Monitoring Single DNA Docking Site Activity with Sequential Modes of an Optoplasmonic Whispering-Gallery Mode Biosensor
Abstract
1. Introduction
2. Materials and Methods
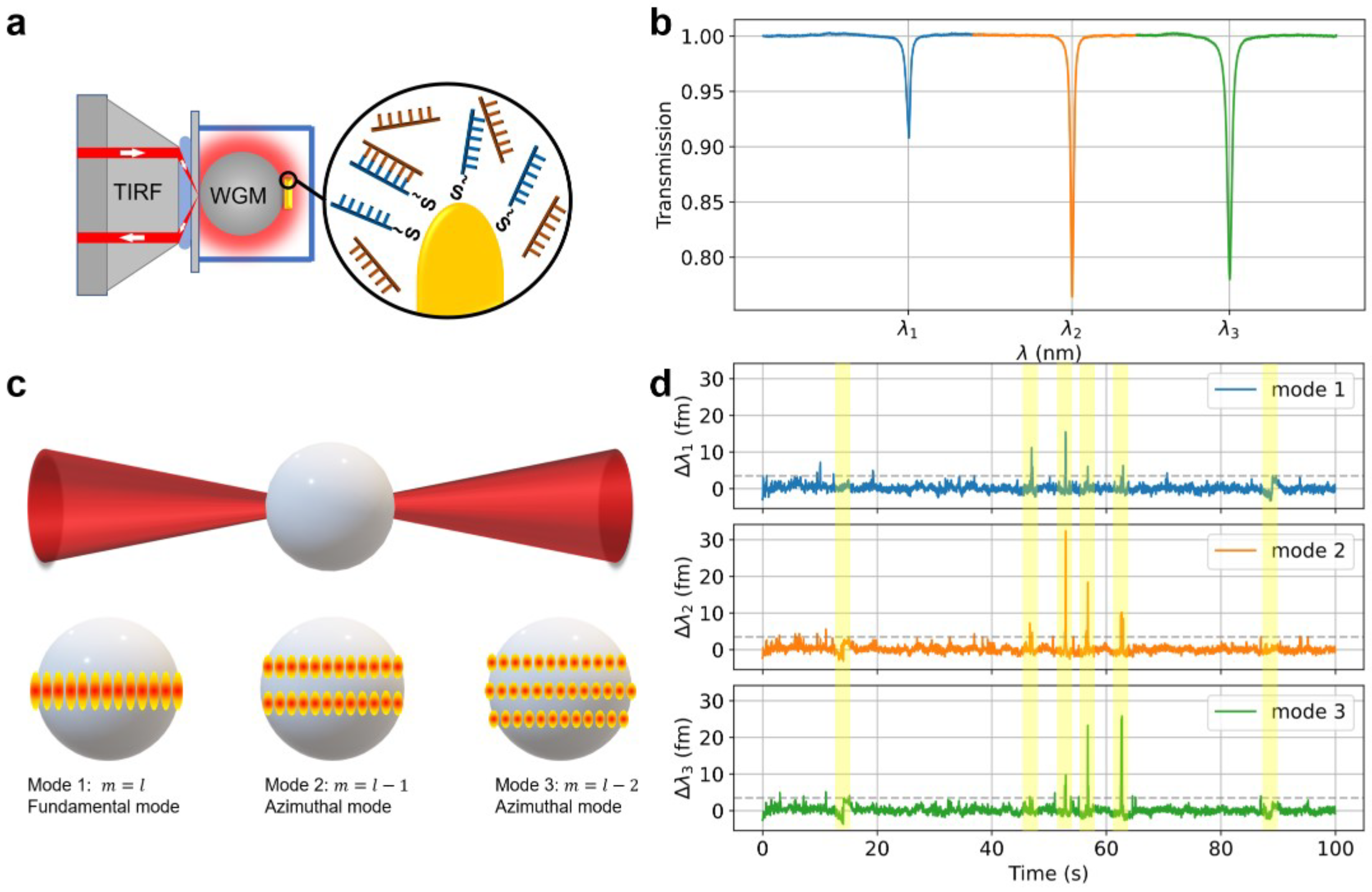
2.1. Optoplasmonic Sensing of DNA Hybridisation
2.2. Sequential Polar Modes of an Optoplasmonic Resonator
3. Results
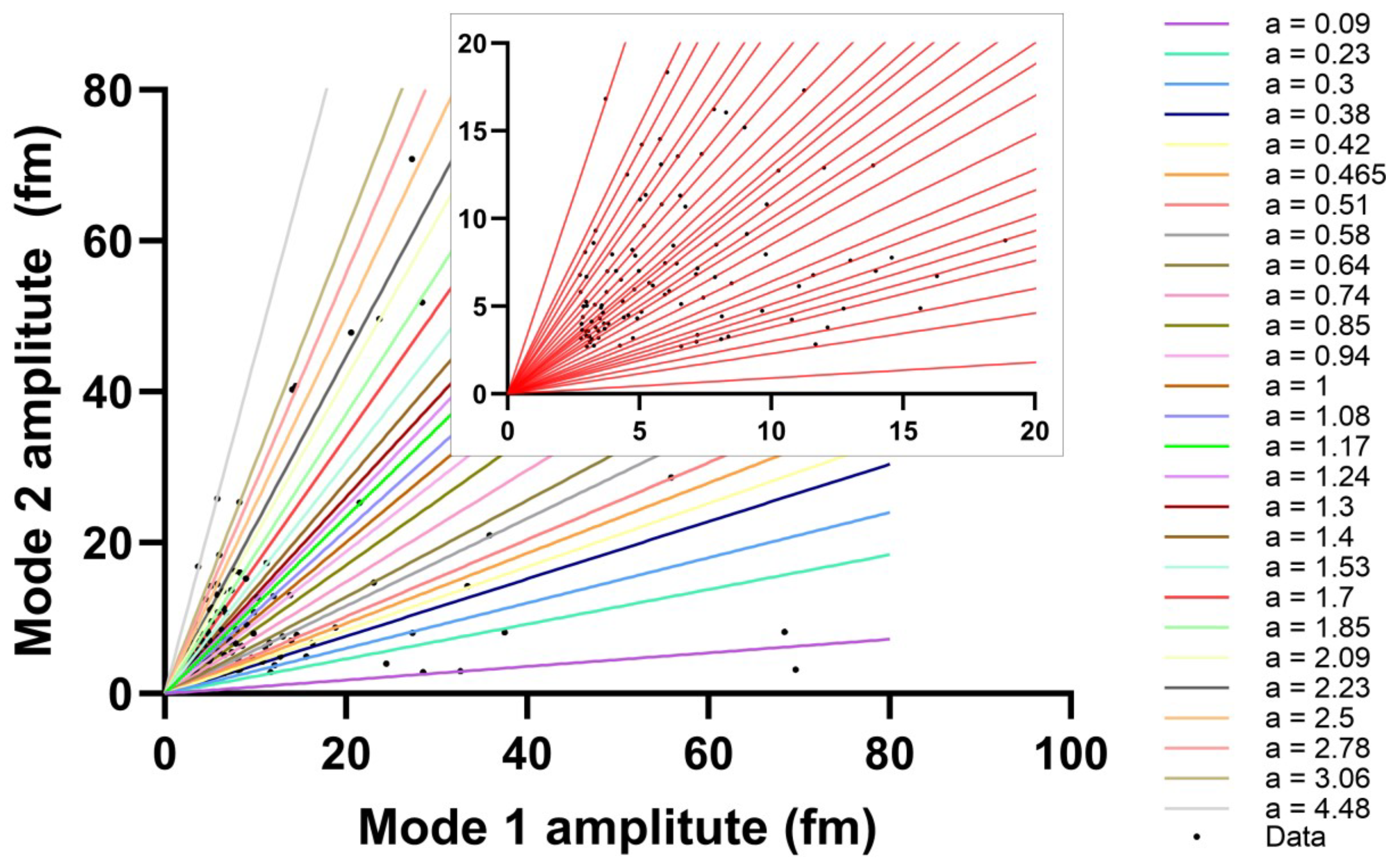
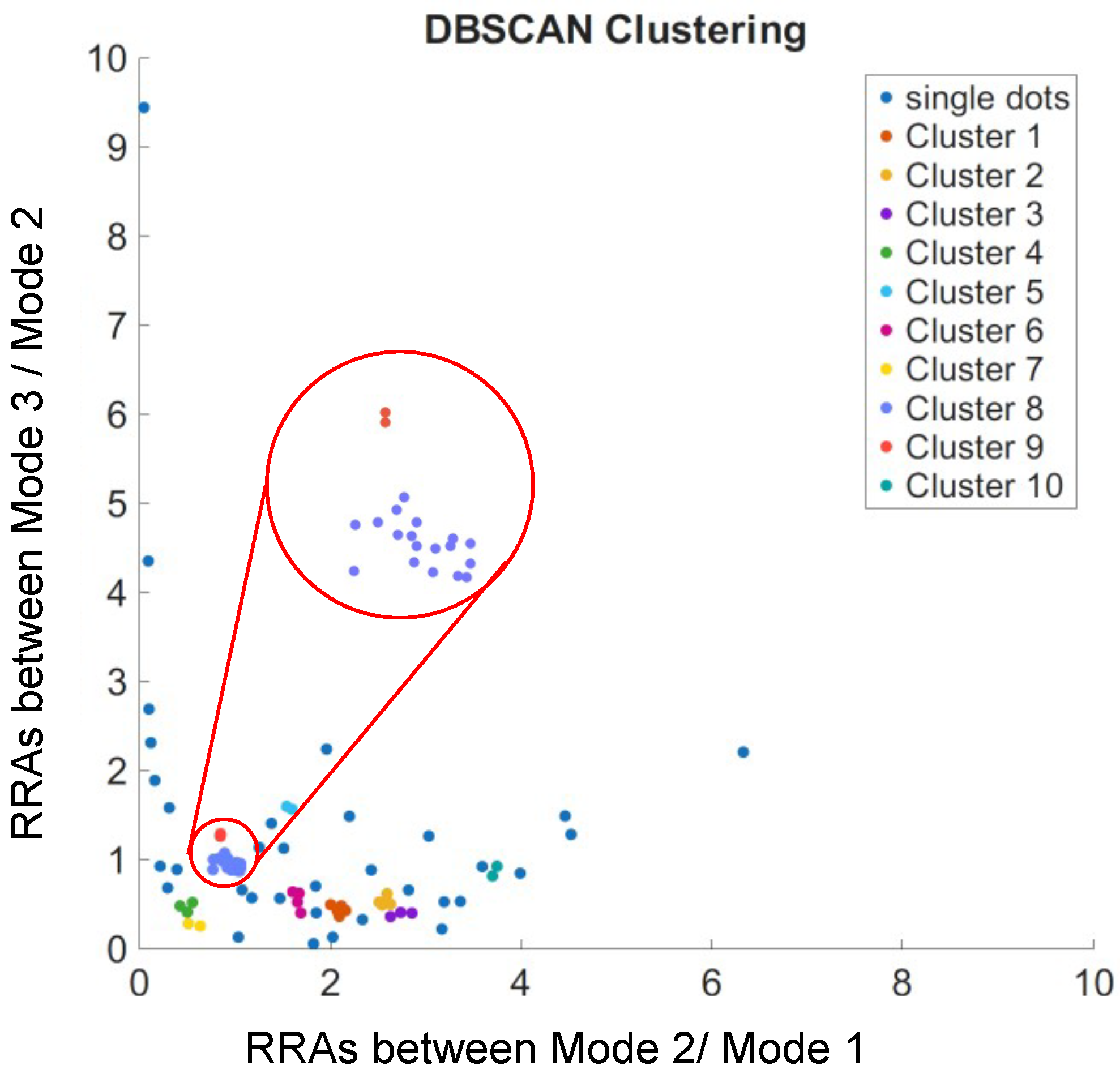
3.1. Repeating Ratios of WGM Mode Amplitudes Among Adjacent Modes
3.2. Tracing of Individual Docking Site Interactions
3.3. Step Signals Correspond to the Disappeared Ratios
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, X.; Kruse, K.; Lu, T.; Wang, J. Nonequilibrium physics in biology. Rev. Mod. Phys. 2019, 91, 045004. [Google Scholar] [CrossRef]
- Lenn, T.; Leake, M.C. Experimental approaches for addressing fundamental biological questions in living, functioning cells with single molecule precision. Open Biol. 2012, 2, 120090. [Google Scholar] [CrossRef]
- Alberts, B.; Barry, J.; Bedinger, P.; Formosa, T.; Jongeneel, C.; Kreuzer, K. Studies on DNA Replication in the Bacteriophage T4 a–.gif System. Cold Spring Harb. Symp. Quant. Biol. 1983, 47, 655–668. [Google Scholar] [CrossRef]
- Nossal, N.G.; Makhov, A.M.; Chastain, P.D.; Jones, C.E.; Griffith, J.D. Architecture of the bacteriophage T4 replication complex revealed with nanoscale biopointers. J. Biol. Chem. 2007, 282, 1098–1108. [Google Scholar] [CrossRef]
- Miller, H.; Zhou, Z.; Shepherd, J.; Wollman, A.J.M.; Leake, M.C. Single-molecule techniques in biophysics: A review of the progress in methods and applications. Rep. Prog. Phys. 2017, 81, 24601. [Google Scholar] [CrossRef]
