1. Introduction
Shear wave elasticity imaging (SWEI), or shear wave elastography, is an ultrasound-based elasticity imaging method that can provide a quantitative characterization of tissue elasticity (shear modulus or Young’s modulus) in a two-dimensional field of view [
1,
2,
3,
4,
5]. This method utilizes acoustic radiation force impulse (ARFI) to remotely generate shear waves within target tissues and employs ultrafast ultrasound imaging [
6] to track the propagation of these shear waves. The speed of the recorded shear wave is then acquired locally and converted to tissue elasticity. To date, this technique has become a standard function of primary commercial ultrasound machines, such as LOGIQ E9/E10/S8/P9 by GE Healthcare, ACUSON S, Sequoia, and X700 by Siemens Healthineers, EPIQ and Affiniti by Pillips, and RS80A and RS85 by Samsung Medison. Clinically, SWEI has been established as an effective tool for diagnosing various diseases, including liver diseases [
7,
8,
9,
10], breast diseases [
11,
12,
13], and thyroid diseases [
14,
15,
16]. It has also been used in examining musculoskeletal organs such as tendons [
17,
18,
19], ligaments [
20,
21,
22], and muscles [
23,
24,
25]. And its application is still expanding to image other organs, such as the kidney [
26,
27], prostate [
28,
29], and pancreas [
30,
31].
The current clinical implementation of SWEI is based on a single-transducer approach, in which an array transducer, either a linear array (e.g., GE ML6-15-D) or a convex array (e.g., GE C1-6-D), is used for both generating and tracking shear waves in tissues under test. Since the same transducer can accomplish both shear wave generation and tracking, this single-transducer approach has the great advantage of being convenient and simple in operation. However, in the attempt to apply SWEI to more challenging situations, such as evaluating the elasticity of small tissues or organs like lymph nodes or coronary arteries, especially in research environments working with small animals, this approach faces critical challenges.
One of these critical challenges is the lack of adequate spatial resolution. Currently, SWEI is primarily implemented at a relatively lower frequency range (not exceeding 10 MHz) in clinical applications. Although this frequency range can meet the requirements of many applications, it cannot provide the sufficient spatial resolution needed for imaging small tissues or organs. A natural solution for improving the spatial resolution and accuracy of SWEI is to adopt high-frequency transducers (whose center frequency is greater than 10 MHz) [
32] despite alternative improvements using phase velocity estimation and a data-driven approach [
33]. While high-frequency SWEI can provide high resolution in shear wave tracking, it poses another critical challenge: low effectiveness in generating shear waves. First, the piezoelectric elements of high-frequency transducers are not designed to sustain high-voltage and long-duration pulses that are required for producing ARFI [
34]. Furthermore, even if ARFI is excited at high frequencies, it will have a lower penetration depth and a lower amplitude compared to low-frequency excitation, due to the lower excitation voltage allowed by high-frequency transducers and the higher attenuation of acoustic intensity at higher frequencies [
35]. The low ARFI penetration depth prevents imaging deep tissue targets, and its low amplitude results in a low signal-to-noise ratio (SNR) of the generated shear wave, both of which make it ineffective for SWEI. To induce shear waves with higher intensity and/or at a deeper depth, low-frequency ultrasound is desired; while to achieve higher imaging resolution, high-frequency ultrasound is required. However, any ultrasound transducer has a limited bandwidth (which is typically indicated by its name), and the frequencies required for optimal shear wave generation and tracking may exceed this bandwidth. Therefore, the limited bandwidth of a single transducer makes it impossible to achieve effective shear wave generation and high-resolution shear wave tracking simultaneously for applications such as imaging small organs or tissue structures deep inside the human or animal body.
To address this challenge and avoid the tradeoff between spatial resolution and shear wave SNR when using the single-transducer approach for SWEI, a dual-transducer approach, in which two transducers of choice can implement shear wave generation and tracking separately, can be an effective solution. The purpose of assigning the tasks of generating and tracking shear waves to two transducers is to achieve optimized performance in each task by choosing a transducer with an appropriate frequency range and other specifications. The shear wave excitation transducer can be a single-element transducer or an array transducer, and it generally should have a low working frequency. The shear wave tracking transducer is usually an array transducer with a higher center frequency and a wider bandwidth.
In medical ultrasound, the dual-transducer idea has initially been adopted for ultrasound image-guided focused ultrasound therapy, which utilizes two transducers, one for imaging and the other for therapy [
36,
37]. In ultrasound elasticity imaging, this dual-transducer idea has been implemented in harmonic motion imaging, where a focused ultrasound transducer is used to generate oscillatory acoustic radiation force (harmonic waves) to excite the target tissue, while an imaging transducer is used to track the dynamic response of the tissue [
38]. In elasticity imaging, this idea has recently also been used in cases where shear waves are generated using mechanical vibrators or actuators, while tracked using ultrasound transducers [
39,
40].
Despite the dual-transducer concept having been technically implemented in several cases of elasticity imaging research, it remains a relatively new idea to SWEI, and its performance has not been evaluated. Among the many factors affecting the output of SWEI, the performance of SWEI is mainly determined by two factors: the effectiveness of shear wave excitation and the quality of shear wave tracking. As a pioneer effort to assess the performance of dual-transducer SWEI, this work will focus on investigating the effects of shear wave tracking frequencies on the performance of SWEI. The performance of SWEI will be evaluated by four measures: SNR, contrast-to-noise ratio (CNR), spatial resolution, and precision in quantitative measurement. The experimental results demonstrate the superior effectiveness of high-frequency tracking over low-frequency tracking in SWEI by comparing the performance of SWEI at three different frequencies.
2. Materials and Methods
2.1. Phantom
The investigations were performed on four tissue-mimicking phantoms composed of 4% weight/volume (w/v) gelatin from porcine skin (Sigma-Aldrich, Co., St. Louis, MO, USA) and 0.2% w/v silica gel (Product Number 288500, Sigma-Aldrich, Co., St. Louis, MO, USA). One of the phantoms is homogeneous and was designed for evaluating the noise level of the imaging system. The other three phantoms each contain a cylindrical inclusion made of 10% w/v gelatin and 0.25% w/v silica gel, and they were designed to assess the performance of the proposed dual-transducer approach for SWEI at different imaging frequencies. The three inclusions are 4.3 mm, 3 mm, and 2.3 mm in diameter, respectively, and they are embedded at depths of 18 mm, 12 mm, and 10 mm from the phantom surface.
