Characterisation of Titanium-Oxide Thin Films for Efficient pH Sensing in Low-Power Electrochemical Systems †
Abstract
1. Introduction
2. Experimental Section
2.1. Layer Deposition Methods
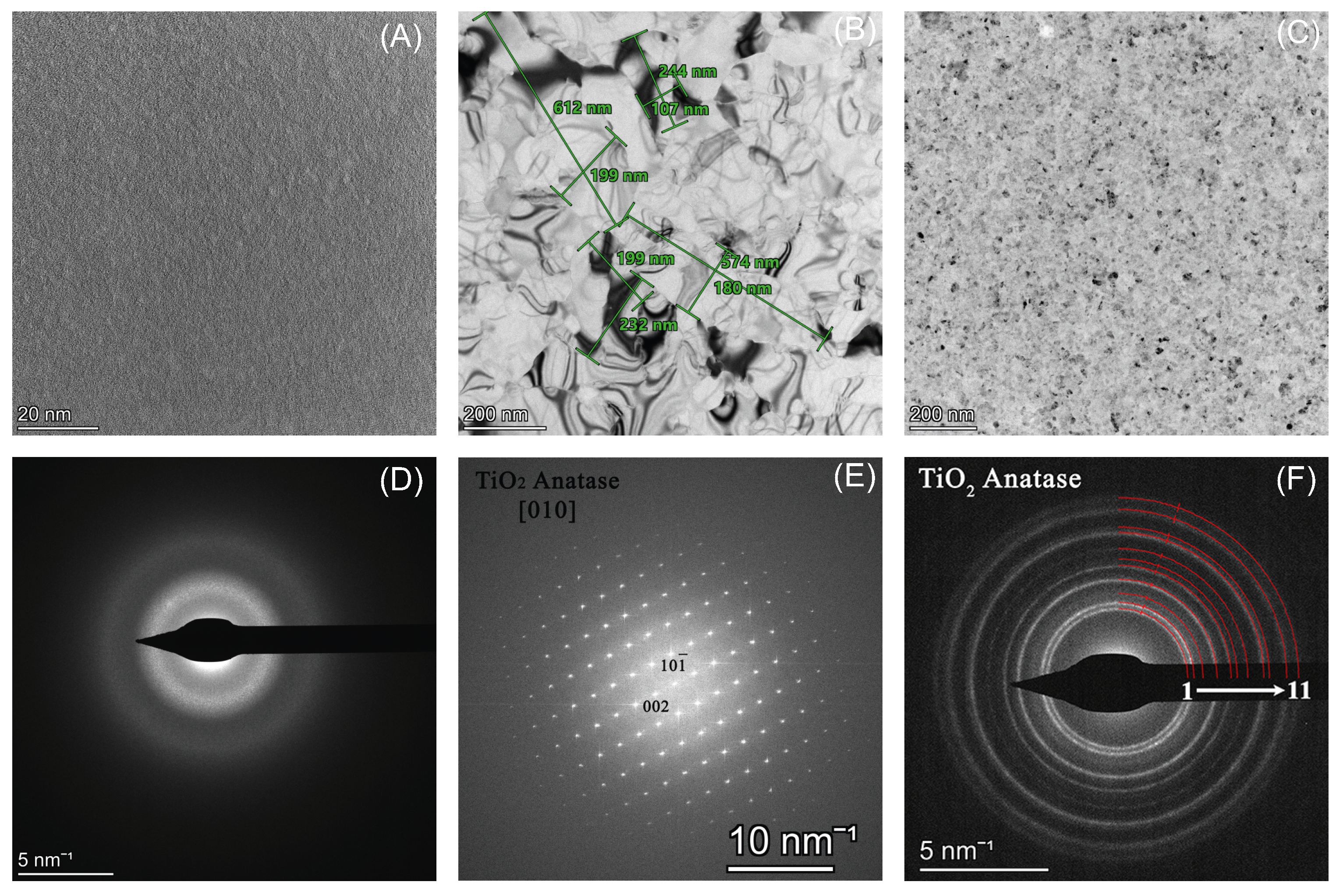
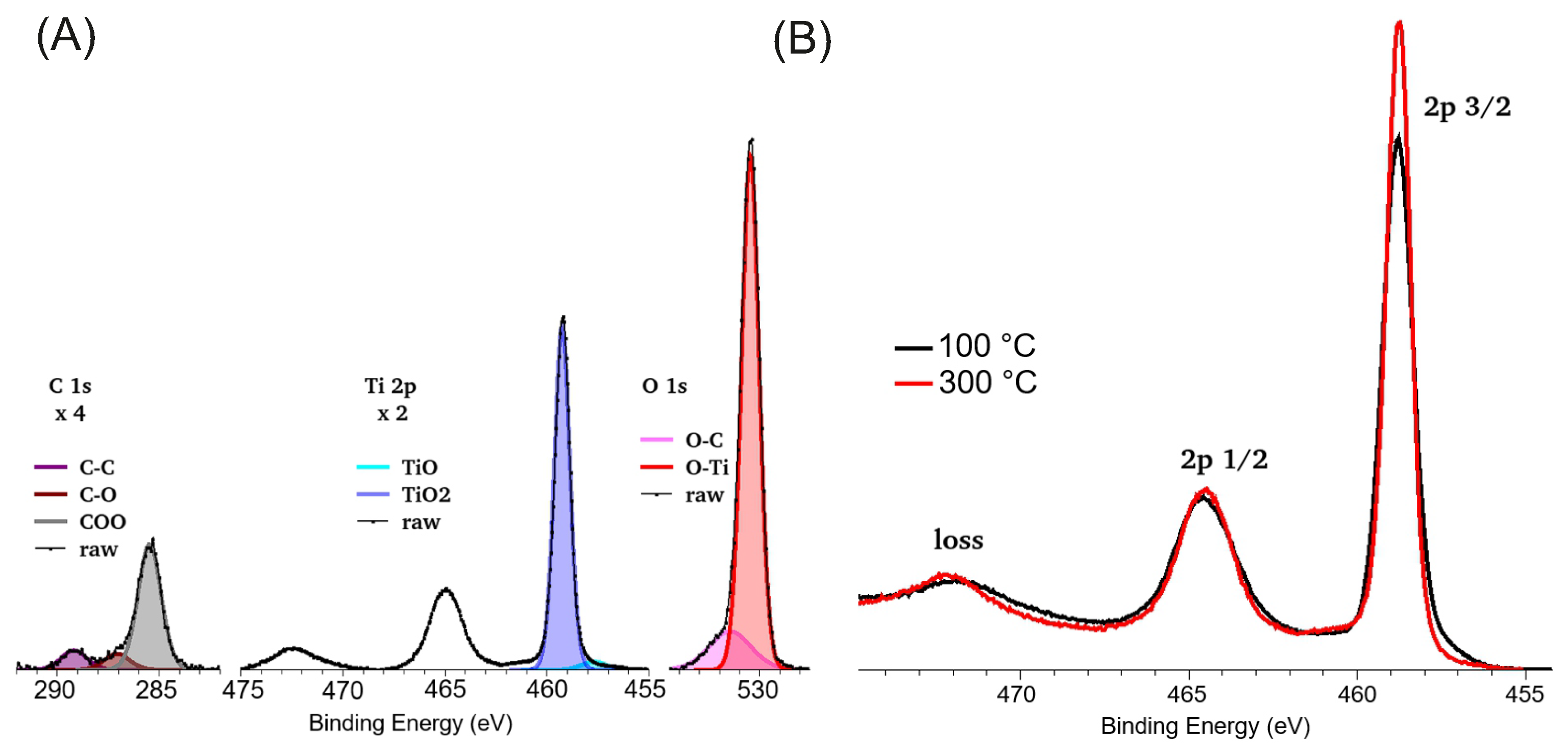
2.2. Characterization of TiOx Layers
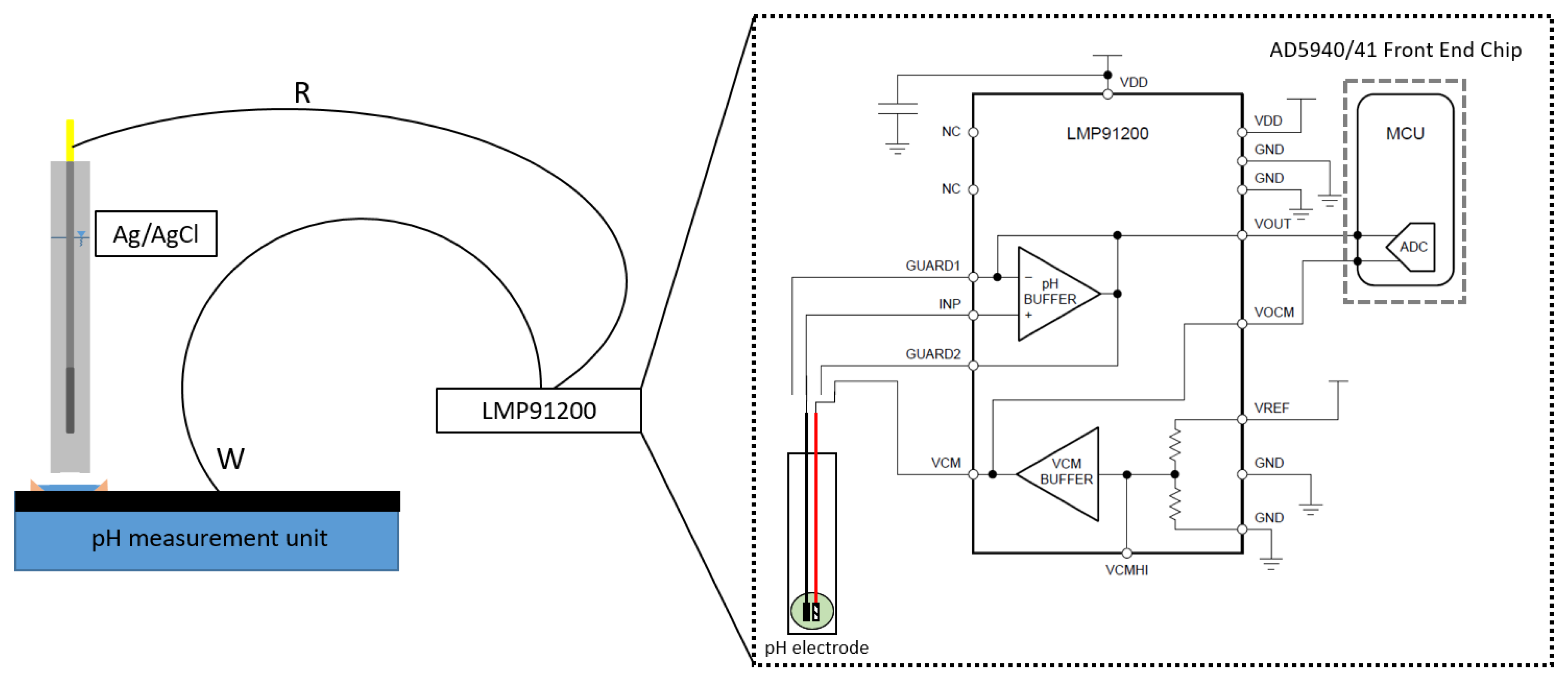
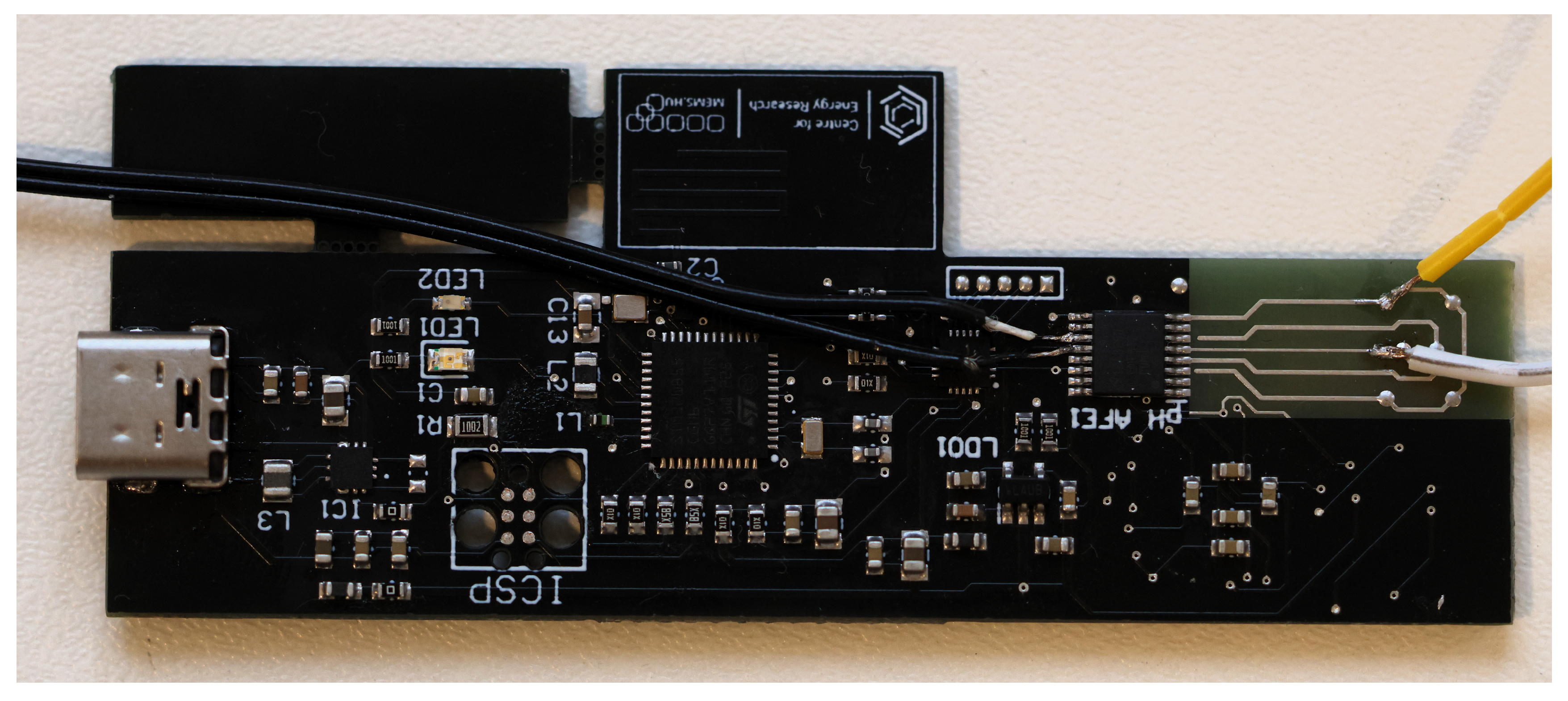
2.3. Manufacturing the Readout Circuit for the Measurements
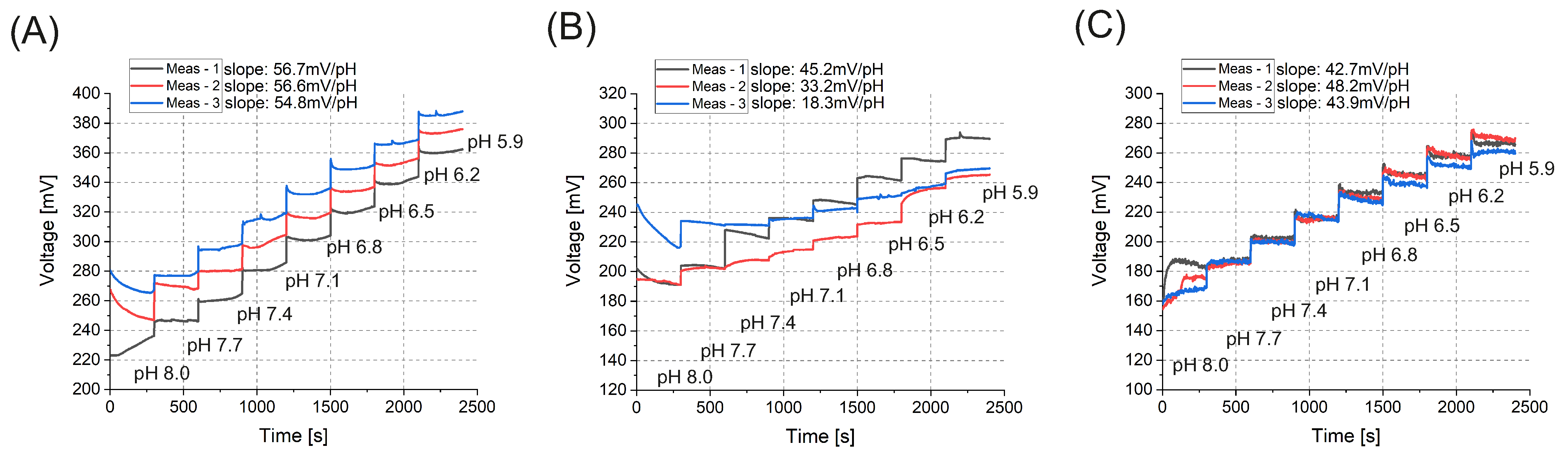
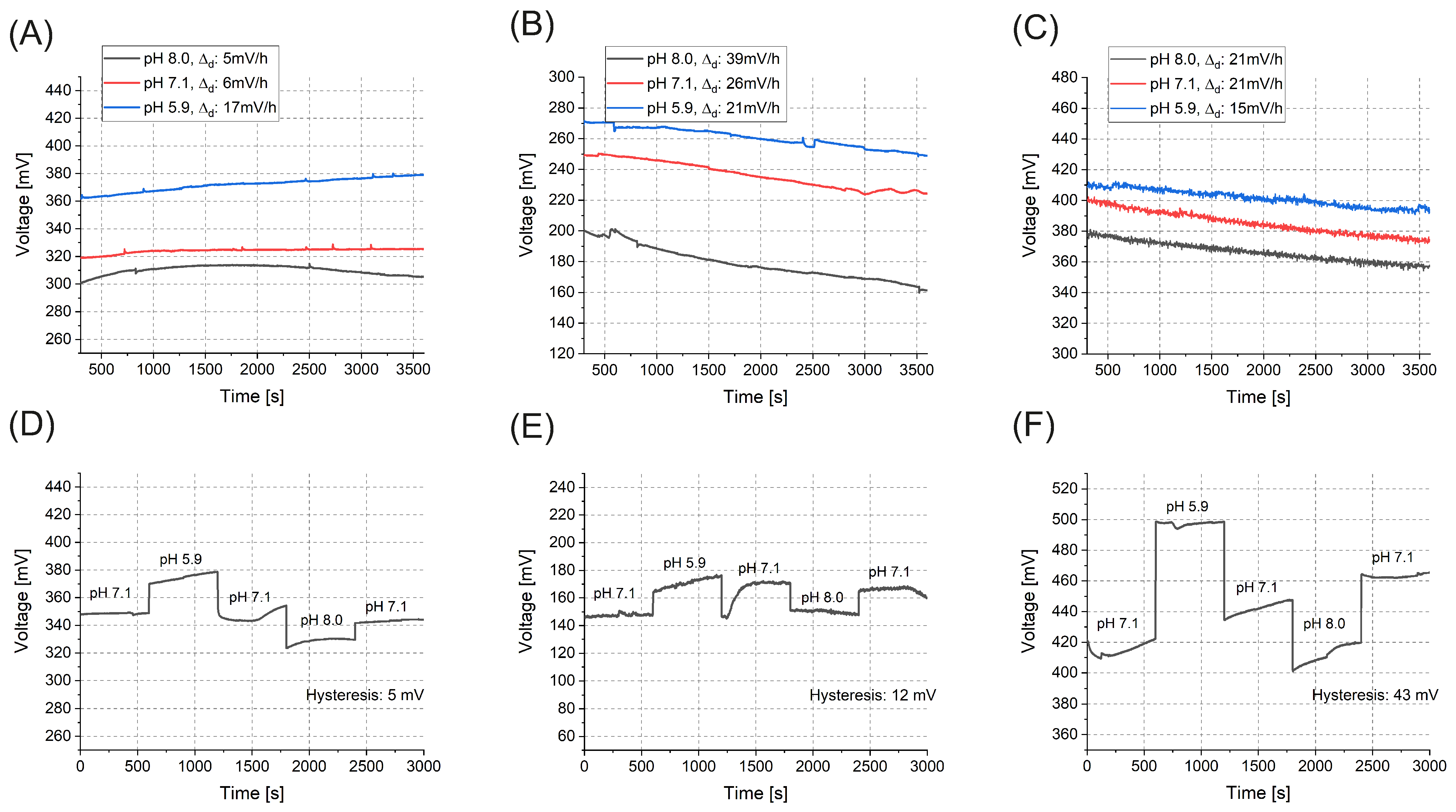
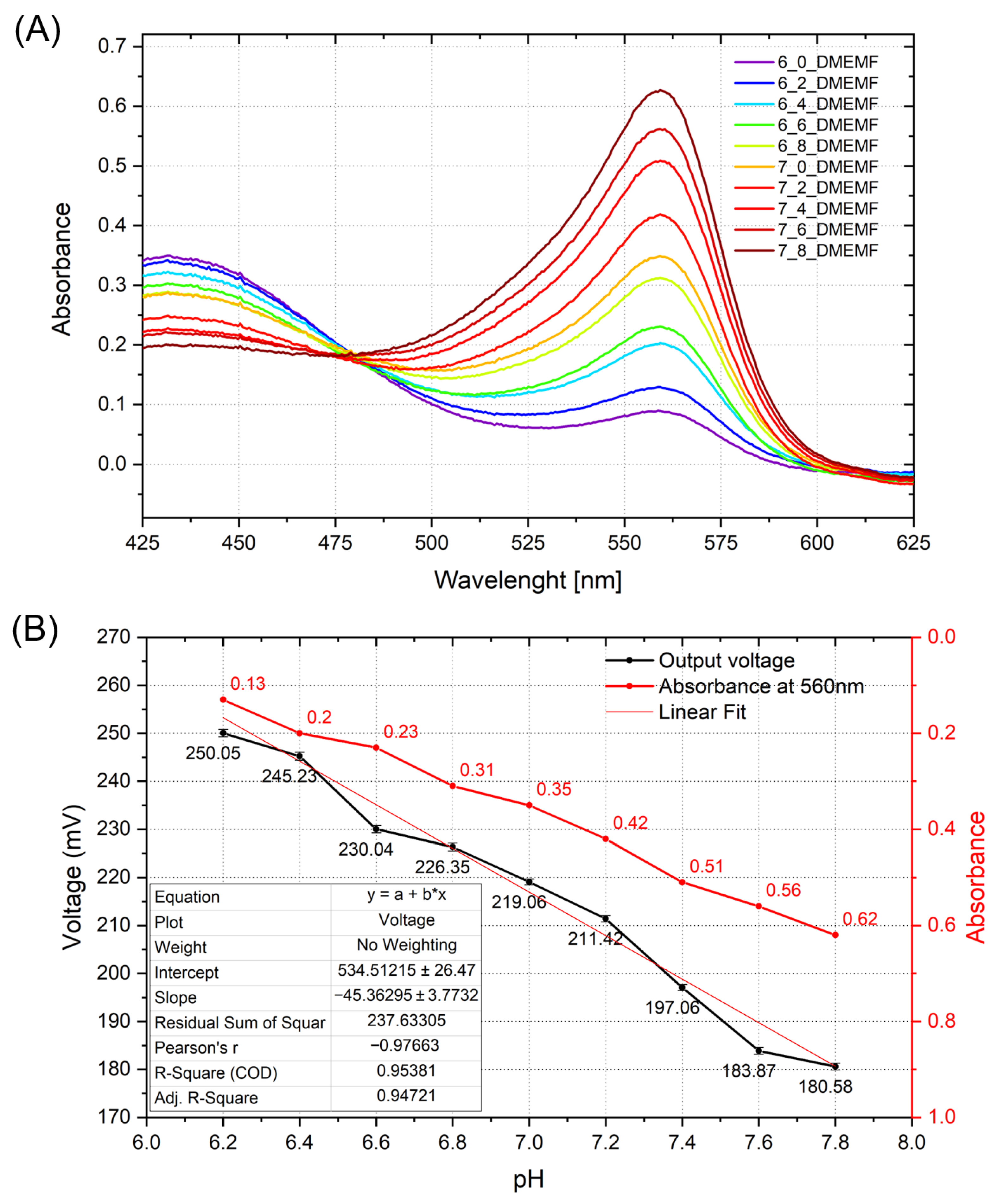
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISFET | Ion Selective Field Effect Transistor |
| EGFET | Extended Gate Field Effect Transistor |
| MOSFET | Metal Oxide Semiconductor FET |
| ALD | Atomic Layer Deposition |
| TEM | Transmission Electron Microscopy |
| HRTEM | High Resolution Transmission Electron Microscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| AFE | Analog Front End |
| DAC | Digital Analog Converter |
| TIA | Trans Impedance Amplifier |
| DMEM | Dulbecco’s Modified Eagle Medium |
References
- Shaegh, S.A.M.; Ferrari, F.D.; Zhang, Y.S.; Nabavinia, M.; Mohammad, N.B.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Sadeghian, R.B.; Nadhman, A.; et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10, 044111. [Google Scholar] [CrossRef]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism – from 2D culture to organ-on-chip. Lab A Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef]
