A Framework to Evaluate Feasibility, Safety, and Accuracy of Wireless Sensors in the Neonatal Intensive Care Unit: Oxygen Saturation Monitoring
Abstract
Highlights
- The study introduces and applies a robust, multidimensional framework for assessing wireless monitoring devices in NICUs—addressing feasibility, safety, and clinical accuracy in real-world conditions.
- Applying this framework to a wireless oximeter revealed a strong performance for skin safety and Bluetooth connectivity of device but issues with signal coverage and accuracy.
- The study’s framework offers a standardized approach for evaluating emerging wireless technologies in neonatal care, encouraging global assessments of feasibility, safety and accuracy.
- The findings of this study suggest future development is needed to address remaining challenges in wireless oximeters, related to their accuracy and signal coverage.
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Outcome
2.2. Study Design
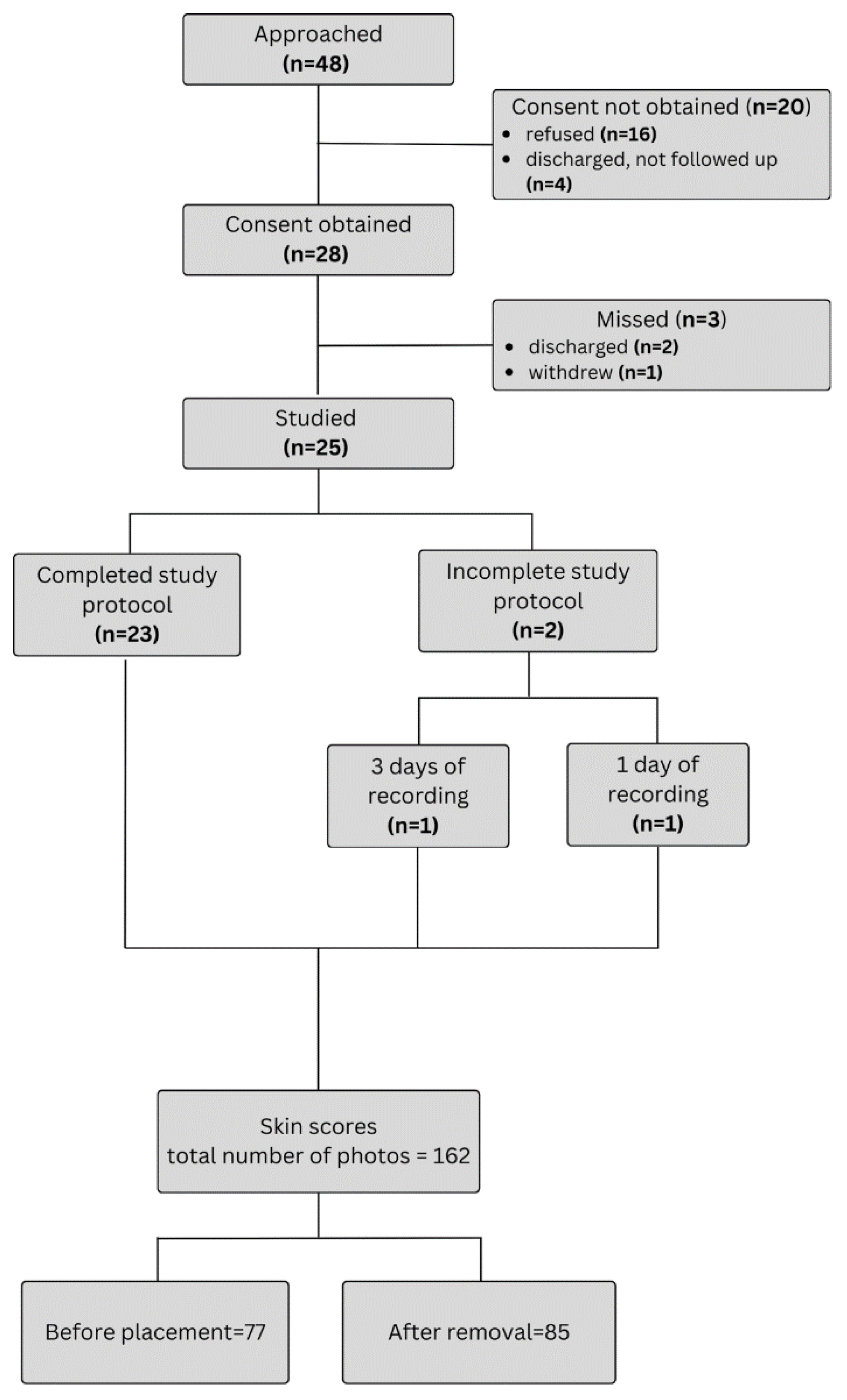
2.3. Study Participants
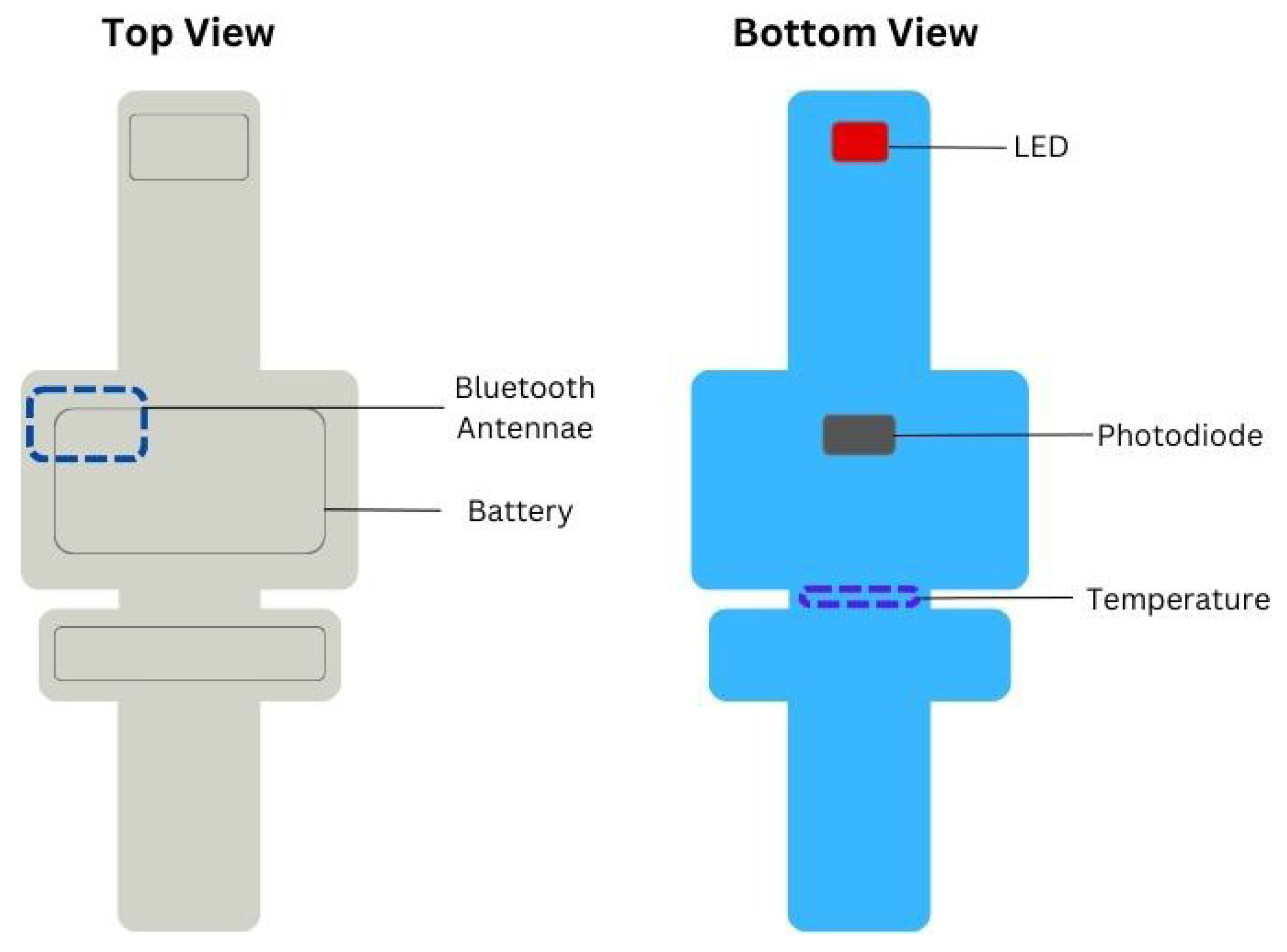
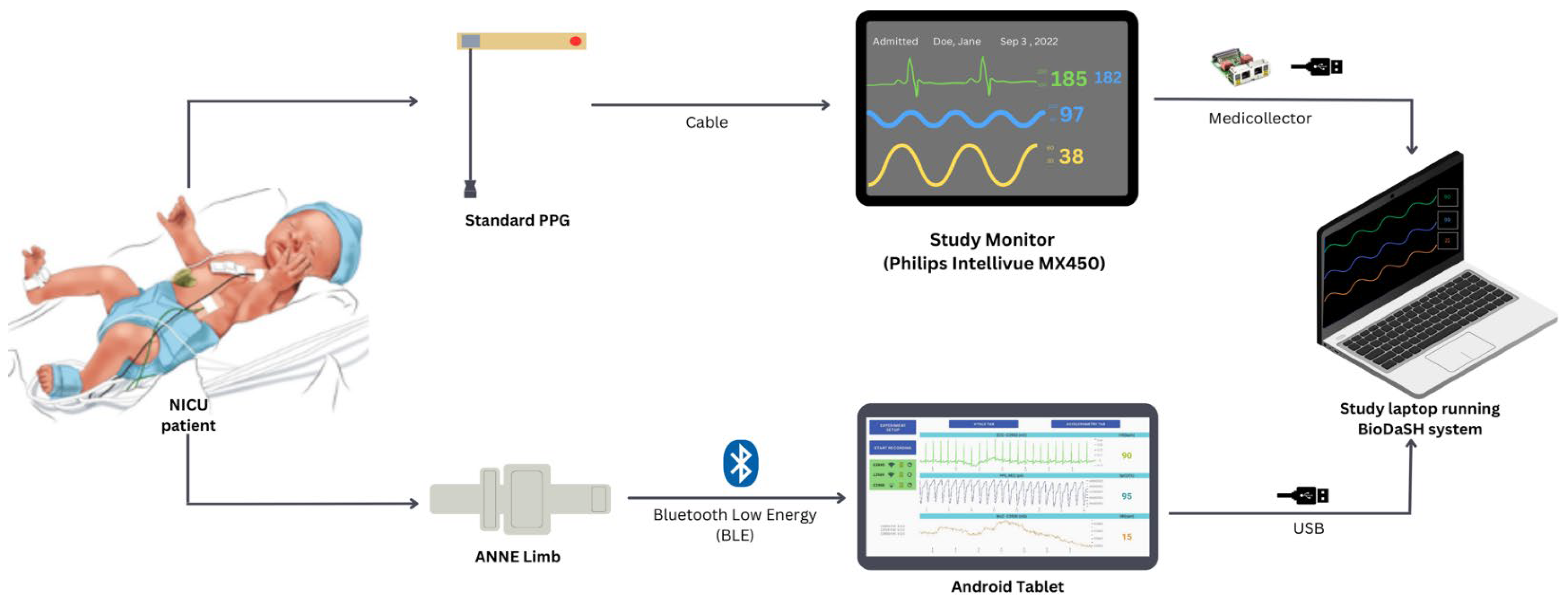
2.4. Study Equipment and Recording
2.5. Signal Pre-Processing
2.6. Data Analysis
2.7. Sample Size and Statistical Analysis
3. Results
