Recent Advancement in Non-Enzymatic Electrochemical Detection of Lactate Based on Metal Nanomaterials: A Review
Abstract
1. Introduction
2. Biofluids Withdrawing Techniques of Wearable Lactate Sensors
2.1. Blood
2.2. Interstitial Fluid
2.3. Saliva
2.4. Tears
2.5. Sweat
3. Non-Enzymatic Lactate Sensing Material
3.1. Bimetallic Nanomaterials
3.2. TMC
3.3. Metal Oxides
3.4. Layered Double Hydroxides
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NELESs | Non-enzymatic lactate electrochemical sensors |
| ISF | Interstitial fluid |
| LOD | Limit of detection |
| RSD | Relative standard deviation |
| PBS | Phosphate buffer solution |
| RI | Reverse iontophoretic |
| N-CNTs | Nitrogen-doped carbon nanotubes |
| LOx | Lactate oxidase |
| TMC | Transition metal chalcogenides |
| SPEs | Screen-printed electrodes |
| LDHs | Layered double hydroxides |
References
- Huang, P.J.; Liu, J. Simultaneous Detection of L—Lactate and D—Glucose Using DNA Aptamers in Human Blood Serum. Angew. Chem. Int. Ed. 2023, 62, e202212879. [Google Scholar] [CrossRef]
- Aburto, C.; Galaz, A.; Bernier, A.; Sandoval, P.Y.; Holtheuer-Gallardo, S.; Ruminot, I.; Soto-Ojeda, I.; Hertenstein, H.; Schweizer, J.A.; Schirmeier, S.; et al. Single-Fluorophore Indicator to Explore Cellular and Sub-cellular Lactate Dynamics. ACS Sens. 2022, 7, 3278–3286. [Google Scholar] [CrossRef]
- Moradi, S.; Firoozbakhtian, A.; Hosseini, M.; Karaman, O.; Kalikeri, S.; Raja, G.G.; Karimi-Maleh, H. Advancements in Wearable Technology for Monitoring Lactate Levels Using Lactate Oxidase Enzyme and Free Enzyme as Analytical Approaches: A Review. Int. J. Biol. Macromol. 2024, 254, 127577. [Google Scholar] [CrossRef]
- Liu, G.; Xia, T.; Liang, X.; Hou, S.; Hou, S. Enzymatic Electrochemical Biosensor from Eu-Doped SnO2 Embedded in MXene for High Performance Sensing Lactate. ChemElectroChem 2022, 9, e202200848. [Google Scholar] [CrossRef]
- Tan, S.C.L.; Ning, Y.; Yu, Y.; Goh, W.P.; Jiang, C.; Liu, L.; Zheng, X.T.; Yang, L. Stretchable Sweat Lactate Sensor with Dual-Signal Read-Outs. Chem. Asian J. 2024, 19, e202400496. [Google Scholar] [CrossRef]
- He, Q.; Wang, C.; Jain, R.; Byrnes, J.; Farquhar, E.R.; Reed, E.; Berezovsky, E.; Chance, M.R.; Lodowski, D.; An, R. An Engineered Lactate Oxidase Based Electrochemical Sensor for Continuous Detection of Biomarker Lactic Acid in Human Sweat and Serum. Heliyon 2024, 10, e34301. [Google Scholar] [CrossRef] [PubMed]
- Olaetxea, I.; Valero, A.; Lopez, E.; Lafuente, H.; Izeta, A.; Jaunarena, I.; Seifert, A. Machine Learning-Assisted Raman Spectroscopy for pH and Lactate Sensing in Body Fluids. Anal. Chem. 2020, 92, 13888–13895. [Google Scholar] [CrossRef] [PubMed]
- Colombi, S.; Macor, L.P.; Ortiz-Membrado, L.; Pérez-Amodio, S.; Jiménez-Piqué, E.; Engel, E.; Pérez-Madrigal, M.M.; García-Torres, J.; Alemán, C. Enzymatic Degradation of Polylactic Acid Fibers Supported on a Hydrogel for Sustained Release of Lactate. ACS Appl. Bio Mater. 2023, 6, 3889–3901. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Y.; Liu, Y.; Zhang, T.; Zhao, Y.; Zang, J.; Yang, Y.; He, R.; Chong, G.; Ruan, S.; et al. Dual Closed-Loop of Catalyzed Lactate Depletion and Immune Response to Potentiate Photothermal Immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 23260–23276. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Ge, J. Multienzyme System in Amorphous Metal–Organic Frameworks for Intracellular Lactate Detection. Nano Lett. 2022, 22, 5029–5036. [Google Scholar] [CrossRef]