2.2. Experiment
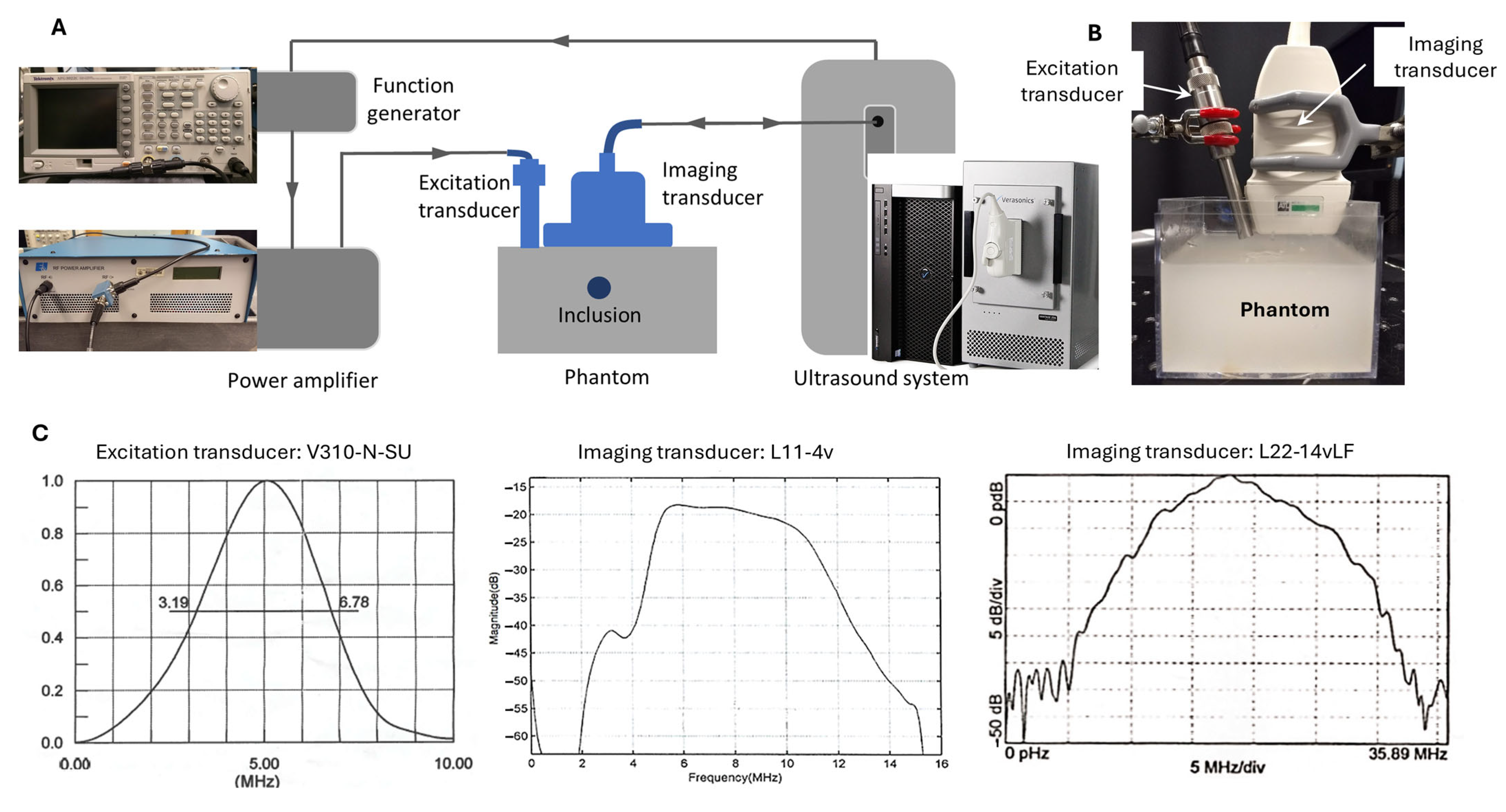
The experimental setup of this study is depicted in
Figure 1. Unlike the conventional SWEI configuration, in which shear wave generation and tracking are performed by the same system (the single-transducer approach), the proposed dual-transducer approach executes shear wave generation and tracking in two separate subsystems. The shear wave generation subsystems consists of a function generator (AFG3022C, Tektronix Inc., Beaverton, OR 97077, USA), a power amplifier (E&I A150, Electronics and Innovation, LTD, Rochester, NY 14623, USA), and an excitation transducer (V310-N-SU, Olympus America Inc., Waltham, MA 02453, USA); its function is to generate an ARFI with enough power to excite shear waves in target tissues. The shear wave tracking subsystem consists of a programmable ultrasound research system (Vantage 128, Verasonics Inc., Redmond, WA, USA) and a linear array imaging transducer; its function is to record the propagating shear waves generated in the target tissues through ultrafast ultrasound imaging [
6]. In operation, these two subsystems were synchronized by a trigger sent from the ultrasound system to the function generator.
For shear wave excitation, a 5 MHz focused single-element transducer was chosen to achieve deeper penetration (due to its low frequency and accordingly low attenuation) and a narrower excitation site (due to its focused nature). To reduce the distance between the excitation site and the imaging transducer, a pencil case style, which is only half the diameter of the typical case style, was chosen for the excitation transducer. To generate an ARFI signal that can effectively produce shear waves in the phantoms of this study, a sine-wave tone burst of 2000 cycles (i.e., 400 µs at 5 MHz) with a peak-to-peak voltage of 1.5 V was generated by the function generator and sent to the amplifier before being fed to the excitation transducer.
To study the effects of ultrasound imaging frequency on SWEI, the generated shear waves were tracked at three frequencies using two linear array transducers: 5 MHz and 10 MHz by L11-4v (Verasonics Inc., Redmond, WA, USA), and 15 MHz by L22-14vLF (Verasonics Inc., Redmond, WA, USA). The selection of these three shear wave tracking frequencies is based on the following facts: (i) the shear wave imaging frequency in clinical practice can be as low as 1–6 MHz [
10], and (ii) the frequency in preclinical studies can be as high as 15–17.5 MHz [
32,
41]. So, the selected three frequencies can be very representative of the entire frequency range used in SWEI. To ensure a fair comparison can be made among these three frequencies, the same experimental parameters (in software settings, hardware settings, and physical configuration) were used during data acquisition, except for the imaging frequency. It should be noted that when acquiring data for evaluating and comparing the noise level of the imaging system at different frequencies, only the imaging subsystem functioned. Therefore, no shear wave was observed, and only system noise was recorded.
