Compact Colocated Bimodal EEG/fNIRS Multi-Distance Sensor
Abstract
1. Introduction
2. System Description
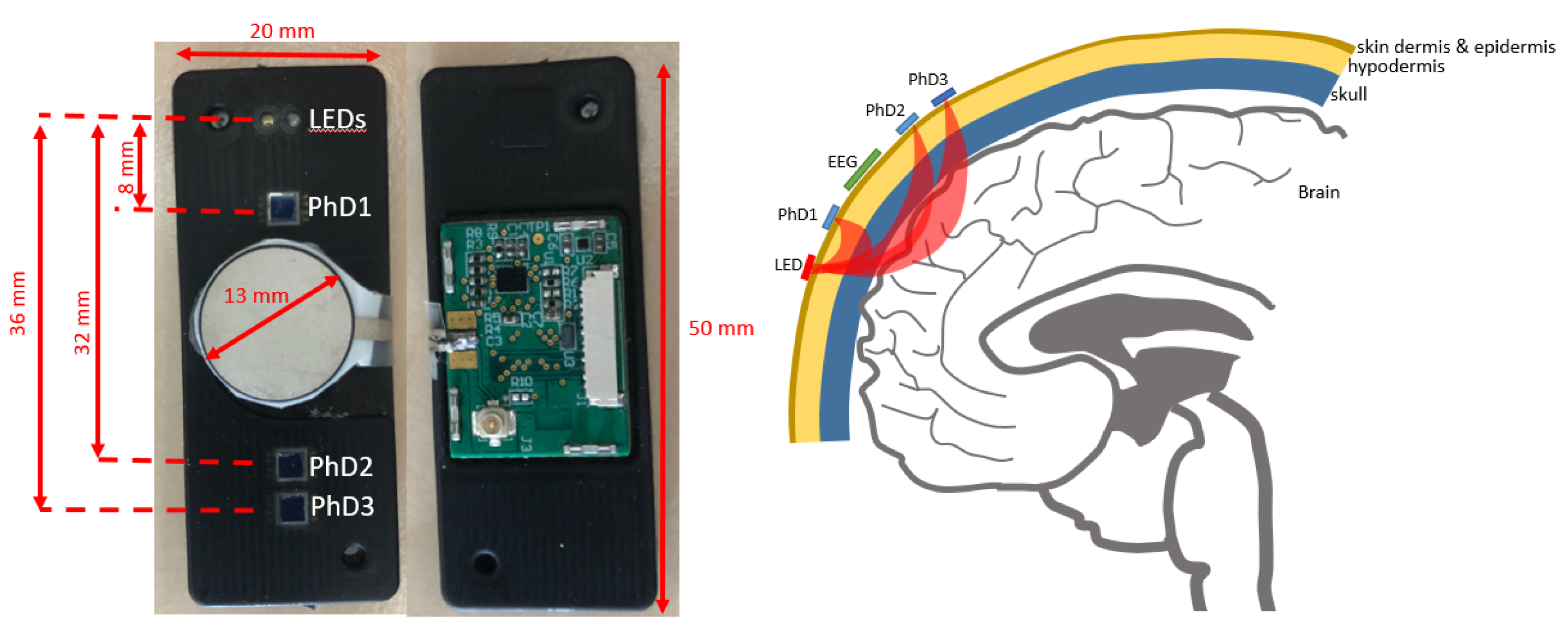
2.1. Optrode Sensor Node
2.2. HubNode
- The main board, PMP-HUB, includes the micro-controller NRF52840 from Nordic Semiconductor, Trondheim, Norway and part of the power management. This board is responsible for the finite-state machine (FSM) EEG/fNIRS measurement sequence, the Serial Peripheral Interface (SPI) setting of the different AFEs, the flash data storage and the Bluetooth pairing with the external control computer.
- The PMP-OPTRODIF board collects up to 8 fNIRS sensors digital information to concentrate the data to the PMP-HUB for storage.
- The PMP-EEG board includes the single ADS1299 AFE. It receives the analog signal of up to 8 EEG electrodes (EEG from the Optrode node or from any commercial EEG electrodes) and the necessary DRL and REF electrodes. As this board is isolated from the rest of the unit thanks to a galvanic isolation coupler (ISO7242 and ISO7341 from Texas Instruments Dallas, TX, USA), it has its own battery and power management. With its 24-bit sigma-delta analog-to-digital converters combined with a programmable gain amplifier (PGA), it offers a very high sensitivity. It can operate from 250 sps to 16 ksps. Its input-referred noise in normal mode at 250 sps is as low as 0.98 Vpp peak to peak. The ADS1299 digitizes the analog signal from EEG electrodes and sends it to the PMP-HUB for storage.