- Capitanio, M.; Pavone, F.S. Interrogating biology with force: Single molecule high-resolution measurements with optical tweezers. Biophys. J. 2013, 105, 1293–1303. [Google Scholar] [CrossRef]
- Yu, B.; Chen, D.; Qu, J.; Niu, H. Fast Fourier domain localization algorithm of a single molecule with nanometer precision. Opt. Lett. 2011, 36, 4317–4319. [Google Scholar] [CrossRef] [PubMed]
- Kufer, S.K.; Strackharn, M.; Stahl, S.W.; Gumpp, H.; Puchner, E.M.; Gaub, H.E. Optically monitoring the mechanical assembly of single molecules. Nat. Nanotechnol. 2009, 4, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hinterdorfer, P.; Dufrêne, Y.F. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 2006, 3, 347–355. [Google Scholar] [CrossRef]
- Weiss, S. Fluorescence spectroscopy of single biomolecules. Science 1999, 283, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, S.C.M.; Masullo, L.A.; Baudrexel, I.; Steen, P.R.; Kowalewski, R.; Eklund, A.S.; Strauss, S.; Unterauer, E.M.; Schlichthaerle, T.; Strauss, M.T.; et al. Ångström-resolution fluorescence microscopy. Nature 2023, 617, 711–716. [Google Scholar] [CrossRef]
- Deniz, A.A.; Mukhopadhyay, S.; Lemke, E.A. Single-molecule biophysics: At the interface of biology, physics and chemistry. J. R. Soc. Interface 2008, 5, 15–45. [Google Scholar] [CrossRef]
- Baaske, M.D.; Foreman, M.R.; Vollmer, F. Single-molecule nucleic acid interactions monitored on a label-free microcavity biosensor platform. Nat. Nanotechnol. 2014, 9, 933–939. [Google Scholar] [CrossRef]
- Baaske, M.D.; Asgari, N.; Punj, D.; Orrit, M. Nanosecond time scale transient optoplasmonic detection of single proteins. Sci. Adv. 2022, 8, eabl5576. [Google Scholar] [CrossRef]
