Advancements and Applications of Lateral Flow Assays (LFAs): A Comprehensive Review
Abstract
1. Introduction
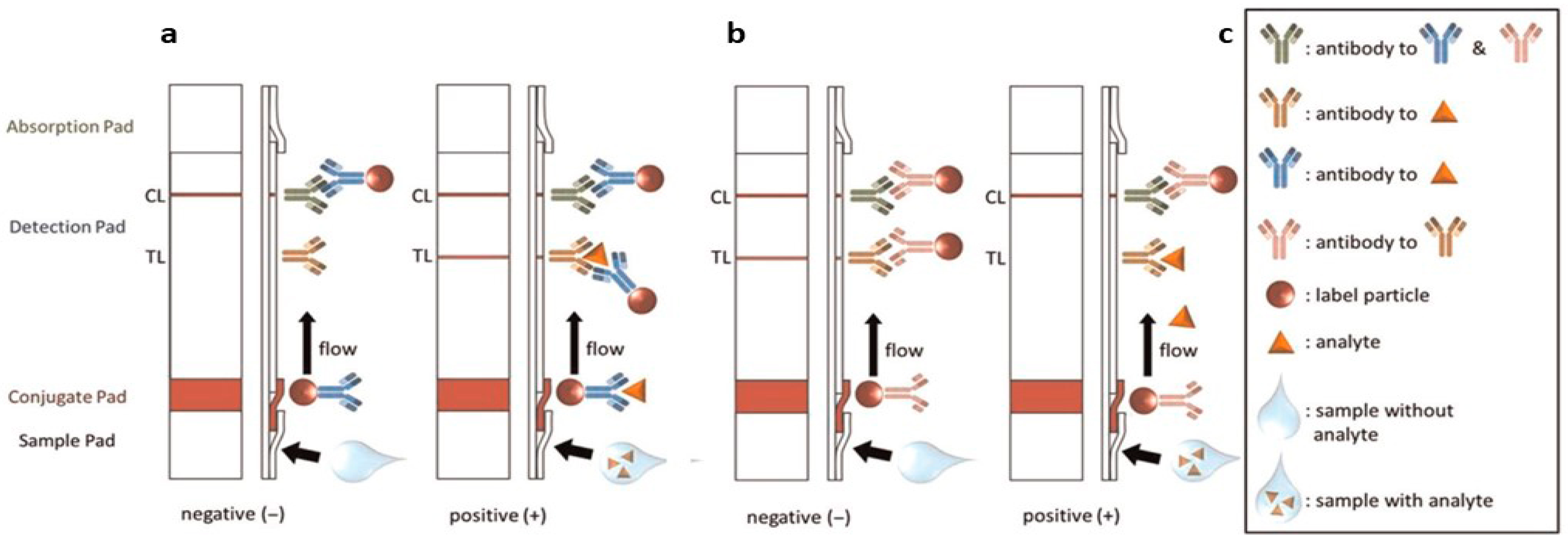
2. Principles of Lateral Flow Assays
3. Recent Advancements in LFA Technology
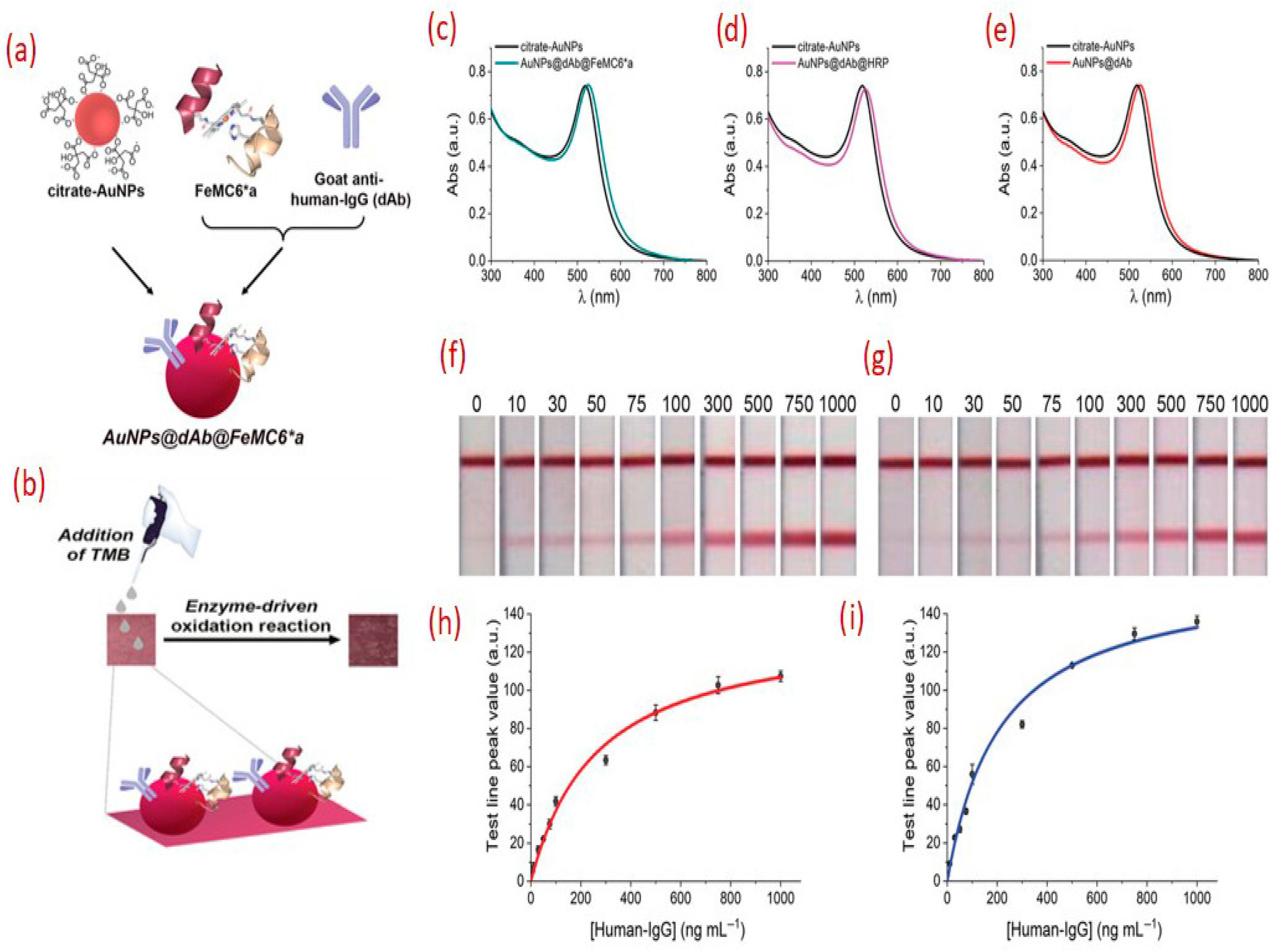
3.1. Sensitivity Enhancement Strategies in Lateral Flow Assays (LFAs)
3.2. Smartphone and Digital Reader Integration in LFA Technology
3.3. Multiplexed LFAs
3.4. Lab-on-a-Chip LFAs
3.5. Aptamer-Based LFAs
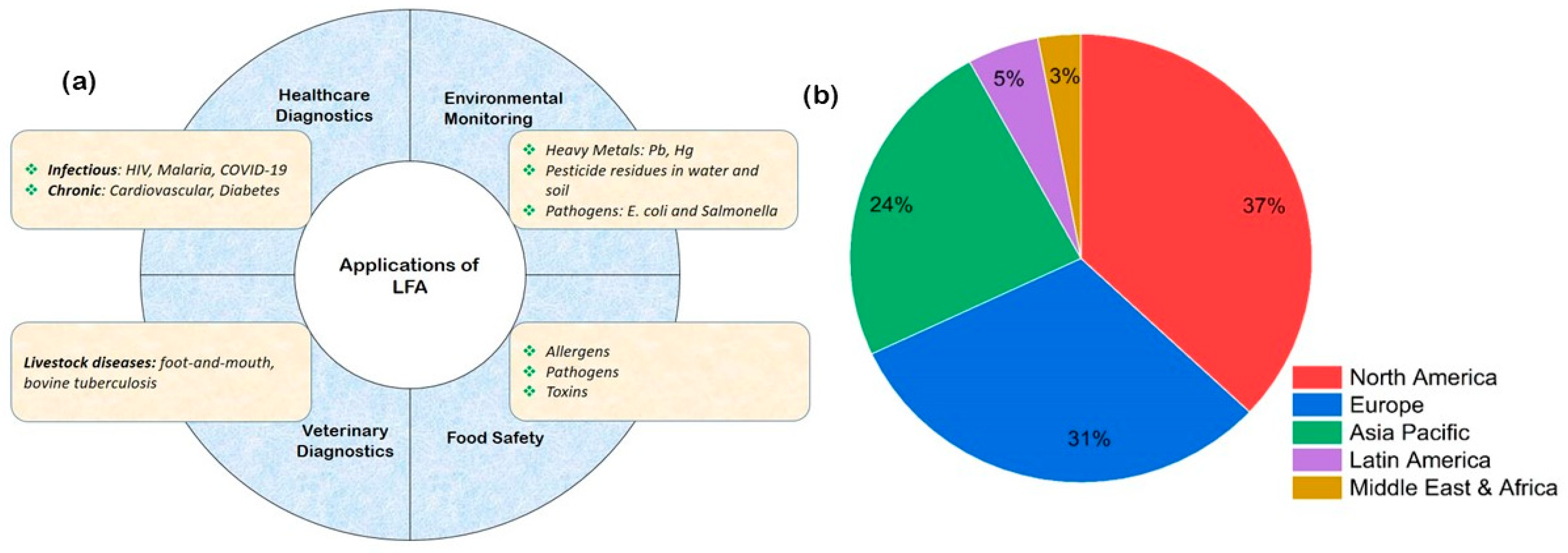
4. Applications of Lateral Flow Assays
4.1. Clinical Diagnostics—Rapid, Reliable, and Revolutionizing Patient Care
4.2. Food Safety—Safeguarding Global Supply Chains
4.3. Drug Testing—Rapid On-Site Screening for Public Safety
4.4. Veterinary Diagnostics—Enhancing Animal Health and Livestock Management
5. Future Prospects
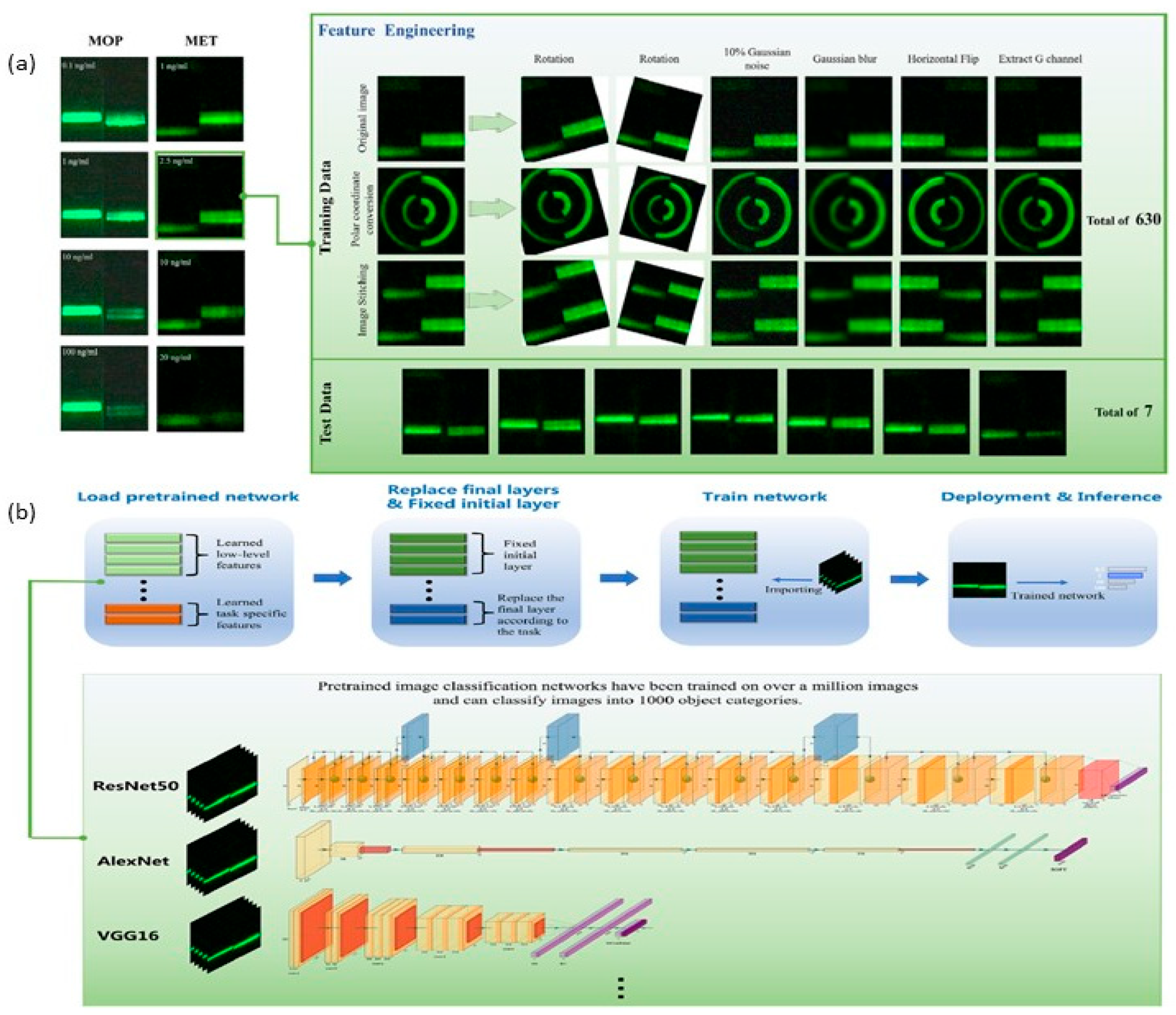
5.1. Integration of Artificial Intelligence
5.2. Development of Environmentally Friendly Materials for LFAs
