Effects of Induced Physical Fatigue on Heart Rate Variability in Healthy Young Adults †
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Data Collection and Analysis
2.4. Statistical Analysis
3. Results
3.1. Condition Effects on Heart Rate Variability (HRV) Measures
3.2. Correlations Between the Total Fatiguing Duration and Baseline HRV Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suarez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- MacIntosh, B.R.; Holash, R.J.; Renaud, J.M. Skeletal muscle fatigue—Regulation of excitation-contraction coupling to avoid metabolic catastrophe. J. Cell Sci. 2012, 125 Pt 9, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Drain, J.R.; Reilly, T.J. Physical employment standards, physical training and musculoskeletal injury in physically demanding occupations. Work 2019, 63, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.L.; Sinden, K.E.; MacPhee, R.S.; Ottawa Paramedic Service Research, T. Identifying the critical physical demanding tasks of paramedic work: Towards the development of a physical employment standard. Appl. Ergon. 2017, 65, 233–239. [Google Scholar] [CrossRef]
- Gledhill, N.; Jamnik, V.K. Characterization of the physical demands of firefighting. Can. J. Sport Sci. 1992, 17, 207–213. [Google Scholar]
- Lucas, S.J.; Helge, J.W.; Schutz, U.H.; Goldman, R.F.; Cotter, J.D. Moving in extreme environments: Extreme loading; carriage versus distance. Extrem. Physiol. Med. 2016, 5, 6. [Google Scholar] [CrossRef]
- Boffey, D.; Harat, I.; Gepner, Y.; Frosti, C.L.; Funk, S.; Hoffman, J.R. The Physiology and Biomechanics of Load Carriage Performance. Mil. Med. 2019, 184, e83–e90. [Google Scholar] [CrossRef]
- Hauschild, V.D.; Lee, T.; Barnes, S.; Forrest, L.; Hauret, K.; Jones, B.H. The Etiology of Injuries in US Army Initial Entry Training. US Army Med. Dep. J. 2018, 22–29. [Google Scholar]
- Armstrong, N.C.D.; Ward, A.; Lomax, M.; Tipton, M.J.; House, J.R. Wearing body armour and backpack loads increase the likelihood of expiratory flow limitation and respiratory muscle fatigue during marching. Ergonomics 2019, 62, 1181–1192. [Google Scholar] [CrossRef]
- Wills, J.A.; Saxby, D.J.; Glassbrook, D.J.; Doyle, T.L.A. Load-Carriage Conditioning Elicits Task-Specific Physical and Psychophysical Improvements in Males. J. Strength Cond. Res. 2019, 33, 2338–2343. [Google Scholar] [CrossRef]
- Vaara, J.P.; Groeller, H.; Drain, J.; Kyrolainen, H.; Pihlainen, K.; Ojanen, T.; Connaboy, C.; Santtila, M.; Agostinelli, P.; Nindl, B.C. Physical training considerations for optimizing performance in essential military tasks. Eur. J. Sport Sci. 2022, 22, 43–57. [Google Scholar] [CrossRef]