- Sun, L.; Gao, W.; Liu, J.; Wang, J.; Li, L.; Yu, H.; Xu, Z.P. O2-Supplying Nanozymes Alleviate Hypoxia and Deplete Lactate to Eliminate Tumors and Activate Antitumor Immunity. ACS Appl. Mate. Interfaces 2022, 14, 56644–56657. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, L.; Guan, T.; Xu, Y.; Guo, C.; Li, D.; He, Y.; Ji, Y. The Real-Time Determination of D- and L-Lactate Based on Optical Weak Measurement. Anal. Methods 2019, 11, 2223–2230. [Google Scholar] [CrossRef]
- Mustafa, Y.L.; Leese, H.S. Fabrication of a Lactate-Specific Molecularly Imprinted Polymer toward Disease Detection. ACS Omega 2023, 8, 8732–8742. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Lawrence, M.M.; Berg, H.; Lyons, M.A.; Shreim, S.; Keating, M.T.; Weidling, J.; Botvinick, E.L. Transcutaneous Flexible Sensor for In Vivo Photonic Detection of pH and Lactate. ACS Sens. 2022, 7, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Kiatamornrak, P.; Boobphahom, S.; Lertussavavivat, T.; Rattanawaleedirojn, P.; Chailapakul, O.; Rodthongkum, N.; Srisawat, N. A Portable Blood Lactate Sensor with a Non-Immobilized Enzyme for Early Sepsis Diagnosis. Analyst 2022, 147, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- Zhang, Z.; Kwok, R.T.K.; Yu, Y.; Tang, B.Z.; Ng, K.M. Sensitive and Specific Detection of l-Lactate Using an AIE-Active Fluorophore. ACS Appl. Mater. Interfaces 2017, 9, 38153–38158. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Mao, H.; Zhou, L.; Wu, Z.; Lu, Y.; Sun, T.; Hui, J.; Ma, G. AuNP/Magnetic Bead-Enhanced Electrochemical Sensor Toward Dual Saliva Alzheimer’s Biomarkers Detection. Sensors 2025, 25, 4088. [Google Scholar] [CrossRef]
- Wu, H.; Wen, Q.; Luan, X.; Yang, W.; Guo, L.; Wei, G. Facile Synthesis of Fe-Doped, Algae Residue-Derived Carbon Aerogels for Electrochemical Dopamine Biosensors. Sensors 2024, 24, 2787. [Google Scholar] [CrossRef]
- Wei, Y.; Li, R.; Lin, M. Gold–Mercury–Platinum Alloy for Light-Enhanced Electrochemical Detection of Hydrogen Peroxide. Sensors 2024, 25, 135. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Montiel, V.R.-V.; Vargas, E.; Teymourian, H.; Wang, J. Wearable and Mobile Sensors for Personalized Nutrition. ACS Sens. 2021, 6, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, Y.; Gao, H.; Xu, L.-P.; Wang, S. Bioinspired Superwettable Electrodes towards Electrochemical Biosensing. Chem. Sci. 2022, 13, 5069–5084. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Yang, A.; Yan, F. Flexible Electrochemical Biosensors for Health Monitoring. ACS Appl. Electron. Mater. 2020, 3, 53–67. [Google Scholar] [CrossRef]
- Gu, W. Application of Nano-Alumina Electrodes in Electrochemical Sensing for Monitoring Exercise-Induced Lactate. Int. J. Electrochem. Sci. 2025, 20, 101124. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Zhang, Q.; Ye, Y.; Cai, Y.; Li, K.; Li, Y.; Huang, X.; Wang, Y. In-Situ Preparation of Lactate-Sensing Membrane for the Noninvasive and Wearable Analysis of Sweat. Biosens. Bioelectron. 2022, 210, 114303. [Google Scholar] [CrossRef]
- Phumma, R.; Phamonpon, W.; Rodthongkum, N.; Ummartyotin, S. Fabrication of Silver Nanoparticle Loaded into Nanocellulose Derived from Hemp and Poly(vinyl alcohol)-Based Composite as an Electrode for Electrochemical Sensors for Lactate Determination. ACS Omega 2024, 9, 10371–10379. [Google Scholar] [CrossRef]
- Xuan, X.; Pérez-Ràfols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable Gold Fiber-Based Wearable Textile Electrochemical Biosensor for Lactate Monitoring in Sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in situ Perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Y.; Wu, H.; Yan, H.; Liu, Y.; Lučev Vasić, Ž.; Pan, H.; Cifrek, M.; Du, M.; Gao, Y. Smartphone-based Electrochemical On-site Quantitative Detection Device for Nonenzyme Lactate Detection. Electroanalysis 2022, 34, 1411–1421. [Google Scholar] [CrossRef]