- Tian, Y.; Shumway, B.R.; Youngbull, A.C.; Li, Y.; Jen, A.K.Y.; Johnson, R.H.; Meldrum, D.R. Dually fluorescent sensing of pH and dissolved oxygen using a membrane made from polymerizable sensing monomers. Sens. Actuators B Chem. 2010, 147, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Mulato, M. Synthesis and electrical characterization of ZnO and TiO2 thin films and their use as sensitive layer of pH field effect transistor sensors. MRS Proc. 2014, 1675, 53–58. [Google Scholar] [CrossRef]
- Yusof, K.A.; Rahman, R.A.; Zulkefle, M.A.; Herman, S.H.; Abdullah, W.F.H. EGFET pH Sensor Performance Dependence on Sputtered TiO2 Sensing Membrane Deposition Temperature. J. Sensors 2016, 2016, 7594531. [Google Scholar] [CrossRef]
- Batista, P.D.; Mulato, M.; de O. Graeff, C.F.; Fernandez, F.J.R.; das C. Marques, F. SnO2 extended gate field-effect transistor as pH sensor. Braz. J. Phys. 2006, 36, 478–481. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Guerra, E.M.; Mulato, M. V2O5/WO3 Mixed Oxide Films as pH-EGFET Sensor: Sequential Re-Usage and Fabrication Volume Analysis. ECS J. Solid State Sci. Technol. 2012, 1, N39–N44. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Mulato, M. ZnO thin films applied as pH sensor in EGFET devices. MRS Proc. 2015, 1805, 685. [Google Scholar] [CrossRef]