- Knapik, J.J.; Harman, E.A.; Steelman, R.A.; Graham, B.S. A systematic review of the effects of physical training on load carriage performance. J. Strength Cond. Res. 2012, 26, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.M.; Pendergrass, T.L.; Lee, I.E.; Hauret, K.G.; Chervak, M.C.; Rhon, D.I. Musculoskeletal Injuries and United States Army Readiness. Part II: Management Challenges and Risk Mitigation Initiatives. Mil. Med. 2020, 185, e1472–e1480. [Google Scholar] [CrossRef]
- Sefton, J.M.; Lohse, K.R.; McAdam, J.S. Prediction of Injuries and Injury Types in Army Basic Training, Infantry, Armor, and Cavalry Trainees Using a Common Fitness Screen. J. Athl. Train. 2016, 51, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Guedes, J.C.; Vaz, M.P.; Pombo, E.; Fernandes, R.J.; Costa, J.T.; Baptista, J.S. Non-Invasive Physiological Monitoring for Physical Exertion and Fatigue Assessment in Military Personnel: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 8815. [Google Scholar] [CrossRef]
- Jeklin, A.T.; Perrotta, A.S.; Davies, H.W.; Bredin, S.S.D.; Paul, D.A.; Warburton, D.E.R. The association between heart rate variability, reaction time, and indicators of workplace fatigue in wildland firefighters. Int. Arch. Occup. Environ. Health 2021, 94, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Stephenson, M.D.; Thompson, A.G.; Merrigan, J.J.; Stone, J.D.; Hagen, J.A. Applying Heart Rate Variability to Monitor Health and Performance in Tactical Personnel: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 8143. [Google Scholar] [CrossRef]
- Vine, C.A.J.; Runswick, O.R.; Blacker, S.D.; Coakley, S.L.; Siddall, A.G.; Myers, S.D. Cognitive, Psychophysiological, and Perceptual Responses to a Repeated Military-Specific Load Carriage Treadmill Simulation. Hum. Factors 2023, 66, 2379–2392. [Google Scholar] [CrossRef]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef]
- Garavaglia, L.; Gulich, D.; Defeo, M.M.; Thomas Mailland, J.; Irurzun, I.M. The effect of age on the heart rate variability of healthy subjects. PLoS ONE 2021, 16, e0255894. [Google Scholar] [CrossRef]
- Hernandez-Vicente, A.; Hernando, D.; Santos-Lozano, A.; Rodriguez-Romo, G.; Vicente-Rodriguez, G.; Pueyo, E.; Bailon, R.; Garatachea, N. Heart Rate Variability and Exceptional Longevity. Front. Physiol. 2020, 11, 566399. [Google Scholar] [CrossRef] [PubMed]
- Sammito, S.; Thielmann, B.; Bockelmann, I. Update: Factors influencing heart rate variability-a narrative review. Front. Physiol. 2024, 15, 1430458. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Regnard, J.; Desmarets, M.; Mauny, F.; Mourot, L.; Fouillot, J.P.; Coulmy, N.; Millet, G. Fatigue shifts and scatters heart rate variability in elite endurance athletes. PLoS ONE 2013, 8, e71588. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yeo, J.C. Effects of load carriage and fatigue on gait characteristics. J. Biomech. 2011, 44, 1259–1263. [Google Scholar] [CrossRef]
