Sensitivity Analysis of Localized Electrochemical Impedance Spectroscopy Towards Tomography-on-a-Chip
Abstract
1. Introduction
- The application of a two- or four-electrode measurement setup is to be compared, and the most effective setup is to be determined.
- To analyze the recorded data in the standard phosphate-buffered saline (PBS) solution.
- A discussion is to be conducted to determine which data processing method achieves the most efficient measurement procedure.
- A description is to be provided, based on electrochemical models, of which frequency range allows detection to be maximized (in the case of the two- or four-electrode measurement technique).
- To establish the conditions for detecting impedance inhomogeneities in chips.
- To observe modifications resulting from the changes to the geometry of the chip.
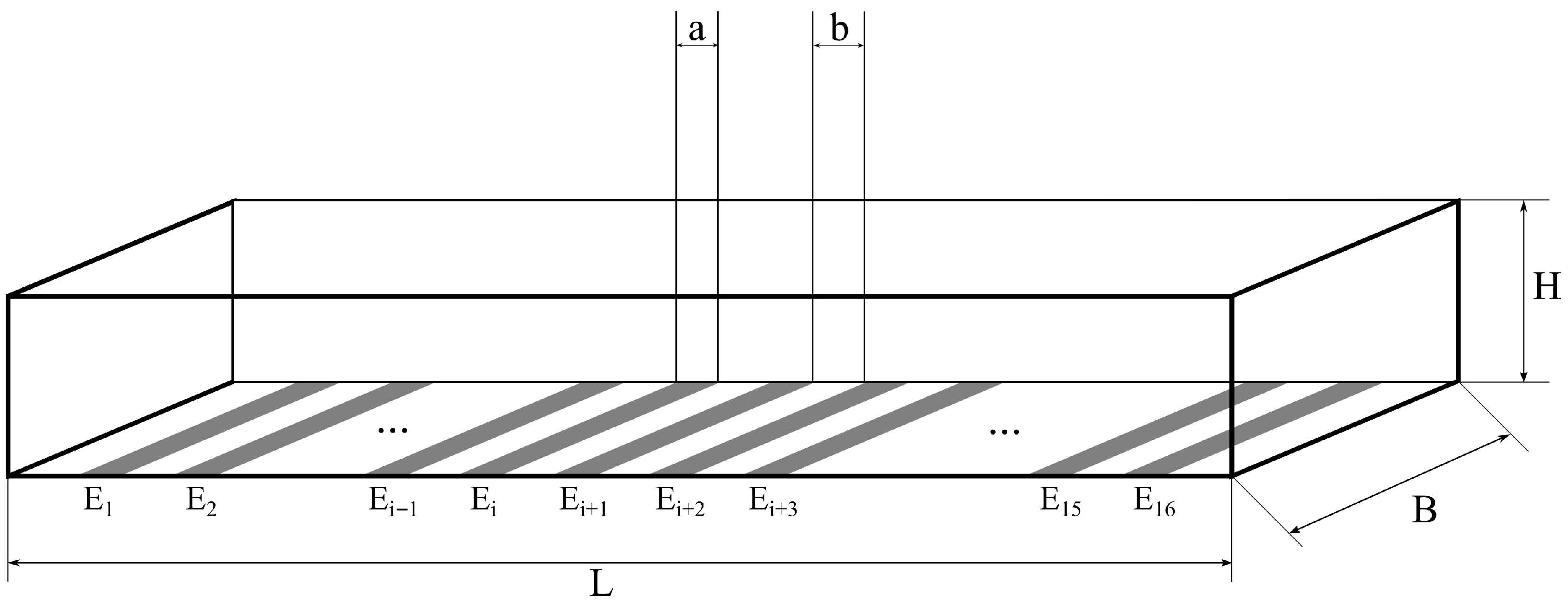
2. Materials and Methods
2.1. Materials
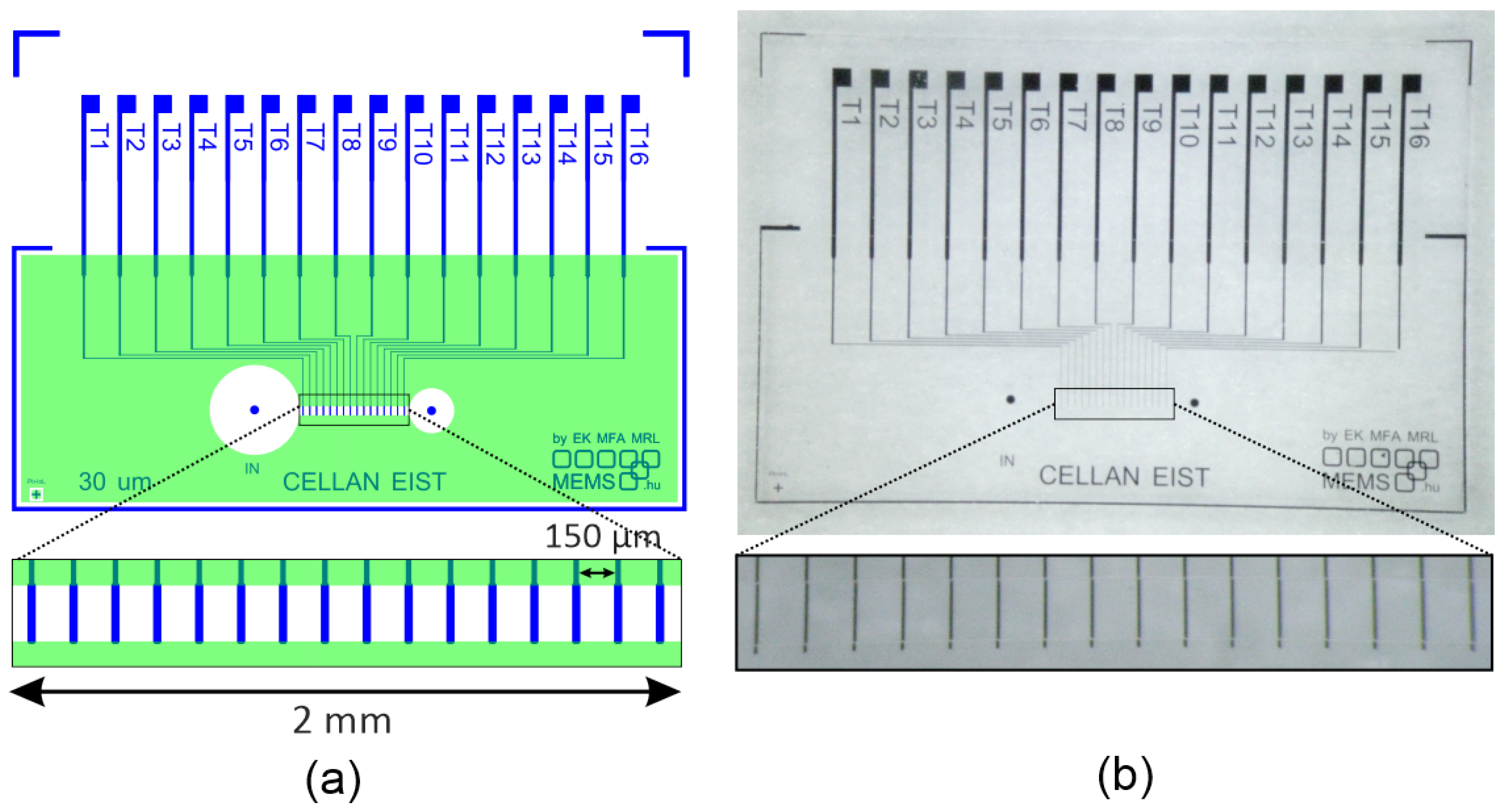
2.2. Sensor Fabrication
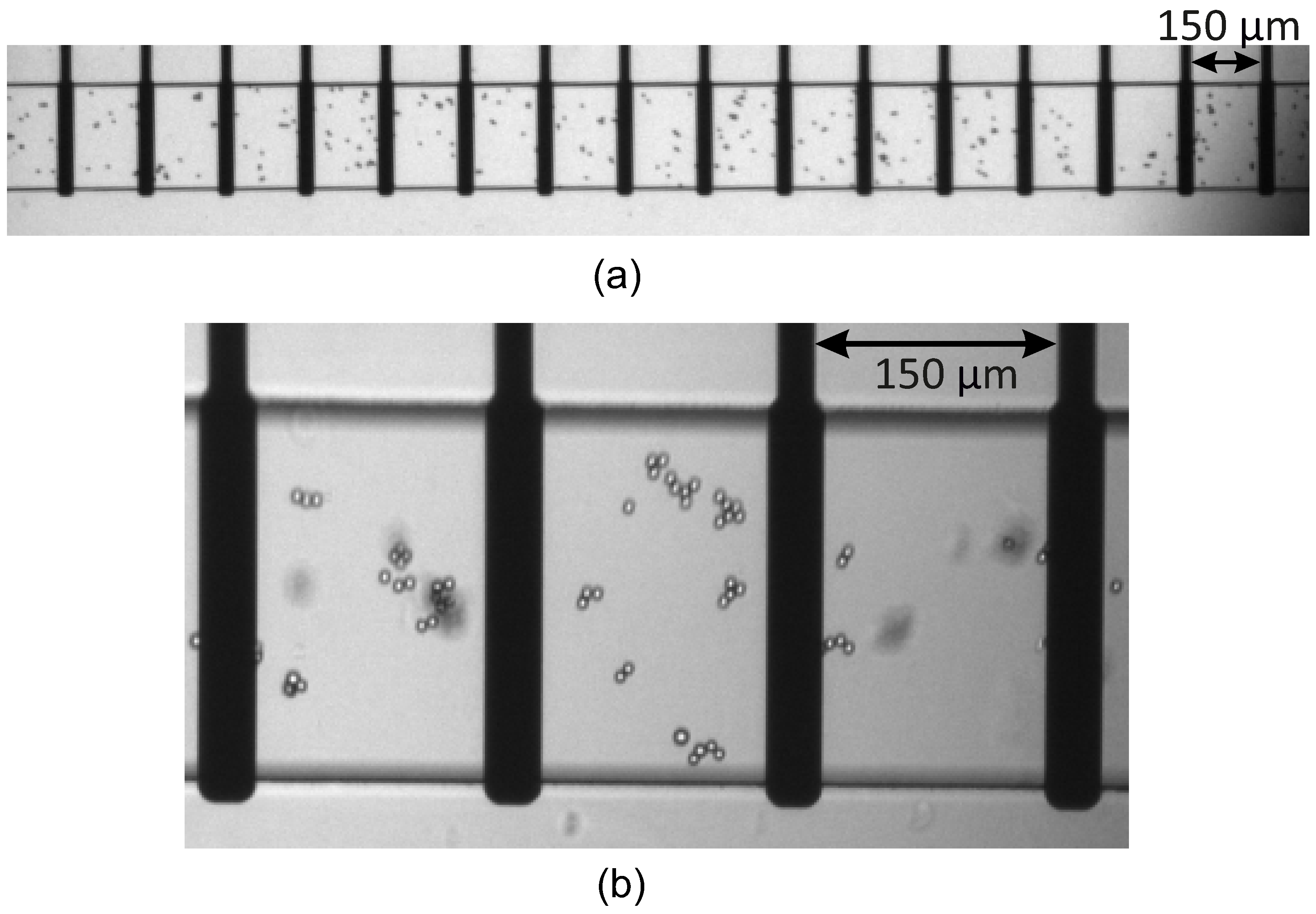
2.3. Microbeads Distributions and Visualisation by Optical Microscopy
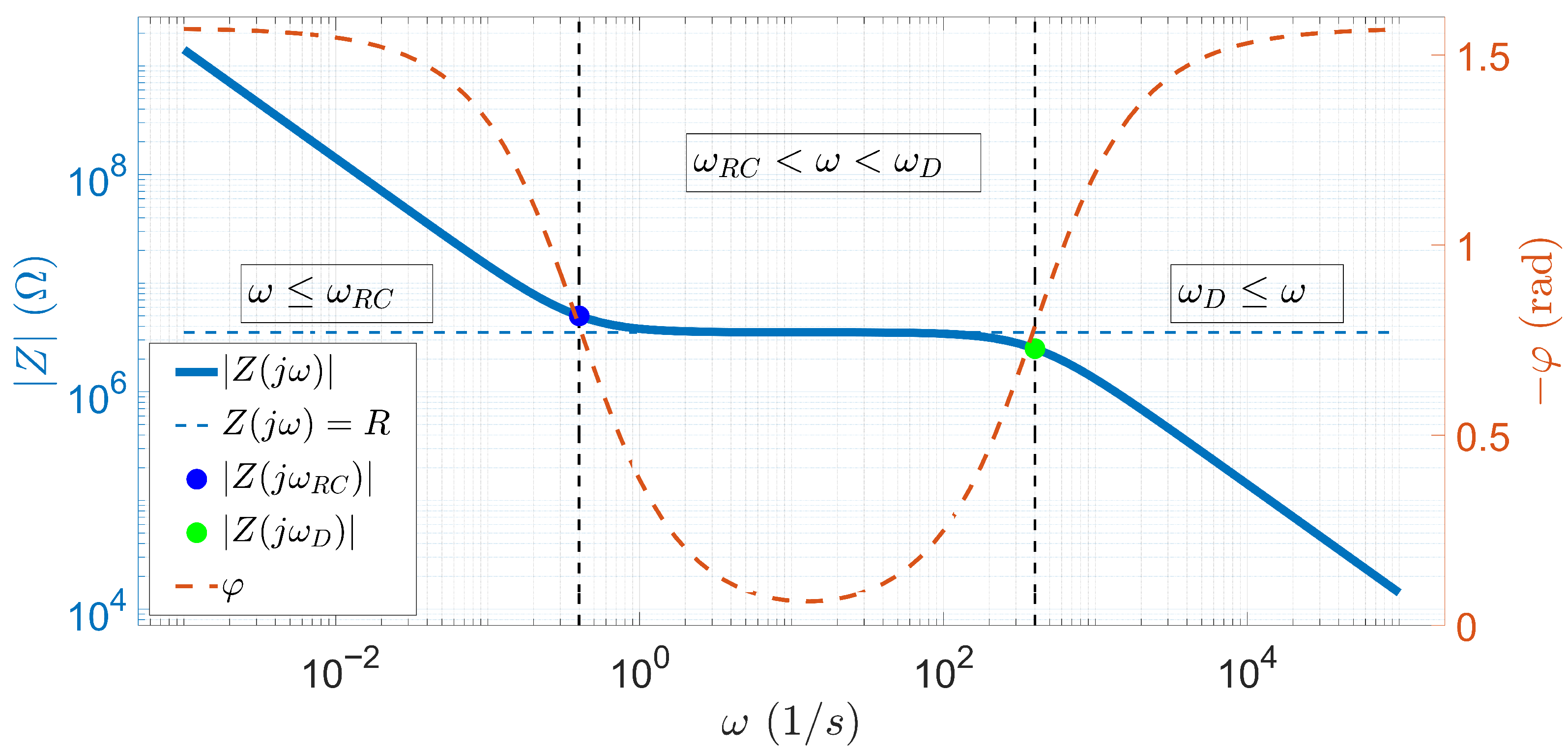
2.4. Basics of EIS Methods
- is the angular frequency obtained from the frequency of the excitation signal as
- R is the resistance of the electrochemical cell, and it can be written as
- is the timescale that characterizes the EDL formation process, and it can be expressed as