2.3. Signal Processing
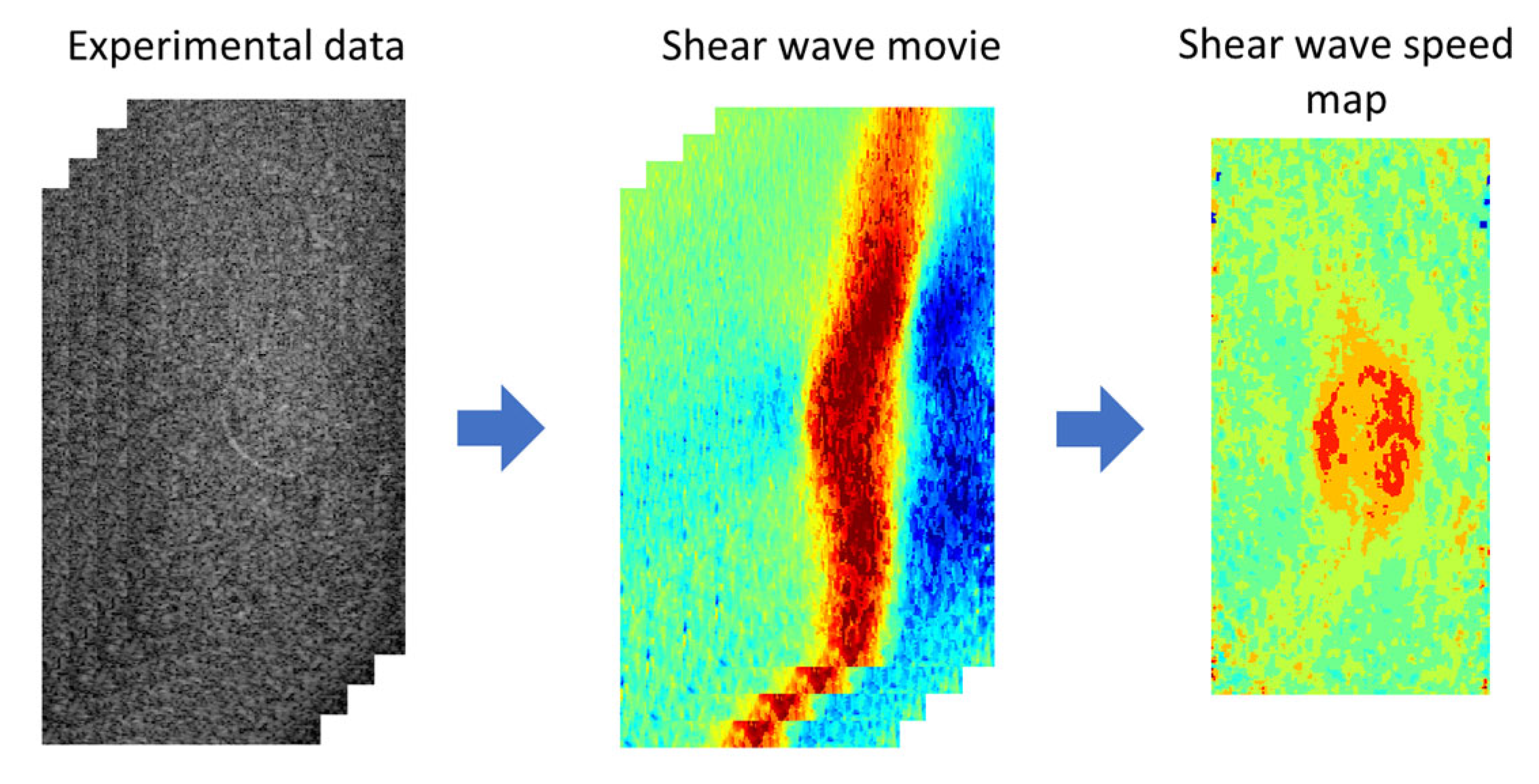
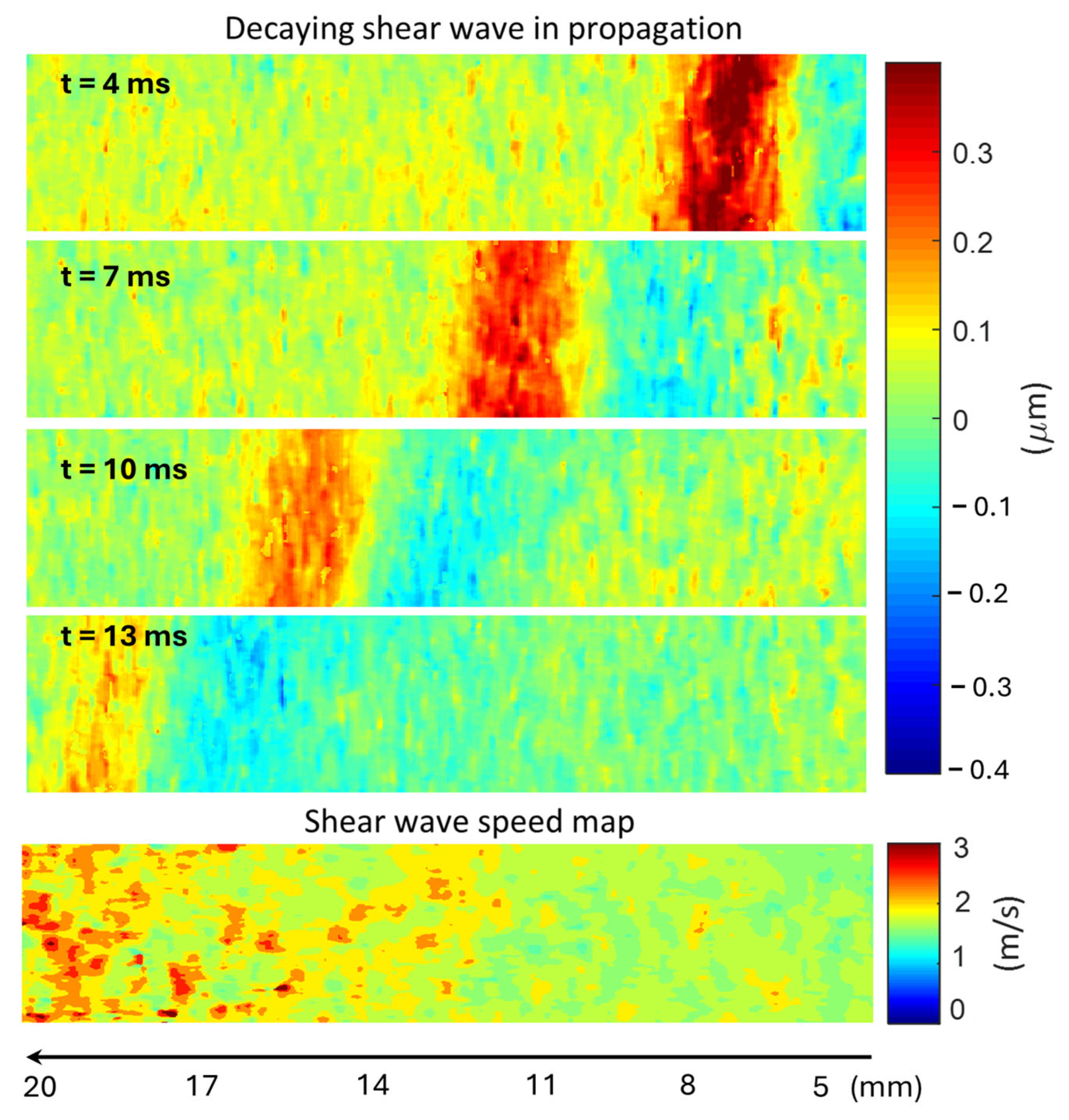
In SWEI, the process of signal processing (
Figure 2) consists of two steps: (i) converting the experimental data to a shear wave movie and (ii) extracting a shear wave speed map from the shear wave movie. In this study, the experimental data are the in-phase quadrature (IQ) data acquired in ultrafast ultrasound imaging, which record the time-varying material displacement (usually invisible to the human eye) of the medium generated by ARFI excitation. In experiments, displacements of the target medium occurred in different directions of the three-dimensional space, but only the axial and lateral displacements were recorded in the acquired IQ data. Because the ARFI excitation was made in the axial direction of the imaging plane, the axial displacement is much larger in amplitude than the lateral displacement. Therefore, in the first step of signal processing, axial displacement was extracted from the acquired IQ data to represent the propagating shear waves. Then, by tracking the movement of the shear waves, a shear wave speed map was obtained for the entire imaging plane. All data processing was implemented offline using MATLAB (2021b, MathWorks Inc., Natick, MA, USA). To ensure a fair comparison, the same parameters were also used in processing data acquired at three different frequencies.