- Sedighi Maman, Z.; Alamdar Yazdi, M.A.; Cavuoto, L.A.; Megahed, F.M. A data-driven approach to modeling physical fatigue in the workplace using wearable sensors. Appl. Ergon. 2017, 65, 515–529. [Google Scholar] [CrossRef]
- Kao, P.C.; Lomasney, C.; Gu, Y.; Clark, J.P.; Yanco, H.A. Effects of induced motor fatigue on walking mechanics and energetics. J. Biomech. 2023, 156, 111688. [Google Scholar] [CrossRef]
- Leard, J.S.; Cirillo, M.A.; Katsnelson, E.; Kimiatek, D.A.; Miller, T.W.; Trebincevic, K.; Garbalosa, J.C. Validity of two alternative systems for measuring vertical jump height. J. Strength Cond. Res. 2007, 21, 1296–1299. [Google Scholar] [PubMed]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.S.; Yi, S.H. Effects of detrending for analysis of heart rate variability and applications to the estimation of depth of anesthesia. J. Korean Phys. Soc. 2004, 4, 561–568. [Google Scholar]
- Xu, B.; Dubois, R.; Pont, O.; Yahia, H. Nonlinear trend removal should be carefully performed in heart rate variability analysis. arXiv 2016. [Google Scholar] [CrossRef]
- Baek, H.J.; Cho, C.H.; Cho, J.; Woo, J.M. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemed. J. E Health 2015, 21, 404–414. [Google Scholar] [CrossRef]
- Shaffer, F.; Shearman, S.; Meehan, Z.M. The promise of ultra-short-term (UST) heart rate variability measurements. Biofeedback 2016, 44, 229–233. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Silva, L.E.; Silva, C.A.; Salgado, H.C.; Fazan, R., Jr. The role of sympathetic and vagal cardiac control on complexity of heart rate dynamics. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H469–H477. [Google Scholar] [CrossRef]
- Eronen, T.; Lipponen, J.A.; Hyrylä, V.V.; Kupari, S.; Mursu, J.; Venojärvi, M.; Tikkanen, H.O.; Tarvainen, M.P. Heart Rate Variability Based Ventilatory Threshold Estimation—Validation of a Commericially Available Algorithm. medRxiv 2024. [Google Scholar] [CrossRef]
- Gronwald, T.; Hoos, O.; Ludyga, S.; Hottenrott, K. Non-linear dynamics of heart rate variability during incremental cycling exercise. Res. Sports Med. 2019, 27, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Beckers, F.; Verheyden, B.; Aubert, A.E. Aging and nonlinear heart rate control in a healthy population. Am. J. Physiol. Heart Circ Physiol. 2006, 290, H2560–H2570. [Google Scholar] [CrossRef]
- Delgado-Bonal, A.; Marshak, A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Parraca, J.A.; Alegrete, J.; Villafaina, S.; Batalha, N.; Fuentes-Garcia, J.P.; Munoz, D.; Fernandes, O. Heart Rate Variability Monitoring during a Padel Match. Int. J. Environ. Res. Public Health 2022, 19, 3623. [Google Scholar] [CrossRef]