- is the Debye time during which the ions diffuse over a distance of the order of the Debye length, and it is given as
- is the product of the Boltzmann constant and room temperature;
- e is the is the elementary charge.
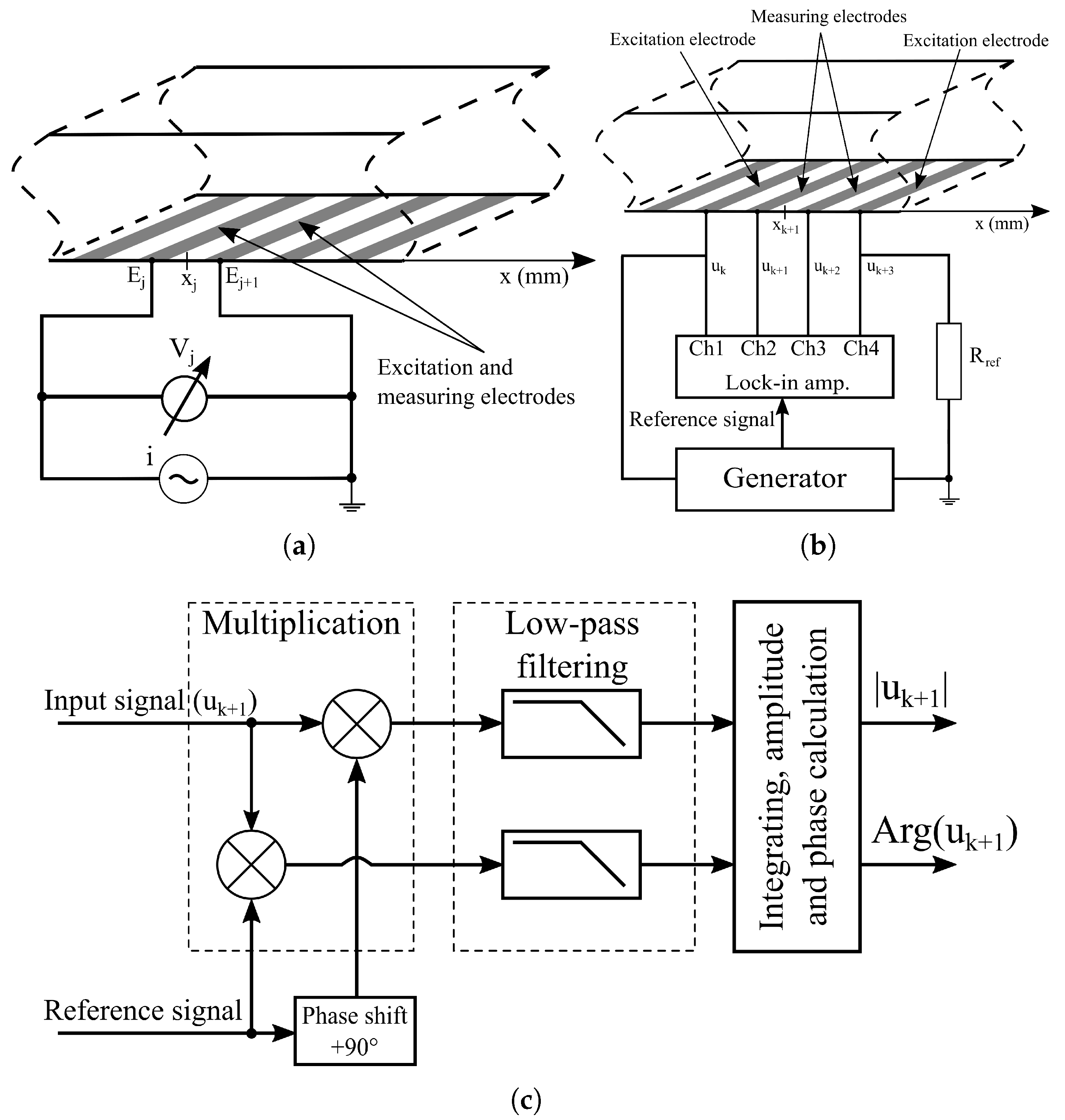
2.5. EIS Measurement Methods
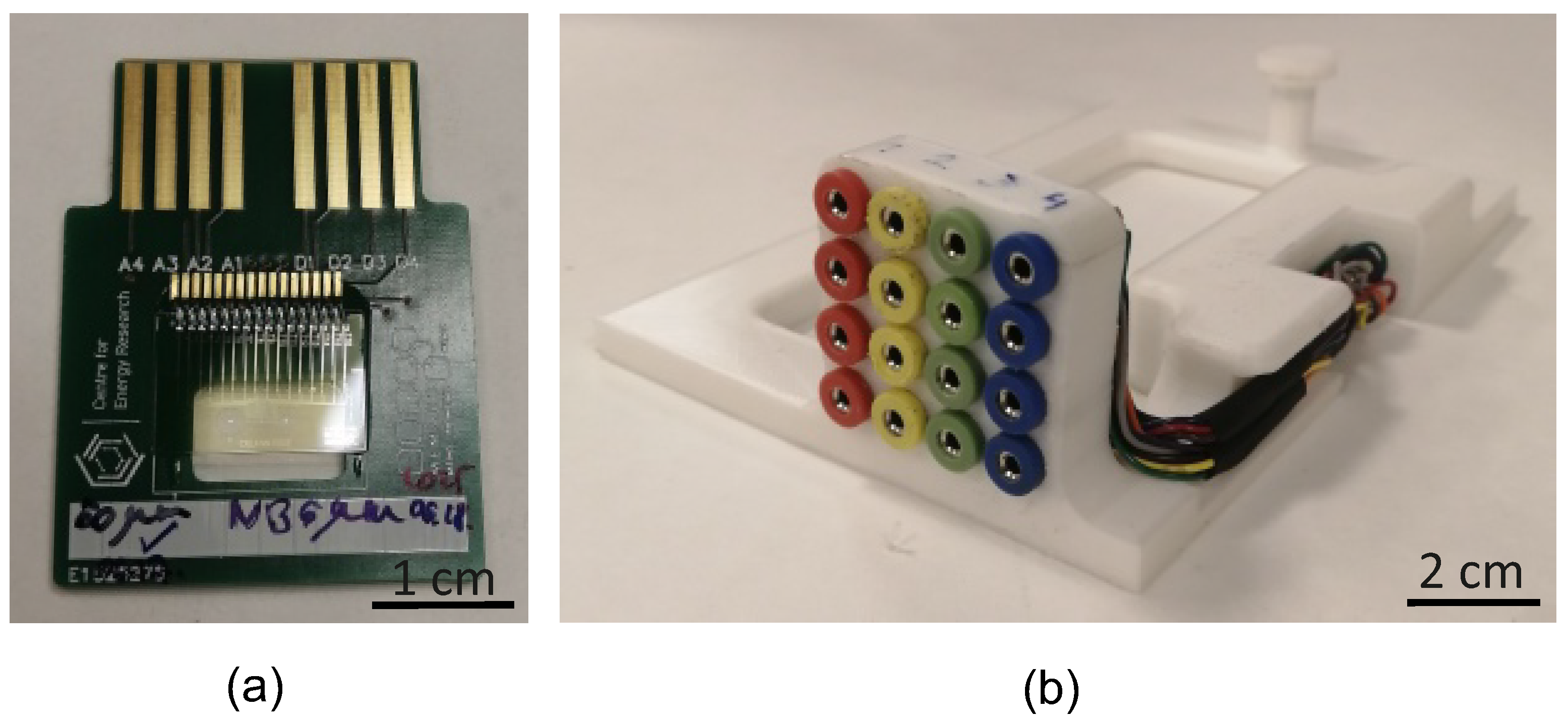
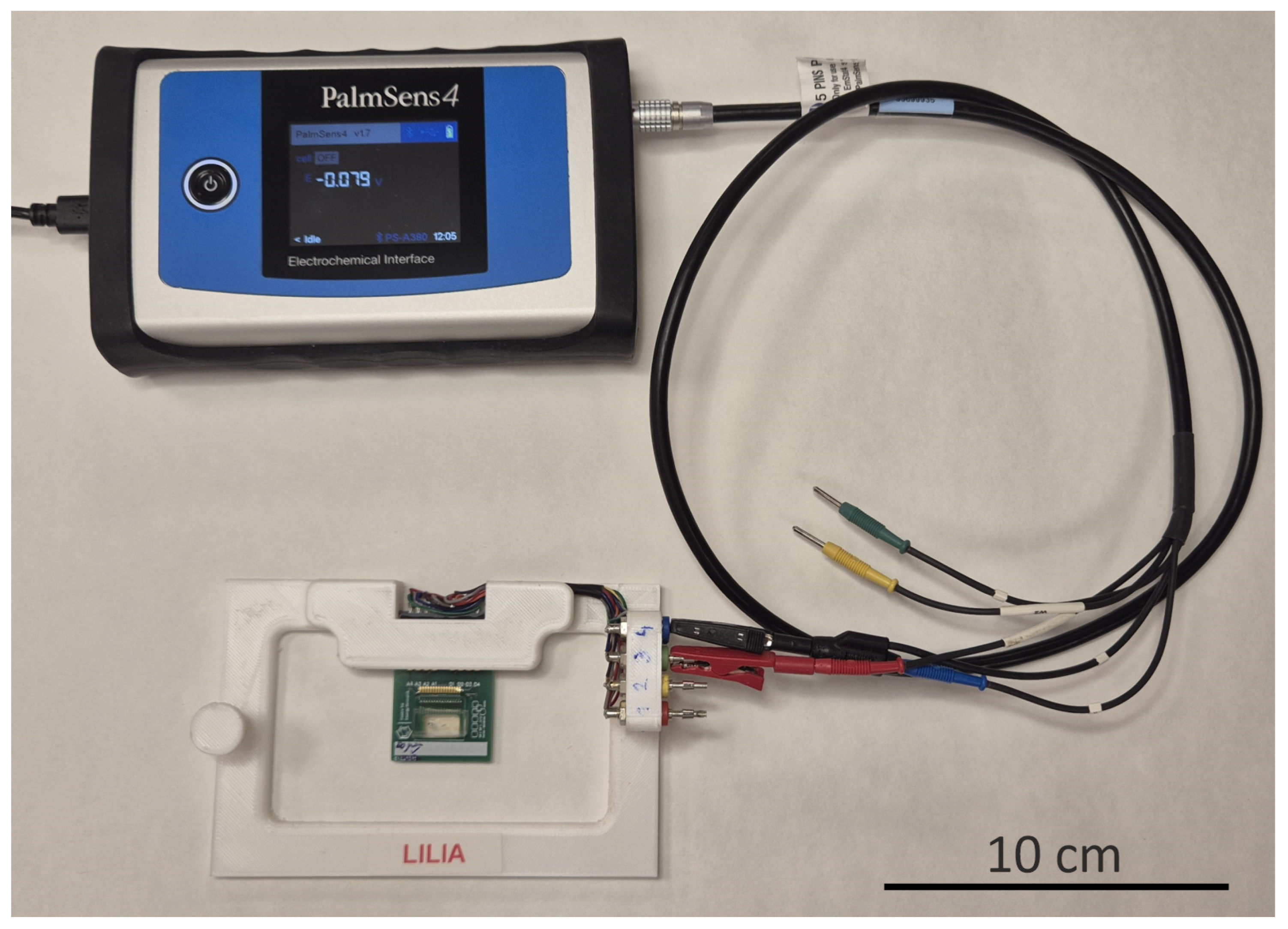
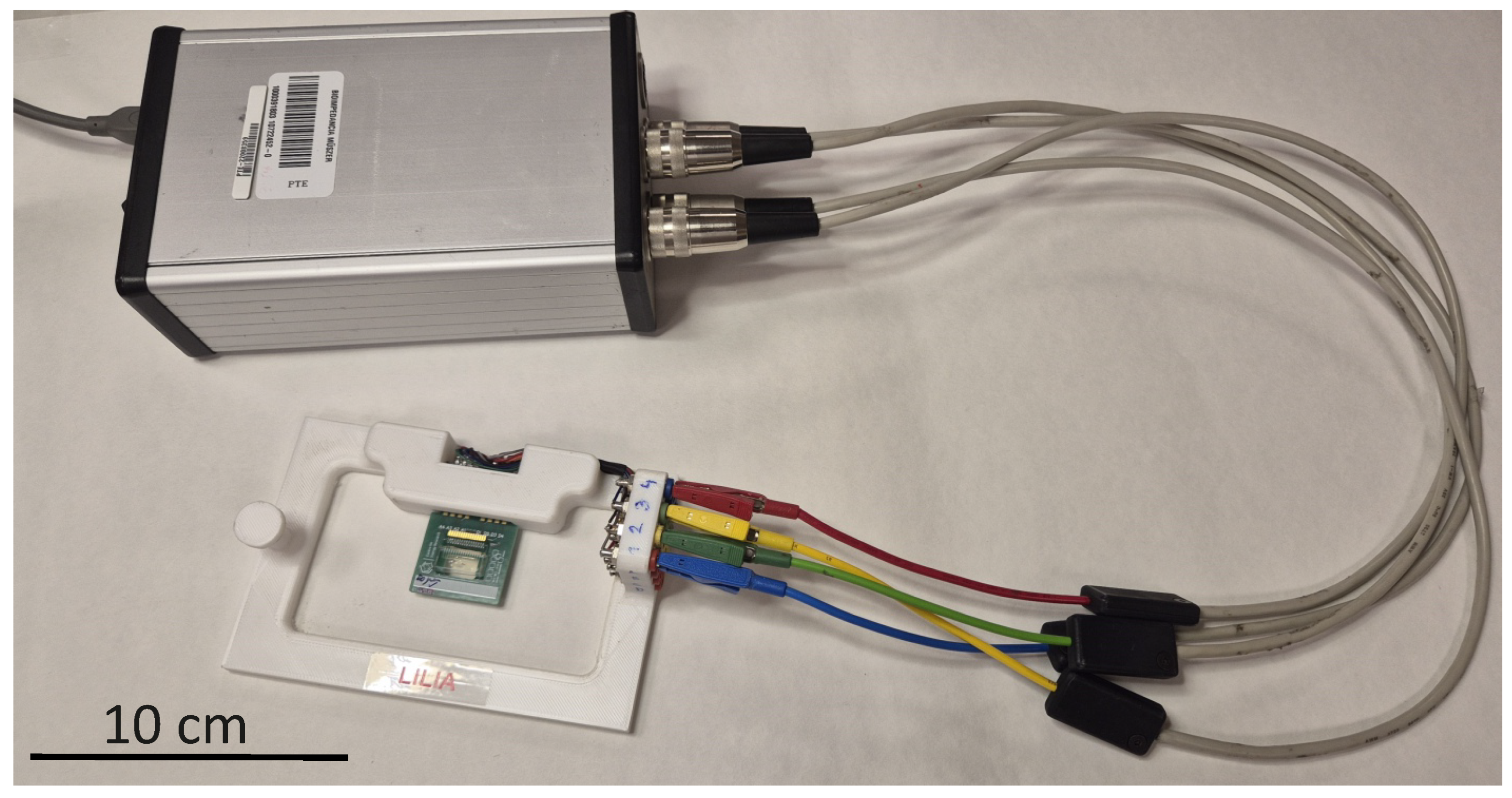
2.6. EIS Instrumentation
2.6.1. Two-Electrode Measurements
2.6.2. Four-Electrode Measurements
- -
- The excitation signal is a monochromatic sinus wave in the frequency range 1 MHz to 100 kHz, with total harmonic distortion plus noise suppression greater than 100 dB .