- A TTL input has been added in order to enable hardware tag events for third-party systems and protocol synchronization. This tag ability is also mandatory for offline post-treatments.
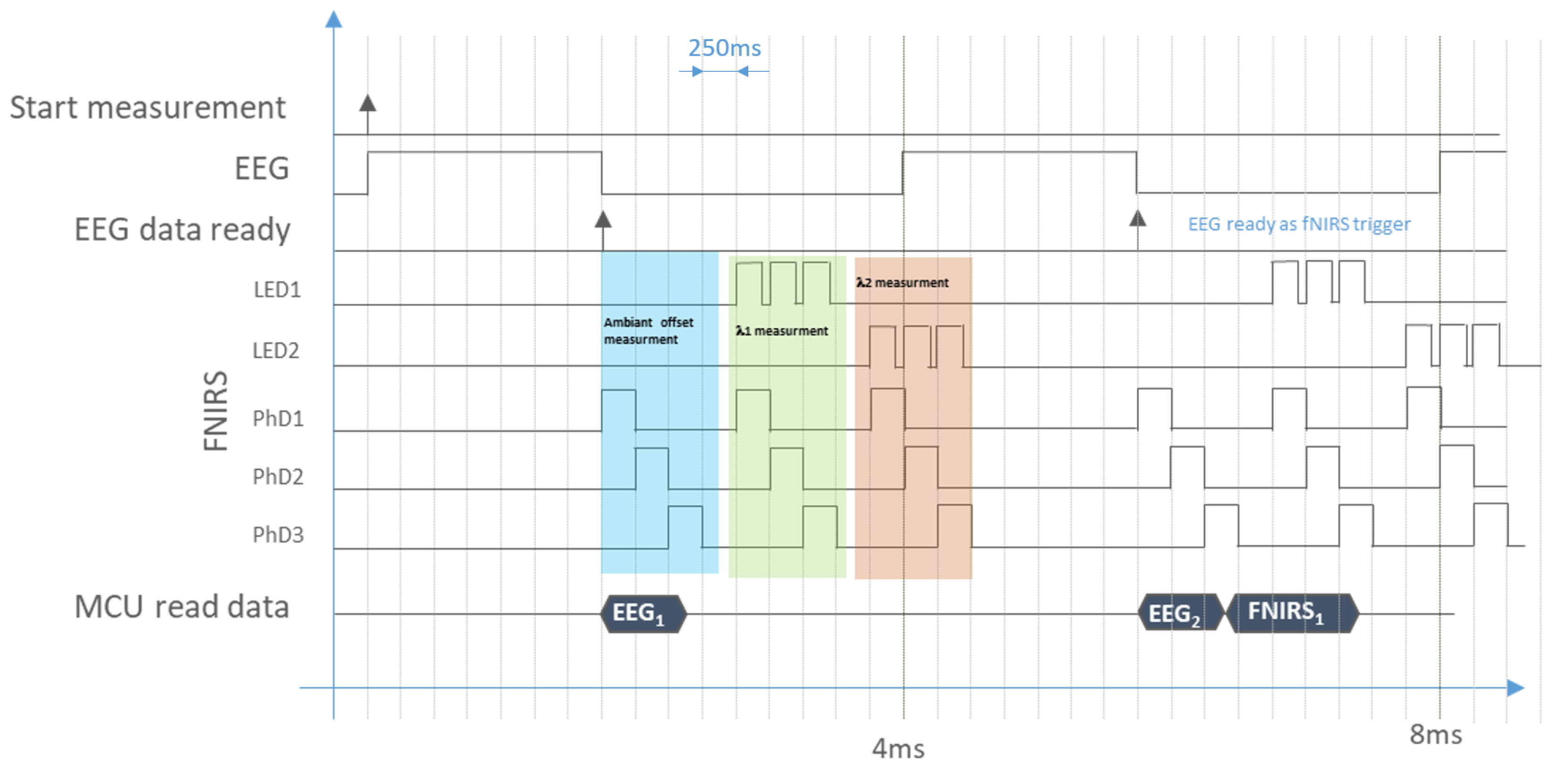
2.3. Finite State Machine (FSM)
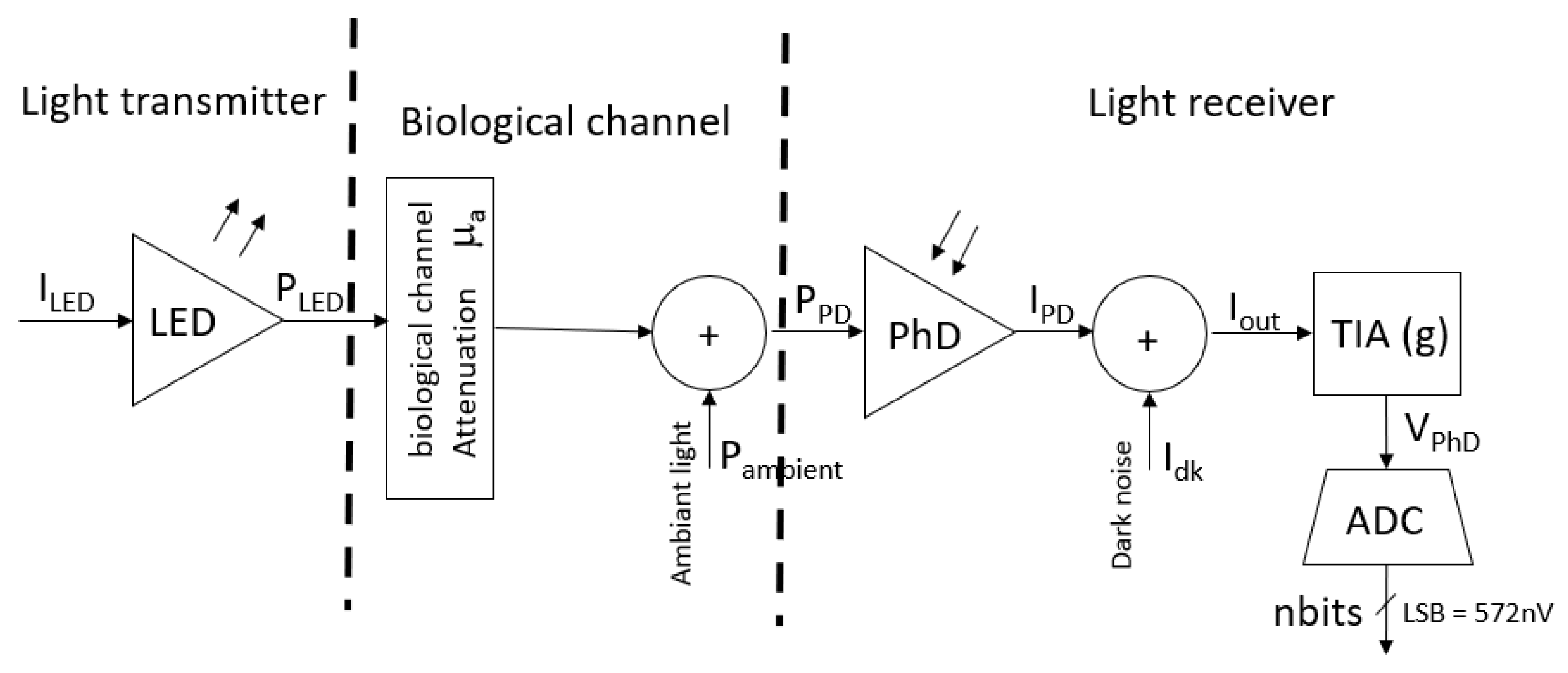
2.4. fNIRS Sensor Calibration
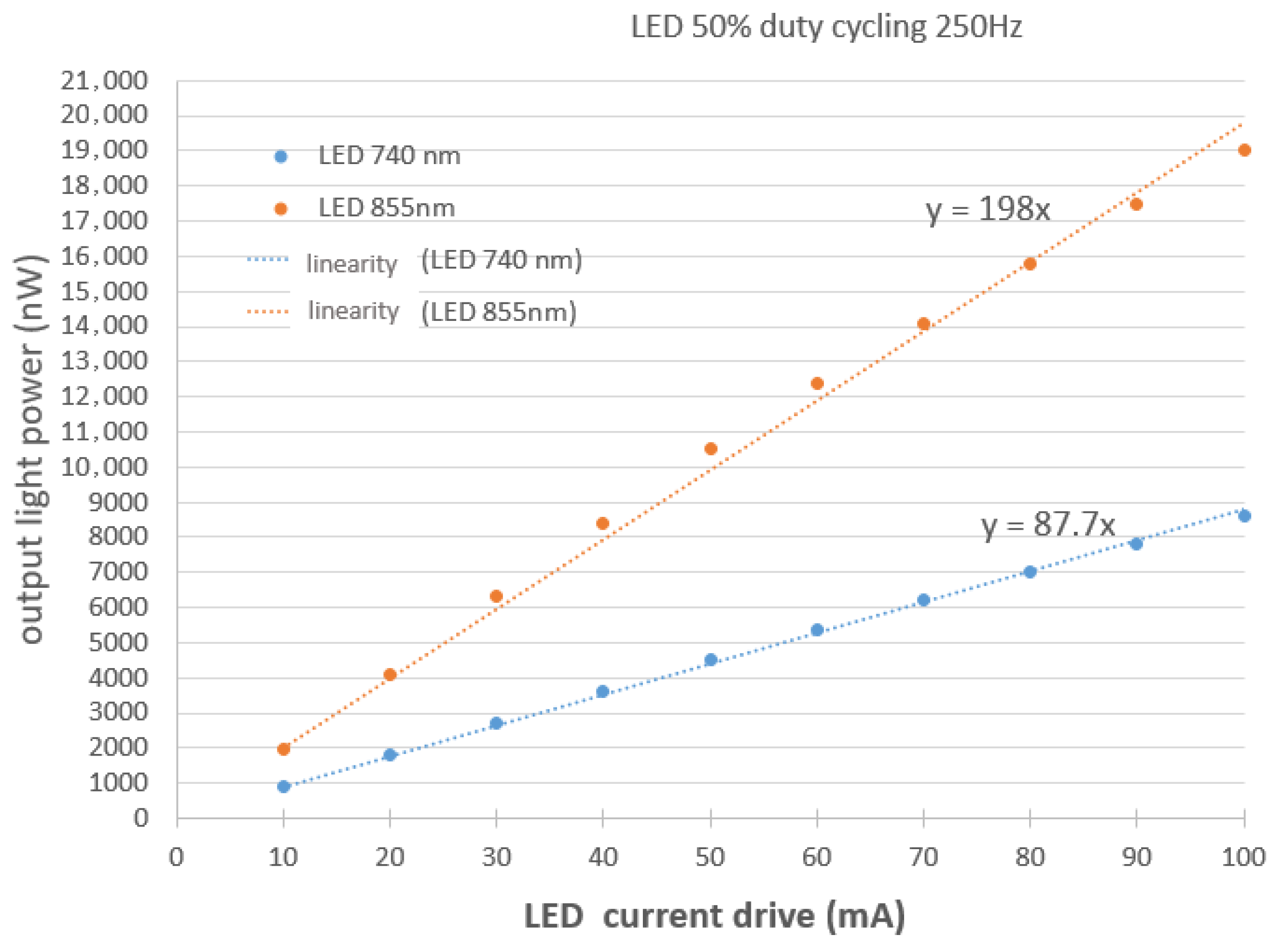
2.4.1. LED Calibration
2.4.2. Photodetector Calibration
3. System Characterization
3.1. Optrode Sensor Characterization
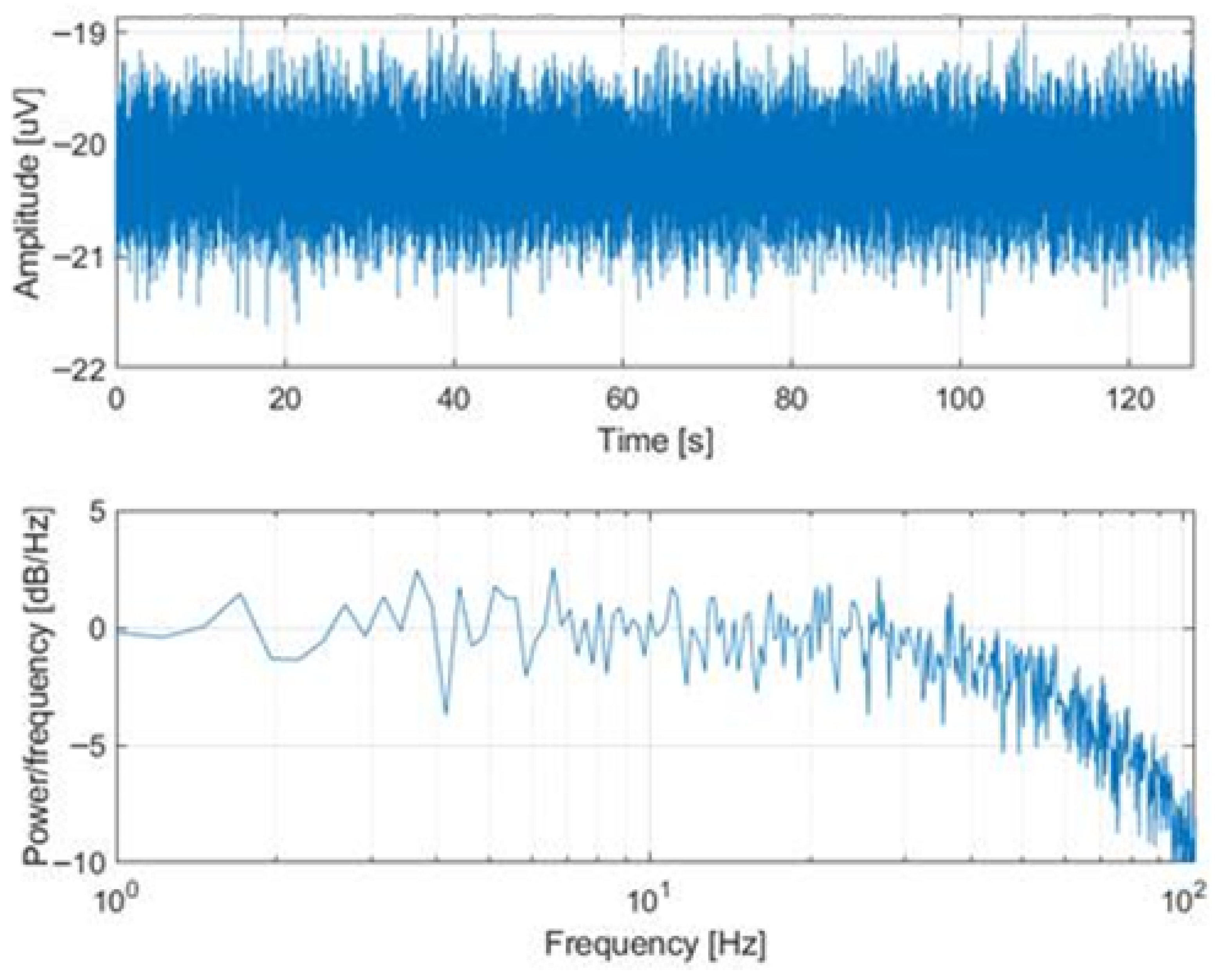
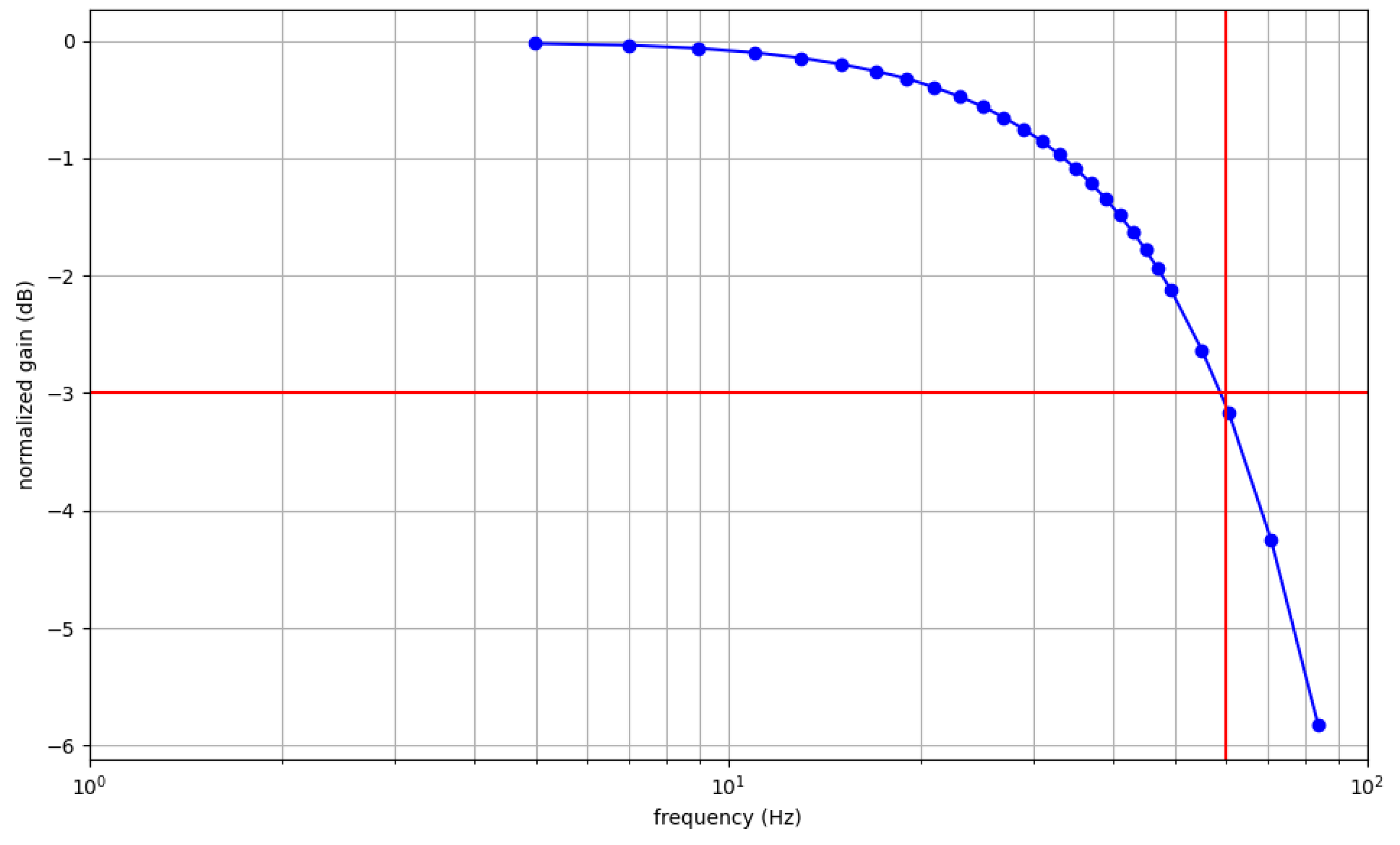
3.1.1. EEG Performances
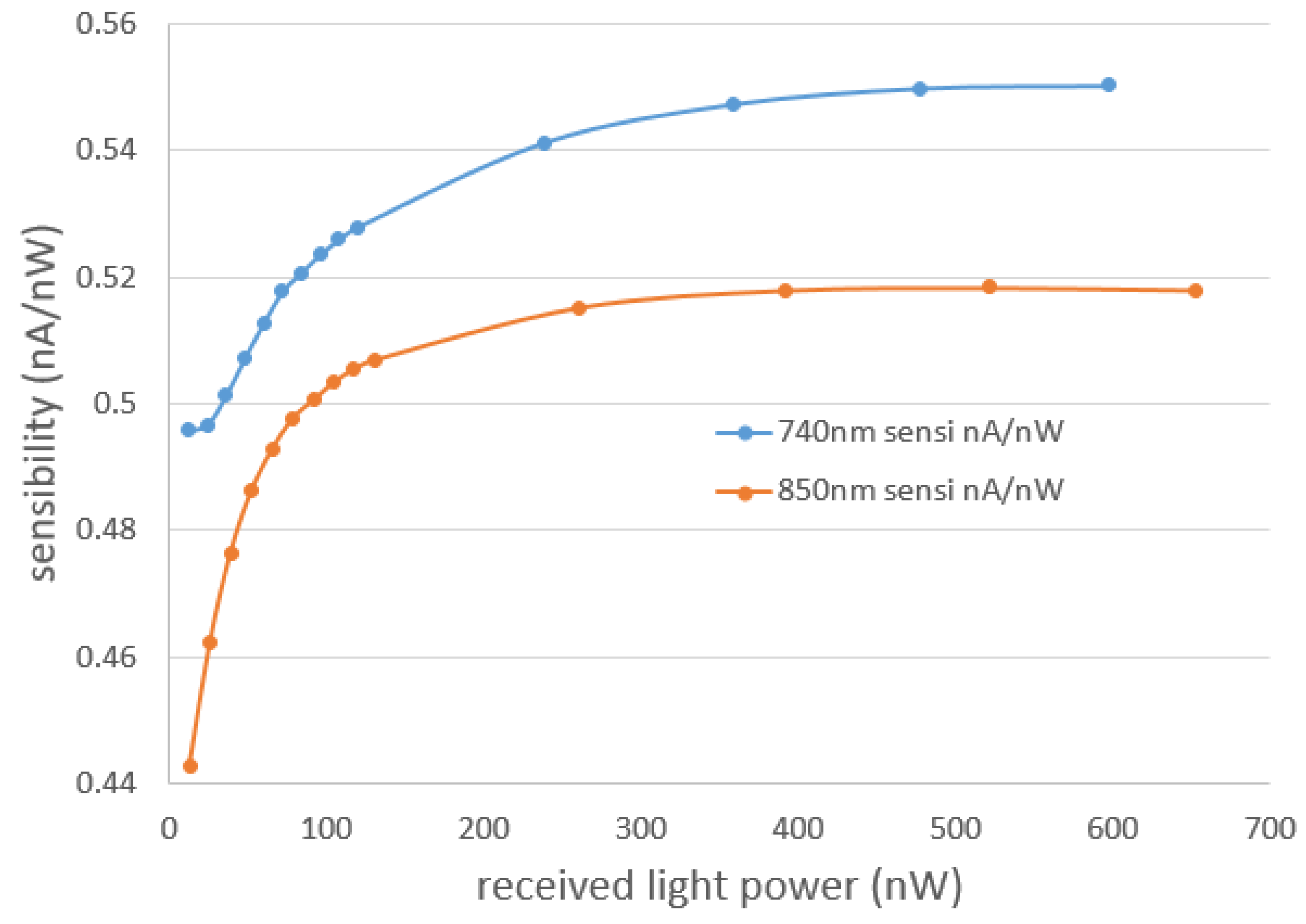
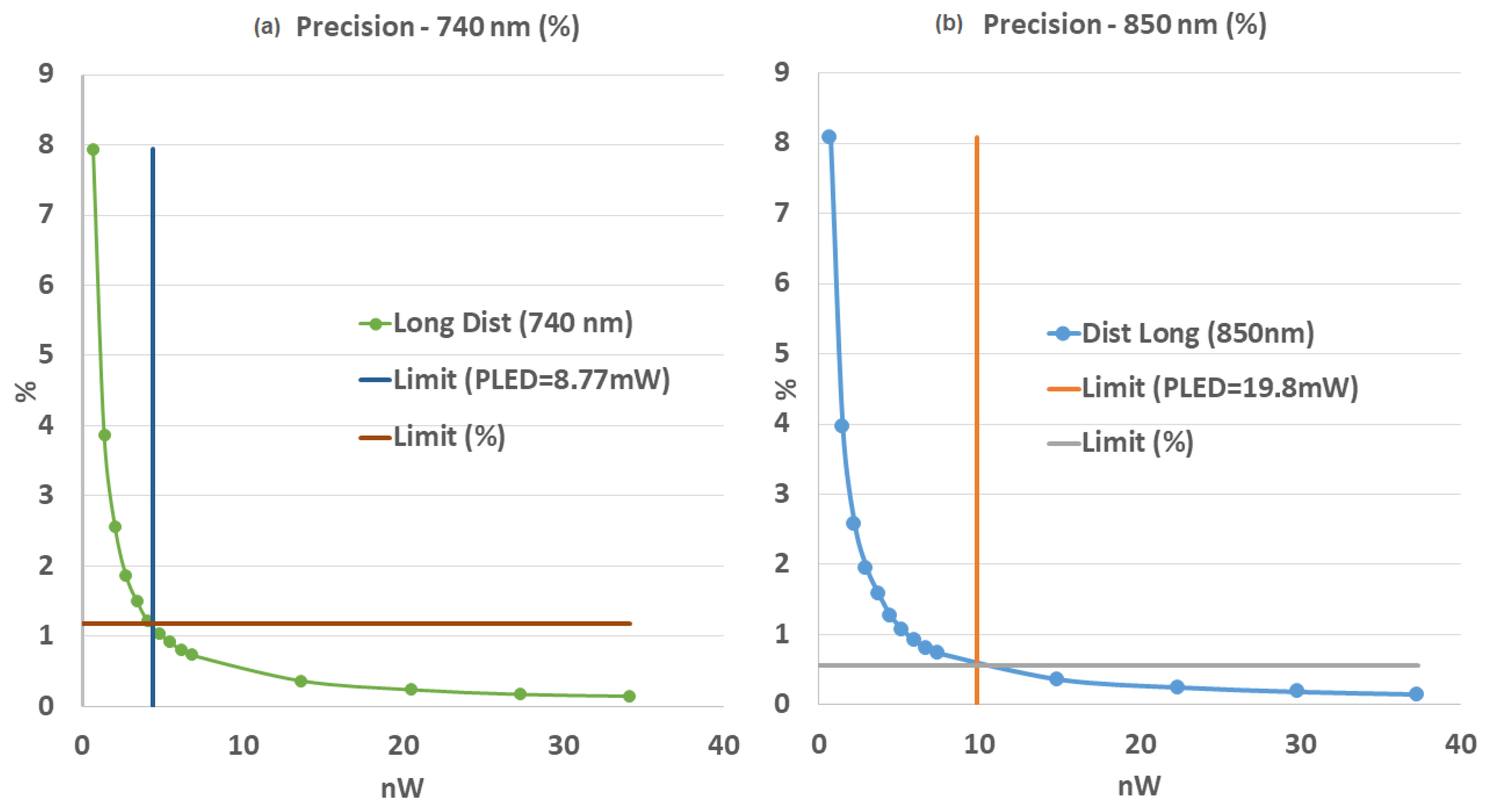
3.1.2. fNIRS Performances
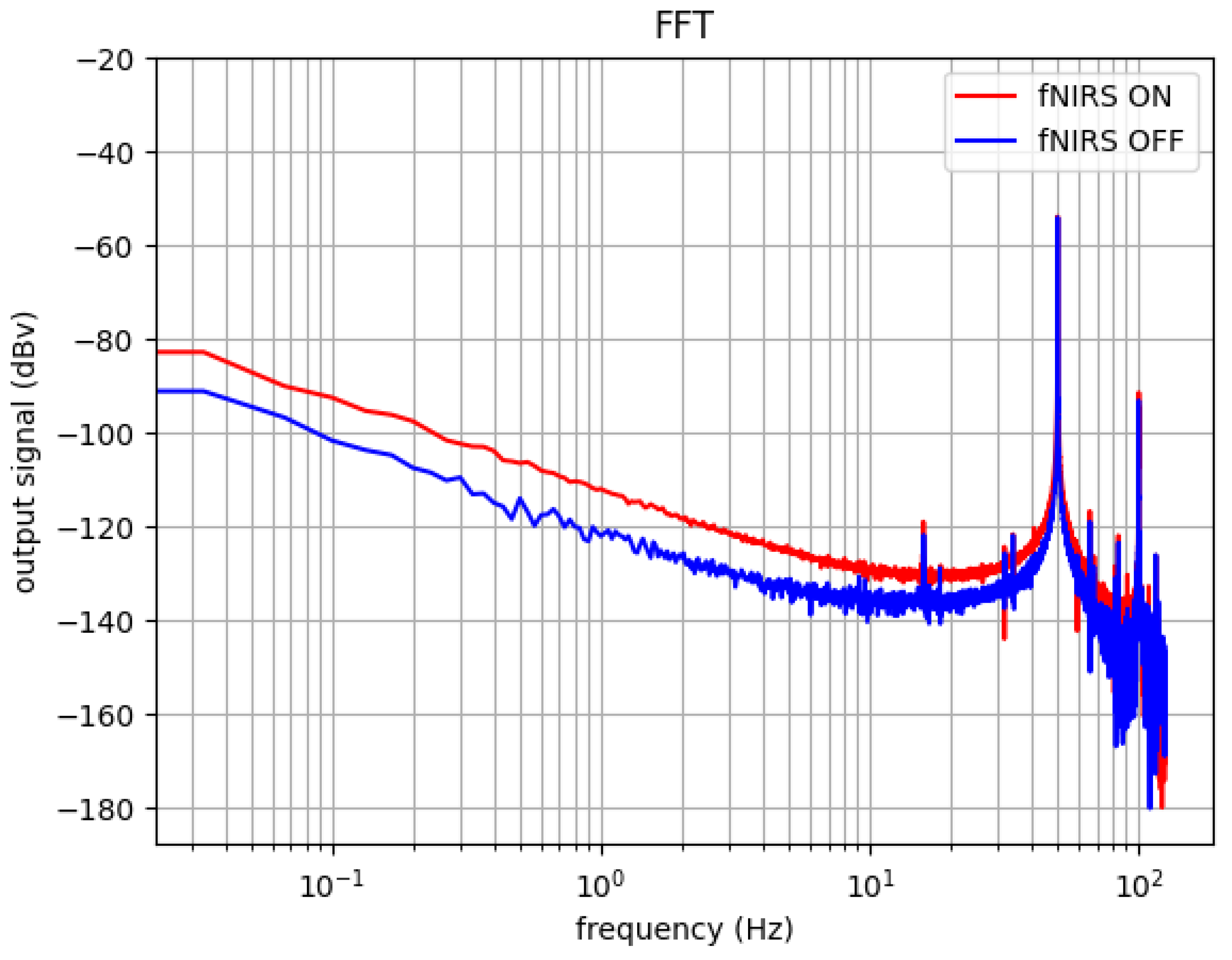
3.2. Crosstalk
4. Results
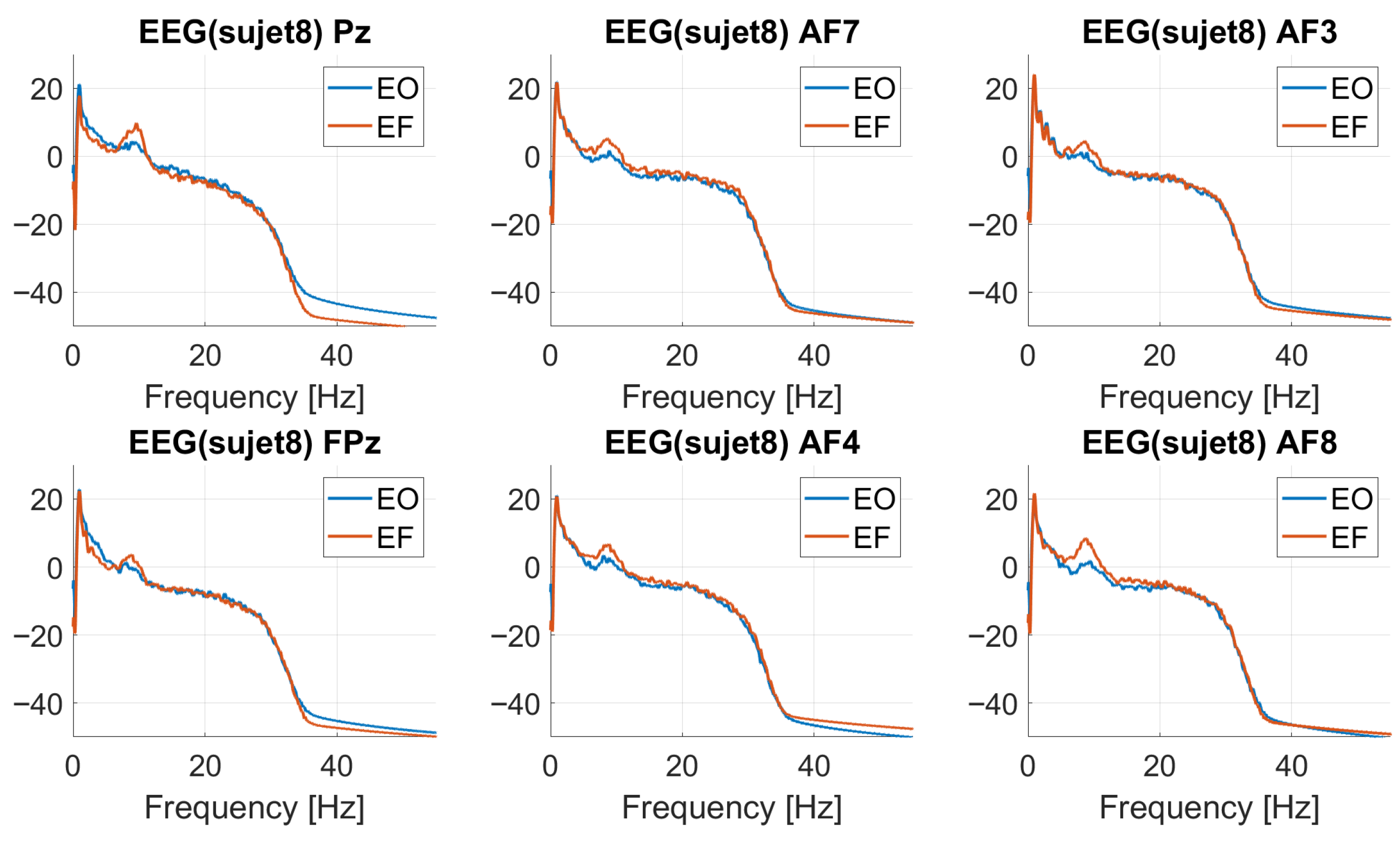
4.1. EEG/Calibration Session
4.2. fNIRS Quality Check
4.3. fNIRS Processing
4.4. N-Back: Protocol
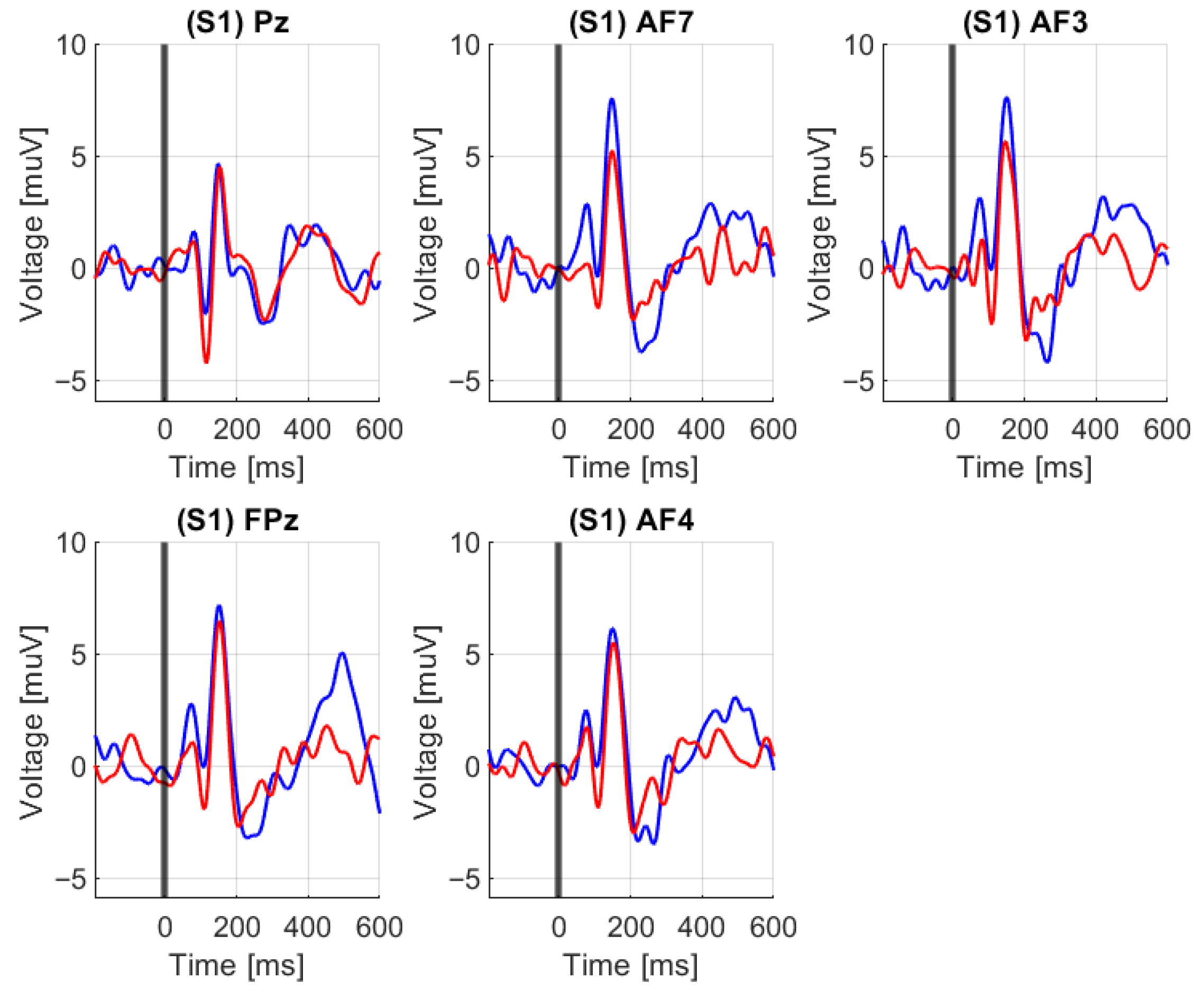
4.4.1. EEG/N-Back: Event-Related Potentials
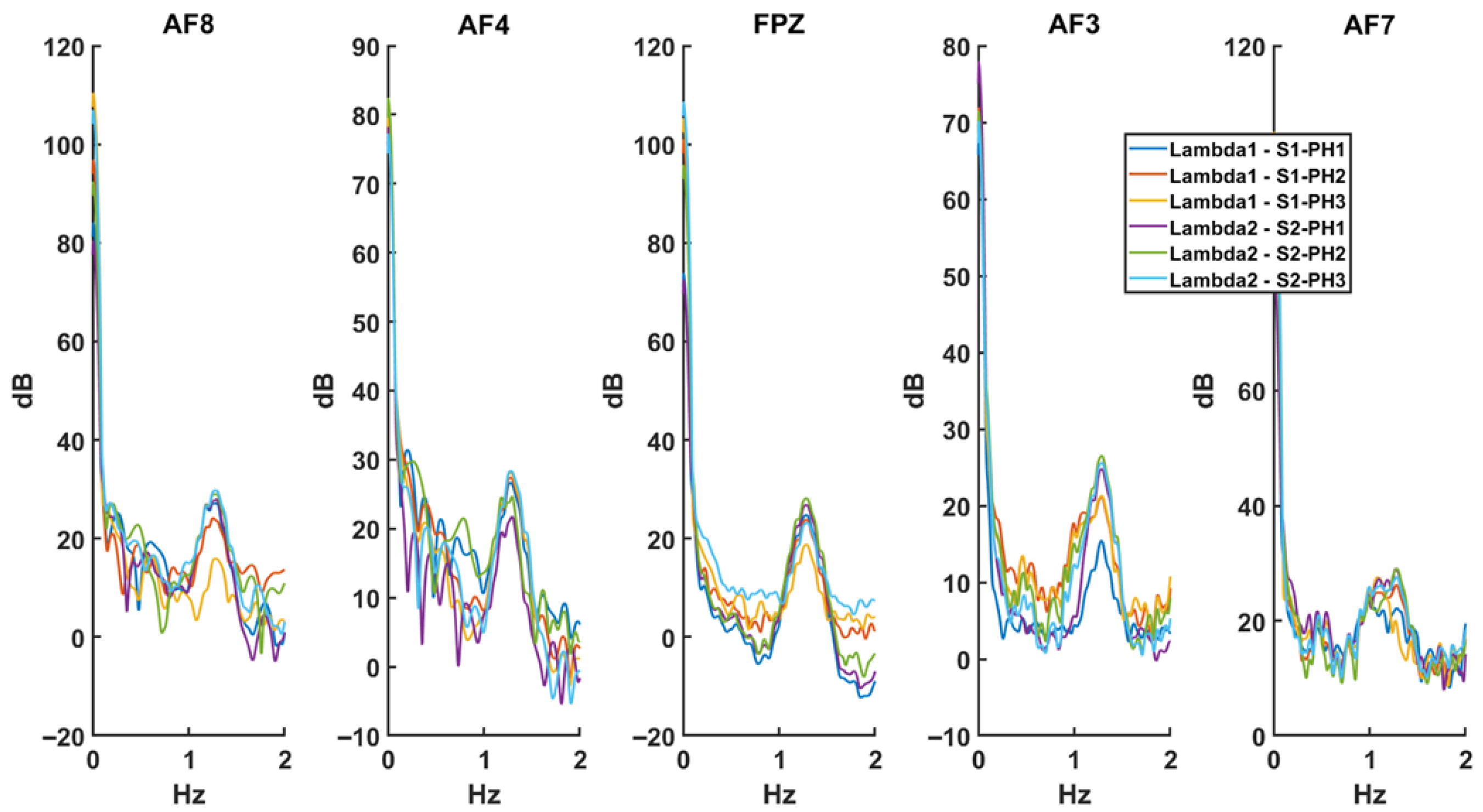
4.4.2. fNIRS Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matusz, P.J.; Dikker, S.; Huth, A.G.; Perrodin, C. Are we ready for real-world neuroscience? J. Cogn. Neurosci. 2019, 31, 327–338. [Google Scholar] [CrossRef]
- Hakim, U.; Felice, S.D.; Pinti, P.; Zhang, X.; Noah, J.; Ono, Y.; Burgess, P.; Hamilton, A.; Hirsch, J.; Tachtsidis, I. Quantification of inter-brain coupling: A review of current methods used in haemodynamic and electrophysiological hyperscanning studies. NeuroImage 2023, 280, 120354. [Google Scholar] [CrossRef]