- Guzik, P.; Piskorski, J.; Krauze, T.; Schneider, R.; Wesseling, K.H.; Wykretowicz, A.; Wysocki, H. Correlations between the Poincare plot and conventional heart rate variability parameters assessed during paced breathing. J. Physiol. Sci. 2007, 57, 63–71. [Google Scholar] [CrossRef]
- Bolea, J.; Pueyo, E.; Orini, M.; Bailon, R. Influence of Heart Rate in Non-linear HRV Indices as a Sampling Rate Effect Evaluated on Supine and Standing. Front. Physiol. 2016, 7, 501. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. xxi. 567p. [Google Scholar]
- Brockmann, L.; Hunt, K.J. Heart rate variability changes with respect to time and exercise intensity during heart-rate-controlled steady-state treadmill running. Sci. Rep. 2023, 13, 8515. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Davis, G.M.O. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Wang, W.; Shao, M.; Du, W.; Xu, Y. Impact of exhaustive exercise on autonomic nervous system activity: Insights from HRV analysis. Front. Physiol. 2024, 15, 1462082. [Google Scholar] [CrossRef] [PubMed]
- Lloria-Varella, J.; Koral, J.; Ravel, A.; Feasson, L.; Murias, J.M.; Busso, T. Neuromuscular and autonomic function is fully recovered within 24 h following a sprint interval training session. Eur. J. Appl. Physiol. 2023, 123, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Leicht, A.S.; Sinclair, W.H.; Spinks, W.L. Effect of exercise mode on heart rate variability during steady state exercise. Eur. J. Appl. Physiol. 2008, 102, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.; Giles, D.; Draper, N.; Hoos, O.; Gronwald, T. A New Detection Method Defining the Aerobic Threshold for Endurance Exercise and Training Prescription Based on Fractal Correlation Properties of Heart Rate Variability. Front. Physiol. 2020, 11, 596567. [Google Scholar] [CrossRef]
- Lewis, M.J.; Short, A.L. Sample entropy of electrocardiographic RR and QT time-series data during rest and exercise. Physiol. Meas. 2007, 28, 731–744. [Google Scholar] [CrossRef]
- Rogers, B.; Giles, D.; Draper, N.; Mourot, L.; Gronwald, T. Detection of the Anaerobic Threshold in Endurance Sports: Validation of a New Method Using Correlation Properties of Heart Rate Variability. J. Funct. Morphol. Kinesiol. 2021, 6, 38. [Google Scholar] [CrossRef]
- Sanno, M.; Willwacher, S.; Epro, G.; Bruggemann, G.P. Positive Work Contribution Shifts from Distal to Proximal Joints during a Prolonged Run. Med. Sci. Sports Exerc. 2018, 50, 2507–2517. [Google Scholar] [CrossRef]
