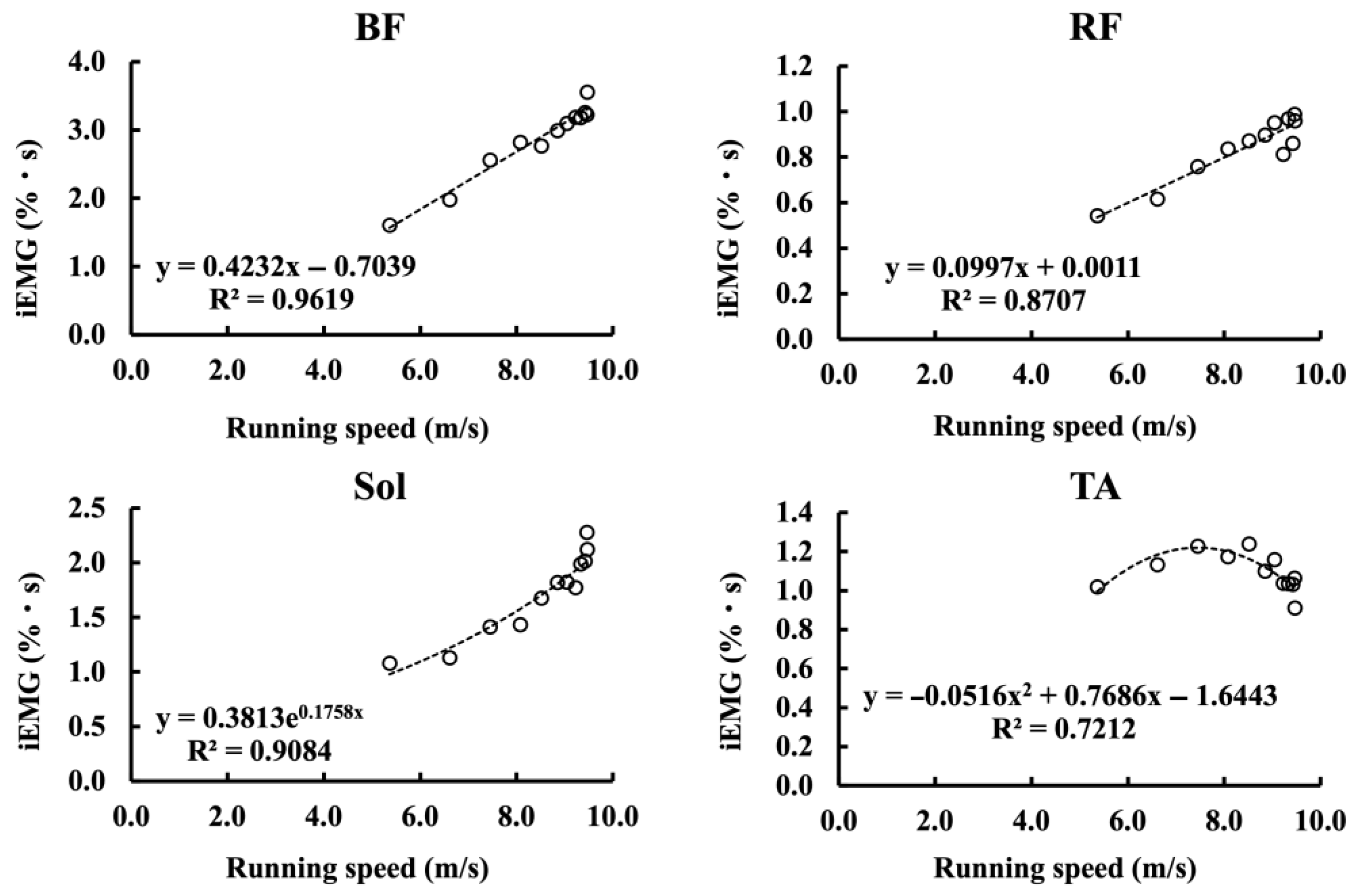
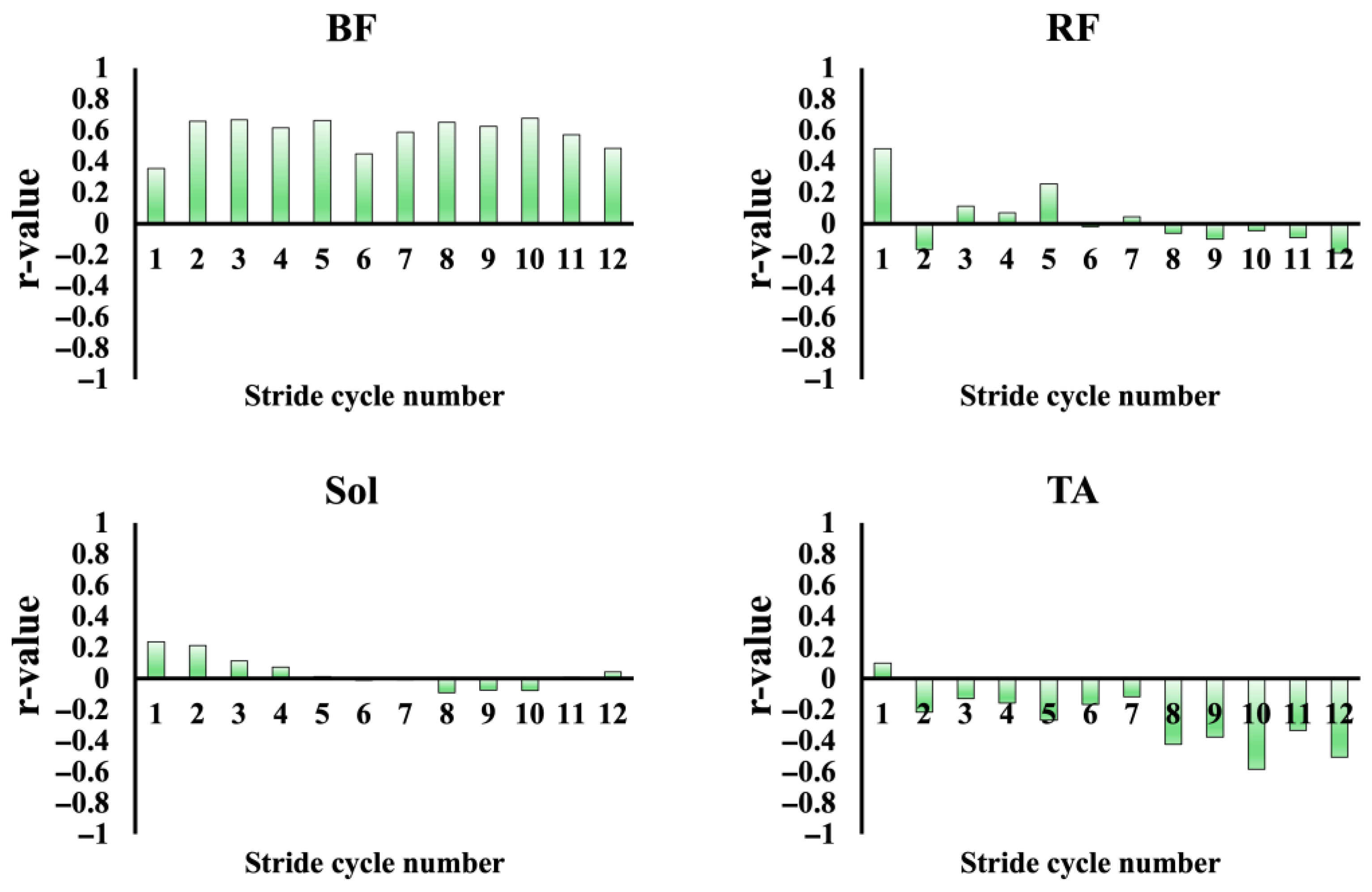
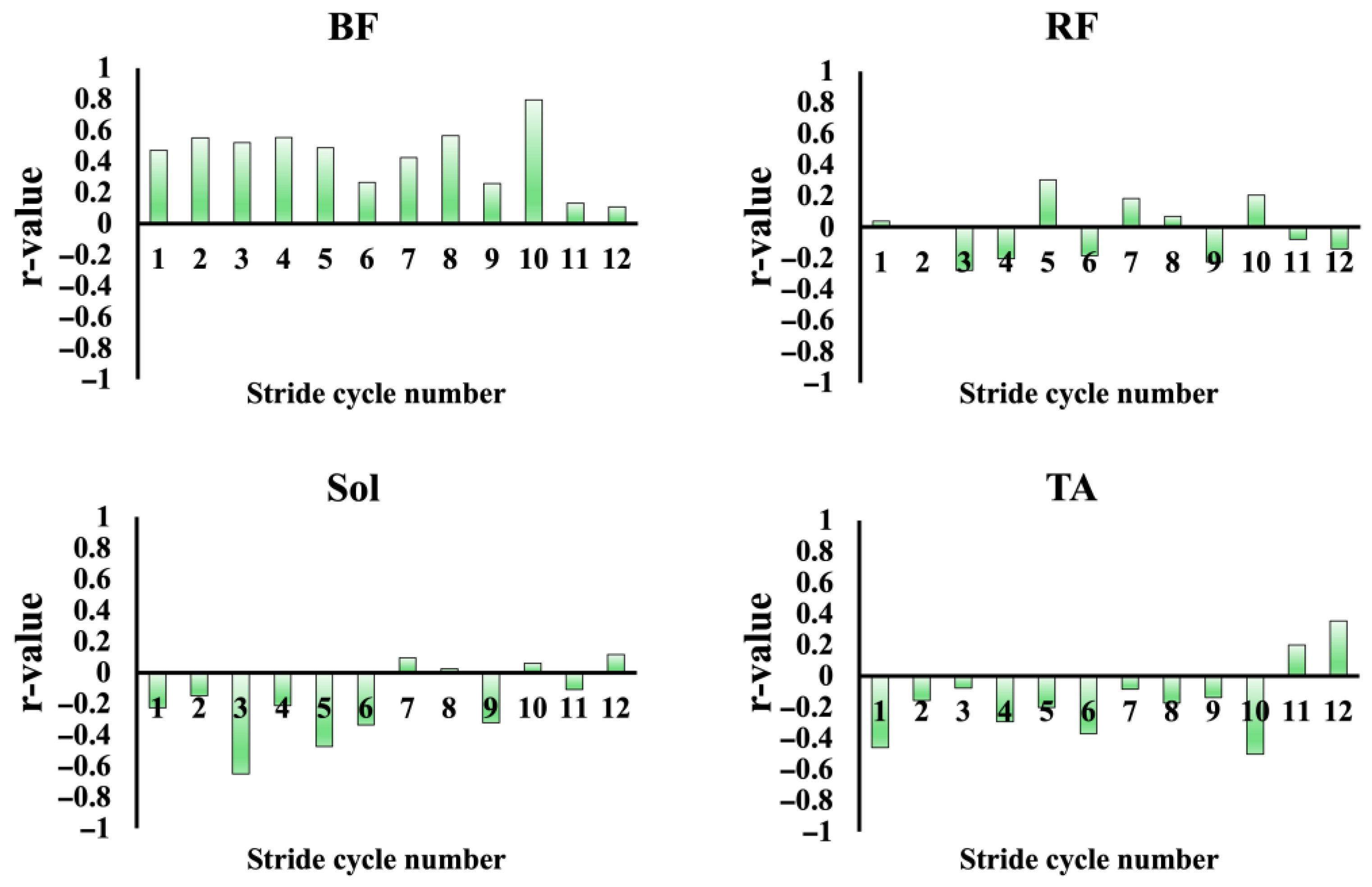
3.4.1. Relationship Between Muscle Activity and Sprint Performance Variables
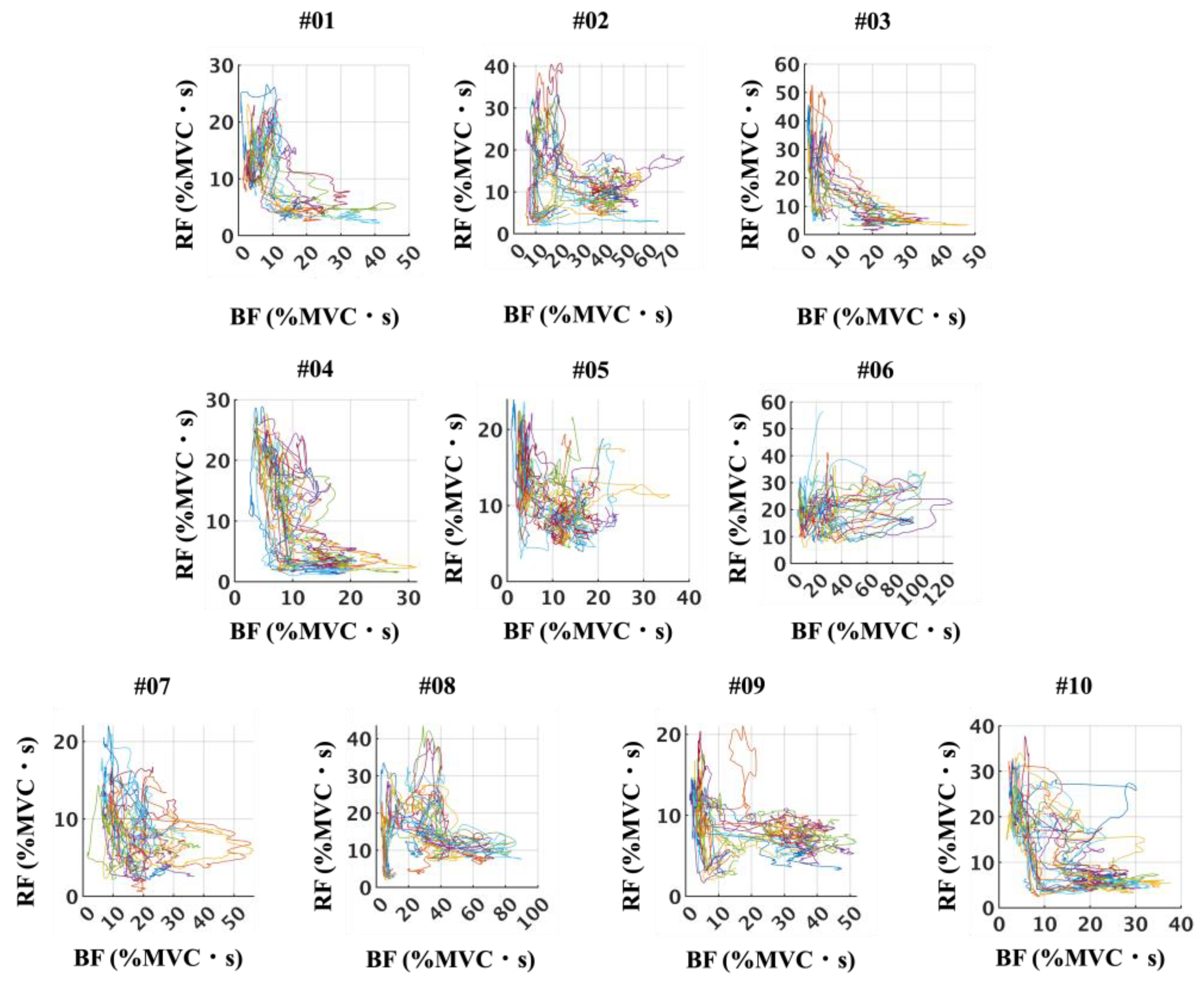
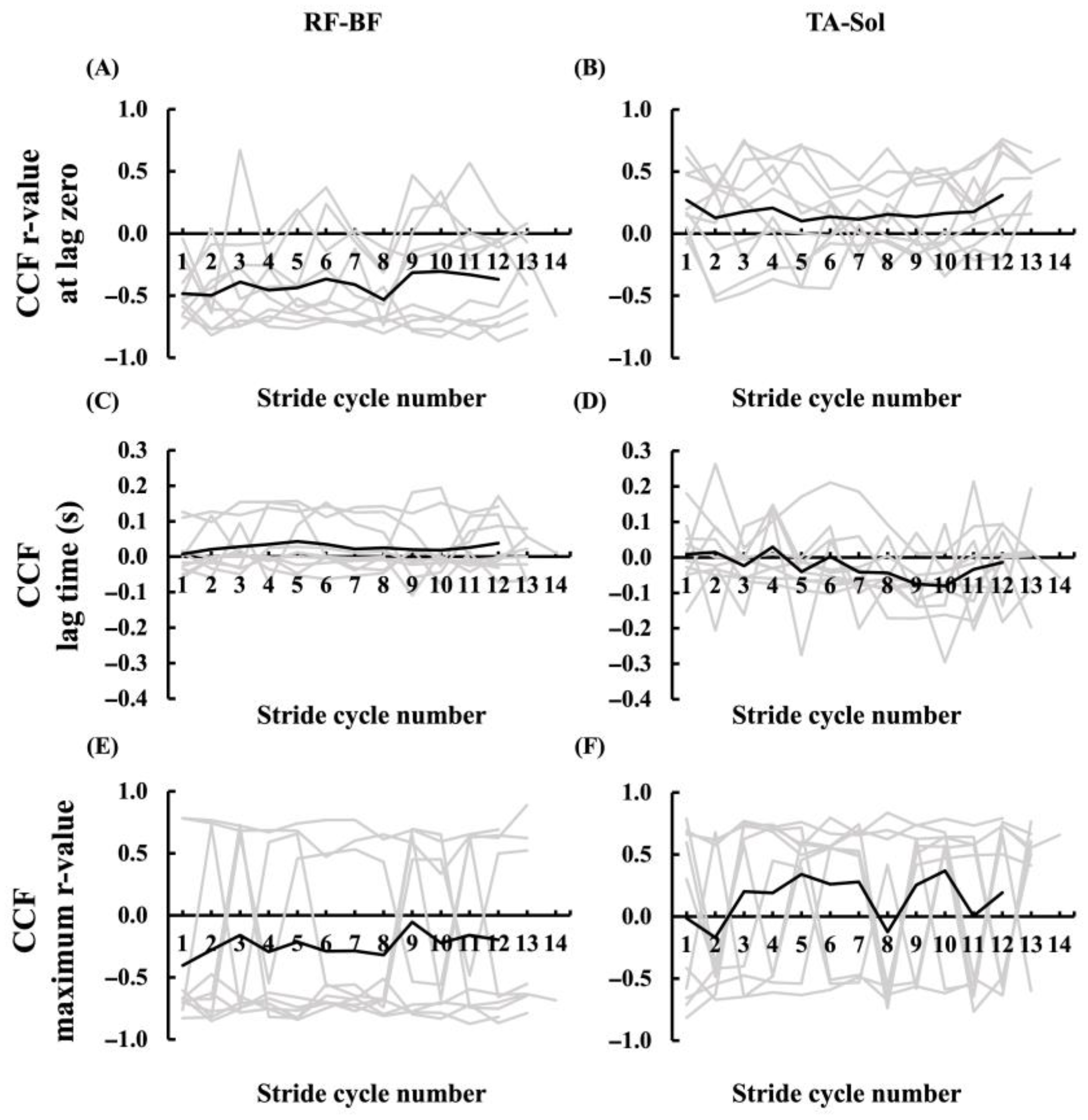
3.4.2. Muscle Activation Pattens of Agonist and Antagonist Muscles
4. Discussion
4.1. Wireless Transmission–Related Attenuation of sEMG Signals
4.2. Relationship Between Muscle Activity and Sprint Performance Variables
4.3. Agonist–Antagonist Coordination Patterns
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, Y.G.; Lee, S.; Park, J.U. Recent Progress in Wireless Sensors for Wearable Electronics. Sensors 2019, 19, 4353. [Google Scholar] [CrossRef]
- Howard, R.M.; Conway, R.; Harrison, A.J. Muscle activity in sprinting: A review. Sports Biomech. 2018, 17, 1–17. [Google Scholar] [CrossRef]
- Morin, J.B.; Gimenez, P.; Edouard, P.; Arnal, P.; Jimenez-Reyes, P.; Samozino, P.; Brughelli, M.; Mendiguchia, J. Sprint Acceleration Mechanics: The Major Role of Hamstrings in Horizontal Force Production. Front. Physiol. 2015, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Kakehata, G.; Goto, Y.; Iso, S.; Kanosue, K. Timing of Rectus Femoris and Biceps Femoris Muscle Activities in Both Legs at Maximal Running Speed. Med. Sci. Sports Exerc. 2021, 53, 643–652. [Google Scholar] [CrossRef]