- Lin, Y.T.; Vermaas, R.; Yan, J.; de Jong, A.M.; Prins, M.W. Click-coupling to Electrostatically Grafted polymers greatly improves the stability of a continuous monitoring sensor with single-molecule resolution. ACS Sens. 2021, 6, 1980–1986. [Google Scholar] [CrossRef]
- Seth, A.; Mittal, E.; Luan, J.; Kolla, S.; Mazer, M.B.; Joshi, H.; Gupta, R.; Rathi, P.; Wang, Z.; Morrissey, J.J.; et al. High-resolution imaging of protein secretion at the single-cell level using plasmon-enhanced FluoroDOT assay. Cell Rep. Methods 2022, 2, 100267. [Google Scholar] [PubMed]
- Subramanian, S.; Jones, H.B.; Frustaci, S.; Winter, S.; van der Kamp, M.W.; Arcus, V.L.; Pudney, C.R.; Vollmer, F. Sensing Enzyme Activation Heat Capacity at the Single-Molecule Level Using Gold-Nanorod-Based Optical Whispering Gallery Modes. ACS Appl. Nano Mater. 2021, 4, 4576–4583. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Winfree, E. Engineering entropy-driven reactions and networks catalyzed by DNA. Science 2009, 324, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Yurke, B.; Turberfield, A.J.; Mills, A.P., Jr.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef]
- Petersen, C.; Heimburg, T.; Mouritsen, O.G.; Zuckermann, M.J. Dielectric response of single-stranded DNA: An experimental and theoretical study. J. Phys. Chem. A 2015, 119, 7491–7502. [Google Scholar] [CrossRef]
- Cammi, R. The Excess Polarizability of Single-Stranded DNA Molecules in Solution: A Linear Response Theory in the Polarizable Continuum Model with an Application to Biosensing. J. Phys. Chem. A 2025, 129, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Eerqing, N.; Wu, H.Y.; Subramanian, S.; Vincent, S.; Vollmer, F. Anomalous DNA hybridisation kinetics on gold nanorods revealed via a dual single-molecule