2.4. Noise Level Evaluation
Noise is an inherent characteristic of an imaging system, although it is not desired. In an output image, noise is a generic term that refers to any type of random fluctuation, which can significantly impact image quality, including the signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and even spatial resolution [
42]. Therefore, it is meaningful and necessary to evaluate the noise level of an imaging system to assist in the development and assessment of imaging methods.
The source and amount of noise depend on the imaging system (including both software and hardware) used and the signal processing methods employed. In this study, we will not investigate the exact sources of noise but rather evaluate the amount of noise to compare the performance of different imaging frequencies. As introduced in the subsection “Signal Processing”, in SWEI, data are stored in three forms: IQ data (acquired experimentally) for recording tissue motion, axial displacement for demonstrating shear wave, and shear wave speed for evaluating material elasticity. The noise originated from the experimental hardware and software of the imaging system, which is initially contained in the IQ data and carried on into the other two types of data. In comparison, the noise (including artifacts) originated from the signal processing and is only contained in the latter two types of data.
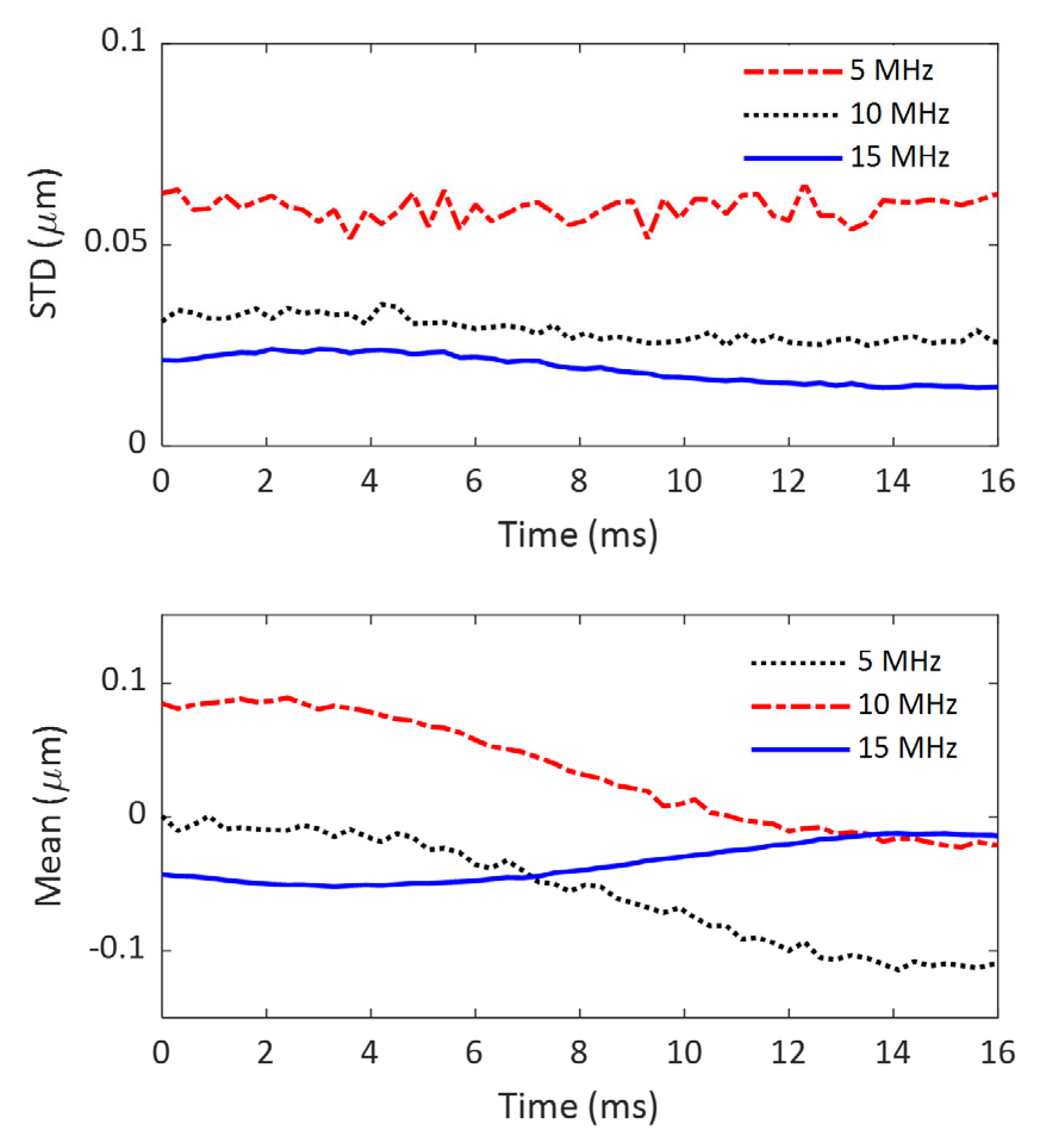
As mentioned in the subsections of “Phantom” and “Experiment”, a homogeneous gelatin phantom was made for noise evaluation, and it was imaged at three different frequencies without external excitation for shear wave generation. To evaluate the noise generated by the imaging system, the acquired IQ data were first converted to B-mode images, and a representative region of interest (ROI) of 10 mm by 10 mm was chosen in the imaging field of view (FOV) for computing the mean signal intensity and the associated standard deviation (STD). Here, the noise of the imaging system is defined as the STD of the ROI with the assumption that noise is distributed uniformly throughout the imaging FOV. To ensure a fair comparison, the ROIs were chosen at the same position in the imaging FOV among the B-mode images obtained at different time points and at different imaging frequencies.
To further evaluate the accumulated noise after the first step of signal processing (converting IQ data to axial displacement), the same ROIs were chosen for computing the means and STDs of axial displacement. To examine the time-varying characteristics of the noise, the evaluation process was performed on all the frames obtained over a 16-ms time period.
In addition to the noise generated by the imaging system and during the first step of signal processing, the noise originating from the second step of signal processing (converting the displacement movie to a shear wave speed map) also contributes to the noise present in shear wave speed maps. In this study, the noise in shear wave speed maps will also be evaluated. However, the evaluation will not be a standalone process, as discussed above, but rather part of the image quality assessment process, which will be described in the next section.