- Kakehata, G.; Goto, Y.; Iso, S.; Kanosue, K. The Timing of Thigh Muscle Activity Is a Factor Limiting Performance in the Deceleration Phase of the 100-m Dash. Med. Sci. Sports Exerc. 2022, 54, 1002–1012. [Google Scholar] [CrossRef]
- Kakehata, G.; Goto, Y.; Yokoyama, H.; Iso, S.; Kanosue, K. Interlimb and Intralimb Coordination of Rectus Femoris and Biceps Femoris Muscles at Different Running Speeds. Med. Sci. Sports Exerc. 2023, 55, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, A.; Ono, T.; Kubota, J.; Okuwaki, T.; Fukubayashi, T. Functional differences in the activity of the hamstring muscles with increasing running speed. J. Sports Sci. 2010, 28, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Schache, A.G.; Dorn, T.W.; Blanch, P.D.; Brown, N.A.; Pandy, M.G. Mechanics of the human hamstring muscles during sprinting. Med. Sci. Sports Exerc. 2012, 44, 647–658. [Google Scholar] [CrossRef]
- Tam, N.; Santos-Concejero, J.; Coetzee, D.R.; Noakes, T.D.; Tucker, R. Muscle co-activation and its influence on running performance and risk of injury in elite Kenyan runners. J. Sports Sci. 2017, 35, 175–181. [Google Scholar] [CrossRef]
- Williams, J.J.; Roshinski, W.C.; Watso, J.C. Upper Leg Muscular Co-Contraction During Maximal-Speed Sprinting in Male Club Ice Hockey Athletes. Sports Med. Open 2025, 11, 1. [Google Scholar] [CrossRef]