- Czeszumski, A.; Liang, S.H.Y.; Dikker, S.; König, P.; Lee, C.P.; Koole, S.L.; Kelsen, B. Cooperative Behavior Evokes Interbrain Synchrony in the Prefrontal and Temporoparietal Cortex: A Systematic Review and Meta-Analysis of fNIRS Hyperscanning Studies. eNeuro 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. Functional Near-Infrared Spectroscopy (fNIRS) for Assessing Cerebral Cortex Function During Human Behavior in Natural/Social Situations: A Concise Review. Organ. Res. Methods 2019, 22, 46–68. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Masataka, N.; Perlovsky, L.; Hiraki, K. Near-infrared spectroscopy (NIRS) in functional research of prefrontal cortex. Front. Hum. Neurosci. 2015, 9, 274. [Google Scholar] [CrossRef]
- Cicalese, P.A.; Li, R.; Ahmadi, M.B.; Wang, C.; Francis, J.T.; Selvaraj, S.; Schulz, P.E.; Zhang, Y. An EEG-fNIRS hybridization technique in the four-class classification of alzheimer’s disease. J. Neurosci. Methods 2020, 336, 108618. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, M.; Bahram Borgheai, S.; Jafari, R.; Constant, N.; Diouf, R.; Shahriari, Y.; Mankodiya, K. Merging fNIRS-EEG Brain Monitoring and Body Motion Capture to Distinguish Parkinsons Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Xie, G.; Li, Z.; Li, X.; Zhang, Y.; Wu, K.; Shao, G.; Lv, B.; Jing, H.; Zhang, C.; et al. Automatic depression diagnosis through hybrid EEG and near-infrared spectroscopy features using support vector machine. Front. Neurosci. 2023, 17, 1205931. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargie, F.; Kiguchi, M.; Badruddin, N.; Dass, S.C.; Hani, A.F.M.; Tang, T.B. Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomed. Opt. Express 2016, 7, 3882–3898. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Zhou, X.; Vidal Rosas, E.; Thomas, A.; Loureiro, R.; Cooper, R.J.; Carlson, T.; Zhao, H. fNIRS-EEG BCIs for Motor Rehabilitation: A Review. Bioengineering 2023, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shore, J.; Wang, M.; Yuan, F.; Buss, A.; Zhao, X. A systematic review on hybrid EEG/fNIRS in brain-computer interface. Biomed. Signal Process. Control 2021, 68, 102595. [Google Scholar] [CrossRef]
- Cao, J.; Garro, E.M.; Zhao, Y. EEG/fNIRS Based Workload Classification Using Functional Brain Connectivity and Machine Learning. Sensors 2022, 22, 7623. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhong, Y.; He, Z.; Pan, J. Improved classification performance of EEG-fNIRS multimodal brain-computer interface based on multi-domain features and multi-level progressive learning. Front. Hum. Neurosci. 2022, 16, 973959. [Google Scholar] [CrossRef]
- Aloui, N.; Planat-Chrétien, A.; Bonnet, S. Artefact subspace reconstruction for both EEG and fNIRS co-registred signals. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 208–211. [Google Scholar] [CrossRef]
- Uchitel, J.; Vidal-Rosas, E.E.; Cooper, R.J.; Zhao, H. Wearable, Integrated EEG–fNIRS Technologies: A Review. Sensors 2021, 21, 6106. [Google Scholar] [CrossRef]