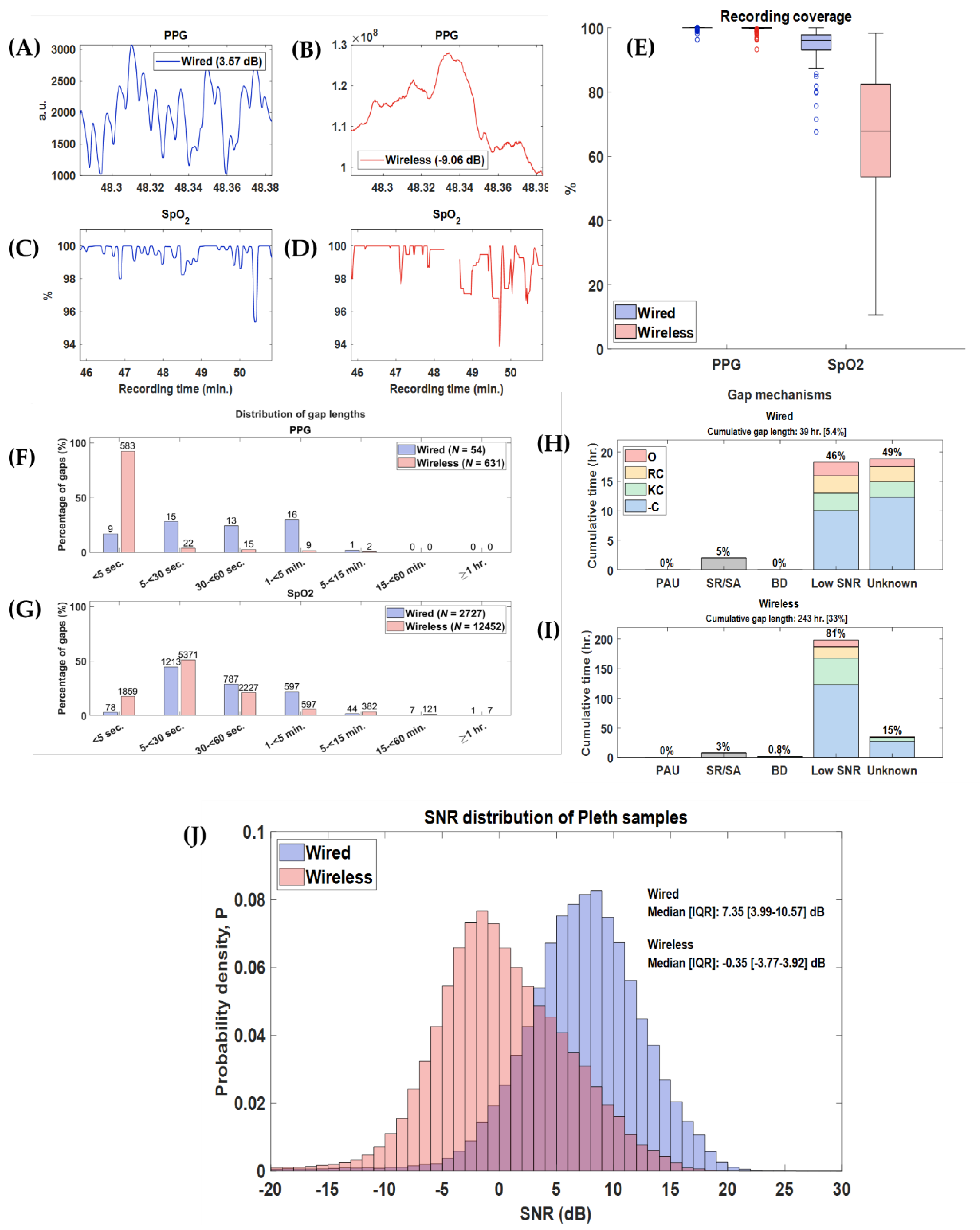
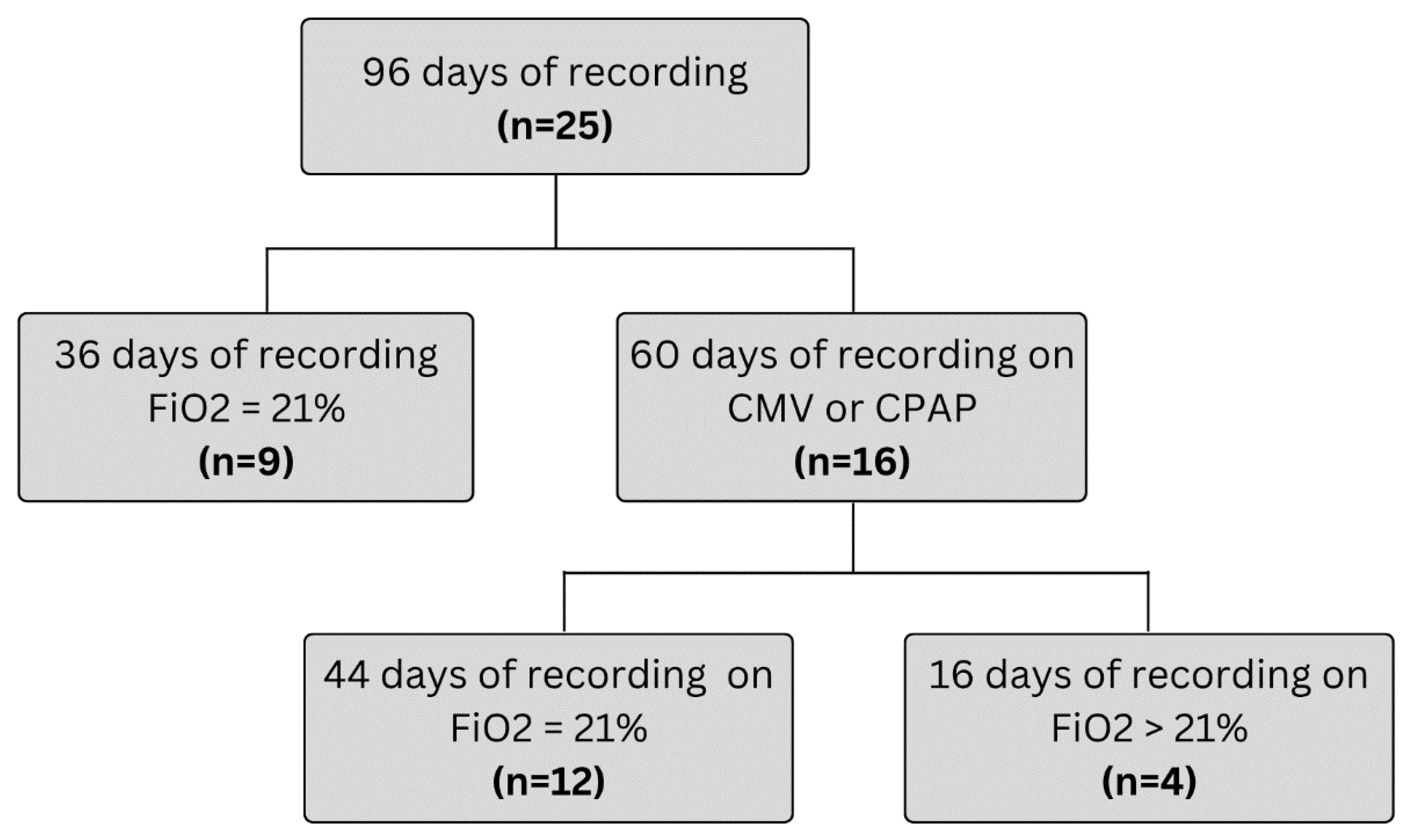
3.1. Feasibility
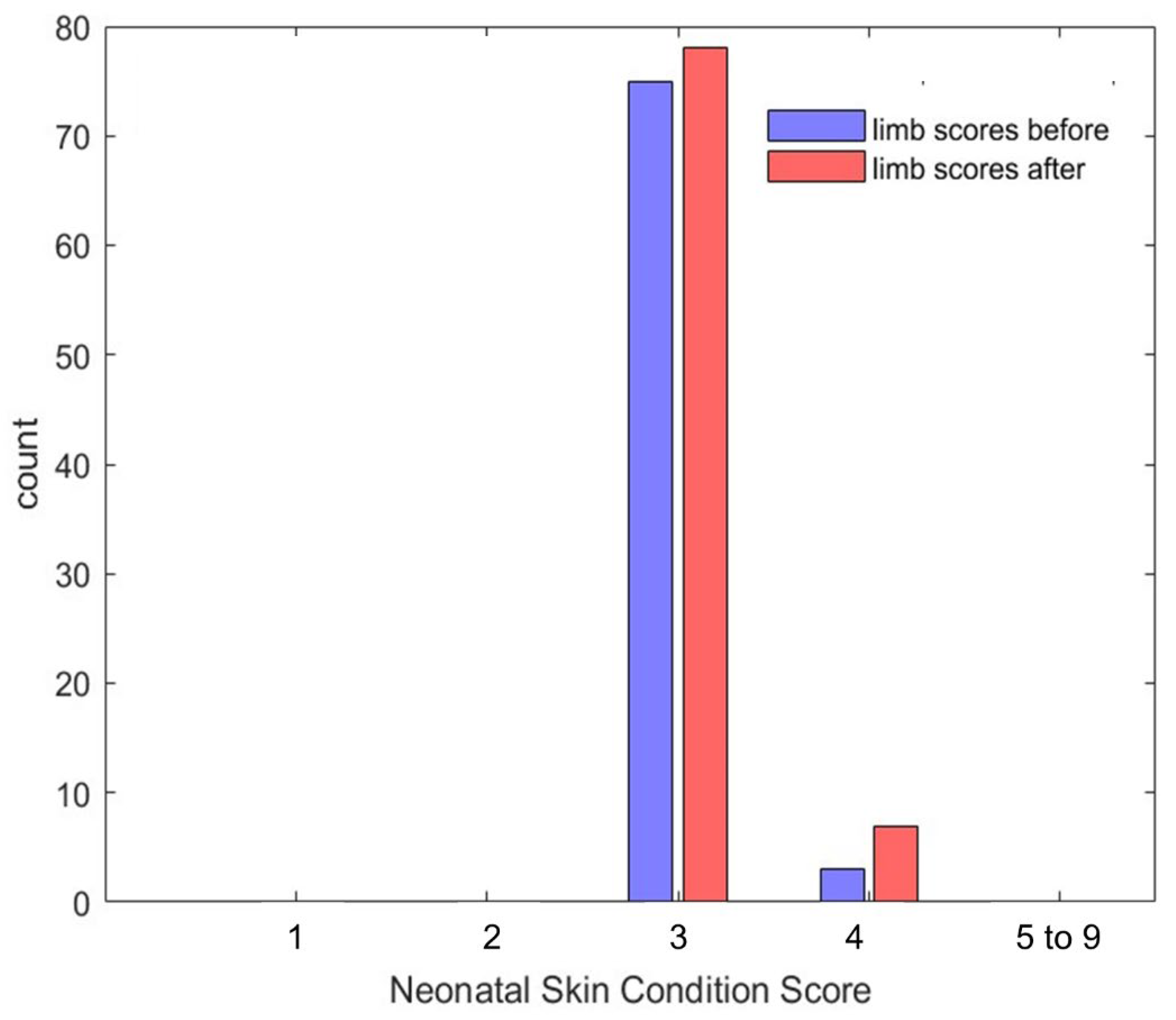
3.2. Safety
3.3. Accuracy
4. Discussion
- (1)
- Recruitment and detailed characterization of a diverse patient population should be included, and data should be collected using a custom platform that enabled time-synchronized recording of vital signs from both the wired and wireless monitoring devices.
- (2)
- Device feasibility should be evaluated by assessing signal coverage across a range of patient activities and analyzing the impact of routine care tasks on device performance. Following this, safety should be assessed through a formal comparative analysis of the neonatal skin after prolonged device wear.
- (3)
- Accuracy must be evaluated on a sample-to-sample basis and further analyzed using the Clarke error grid to provide clinically meaningful insights.
4.1. Comparison to Existing Studies
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NICU | Neonatal intensive care unit |
| SpO2 | Oxygen saturation |
| PPG | Photoplethysmography |
| FiO2 | Fraction of inspired oxygen |
| BioDAsh | Biosensor data aggregation and synchronization system |
| GA | Gestational age |
| PMA | Postmenstrual age |
| DoL | Days of life |
| BW | Birthweight |
| CW | Current weight |
| dB | Decibels |
| EGA | Error grid analysis |
| CPAP | Continuous positive airway pressure |
| SNR | Signal to noise ratio |
| NSCS | Neonatal skin condition score |
| LoA | Limits of agreement |
| MoE | Margin of error |
References
- Kumar, N.; Akangire, G.; Sullivan, B.; Fairchild, K.; Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: Big data to the forefront. Pediatr. Res. 2019, 87, 210–220. [Google Scholar] [CrossRef]
- Pesola, G.R.; Sankari, A. Oxygenation Status and Pulse Oximeter Analysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK592401/ (accessed on 7 March 2025).
