A Novel BODIPY-Derived Fluorescent Sensor for Sulfite Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Instrument
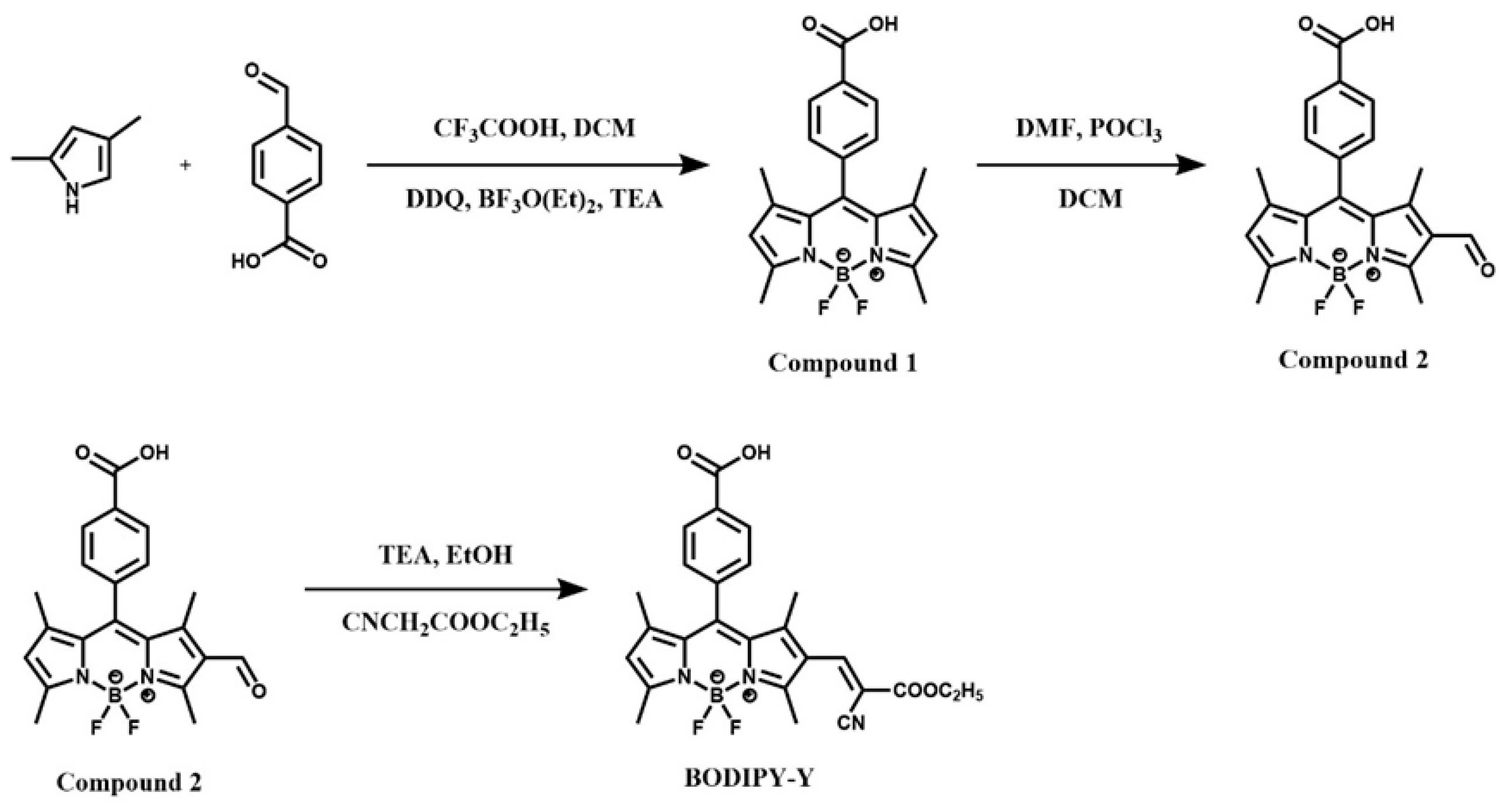
2.2. Synthesis of Compound 1
2.3. Synthesis of Compound 2
2.4. Synthesis of the Fluorescent Probe BODIPY-Y
2.5. Fluorescence Measurement Conditions
2.6. Cell Cytotoxic and Imaging
3. Results
3.1. Synthesis of the Probe BODIPY-Y
3.2. Spectral Characteristics of Probe BODIPY-Y for SO32−
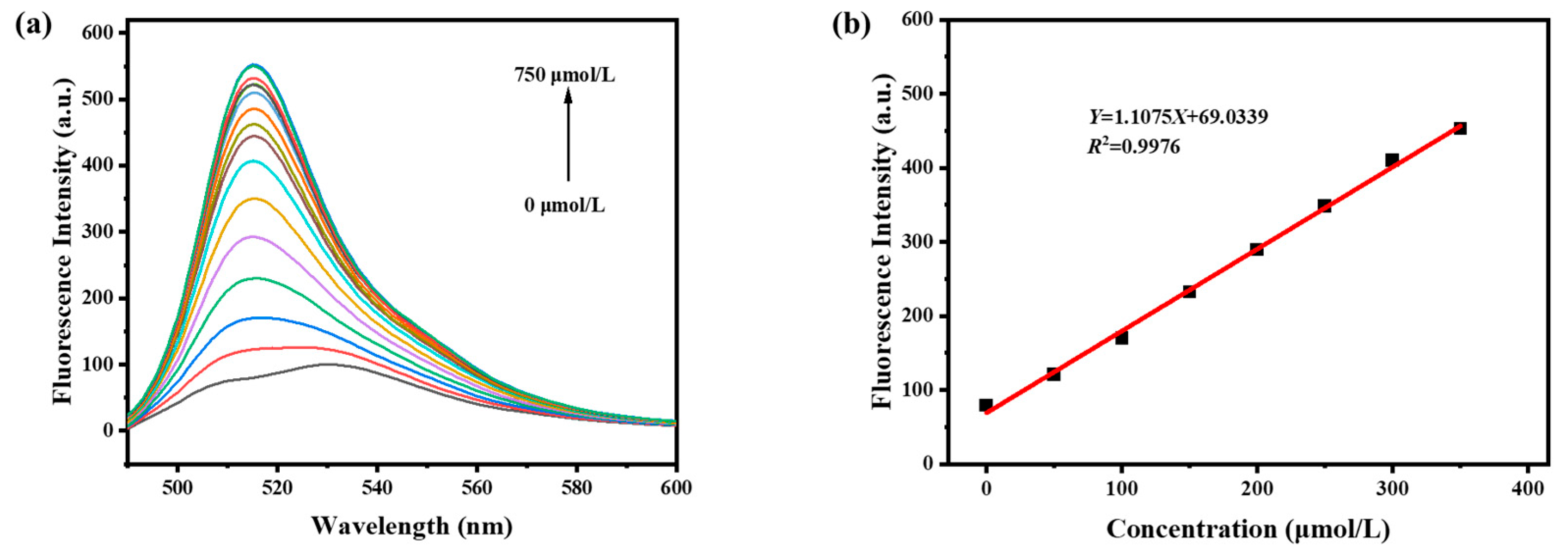
3.3. Sensitivity of Probe BODIPY-Y to SO32−
3.4. Stability and Reactivity of Probe BODIPY-Y on SO32−
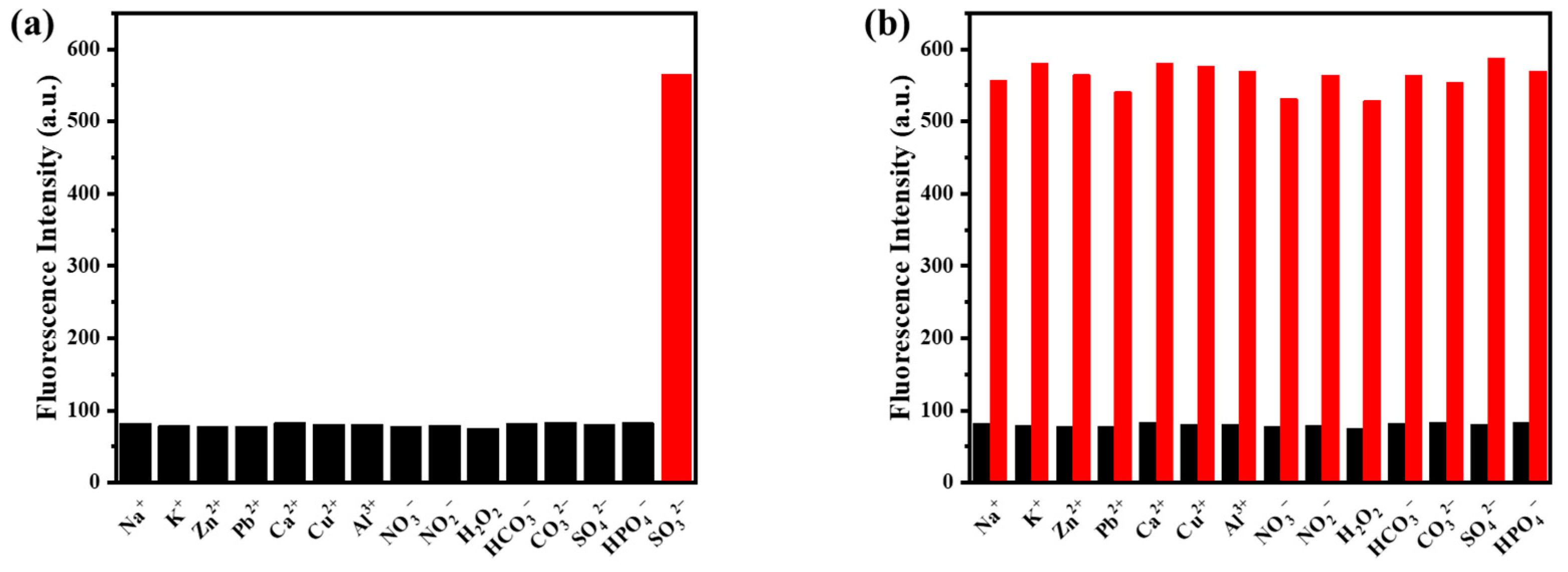
3.5. Selective Response of Probe BODIPY-Y to SO32−
3.6. Reaction Mechanism
3.7. Cytotoxicity
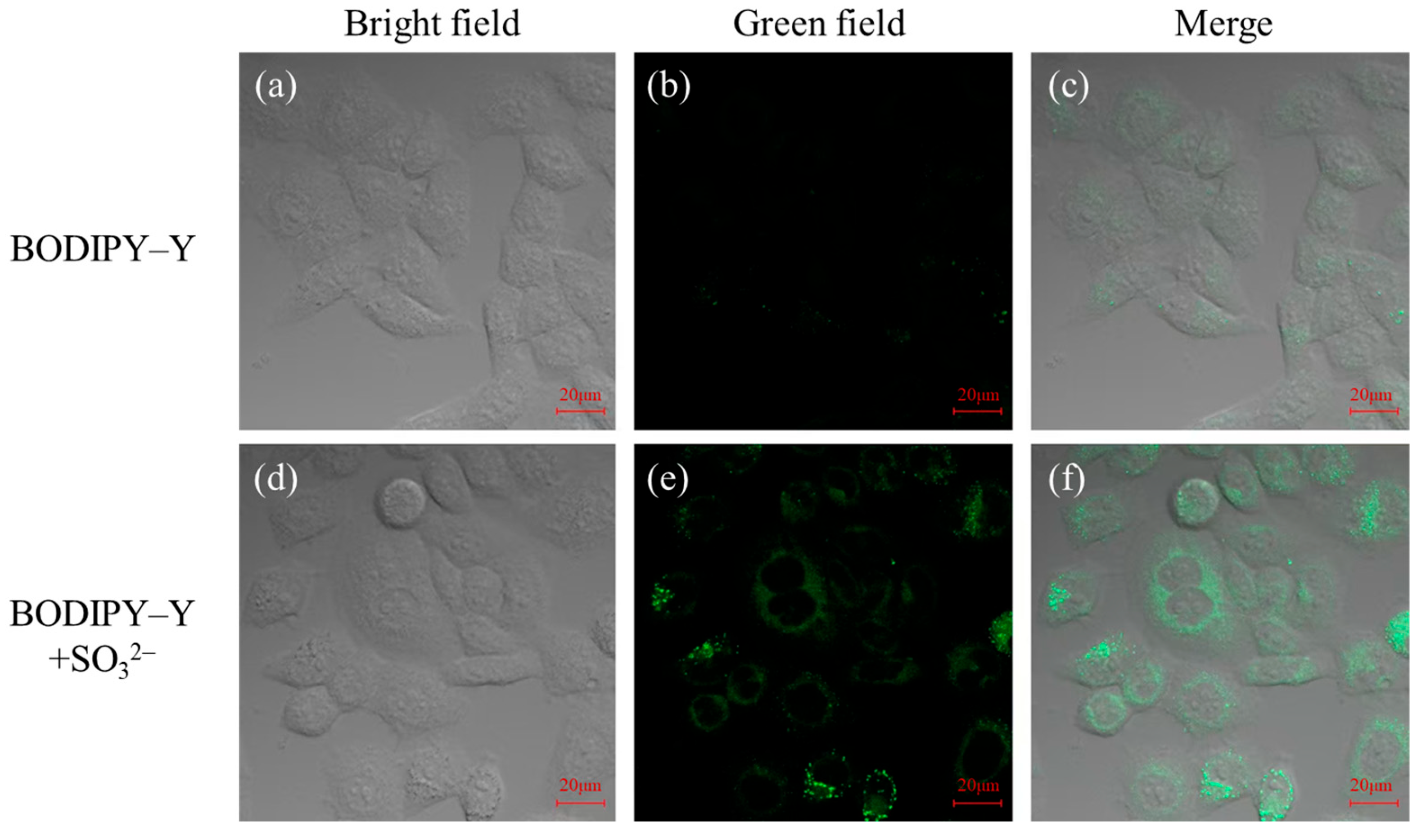
3.8. Biological Imaging
3.9. Detection of SO32− in Food
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.-Y.; Yang, X.-P.; Xu, Y.; Wang, H.-Y.; Luo, F.; Fu, G.-M.; Yan, D.-W.; Lai, M.; Ke, Y.; Ye, Y.; et al. Rational design of a rapidly responsive and highly selective fluorescent probe for SO2 derivatives detection and imaging. Food Chem. 2024, 439, 138151. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-L.; Wang, M.-H.; Yang, Y.-H.; Ji, X.; Wang, J.-Y. Viscosity & SO2-sensitive dual colorimetric effect fluorescent sensor enabled imaging detection within plant onion and biological system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 298, 122775. [Google Scholar] [CrossRef]
- Tian, L.; Sun, X.-F.; Zhou, L.-F.; Zhong, K.-L.; Li, S.-D.; Yan, X.-M.; Tang, L.-J. Reversible colorimetric and NIR fluorescent probe for sensing SO2/H2O2 in living cells and food samples. Food Chem. 2023, 407, 135031. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Di Taranto, A.; Berardi, G.; Vita, V.; Marchesani, G.; Chiaravalle, A.E.; Iammarino, M. Sulfites in meat: Occurrence, activity, toxicity, regulation, and detection. A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2701–2720. [Google Scholar] [CrossRef]