2.5. Image Quality Assessment
In SWEI, the output image of the target tissue can be a shear wave speed map, a shear wave modulus map, or a Young’s modulus map. Because the latter two types of images can be obtained from the shear wave speed map through simple conversion, the shear wave speed map will be used in this work for image quality assessment. The image quality of the speed maps obtained at the three frequencies investigated in this work (5 MHz, 10 MHz, and 15 MHz) was assessed using four measures: SNR, CNR, spatial resolution, and measurement precision.
2.5.1. Signal-to-Noise Ratio
In a shear wave speed map, the signal is defined as the mean speed of an ROI, and the noise is the STD of the same ROI. So, the SNR can be simply obtained by computing the mean signal intensity over a specific ROI and dividing this by the associated STD. In each shear wave speed map, two ROIs of the same size, which are close to each other, were chosen for evaluating SNR: one from the background of the phantom and the other from the inclusion. For comparison, these two ROIs are in the same position in the shear wave speed maps obtained at different imaging frequencies for the same phantom. However, the size and location of the ROI will vary among the three phantoms, which have different inclusion sizes (4.3 mm, 3 mm, and 2.3 mm).
To compute SNRs, the mean values (
,
) and STDs (
,
) of the background and inclusion of each phantom were first calculated for the ROIs chosen. Based on these measurements, the SNRs (
,
) of the background and inclusion of the phantom were then calculated using the following equations.
2.5.2. Contrast-to-Noise Ratio
In SWEI, a high SNR is adequate for imaging homogeneous tissue (e.g., a fibrotic liver), but it is insufficient for identifying local inhomogeneous features of the target tissue (e.g., a tumor in the liver). To identify these local features or objects within an image, they need to have different values from their surrounding areas, and the difference should be larger than the local noise. In other words, high CNR is needed for imaging inhomogeneous tissues. In this study, contrast is defined as the difference between the mean speeds of the inclusion (
) and background (
) of a phantom. Because the noise level of the inclusion (
) is usually different from that of the background of the phantom (
), two CNRs, one based on the noise level of the background (
) and the other based on the noise level of the inclusion of the phantom (
), will be computed for comparison of the effect of imaging frequency on CNR using the following two equations.
2.5.3. Spatial Resolution
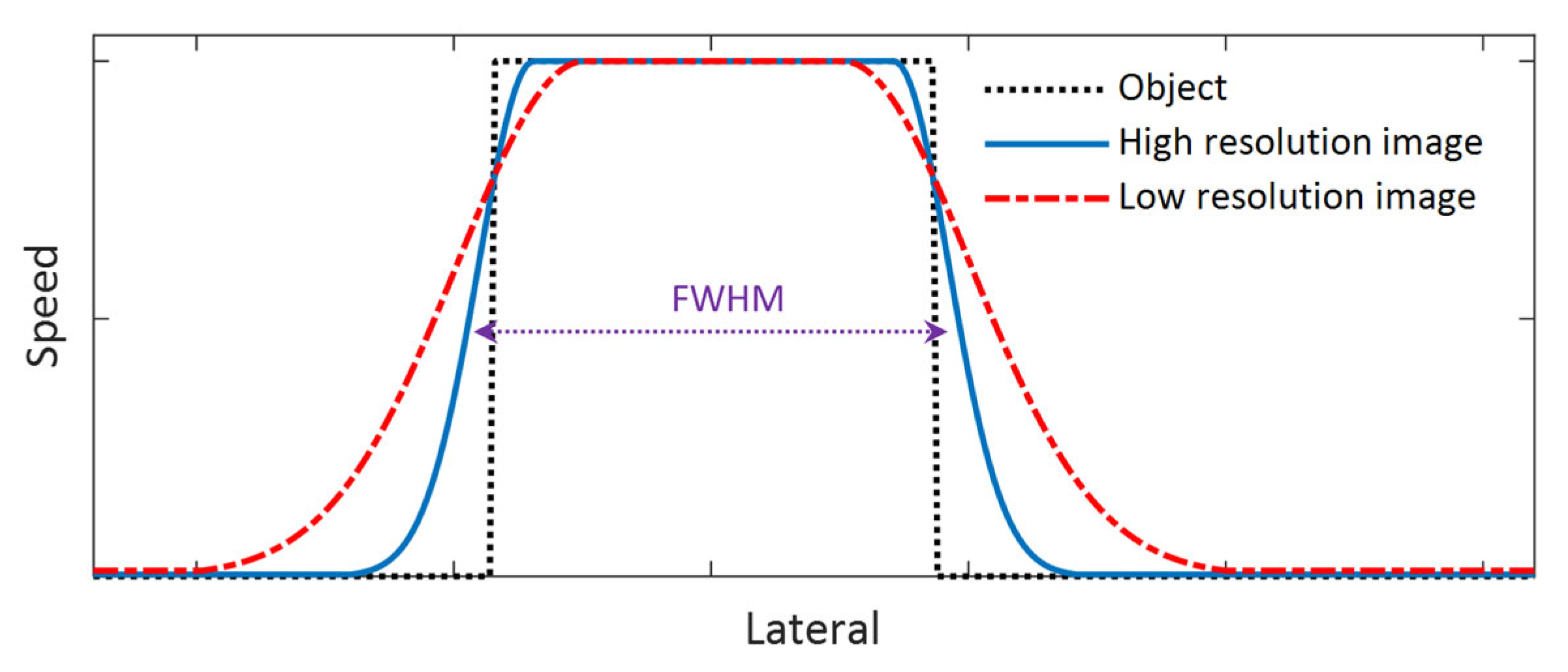
Spatial resolution can be thought of as the ability of a medical imaging system to accurately depict two distinct events in space as separate. It can also be thought of as the degree of smearing, or blurring, a medical imaging system introduces to a single event in space [
42]. Based on these two perspectives, there are two primary methods to assess spatial resolution. The first method assesses spatial resolution using a tool that contains more than one distinct object or event. A typical example of such a tool is a bar phantom, which contains several groups of parallel lines with different widths. The spatial resolution of an imaging system is characterized by the group of parallel lines with the smallest width that the imaging system can resolve. The second method for assessing spatial resolution uses only one object or event. Theoretically, given the point spread function (PSF) or line spread function (LSF) of an imaging system, the spatial resolution can be quantified by its full width at half maximum (FWHM) [
42]. However, it is practically hard to acquire the PSF or LSF of an imaging system. An alternative method to assess the spatial resolution of an imaging system using a single object, or even to use the edge spread function, which is the convolution of the LFS with a step function [
43]. Since the sharp change in material, which leads to a rapid variation in shear wave speed, occurs at the boundary between the inclusion and the background of the phantom, edges can be defined and utilized to characterize the spatial resolution.
