Monitoring of Ammonia in Biomass Combustion Flue Gas Using a Zeolite-Based Capacitive Sensor
Abstract
1. Introduction
2. Materials and Methods
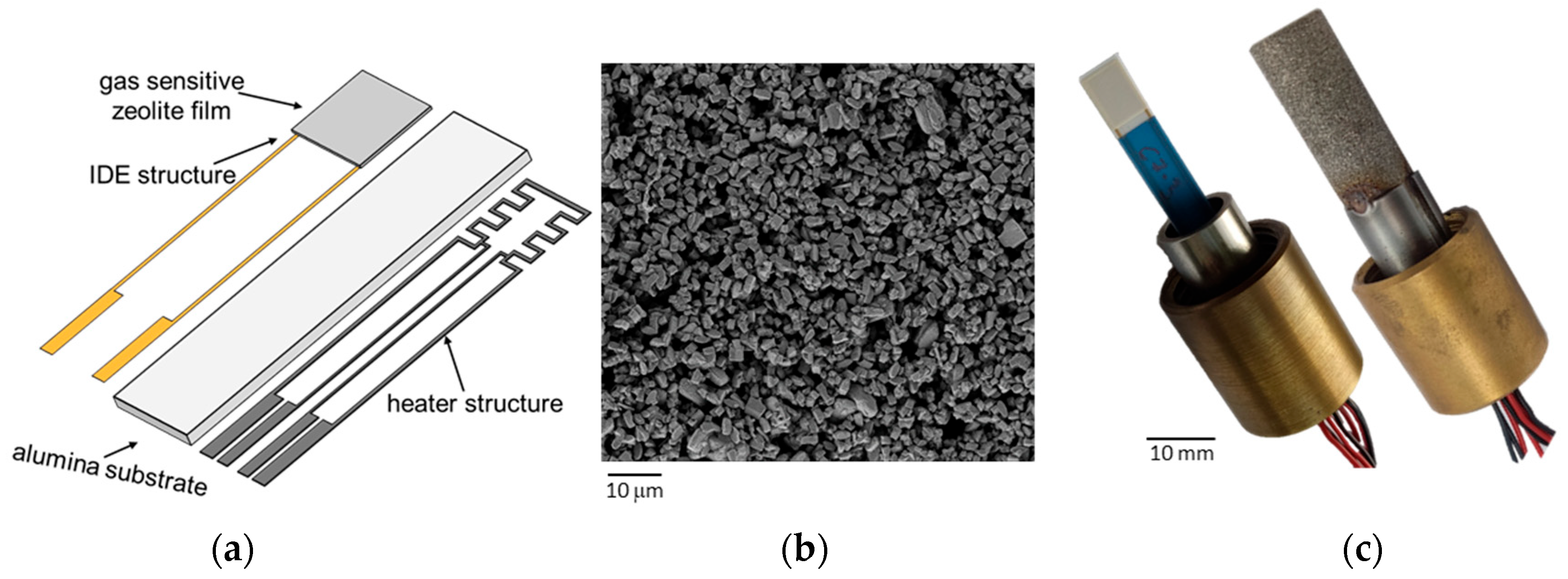
2.1. Sensor Setup
2.2. Experimental Setup in the Laboratory
2.3. Experimental Setup for Measurements in Flue Gas
3. Results
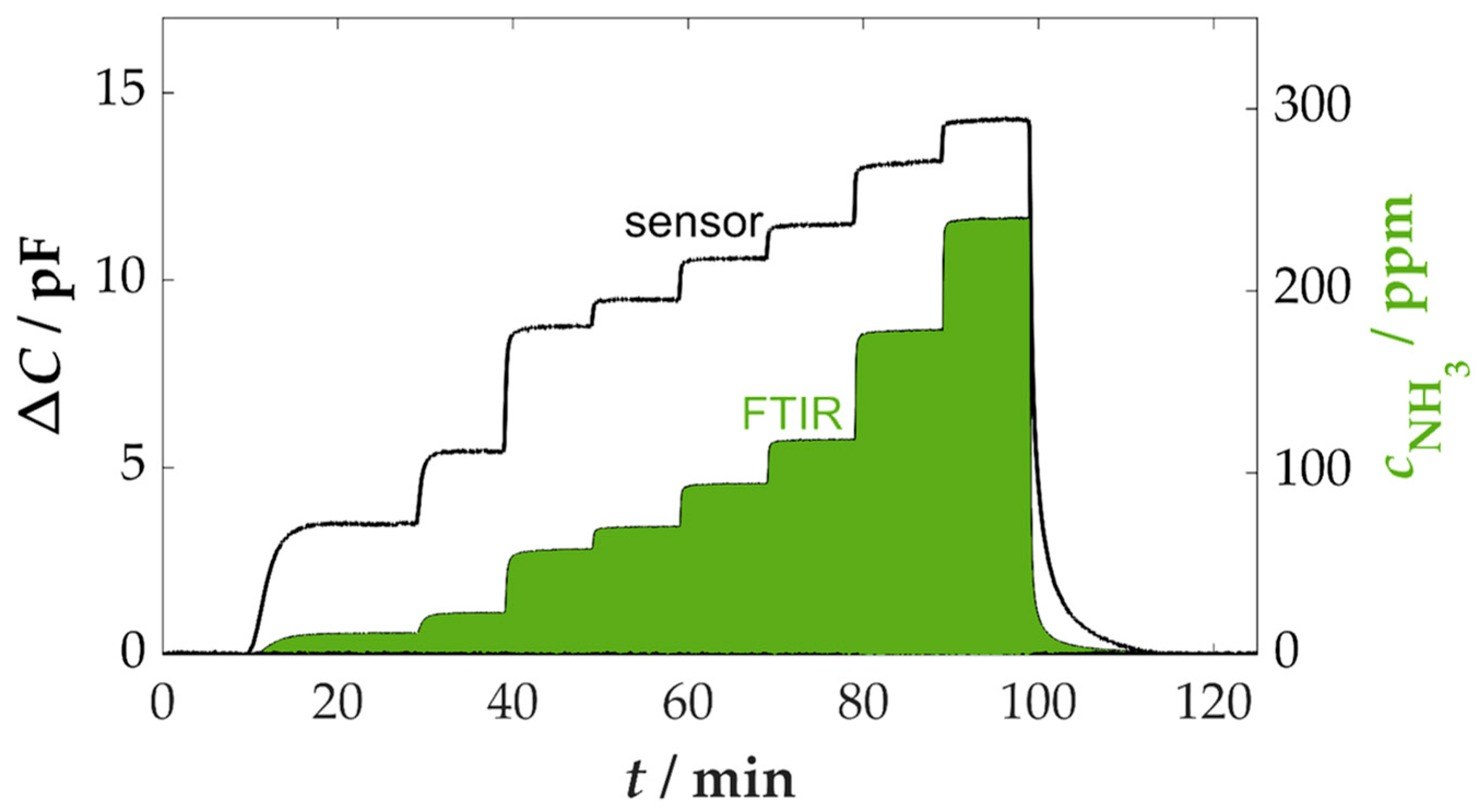
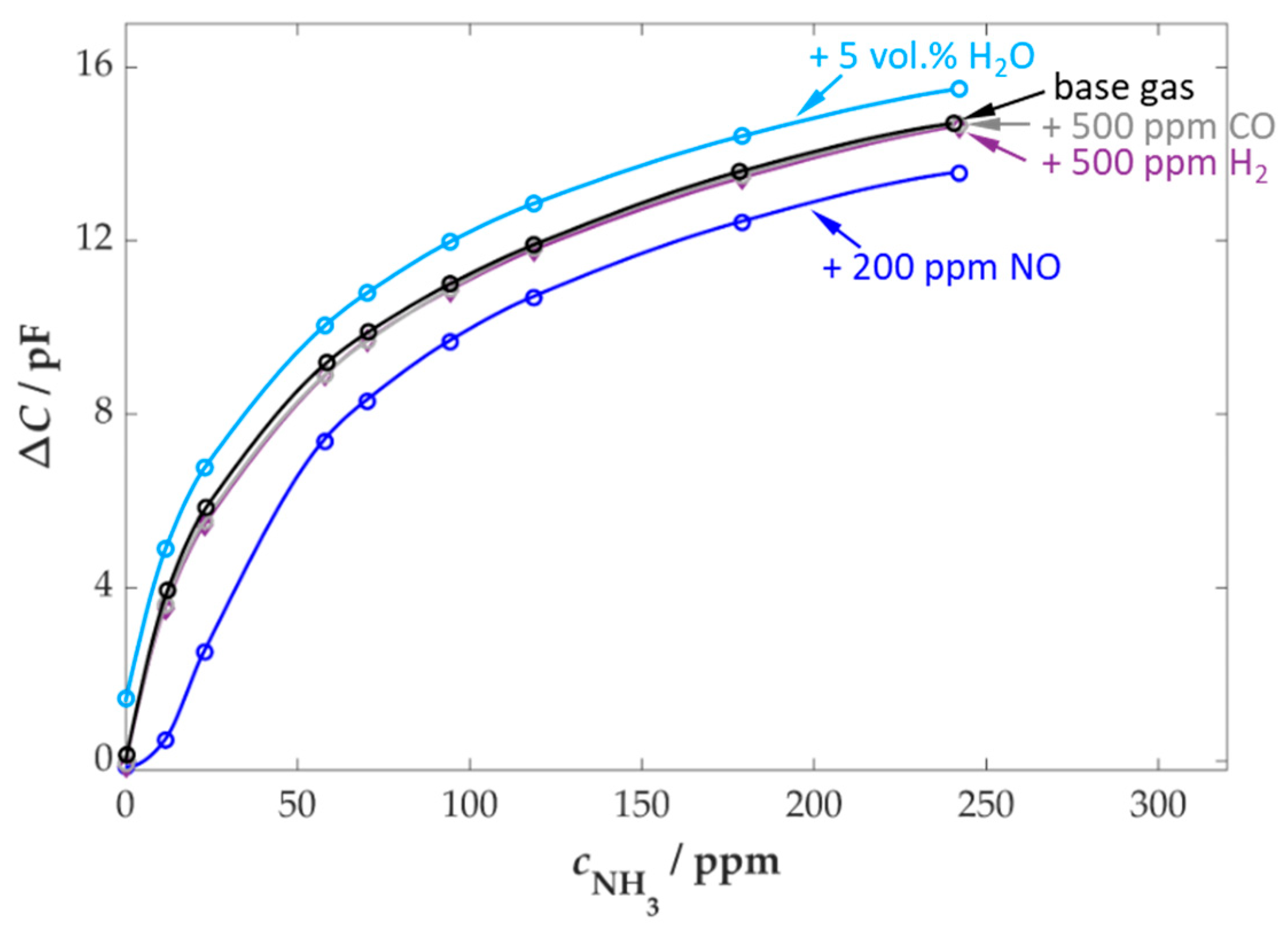
3.1. Ammonia Sensitivity and Cross-Sensitivities
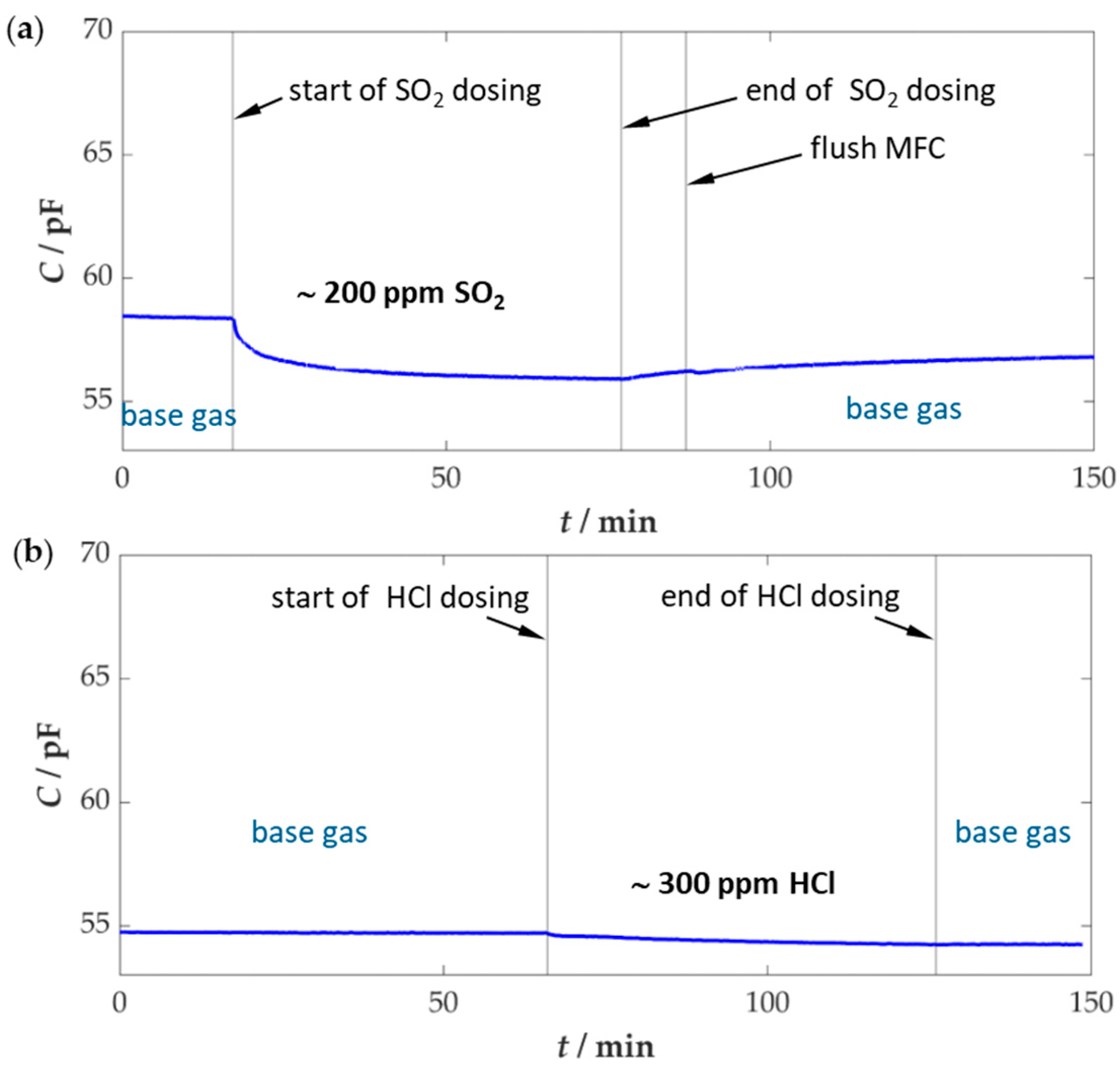
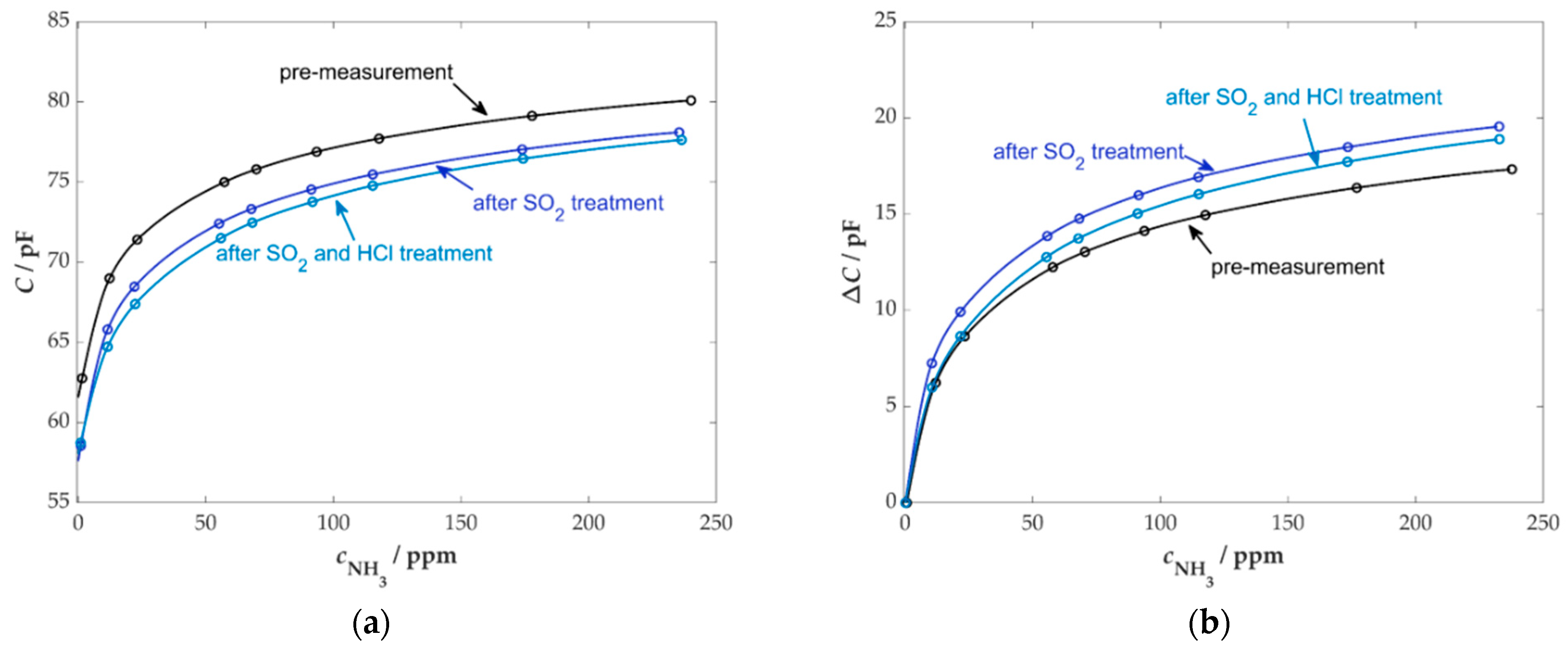
3.2. Sensor Poisoning with SO2 and HCl
3.3. Long-Term Stability
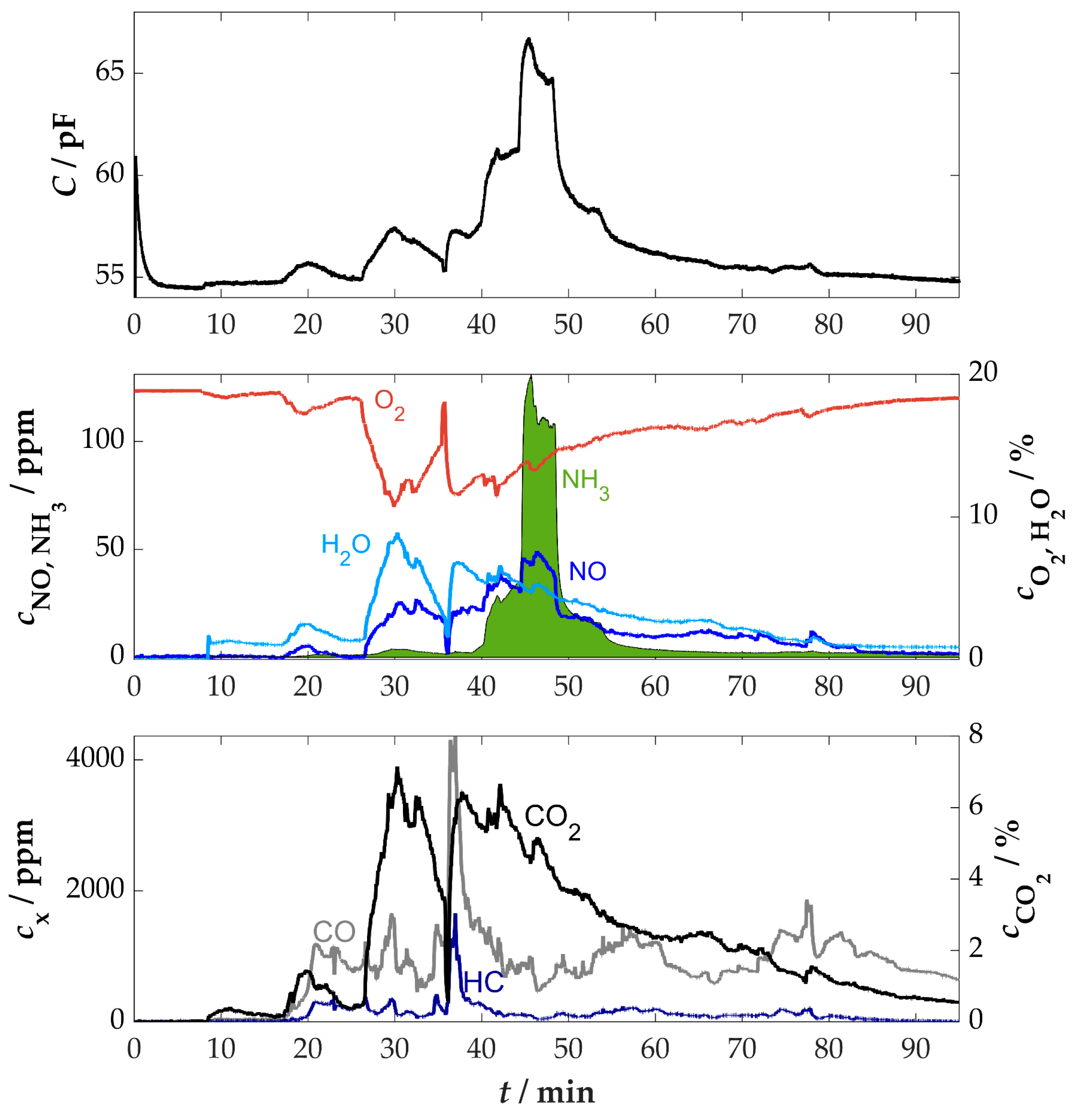
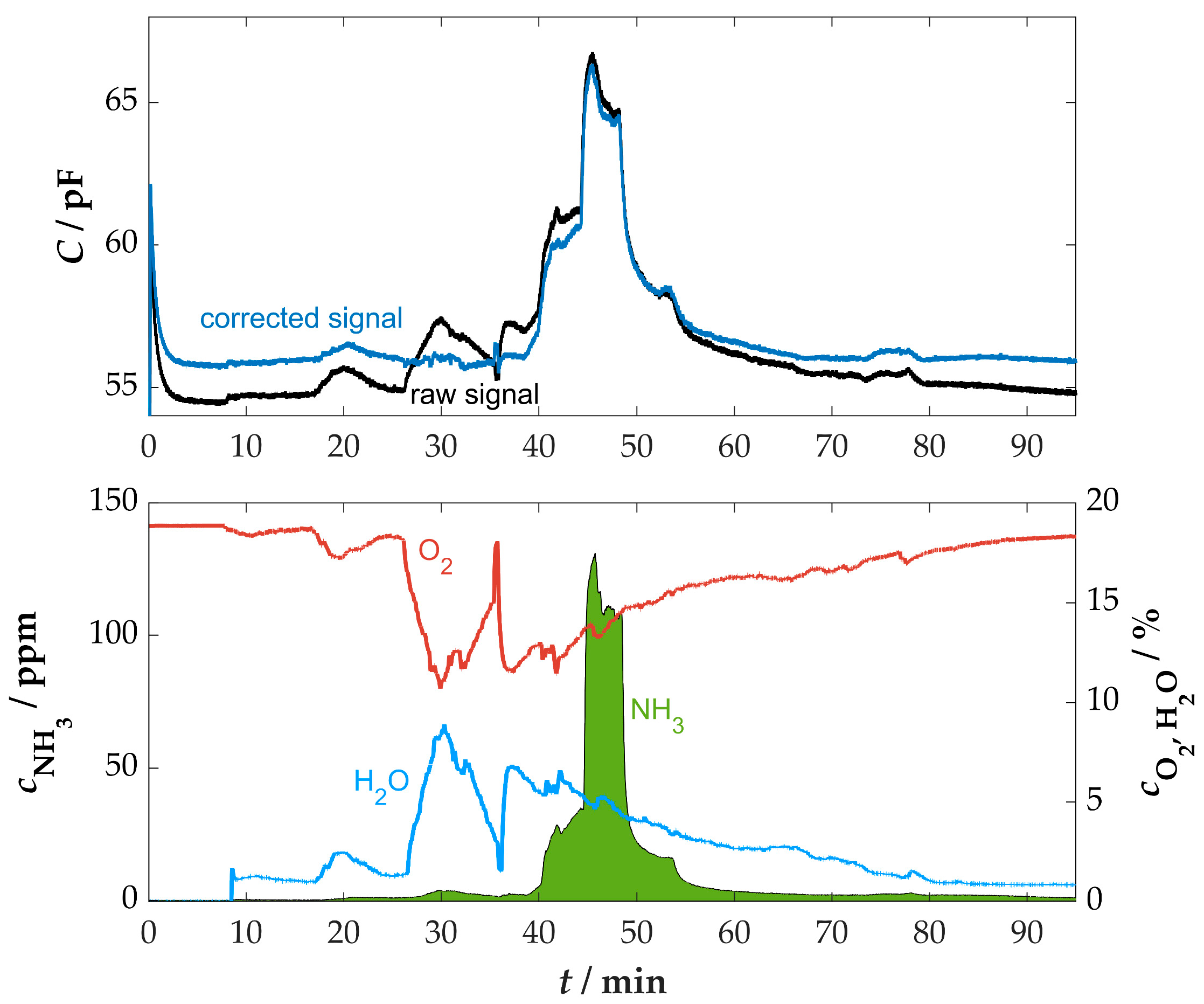
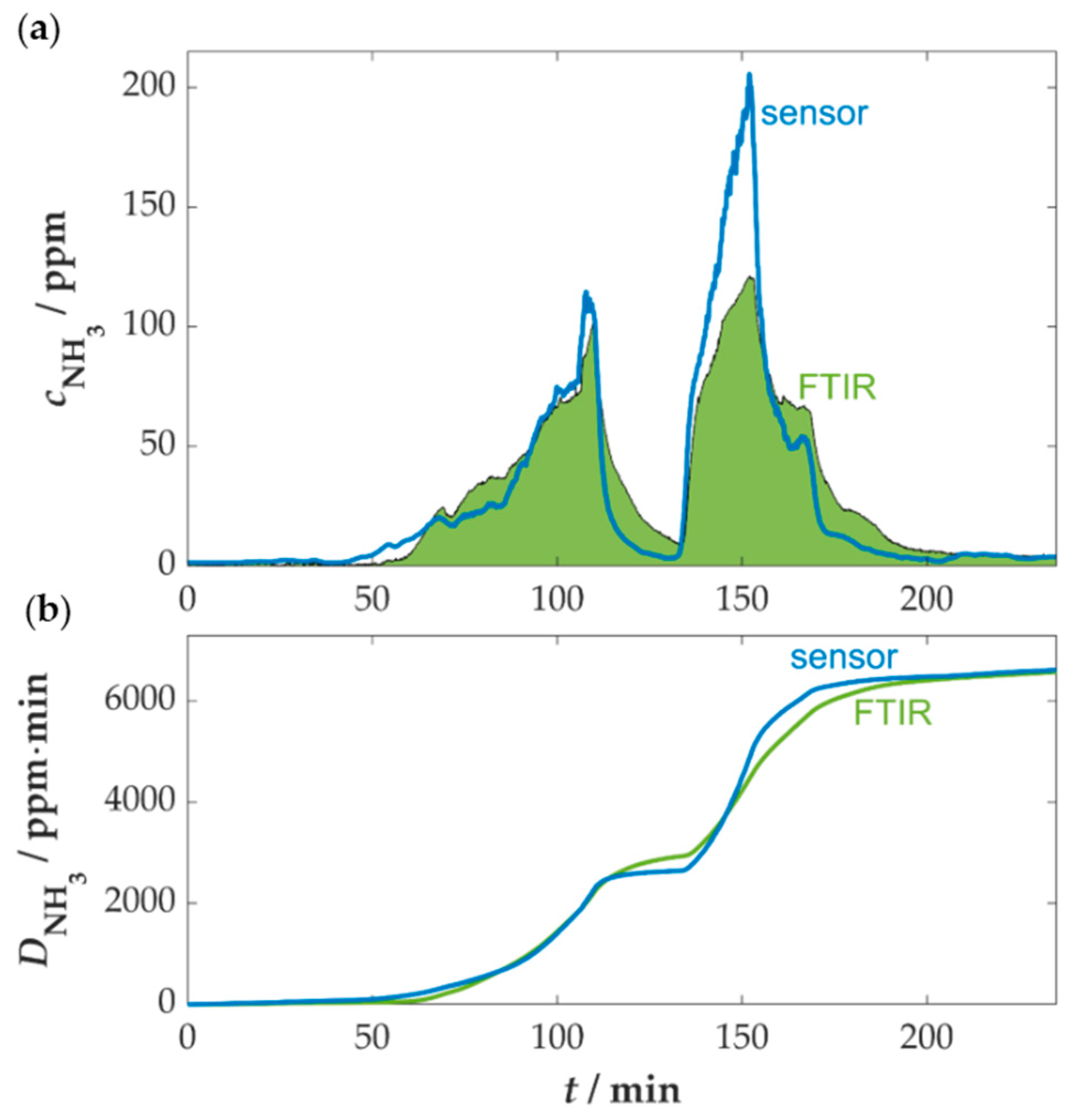
3.4. Measurement at a Firewood Furnace Under Dynamic Conditions
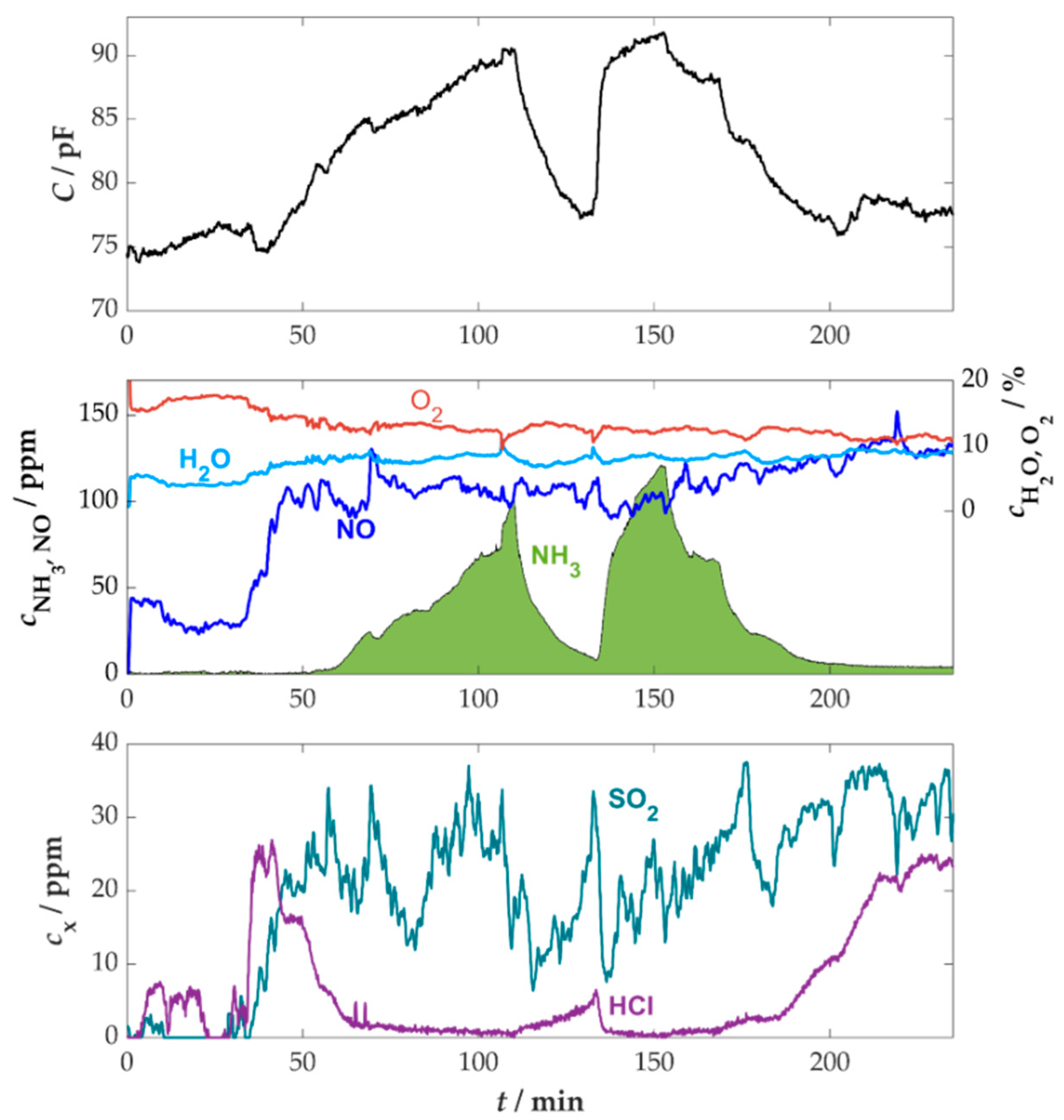
3.5. Measurement on Multi-Fuel Boilers with Straw Pellets Under Stationary Conditions
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BImschV | Verordnung zur Durchführung des Bundes-Immisionsschutzgesetzes |
| SCR | Selective catalytic reduction |
| MOS | Metal-oxide semiconductor |
| NDIR | Non-dispersive infrared spectroscopy |
| SAW | Surface acoustic wave |
| FTIR | Fourier-transform infrared spectroscopy |
| ZSM5 | Zeolite Socony Mobil-5 |
| MFI | Metal framework inverted |
| SEM | Scanning electron microscope |
| IDE | Interdigital electrode |
| DBFZ | Deutsches Biomasseforschungszentrum |
| MFC | Mass flow controller |
Appendix A
Appendix A.1
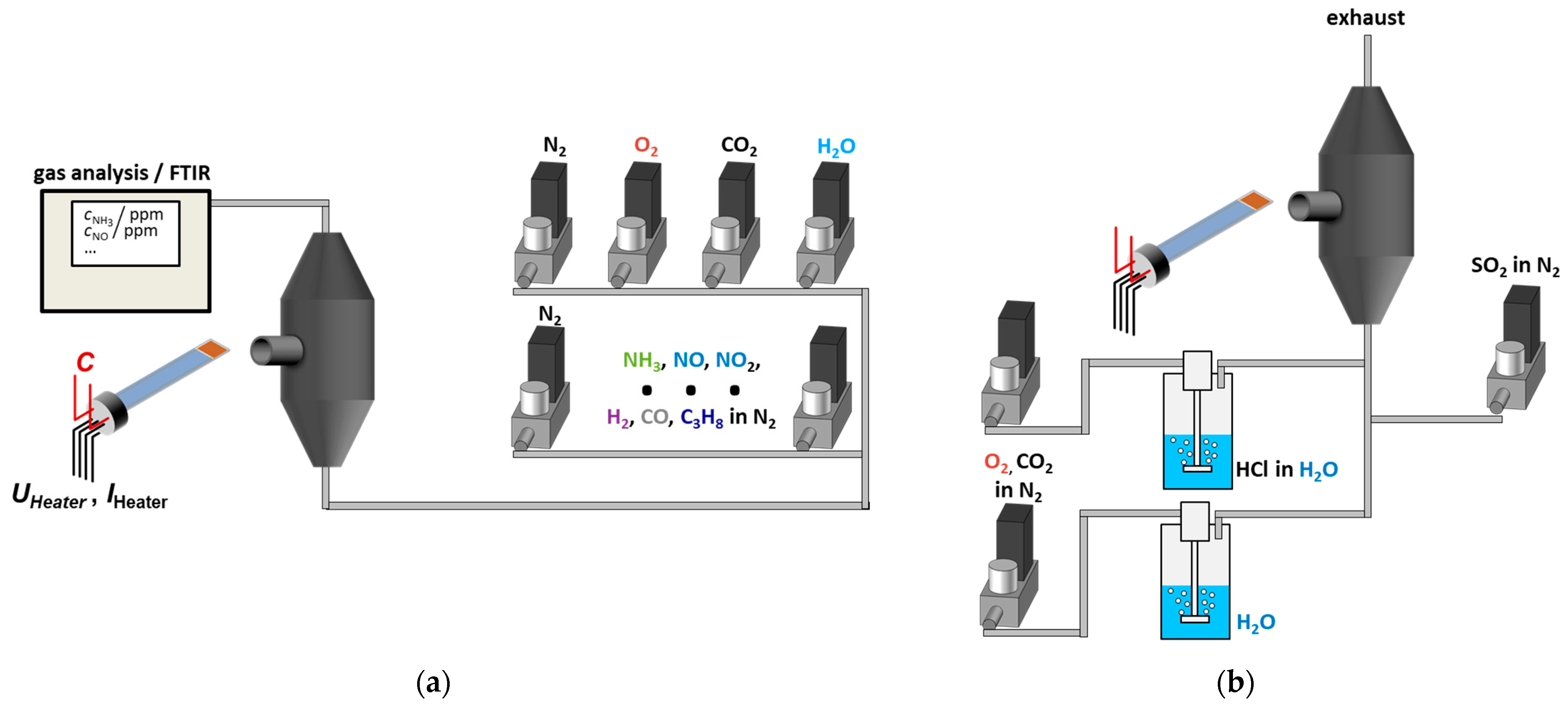
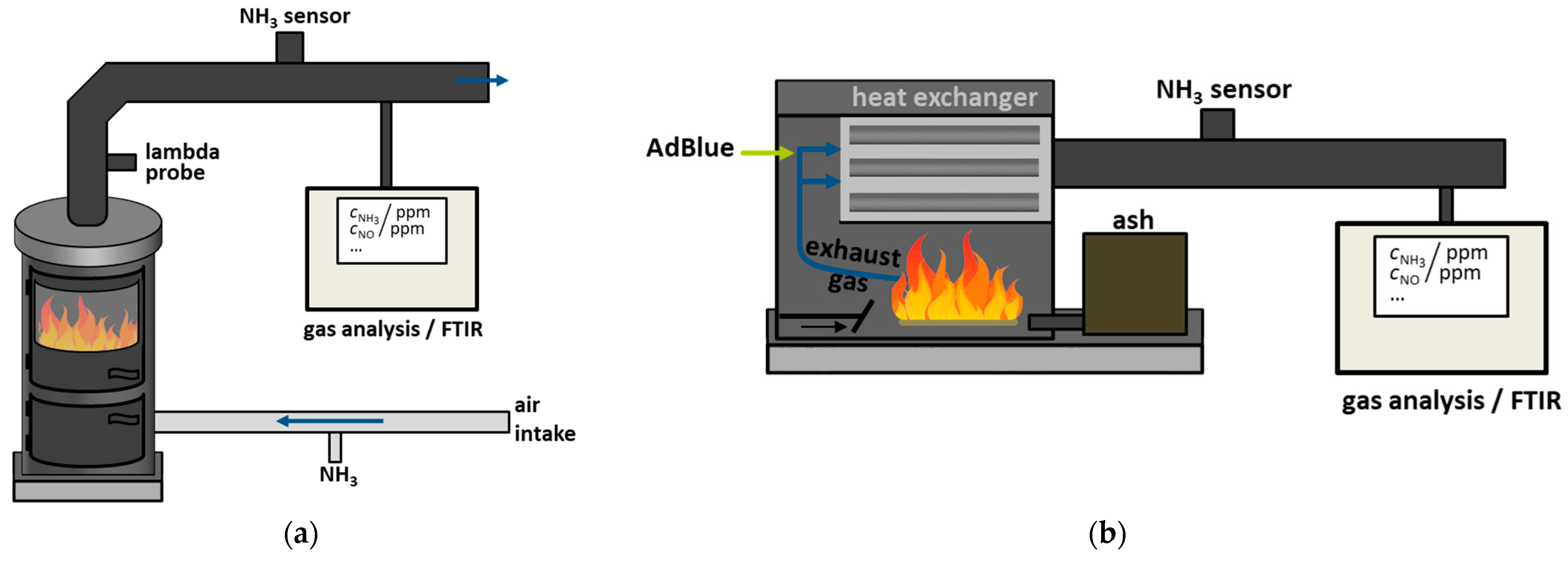
Appendix A.2
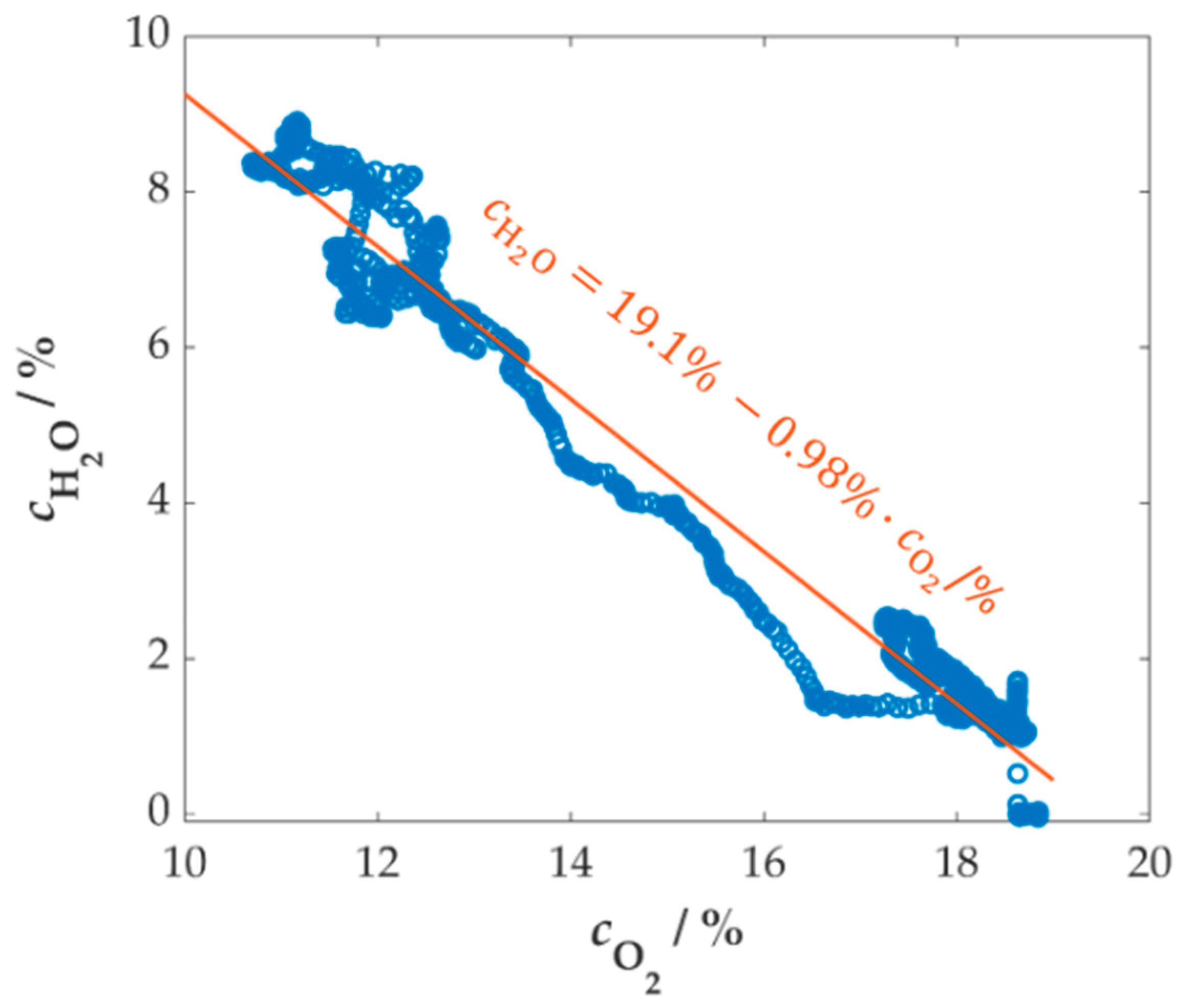

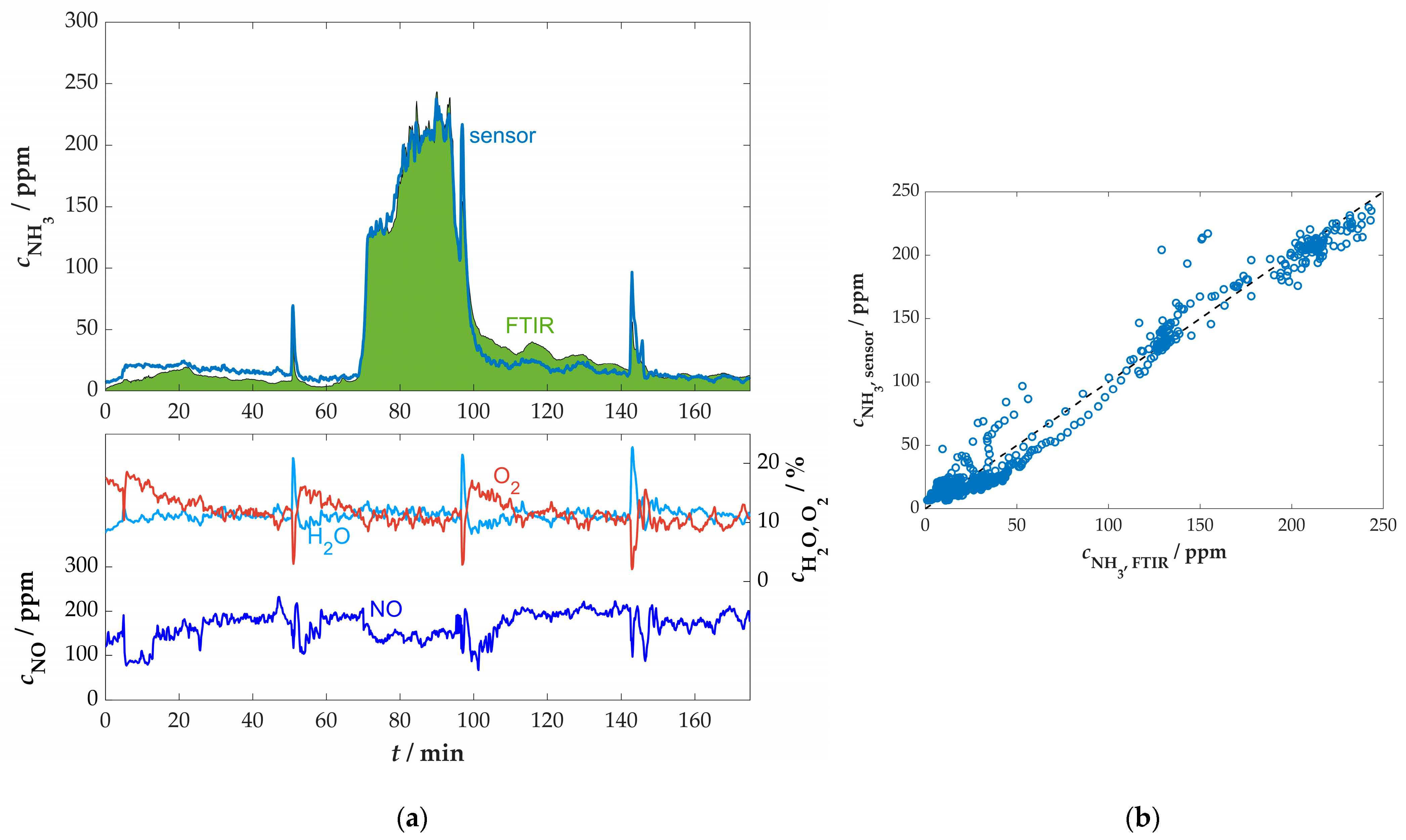
References
- König, M.; Eisinger, K.; Hartmann, I.; Müller, M. Combined removal of particulate matter and nitrogen oxides from the exhaust gas of small-scale biomass combustion. Biomass Convers. Biorefin. 2019, 9, 201–212. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef] [PubMed]