- Chang, A.S.; Memon, N.N.; Amin, S.; Chang, F.; Aftab, U.; Abro, M.I.; dad Chandio, A.; Shah, A.A.; Ibupoto, M.H.; Ansari, M.A.; et al. Facile Non-enzymatic Lactic Acid Sensor Based on Cobalt Oxide Nanostructures. Electroanalysis 2019, 31, 1296–1303. [Google Scholar] [CrossRef]
- Jeganathan, C.; Mitsuboshi, H.; Yamamoto, H.; Motoyama, Y.; Kokado, K.; Hara, M.; Yoshimura, M. Hydrogen-Substituted Graphdiyne Encapsulated Cu2O Nanowires as Binder-Free Electrodes for Non-enzymatic Glucose Sensing. ACS Appl. Nano Mater. 2024, 7, 20665–20677. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, J.; Liu, C.; Xu, T.; Zhang, X. Machine Learning with Neural Networks to Enhance Selectivity of Nonenzymatic Electrochemical Biosensors in Multianalyte Mixtures. ACS Appl. Mater. Interfaces 2022, 14, 52684–52690. [Google Scholar] [CrossRef]
- Lin, S.-H.; Lefeuvre, E.; Wang, H.-Y. Battery-Less Lactate Monitoring System Using a Non-enzymatic Sensor with Selectivity. Taiwan Inst. Chem. Eng. 2024, 160, 105393. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical Non-enzymatic Glucose Sensors: Recent Progress and Perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef]
- Manivel, P.; Suryanarayanan, V.; Nesakumar, N.; Velayutham, D.; Madasamy, K.; Kathiresan, M.; Kulandaisamy, A.J.; Rayappan, J.B.B. A Novel Electrochemical Sensor Based on a Nickel-Metal Organic Framework for Efficient Electrocatalytic Oxidation and Rapid Detection of Lactate. New J. Chem. 2018, 42, 11839–11846. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Zhong, Y.; Li, R.; Deng, W.; Xiao, X.; Xu, Y.; Zhang, J.; Hu, X.; Wang, Y. A Review of Nanomaterials for Biosensing Applications. J. Mater. Chem. B 2024, 12, 1168–1193. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Tong, J.; Chen, X.; Deng, W.; Chen, Z.; Xiao, X.; Yin, Y.; Zhou, Q.; Gao, Y.; et al. Advanced NanoMaterials for Electrochemical Sensors: Application in Wearable Tear Glucose Sensing Technology. J. Mater. Chem. B 2024, 12, 6774–6804. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent Advances in Non-enzymatic Electrochemical Glucose Sensors Based on Non-Precious Transition Metal Materials: Opportunities and Challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Wu, J.; Li, H.; Peng, H.; Zhang, J.; Ding, K.; Wang, X.; Hou, C.; Zhang, H.; et al. Smart Wearable Sensor Fuels Noninvasive Body Fluid Analysis. ACS Appl. Mater. Interfaces 2025, 17, 13279–13301. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; RoyChoudhury, S.; Jalal, A.H.; Umasankar, Y.; Forouzanfar, S.; Akter, N.; Bhansali, S.; Pala, N. Lactate Biosensing: The Emerging Point-of-Care and Personal Health Monitoring. Biosens. Bioelectron. 2018, 117, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ausri, I.R.; Wang, Z.; Derry, C.; Tang, X.S. Towards a Transdermal Membrane Biosensor for the Detection of Lactate in Body Fluids. Sens. Actuators B Chem. 2020, 308, 127645. [Google Scholar] [CrossRef]
- De la Paz, E.; Saha, T.; Del Cano, R.; Seker, S.; Kshirsagar, N.; Wang, J. Non-Invasive Monitoring of Interstitial Fluid Lactate through an Epidermal Iontophoretic Device. Talanta 2023, 254, 124122. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Z.; Dong, H.; Liu, Y.; Chu, Z.; Mou, Y.; Jin, W. MnFe@N-CNTs Based Lactate Biomicrochips for Nonintrusive and Onsite Periodontitis Diagnosis. ACS Appl. Mater. Interfaces 2024, 16, 20221–20231. [Google Scholar] [CrossRef]