imaging and optoplasmonic sensing platform. Nanoscale Horizons 2023, 8, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Keng, D.; Tan, X.; Arnold, S. Whispering gallery micro-global positioning system for nanoparticle sizing in real time. Appl. Phys. Lett. 2014, 105, 071105. [Google Scholar] [CrossRef]
- Foreman, M.R.; Vollmer, F. Theory of resonance shifts of whispering gallery modes by arbitrary plasmonic nanoparticles. New J. Phys. 2013, 15, 083006. [Google Scholar] [CrossRef]
- Novotny, L.; Hecht, B. Principles of Nano-Optics; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Methods 2008, 5, 591–596. [Google Scholar] [CrossRef]
- Houghton, M.C.; Toropov, N.A.; Yu, D.; Bagby, S.; Vollmer, F. Single molecule thermodynamic penalties applied to enzymes by whispering gallery mode biosensors. Adv. Sci. 2024, 11, 2403195. [Google Scholar] [CrossRef]
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Jäger, M.; Nir, E.; Weiss, S. Site-specific labeling of proteins for single-molecule FRET by combining chemical and enzymatic modification. Protein Sci. 2006, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, S.; Ding, S.; Zhang, L.; Yi, C.; Chen, Y.; Liu, G.S.; Chen, Z.; Xiao, W.; Chen, L.; et al. Algorithm assisted comprehensive optimization of SPR sensors towards single molecule detection. Biosens. Bioelectron. 2025, 289, 117846. [Google Scholar] [CrossRef]
- Booth, L.S.; Browne, E.V.; Mauranyapin, N.P.; Madsen, L.S.; Barfoot, S.; Mark, A.; Bowen, W.P. Modelling of the dynamic polarizability of macromolecules for single-molecule optical biosensing. Sci. Rep. 2022, 12, 1995. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Yu, D. Optical Whispering Gallery Modes for Biosensing: From Physical Principles to Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Zossimova, E.; Jones, C.; Perera, K.M.K.; Pedireddy, S.; Walter, M.; Vollmer, F. Whispering Gallery Mode Sensing through the Lens of Quantum Optics, Artificial Intelligence, and Nanoscale Catalysis. Appl. Phys. Lett. 2024, 125, 030501. [Google Scholar] [CrossRef]




| ssDNA | Sequence (5′-3′) | |
|---|---|---|
| Set I | P1 | [ThiolC6] TTT TAT ACA TCT A |
| ImP1*D | [DY782] CTA GAT GTA T | |
| Set II | T22 | [ThiolC6] TTT TGA GAT AAA CGA GAA GGA TTG AT |
| ImT22*D | [DY782] ATC AGT CCT TTT CCT TTA TCT C (3 mismatched) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eerqing, N.; Zossimova, E.; Subramanian, S.; Wu, H.-Y.; Vollmer, F. Monitoring Single DNA Docking Site Activity with Sequential Modes of an Optoplasmonic Whispering-Gallery Mode Biosensor. Sensors 2025, 25, 6059. https://doi.org/10.3390/s25196059
Eerqing N, Zossimova E, Subramanian S, Wu H-Y, Vollmer F. Monitoring Single DNA Docking Site Activity with Sequential Modes of an Optoplasmonic Whispering-Gallery Mode Biosensor. Sensors. 2025; 25(19):6059. https://doi.org/10.3390/s25196059
Chicago/Turabian StyleEerqing, Narima, Ekaterina Zossimova, Sivaraman Subramanian, Hsin-Yu Wu, and Frank Vollmer. 2025. "Monitoring Single DNA Docking Site Activity with Sequential Modes of an Optoplasmonic Whispering-Gallery Mode Biosensor" Sensors 25, no. 19: 6059. https://doi.org/10.3390/s25196059
APA StyleEerqing, N., Zossimova, E., Subramanian, S., Wu, H.-Y., & Vollmer, F. (2025). Monitoring Single DNA Docking Site Activity with Sequential Modes of an Optoplasmonic Whispering-Gallery Mode Biosensor. Sensors, 25(19), 6059. https://doi.org/10.3390/s25196059