- Chan, K.Y.Y.; Miller, S.L.; Schmölzer, G.M.; Stojanovska, V.; Polglase, G.R. Respiratory Support of the Preterm Neonate: Lessons About Ventilation-Induced Brain Injury From Large Animal Models. Front. Neurol. 2020, 11, 862. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Bekker, L.; Himmelreich, J.C.L.; De Clercq, L.; Karregat, E.P.M.; E. Sleeswijk, M.; Lucassen, W.A.M. Performance of popular pulse oximeters compared with simultaneous arterial oxygen saturation or clinical-grade pulse oximetry: A cross-sectional validation study in intensive care patients. BMJ Open Respir Res. 2021, 8, e000939. [CrossRef]
- Petterson, M.T.; Begnoche, V.L.; Graybeal, J.M. The Effect of Motion on Pulse Oximetry and Its Clinical Significance. Anesthesia Analg. 2007, 105, S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Al Maghaireh, D.F.; Abdullah, K.L.; Chan, C.M.; Piaw, C.Y.; Al Kawafha, M.M. Systematic review of qualitative studies exploring parental experiences in the Neonatal Intensive Care Unit. J. Clin. Nurs. 2016, 25, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Bonner, O.; Beardsall, K.; Crilly, N.; Lasenby, J. ‘There were more wires than him’: The potential for wireless patient monitoring in neonatal intensive care. BMJ Innov. 2017, 3, 12–18. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Díaz-Rossello, J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. 2016, 8, CD002771. [Google Scholar] [CrossRef] [PubMed]
- Mehrpisheh, S.; Doorandish, Z.; Farhadi, R.; Ahmadi, M.; Moafi, M.; Elyasi, F. The Effectiveness of Kangaroo Mother Care (KMC) on attachment of mothers with premature infants. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2022, 15, 100149. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef]
- Trevisanuto, D.; Ferrarese, P.; Cantarutti, F.; Zanardo, V. Skin lesions caused by oxygen saturation monitor probe: Pathogenetic considerations concerning 2 neonatal cases. Pediatr. Medica Chir. Med. Surg. Pediatr. 1995, 17, 373–374. [Google Scholar]
- Quaresima, V.; Ferrari, M.; Scholkmann, F. Ninety years of pulse oximetry: History, current status, and outlook. J. Biomed. Opt. 2024, 29, S33307. [Google Scholar] [CrossRef]
- Xu, S.; Rwei, A.Y.; Vwalika, B.; Chisembele, M.P.; Stringer, J.S.A.; Ginsburg, A.S.; A. Rogers, J. Wireless skin sensors for physiological monitoring of infants in low-income and middle-income countries. Lancet Digit. Health 2021, 3, e266–e273. [Google Scholar] [CrossRef]
- Bonafide, C.P.; Localio, A.R.; Ferro, D.F.; Orenstein, E.W.; Jamison, D.T.; Lavanchy, C.; Foglia, E.E. Accuracy of Pulse Oximetry-Based Home Baby Monitors. JAMA 2018, 320, 717–719. [Google Scholar] [CrossRef]
- Kovesi, T.; Saban, J.; Haddad, J.F.; Reddy, D.; Webster, R.; Udupa, S. The Accuracy of Readily Available Consumer-Grade Oxygen Saturation Monitors in Pediatric Patients. Respir. Care 2024, 69, 387–394. [Google Scholar] [CrossRef]
- Afeerah, M.; Zarmina, E. Media Review: The Owlet Smart Sock—A “must have” for the baby registry? J. Clin. Sleep Med. 2020, 16, 839–840. [Google Scholar] [CrossRef]
- Senechal, E.; Jeanne, E.; Tao, L.; Kearney, R.; Shalish, W.; Sant’aNna, G. Wireless monitoring devices in hospitalized children: A scoping review. Eur. J. Pediatr. 2023, 182, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.U.; Rwei, A.Y.; Hourlier-Fargette, A.; Xu, S.; Lee, K.; Dunne, E.C.; Xie, Z.; Liu, C.; Carlini, A.; Kim, D.H.; et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 2020, 26, 418–429. [Google Scholar] [CrossRef]