- Luo, L.-M.; Chen, S.; Jin, H.-F.; Tang, C.-S.; Du, J.-B. Endogenous generation of sulfur dioxide in rat tissues. Biochem. Biophys. Res. Commun. 2011, 415, 61–67. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Zhang, H.; Lv, B.-Y.; Tang, C.-S.; Du, J.-B.; Jin, H.-F. Sulfur Dioxide: Endogenous Generation, Biological Effects, Detection, and Therapeutic Potential. Antioxid. Redox Signal. 2021, 36, 256–274. [Google Scholar] [CrossRef]
- Meng, Z.-Q.; Yang, Z.-H.; Li, J.-L.; Zhang, Q.-X. The vasorelaxant effect and its mechanisms of sodium bisulfite as a sulfur dioxide donor. Chemosphere 2012, 89, 579–584. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Mohammadi, M.J.; Sulistiyani, S.; Ramírez-Coronel, A.A.; Kiani, F.; Jalil, A.T.; Almulla, A.F.; Asban, P.; Farhadi, M.; Derikondi, M. Effects of sulfur dioxide inhalation on human health: A review. Rev. Environ. Health 2024, 39, 331–337. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhang, Y.-J.; Han, X.; Qi, H.-C.; Ma, F.-X.; Chen, K. Pressure-Compensated Fiber-Optic Photoacoustic Sensors for Trace SO2 Analysis in Gas Insulation Equipment. Anal. Chem. 2024, 96, 10995–11001. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, J.-X.; Zhou, J.; Lin, X.-J.; Huang, K.; Jiang, X.; Yu, H.-M.; Xiong, X.-L. A microplasma converter-based spectrophotometry and visual colorimetry for nonchromatographic speciation analysis of H2S/SO2 or S2−/SO32− in environmental water samples. Microchem. J. 2022, 183, 108100. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Lai, M.; Gao, Z.-T.; Li, H.-X.; Yang, X.-P.; Wei, Y.-W.; Luo, F.; Yu, Z.-J.; Zhang, D.; Ji, X.-M. An ICT-based ratiometric fluorescent probe for tracking and imaging SO2 during Cd-induced stress in plants. Sens. Actuators B Chem. 2023, 395, 134499. [Google Scholar] [CrossRef]
