EEG Spectral Analysis in Chronic Pain During Rest and Cognitive Reasoning
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
CP Patients
2.2. Healthy Controls
2.3. EEG Recording Procedure
2.4. Data Acquisition and Preprocessing
2.5. Power Analysis
2.6. Statistical Analysis
2.7. Task/Resting State Index
3. Results
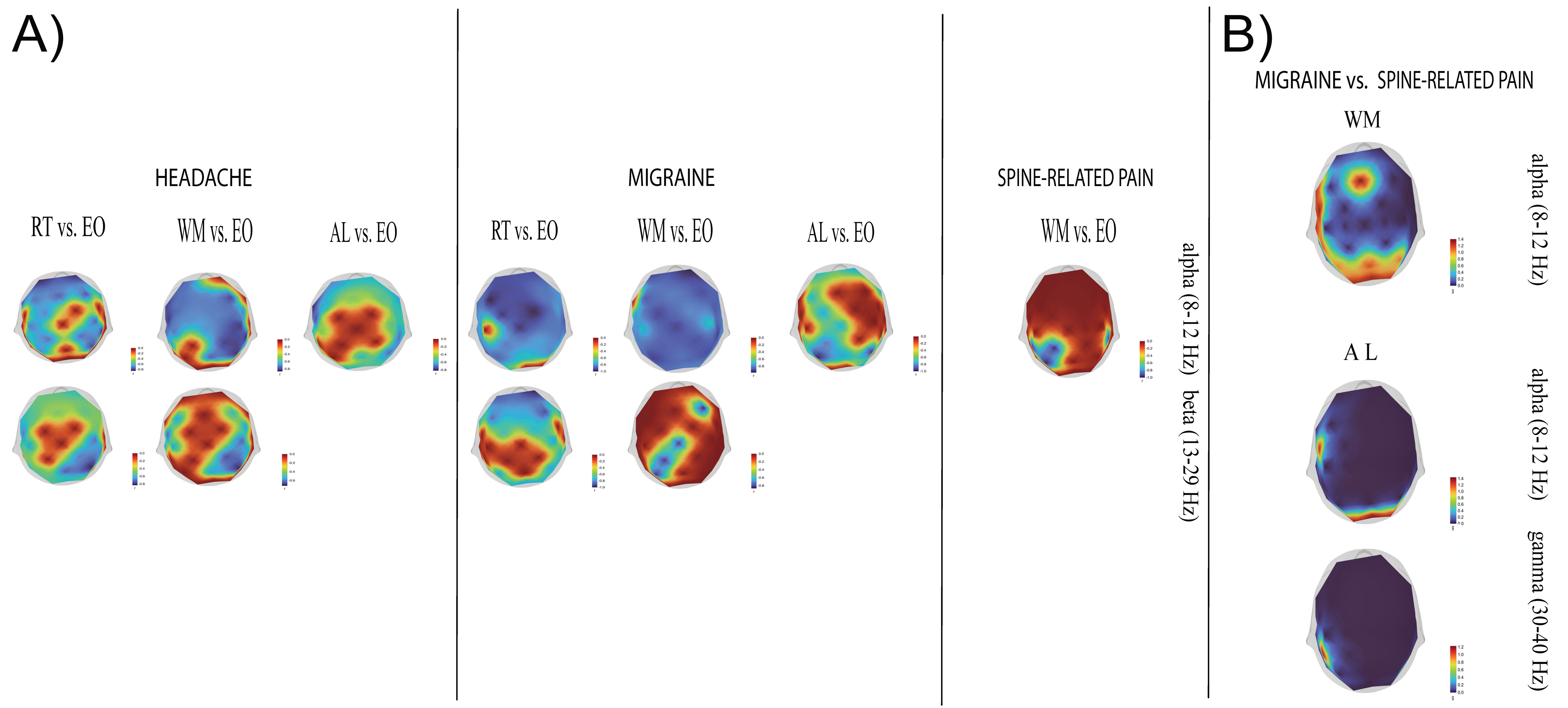
3.1. Cognitive Oscillatory Dynamics Across Three Different Pain Subgroups
3.1.1. Comparison Between VCM and EO for Each Pain Subgroup