- Lin, C.-E.; Hiraka, K.; Matloff, D.; Johns, J.; Deng, A.; Sode, K.; La Belle, J. Development toward a Novel Integrated Tear Lactate Sensor Using Schirmer Test Strip and Engineered Lactate Oxidase. Sens. Actuators B Chem. 2018, 270, 525–529. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Cao, X.Q.; Hua, Y.; Yu, C.M. A Bifunctional Wearable Sensor Based on a Nanoporous Membrane for Simultaneous Detection of Sweat Lactate and Temperature. Anal. Chem. 2024, 96, 3087–3095. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing Analytes in Biofluids for Peripheral Biochemical Monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Mirzaei, Y.; Gholami, A.; Bordbar, M.M. A Distance-Based Paper Sensor for Rapid Detection of Blood Lactate Concentration Using Gold Nanoparticles Synthesized by Satureja Hortensis. Sens. Actuators B Chem. 2021, 345, 130445. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Cheng, Y.; Lee, C.; Tsai, H. An Inkjet-Printed Flexible Non-Enzymatic Lactate Sensor for Clinical Blood Plasma Test. IEEE Electron. Device Lett. 2020, 41, 597–600. [Google Scholar] [CrossRef]
- Li, H.; Gu, S.; Zhang, Q.; Song, E.; Kuang, T.; Chen, F.; Yu, X.; Chang, L. Recent Advances in Biofluid Detection with Micro/nanostructured Bioelectronic Devices. Nanoscale 2021, 13, 3436–3453. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Harvesting and Manipulating Sweat and Interstitial Fluid in Microfluidic Devices. Lab Chip 2024, 24, 1244–1265. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the Biosensors for Lactate and Pyruvate Detection for Medical Applications: A Review. TrAC Trends Anal. Chem. 2019, 110, 160–172. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors Based on Electrochemical Lactate Detection: A Comprehensive Review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of Lactic Acid with Special Emphasis on Biosensing Methods: A Review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Imanzadeh, H.; Amiri, M.; Nozari-Asbemarz, M. A Novel NiO/C@rGO Nanocomposite Derived from Ni(gallate): A Non-Enzymatic Electrochemical Glucose Sensor. Microchem. J. 2024, 199, 110106. [Google Scholar] [CrossRef]
- Lu, Z.; Ke, X.; Zhao, Z.; Huang, J.; Liu, C.; Wang, J.; Xu, R.; Mei, Y.; Huang, G. Fabrication of NiCo Bimetallic MOF Films on 3D Foam with Assistance of Atomic Layer Deposition for Non-Invasive Lactic Acid Sensing. ACS Appl. Mater. Interfaces 2024, 16, 14218–14228. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Shankar, A.; Maduraiveeran, G. Hollow Sphere Nickel Sulfide Nanostructures-Based Enzyme Mimic ElectroChemical Sensor Platform for Lactic Acid in Human Urine. Mikrochim. Acta 2020, 187, 468. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, M.; Maduraiveeran, G. Ultra-Fine Nickel Sulfide Nanoclusters @ Nickel Sulfide Microsphere as Enzyme-Free Electrode Materials for Sensitive Detection of Lactic Acid. J. Electroanal. Chem. 2020, 874, 114465. [Google Scholar] [CrossRef]
- Tao, B.; Ren, X.; Liu, X.; Miao, F. NiS-Modified Zeolite Imidazolic Acid Frame-67 as a Bi-Functional Catalyst: A Non-enzymatic Lactic Acid Sensor and Supercapacitor. Vacuum 2024, 224, 113181. [Google Scholar] [CrossRef]
- Xiao, H.; Cao, L.; Qin, H.; Wei, S.; Gu, M.; Zhao, F.; Chen, Z. Non-enzymatic Lactic Acid Sensor Based on AuPtNPs Functionalized MoS2 Nanosheet as Electrode Modified Materials. J. Electroanal. Chem. 2021, 903, 115806. [Google Scholar] [CrossRef]