- Schmitt, L.; Regnard, J.; Millet, G.P. Monitoring Fatigue Status with HRV Measures in Elite Athletes: An Avenue Beyond RMSSD? Front. Physiol. 2015, 6, 343. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Hautala, A.J.; Makikallio, T.H.; Laukkanen, R.T.; Nissila, S.; Hughson, R.L.; Huikuri, H.V. Effects of aerobic training on heart rate dynamics in sedentary subjects. J. Appl. Physiol. (1985) 2003, 95, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Macartney, M.J.; Larsen, P.; Gibson, N.; Michael, S.; Drain, J.; Peoples, G.E.; Groeller, H. Overnight sleeping heart rate variability of Army recruits during a 12-week basic military training course. Eur. J. Appl. Physiol. 2022, 122, 2135–2144. [Google Scholar] [CrossRef]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Froelicher, V.; Plews, D. Heart Rate Variability: An Old Metric with New Meaning in the Era of Using mHealth technologies for Health and Exercise Training Guidance. Part Two: Prognosis and Training. Arrhythm. Electrophysiol. Rev. 2018, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Finni, T.; Zou, L.; Perhonen, M.; Sedliak, M.; Alen, M.; Cheng, S. Effects of strength training on work capacity and parasympathetic heart rate modulation during exercise in physically inactive men. Int. J. Sports Med. 2009, 30, 719–724. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Teisala, T.; Mutikainen, S.; Tolvanen, A.; Rottensteiner, M.; Leskinen, T.; Kaprio, J.; Kolehmainen, M.; Rusko, H.; Kujala, U.M. Associations of physical activity, fitness, and body composition with heart rate variability-based indicators of stress and recovery on workdays: A cross-sectional study. J. Occup. Med. Toxicol. 2014, 9, 16. [Google Scholar] [CrossRef]
- Lundstrom, C.J.; Foreman, N.A.; Biltz, G. Practices and Applications of Heart Rate Variability Monitoring in Endurance Athletes. Int. J. Sports Med. 2023, 44, 9–19. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef]
- Kao, P.C.; Lomasney, C. Walking Stability and Kinematic Variability Following Motor Fatigue Induced by Incline Treadmill Walking. Sensors 2025, 25, 1489. [Google Scholar] [CrossRef]
- Vescovi, J.D.; Mandic, I.; Watson, G. Relationships between heart rate variability and indirect indicators of hydration status in elite male field hockey players. Int. J. Sports Sci. Coach. 2022, 17, 619–625. [Google Scholar] [CrossRef]
- Immanuel, S.; Teferra, M.N.; Baumert, M.; Bidargaddi, N. Heart Rate Variability for Evaluating Psychological Stress Changes in Healthy Adults: A Scoping Review. Neuropsychobiology 2023, 82, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Sajjadieh, A.; Shahsavari, A.; Safaei, A.; Penzel, T.; Schoebel, C.; Fietze, I.; Mozafarian, N.; Amra, B.; Kelishadi, R. The Association of Sleep Duration and Quality with Heart Rate Variability and Blood Pressure. Tanaffos 2020, 19, 135–143. [Google Scholar] [PubMed]