- Fulati, A.; Ali, S.M.U.; Riaz, M.; Amin, G.; Nur, O.; Willander, M. Miniaturized pH Sensors Based on Zinc Oxide Nanotubes/Nanorods. Sensors 2009, 9, 8911–8923. [Google Scholar] [CrossRef]
- Alvarez-Serna, B.E.; Ramírez-Chavarría, R.G. EGFET-based pH Sensor coupled with Low-cost Electrochemical Screen-printed Electrodes. J. Physics Conf. Ser. 2021, 1723, 012024. [Google Scholar] [CrossRef]
- da Silva, L.L.F.; de Moraes Cruz, C.A.; Silveira, F.; de Oliveira Moura, T.D. Active ISFET Sensor Readout Circuit. In Proceedings of the 2017 2nd International Symposium on Instrumentation Systems, Circuits and Transducers (INSCIT), Fortaleza, Brazil, 28 August–1 September 2017. [Google Scholar] [CrossRef]
- Asgari, M.; shin Lee, K.; Ida, N. Highly Linear Bridge-based ISFET pH Sensor Readout Circuit. In Proceedings of the 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS), Boston, MA, USA, 6–9 August 2017. [Google Scholar] [CrossRef]
- Cruz, F.R.G.; Magsipoc, C.M.; Alinea, F.E.B.; Baronia, M.E.P.; Jumahadi, M.M.; Garcia, R.G.; Chung, W.Y. Application Specific Integrated Circuit (ASIC) for Ion Sensitive Field Effect Transistor (ISFET) L-Asparagine Biosensor. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Guan, Z. Design of a Integrated readout Circuit for pH Sensor with Chopper Instrumentation Amplifier. Advances in Engineering Research; Atlantis Press: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef][Green Version]