- Hupa, M.; Karlström, O.; Vainio, E. Biomass combustion technology development—It is all about chemical details. Proc. Combust. Inst. 2017, 36, 113–134. [Google Scholar] [CrossRef]
- Mladenovic, M.; Dakic, D.; Nemoda, S.; Paprika, M.; Komatina, M.; Repic, B.; Eric, A. The combustion of biomass—The impact of its types and combustion technologies on the emission of nitrogen oxide. Hem. Ind. 2016, 70, 287–298. [Google Scholar] [CrossRef]
- Bowman, C.T. Control of combustion-generated nitrogen oxide emissions: Technology driven by regulation. Symp. Int. Combust. 1992, 24, 859–878. [Google Scholar] [CrossRef]
- De Vries, W. Impacts of nitrogen emissions on ecosystems and human health: A mini review. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Theodorakidou, M.; Lambrou, G.I. Public Health Issues from the Exposure to Nitrogen Oxides: A Brief Review. ARC J. Public Health Community Med. 2017, 2, 44–56. [Google Scholar] [CrossRef]
- Ciupek, B.; Urbaniak, R.; Kinalska, D.; Nadolny, Z. Flue Gas Recirculation System for Biomass Heating Boilers—Research and Technical Applications for Reductions in Nitrogen Oxides (NOx) Emissions. Energies 2024, 17, 259. [Google Scholar] [CrossRef]
- Elkaee, S.; Phule, A.D.; Yang, J.H. Advancements in (SCR) technologies for NOx reduction: A comprehensive review of reducing agents. Process Saf. Environ. Prot. 2024, 184, 854–880. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrøm, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef]
- Sala, R.; Bielaczyc, P.; Brzezanski, M. Concept of Vaporized Urea Dosing in Selective Catalytic Reduction. Catalysts 2017, 7, 307. [Google Scholar] [CrossRef]
- Börnhorst, M.; Deutschmann, O. Advances and challenges of ammonia delivery by urea-water sprays in SCR systems. Prog. Energy Combust. Sci. 2021, 87, 100949. [Google Scholar] [CrossRef]