- Riganello, F.; Prada, V.; Soddu, A.; di Perri, C.; Sannita, W.G. Circadian Rhythms and Measures of CNS/Autonomic Interaction. Int. J. Environ. Res. Public Health 2019, 16, 2336. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Warth, M.; Ellis, R.J.; Bach, C.; Hillecke, T.K.; Thayer, J.F. Body mass index is related to autonomic nervous system activity as measured by heart rate variability—A replication using short term measurements. J. Nutr. Health Aging 2014, 18, 300–302. [Google Scholar] [CrossRef] [PubMed]


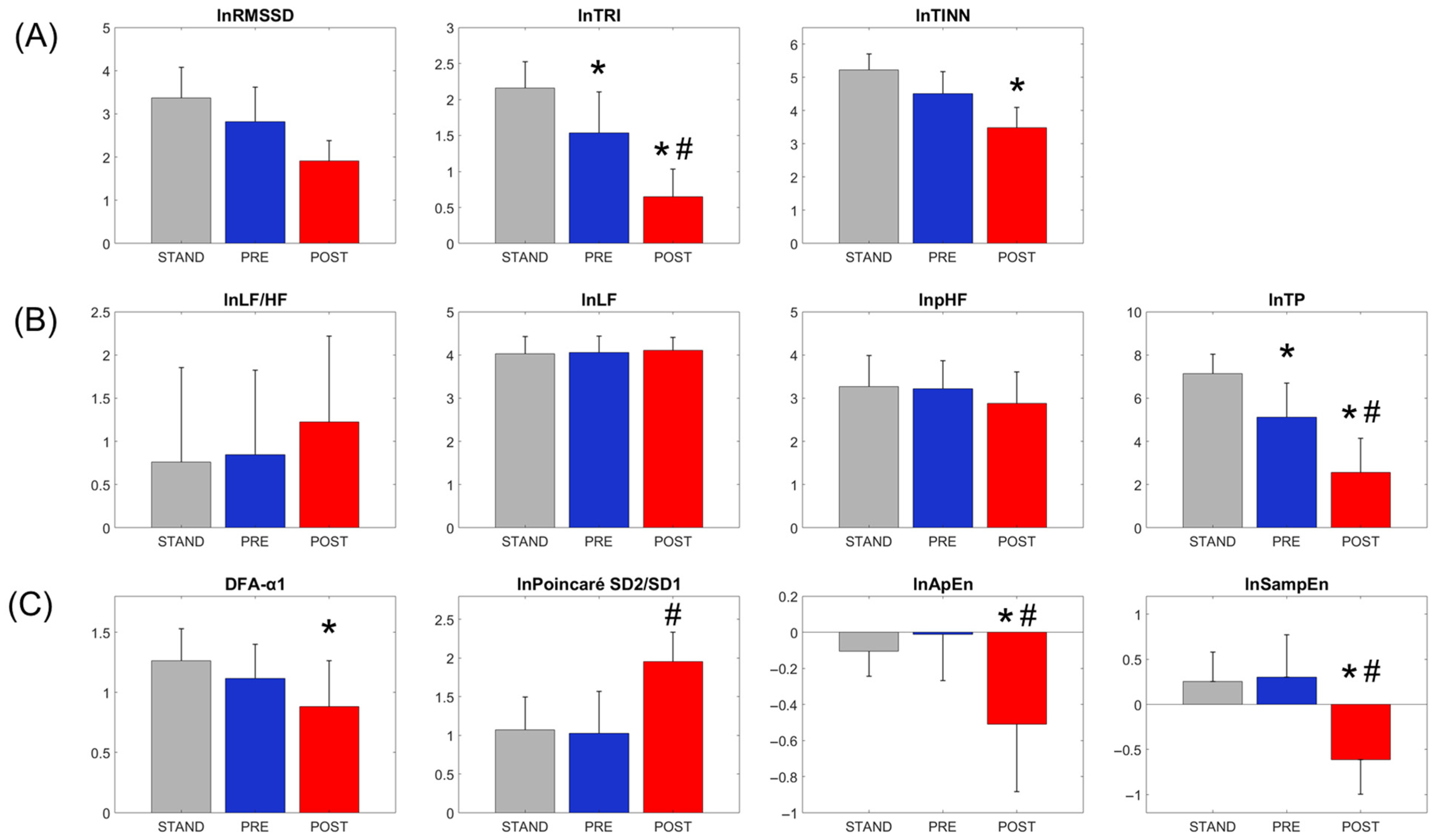
| Raw Cardiorespiratory Parameters | Conditions | Condition Effect ANOVA, p-Value (Power; Partial η2) | Post Hoc Comparison (THSD, p < 0.05; Cohen’s d) | ||
|---|---|---|---|---|---|
| STAND | PRE | POST | |||
| Inter-beat RR interval (RR, ms) | 726.6 ± 175.4 | 590.5 ± 103.3 | 423.7 ± 62.8 | — | — |
| Natural log of RR interval (lnRR) | 6.56 ± 0.22 | 6.37 ± 0.17 * | 6.04 ± 0.14 *# | <0.001 (1.000; 0.902) | POST vs. STAND (d = 5.85) POST vs. PRE (d = 3.66) PRE vs. STAND (d = 2.20) |
| Heart rate (bpm) | 86.6 ± 18.3 | 104.4 ± 17.3 * | 144.1 ± 18.5 *# | <0.001 (1.000; 0.930) | POST vs. STAND (d = 6.95) POST vs. PRE (d = 4.80) PRE vs. STAND (d = 2.15) |
| Respiratory rate (breaths/min) | 17.7 ± 3.5 | 27.1 ± 5.7 * | 33.8 ± 5.6 *# | <0.001 (1.000; 0.817) | POST vs. STAND (d = 2.98) POST vs. PRE (d = 1.57) PRE vs. STAND (d = 0.97) |
| Heart Rate Variability (HRV) Parameters | Conditions | Condition Effect ANOVA, p-Value (Power; Partial η2) | Post Hoc Comparison (THSD, p < 0.05; Cohen’s d) | ||
|---|---|---|---|---|---|
| STAND | PRE | POST | |||
| Time-domain measures | |||||
| Root mean square of successive RR interval differences (RMSSD, ms) | 37.7 ± 32.3 | 22.6 ± 18.5 | 7.7 ± 4.6 | — | — |
| Natural log of RMSSD (lnRMSSD)—unadjusted | 3.37 ± 0.70 | 2.82 ± 0.79 | 1.91 ± 0.47 | <0.001 (1.000; 0.704) | POST vs. STAND (d = 2.97) POST vs. PRE (d = 1.85) PRE vs. STAND (d = 1.12) |
| lnRMSSD—adjusted for lnRR | — | — | — | 0.948 | — |
| Triangular index of RR intervals (TRI) | 9.3 ± 4.0 | 5.44 ± 3.24 | 2.10 ± 1.27 | — | — |
| Natural log of TRI (lnTRI)—unadjusted | 2.16 ± 0.36 | 1.54 ± 0.57 | 0.65 ± 0.38 | <0.001 (1.000; 0.659) | POST vs. STAND (d = 5.44) POST vs. PRE (d = 3.20) PRE vs. STAND (d = 2.24) |
| lnTRI—adjusted for lnRR | — | — | — | 0.003 (0.885; 0.282) | POST vs. STAND (d = 3.77) POST vs. PRE (d = 2.15) PRE vs. STAND (d = 1.62) |
| Triangular interpolation of RR intervals (TINN, ms) | 208.0 ± 115.3 | 110.8 ± 70.2 | 41.0 ± 39.0 | — | — |