- Bhardwaj, R.; Majumder, S.; Ajmera, P.K.; Sinha, S.; Sharma, R.; Mukhiya, R.; Narang, P. Temperature Compensation of ISFET Based pH Sensor Using Artificial Neural Networks. In Proceedings of the IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Batu Ferringhi, Malaysia, 23–25 August 2017. [Google Scholar] [CrossRef]
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sensors Actuators B-Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Pullano, S.A.; Critello, C.D.; Mahbub, I.; Tasneem, N.T.; Shamsir, S.; Islam, S.K.; Greco, M.; Fiorillo, A.S. EGFET-Based Sensors for Bioanalytical Applications: A Review. Sensors 2018, 18, 4042. [Google Scholar] [CrossRef]
- Li, J.Y.; Chang, S.P.; Chang, S.J.; Tsai, T.Y. Sensitivity of EGFET pH sensors with TiO2 nanowires. ECS Solid State Lett. 2014, 3, 123–126. [Google Scholar] [CrossRef]
- Mokhtarifar, N.; Goldschmidtboeing, F.; Woias, P. Low-cost EGFET-based pH-sensor Using Encapsulated ITO/PET-electrodes. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018. [Google Scholar] [CrossRef]
- Mokhtarifar, N.; Goldschmidtboeing, F.; Woias, P. ITO/Glass as Extended-Gate of FET: A Low-Cost Method for Differential pH-Sensing in Alkaline Solutions. J. Electrochem. Soc. 2019, 166, B896. [Google Scholar] [CrossRef]