- Ha, U.; Lee, J.; Kim, M.; Roh, T.; Choi, S.; Yoo, H.J. An EEG-NIRS Multimodal SoC for Accurate Anesthesia Depth Monitoring. IEEE J. Solid-State Circuits 2018, 53, 1830–1843. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Xia, J.; Jin, H.; Dong, S.; Luo, J. Hybrid Integrated Wearable Patch for Brain EEG-fNIRS Monitoring. Sensors 2024, 24, 4847. [Google Scholar] [CrossRef]
- Cui, W.; Lin, K.; Liu, G.; Sun, Y.; Cai, J. A Wireless Integrated EEG-fNIRS System for Brain Function Monitoring. IEEE Sensors J. 2024, 24, 2125–2133. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef]
- Guglielmini, S.; Bopp, G.; Marcar, V.L.; Scholkmann, F.; Wolf, M. Systemic physiology augmented functional near-infrared spectroscopy hyperscanning: A first evaluation investigating entrainment of spontaneous activity of brain and body physiology between subjects. Neurophotonics 2022, 9, 026601. [Google Scholar] [CrossRef]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer-Lambert law revisited. Phys. Med. Biol. 2006, 51, N91. [Google Scholar] [CrossRef]
- Suzuki, S.; Takasaki, S.; Ozaki, T.; Kobayashi, Y. Tissue oxygenation monitor using NIR spatially resolved spectroscopy. In Proceedings of the Optical Tomography and Spectroscopy of Tissue III, San Jose, CA, USA, 24–28 January 1999; Chance, B., Alfano, R.R., Tromberg, B.J., Eds.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 1999; Volume 3597, pp. 582–592. [Google Scholar] [CrossRef]