- -
- The excitation signal is provided by a voltage generator (the maximum noise level is 1.5 ).
- -
- The amplitude of the signal is manually damped between 0 and 60 dB. The default excitation (0 dB) is (the excitation current is limited to 1 mA).
- -
- The number of frequency points varies between 3 and 100 in the decades selected by the user.
- -
- The digital lock-in algorithm uses 32-bit AD and DA converters for excitation and measurement.
- -
- The measurement results (amplitude and phase) are calculated using IEEE 754 64-bit double precision floating-point operations [60].
- -
- The dynamic range of the measurements is at least 160 dB.
- -
- The accuracy (defined by the physical model, phantom [48]) is at least 1% for the resistive and at least 5% for the capacitive components.
- -
- Battery operation with a maximum of 6.5 h.
- -
- Currently, the main disadvantage of the technology is the offline data evaluation.
2.7. Data Collection and Evaluation Methods
2.8. Experimental Methodology
- Filling the microfluidic chip with PBS solution;
- Two-electrode impedance scanning for PBS solution;
- Four-electrode impedance scanning for PBS solution;
- Filling the chip with microbead solution;
- Covering the chip with paraffin oil;
- Two-electrode impedance scanning for microbead solution;
- Four-electrode impedance scanning for microbead solution;
- Cleaning the chip, returning to step (4), and repeating steps (5) and (6) after completion.
3. Results and Discussion
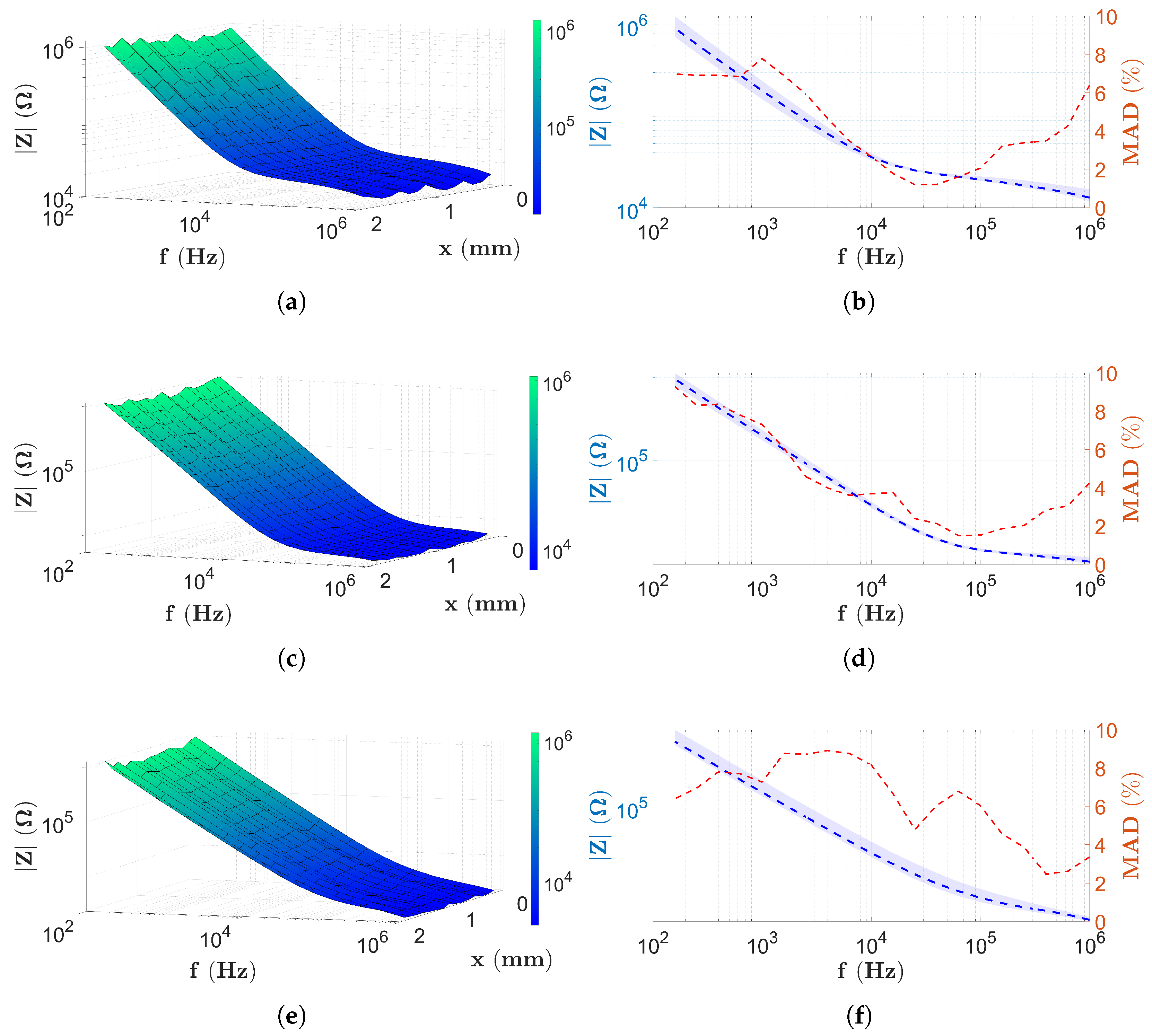
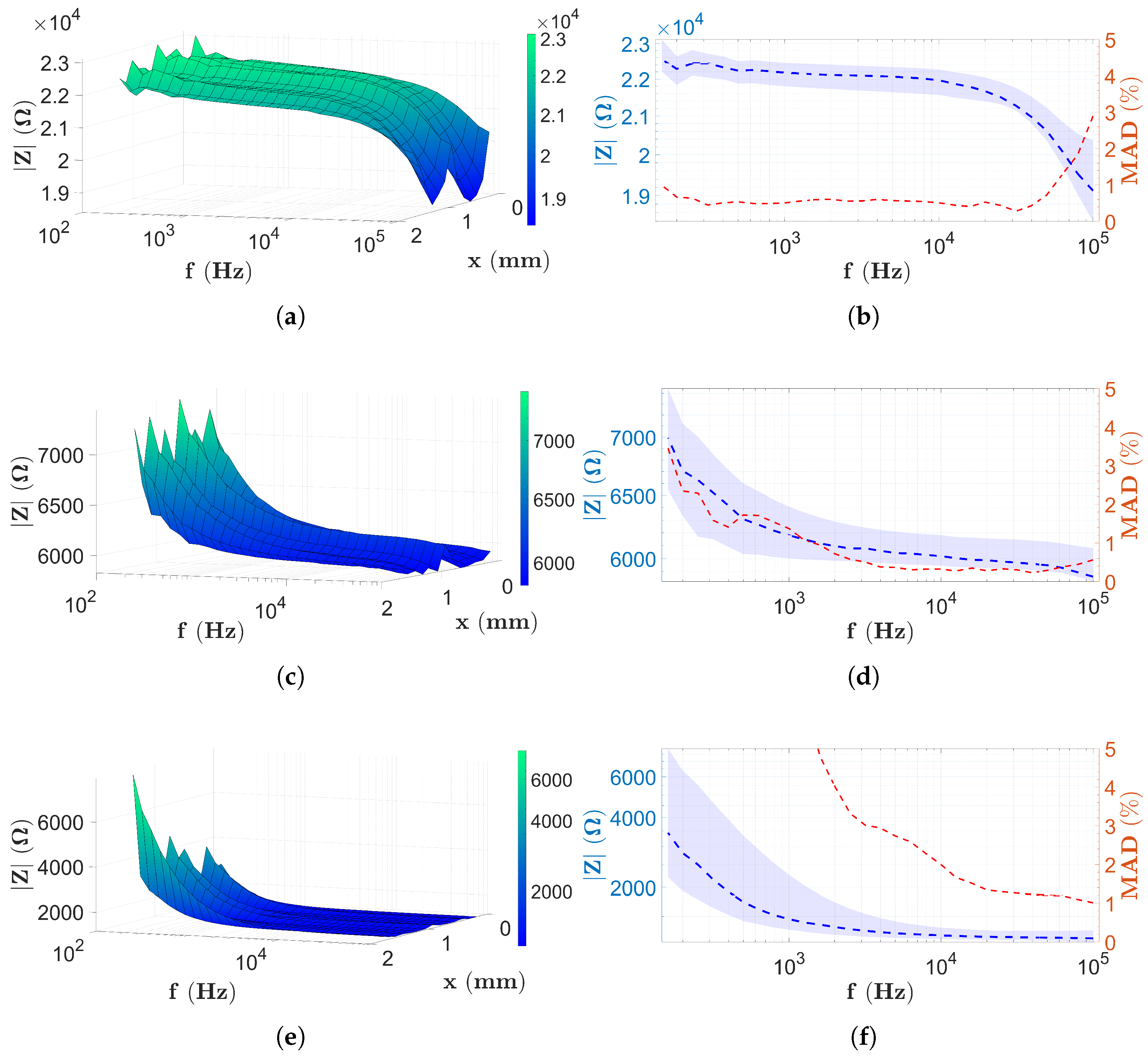
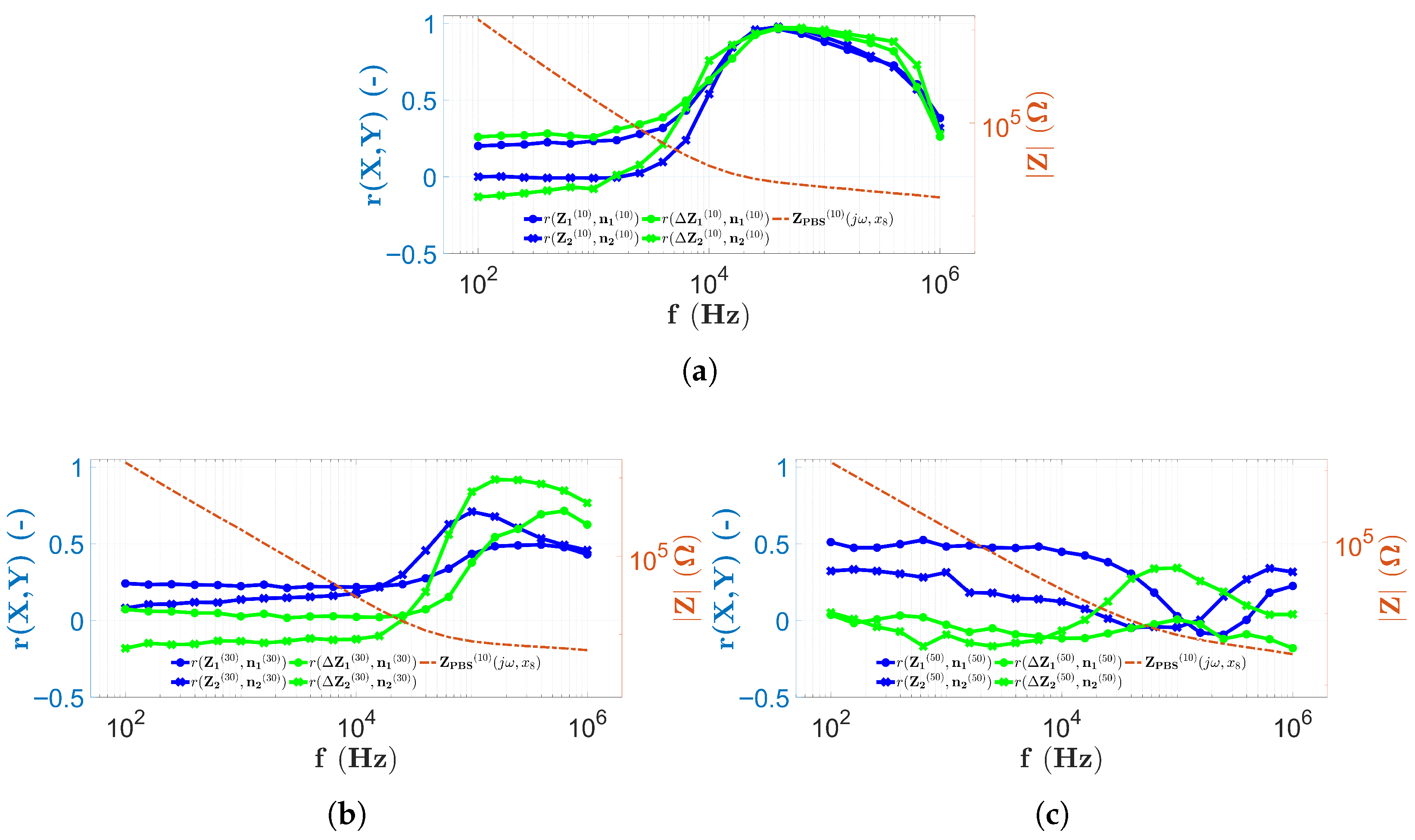
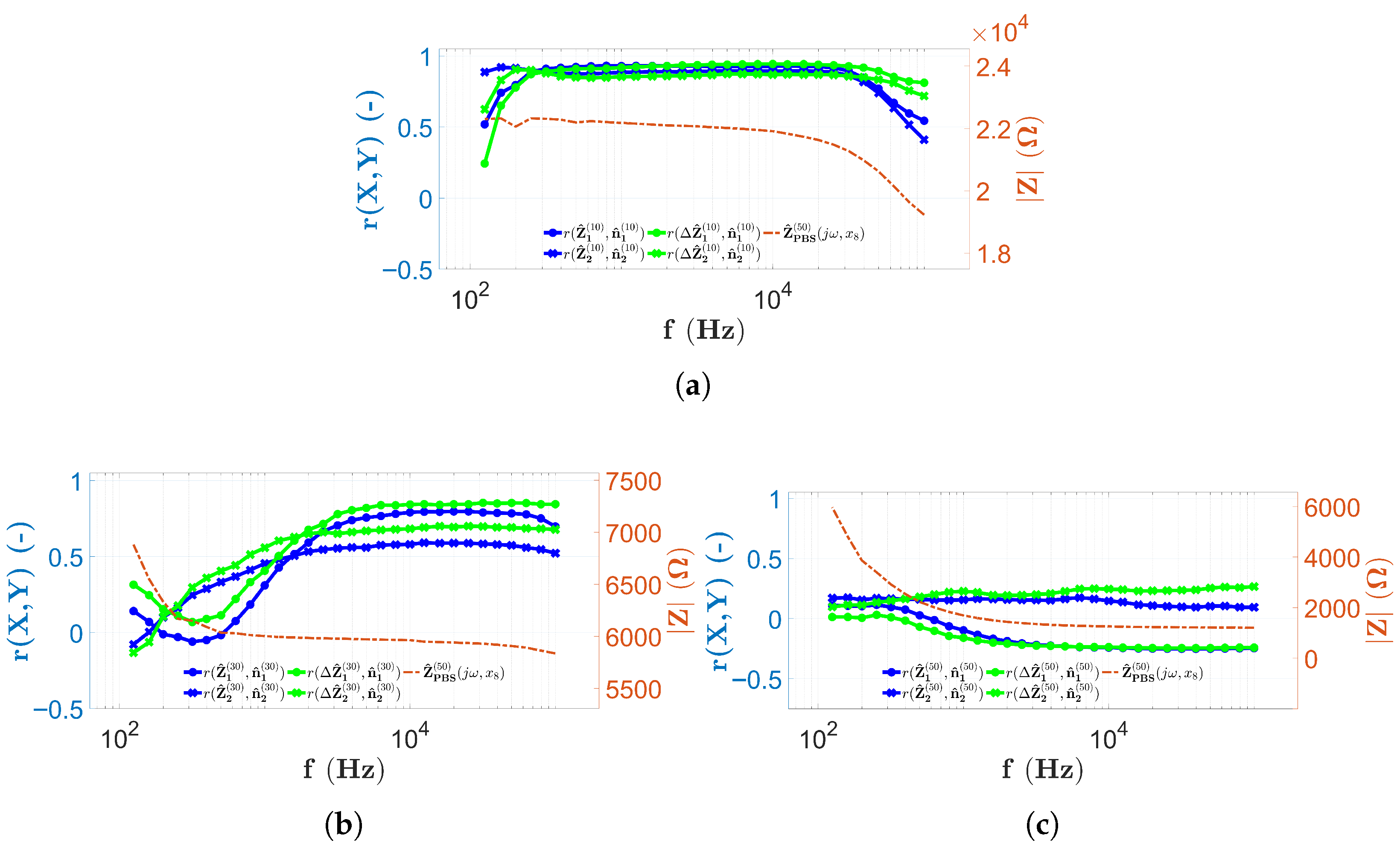
3.1. The Results in Case of the PBS Solution
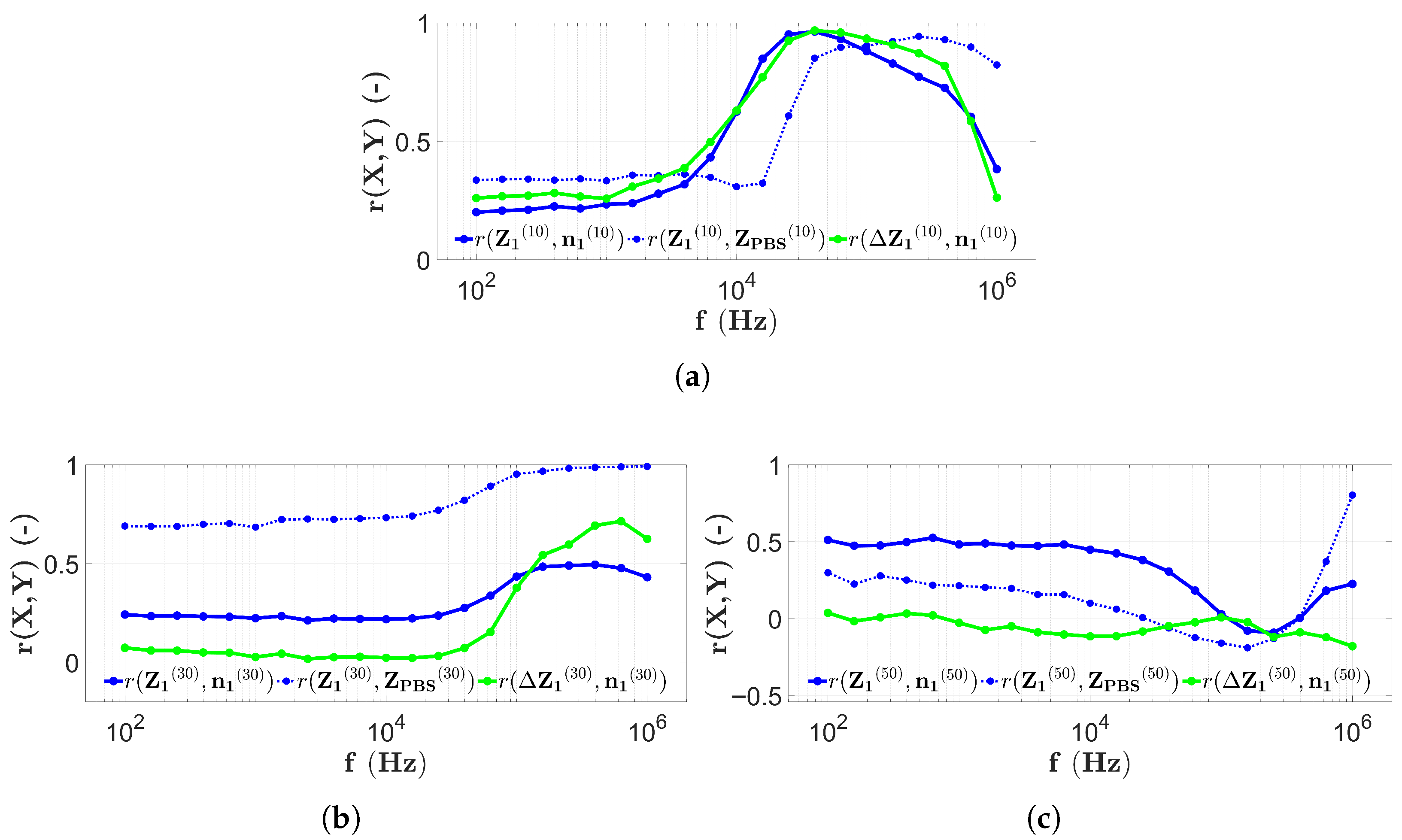
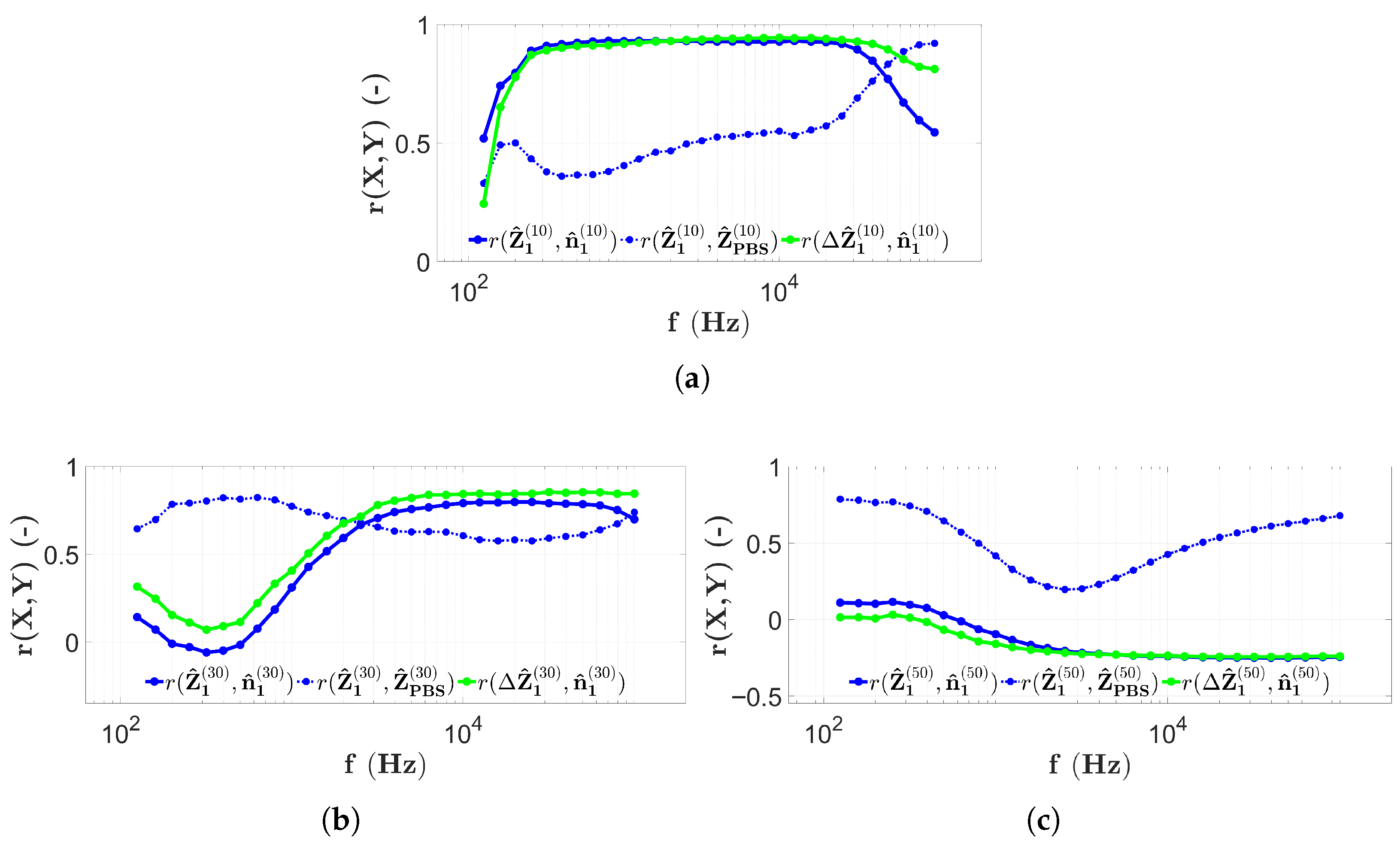
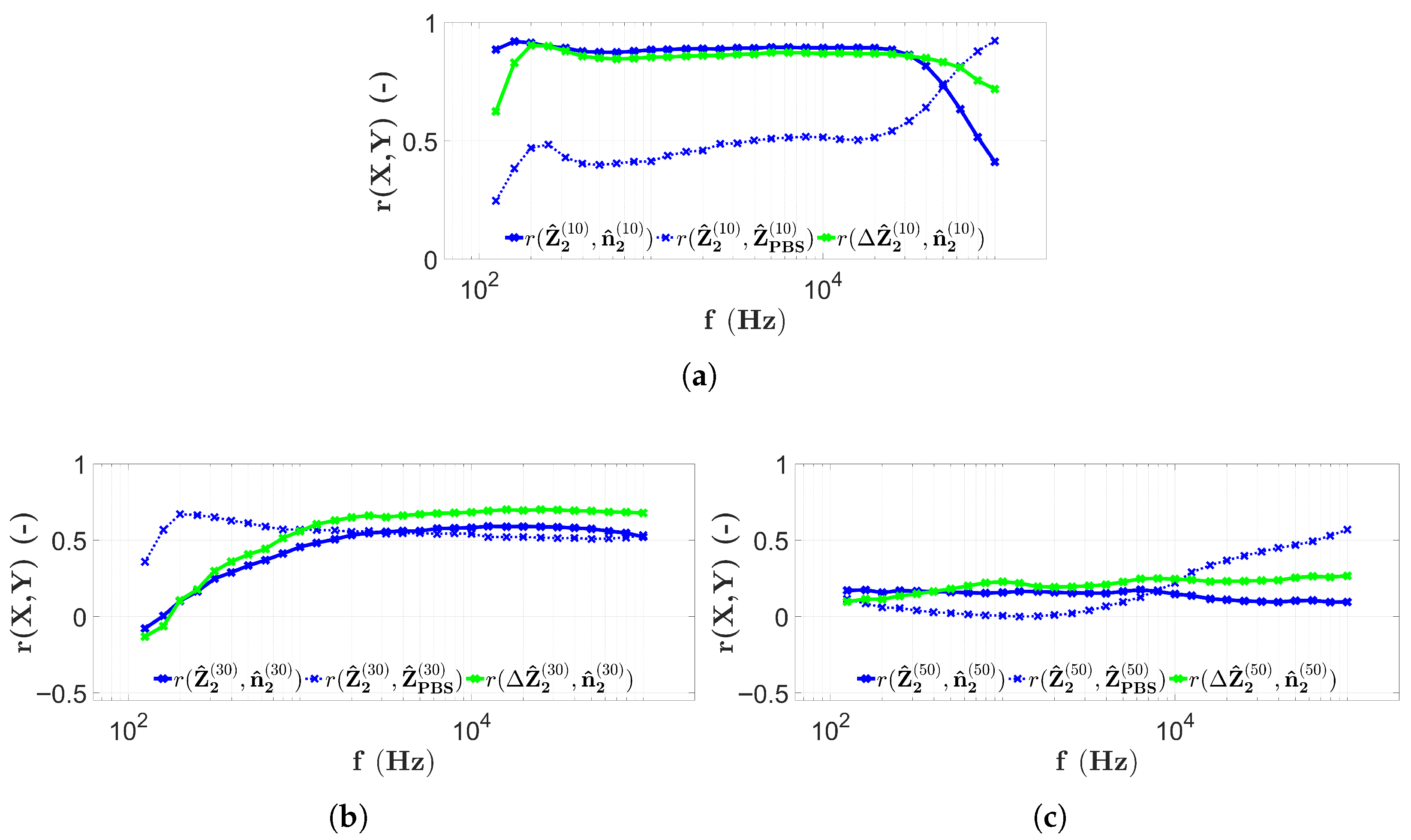
3.2. Results with Two-Electrode Measurements
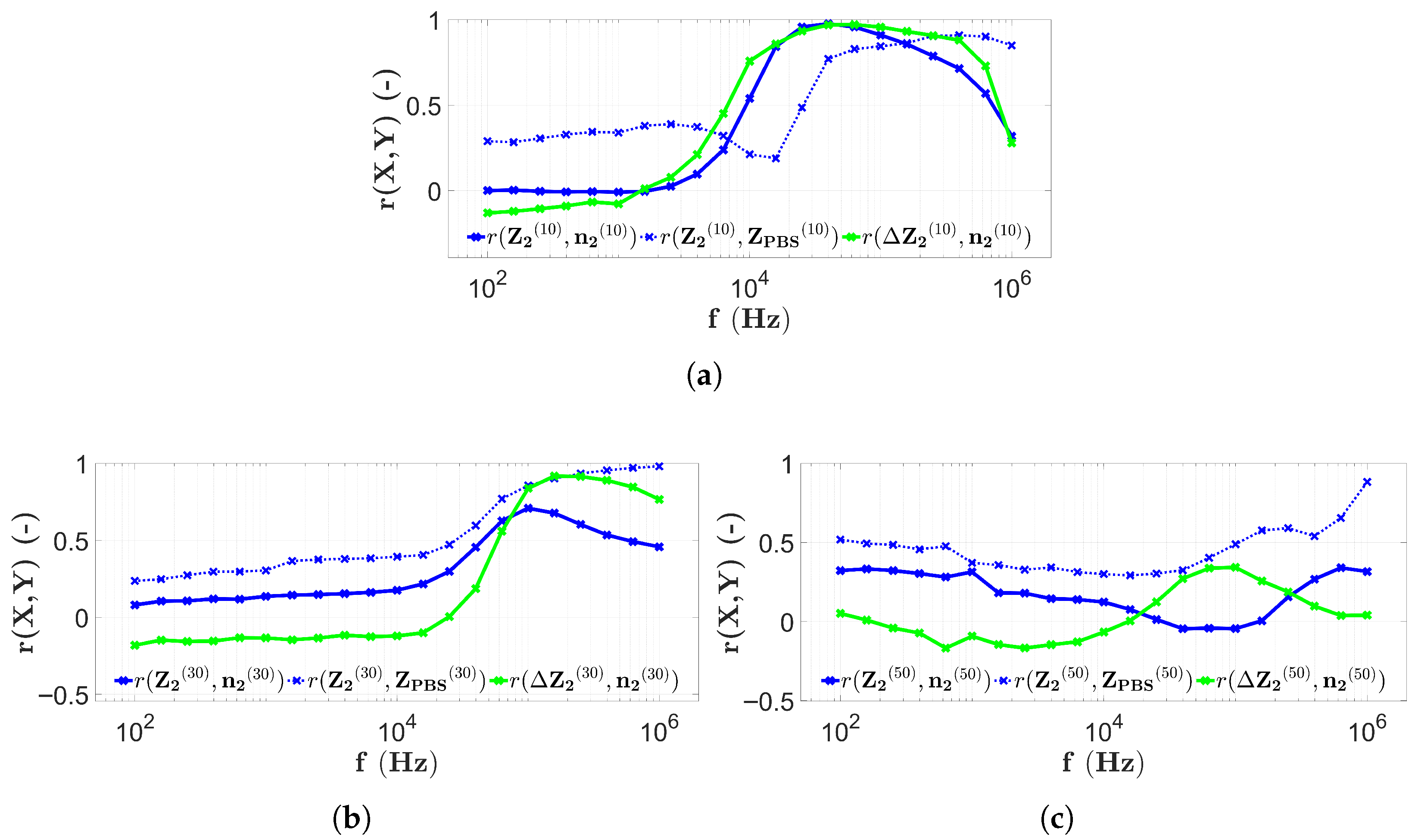
3.3. Results with Four-Electrode Measurements
3.4. Discussion
4. Conclusions and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ECIS | Electric Cell-substrate Impedance Sensing |
| EDL | Electric Double Layer |
| EIS | Elecrochemical impedance spectroscopy |
| EIT | Electrical impedance tomography |
| OoC | Organ-on-Chip |
| PBS | Phosphate buffered saline |
| PCB | Printed circuit board |
| PDMS | Polydimethylsiloxane |
| PDMS-b-PEO | Poly(dimethylsiloxane-b-ethylene oxide) |
| TEER | Trans-Epithelial/Endothelial Electrical 45 Resistance |
References
- Kumar, D.; Nadda, R.; Repaka, R. Advances and challenges in organ-on-chip technology: Toward mimicking human physiology and disease in vitro. Med. Biol. Eng. Comput. 2024, 62, 1925–1957. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2020, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Rim, Y.A.; Sohn, Y.; Nam, Y.; Ju, J.H. Replacing Animal Testing with Stem Cell-Organoids: Advantages and Limitations. Stem Cell Rev. Rep. 2024, 20, 1375–1386. [Google Scholar] [CrossRef]
- Gunasekaran, B.M.; Srinivasan, S.; Ezhilan, M.; Rajagopal, V.; Nesakumar, N. Advancements in Organ-on-a-Chip Systems: Materials, Characterization, and Applications. ChemistrySelect 2024, 9, e202403611. [Google Scholar] [CrossRef]