- Kim, S.; Yang, W.S.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Seo, S.-J.; Park, Y.M. Highly Sensitive Non-enzymatic Lactate Biosensor Driven by Porous Nanostructured Nickel Oxide. Ceram. Int. 2019, 45, 23370–23376. [Google Scholar] [CrossRef]
- Nasiri, R.; Guagliano, G.; Van Gastel, D.; Sanei, R.; Madadelahi, M.; Tanriverdi, S.; Jain, S.; Fayazbaksh, F.; Lee, S.W.; Zhu, Y.; et al. Electrochemical Dual-Sensing of Lactate and Glucose Using NiO Nanoparticles with Cross-Sensitivity Calibration. Talanta 2026, 297, 128678. [Google Scholar] [CrossRef]
- Sajna, M.S.; Cabibihan, J.-J.; Malik, R.A.; Kumar Sadasivuni, K.; Geetha, M.; Khalid Alahmad, J.; Anwar Hijazi, D.; Alsaedi, F. Nonenzymatic Electrochemical Lactic Acid Sensor Using CuO Nanocomposite. Mater. Sci. Eng. B 2023, 288, 116217. [Google Scholar] [CrossRef]
- Tsou, K.L.; Chen, K.Y.; Chou, Y.D.; Cheng, Y.T.; Tsai, H.E.; Lee, C.K. Inkjet-Printed Flexible Non-enzymatic Lactate Sensor with High Sensitivity and Low Interference Using a Stacked NiOx/NiOx-Nafion Nanocomposite Electrode with Clinical Blood Test Verification. Talanta 2022, 249, 123598. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Tsao, P.-K.; Rinawati, M.; Chen, K.-J.; Chen, K.-Y.; Chang, C.Y.; Yeh, M.-H. Designing ZIF-67 Derived NiCo Layered Double Hydroxides with 3D Hierarchical Structure for Enzyme-Free Electrochemical Lactate Monitoring in Human Sweat. Chem. Eng. J. 2022, 427, 131687. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Tsao, P.-K.; Chen, K.-J.; Lin, Y.-C.; Aulia, S.; Chang, L.-Y.; Ho, K.-C.; Chang, C.Y.; Mizuguchi, H.; Yeh, M.-H. Designing Bimetallic Ni-Based Layered Double Hydroxides for Enzyme-Free Electrochemical Lactate Biosensors. Sens. Actuators B Chem. 2021, 346, 130505. [Google Scholar] [CrossRef]
- Shin, K.Y.; Kim, Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Bimetal-Decorated Resistive Gas Sensors: A Review. J. Mater. Chem. C 2025, 13, 9930–9950. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Tian, X.; Jiang, Y.; Ma, H.-Y.; Wang, W.; Zhang, W.-S.; Martin, J.S.; Yan, Y.; Qin, D.-D.; Han, D.-X.; et al. Advances in Bimetallic Materials and Bimetallic Oxide Nanozymes: Synthesis, Classification, Catalytic Mechanism and Application in Analytical Chemistry. TrAC Trends Anal. Chem. 2024, 176, 117757. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Mengesha, A.M.; Melkamu, W.W.; Abebe, A. Biogenic Ag-Based Bimetallic Nanoparticle for Extraordinary Medicinal and Photocatalytic Application. Results Chem. 2025, 16, 102395. [Google Scholar] [CrossRef]
- Kim, S.E.; Muthurasu, A. Highly Oriented Nitrogen-doped Carbon Nanotube Integrated Bimetallic Cobalt Copper Organic Framework for Non-enzymatic Electrochemical Glucose and Hydrogen Peroxide Sensor. Electroanalysis 2021, 33, 1333–1345. [Google Scholar] [CrossRef]
- Shaban, S.M.; Kim, S.; Basiony, N.M.E.; Kappen, J.; Mostafa, M.H.; Elbalaawy, A.Y.; Elmasry, M.R.; Shin, J.; Jeon, I.; Kim, D.H. Ecofriendly Sunlight-Mediated Nontoxic Bimetallic Nanoparticles: Synthesis, Reusable Catalytic Membrane, and Biosensor Applications. Adv. Sci. 2025, 12, e2503120. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zhao, P.; Fei, J.; Xie, Y. A Review on Pd-M Bimetallic Electrochemical Sensors: Techniques, Performance, and Applications. Talanta 2025, 282, 126989. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Recent Progress of Ultrathin 2D Pd-Based Nanomaterials for Fuel Cell Electrocatalysis. Small 2021, 17, 2005092. [Google Scholar] [CrossRef]
- Dickinson, R.G.; Pauling, L. The Crystal Structure of Molybdenite. J. Am. Chem. Soc. 1923, 45, 1466–1471. [Google Scholar] [CrossRef]