- Yang, Y.-B.; Li, J.-H.; Zhang, Z.-H.; Wang, J.-N.; Lin, G.-Y. Quantitative analysis of SO2, NO2 and NO mixed gases based on ultraviolet absorption spectrum. Analyst 2023, 148, 6341–6349. [Google Scholar] [CrossRef]
- Wen, X.-Y.; Li, F.; Liu, F.-R.; Fan, Z.-F. A novel ratiometric sensor prepared based aggregation-induced emission for ultrafast detection of SO2 derivatives in food samples and living cells. Anal. Chim. Acta 2022, 1229, 340385. [Google Scholar] [CrossRef]
- Prasertying, P.; Ninlapath, T.; Jantawong, N.; Wongpakdee, T.; Sonsa-ard, T.; Uraisin, K.; Saetear, P.; Wilairat, P.; Nacapricha, D. Disposable Microchamber with a Microfluidic Paper-Based Lid for Generation and Membrane Separation of SO2 Gas Employing an In Situ Electrochemical Gas Sensor for Quantifying Sulfite in Wine. Anal. Chem. 2022, 94, 7892–7900. [Google Scholar] [CrossRef]
- Gajdár, J.; Herzog, G.; Etienne, M. Amperometric Sensor for Selective On-Site Analysis of Free Sulfite in Wines. ACS Sens. 2022, 7, 2209–2217. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Zheng, D.-B.; Zhang, T.-R.; Cao, Z.-X.; Zhou, H.-C.; Deng, Y.; Peng, C. A rationally designed fluorescent probe for sulfur dioxide and its derivatives: Applications in food analysis and bioimaging. Anal. Bioanal. Chem. 2024, 416, 533–543. [Google Scholar] [CrossRef]
- Liu, X.-N.; Shi, T.-Z.; Xu, C.-Y.; Zhu, M.-Q.; Wang, Y. A highly selective and sensitive ICT-based Cu2+ fluorescent probe and its application in bioimaging. Ecotoxicol. Environ. Saf. 2023, 262, 115127. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Lu, H.-Z.; Wei, Y.-F.; Jin, L.-X.; Zhang, Q.; Liu, B. A simple “turn-on” fluorescent probe capable of recognition cysteine with rapid response and high sensing in living cells and zebrafish. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 275, 121167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wang, P.-P.; Niu, L.-F.; Liu, C.-H.; Xiao, Y.-Z.; Tang, Y.; Chen, Y. Carbazole-thiophene based fluorescent probe for selective detection of Cu2+ and its live cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121257. [Google Scholar] [CrossRef]