- Yuan, S.; Yuan, H.; Hay, D.C.; Hu, H.; Wang, C. Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology. Biosensors 2024, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Gangwal, A.; Lavecchia, A. Artificial intelligence in preclinical research: Enhancing digital twins and organ-on-chip to reduce animal testing. Drug Discov. Today 2025, 30, 104360. [Google Scholar] [CrossRef]
- Alghannam, F.; Alayed, M.; Alfihed, S.; Sakr, M.A.; Almutairi, D.; Alshamrani, N.; Al Fayez, N. Recent Progress in PDMS-Based Microfluidics Toward Integrated Organ-on-a-Chip Biosensors and Personalized Medicine. Biosensors 2025, 15, 76. [Google Scholar] [CrossRef]
- Tawade, P.; Mastrangeli, M. Integrated Electrochemical and Optical Biosensing in Organs-on-Chip. ChemBioChem 2023, 25, e202300560. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent Probes for the Visualization of Cell Viability. Accounts Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef]
- Schneckenburger, H.; Weber, P.; Wagner, M.; Schickinger, S.; Richter, V.; Bruns, T.; Strauss, W.; Witting, R. Light exposure and cell viability in fluorescence microscopy. J. Microsc. 2011, 245, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Jan, M.S.; Chou, C.K.; Chen, P.H.; Ke, N.J. Is Green Fluorescent Protein Toxic to the Living Cells? Biochem. Biophys. Res. Commun. 1999, 260, 712–717. [Google Scholar] [CrossRef]
- Zhu, Y.; Mandal, K.; Hernandez, A.L.; Kawakita, S.; Huang, W.; Bandaru, P.; Ahadian, S.; Kim, H.J.; Jucaud, V.; Dokmeci, M.R.; et al. State of the art in integrated biosensors for organ-on-a-chip applications. Curr. Opin. Biomed. Eng. 2021, 19, 100309. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef]
- Yang, P.; Zheng, F.; Li, Q.; Tian, T.; Zhang, G.; Wu, L.; Mao, H. An easy-repeat method to build a blood–brain barrier model on a chip with independent TEER detection module. Chin. J. Anal. Chem. 2021, 50, 97–101. [Google Scholar] [CrossRef]
- Henry, O.Y.F.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab A Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab A Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef]
- Malik, M.; Steele, S.A.; Mitra, D.; Long, C.J.; Hickman, J.J. Trans-epithelial/endothelial electrical resistance (TEER): Current state of integrated TEER measurements in organ-on-a-chip devices. Curr. Opin. Biomed. Eng. 2025, 34, 100588. [Google Scholar] [CrossRef]
- Schaller, L.; Hofmann, K.; Geiger, F.; Dietrich, A. Electrical cell-substrate impedance sensing (ECIS) in lung biology and disease. Appl. Res. 2024, 3, e202400059. [Google Scholar] [CrossRef]
- Tran, T.B.; Cho, S.; Min, J. Hydrogel-based diffusion chip with electric cell-substrate impedance sensing (ECIS) integration for cell viability assay and drug toxicity screening. Biosens. Bioelectron. 2013, 50, 453–459. [Google Scholar] [CrossRef]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, Z.; Zhang, Z.; Xu, F.; Marchisio, M.A.; Wang, Z.; Pan, D.; Zhao, X.; Huang, Q.A. Design and 3D modeling investigation of a microfluidic electrode array for electrical impedance measurement of single yeast cells. Electrophoresis 2021, 42, 1996–2009. [Google Scholar] [CrossRef]