3.1.2. Comparison of Power Changes Produced Under Cognitive Load Between Pain Subtypes
3.1.3. Task/Resting-State Index
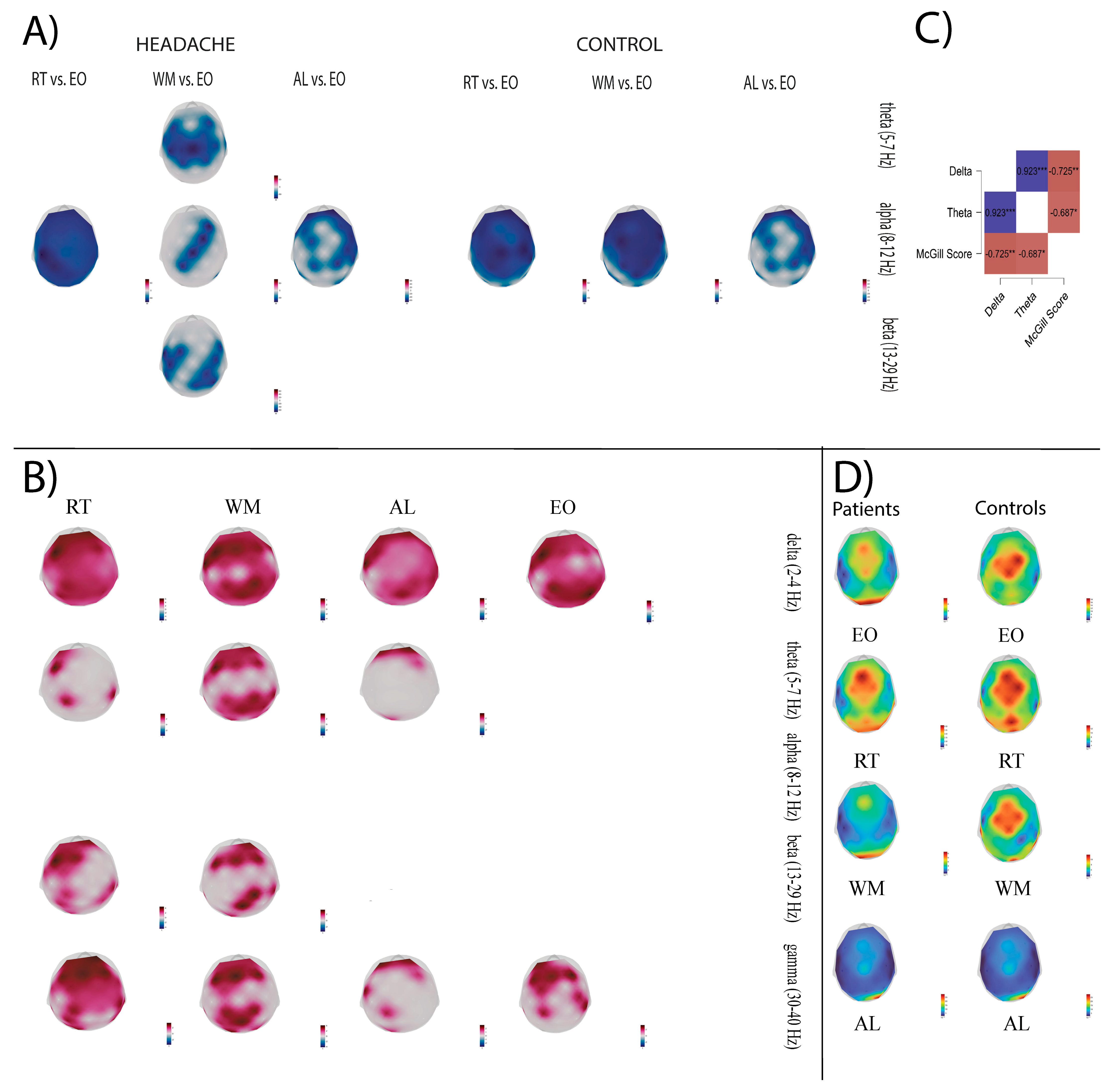
3.2. Global Brain Activity Contrasts Between Headache and Control Groups
3.2.1. Comparison of Power Between VCMs and EO in the Headache and Control Group
3.2.2. Brain Activity Comparison Between Headache and Control Group
- Resting state.
- Delta band during VCMs.
- Theta band during VCMs.
- Beta band during VCMs.
- Gamma band during VCMs.