- Kido, A.; Tanaka, N.; Stein, R.B. Spinal reciprocal inhibition in human locomotion. J. Appl. Physiol. 2004, 96, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Lestienne, F. Effects of inertial load and velocity on the braking process of voluntary limb movements. Exp. Brain Res. 1979, 35, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M. The coactivation of antagonist muscles. Can. J. Physiol. Pharmacol. 1981, 59, 733–747. [Google Scholar] [CrossRef]
- Benecke, R.; Meinck, H.M.; Conrad, B. Rapid goal-directed elbow flexion movements: Limitations of the speed control system due to neural constraints. Exp. Brain Res. 1985, 59, 470–477. [Google Scholar] [CrossRef]
- Heuer, H. Control of the dominant and nondominant hand: Exploitation and taming of nonmuscular forces. Exp. Brain Res. 2007, 178, 363–373. [Google Scholar] [CrossRef]
- Tyler, A.E.; Hutton, R.S. Was Sherrington right about co-contractions? Brain Res. 1986, 370, 171–175. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Nagahara, R.; Mizutani, M.; Matsuo, A.; Kanehisa, H.; Fukunaga, T. Association of Sprint Performance With Ground Reaction Forces During Acceleration and Maximal Speed Phases in a Single Sprint. J. Appl. Biomech. 2018, 34, 104–110. [Google Scholar] [CrossRef]