- León, S.E.D.; Pupovac, A.; McArthur, S.L. Three-dimensional (3D) cell culture monitoring: Opportunities and challenges for impedance spectroscopy. Biotechnol. Bioeng. 2020, 117, 1230–1240. [Google Scholar] [CrossRef]
- Chen, Y.S.; Huang, C.H.; Pai, P.C.; Seo, J.; Lei, K.F. A Review on Microfluidics-Based Impedance Biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef]
- Rapier, C.E.; Jagadeesan, S.; Vatine, G.; Ben-Yoav, H. Microfluidic channel sensory system for electro-addressing cell location, determining confluency, and quantifying a general number of cells. Sci. Rep. 2022, 12, 3248. [Google Scholar] [CrossRef]
- Ngoc Le, H.T.; Kim, J.; Park, J.; Cho, S. A Review of Electrical Impedance Characterization of Cells for Label-Free and Real-Time Assays. BioChip J. 2019, 13, 295–305. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Ren, T.; Wen, J.; Li, J.; Tang, T. Recent Advances in Microfluidic Impedance Detection: Principle, Design and Applications. Micromachines 2025, 16, 683. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Geng, Y.; Zhang, Z.; Fu, J.; Ouyang, J.; Zhu, Z. Equivalent Circuit Modeling and Analysis for Microfluidic Electrical Impedance Monitoring of Single-Cell Growth. Biosensors 2025, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, S.; Liu, K.; Chen, B.; Wu, H.; Jia, J.; Yao, J. A hybrid genetic algorithm and Levenberg–Marquardt (GA–LM) method for cell suspension measurement with electrical impedance spectroscopy. Rev. Sci. Instrum. 2020, 91, 124104. [Google Scholar] [CrossRef]
- Gershon, H.; Gershon, D. The budding yeast, Saccharomyces cerevisiae, as a model for aging research: A critical review. Mech. Ageing Dev. 2000, 120, 1–22. [Google Scholar] [CrossRef]
- Van den Eeckhoudt, R.; Christiaens, A.S.; Ceyssens, F.; Vangalis, V.; Verstrepen, K.J.; Boon, N.; Tavernier, F.; Kraft, M.; Taurino, I. Full-electric microfluidic platform to capture, analyze and selectively release single cells. Lab A Chip 2023, 23, 4276–4286. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Liu, K.; Lan, T.; Wang, Z.; Zhu, Z. Recent advances in electrical impedance sensing technology for single-cell analysis. Biosensors 2021, 11, 470. [Google Scholar] [CrossRef]
- Tsai, S.L.; Wang, M.H. 24 h observation of a single HeLa cell by impedance measurement and numerical modeling. Sens. Actuators B Chem. 2016, 229, 225–231. [Google Scholar] [CrossRef]
- Mansoorifar, A.; Koklu, A.; Ma, S.; Raj, G.V.; Beskok, A. Electrical impedance measurements of biological cells in response to external stimuli. Anal. Chem. 2018, 90, 4320–4327. [Google Scholar] [CrossRef]
- Kargupta, R.; Puttaswamy, S.; Lee, A.J.; Butler, T.E.; Li, Z.; Chakraborty, S.; Sengupta, S. Rapid culture-based detection of living mycobacteria using microchannel electrical impedance spectroscopy (m-EIS). Biol. Res. 2017, 50, 21. [Google Scholar] [CrossRef] [PubMed]
- Pitruzzello, G.; Johnson, S.; Krauss, T.F. Exploring the fundamental limit of antimicrobial susceptibility by near-single-cell electrical impedance spectroscopy. Biosens. Bioelectron. 2023, 224, 115056. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.D.; Keidar, M. Progressive Approaches in Oncological Diagnosis and Surveillance: Real-Time Impedance-Based Techniques and Advanced Algorithms. Bioelectromagnetics 2025, 46, e22540. [Google Scholar] [CrossRef]