- Liu, W.; Gao, Y.; You, Y.; Jiang, C.; Hua, T.; Xia, B. Optimization control strategy for diesel urea-selective catalytic reduction (SCR) urea injection based on the interior point method (IP) + higher order SCR model. J. Environ. Chem. Eng. 2024, 12, 114423. [Google Scholar] [CrossRef]
- König, M.; Müller, M.; Hartmann, I. Emission reduction process for the energetic use of biogenic residues. IOP Conf. Ser. Earth Environ. Sci. 2021, 642, 012006. [Google Scholar] [CrossRef]
- Vierundvierzigste Verordnung zur Durchführung des Bundes-Immissionsschutzgesetzes: 44. BImSchV. 2019. Available online: https://www.gesetze-im-internet.de/bimschv_44/BJNR080410019.html (accessed on 22 July 2025).
- Mladenović, M.; Paprika, M.; Marinković, A. Denitrification techniques for biomass combustion. Renew. Sustain. Energy Rev. 2018, 82, 3350–3364. [Google Scholar] [CrossRef]
- Walberer, J.; Giovanny, M.; Meiller, M.; Weih, C.; Hornung, A. Development and Tests of a Combined Filter for NOx, Particulates, and SO2 Reduction. Chem. Eng. Technol. 2018, 41, 2150–2158. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Zeng, T.; von Sonntag, J.; Weller, N.; Pilz, A.; Lenz, V.; Nelles, M. CO, NOx, PCDD/F, and Total Particulate Matter Emissions from Two Small Scale Combustion Appliances Using Agricultural Biomass Type Test Fuels. Energy Fuels 2017, 31, 7540–7551. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Meng, X.; Vorobiev, N.; Schiemann, M.; Levendis, Y.A. Carbon, sulfur and nitrogen oxide emissions from combustion of pulverized raw and torrefied biomass. Fuel 2017, 188, 310–323. [Google Scholar] [CrossRef]
- Moos, R. A Brief Overview on Automotive Exhaust Gas Sensors Based on Electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2, 401–413. [Google Scholar] [CrossRef]
- Frobert, A.; Raux, S.; Creff, Y.; Jeudy, E. About Cross-Sensitivities of NOx Sensors in SCR Operation. SAE Tech. Pap. 2013, 1, 1512. [Google Scholar] [CrossRef]