- Pullano, S.A.; Tasneem, N.T.; Mahbub, I.; Shamsir, S.; Greco, M.; Islam, S.K.; Fiorillo, A.S. Deep Submicron EGFET Based on Transistor Association Technique for Chemical Sensing. Sensors 2019, 19, 1063. [Google Scholar] [CrossRef]
- Huang, W.D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Santos, L.; Neto, J.P.; Crespo, A.; Nunes, D.; Costa, N.; Fonseca, I.M.; Barquinha, P.; Pereira, L.; Silva, J.; Martins, R.; et al. WO3 Nanoparticle-Based Conformable pH Sensor. ACS Appl. Mater. Interfaces 2014, 6, 12226–12234. [Google Scholar] [CrossRef]
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Szwagierczak, D.; Socha, R.P. Fabrication of thick film sensitive RuO2-TiO2 and Ag/AgCl/KCl reference electrodes and their application for pH measurements. Sens. Actuators B Chem. 2014, 204, 57–67. [Google Scholar] [CrossRef]
- Chou, J.C.; Liao, L.P. Study of TiO2 Thin Films for Ion Sensitive Field Effect Transistor Application with RF Sputtering Deposition. Jpn. J. Appl. Phys. 2004, 43, 61–65. [Google Scholar] [CrossRef]
- Jović, M.; Hidalgo-Acosta, J.C.; Lesch, A.; Costa Bassetto, V.; Smirnov, E.; Cortés-Salazar, F.; Girault, H.H. Large-scale layer-by-layer inkjet printing of flexible iridium-oxide based pH sensors. J. Electroanal. Chem. 2018, 819, 384–390. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Gao, H.; Xu, K.; Zhang, X. Fabrication of Functional Super-Hydrophilic TiO2 Thin Film for pH Detection. Chemosensors 2022, 10, 182. [Google Scholar] [CrossRef]