- Hou, S.-M.; Wang, Y.; Zhang, Y.-K.; Wang, W.-J.; Zhou, X. A reversible turn-on fluorescent probe for quantitative imaging and dynamic monitoring of cellular glutathione. Anal. Chim. Acta 2022, 1214, 339957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-X.; Wang, B.-B.; Bai, M.-Q.; Dong, M.-D.; Tang, X. Fluorescent probe for highly selective detection of cysteine in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 294, 122523. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Liu, S.-T.; Xu, L.-R.; Li, Z.-C.; Zhang, X.-L.; Kang, H.; Liu, Y.-F.; Yu, J.; Jing, J.; Niu, G.-L.; et al. A novel ratiometric fluorescent probe with high selectivity for lysosomal nitric oxide imaging. Anal. Chim. Acta 2024, 1297, 342303. [Google Scholar] [CrossRef]
- Peng, J.-L.; Ju, Q.-K.; An, B.-S.; Yin, Z.-J.; Wei, N.-N.; Zhang, Y.-R. A super sensitive fluorescent probe for imaging endogenous hydrogen sulfide in living cells. Talanta 2022, 250, 123741. [Google Scholar] [CrossRef]
- Liang, Z.-Y.; Wei, N.; Guo, X.-F.; Wang, H. A new quinoline based probe with large Stokes shift and high sensitivity for formaldehyde and its bioimaging applications. Anal. Chim. Acta 2023, 1239, 340723. [Google Scholar] [CrossRef]
- Yang, K.-Q.; Tang, H.-D.; Zhang, Y.-P.; Wu, Y.; Su, L.-C.; Zhang, X.; Du, W.; Zhang, J.-P.; Wang, G.-Y.; Liu, D.-J.; et al. NIR-II Ratiometric Fluorescent Nanoplatform for Real-Time Monitoring and Evaluating Cancer Sonodynamic Therapy Efficacy. Adv. Opt. Mater. 2024, 12, 2303258. [Google Scholar] [CrossRef]