3.3. Relationship Between Cortical Oscillatory Dynamics and Clinical Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Reckziegel, D.; Vachon-Presseau, E.; Petre, B.; Schnitzer, T.J.; Baliki, M.N.; Apkarian, A.V. Deconstructing biomarkers for chronic pain: Context and hypothesis dependent biomarker types in relation to chronic pain. Pain 2019, 160, S37–S48. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central sensitization, chronic pain, and other symptoms: Better understanding, better management. Clevel. Clin. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef]
- Baliki, M.N.; Apkarian, A.V. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 2015, 87, 474–491. [Google Scholar] [CrossRef]
- Farmer, M.A.; Baliki, M.N.; Apkarian, A.V. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012, 520, 197–203. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Kim, K.H. Current understanding of nociplastic pain. Korean J. Pain 2024, 37, 107–118. [Google Scholar] [CrossRef]
- Danno, D.; Imai, N.; Kitamura, S.; Ishizaki, K.; Kikui, S.; Takeshima, T. Efficacy of galcanezumab in migraine central sensitization. Sci. Rep. 2024, 14, 21824. [Google Scholar] [CrossRef]
- Lepri, B.; Romani, D.; Storari, L.; Barbari, V. Effectiveness of Pain Neuroscience Education in Patients with Chronic Musculoskeletal Pain and Central Sensitization: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4098. [Google Scholar] [CrossRef] [PubMed]
- Rueda, M.R.; Moyano, S.; Rico-Picó, J. Attention: The grounds of self-regulated cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2023, 14, e1582. [Google Scholar] [CrossRef] [PubMed]
- Hauck, M.; Lorenz, J.; Engel, A.K. Attention to painful stimulation enhances γ-band activity and synchronization in human sensorimotor cortex. J. Neurosci. 2007, 27, 9270–9277. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Eccleston, C.; Crombez, G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol. Bull. 1999, 125, 356–366. [Google Scholar] [CrossRef]
- Oosterman, J.M.; Derksen, L.C.; van Wijck, A.J.M.; Veldhuijzen, D.S.; Kessels, R.P.C. Memory Functions in Chronic Pain. Clin. J. Pain 2011, 27, 70–75. [Google Scholar] [CrossRef]
- Higgins, D.M.; Martin, A.M.; Baker, D.G.; Vasterling, J.J.; Risbrough, V. The Relationship between Chronic Pain and Neurocognitive Function. Clin. J. Pain 2017, 34, 262–275. [Google Scholar] [CrossRef]
- Berryman, C.; Stanton, T.R.; Jane Bowering, K.; Tabor, A.; McFarlane, A.; Lorimer Moseley, G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain 2013, 154, 1181–1196. [Google Scholar] [CrossRef]
- Berryman, C.; Stanton, T.R.; Bowering, K.J.; Tabor, A.; McFarlane, A.; Moseley, G.L. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin. Psychol. Rev. 2014, 34, 563–579. [Google Scholar] [CrossRef]
- Sobott, N.; Crowther, M.E.; Vincent, G.E.; Belavý, D.L.; Buntine, P.; Ferguson, S.A.; Mundell, N.L.; Sprajcer, M.; Tagliaferri, S.D.; Tait, J.L.; et al. Chronic low back pain is associated with compromised cognitive function: A systematic review and meta-analysis. J. Pain 2025, 33, 105475. [Google Scholar] [CrossRef]
- Mussigmann, T.; Bardel, B.; Lefaucheur, J.P. Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. Neuroimage 2022, 258, 119351. [Google Scholar] [CrossRef]
- Zebhauser, P.T.; Hohn, V.D.; Ploner, M. Resting-state electroencephalography and magnetoencephalography as biomarkers of chronic pain: A systematic review. Pain 2023, 164, 1200–1221. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Suen, H.; Ullah, R.; Purcell, M.; Diggin, S.; McCaughey, E.; Vuckovic, A. Electroencephalography Longitudinal Markers of Central Neuropathic Pain Intensity in Spinal Cord Injury: A Home-Based Pilot Study. Biomedicines 2024, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kanie, Y.; Yoshimoto, S.; Yamamoto, N.; Furuya, M.; Fujimori, T.; Okada, S. Classification accuracy of pain intensity induced by leg blood flow restriction during walking using machine learning based on electroencephalography. Sci. Rep. 2025, 15, 27955. [Google Scholar] [CrossRef] [PubMed]
- Shirvalkar, P.; Prosky, J.; Chin, G.; Ahmadipour, P.; Sani, O.G.; Desai, M.; Schmitgen, A.; Dawes, H.; Shanechi, M.M.; Starr, P.A.; et al. First-in-human prediction of chronic pain state using intracranial neural biomarkers. Nat. Neurosci. 2023, 26, 1090–1099. [Google Scholar] [CrossRef]
- You, L.; Yang, B.; Lu, X.; Yang, A.; Zhang, Y.; Bi, X.; Zhou, S. Similarities and differences between chronic primary pain and depression in brain activities: Evidence from resting-state microstates and auditory Oddball task. Behav. Brain Res. 2025, 477, 115319. [Google Scholar] [CrossRef]
- Camargo, L.; Pacheco-Barrios, K.; Marques, L.M.; Caumo, W.; Fregni, F. Adaptive and Compensatory Neural Signatures in Fibromyalgia: An Analysis of Resting-State and Stimulus-Evoked EEG Oscillations. Biomedicines 2024, 12, 1428. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing Clinically Significant Values for Identifying Central Sensitivity Syndromes in an Outpatient Chronic Pain Sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Robbins, T.W.; James, M.; Owen, A.M.; Sahakian, B.J.; Lawrence, A.D.; McInnes, L.; Rabbitt, P.M. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J. Int. Neuropsychol. Soc. 1998, 4, 474–490. [Google Scholar]
- Robbins, T.W.; James, M.; Owen, A.M.; Sahakian, B.J.; McInnes, L.; Rabbitt, P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia 1994, 5, 266–281. [Google Scholar] [CrossRef]
- Dinh, S.T.; Nickel, M.M.; Tiemann, L.; May, E.S.; Heitmann, H.; Hohn, V.D.; Edenharter, G.; Utpadel-Fischler, D.; Tölle, T.R.; Sauseng, P.; et al. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. Pain 2019, 160, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef] [PubMed]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar]
- JASP Team. JASP, Version 0.16.4; JASP Team: Amsterdam, The Netherlands, 2022.