- Kamarozaman, N.S.; Zainal, N.; Rosli, A.B.; Zulkefle, M.A.; Him, N.R.N.; Abdullah, W.F.H.; Herman, S.H.; Zulkifli, Z. Highly Sensitive and Selective Sol-Gel Spin-Coated Composite TiO2–PANI Thin Films for EGFET-pH Sensor. Gels 2022, 8, 690. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.A.; Zulkefle, M.A.H.; Abdullah, W.F.H.; Rusop, M.; Herman, S.H. Characteristics of TiO2/ZnO bilayer film towards pH sensitivity prepared by different spin coating deposition process. AIP Conf. Proc. 2016, 1733, 020059. [Google Scholar] [CrossRef]
- Khizir, H.A.; Abbas, T.A.H. Hydrothermal synthesis of TiO2 nanorods as sensing membrane for extended-gate field-effect transistor (EGFET) pH sensing applications. Sens. Actuators A. Phys. 2021, 333, 113231. [Google Scholar] [CrossRef]
- Rosdan, M.; Herman, S.; Abdullah, W.; Kamarozaman, N.; Syono, M. Sputtered Titanium Dioxide Thin Film for Extended-Gate FET Sensor Application. In Proceedings of the RSM 2013 IEEE Regional Symposium on Micro and Nanoelectronics, Daerah Langkawi, Malaysia, 25–27 September 2013; pp. 219–222. [Google Scholar] [CrossRef]
- Bertel, L.; Miranda, D.A.; García-Martín, J.M. Nanostructured Titanium Dioxide Surfaces for Electrochemical Biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef]