- Roy, S.; Joseph, A.; Zhang, X.; Bhattacharyya, S.; Puthirath, A.B.; Biswas, A.; Tiwary, C.S.; Vajtai, R.; Ajayan, P.M. Engineered Two-Dimensional Transition Metal Dichalcogenides for Energy Conversion and Storage. Chem. Rev. 2024, 124, 9376–9456. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Q.; Zhao, X.; Liu, M.; Wee, A.T.S. Defect Engineering of Two-Dimensional Transition-Metal Dichalcogenides: Applications, Challenges, and Opportunities. ACS Nano 2021, 15, 2165–2181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Aljarb, A.; Shi, Y.; Li, L.J. Epitaxial Growth of Two-Dimensional Layered Transition-Metal Dichalcogenides: Growth Mechanism, Controllability, and Scalability. Chem. Rev. 2018, 118, 6134–6150. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; He, Q.; Huang, Y.; Duan, X.; Lin, Z. Solution-Processable and Printable Two-Dimensional Transition Metal Dichalcogenide Inks. Chem. Rev. 2024, 124, 5795–5845. [Google Scholar] [CrossRef] [PubMed]
- Sovizi, S.; Angizi, S.; Ahmad Alem, S.A.; Goodarzi, R.; Taji Boyuk, M.R.R.; Ghanbari, H.; Szoszkiewicz, R.; Simchi, A.; Kruse, P. Plasma Processing and Treatment of 2D Transition Metal Dichalcogenides: Tuning Properties and Defect Engineering. Chem. Rev. 2023, 123, 13869–13951. [Google Scholar] [CrossRef]
- Dhakal, K.P.; Roy, S.; Jang, H.; Chen, X.; Yun, W.S.; Kim, H.; Lee, J.; Kim, J.; Ahn, J.-H. Local Strain Induced Band Gap Modulation and Photoluminescence Enhancement of Multilayer Transition Metal Dichalcogenides. Chem. Mater. 2017, 29, 5124–5133. [Google Scholar] [CrossRef]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.A.; Wang, Q.H.; Chae, J. Low Cytotoxicity and Genotoxicity of Two-Dimensional MoS2 and WS2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef]
- Chen, X.; Ahn, J.H. Biodegradable and Bioabsorbable Sensors Based on Two-Dimensional Materials. J. Mater. Chem. B 2020, 8, 1082–1092. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Jiang, L.; Gao, J.; Tang, Z.; Xu, Y.; Chen, X.; Zhang, H. Transition Metal Dichalcogenides for Sensing and Oncotherapy: Status, Challenges, and Perspective. Adv. Funct. Mater. 2021, 31, 2004408. [Google Scholar] [CrossRef]
- Chitare, Y.M.; Jadhav, S.B.; Pawaskar, P.N.; Magdum, V.V.; Gunjakar, J.L.; Lokhande, C.D. Metal Oxide-Based Composites in Nonenzymatic Electrochemical Glucose Sensors. Ind. Eng. Chem. Res. 2021, 60, 18195–18217. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Annaraj, J.; Chang, P.-L.; Selvaraj, M.; Singh, G.; Arumugam, B. Bioflocculant Polymer Reduced CuO/NiO Binary Transition Metal Oxide Nanocomposite: Application as an Effective Non-enzymatic Glucose Sensor. Inorg. Chem. Commun. 2024, 170, 113250. [Google Scholar] [CrossRef]
- Jannath, K.A.; Karim, M.M.; Saputra, H.A.; Seo, K.D.; Kim, K.B.; Shim, Y.B. A Review on the Recent Advancements in NanoMaterials for Nonenzymatic Lactate Sensing. Bull. Korean Chem. Soc. 2023, 44, 407–419. [Google Scholar] [CrossRef]
- Ratnam, K.V.; Manjunatha, H.; Janardan, S.; Babu Naidu, K.C.; Ramesh, S. Nonenzymatic Electrochemical Sensor Based on Metal Oxide, MO (M = Cu, Ni, Zn, and Fe) Nanomaterials for Neurotransmitters: An Abridged Review. Sens. Int. 2020, 1, 100047. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, V.T. Copper Oxide Nanomaterials Prepared by Solution Methods, Some Properties, and Potential Applications: A Brief Review. Int. Sch. Res. Not. 2014, 2014, 856592. [Google Scholar] [CrossRef]
- Okoye, P.C.; Azi, S.O.; Qahtan, T.F.; Owolabi, T.O.; Saleh, T.A. Synthesis, Properties, and Applications of Doped and Undoped CuO and Cu2O Nanomaterials. Mater. Today Chem. 2023, 30, 101513. [Google Scholar] [CrossRef]