5.3. Application of LFAs in Emerging Fields—Liquid Biopsy and Personalized Medicine
5.4. Expansion of LFAs for Bacteria and Bioterrorism Threats
5.5. Improved Manufacturing Processes for Cost-Effective Production at Scale
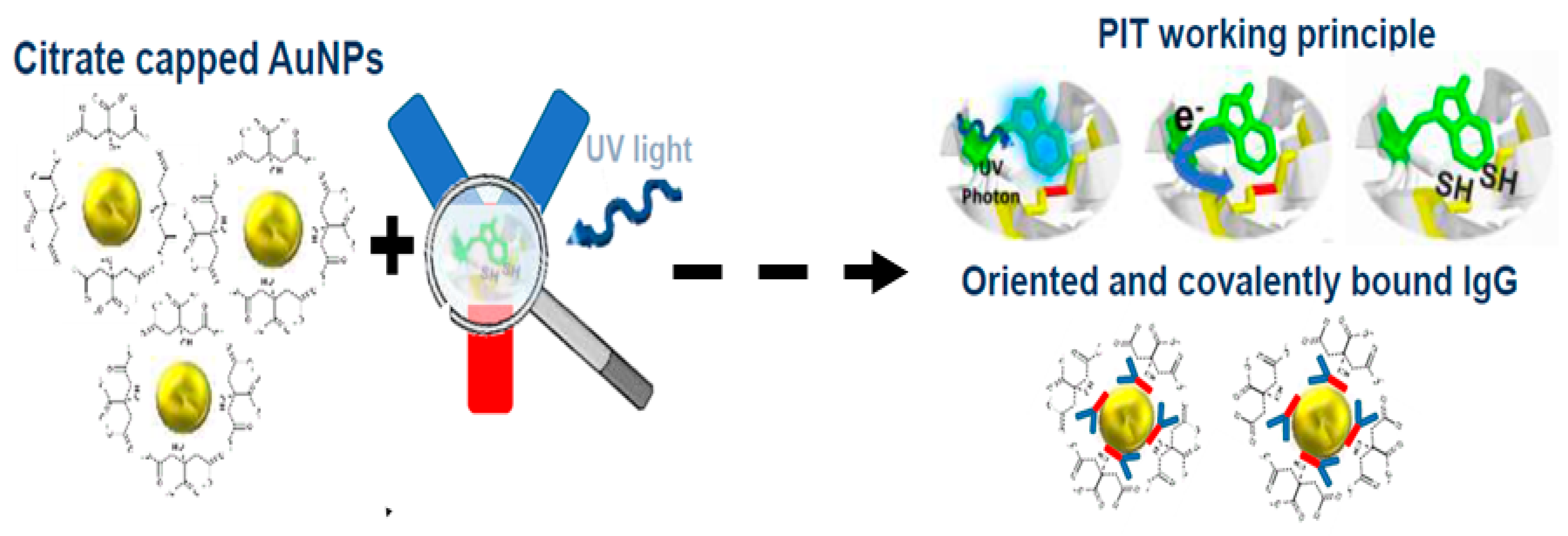
5.6. Integration of UV-Irradiation Functionalization
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plotz, C.M.; Singer, J.M. The Latex Fixation Test. I. Application to the Serologic Diagnosis of Rheumatoid Arthritis. Am. J. Med. 1956, 21, 888–892. [Google Scholar]
- Berson, S.A.; Yalow, R.S. Quantitative Aspects of the Reaction between Insulin and Insulin-Binding Antibody. J. Clin. Investig. 1959, 38, 1996–2016. [Google Scholar] [CrossRef]
- O’Farrell, B. Evolution in Lateral Flow–Based Immunoassay Systems. Lateral Flow Immunoass. 2008, 1–33. [Google Scholar] [CrossRef]
- Lateral Flow Assay Market Size to Hit USD 24.39 Bn by 2034. Available online: https://www.precedenceresearch.com/lateral-flow-assay-market (accessed on 13 March 2025).