Because the primary purpose of this study is to compare the performance of different shear wave tracking frequencies in SWEI, we will not quantify the spatial resolution of the system; instead, we will compare the performance of each imaging frequency in detecting the edge of the inclusion. To do this, we will compare the speed profiles of the shear wave speed map that laterally crosses the center of the inclusion phantom and the spatial resolution will be assessed based on two measures: the slope of the rising and falling sections of the speed profile (corresponding to the edge of the object) and the FWHM of the speed profiles as shown in
Figure 3. A larger slope can be observed at the rising and falling sections of the speed profile for an image with higher spatial resolution and vice versa. In addition, the FWHM will be computed for all the phantoms, and it is defined as the lateral width of the inclusion at a height equal to the mean speed of the inclusion and that of the background of the phantom. For this measure, a larger FWHM results from a lower spatial resolution and vice versa.
2.5.4. Measurement Precision
Medical imaging methods/techniques can be generally categorized into two types: qualitative imaging and quantitative imaging. Qualitative imaging, to which most current imaging techniques belong, can provide anatomical and/or functional information about target tissues; however, the image intensity is not a meaningful physical property of the target tissue. Quantitative imaging, however, can provide quantitative evaluation of a specific property of the target tissue, in addition to anatomical and/or functional information. Elasticity imaging, for example, images tissue by evaluating its elasticity (either shear modulus or Young’s modulus) at each pixel.
Like any quantitative measurement technique, measurement accuracy and precision are essential criteria for assessing the effectiveness of SWEI. Accuracy is a measure of the difference between the actual value of a quantity and its measurements. This difference is typically evaluated by error, and a larger error indicates lower accuracy, and vice versa. Because the accuracy of SWEI has been assessed using a widely accepted and reliable method for measuring material elasticity, such as mechanical compression testing (the gold standard method) [
44,
45], it will not be the focus of this work. Precision is a measure of the difference between different measurements of the same quantity, and it is an essential indicator of the reproducibility of an imaging method. In this work, the shear wave speeds in the background and the inclusion of a phantom were measured three times at each imaging frequency. These measurements will be used to qualitatively assess the effect of imaging frequency on measurement precision.
4. Discussion
4.1. On the Accuracy of Inclusion Size Measurement in the Depth Direction
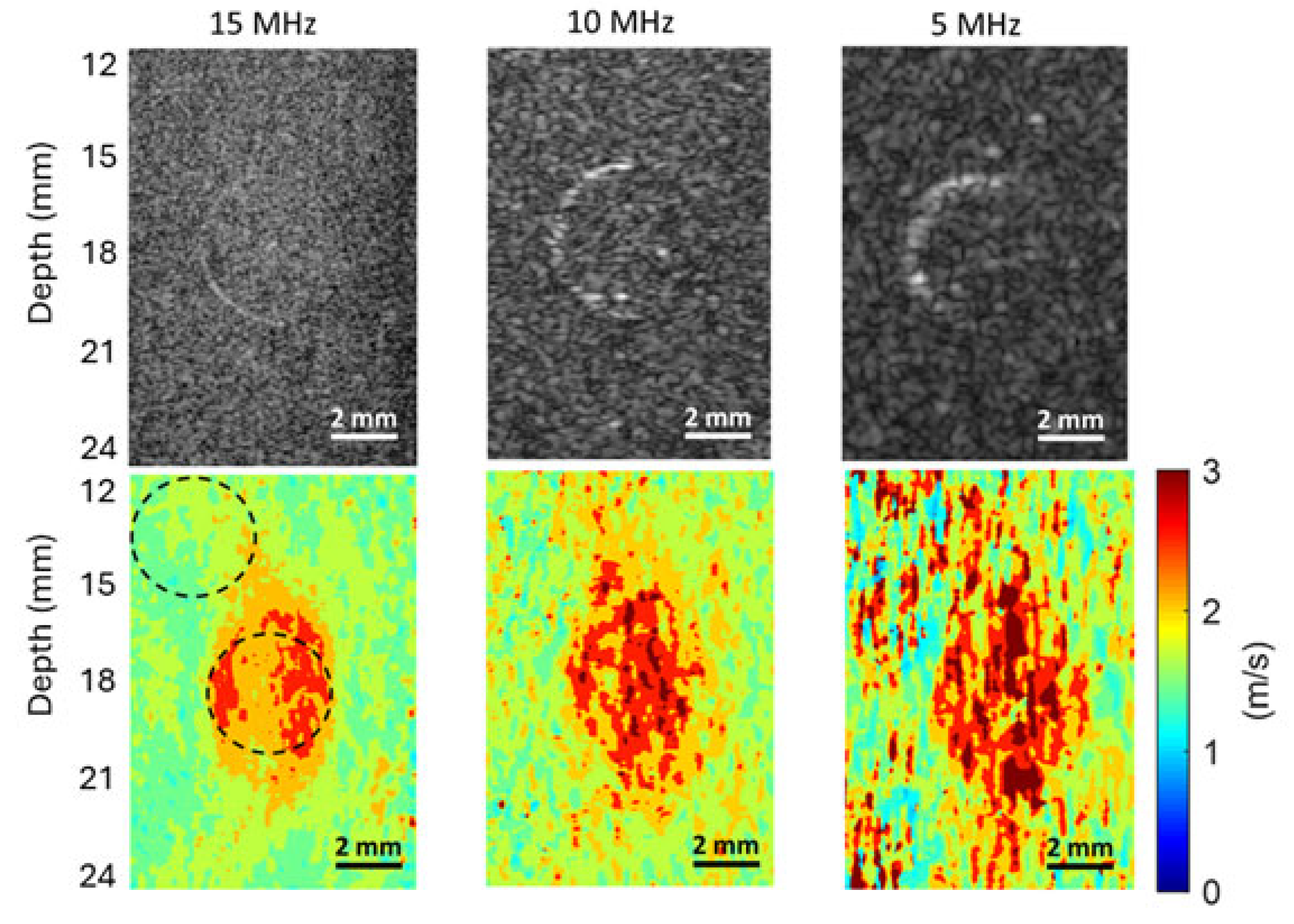
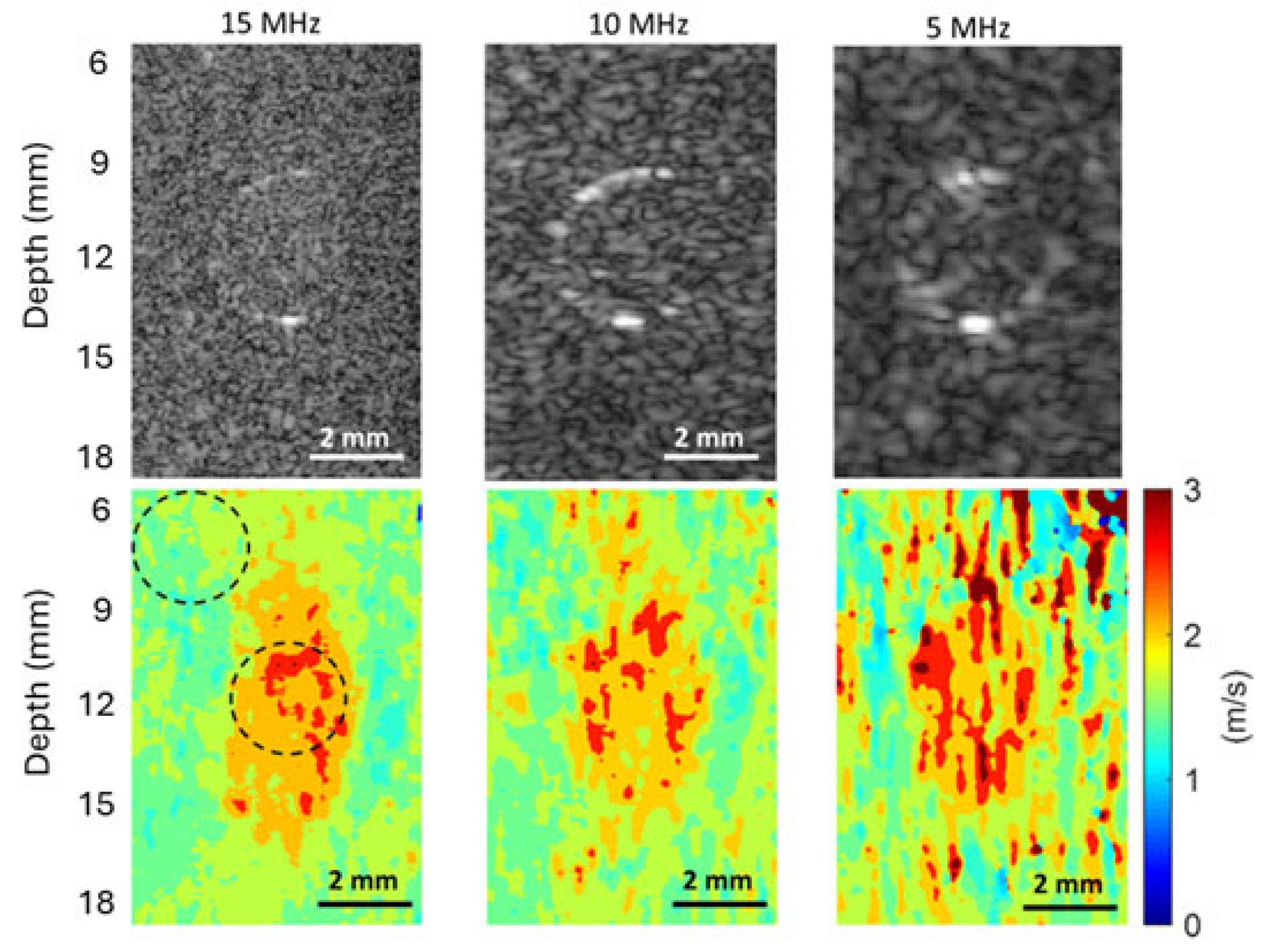
From
Figure 7,
Figure 8 and
Figure 9, we can observe that the elasticity images (represented by speed maps of inclusion) of all three inclusions are not circular, as expected, but elongated in the depth (vertical) direction. This phenomenon, that the vertical dimension of elasticity observed is larger than expected, is common in SWEI [
46,
47,
48], and it is an artifact related to the mechanism of this imaging method. Many factors can contribute to this artifact, and the primary causes include the physical process of wave interaction and the digital process of signal processing.
First, the interaction of shear waves and inclusions can cause nonplanar waves near the top and bottom boundaries between the inclusions and the background. In SWEI, when shear waves, which are assumed to be a planar wave in the vertical plane and propagating horizontally, encounter a surface that is neither perpendicular nor parallel to their propagation direction, the wavefront will not be planar anymore. These nonplanar (tilted) waves normally exist near the top and bottom regions of the inclusion. Because SWEI assumes shear waves are planar during the signal processing process to generate the elasticity map, artifacts will occur in the regions of these tilted waves (the top and bottom regions of inclusions), generating vertically elongated inclusion maps.