- Harrell, M.; Dobson, N.; Olsen, C.; Ahmed, A.; Hunt, C. Inpatient comparison of wireless and wired pulse oximetry in neonates. J. Neonatal-Perinatal Med. 2022, 15, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Day, H.; Petersen, B.; Marchant, T.; Jones, C.; Singh, Y.; Chan, B. Accuracy of wireless pulse oximeter on preterm or< 2.5 kg infants. Am. J. Perinatol. 2024, 41.S 01, e1606–e1612. [Google Scholar]
- Senechal, E.; Radeschi, D.; Tao, L.; Lv, S.; Jeanne, E.; Kearney, R.; Shalish, W.; Anna, G.S. The use of wireless sensors in the neonatal intensive care unit: A study protocol. PeerJ 2023, 11, e15578. [Google Scholar] [CrossRef]
- Radeschi, D.J.; Senechal, E.; Tao, L.; Lv, S.; Shalish, W.; Sant’anna, G.; Kearney, R.E. Comparison of Wired and Wireless Heart Rate Monitoring in the Neonatal Intensive Care Unit. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia; 2023; pp. 1–4. [Google Scholar]
- Lund, C.H.; Osborne, J.W. Validity and Reliability of the Neonatal Skin Condition Score. J. Obstet. Gynecol. Neonatal Nurs. 2004, 33, 320–327. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. Stat 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Martin Bland, J.; Altman Douglas, G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Aggarwal, R.; Gunaseelan, V.; Manual, D.; Sanker, M.; Prabaaker, S. Clinical Evaluation of a Wireless Device for Monitoring Vitals in Newborn Babies. Indian J. Pediatr. 2023, 90, 1110–1115. [Google Scholar] [CrossRef]
- Coleman, J.; Ginsburg, A.S.; Macharia, W.; Ochieng, R.; Chomba, D.; Zhou, G.; Dunsmuir, D.; Xu, S.; Ansermino, J.M.; Frasch, M.G. Evaluation of Sibel’s Advanced Neonatal Epidermal (ANNE) wireless continuous physiological monitor in Nairobi, Kenya. PLoS ONE 2022, 17, e0267026. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Nia, S.Z.; Chomba, D.; Parsimei, M.; Dunsmuir, D.; Waiyego, M.; Coleman, J.; Ochieng, R.; Zhou, G.; Macharia, W.M.; et al. Clinical feasibility of an advanced neonatal epidermal multiparameter continuous monitoring technology in a large public maternity hospital in Nairobi, Kenya. Sci. Rep. 2022, 12, 11722. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.U.; Stewart, S.; Verma, A.; Hoen, H.; Stein, M.L.; Wright, G.; Ramamoorthy, C. Accuracy of a portable pulse oximeter in monitoring hypoxemic infants with cyanotic heart disease. Cardiol. Young 2019, 29, 1025–1029. [Google Scholar] [CrossRef]
- Keerthy, S.; Nagesh, N.K. Efficacious Continuous Monitoring of Infants Using Wireless Remote Monitoring Technology. Indian J. Pediatr. 2022, 89, 771–775. [Google Scholar] [CrossRef]
- Swamy, S.K.N.; Stockwell, S.J.; Liu, C.; Henry, C.; Shipley, L.; Ward, C.; Mirahmadi, S.; Correia, R.; Morgan, S.P.; Crowe, J.A.; et al. Comparing peripheral limb and forehead vital sign monitoring in newborn infants at birth. Pediatr. Res. 2024, 1–6. [Google Scholar] [CrossRef]
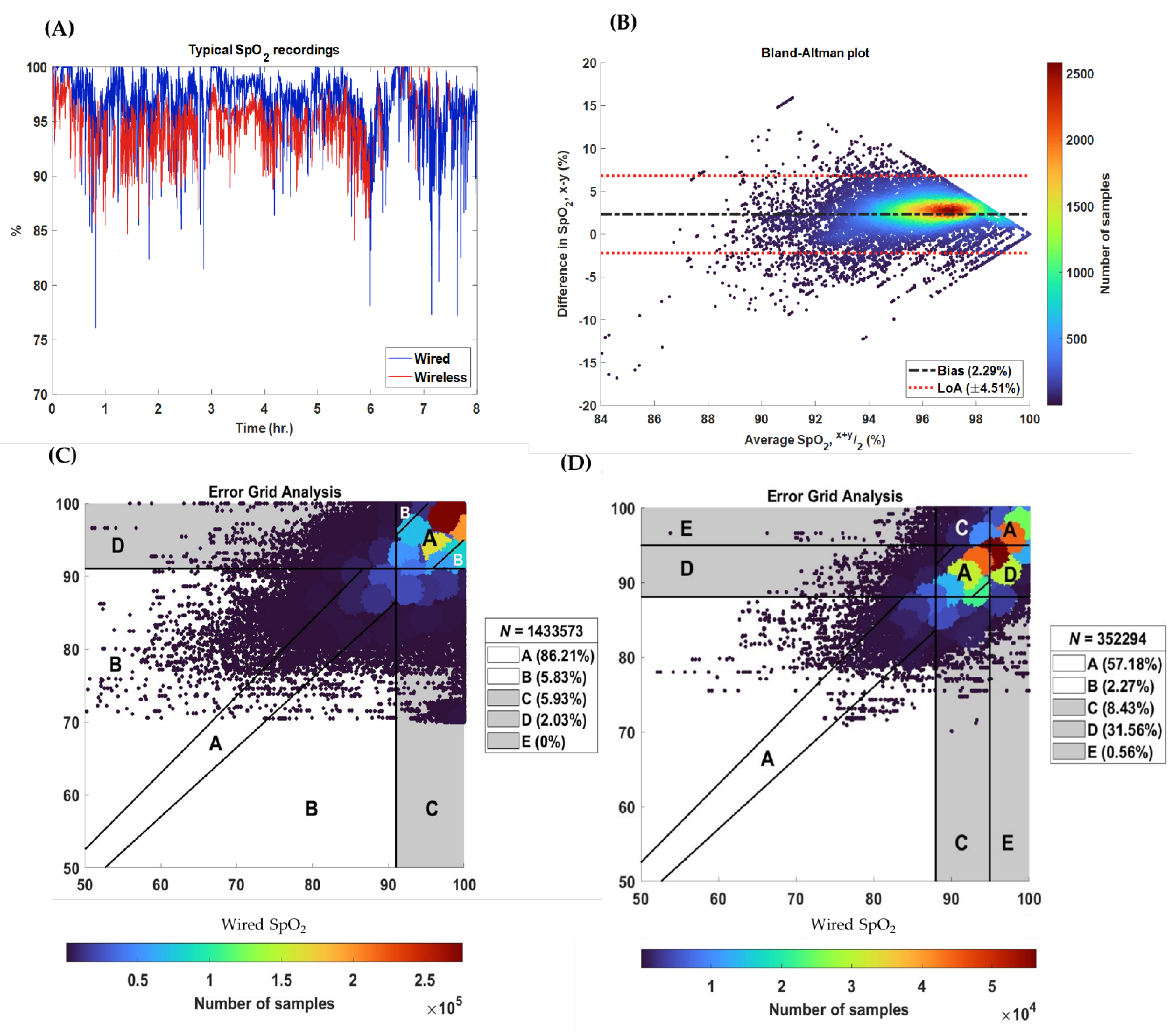
| Region | Interpretation |
|---|---|
| A | Values within 5% of the reference device and yielding the same clinical outcomes |
| B | Values greater than 5% of the reference device but still yielding the same clinical outcomes |
| C | Values that would result in unnecessary treatment (i.e., false positives) |
| D | Values that would result in failure-to-treat (i.e., false negatives) |
| E | Values that would result in the reverse treatment |
| All (n = 25) | CPAP (n = 13) | Room Air (n = 9) | CMV (n = 3) | |
|---|---|---|---|---|
| Gestational Age (weeks) | 28.4 [26.1–30.7] (25–40.7) | 28.4 [26.6–29.3] (25.3–32) | 30.7 [28.3–35.8] (26.1–40.7) | 25.6 [25.1–26.0] (25–26.1) |