- Nagahara, R.; Kanehisa, H.; Matsuo, A.; Fukunaga, T. Are peak ground reaction forces related to better sprint acceleration performance? Sports Biomech. 2021, 20, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Carius, D.; Kugler, P.; Kuhwald, H.M.; Wollny, R. Absolute and relative intrasession reliability of surface EMG variables for voluntary precise forearm movements. J. Electromyogr. Kinesiol. 2015, 25, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Thelen, D.G.; Chumanov, E.S.; Best, T.M.; Swanson, S.C.; Heiderscheit, B.C. Simulation of biceps femoris musculotendon mechanics during the swing phase of sprinting. Med. Sci. Sports Exerc. 2005, 37, 1931–1938. [Google Scholar] [CrossRef]
- Chumanov, E.S.; Heiderscheit, B.C.; Thelen, D.G. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J. Biomech. 2007, 40, 3555–3562. [Google Scholar] [CrossRef]
- Schache, A.G.; Kim, H.J.; Morgan, D.L.; Pandy, M.G. Hamstring muscle forces prior to and immediately following an acute sprinting-related muscle strain injury. Gait Posture 2010, 32, 136–140. [Google Scholar] [CrossRef]
- Schache, A.G.; Blanch, P.D.; Dorn, T.W.; Brown, N.A.; Rosemond, D.; Pandy, M.G. Effect of running speed on lower limb joint kinetics. Med. Sci. Sports Exerc. 2011, 43, 1260–1271. [Google Scholar] [CrossRef]