- Brigadoi, S.; Cooper, R. How short is short? Optimum source–detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics 2015, 2, 025005. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, Y.; Kumar, A.; Kim, M.; Lee, H.N. Dry Electrode-Based Fully Isolated EEG/fNIRS Hybrid Brain-Monitoring System. IEEE Trans. Biomed. Eng. 2019, 66, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M.; Hogervorst, M.A.; van Erp, J.B.F.; Heffelaar, T.; Zimmerman, P.H.; Oostenveld, R. Estimating workload using EEG spectral power and ERPs in the N-Back task. J. Neural Eng. 2012, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Alday, P.; Schlesewsky, M.; Bornkessel-Schlesewsky, I. Toward a reliable, automated method of individual alpha frequency (IAF) quantification. Psychophysiology 2018, 55, e13064. [Google Scholar] [CrossRef]
- Wyser, D.; Mattille, M.; Wolf, M.; Lambercy, O.; Scholkmann, F.; Gassert, R. Short-channel regression in functional near-infrared spectroscopy is more effective when considering heterogeneous scalp hemodynamics. Neurophotonics 2020, 7, 035011. [Google Scholar] [CrossRef]
- Perdue, K.L.; Westerlund, A.; McCormick, S.A.; Nelson, C.A., III. Extraction of heart rate from functional near-infrared spectroscopy in infants. J. Biomed. Opt. 2014, 19, 067010. [Google Scholar] [CrossRef]
- Saager, R.B.; Berger, A.J. Direct characterization and removal of interfering absorption trends in two-layer turbid media. J. Opt. Soc. Am. A 2005, 22, 1874–1882. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, F.; Xu, X.; Duan, L.; Liu, H.; Tian, F.; Zhu, C.Z. Multiregional functional near-infrared spectroscopy reveals globally symmetrical and frequency-specific patterns of superficial interference. Biomed. Opt. Express 2015, 6, 2786–2802. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A motion correction method for fNIRS. NeuroImage 2019, 184, 171–179. [Google Scholar] [CrossRef]
- Matcher, S.J.; Cope, M.; Delpy, D.T. Quantification of tissue chromophore concentration via water-peak measurements in near-infrared spectroscopy. In Proceedings of the Photon Migration and Imaging in Random Media and Tissues, Los Angeles, CA, USA, 17–19 January 1993; SPIE: Bellingham, WA, USA, 1993; Volume 1888, pp. 239–247. [Google Scholar]
- Mohamed, M.; Jo, E.; Mohamed, N.; Kim, M.; Yun, J.-d.; Kim, J.G. Development of an Integrated EEG/fNIRS Brain Function Monitoring System. Sensors 2021, 21, 7703. [Google Scholar] [CrossRef]



| PhD2/PhD1 @740 nm | PhD3/PhD1 @740 nm | PhD2/PhD1 @850 nm | PhD3/PhD1 @850 nm | |
|---|---|---|---|---|
| 10 | 106% | 111% | 110% | 117% |
| 25 * | 106% | 112% | 110% | 117% |
| 50 | 106% | 112% | 110% | 118% |
| 100 | 106% | 112% | 110% | 118% |
| 500 | 106% | 112% | 109% | 118% |
| 1000 | 106% | 112% | 109% | 118% |
| 2000 ** | 107% | 113% | 111% | 119% |
| PhD1 | PhD2 | PhD3 | |
|---|---|---|---|
| 10 | 120% | 116% | 114% |
| 25 * | 120% | 117% | 115% |
| 50 | 121% | 117% | 115% |
| 100 | 121% | 117% | 115% |
| 500 | 120% | 116% | 114% |
| 1000 | 120% | 117% | 114% |
| 2000 ** | 120% | 116% | 114% |
| [19] | [26] | [35] | [17] | This Work | |
|---|---|---|---|---|---|