- Pfurtscheller, G. Graphical display and statistical evaluation of event-related desynchronization (ERD). Electroencephalogr. Clin. Neurophysiol. 1977, 43, 757–760. [Google Scholar] [CrossRef]
- Michels, L.; Bucher, K.; Lüchinger, R.; Klaver, P.; Martin, E.; Jeanmonod, D.; Brandeis, D. Simultaneous EEG-fMRI during a working memory task: Modulations in low and high frequency bands. PLoS ONE 2010, 5, e10298. [Google Scholar] [CrossRef]
- Chafee, M.V.; Goldman-Rakic, P.S. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J. Neurophysiol. 1998, 79, 2919–2940. [Google Scholar] [CrossRef]
- Rottschy, C.; Caspers, S.; Roski, C.; Reetz, K.; Dogan, I.; Schulz, J.B.; Zilles, K.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Differentiated parietal connectivity of frontal regions for “what” and ”where” memory. Brain Struct. Funct. 2013, 218, 1551–1567. [Google Scholar] [CrossRef]
- Ruspantini, I.; Mäki, H.; Korhonen, R.; D’Ausilio, A.; Ilmoniemi, R.J. The functional role of the ventral premotor cortex in a visually paced finger tapping task: A TMS study. Behav. Brain Res. 2011, 220, 325–330. [Google Scholar] [CrossRef]
- Proskovec, A.L.; Wiesman, A.I.; Heinrichs-Graham, E.; Wilson, T.W. Beta Oscillatory Dynamics in the Prefrontal and Superior Temporal Cortices Predict Spatial Working Memory Performance. Sci. Rep. 2018, 8, 8488. [Google Scholar] [CrossRef]
- Sochůrková, D.; Rektor, I.; Jurák, P.; Stancák, A. Intracerebral recording of cortical activity related to self-paced voluntary movements: A Bereitschaftspotential and event-related desynchronization/synchronization. SEEG study. Exp. Brain Res. 2006, 173, 637–649. [Google Scholar] [CrossRef]
- Boiten, F.; Sergeant, J.; Geuze, R. Event-related desynchronization: The effects of energetic and computational demands. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 302–309. [Google Scholar] [CrossRef]
- Dujardin, K.; Bourriez, J.L.; Guieu, J.D. Event-related desynchronization (ERD) patterns during memory processes: Effects of aging and task difficulty. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Russegger, H.; Pachinger, T.; Schwaiger, J. Induced alpha band power changes in the human EEG and attention. Neurosci. Lett. 1998, 244, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; van Ede, F.; Kuo, B.C. Alpha Oscillations Track Content-Specific Working Memory Capacity. J. Neurosci. 2022, 42, 7285–7293. [Google Scholar] [CrossRef]
- Kim, S.J.; Yang, K.; Kim, D. Quantitative electroencephalography as a potential biomarker in migraine. Brain Behav. 2023, 13, e3282. [Google Scholar] [CrossRef]
- Bridge, H.; Stagg, C.J.; Near, J.; Lau, C.I.; Zisner, A.; Cader, M.Z. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 2015, 35, 1025–1030. [Google Scholar] [CrossRef]
- Wu, X.; Han, S.; Yang, Y.; Dai, H.; Wu, P.; Zhao, H.; Jin, X.; Li, Y. Decreased Brain GABA Levels in Patients with Migraine Without Aura: An Exploratory Proton Magnetic Resonance Spectroscopy Study. Neuroscience 2022, 488, 10–19. [Google Scholar] [CrossRef]
- Schreckenberger, M.; Lange-Asschenfeldt, C.; Lochmann, M.; Mann, K.; Siessmeier, T.; Buchholz, H.G.; Bartenstein, P.; Gründer, G. The thalamus as the generator and modulator of EEG alpha rhythm: A combined PET/EEG study with lorazepam challenge in humans. Neuroimage 2004, 22, 637–644. [Google Scholar] [CrossRef]
- Huang, J.; Wilkins, A. The Functional Network of the Visual Cortex Is Altered in Migraine. Vision 2021, 5, 57. [Google Scholar] [CrossRef]