- Tang, L.Y.; Li, P.P.; Han, Y.Y.; Yang, G.Y.; Xin, H.T.; Zhao, S.F.; Guan, R.F.; Liu, Z.Q.; Cao, D.X. A fluorescein-based fluorescent probe for real-time monitoring hypochlorite. J. Photochem. Photobiol. A Chem. 2023, 438, 114511. [Google Scholar] [CrossRef]
- Zhou, Z.-L.; Fang, C.; Yu, F.-J.; Shen, Y.-M.; Xu, H.; Li, H.-T.; Zhang, Y.-Y. Visualization of cysteine in AD mouse with a high-quantum yield NIR fluorescent probe. Talanta 2024, 278, 126482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Qiao, L.-L.; Zhang, Z.-Q.; Liu, Y.-F.; Li, L.-S.; Shen, H.-B.; Zhao, M.-X. A mitochondrial-targetable fluorescent probe based on high-quality InP quantum dots for the imaging of living cells. Mater. Des. 2022, 219, 110736. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.-H.; Pei, Z.-C.; Pei, Y.-X. Lactosylation leads to a water-soluble fluorescent probe for detection of S2− in water. Microchem. J. 2022, 181, 107800. [Google Scholar] [CrossRef]
- Song, J.; Yu, J.-Y.; Sun, K.; Chen, Z.-X.; Xing, X.-X.; Yang, Y.-M.; Sun, C.-Y.; Wang, Z.-F. A high quantum yield xanthene-based fluorescent probe for the specific detection of tyrosinase and cell imaging. J. Photochem. Photobiol. A Chem. 2023, 441, 114693. [Google Scholar] [CrossRef]
- Hu, N.; Zeng, H.; Shi, S.; Yao, W.; Ji, D.; Guo, H.; Luo, L.; Jin, T.; Yu, Q.; Xu, K.; et al. The preparation of a chitosan-based novel fluorescent macromolecular probe and its application in the detection of hypochlorite. Mater. Today Chem. 2023, 29, 101420. [Google Scholar] [CrossRef]
- Wu, J.-J.; Pan, J.; Ye, Z.; Zeng, L.-T.; Su, D.-D. A smart fluorescent probe for discriminative detection of hydrazine and bisulfite from different emission channels. Sens. Actuators B Chem. 2018, 274, 274–284. [Google Scholar] [CrossRef]
- Du, Y.-T.; Pan, C.-X.; Cao, C.-J. A mitochondria-targetable fluorescent probe for sulfur dioxide detection and visualisation in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 290, 122275. [Google Scholar] [CrossRef]