- Murugan, B.; Rahman, M.Z.; Fatimah, I.; Anita Lett, J.; Annaraj, J.; Kaus, N.H.M.; Al-Anber, M.A.; Sagadevan, S. Green Synthesis of CuO Nanoparticles for Biological Applications. Inorg. Chem. Commun. 2023, 155, 111088. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered Double Hydroxide-Based Nanomaterials for Biomedical Applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Mousty, C.; Farhat, H. Recent Advances in Layered Double Hydroxides-Based Electrochemical Sensors: Insight in Transition Metal Contribution. Electroanalysis 2023, 35, e202200527. [Google Scholar] [CrossRef]
- Ni, G.; Cheng, J.; Dai, X.; Guo, Z.; Ling, X.; Yu, T.; Sun, Z. Integrating Ultrathin Polypyrrole Framework on Nickel-Cobalt Layered Double Hydroxide as an Amperometric Sensor for Non-enzymatic Glucose Determination. Electroanalysis 2018, 30, 2366–2373. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Fan, S.; Wu, F.; Hu, B.; Meng, F. Highly Sensitive Nonenzymatic Glucose Sensor Based on 3D Ultrathin NiFe Layered Double Hydroxide Nanosheets. Electroanalysis 2017, 29, 1755–1761. [Google Scholar] [CrossRef]
- Dou, L.; Xiao, K. High Entropy Layered Double Hydroxide Nanozyme for Sensitive Detection of Tetracycline. ACS Appl. Nano Mater. 2025, 8, 2456–2465. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Liang, W.; Chen, L.; Li, Y.; He, X. Non-enzymatic Glucose Sensor with High Sensitivity Based on Cu-Al Layered Double Hydroxides. Sens. Actuators B Chem. 2018, 273, 41–47. [Google Scholar] [CrossRef]
- Li, J.; Zhao, N.; Liu, X.; Chang, X.; Zheng, W.; Zhang, J. Two-Dimensional Layered Double Hydroxides for Advanced Sensors. Coordin. Chem. Rev. 2025, 523, 216262. [Google Scholar] [CrossRef]
- Ahmad, S.; Wazir, M.B.; Daud, M.; Kui Cheng, C.; Ul Hassan Shah, M.; Al-Harthi, M.A. Recent Advancement in Ionic Liquid Modified Layered Double Hydroxide (IL-LDH): Progress, Challenges, and Future Prospects. Inorg. Chem. Commun. 2023, 158, 111591. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.; Sridhar, D.; Xu, B.B.; Algadi, H.; El-Bahy, Z.M.; Ma, Y.; Li, T.; Guo, Z. Progress of Layered Double Hydroxide-Based Materials for Supercapacitors. Mater. Chem. Front. 2023, 7, 1520–1561. [Google Scholar] [CrossRef]
- Dong, J.; Ouyang, X.; Huo, B.; Deng, D.; Yan, X.; Luo, L. CuO/CoZn-Layered Double-Hydroxide Nanowires on Carbon Cloth as an Enzyme-Free H2O2 Sensor. ACS Appl. Nano Mater. 2024, 7, 6564–6573. [Google Scholar] [CrossRef]
- Shishegari, N.; Sabahi, A.; Manteghi, F.; Ghaffarinejad, A.; Tehrani, Z. Non-enzymatic Sensor Based on Nitrogen-Doped Graphene Modified with Pd Nano-Particles and NiAl Layered Double Hydroxide for Glucose Determination in Blood. J. Electroanal. Chem. 2020, 871, 114285. [Google Scholar] [CrossRef]
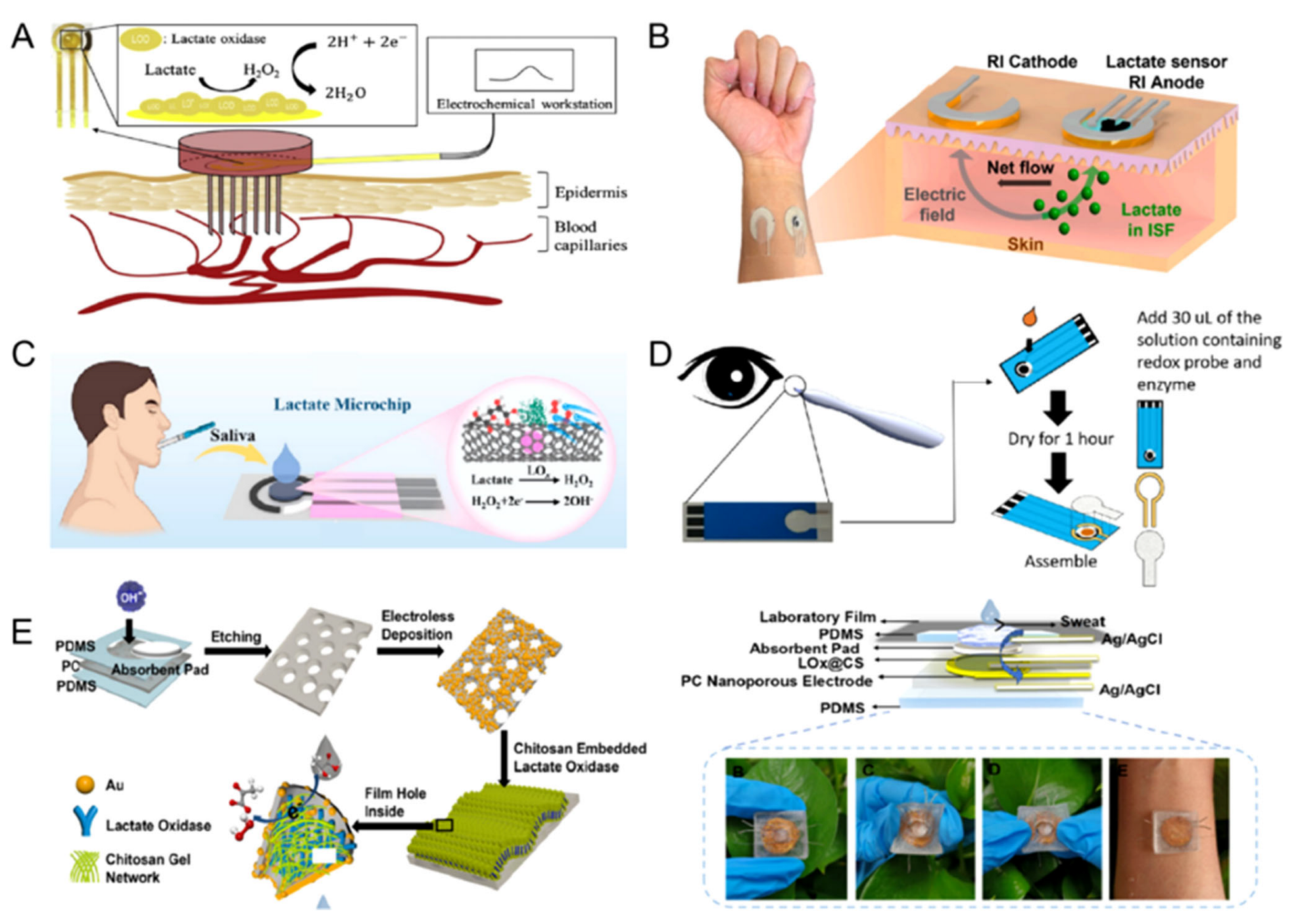

| Electrolyte | Sensitivity | Linear Range | Limit of Detection (LOD) | Response Time | Stability | Reproducibility (Relative Standard Deviation, RSD) | Application (Biological Samples) | Ref |
|---|---|---|---|---|---|---|---|---|
| Blood plasma | 0.337 μA mM−1 | 5–30 mM | - | 15 s | 8 h | - | Blood plasma | [46] |
| Phosphate buffer solutions (PBS, 0.1 M, pH 7.4) | - | 0–5 mM | 0.15 mM | - | ~1 h and 35 min | 6% | ISF | [47] |
| 10 mM PBS (pH 6.5) containing 0.1 M KCl | 85.17 μA/(mM cm2) | 0.1–3.7 mM | - | 30 s | 2 weeks | 6.41% | Saliva | [48] |
| Simulated tear fluid | - | 0.39–16.60 mM | - | - | 8 weeks at 25 °C | - | Tears | [49] |
| PBS (0.1 M, pH 7.4) | 0.0824 μA·mM−1 | 0.01–35 mM | 0.144 μM | - | 13 days | 3.34% | Sweat | [50] |