| EEG noise floor (Vrms) | 0.89 | 0.14 | 0.29 | 0.44 | 0.345 |
| EEG sampling rate (Hz) | 16k | 250 | 250 | 2k | 250 |
| fNIRS sampling rate (Hz) | 100 | 5 | 8 | 10 | 250 |
| EEG resolution (bits) | 24 | 24 | 24 | 12 | 24 |
| fNIRS resolution (bits) | 24 | 16 | 24 | 12 | 22 |
| short and long distance fNIRs | 0 + 1 | 0 + 1 | 0 + 1 | 0 + 2 | 1 + 2 |
| Dry EEG electrode | yes | yes | no | yes | yes |
| Co-located EEG/fNIRS | yes | no | no | no | yes |
| Crosstalk suppression | yes | yes | yes | no | yes |
| Sensor dimension (mm2) | 78.54 | na | 716 × 60 | na | 50 × 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameau, F.; Planat-Chrétien, A.; Gharbi, S.; Prada-Mejia, R.; Thomas, S.; Bonnet, S.; Rascle, A. Compact Colocated Bimodal EEG/fNIRS Multi-Distance Sensor. Sensors 2025, 25, 5520. https://doi.org/10.3390/s25175520
Hameau F, Planat-Chrétien A, Gharbi S, Prada-Mejia R, Thomas S, Bonnet S, Rascle A. Compact Colocated Bimodal EEG/fNIRS Multi-Distance Sensor. Sensors. 2025; 25(17):5520. https://doi.org/10.3390/s25175520
Chicago/Turabian StyleHameau, Frédéric, Anne Planat-Chrétien, Sadok Gharbi, Robinson Prada-Mejia, Simon Thomas, Stéphane Bonnet, and Angélique Rascle. 2025. "Compact Colocated Bimodal EEG/fNIRS Multi-Distance Sensor" Sensors 25, no. 17: 5520. https://doi.org/10.3390/s25175520
APA StyleHameau, F., Planat-Chrétien, A., Gharbi, S., Prada-Mejia, R., Thomas, S., Bonnet, S., & Rascle, A. (2025). Compact Colocated Bimodal EEG/fNIRS Multi-Distance Sensor. Sensors, 25(17), 5520. https://doi.org/10.3390/s25175520