- Market Data Forecast. Lateral Flow Assay Market Size & Share Report, 2033. Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/lateral-flow-assay-market (accessed on 18 August 2025).
- Strengthening Diagnostics Capacity. Available online: https://www.who.int/activities/strengthening-diagnostics-capacity (accessed on 27 October 2024).
- Wang, X.; Xue, C.-H.; Yang, D.; Jia, S.-T.; Ding, Y.-R.; Lei, L.; Gao, K.-Y.; Jia, T.-T. Modification of a nitrocellulose membrane with nanofibers for sensitivity enhancement in lateral flow test strips. RSC Adv. 2021, 11, 26493–26501. [Google Scholar] [CrossRef]
- Dey, M.K.; Iftesum, M.; Devireddy, R.; Gartia, M.R. New Technologies and Reagents in Lateral Flow Assay (LFA) Designs for Enhancing Accuracy and Sensitivity. Anal. Methods 2023, 15, 4351–4376. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Álvarez-Diduk, R.; Parolo, C.; Piper, A.; Merkoçi, A. Toward Next Generation Lateral Flow Assays: Integration of Nanomaterials. Chem. Rev. 2022, 122, 14881–14910. [Google Scholar] [CrossRef]
- Fu, H.E.; Koo, T.M.; Kim, M.S.; Ko, M.J.; Park, B.C.; Oh, K.; Cho, Y.; Jung, J.-W.; Kim, S.; Jang, W.S.; et al. Magnetic-Fluorescent Nanocluster Lateral Flow Assay for Rotavirus Detection. ACS Appl. Nano Mater. 2023, 6, 5789–5798. [Google Scholar] [CrossRef]
- Ye, H.; Xia, X. Enhancing the sensitivity of colorimetric lateral flow assay (CLFA) through signal amplification techniques. J. Mater. Chem. B 2018, 6, 7102–7111. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Liu, G. Signal Amplification Strategies for Paper-Based Analytical Devices. Biosens. Bioelectron. 2019, 136, 60–75. [Google Scholar] [CrossRef]
- Díaz-González, M.; De La Escosura-Muñiz, A. Strip modification and alternative architectures for signal amplification in nanoparticle-based lateral flow assays. Anal. Bioanal. Chem. 2021, 413, 4111–4117. [Google Scholar] [CrossRef]
- Jie, H.; Wang, Y.; Zhao, M.; Wang, X.; Wang, Z.; Zeng, L.; Cao, X.; Xu, T.; Xia, F.; Liu, Q. Automatic Ultrasensitive Lateral Flow Immunoassay Based on a Color-Enhanced Signal Amplification Strategy. Biosens. Bioelectron. 2024, 256, 116262. [Google Scholar] [CrossRef]
- Jin, B.; Yang, Y.; He, R.; Park, Y.I.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B Chem. 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Althomali, R.H.; Elov, B.B.; Hussn, M.; Sapaev, I.B.; Obaid, R.F.; Jabbar, H.S.; Romero-Parra, R.M.; Zearah, S.A.; Albahash, Z.F. Biomedical applications of smartphone-based lateral flow detection systems as a diagnosis tool. Microchem. J. 2023, 193, 109159. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- An Introduction to the Lateral Flow Test: Strengths, Limitations and Applications. Diagnostics from Technology Networks. Available online: http://www.technologynetworks.com/diagnostics/articles/an-introduction-to-the-lateral-flow-test-strengths-limitations-and-applications-370382 (accessed on 26 March 2025).