- Higashihara, A.; Nagano, Y.; Ono, T.; Fukubayashi, T. Relationship between the peak time of hamstring stretch and activation during sprinting. Eur. J. Sport. Sci. 2016, 16, 36–41. [Google Scholar] [CrossRef]
- Mann, R.; Sprague, P. A kinetic analysis of the ground leg during sprint running. Res. Q. Exerc. Sport 1980, 51, 334–348. [Google Scholar] [CrossRef]
- Mattes, K.; Wolff, S.; Alizadeh, S. Kinematic Stride Characteristics of Maximal Sprint Running of Elite Sprinters—Verification of the “Swing-Pull Technique”. J. Hum. Kinet. 2021, 77, 15–24. [Google Scholar] [CrossRef]
- Smith, J.L.; Betts, B.; Edgerton, V.R.; Zernicke, R.F. Rapid ankle extension during paw shakes: Selective recruitment of fast ankle extensors. J. Neurophysiol. 1980, 43, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Bondy, B.; Prilutsky, B.I.; Cymbalyuk, G. Control of transitions between locomotor-like and paw shake-like rhythms in a model of a multistable central pattern generator. J. Neurophysiol. 2018, 120, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Prilutsky, B.I.; Parker, J.; Cymbalyuk, G.S.; Klishko, A.N. Emergence of Extreme Paw Accelerations During Cat Paw Shaking: Interactions of Spinal Central Pattern Generator, Hindlimb Mechanics and Muscle Length-Depended Feedback. Front. Integr. Neurosci. 2022, 16, 810139. [Google Scholar] [CrossRef] [PubMed]