- Stupin, D.D.; Kuzina, E.A.; Abelit, A.A.; Emelyanov, A.K.; Nikolaev, D.M.; Ryazantsev, M.N.; Koniakhin, S.V.; Dubina, M.V. Bioimpedance spectroscopy: Basics and applications. ACS Biomater. Sci. Eng. 2021, 7, 1962–1986. [Google Scholar] [CrossRef]
- Schwarz, M.; Jendrusch, M.; Constantinou, I. Spatially resolved electrical impedance methods for cell and particle characterization. Electrophoresis 2019, 41, 65–80. [Google Scholar] [CrossRef]
- Peng, P.; Fu, Y.; Che, B.; Li, X.; Liu, L.; Sun, J.; Luo, T.; Deng, L. Development and evaluation of an electrical impedance tomography (EIT) sensor for real-time monitoring of hemolysis dynamics. Anal. Chim. Acta 2025, 1350, 343812. [Google Scholar] [CrossRef] [PubMed]
- Weiz, S.M.; Jha, P.; Lee, K.; Herzer, R.; Schmidt, O.G.; Medina-Sánchez, M. Single-Cell Impedance Tomography Using Rolled-Up Microtubular Sensors. Adv. Mater. Technol. 2023, 8, 2300724. [Google Scholar] [CrossRef]
- Kokabi, M.; Rather, G.M.; Javanmard, M. Ionic Cell Microscopy: A new modality for visualizing cells using microfluidic impedance cytometry and generative artificial intelligence. Biosens. Bioelectron. X 2025, 24, 100619. [Google Scholar] [CrossRef]
- Ngamdi, N.; Sriwilai, J.; Trongwongsa, T.; Ouypornkochagorn, T. A Development of Electrode Probes for Imaging Precancerous Lesions with Electrical Impedance Tomography Technique: A Phantom Study. J. Appl. Sci. Eng. 2023, 27, 1901–1910. [Google Scholar]
- Um, S.; Lee, J.; Yoo, H.J. A 3.8-mW 1.9-mΩ/√Hz Electrical Impedance Tomography IC with High Input Impedance and Loading Effect Calibration for 3-D Early Breast Cancer Detect System. IEEE J. Solid-State Circuits 2024, 59, 2019–2028. [Google Scholar] [CrossRef]
- Deng, Q.; He, J.; Zhu, C.; Wang, H.; Liu, K.; Sun, B.; Yao, J. Three-Dimensional Bioelectrical Impedance Spectroscopic Tomography for Visualization of Breast Tumor. IEEE Sens. J. 2023, 23, 19598–19605. [Google Scholar] [CrossRef]
- Sari, Z.; Klincsik, M.; Odry, P.; Tadic, V.; Toth, A.; Vizvari, Z. Lumped Element Method Based Conductivity Reconstruction Algorithm for Localization Using Symmetric Discrete Operators on Coarse Meshes. Symmetry 2023, 15, 1008. [Google Scholar] [CrossRef]
- Vizvari, Z.; Gyorfi, N.; Odry, A.; Sari, Z.; Klincsik, M.; Gergics, M.; Kovacs, L.; Kovacs, A.; Pal, J.; Karadi, Z.; et al. Physical Validation of a Residual Impedance Rejection Method during Ultra-Low Frequency Bio-Impedance Spectral Measurements. Sensors 2020, 20, 4686. [Google Scholar] [CrossRef]
- Kuljic, B.; Vizvari, Z.; Gyorfi, N.; Klincsik, M.; Sari, Z.; Kovacs, F.; Juhos, K.; Szakall, T.; Odry, A.; Kovacs, L.; et al. An Effective and Robust Parameter Estimation Method in a Self-Developed, Ultra-Low Frequency Impedance Spectroscopy Technique for Large Impedances. Electronics 2024, 13, 3300. [Google Scholar] [CrossRef]
- Vizvari, Z.; Gyorfi, N.; Maczko, G.; Varga, R.; Jakabfi-Csepregi, R.; Sari, Z.; Furedi, A.; Bajtai, E.; Vajda, F.; Tadic, V.; et al. Reproducibility analysis of bioimpedance-based self-developed live cell assays. Sci. Rep. 2024, 14, 16380. [Google Scholar] [CrossRef]