| Corrected gestational age (weeks) | 33.3 [31.3–36.1] (26.3–45.9) | 33.1 [31.2–33.8] (26.3–36.7) | 36.3 [35.1–40.8] (32.1–45.9) | 27.3 [27.0–29.1] (26.9–29.7) |
| Birthweight (g) | 1110 [780–1397] (600–3480) | 1200 [806–1356] (600–1790) | 1360 [795–2095] (605–3480) | 780 [728–1020] (710–1100) |
| Current weight (g) | 1450 [1151–1930] (750–3990) | 1450 [1225–1795] (810–2685) | 1870 [1378–3028] (1150–3990) | 1000 [813–1030] (750–1040) |
| Infants Studied n = 25 | |
|---|---|
| Apneas and Bradycardia events | 10 (40) |
| Anemia | 8 (32) |
| Hyperbilirubinemia | 8 (32) |
| Respiratory Distress Syndrome | 8 (32) |
| Intraventricular Hemorrhage | 5 (20) |
| Lung Immaturity | 4 (16) |
| Intrauterine Growth Restriction | 2 (8) |
| Patent Ductus Arteriosus | 2 (8) |
| Bronchopulmonary Dysplasia | 1 (4) |
| Cholestasis | 1 (4) |
| Feeding Intolerance | 1 (4) |
| Gastric Perforation w/ileostomy | 1 (4) |
| Gastro-Esophageal Reflux | 1 (4) |
| Suspected Neonatal Sepsis | 1 (4) |
| Urinary Tract Infection | 1 (4) |
| Neonatal Skin Condition Score (NSCS) | |||||
| Time/Score | 3 | 4 | 5–9 | Sum | |
| Day 1 | Before | 18 (11) | 1 (<1) | 0 | 19 |
| After | 22 (14) | 1 (<1) | 0 | 23 | |
| Day 2 | Before | 20 (12) | 0 (0) | 0 | 20 |
| After | 20 (12) | 1 (<1) | 0 | 21 | |
| Day 3 | Before | 18 (11) | 1 (<1) | 0 | 19 |
| After | 19 (12) | 2 (1) | 0 | 21 | |
| Day 4 | Before | 18 (11) | 1 (1) | 0 | 19 |
| After | 17 (11) | 3 (2) | 0 | 20 | |
| Total | 152 (94) | 10 (6) | 0 | 162 (100) | |
| NSCS (After-Before) | |||||
| −1 | 0 | 1 | Sum | ||
| Day 1 | 1 | 17 | 1 | 19 | |
| Day 2 | 0 | 18 | 1 | 19 | |
| Day 3 | 1 | 14 | 2 | 17 | |
| Day 4 | 1 | 14 | 3 | 18 | |
| Total | 3 | 63 | 7 | 73 (100) | |
| Metric (%) | Median [IQR] | Effect Size |
|---|---|---|
| Bias, | 1.34 [0.53 to 2.20] | ±0.64 |
| Margin of error | 4.83 [4.05 to 5.96] | ±1.19 |
| Upper 95%-limit of agreement | 6.41 [5.2 to 7.77] | |
| Lower 95%-limit of agreement | −3.63 [−4.82 to −2.59] | |
| Mean absolute error | 2.2 [1.18 to 2.93] | ±0.5 |
| Study Component | Recommendations |
|---|---|
| Population |
|
| Data acquisition |
|
| Analysis |
|
| Reporting |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senechal, E.; Radeschi, D.; Jeanne, E.; Ruiz, A.S.; Dulmage, B.; Shalish, W.; Kearney, R.E.; Sant’Anna, G. A Framework to Evaluate Feasibility, Safety, and Accuracy of Wireless Sensors in the Neonatal Intensive Care Unit: Oxygen Saturation Monitoring. Sensors 2025, 25, 5647. https://doi.org/10.3390/s25185647
Senechal E, Radeschi D, Jeanne E, Ruiz AS, Dulmage B, Shalish W, Kearney RE, Sant’Anna G. A Framework to Evaluate Feasibility, Safety, and Accuracy of Wireless Sensors in the Neonatal Intensive Care Unit: Oxygen Saturation Monitoring. Sensors. 2025; 25(18):5647. https://doi.org/10.3390/s25185647
Chicago/Turabian StyleSenechal, Eva, Daniel Radeschi, Emily Jeanne, Ana Saveedra Ruiz, Brittany Dulmage, Wissam Shalish, Robert E. Kearney, and Guilherme Sant’Anna. 2025. "A Framework to Evaluate Feasibility, Safety, and Accuracy of Wireless Sensors in the Neonatal Intensive Care Unit: Oxygen Saturation Monitoring" Sensors 25, no. 18: 5647. https://doi.org/10.3390/s25185647
APA StyleSenechal, E., Radeschi, D., Jeanne, E., Ruiz, A. S., Dulmage, B., Shalish, W., Kearney, R. E., & Sant’Anna, G. (2025). A Framework to Evaluate Feasibility, Safety, and Accuracy of Wireless Sensors in the Neonatal Intensive Care Unit: Oxygen Saturation Monitoring. Sensors, 25(18), 5647. https://doi.org/10.3390/s25185647