- de Tommaso, M.; Trotta, G.; Vecchio, E.; Ricci, K.; Siugzdaite, R.; Stramaglia, S. Brain networking analysis in migraine with and without aura. J. Headache Pain 2017, 18, 98. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, B.Y.; Cho, S.; Kim, S.T.; Park, H.; Chung, C.S. Increased connectivity of pain matrix in chronic migraine: A resting-state functional MRI study. J. Headache Pain 2019, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Yang, S.; Hou, Q.; Rudder, P.; Tanglay, O.; Young, I.; Peng, T.; He, W.; Yang, L.; Osipowicz, K.; et al. Functional connectivity of the language area in migraine: A preliminary classification model. BMC Neurol. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- London, R.E.; Benwell, C.S.Y.; Cecere, R.; Quak, M.; Thut, G.; Talsma, D. EEG alpha power predicts the temporal sensitivity of multisensory perception. Eur. J. Neurosci. 2022, 11–12, 3241–3255. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.S.; de Queirós, F.C.; Montoya, P.; Santos, C.L.; do Nascimento, M.A.; Ito, C.H.; Silva, M.; Nunes Santos, D.B.; Benevides, S.; Miranda, J.G.; et al. Electroencephalographic Patterns in Chronic Pain: A Systematic Review of the Literature. PLoS ONE 2016, 11, e0149085. [Google Scholar] [CrossRef]
- De Pascalis, V.; Scacchia, P.; Papi, B.; Corr, P.J. Changes of EEG band oscillations to tonic cold pain and the behavioral inhibition and fight-flight-freeze systems. Personal. Neurosci. 2019, 2, e12. [Google Scholar] [CrossRef]
- Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef]
- de Vries, I.E.J.; Savran, E.; van Driel, J.; Olivers, C.N.L. Oscillatory Mechanisms of Preparing for Visual Distraction. J. Cogn. Neurosci. 2019, 31, 1873–1894. [Google Scholar] [CrossRef]
- Riddle, J.; Scimeca, J.M.; Cellier, D.; Dhanani, S.; D’Esposito, M. Causal Evidence for a Role of Theta and Alpha Oscillations in the Control of Working Memory. Curr. Biol. 2020, 30, 1748–1754.e4. [Google Scholar] [CrossRef] [PubMed]
- Hoegh, M.; Seminowicz, D.A.; Graven-Nielsen, T. Delayed effects of attention on pain sensitivity and conditioned pain modulation. Eur. J. Pain 2019, 23, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Kucyi, A.; Salomons, T.V.; Davis, K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc. Natl. Acad. Sci. USA 2013, 110, 18692–18697. [Google Scholar] [CrossRef] [PubMed]
- Mac Goris, J.L.; Todd, J.; Clarke, P.J.F.; Hughes, A.M.; Vögele, C.; Van Ryckeghem, D.M.L. The role of attention bias malleability in experiencing pain and associated disability. PeerJ 2024, 12, e17430. [Google Scholar] [CrossRef]
- Magosso, E.; Borra, D. The strength of anticipated distractors shapes EEG alpha and theta oscillations in a Working Memory task. Neuroimage 2024, 300, 120835. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Wessel, J.R.; Aron, A.R. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron 2017, 93, 259–280. [Google Scholar] [CrossRef]
- Di Dona, G.; Ronconi, L. Beta oscillations in vision: A (preconscious) neural mechanism for the dorsal visual stream? Front. Psychol. 2023, 14, 1296483. [Google Scholar] [CrossRef]
- May, E.S.; Nickel, M.M.; Ta Dinh, S.; Tiemann, L.; Heitmann, H.; Voth, I.; Tölle, T.R.; Gross, J.; Ploner, M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2018, 40, 293–305. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef]
- Popov, T.; Jensen, O.; Schoffelen, J.M. Dorsal and ventral cortices are coupled by cross-frequency interactions during working memory. Neuroimage 2018, 178, 277–286. [Google Scholar] [CrossRef]
- Brzezicka, A.; Kamiński, J.; Reed, C.M.; Chung, J.M.; Mamelak, A.N.; Rutishauser, U. Working Memory Load-related Theta Power Decreases in Dorsolateral Prefrontal Cortex Predict Individual Differences in Performance. J. Cogn. Neurosci. 2019, 31, 1290–1307. [Google Scholar] [CrossRef]
- de Vries, I.E.J.; van Driel, J.; Karacaoglu, M.; Olivers, C.N.L. Priority Switches in Visual Working Memory are Supported by Frontal Delta and Posterior Alpha Interactions. Cereb Cortex 2018, 28, 4090–4104. [Google Scholar] [CrossRef] [PubMed]