- Pla, B.; Piqueras, P.; Bares, P.; Aronis, A. NOx sensor cross sensitivity model and simultaneous prediction of NOx and NH3 slip from automotive catalytic converters under real driving conditions. Int. J. Engine Res. 2021, 22, 3209–3218. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Priedniece, V.; Kirsanovs, V.; Freimanis, R.; Veidenbergs, I.; Blumberga, D. Emissions and Efficiency Limits of Small-Scale Biomass Heating Systems: Regulations, Standards, and Ecolabels. Environ. Clim. Technol. 2022, 26, 1032–1043. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent Advances in Ammonia Gas Sensors Based on Carbon Nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Qian, F.; Yin, X.; Zhang, J.; Luo, C.; Li, J.; Xu, X.; Wang, C. A review of high-temperature solid-state ammonia sensors. J. Mater. Sci. 2023, 58, 10600–10634. [Google Scholar] [CrossRef]
- Donker, N.; Schönauer-Kamin, D.; Moos, R. Mixed-Potential Ammonia Sensor Based on a Dense Yttria-Stabilized Zirconia Film Manufactured at Room Temperature by Powder Aerosol Deposition. Sensors 2024, 24, 811. [Google Scholar] [CrossRef]
- Da Wang, Y.; Yao, S.; Shost, M.; Yoo, J.-H.; Cabush, D.; Racine, D.; Cloudt, R.; Willems, F. Ammonia Sensor for Closed-Loop SCR Control. SAE Int. J. Passeng. Cars—Electron. Electr. Syst. 2009, 1, 323–333. [Google Scholar] [CrossRef]
- Riegel, J.; Neumann, H.; Wiedenmann, H.-M. Exhaust gas sensors for automotive emission control. Solid State Ion. 2002, 152–153, 783–800. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Zhang, C.; Shi, S.Q.; Zhang, H. High-Temperature Gas Sensors for Harsh Environment Applications: A Review. Clean-Soil Air Water 2019, 47, 1800491. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Shynybekov, Y.; Soltabayev, B.; Yergaliuly, G.; Mentbayeva, A. The Cross-Sensitivity of Chemiresistive Gas Sensors: Nature, Methods, and Peculiarities: A Systematic Review. ACS Sens. 2024, 9, 6358–6371. [Google Scholar] [CrossRef] [PubMed]