- Ren, H.-X.; Huo, F.-J.; Wu, X.; Liu, X.-G.; Yin, C.-X. An ESIPT-induced NIR fluorescent probe to visualize mitochondrial sulfur dioxide during oxidative stress in vivo. Chem. Commun. 2021, 57, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Li, N.; Chen, Y.-S.; Yu, H.-Y.; Miao, J.-Y.; Zhao, B.-X. A quinoline-coumarin near-infrared ratiometric fluorescent probe for detection of sulfur dioxide derivatives. Anal. Chim. Acta 2022, 1211, 339908. [Google Scholar] [CrossRef]
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent advances of BODIPY based derivatives for optoelectronic applications. Coordin. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Das, S.; Dey, S.; Patra, S.; Bera, A.; Ghosh, T.; Prasad, B.; Sayala, K.D.; Maji, K.; Bedi, A.; Debnath, S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules 2023, 13, 1723. [Google Scholar] [CrossRef]
- Yan, M.-M.; He, D.-M.; Zhang, L.-S.; Sun, P.-J.; Sun, Y.-Q.; Qu, L.-B.; Li, Z.-H. Explorations into the meso-substituted BODIPY-based fluorescent probes for biomedical sensing and imaging. TrAC Trends Anal. Chem. 2022, 157, 116771. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.-X.; Zhang, D.-L.; Xi, Y.; Yu, S.-R.; Zhong, H.-M.; He, K.-D.; Li, D.; Wei, W.-T.; Cao, Y.-T.; et al. A BODIPY-Hemicyanine-Based Water-Soluble Dual-Color Fluorescence Probe for Colorimetric Monitoring of Intracellular Endogenous Sulfur Dioxide and Bioimaging Applications. ChemistrySelect 2020, 5, 3033–3040. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Bodipy Dyes with Tunable Redox Potentials and Functional Groups for Further Tethering: Preparation, Electrochemical, and Spectroscopic Characterization. J. Am. Chem. Soc. 2010, 132, 17560–17569. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Yang, L.; Yang, N.; Gu, P.; Zhang, Y.; Gong, X.; Zhang, S.; Li, J.; Ji, L.; He, G. A novel naphthalimide-based fluorescent probe for the colorimetric and ratiometric detection of SO2 derivatives in biological imaging. Bioorg. Chem. 2022, 123, 105801. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Yan, J.-L.; Wu, W.-N.; Zhao, X.-L.; Wang, Y.; Fan, Y.-C.; Xu, Z.-H. A dual-response fluorescent probe for SO2 and viscosity and imaging application in lysosomes and zebrafish. Microchem. J. 2022, 181, 107653. [Google Scholar] [CrossRef]
- Zhong, K.; Li, Y.; Zhou, L.; Sun, X.; Tang, L.; Zhang, N.; Tang, Y. A benzopyran-hemicyanine-based mitochondria-targeted NIR fluorescent probe for detection of SO2 derivatives in food samples and living cells. Microchem. J. 2024, 207, 112037. [Google Scholar] [CrossRef]
- Dutta, K.; Chattaraj, S.; Pal, R.; Jonnalgadda, P.N.; Patro, B.S.; Mula, S.; Chakraborty, G. A Kumujian-C based highly selective fluorescence turn-on probe enables the detection of sulfite in real samples and living cells. New J. Chem. 2024, 48, 9961–9969. [Google Scholar] [CrossRef]
- Liu, F.-T.; Han, W.-W.; Ren, H.; Wang, R.-N.; Yang, W.-J.; Miao, J.-Y.; Zhao, B.-X.; Lin, Z.-M. A dicyanoisophorone-quinolinium-based near-infrared-emission fluorescent probe for ratiometric sensing of bisulfite/sulfite in living cells. Sens. Actuators B Chem. 2023, 380, 133305. [Google Scholar] [CrossRef]
- Cui, R.; Gao, Y.; Ge, H.; Shi, G.; Li, Y.; Liu, H.; Ma, C.; Ge, Y.; Liu, C. A turn-on fluorescent probe based on indolizine for the detection of sulfite. New J. Chem. 2022, 46, 8088–8093. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Zhou, L.; Tian, L.; Zhong, K.; Zhang, J.; Yan, X.; Tang, L. Novel Colorimetric and NIR Fluorescent Probe for Bisulfite/Sulfite Detection in Food and Water Samples and Living Cells Based on the PET Mechanism. J. Agric. Food Chem. 2022, 70, 10899. [Google Scholar] [CrossRef]