| Biofluid | Withdrawing Techniques | Components | Lactate Concentration | Potential Interferents | Detection Challenges | Can Require Stimulation | Ref |
|---|---|---|---|---|---|---|---|
| Blood |
| Plasma, blood cells | 0.5–2 mM | Uric acid, glucose, Na+, K+, Ca+, urea, ascorbic acid | Difficult to collect via non-invasive methods | No | [45,46,51,52,53] |
| ISF |
| Similar to plasma | 1–2 mM | Similar to blood | Most of ISF is gelatinous, which is difficult to collect | No | [45,47,51,54,55] |
| Saliva |
| Dilute secretion (99% water), contains many different enzymes, electrolytes and other components | 0.11–0.56 mM | Similar to blood | Interference from daily dietary secretions and the accumulation of oral bacteria | No | [42,45,48,51,54] |
| Sweat |
| Perspiration contains a large amount of water and a small amount of electrolyte, glucose, lactate, and other substances. | 10–25 mM | Similar to Blood | High evaporability of sweat and contamination on the skin surface (such as dust, oil) | Yes | [42,45,50,51,54,55] |
| Tears |
| Tears contain many elements such as lysozyme, immunoglobulin, sugar, and inorganic salts | 1–5 mM | Similar to Blood | Difficult to collect and high requirements on the safety of sensors | No | [45,49,51,54] |
| Classification | Sensing Material | Electrolyte | Sensitivity | Linear Range | LOD | Stability | Reproducibility (RSD) | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
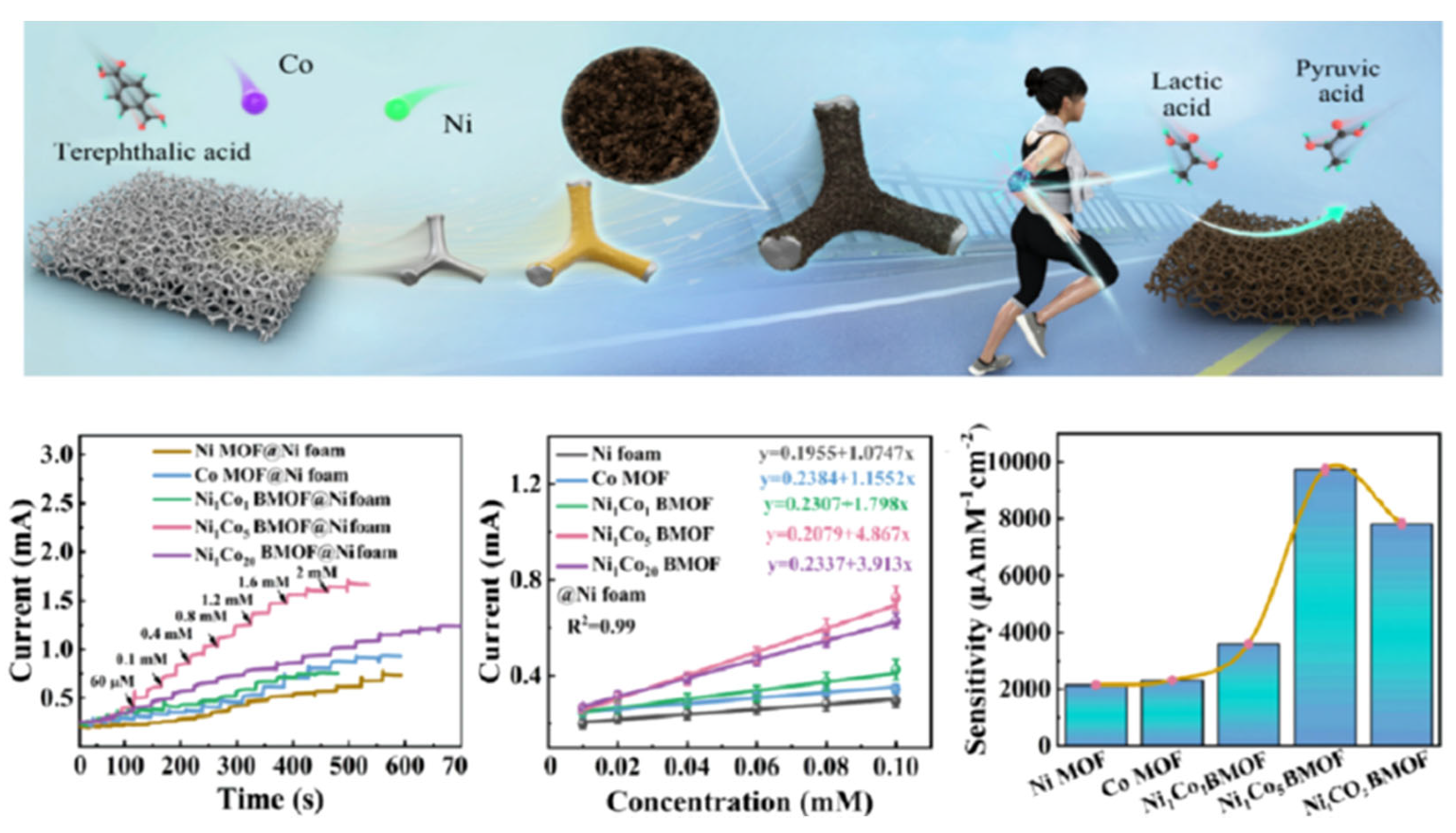
| Bimetallic nanomaterials | NixCoy BMOF@Ni foams | 0.1 M NaOH | 9030 μA mM−1 cm−2 | 0.01−2.2 mM | 0.16 μM | - | - | - | [60] |
| TMC | NiF/HS-NiS | 1.0 M KOH | 0.655 μA μM−1 cm−2 | 0.5−88.5 μM | 0.023 μM | 5000 s | 2.3% | Urine | [61] |
| NiS-NC@NiS-MS | 1.0 M KOH | 0.39 μA μM−1 | 0.5−85.5 μM | 0.5 μM | 5000 s | 2.3% | Urine | [62] | |
| ZIF-67/NiS composite | 1.0 M KOH | 1.34 μA μM−1 cm−2 | 5 μM–25 μM | 0.8 μM | 30 days | 2.2% | - | [63] | |
| MoS2-AuPt | 0.01 M PBS (pH 7.4) | - | 0.005–3 mM | 0.33 μM | 30 days | 0.8% | Sweat | [64] | |
| Metal oxides | Porous NiO | 0.1 M NaOH | 62.35 μA mM−1 cm−2 | - | 27 μM | - | - | - | [65] |
| NiO nanoparticles | 0.1 M NaOH–KCl | 1.564 μA mM−1 | 0.1–5 mM | 0.03 mM | - | - | - | [66] | |
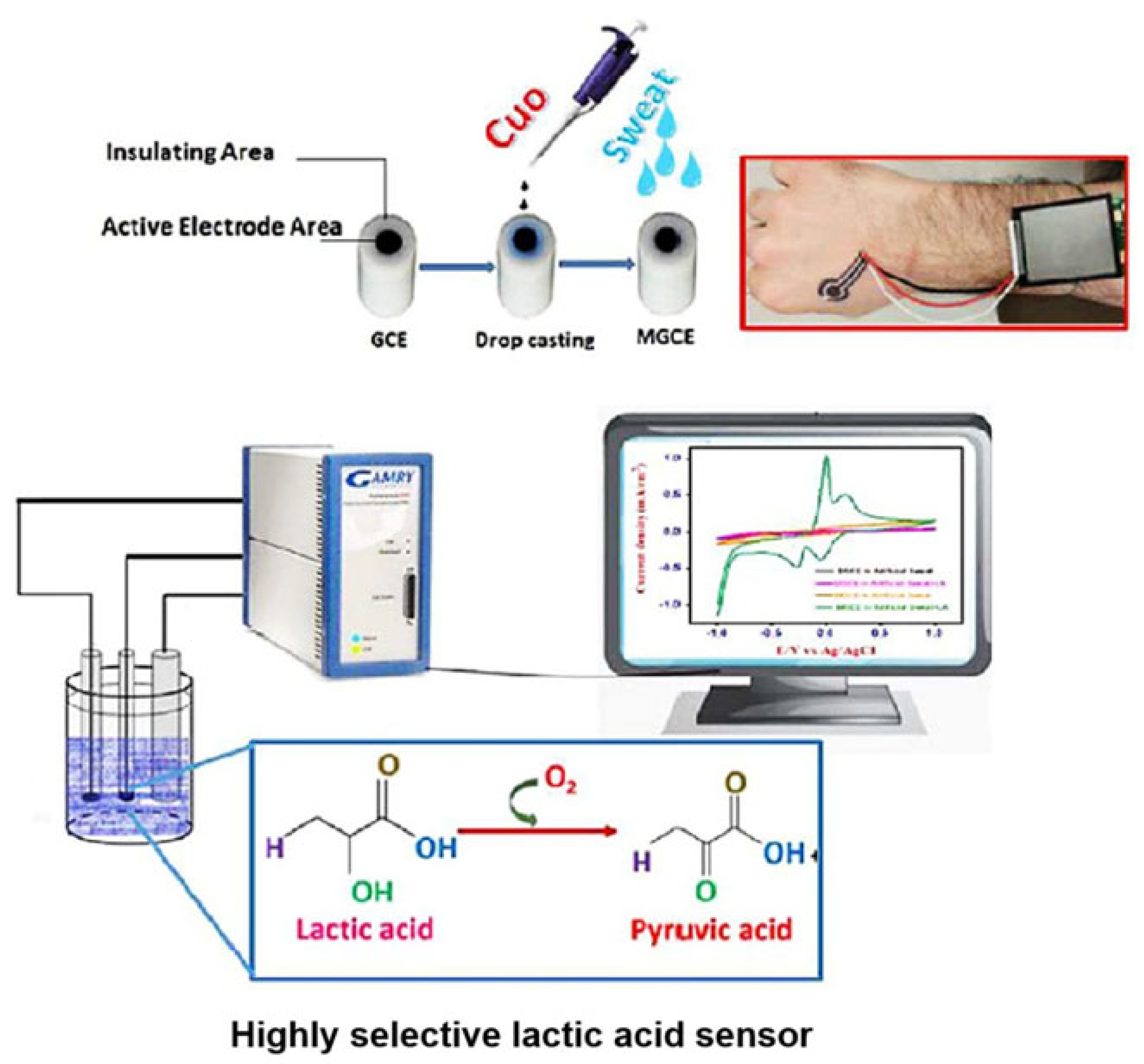
| CuO nanoparticles | Artificial Sweat (pH 7.4) | 14.47 mA mM−1 cm−2 | 0.05–2.5 mM | 0.027 mM | - | 1.12% | Sweat | [67] | |
| NiOx/NiOx-Nafion | 0.01 M PBS (pH 7.4) | 20.56 nA mM−1 mm−2 | 0.5–4 mM | 0.27 mM | - | - | Blood plasma | [68] | |
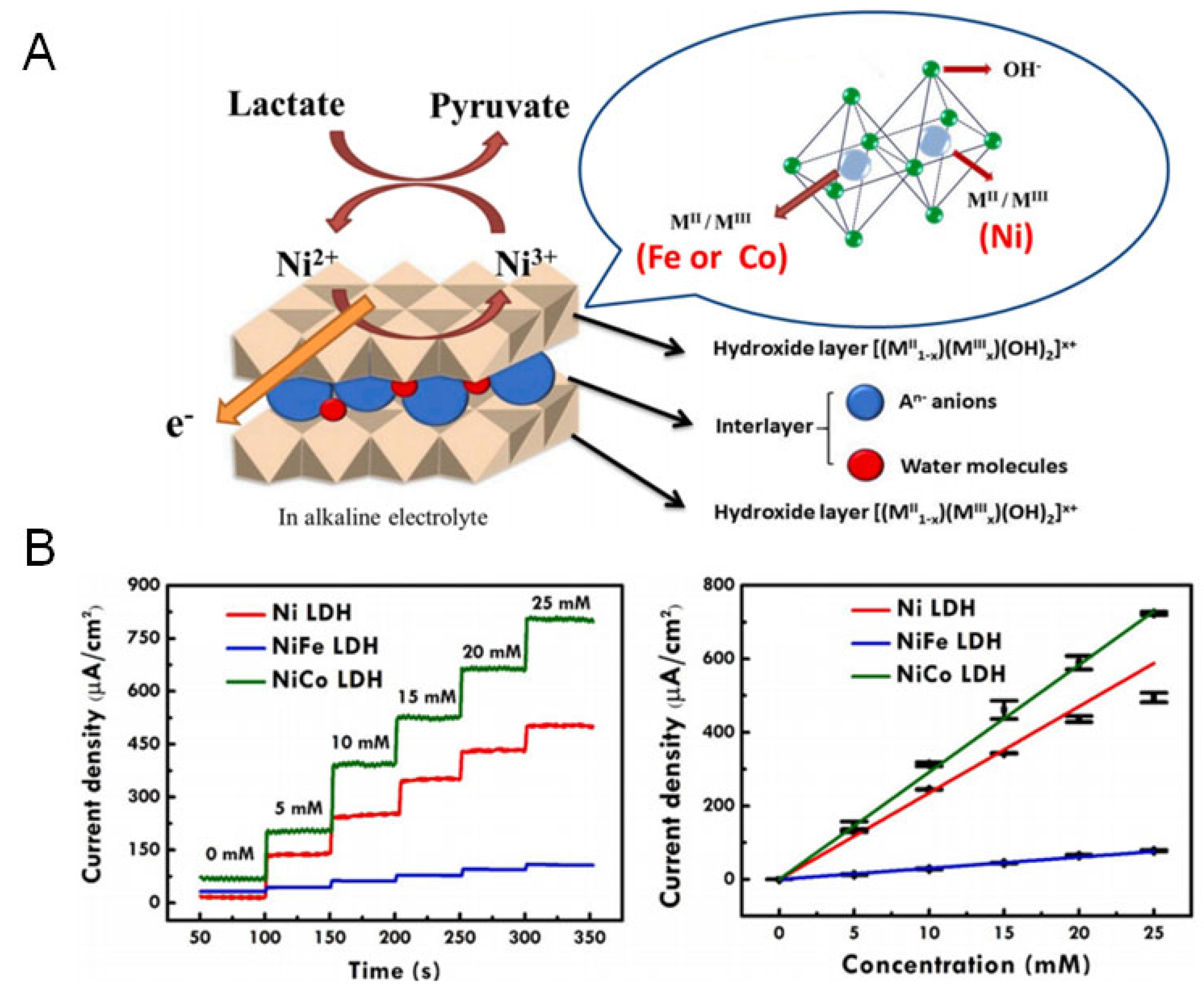
| Layered double hydroxides | ZIF-67 derived NiCo LDH | 0.1 M NaOH | 83.98 μA mM−1 cm−2 | 2–26 mM | 0.399 mM | 28 days | - | Sweat | [69] |
| NiCo LDH | 0.1 M NaOH | 30.59 μA mM−1 cm−2 | 5–25 mM | 0.53 mM | 28 days | - | - | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, G. Recent Advancement in Non-Enzymatic Electrochemical Detection of Lactate Based on Metal Nanomaterials: A Review. Sensors 2025, 25, 6194. https://doi.org/10.3390/s25196194
Wang C, Li G. Recent Advancement in Non-Enzymatic Electrochemical Detection of Lactate Based on Metal Nanomaterials: A Review. Sensors. 2025; 25(19):6194. https://doi.org/10.3390/s25196194
Chicago/Turabian StyleWang, Chenxin, and Guanglei Li. 2025. "Recent Advancement in Non-Enzymatic Electrochemical Detection of Lactate Based on Metal Nanomaterials: A Review" Sensors 25, no. 19: 6194. https://doi.org/10.3390/s25196194
APA StyleWang, C., & Li, G. (2025). Recent Advancement in Non-Enzymatic Electrochemical Detection of Lactate Based on Metal Nanomaterials: A Review. Sensors, 25(19), 6194. https://doi.org/10.3390/s25196194