- Docquier, N.; Candel, S. Combustion control and sensors: A review. Prog. Energy Combust. Sci. 2002, 28, 107–150. [Google Scholar] [CrossRef]
- Shooshtari, M. Ammonia gas sensors based on multi-wall carbon nanofiber field effect transistors by using gate modulation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135563. [Google Scholar] [CrossRef]
- Szabo, N.; Lee, C.; Trimboli, J.; Figueroa, O.; Ramamoorthy, R.; Midlam-Mohler, S.; Soliman, A.; Verweij, H.; Dutta, P.; Akbar, S. Ceramic-based chemical sensors, probes and field-tests in automobile engines. J. Mater. Sci. 2003, 38, 4239–4245. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef]
- Wöhrl, T.; Kita, J.; Moos, R.; Hagen, G. Capacitive, Highly Selective Zeolite-Based Ammonia Sensor for Flue Gas Applications. Chemosensors 2023, 11, 413. [Google Scholar] [CrossRef]
- Wöhrl, T.; Moos, R.; Hagen, G. Analyzing the cross-sensitivities of a zeolite-based ammonia sensor for SCR systems for application in the flue gas of biogenic waste combustion. Sens. Actuators B Chem. 2025, 436, 137727. [Google Scholar] [CrossRef]
- Simon, U.; Sanders, D.; Jockel, J.; Heppel, C.; Brinz, T. Design strategies for multielectrode arrays applicable for high-throughput impedance spectroscopy on novel gas sensor materials. J. Comb. Chem. 2002, 4, 511–515. [Google Scholar] [CrossRef]
- Simons, T.; Simon, U. Zeolite H-ZSM-5: A Microporous Proton Conductor for the in situ Monitoring of DeNOx -SCR. MRS Proc. 2011, 1330, 303. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Ozin, G.A.; Kuperman, A.; Stein, A. Advanced Zeolite, Materials Science. Angew. Chem. Int. Ed. Engl. 1989, 28, 359–376. [Google Scholar] [CrossRef]