- Jung, Y.; Heo, Y.; Lee, J.J.; Deering, A.; Bae, E. Smartphone-based lateral flow imaging system for detection of food-borne bacteria E.coli O157:H7. J. Microbiol. Methods 2020, 168, 105800. [Google Scholar] [CrossRef]
- Wang, W.; Srivastava, S.; Garg, A.; Xiao, C.; Hawks, S.; Pan, J.; Duggal, N.; Isaacman-VanWertz, G.; Zhou, W.; Marr, L.C.; et al. Digital Surface-Enhanced Raman Spectroscopy–Lateral Flow Test Dipstick: Ultrasensitive, Rapid Virus Quantification in Environmental Dust. Environ. Sci. Technol. 2024, 58, 4926–4936. [Google Scholar] [CrossRef]
- Ruiz-Vega, G.; Kitsara, M.; Pellitero, M.A.; Baldrich, E.; del Campo, F.J. Electrochemical Lateral Flow Devices: Towards Rapid Immunomagnetic Assays. ChemElectroChem 2017, 4, 880–889. [Google Scholar] [CrossRef]
- Mak, W.C.; Beni, V.; Turner, A.P.F. Lateral-flow technology: From visual to instrumental. TrAC Trends Anal. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Xu, H.; Peng, Y.; Yao, L.; Chen, Z.; Yang, J.; Adeloju, S.; Chen, W. One-Pot Rpa-Crispr/Cas12a Integrated Dual-Mode Electrochemical Lateral Flow Strip for Ultrasensitive and Precise Detection of Salmonella. Biosens. Bioelectron. 2025, 285, 117529. [Google Scholar] [CrossRef]
- Sanattalab, E.; Ayni, E.; Kaya, K.; Yildirim-Tirgil, N. Applications of Magnetic Nanocomposites and Their Role in Advancing Lateral Flow Assays. ChemistrySelect 2025, 10, e202405827. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, X.; Zhu, J.; Zhang, Y.; Zhou, R.; Wang, T.; Katona, J.; Zhang, D.; Zou, X. Magnetic nanoprobe-enabled lateral flow assays in the applications of food safety and in vitro diagnostic. Coord. Chem. Rev. 2025, 534, 216588. [Google Scholar] [CrossRef]
- Atta, S.; Thorsen, T.; Zhao, Y.; Sanchez, S.; Hill, H.J.; Berner, V.K.; Gates-Hollingsworth, M.A.; Devadhasan, J.P.; Summers, A.J.; Gu, J.; et al. Magneto-Plasmonics-Enhanced Colorimetric Lateral Flow Immunoassay Using Magnetic-Gold Nanostars. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Parolo, C.; De La Escosura-Muñiz, A.; Merkoçi, A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef]
- Qiao, W.; He, B.; Yang, J.; Ren, W.; Zhao, R.; Zhang, Y.; Bai, C.; Suo, Z.; Xu, Y.; Wei, M.; et al. Pt@AuNF nanozyme and horseradish peroxidase-based lateral flow immunoassay dual enzymes signal amplification strategy for sensitive detection of zearalenone. Int. J. Biol. Macromol. 2024, 254, 127746. [Google Scholar] [CrossRef]
- Bishop, J.D.; Hsieh, H.V.; Gasperino, D.J.; Weigl, B.H. Sensitivity enhancement in lateral flow assays: A systems perspective. Lab Chip 2019, 19, 2486–2499. [Google Scholar] [CrossRef]
- Chan, C.P.Y.; Sum, K.W.; Cheung, K.Y.; Glatz, J.F.C.; Sanderson, J.E.; Hempel, A.; Lehmann, M.; Renneberg, I.; Renneberg, R. Development of a quantitative lateral-flow assay for rapid detection of fatty acid-binding protein. J. Immunol. Methods 2003, 279, 91–100. [Google Scholar] [CrossRef]