- Zelmann, R.; Lina, J.M.; Schulze-Bonhage, A.; Gotman, J.; Jacobs, J. Scalp EEG is not a blur: It can see high frequency oscillations although their generators are small. Brain Topogr. 2014, 27, 683–704. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Front. Hum. Neurosci. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Whitham, E.M.; Lewis, T.; Pope, K.J.; Fitzgibbon, S.P.; Clark, C.R.; Loveless, S.; DeLosAngeles, D.; Wallace, A.K.; Broberg, M.; Willoughby, J.O. Thinking activates EMG in scalp electrical recordings. Clin. Neurophysiol. 2008, 119, 1166–1175. [Google Scholar] [CrossRef]
- Gaižauskaitė, R.; Gladutytė, L.; Zelionkaitė, I.; Čėsnaitė, E.; Busch, N.A.; Grikšienė, R. The search for the relationship between female hormonal status, alpha oscillations, and aperiodic features of resting state EEG. Int. J. Psychophysiol. 2024, 198, 112312. [Google Scholar] [CrossRef]
- Gaižauskaitė, R.; Gladutytė, L.; Zelionkaitė, I.; Grikšienė, R. Exploring the role of sex, sex steroids, menstrual cycle, and hormonal contraception use in visual working memory: Insights from behavioral and EEG analyses. Int. J. Psychophysiol. 2025, 209, 112520. [Google Scholar] [CrossRef]
- Ramos-Loyo, J.; González-Garrido, A.A.; Llamas-Alonso, L.A.; Sequeira, H. Sex differences in cognitive processing: An integrative review of electrophysiological findings. Biol. Psychol. 2022, 172, 108370. [Google Scholar] [CrossRef]
- Sorge, R.E.; Totsch, S.K. Sex Differences in Pain. J. Neurosci. Res. 2016, 95, 1271–1281. [Google Scholar] [CrossRef]
- Birkinshaw, H.; Friedrich, C.M.; Cole, P.; Eccleston, C.; Serfaty, M.; Stewart, G.; White, S.; Moore, R.A.; Phillippo, D.; Pincus, T. Antidepressants for pain management in adults with chronic pain: A network meta-analysis. Cochrane Libr. 2023, 5, CD014682. [Google Scholar] [CrossRef]
- Narayan, S.W.; Naganathan, V.; Vizza, L.; Underwood, M.; Ivers, R.; McLachlan, A.J.; Zhou, L.; Singh, R.; Tao, S.; Xi, X. Efficacy and Safety of Antidepressants for Pain in Older Adults: A Systematic Review and Meta-analysis. Br. J. Clin. Pharmacol. 2024, 90, 3097–3118. [Google Scholar] [CrossRef]
- Ferreira, G.E.; Abdel-Shaheed, C.; Underwood, M.; Finnerup, N.B.; Day, R.O.; McLachlan, A.; Eldabe, S.; Zadro, J.R.; Maher, C.G. Efficacy, safety, and tolerability of antidepressants for pain in adults: Overview of systematic reviews. BMJ 2023, 380, e072415. [Google Scholar] [CrossRef]
- Zebhauser, P.T.; Bott, F.; Ávila, C.G.; Heitmann, H.; May, E.S.; Tiemann, L.; Baki, E.; Tölle, T.R.; Ploner, M. Effects of centrally acting analgesics on resting-state electroencephalography biomarker candidates of chronic pain. J. Pain 2025, 28, 104788. [Google Scholar] [CrossRef]


| Patients | McGill Score | CSI Score | Age | Sex | Chronic Pain Type | Medication Class/Therapeutic Approach | Risk Behaviors/Factors for Pain | Pain Duration (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 40 | 54 | F | Tension-type headache | Supplements/Herbal Remedies | Smoker Prolonged Standing | 60 |
| 2 | 25 | 9 | 44 | M | Tension-type headache | Supplements/Herbal Remedies Acupuncture | Occasional Alcohol Consumption | 300 |
| 3 | 20 | 26 | 48 | F | Tension-type headache | Homeopathy | Smoker Occasional Alcohol Consumption | 90 |
| 4 | 30 | 19 | 31 | F | Migraine without aura | None | Overweight | 60 |
| 5 | 23 | 37 | 43 | F | Tension-type headache | Tricyclic Antidepressants | Smoker | 180 |
| 6 | 16 | 59 | 52 | F | Tension-type headache | Tricyclic Antidepressants Analgesics | None declared | 66 |
| 7 | 42 | 43 | 31 | M | Tension-type headache | Tricyclic Antidepressants SNRIs SSRIs Other Analgesics | Smoker | 131 |
| 8 | 15 | 50 | 68 | F | Migraine (possibly with aura) | None | None declared | 108 |
| 9 | 40 | 25 | 50 | F | Migraine without aura | Tricyclic Antidepressants Supplements/Herbal Remedies | None declared | 204 |
| 10 | 25 | 52 | 44 | M | Cluster-type Headache | Supplements/Herbal Remedies | Smoker Occasional alcohol consumption | 6 |
| 11 | 10 | 21 | 43 | F | Arnold Neuralgia | Supplements/Herbal Remedies Tricyclic Antidepressants | None declared | 3 |
| 12 | 26 | 31 | 24 | M | Tension-type headache | Ergot Derivates Supplements/Herbal Remedies Tricyclic Antidepressants Other Analgesics | None declared | 4 |