- Brüggemann, T.C.; Keil, F.J. Theoretical Investigation of the Mechanism of the Selective Catalytic Reduction of Nitric Oxide with Ammonia on H-Form Zeolites. J. Phys. Chem. C 2008, 112, 17378–17387. [Google Scholar] [CrossRef]
- Devadas, M.; Kröcher, O.; Wokaun, A. Catalytic investigation of Fe-ZSM5 in the selective catalytic reduction of NOx with NH3. React. Kinet. Catal. Lett. 2005, 86, 347–354. [Google Scholar] [CrossRef]
- Ma, A.-Z.; Grünert, W. Selective catalytic reduction of NO by ammonia over Fe-ZSM-5 catalysts. Chem. Commun. 1999, 1, 71–72. [Google Scholar] [CrossRef]
- Pullano, S.A.; Falcone, F.; Critello, D.C.; Bianco, M.G.; Menniti, M.; Fiorillo, A.S. An Affordable Fabrication of a Zeolite-Based Capacitor for Gas Sensing. Sensors 2020, 20, 2143. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Dutta, P.K. Exploitation of unique properties of zeolites in the development of gas sensors. Sensors 2012, 12, 5170–5194. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. Effect of Structural and Preparation Parameters on the Activity and Hydrothermal Stability of Metal-Exchanged ZSM-5 in the Selective Catalytic Reduction of NO by NH3. Ind. Eng. Chem. Res. 2011, 50, 4308–4319. [Google Scholar] [CrossRef]
- Wöhrl, T.; Herrmann, J.; Kita, J.; Moos, R.; Hagen, G. Methods to investigate the temperature distribution of heated ceramic gas sensors for high-temperature applications. J. Sens. Sens. Syst. 2023, 12, 205–214. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Barsukov, Y.; Macdonald, J.R. Electrochemical Impedance Spectroscopy. Charact. Mater. 2012, 2, 898–913. [Google Scholar] [CrossRef]
- Hagen, G.; Herrmann, J.; Zhang, X.; Kohler, H.; Hartmann, I.; Moos, R. Application of a Robust Thermoelectric Gas Sensor in Firewood Combustion Exhausts. Sensors 2023, 23, 2930. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Kidner, N.J.; Homrighaus, Z.J.; Mason, T.O.; Garboczi, E.J. Modeling interdigital electrode structures for the dielectric characterization of electroceramic thin films. Thin Solid Film. 2006, 496, 539–545. [Google Scholar] [CrossRef]