Second, the horizontal window or kernel used in most SWEI signal processes can also shorten the lateral dimension of inclusions. Again, SWEI assumes that shear waves are planar and propagate horizontally, thus a cornel of certain width is selected in the horizontal direction when extracting shear wave speed. Typically, a larger kernel can provide a smoother elasticity map, but it can also lead to an underestimation of the horizontal dimension of the inclusion. In this study, we believe both physical and digital causes together contribute to the elongated inclusions in the elasticity maps, with the physical cause as the primary cause.
4.2. Limitations and Challenges of the Dual-Transducer Approach
In the proposed dual-transducer SWEI, a higher frequency is usually used for tracking shear wave motions. Although high-frequency ultrasound increases the spatial resolution, it also limits the depth of target tissues to be imaged. Typically, high-frequency imaging is used for imaging targets near the interface instead of deep targets. This is also the reason that we generate inclusions of different sizes at different depths of the phantom in this study.
A common challenge of SWEI is the decreasing quality of the elasticity map as the shear wave propagates away from the excitation site. The main cause of this image quality degradation is the decreased SNR of shear waves due to the attenuation during propagation. With the dual-transducer approach, this problem can be remedied using an appropriately selected shear wave generation transducer and related working parameters such as excitation frequency and duration.
As in any other SWEI technique, challenges will undoubtedly occur when applying the dual-transducer method to measure biological tissues. These challenges mainly arise from the complex nature of biological tissues, including their heterogeneity, viscoelasticity, and high attenuation of acoustic waves. Since these challenges are not unique to the dual-transducer approach, we can refer to the literature for potential solutions. Given the flexibility the dual-transducer approach provides, it is highly possible that these challenges can be better addressed compared with the current single-transducer approach.
4.3. Considerations for SWEI Approach Selection
Including this work, the literature presents two experimental configurations for SWEI: the single-transducer approach and the dual-transducer approach. In the single-transducer SWEI, shear waves are generated and tracked using the same transducer. Within the frequency coverage of the transducer, proper frequencies should be chosen for shear wave generation and tracking, respectively. Typically, a relatively lower frequency will be used for shear wave generation because low-frequency ultrasound allows for higher power, which will increase the depth of penetration and SNR in the shear waves; a relatively higher frequency will be used for shear wave tracking primarily due to its higher spatial resolution in detecting wave motion. The biggest advantages of this single-transducer approach include its ease of use (only one transducer is needed) and its wide availability (available on most commercial ultrasound machines). So, in practice, the single-transducer approach should be chosen for a defined task as long as the frequency coverage of the transducer satisfies the task requirements. However, if the transducer fails in either shear wave generation or tracking for a defined task, the dual-transducer approach should be adopted. The example scenarios include, but are not limited to, the case where the desired shear wave penetration and the shear wave motion tracking requirements cannot be achieved using a single transducer.
As mentioned earlier in this study, the primary purpose and advantage of the dual-transducer configuration of SWEI is to optimize the performance of both shear wave generation and tracking for a defined application. For example, in imaging small inclusions or target tissues, this approach enables the use of high-frequency ultrasound for tracking shear waves. In the meantime, this approach can also enable high tissue penetration.