| 13 | 33 | 56 | 44 | F | Tension-type headache | Supplements/Herbal Remedies Tricyclic Antidepressants Anticonvulsants | None declared | 60 |
| 14 | 32 | 36 | 31 | F | Transformed Migraine | Triptans Tricyclic Antidepressants Other Analgesics | Analgetic Abuse | 6 |
| 15 | 20 | 42 | 30 | F | Tension-type headache | NSAIDs Supplements/Herbal Remedies | None declared | 0.75 |
| 16 | 30 | 37 | 29 | F | Tension-type headache | Supplements/Herbal Remedies Tricyclic Antidepressants | None declared | 18 |
| 17 | 7 | 38 | 68 | F | S1 Radiculopathy Dorsal Spondylosis Cervical C4-C7 disk protrusions | Supplements/Herbal Remedies | None declared | 48 |
| 18 | 10 | 18 | 49 | F | Tension-type headache | NSAIDs Supplements/Herbal Remedies | None declared | 60 |
| 19 | 10 | 33 | 47 | F | Degenerative C5-C6 disk hernia | N/A | N/A | 12 |
| 20 | 20 | 23 | 33 | F | Transformed Migraine | Supplements/Herbal Remedies | None declared | 6 |
| 21 | 28 | 30 | 35 | F | Migraine without aura | Tricyclic Antidepressants | Smoker | 24 |
| 22 | 5 | 2 | 25 | M | Tension-type headache | NSAIDs | Smoker Energy Drinks Psychoactive Drugs | 48 |
| 23 | 16 | 40 | 48 | M | Tension-type headache | Supplements/Herbal Remedies Tricyclic Antidepressants | None declared | 1 |
| 24 | 55 | 36 | 47 | F | Migraine without aura | Triptans Tricyclic Antidepressants | Smoker | 396 |
| 25 | 18 | 58 | 42 | M | Tension-type headache | Tricyclic Antidepressants SSRIs | Recent suppression of caffeine excess Recent suppression of smoking | 18 |
| 26 | 39 | 19 | 69 | F | Arnold Neuralgia | Anticonvulsivants | None declared | 36 |
| 27 | 32 | 61 | 35 | F | Tension-type headache | Tricyclic Antidepressants | Smoker Night Shifts | 12 |
| 28 | 40 | 62 | 23 | F | Migraine | NSAIDs | None declared | 60 |
| 29 | 36 | 71 | 70 | F | Tension-type headache | Supplements/Herbal Remedies | Obesity | 36 |
| 30 | 37 | 63 | 35 | M | Degenerative C7-C8 disk disease | NSAIDs | None declared | 12 |
| 31 | 14 | 35 | 51 | M | Tension-type headache | Tricyclic Antidepressants Anticonvulsivants | None declared | 132 |
| 32 | 41 | 73 | 41 | F | Tension-type headache | None | Night Shifts | 60 |
| 33 | 26 | 34 | 34 | F | Mixed tension headache Pharmacologically induced Headache (Multiclass drug-resistant pain) | Triptans Non-NSAID analgesics | None declared | 96 |
| 34 | 27 | 34 | 60 | F | Tension-type headache with migraine elements | Supplements/Herbal Remedies Non-NSAID analgesics | Occasional alcohol consumption Occasional smoking | 180 |
| 35 | 25 | 46 | 51 | F | Migraine and Hypnic Headache | NSAIDs Supplements/Herbal Remedies Triptans | Emotional Distress | 120 |
| 36 | 41 | 46 | 38 | M | Tension-type headache | Opioids (Multiclass drug-resistant pain) | None declared | 36 |
| 37 | 34 | 33 | 72 | F | Degenerative C3-C6 disk disease | None | None declared | 60 |
| 38 | 9 | 27 | 34 | F | Tension-type headache | None | None declared | 60 |
| 39 | 10 | 30 | 28 | F | Migraine | Supplements/Herbal Remedies (Multiclass drug-resistant pain) | None declared | 42 |
| 40 | 20 | 12 | 41 | F | Tension-type headache | SNRIs | None declared | 168 |
| 41 | 51 | 47 | 36 | F | Transformed Migraine | Triptans | Analgetic abuse | 264 |
| 42 | 13 | 12 | 24 | M | Low Back Pain | None | None declared | 60 |
| 43 | 28 | 35 | 32 | M | Migraine with aura | None | None declared | 214 |
| 44 | 27 | 34 | 30 | F | Tension-type headache | None | Smoker Alcohol consumption | 12 |
| 45 | 5 | 1 | 63 | M | Tension-type headache | Supplements/Herbal Remedies | Alcohol consumption | 36 |
| 46 | 18 | 10 | 37 | M | Tension-type headache | None | None declared | 1 |
| 47 | 39 | 33 | 46 | M | Chronic headache | None | Emotional Distress | 516 |
| 48 | 32 | 58 | 24 | M | Migraine with aura | Supplements/Herbal Remedies | Smoker Alcohol consumption | 18 |