- Mamishev, A.V.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–845. [Google Scholar] [CrossRef]
- Minissale, M.; Faure, J.-B.; Dunand, A.; Angot, T.; de Temmerman, G.; Bisson, R. Sticking Probability of Ammonia Molecules on Tungsten and 316L Stainless Steel Surfaces. J. Phys. Chem. C 2020, 124, 17566–17577. [Google Scholar] [CrossRef]
- Vaittinen, O.; Metsälä, M.; Persijn, S.; Vainio, M.; Halonen, L. Adsorption of ammonia on treated stainless steel and polymer surfaces. Appl. Phys. B 2014, 115, 185–196. [Google Scholar] [CrossRef]
- Hayhurst, D.T. Gas adsorption by some natural zeolites. Chem. Eng. Commun. 1980, 4, 729–735. [Google Scholar] [CrossRef]
- Hunger, B.; Heuchel, M.; Matysik, S.; Beck, K.; Einicke, W.D. Adsorption of water on ZSM-5 zeolites. Thermochim. Acta 1995, 269–270, 599–611. [Google Scholar] [CrossRef]
- Pérez-Page, M.; Makel, J.; Guan, K.; Zhang, S.; Tringe, J.; Castro, R.H.; Stroeve, P. Gas adsorption properties of ZSM-5 zeolites heated to extreme temperatures. Ceram. Int. 2016, 42, 15423–15431. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Molecular simulations of zeolites: Adsorption, diffusion, and shape selectivity. Chem. Rev. 2008, 108, 4125–4184. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Characterization of Fe-ZSM-5 Catalyst for Selective Catalytic Reduction of Nitric Oxide by Ammonia. J. Catal. 2000, 194, 80–90. [Google Scholar] [CrossRef]
- Shi, X.; He, H.; Xie, L. The effect of Fe species distribution and acidity of Fe-ZSM-5 on the hydrothermal stability and SO2 and hydrocarbons durability in NH3-SCR reaction. Chin. J. Catal. 2015, 36, 649–656. [Google Scholar] [CrossRef]
- Park, G.G.; Jeong Chae, H.; Nam, I.-S.; Woo Choung, J.; Ho Choi, K. Deactivation of mordenite-type zeolite catalyst by HCl for the reduction of NOx with NH3. Microporous Mesoporous Mater. 2001, 48, 337–343. [Google Scholar] [CrossRef]