- Hoy, M.G.; Zernicke, R.F.; Smith, J.L. Contrasting roles of inertial and muscle moments at knee and ankle during paw-shake response. J. Neurophysiol. 1985, 54, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Romano, C.; Schieppati, M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J. Physiol. 1989, 409, 451–471. [Google Scholar] [CrossRef]
- Tamaki, H.; Kitada, K.; Akamine, T.; Sakou, T.; Kurata, H. Electromyogram patterns during plantarflexions at various angular velocities and knee angles in human triceps surae muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]



| Participant Number | Height (m) | Body Mass (kg) | Age (yr) | Maximal Running Speed (m·s−1) | Maximal Cycle Frequency (Hz) | Maximal Cycle Stride Length (m) | Maximal Mean Anteroposterior (N/kg) | Sprint Event | Personal Record (in Seconds) |
|---|---|---|---|---|---|---|---|---|---|
| #01 | 1.732 | 65.3 | 27 | 9.18 | 2.36 | 4.22 | 5.18 | 110 mH | 15.49 |
| #02 | 1.663 | 64.4 | 26 | 10.05 | 2.46 | 4.37 | 5.99 | 100 m | 10.53 |
| #03 | 1.724 | 74.0 | 25 | 10.02 | 2.42 | 4.30 | 5.27 | 200 m | 21.42 |
| #04 | 1.708 | 64.7 | 21 | 9.30 | 2.26 | 4.74 | 6.20 | 400 mH | 58.1 |
| #05 | 1.628 | 59.3 | 22 | 8.23 | 2.13 | 4.06 | 5.28 | 400 mH | 64.28 |
| #06 | 1.714 | 66.1 | 19 | 9.07 | 2.38 | 4.25 | 4.77 | 110 mH | 15.74 |