- Lin, Z.L.S.; Tian, Y.; Bokkelen, A.V.; Valerio, M.; Gomez, M.A. Oxygen Vacancies Altering the Trapping in the Proton Conduction Landscape of Doped Barium Zirconate. J. Phys. Chem. C 2020, 124, 27954–27964. [Google Scholar] [CrossRef]
- Dong, K.; Liang, J.; Wang, Y.; Ren, Y.; Xu, Z.; Zhou, H.; Li, L.; Liu, Q.; Luo, Y.; Li, T.; et al. Plasma-induced defective TiO2-x with oxygen vacancies: A high-active and robust bifunctional catalyst toward H2O2 electrosynthesis. Chem Catal. 2021, 1, 1437–1448. [Google Scholar] [CrossRef]
- Goers, L.; Ainsworth, C.; Goey, C.H.; Kontoravdi, C.; Freemont, P.S.; Polizzi1, K.M. Whole-Cell Escherichia coli Lactate Biosensor for Monitoring Mammalian Cell Cultures During Biopharmaceutical Production. Biotechnol. Bioeng. 2017, 114, 1290–1300. [Google Scholar] [CrossRef]
- Schmiedeknecht, K.; Kaufmann, A.; Bauer, S.; VenegasSolis, F. L-lactate as an indicator for cellular metabolic status: An easy and cost-effective colorimetric L-lactate assay. PLoS ONE 2022, 17, e0271818. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Park, K.C.; Swietach, P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun. Biol. 2019, 2, 144. [Google Scholar] [CrossRef] [PubMed]