- Gyorfi, N.; Gal, A.R.; Fincsur, A.; Kalmar-Nagy, K.; Mintal, K.; Hormay, E.; Miseta, A.; Tornoczky, T.; Nemeth, A.K.; Bogner, P.; et al. Novel Noninvasive Paraclinical Study Method for Investigation of Liver Diseases. Biomedicines 2023, 11, 2449. [Google Scholar] [CrossRef] [PubMed]
- Kovács, F.; Ákos, O.; Vizvári, Z.; Kabalan, S.; Papdi, E.; Odry, P.; Juhos, K. A novel approach to water stress assessment in plants: New bioimpedance method with PSO-optimized Cole-Cole impedance modeling. Comput. Electron. Agric. 2025, 234, 110167. [Google Scholar] [CrossRef]
- Bató, L.; Fürjes, P. Vertical Microfluidic Trapping System for Capturing and Simultaneous Electrochemical Detection of Cells. Sensors 2024, 24, 6638. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Bazant, M.Z.; Thornton, K.; Ajdari, A. Diffuse-charge dynamics in electrochemical systems. Phys. Rev. E 2004, 70, 021506. [Google Scholar] [CrossRef] [PubMed]
- Barnaveli, A.; van Roij, R. Asymmetric rectified electric fields: Nonlinearities and equivalent circuits. Soft Matter 2024, 20, 704–716. [Google Scholar] [CrossRef]
- Amini, M.; Hisdal, J.; Kalvøy, H. Applications of bioimpedance measurement techniques in tissue engineering. J. Electr. Bioimpedance 2018, 9, 142–158. [Google Scholar] [CrossRef]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, Recent Advances, and Future Challenges in Bioimpedance Devices for Healthcare Applications. J. Sens. 2019, 2019, 9210258:1–9210258:42. [Google Scholar] [CrossRef]
- Available online: https://www.palmsens.com/version/palmsens4 (accessed on 13 October 2025).
- IEEE Std 754-2019 (Revision of IEEE 754-2008); IEEE Standard for Floating-Point Arithmetic. IEEE: Piscataway, NJ, USA, 2019; pp. 1–84. [CrossRef]
- Arachchige, C.N.P.G.; Prendergast, L.A.; Staudte, R.G. Robust analogs to the coefficient of variation. J. Appl. Stat. 2020, 49, 268–290. [Google Scholar] [CrossRef]
- Donna, L.; Mohr, F.L.; William, J.; Wilson, F.L.; Freund, R.J. Statistical Methods; Elsevier Science Publishing Co., Ltd.: Amsterdam, The Netherlands, 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bató, L.; Fürjes, P.; Bozorádi, J.M.; Tadić, V.; Odry, P.; Vizvári, Z. Sensitivity Analysis of Localized Electrochemical Impedance Spectroscopy Towards Tomography-on-a-Chip. Sensors 2025, 25, 6393. https://doi.org/10.3390/s25206393
Bató L, Fürjes P, Bozorádi JM, Tadić V, Odry P, Vizvári Z. Sensitivity Analysis of Localized Electrochemical Impedance Spectroscopy Towards Tomography-on-a-Chip. Sensors. 2025; 25(20):6393. https://doi.org/10.3390/s25206393
Chicago/Turabian StyleBató, Lilia, Péter Fürjes, János M. Bozorádi, Vladimir Tadić, Péter Odry, and Zoltán Vizvári. 2025. "Sensitivity Analysis of Localized Electrochemical Impedance Spectroscopy Towards Tomography-on-a-Chip" Sensors 25, no. 20: 6393. https://doi.org/10.3390/s25206393
APA StyleBató, L., Fürjes, P., Bozorádi, J. M., Tadić, V., Odry, P., & Vizvári, Z. (2025). Sensitivity Analysis of Localized Electrochemical Impedance Spectroscopy Towards Tomography-on-a-Chip. Sensors, 25(20), 6393. https://doi.org/10.3390/s25206393