- Spivey, J.J.; Butt, J.B. Literature review: Deactivation of catalysts in the oxidation of volatile organic compounds. Catal. Today 1992, 11, 465–500. [Google Scholar] [CrossRef]
- Antenucci, D.; Delaude, L.; Fransolet, A.-M.; Laszlo, P. Acidic degradation of zeolite catalysts in the course of aromatic chlorination using sulfuryl chloride. J. Catal. 1992, 135, 92–98. [Google Scholar] [CrossRef]
- de Lucas, A.; Canizares, P.; Durán, A.; Carrero, A. Dealumination of HZSM-5 zeolites: Effect of steaming on acidity and aromatization activity. Appl. Catal. A Gen. 1997, 154, 221–240. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion, Chemistry, and Carbon Neutrality. Chem. Rev. 2023, 123, 5139–5219. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, A.; Miklos, A.; Hess, P. Effects of adsorption-desorption processes on the response time and accuracy of photoacoustic detection of ammonia. Appl. Opt. 2001, 40, 2571–2578. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wöhrl, T.; König, M.; Moos, R.; Hagen, G. Monitoring of Ammonia in Biomass Combustion Flue Gas Using a Zeolite-Based Capacitive Sensor. Sensors 2025, 25, 5519. https://doi.org/10.3390/s25175519
Wöhrl T, König M, Moos R, Hagen G. Monitoring of Ammonia in Biomass Combustion Flue Gas Using a Zeolite-Based Capacitive Sensor. Sensors. 2025; 25(17):5519. https://doi.org/10.3390/s25175519
Chicago/Turabian StyleWöhrl, Thomas, Mario König, Ralf Moos, and Gunter Hagen. 2025. "Monitoring of Ammonia in Biomass Combustion Flue Gas Using a Zeolite-Based Capacitive Sensor" Sensors 25, no. 17: 5519. https://doi.org/10.3390/s25175519
APA StyleWöhrl, T., König, M., Moos, R., & Hagen, G. (2025). Monitoring of Ammonia in Biomass Combustion Flue Gas Using a Zeolite-Based Capacitive Sensor. Sensors, 25(17), 5519. https://doi.org/10.3390/s25175519