| Shapiro–Wilk Test | Significance Testing (Statistical Test) | Shapiro–Wilk Test | Significance Testing (Statistical Test/Mean) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition | Headache (n = 28) | Migraine (n = 13) | Spine-Related Pain (n = 7) | Headache Subgroup (n = 12) | Control (n = 12) | ||||
| Age (Mean± SD) | 42.393 11.272 | 37.615 12.862 | 51.143 18.792 | Headache—0.651; Migraine—0.075; Spine—0.349 | 0.168 (Kruskal–Wallis Test) * | 34.333 7.475 | 30.083 8.512 | Headache Subgroup—0.364 Control—0.010 | <0.164 (Mann–Whitney U test) |
| Sex (M/F) | 12/16 | 2/11 | 2/5 | N/A | Headache vs. Migraine = 0.156; Headache vs. Spine = 0.676; Migraine vs. Spine = 0.587 (Fisher’s Exact Test, all three comparisons) | 8/4 | 8/4 | N/A | 1.00 (Fisher’s exact test) |
| McGill Score (Mean SD) | 23.500 10.655 | 31.231 12.950 | 21.429 14.432 | Headache—0.492; Migraine—0.863; Spine—0.036 | 0.163 (Kruskal–Wallis Test) | 27.000 10.436 | N/A | N/A | N/A |
| CSI (Mean SD) | 36.464 19.259 | 38.231 13.430 | 31.286 16.760 | Headache—0.554; Migraine—0.650; Spine—0.418 | 0.608 (Kruskal–Wallis Test) * | 31.083 15.826 | N/A | N/A | N/A |
| Average Pain Duration (Months, Mean SD) | 85.741 110.125 | 119.077 121.586 | 33 24.062 | Headache—0.001; Migraine—0.037; Spine—0.492 | 0.225 (Kruskal–Wallis Test) | 96.896 157.821 | N/A | N/A | N/A |
| Headache | Migraine | Spine-Related Pain | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction Time | Alpha | Beta | CSI Score | McGill Score | Pain Duration (months) | Alpha | Beta | CSI Score | McGill Score | Pain Duration (months) | Alpha | Beta | CSI Score | McGill Score | Pain Duration (months) |
| Alpha | 0.672 *** | 0.753 ** | 0.657 * | ||||||||||||
| Beta | 0.672 *** | 0.753 ** | 0.566 * | ||||||||||||
| CSI Score | 0.504 ** | ||||||||||||||
| McGill Score | 0.504 ** | −0.382 * | 0.657 * | 0.566 * | |||||||||||
| Pain Duration (months) | 0.382 * | ||||||||||||||
| Working Memory | |||||||||||||||
| Alpha | 0.588 ** | 0.802 ** | 0.687 ** | 0.857 * | |||||||||||
| Beta | 0.588 ** | 0.802 ** | 0.659 * | 0.857 * | |||||||||||
| CSI Score | 0.504 ** | ||||||||||||||
| McGill Score | 0.504 ** | 0.382 * | 0.687 ** | 0.659 * | |||||||||||
| Pain Duration (months) | −0.382 * | ||||||||||||||
| Multitasking | |||||||||||||||
| Alpha | 0.758 *** | 0.802 ** | 0.786 * | ||||||||||||
| Beta | 0.758 *** | 0.802 ** | 0.582 * | 0.786 * | |||||||||||
| CSI Score | 0.504 ** | ||||||||||||||
| McGill Score | 0.504 ** | 0.382 * | 0.582 * | ||||||||||||
| Pain Duration (months) | 0.382 * | ||||||||||||||
| Resting-State | |||||||||||||||
| Alpha | 0.563 ** | 0.824 *** | 0.929 ** | ||||||||||||
| Beta | 0.563 ** | 0.824 *** | 0.929 ** | ||||||||||||
| CSI Score | 0.504 ** | ||||||||||||||
| McGill Score | 0.504 ** | 0.382 * | |||||||||||||
| Pain Duration (months) | 0.382 * | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chertic, D.; Dăbală, V.; Livinț-Popa, L.; Balea, M.; Drăghici, N.C.; Strilciuc, Ș.; Cherecheș, R.; Văcăraș, V.; Mureșanu, D.F. EEG Spectral Analysis in Chronic Pain During Rest and Cognitive Reasoning. Sensors 2025, 25, 6230. https://doi.org/10.3390/s25196230
Chertic D, Dăbală V, Livinț-Popa L, Balea M, Drăghici NC, Strilciuc Ș, Cherecheș R, Văcăraș V, Mureșanu DF. EEG Spectral Analysis in Chronic Pain During Rest and Cognitive Reasoning. Sensors. 2025; 25(19):6230. https://doi.org/10.3390/s25196230
Chicago/Turabian StyleChertic, Diana, Victor Dăbală, Livia Livinț-Popa, Maria Balea, Nicu Cătălin Drăghici, Ștefan Strilciuc, Răzvan Cherecheș, Vitalie Văcăraș, and Dafin F. Mureșanu. 2025. "EEG Spectral Analysis in Chronic Pain During Rest and Cognitive Reasoning" Sensors 25, no. 19: 6230. https://doi.org/10.3390/s25196230
APA StyleChertic, D., Dăbală, V., Livinț-Popa, L., Balea, M., Drăghici, N. C., Strilciuc, Ș., Cherecheș, R., Văcăraș, V., & Mureșanu, D. F. (2025). EEG Spectral Analysis in Chronic Pain During Rest and Cognitive Reasoning. Sensors, 25(19), 6230. https://doi.org/10.3390/s25196230