- Szomor, Z.; Bató, L.; Stágl, S.; Hakkel, O.; Sulyok, A.; Dücső, C.; Baji, Z.; Fürjes, P. Non-Stoichiometric Titanium-Oxide Gate Electrodes for EGFET Based pH Sensors. Proceedings 2023, 97, 193. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szomor, Z.; Bató, L.; Hakkel, O.; Dücső, C.; Baji, Z.; Sulyok, A.; Dodony, E.; Balázsi, K.; Bozorádi, J.M.; Szabó, Z.; et al. Characterisation of Titanium-Oxide Thin Films for Efficient pH Sensing in Low-Power Electrochemical Systems. Sensors 2025, 25, 6113. https://doi.org/10.3390/s25196113
Szomor Z, Bató L, Hakkel O, Dücső C, Baji Z, Sulyok A, Dodony E, Balázsi K, Bozorádi JM, Szabó Z, et al. Characterisation of Titanium-Oxide Thin Films for Efficient pH Sensing in Low-Power Electrochemical Systems. Sensors. 2025; 25(19):6113. https://doi.org/10.3390/s25196113
Chicago/Turabian StyleSzomor, Zsombor, Lilia Bató, Orsolya Hakkel, Csaba Dücső, Zsófia Baji, Attila Sulyok, Erzsébet Dodony, Katalin Balázsi, János M. Bozorádi, Zoltán Szabó, and et al. 2025. "Characterisation of Titanium-Oxide Thin Films for Efficient pH Sensing in Low-Power Electrochemical Systems" Sensors 25, no. 19: 6113. https://doi.org/10.3390/s25196113
APA StyleSzomor, Z., Bató, L., Hakkel, O., Dücső, C., Baji, Z., Sulyok, A., Dodony, E., Balázsi, K., Bozorádi, J. M., Szabó, Z., & Fürjes, P. (2025). Characterisation of Titanium-Oxide Thin Films for Efficient pH Sensing in Low-Power Electrochemical Systems. Sensors, 25(19), 6113. https://doi.org/10.3390/s25196113