| Natural log of TINN (lnTINN)—unadjusted | 5.22 ± 0.48 | 4.51 ± 0.67 | 3.48 ± 0.62 | <0.001 (1.000; 0.775) | POST vs. STAND (d = 3.59) POST vs. PRE (d = 2.12) PRE vs. STAND (d = 1.47) |
| lnTINN—adjusted for lnRR | — | — | — | 0.028 (0.696; 0.126) | POST vs. STAND (d = 1.41) |
| Frequency-domain measures | |||||
| Ratio between LF and HF band powers (LF/HF) | 3.53 ± 3.29 | 3.49 ± 3.18 | 5.28 ± 4.96 | — | — |
| Natural log of the ratio between LF and HF band powers (lnLF/HF)—unadjusted | 0.76 ± 1.09 | 0.84 ± 0.98 | 1.23 ± 0.99 | 0.314 | — |
| lnLF/HF—adjusted for breathing rate | — | — | — | 0.790 | — |
| Percentage of low-frequency band power to the total power (pLF, %) | 60.0 ± 20.5 | 61.2 ± 18.0 | 63.2 ± 17.4 | — | — |
| Natural log of the percentage of low-frequency band power to the total power (lnpLF)—unadjusted | 4.03 ± 0.40 | 4.06 ± 0.37 | 4.11 ± 0.30 | 0.781 | — |
| lnpLF—adjusted for breathing rate | — | — | — | 0.967 | — |
| Percentage of high-frequency band power to the total power (pHF, %) | 32.7 ± 20.2 | 30.0 ± 17.6 | 22.4 ± 14.6 | — | — |
| Natural log of the percentage of high-frequency band power to the total power (lnpHF)—unadjusted | 3.27 ± 0.72 | 3.22 ± 0.66 | 2.88 ± 0.73 | 0.176 | — |
| lnpHF—adjusted for breathing rate | — | — | — | 0.659 | — |
| Total power (TP, ms2) | 1920.6 ± 2143.8 | 504.4 ± 800.2 | 61.4 ± 186.3 | — | — |
| Natural log of the Total power (lnTP)—unadjusted | 7.13 ± 0.91 | 5.12 ± 1.57 | 2.57 ± 1.58 | <0.001 (1.000; 0.859) | POST vs. STAND (d = 4.79) POST vs. PRE (d = 2.68) PRE vs. STAND (d = 2.12) |
| lnTP—adjusted for breathing rate | — | — | — | <0.001 (0.999; 0.474) | POST vs. STAND (d = 3.90) POST vs. PRE (d = 2.32) PRE vs. STAND (d = 1.58) |
| Non-linear measures | |||||
| Short-term scaling exponent of detrended fluctuation (DFA-α1) | 1.26 ± 0.26 | 1.12 ± 0.28 | 0.88 ± 0.38 | 0.001 (0.939; 0.295) | POST vs. STAND (d = 1.25) |
| Poincaré SD2/SD1 ratio | 3.16 ± 1.24 | 3.26 ± 2.27 | 7.56 ± 2.85 | — | — |
| Natural log of Poincaré SD2/SD1 (lnPoincaré SD2/SD1)—unadjusted | 1.07 ± 0.43 | 1.02 ± 0.54 | 1.96 ± 0.38 | <0.001 (1.000; 0.576) | POST vs. STAND (d = 1.91) POST vs. PRE (d = 2.02) |
| lnPoincaré SD2/SD1—adjusted for lnRR | — | — | — | 0.002 (0.926; 0.208) | POST vs. PRE (d = 1.43) |
| Approximate entropy (ApEn) | 0.91 ± 0.12 | 1.01 ± 0.19 | 0.64 ± 0.23 | — | — |
| Natural log of Approximate entropy (lnApEn)—unadjusted | −0.10 ± 0.14 | −0.01 ± 0.26 | −0.51 ± 0.38 | <0.001 (0.999; 0.471) | POST vs. STAND (d = 1.73) POST vs. PRE (d = 1.41) |
| lnApEn—adjusted for lnRR | — | — | — | <0.001 (0.989; 0.283) | POST vs. STAND (d = 1.86) POST vs. PRE (d = 1.57) |
| Sample entropy (SampEn) | 1.35 ± 0.39 | 1.46 ± 0.45 | 0.58 ± 0.23 | — | — |