- Hnasko, R.; Lin, A.V.; McGarvey, J.A. Rapid Detection of Staphylococcal Enterotoxin-B by Lateral Flow Assay. Monoclon. Antibodies Immunodiagn. Immunother. 2019, 38, 209–212. [Google Scholar] [CrossRef]
- Saxena, K.; Toley, B. Novel Oxidative Coupling-Based Chromogenic Substrates for Horseradish Peroxidase-Enhanced Lateral Flow Immunoassays: A Highly Sensitive and Economical Alternative to Conventional Substrates. ChemRxiv 2023, 1, 444. [Google Scholar] [CrossRef]
- Ye, L.; Xu, X.; Qu, A.; Liu, L.; Xu, C.; Kuang, H. Quantitative assessment of the breast cancer marker HER2 using a gold nanoparticle-based lateral flow immunoassay. Nano Res. 2024, 17, 5452–5460. [Google Scholar] [CrossRef]
- Renzi, E.; Piper, A.; Nastri, F.; Merkoçi, A.; Lombardi, A. An Artificial Miniaturized Peroxidase for Signal Amplification in Lateral Flow Immunoassays. Small 2023, 19, 2207949. [Google Scholar] [CrossRef]
- Omidfar, K.; Riahi, F.; Kashanian, S. Lateral Flow Assay: A Summary of Recent Progress for Improving Assay Performance. Biosensors 2023, 13, 837. [Google Scholar] [CrossRef]
- Park, J. Lateral Flow Immunoassay Reader Technologies for Quantitative Point-of-Care Testing. Sensors 2022, 22, 7398. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, Y.; Liang, S.; Zhang, J. Detection of Hg(II) in adsorption experiment by a lateral flow biosensor based on streptavidin-biotinylated DNA probes modified gold nanoparticles and smartphone reader. Environ. Pollut. 2020, 266, 115389. [Google Scholar] [CrossRef]
- Yu, L.; Shi, Z.; Fang, C.; Zhang, Y.; Liu, Y.; Li, C. Disposable lateral flow-through strip for smartphone-camera to quantitatively detect alkaline phosphatase activity in milk. Biosens. Bioelectron. 2015, 69, 307–315. [Google Scholar] [CrossRef]
- Lee, S.; O’Dell, D.; Hohenstein, J.; Colt, S.; Mehta, S.; Erickson, D. NutriPhone: A mobile platform for low-cost point-of-care quantification of vitamin B12 concentrations. Sci. Rep. 2016, 6, 28237. [Google Scholar] [CrossRef]
- RDT Digital Companion Apps. FIND. Available online: https://www.finddx.org/what-we-do/cross-cutting-workstreams/digital-health/rdt-digital-companion-apps/ (accessed on 18 August 2025).
- Lee, S.; Kim, S.; Yoon, D.S.; Park, J.S.; Woo, H.; Lee, D.; Cho, S.-Y.; Park, C.; Yoo, Y.K.; Lee, K.-B.; et al. Sample-to-answer platform for the clinical evaluation of COVID-19 using a deep learning-assisted smartphone-based assay. Nat. Commun. 2023, 14, 2361. [Google Scholar] [CrossRef]
- Target Product Profile for Readers of Rapid Diagnostic Tests. Available online: https://www.who.int/publications/i/item/9789240067172 (accessed on 15 April 2025).
- Sofia® Influenza A+B FIA. Available online: https://www.quidelortho.com/in/en/products/sofia-platform/sofia-influenza-a-b-fia (accessed on 15 April 2025).