4.4. The Effect of Shear Wave Tracking Frequency on SWEI Performance
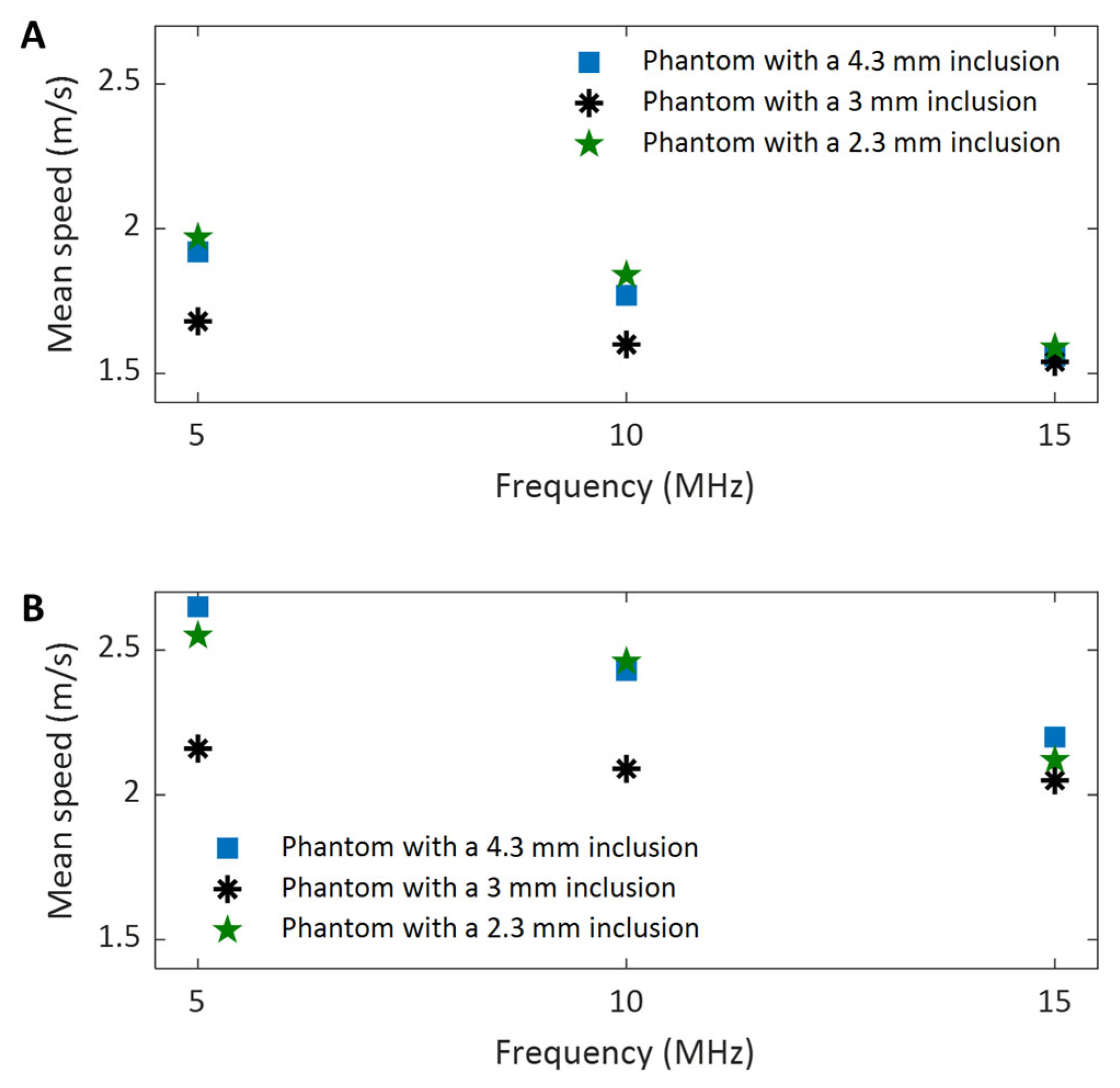
In evaluating the performance of the proposed dual-transducer approach, this study demonstrates that high shear wave tracking frequency outperforms low frequency in SWEI across all four image quality assessment criteria used: SNR (
Figure 10), CNR (
Figure 11), spatial resolution (
Figure 13), and measurement precision (
Figure 14). Although it is difficult to provide a definitive explanation without purposefully designed additional research, we believe the most likely reason is the small wavelength and thus high spatial resolution in B-mode imaging that high tracking frequency offers. In SWEI, particle displacements caused by shear wave motion are very small (at the micrometer level, as shown in
Figure 6) and are extracted using correlation-based methods from B-mode images obtained with imaging transducers. As shown in
Figure 7,
Figure 8 and
Figure 9, higher frequency B-mode imaging produces smaller speckles, and these smaller speckles can result in more accurate measurements of shear wave particle motions. Because all the signal processing steps leading to an elasticity map, including shear wave speed calculation and elasticity calculation, depend on the extracted particle motions of shear waves, high-accuracy particle motions produced in high-frequency tracking will subsequently lead to improved performance in the elasticity images.
4.5. Future Work
As stated in the Introduction of this work, the performance of SWEI is determined by two main factors: the effectiveness of shear wave excitation and the quality of shear wave tracking/recording. In this study, we demonstrated the role of imaging frequency in the performance of SWEI, but we did not investigate the role of shear wave excitation. In future work, the effects of shear wave excitation parameters, such as duration, amplitude, and beam width of ARFI, on the quality of shear wave speed maps should be investigated. Such an investigation could provide guidelines for choosing optimal shear wave excitation parameters for specific applications. Together with the results presented in this work, the knowledge of how to choose optimal excitation parameters for a defined application will lead to a custom-designed SWEI, which can produce the best possible results.
5. Conclusions
To meet the need for characterizing the elasticity of small biological tissues or organs, or for identifying small tissue structures within surrounding tissue with different stiffness, we proposed and assessed a dual-transducer approach for high-frequency SWEI in this study. In this approach, two ultrasound transducers and, accordingly, two subsystems are proposed for shear wave generation and tracking, respectively, as opposed to a single transducer in conventional SWEI. This proposal is to address the limitations of the conventional SWEI, where a single transducer is used for both shear wave generation and tracking, but the limited bandwidth of a single transducer does not allow for simultaneous high-frequency imaging and low-frequency excitation that are required for specific applications such as elasticity imaging for lymph nodes, coronary arteries, and tissues of interest in small animals. Then, we experimentally demonstrated the effectiveness of the proposed dual-transducer approach by investigating the effect of shear wave tracking frequency on the performance of SWEI. By comparing the SNR, CNR, spatial resolution, and measurement precision of the results obtained at three different frequencies, we demonstrated that higher-frequency shear wave tracking can achieve higher SNR, CNR, spatial resolution, and measurement precision in SWEI.