| Natural log of Sample entropy (lnSampEn)—unadjusted | 0.25 ± 0.33 | 0.30 ± 0.47 | −0.61 ± 0.39 | <0.001 (1.000; 0.621) | POST vs. STAND (d = 2.22) POST vs. PRE (d = 2.10) |
| lnSampEn—adjusted for lnRR | — | — | — | <0.001 (0.989; 0.285) | POST vs. STAND (d = 1.85) POST vs. PRE (d = 1.42) |
| Raw Cardiorespiratory Parameters and Heart Rate Variability (HRV) Parameters | Stand | PRE | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| lnRR | 0.466 † | 0.019 | 0.417 † | 0.034 |
| Heart rate | −0.434 † | 0.028 | −0.414 † | 0.039 |
| Respiratory rate | 0.012 | 0.480 | −0.240 | 0.154 |
| Time-domain measures | ||||
| lnRMSSD—unadjusted | 0.293 | 0.105 | 0.390 † | 0.045 |
| lnRMSSD—adjusted for lnRR | −0.189 | 0.220 | 0.112 | 0.325 |
| lnTRI—unadjusted | 0.175 | 0.231 | 0.416 † | 0.034 |
| lnTRI—adjusted for lnRR | −0.229 | 0.173 | 0.129 | 0.299 |
| lnTINN—unadjusted | 0.088 | 0.356 | 0.241 | 0.154 |
| lnTINN—adjusted for lnRR | −0.258 | 0.143 | −0.096 | 0.347 |
| Frequency-domain measures | ||||
| lnLF/HF—unadjusted | −0.500 † | 0.012 | −0.164 | 0.244 |
| lnLF/HF—adjusted for breathing rate | −0.436 † | 0.031 | −0.143 | 0.279 |
| lnpLF—unadjusted | −0.488 † | 0.015 | −0.098 | 0.341 |
| lnpLF—adjusted for breathing rate | −0.488 † | 0.017 | −0.097 | 0.346 |
| lnpHF—unadjusted | 0.488 † | 0.014 | 0.191 | 0.210 |
| lnpHF—adjusted for breathing rate | 0.492 † | 0.016 | 0.194 | 0.213 |
| lnTP—unadjusted | 0.011 | 0.482 | 0.423 † | 0.031 |
| lnTP—adjusted for breathing rate | 0.009 | 0.485 | 0.436 † | 0.031 |
| Non-linear measures | ||||
| DFA-α1 | −0.335 | 0.074 | −0.327 | 0.080 |
| lnPoincaré SD2/SD1—unadjusted | −0.403 † | 0.039 | −0.555 † | 0.006 |
| lnPoincaré SD2/SD1—adjusted for lnRR | −0.067 | 0.393 | −0.485 † | 0.018 |
| lnApEn—unadjusted | −0.030 | 0.449 | 0.570 † | 0.004 |
| lnApEn—adjusted for lnRR | −0.015 | 0.476 | 0.582 † | 0.004 |
| lnSampEn—unadjusted | 0.489 † | 0.014 | 0.580 † | 0.004 |
| lnSampEn—adjusted for lnRR | 0.276 | 0.126 | 0.555 † | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, P.-C.; Cornell, D.J. Effects of Induced Physical Fatigue on Heart Rate Variability in Healthy Young Adults. Sensors 2025, 25, 5572. https://doi.org/10.3390/s25175572
Kao P-C, Cornell DJ. Effects of Induced Physical Fatigue on Heart Rate Variability in Healthy Young Adults. Sensors. 2025; 25(17):5572. https://doi.org/10.3390/s25175572
Chicago/Turabian StyleKao, Pei-Chun, and David J. Cornell. 2025. "Effects of Induced Physical Fatigue on Heart Rate Variability in Healthy Young Adults" Sensors 25, no. 17: 5572. https://doi.org/10.3390/s25175572
APA StyleKao, P.-C., & Cornell, D. J. (2025). Effects of Induced Physical Fatigue on Heart Rate Variability in Healthy Young Adults. Sensors, 25(17), 5572. https://doi.org/10.3390/s25175572