| #07 | 1.629 | 68.0 | 21 | 9.91 | 2.53 | 4.18 | 5.52 | 100 m | 10.83 |
| #08 | 1.678 | 68.9 | 20 | 9.92 | 2.46 | 4.32 | 5.38 | 100 m | 11.46 |
| #09 | 1.828 | 71.0 | 19 | 10.01 | 2.24 | 4.74 | 6.50 | 100 m | 11.16 |
| #10 | 1.77 | 66.6 | 18 | 9.48 | 2.29 | 4.45 | 5.85 | 400 mH | 53.32 |
| mean | 1.707 | 66.83 | 21.8 | 9.52 | 2.35 | 4.36 | 5.59 | ||
| SD | 0.059 | 3.80 | 2.99 | 0.56 | 0.12 | 0.21 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokota, K.; Tamaki, H. Application of Wireless EMG Sensors for Assessing Agonist–Antagonist Muscle Activity During 50-m Sprinting in Athletes. Sensors 2025, 25, 6395. https://doi.org/10.3390/s25206395
Yokota K, Tamaki H. Application of Wireless EMG Sensors for Assessing Agonist–Antagonist Muscle Activity During 50-m Sprinting in Athletes. Sensors. 2025; 25(20):6395. https://doi.org/10.3390/s25206395
Chicago/Turabian StyleYokota, Kanta, and Hiroyuki Tamaki. 2025. "Application of Wireless EMG Sensors for Assessing Agonist–Antagonist Muscle Activity During 50-m Sprinting in Athletes" Sensors 25, no. 20: 6395. https://doi.org/10.3390/s25206395
APA StyleYokota, K., & Tamaki, H. (2025). Application of Wireless EMG Sensors for Assessing Agonist–Antagonist Muscle Activity During 50-m Sprinting in Athletes. Sensors, 25(20), 6395. https://doi.org/10.3390/s25206395