- Huang, Y.; Xin, H.; Lin, Q.; Yang, G.; Zhang, Y.; Cao, D.; Yu, X. A fluorescent probe for detecting bisulfite/sulfite in lipid droplets and tracking the dynamics of lipid droplets. Talanta 2024, 279, 126605. [Google Scholar] [CrossRef]
- Liang, T.; Liu, S.; Shen, T.; Chen, X.; Li, X.; Yan, X.; Sun, X.; Tian, M.; Wu, C.; Sun, X.; et al. Chromene-derived red-fluorescent probes for sulfite detection in food and living cells based on an integrated ICT&PET platform. Sens. Actuators B Chem. 2024, 413, 135864. [Google Scholar] [CrossRef]
- Yang, W.; Fang, X.; Chen, C.; Zhang, Y.; Zhang, W.; Qian, J. Coumarin-based fluorescent probes for colorimetric and ratiometric fluorescent detection of sulfite: Structure-activity relationship. Tetrahedron 2024, 159, 134017. [Google Scholar] [CrossRef]




| Samples | Na2SO3 Spiked (μmol/L) | Found Mean (μmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| White sugar | 0 | 15.93 | - | - |
| 25 | 35.4 | 77.9 | 0.7 | |
| 50 | 62.23 | 93.6 | 1.4 | |
| 75 | 89.53 | 98.1 | 1.4 | |
| Red wine | 0 | 7.3 | - | - |
| 25 | 30.79 | 94.0 | 4.9 | |
| 50 | 52.33 | 90.1 | 1.6 | |
| 75 | 71.11 | 85.1 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Liu, Y.; Fang, W.; Liu, H.; Liu, Z. A Novel BODIPY-Derived Fluorescent Sensor for Sulfite Monitoring. Sensors 2025, 25, 6332. https://doi.org/10.3390/s25206332
Qu J, Liu Y, Fang W, Liu H, Liu Z. A Novel BODIPY-Derived Fluorescent Sensor for Sulfite Monitoring. Sensors. 2025; 25(20):6332. https://doi.org/10.3390/s25206332
Chicago/Turabian StyleQu, Junyu, Yixuan Liu, Wenqiang Fang, Huitao Liu, and Zhenbo Liu. 2025. "A Novel BODIPY-Derived Fluorescent Sensor for Sulfite Monitoring" Sensors 25, no. 20: 6332. https://doi.org/10.3390/s25206332
APA StyleQu, J., Liu, Y., Fang, W., Liu, H., & Liu, Z. (2025). A Novel BODIPY-Derived Fluorescent Sensor for Sulfite Monitoring. Sensors, 25(20), 6332. https://doi.org/10.3390/s25206332