- Ogunfowokan, O.; Ogunfowokan, B.A.; Nwajei, A.I. Sensitivity and specificity of malaria rapid diagnostic test (mRDT CareStatTM) compared with microscopy amongst under five children attending a primary care clinic in southern Nigeria. Afr. J. Prim. Health Care Fam. Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Cecere, G.; Corrocher, N.; Battaglia, R.D. Innovation and competition in the smartphone industry: Is there a dominant design? Telecommun. Policy 2015, 39, 162–175. [Google Scholar] [CrossRef]
- Petersen, K.Y. Ambient Adaptive Lighting. Arch. Res. Finl. 2022, 3, 184–199. [Google Scholar] [CrossRef]
- Hay Burgess, D.C.; Wasserman, J.; Dahl, C.A. Global health diagnostics. Nature 2006, 444 (Suppl. S1), 1–2. [Google Scholar] [CrossRef]
- Olowoyo, K.S.; Esan, D.T.; Adeyanju, B.T.; Olawade, D.B.; Oyinloye, B.E.; Olowoyo, P. Telemedicine as a tool to prevent multi-drug resistant tuberculosis in poor resource settings: Lessons from Nigeria. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 35, 100423. [Google Scholar] [CrossRef]
- Singh, P.R.; Singh, V.K.; Yadav, R.; Chaurasia, S.N. 6G networks for artificial intelligence-enabled smart cities applications: A scoping review. Telemat. Inform. Rep. 2023, 9, 100044. [Google Scholar] [CrossRef]
- Lateral Flow Assays Market Size | Industry Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/lateral-flow-assay-market (accessed on 18 August 2025).
- Lamprou, E.; Kalligosfyri, P.M.; Kalogianni, D.P. Beyond Traditional Lateral Flow Assays: Enhancing Performance Through Multianalytical Strategies. Biosensors 2025, 15, 68. [Google Scholar] [CrossRef]
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Multiplexed detection of biomarkers in lateral-flow immunoassays. Analyst 2020, 145, 2828–2840. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Zhao, Z.; Njumbe Ediage, E.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Liu, L.; Kuang, H.; Li, A.; Xu, C. Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv. 2016, 6, 7798–7805. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Wang, M.-Z.; Chen, Z.-L.; Fang, J.-H.; Fang, M.-M.; Liu, J.; Yu, X.-P. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal. Bioanal. Chem. 2009, 395, 2591–2599. [Google Scholar] [CrossRef]
- Nörz, D.; Hoffmann, A.; Aepfelbacher, M.; Pfefferle, S.; Lütgehetmann, M. Clinical Evaluation of a Fully Automated, Laboratory-Developed Multiplex RT-PCR Assay Integrating Dual-Target SARS-CoV-2 and Influenza A/B Detection on a High-Throughput Platform. J. Med. Microbiol. 2021, 70, 001295. [Google Scholar] [CrossRef]
- Liu, B.; Gong, H.; Wang, Y.; Zhang, X.; Li, P.; Qiu, Y.; Wang, L.; Hua, X.; Guo, Y.; Wang, M.; et al. A Gold Immunochromatographic Assay for Simultaneous Detection of Parathion and Triazophos in Agricultural Products. Anal. Methods 2018, 104, 422–428. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Shi, Y.-B.; Zou, Q.; Sun, J.-H.; Chen, Z.-F.; Wang, H.; Li, S.-Q.; Yan, Y.-X. Development of a rapid and simultaneous immunochromatographic assay for the determination of zearalenone and fumonisin B1 in corn, wheat and feedstuff samples. Food Control. 2013, 31, 180–188. [Google Scholar] [CrossRef]
- Xing, C.; Liu, L.; Song, S.; Feng, M.; Kuang, H.; Xu, C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453. [Google Scholar] [CrossRef]
- Enhancing a SARS-CoV-2 Nucleocapsid Antigen Test Sensitivity with Cost Efficient Strategy Through a Cotton Intermembrane Insertion | Scientific Reports. Available online: https://www.nature.com/articles/s41598-023-31641-5 (accessed on 18 August 2025).
- Taranova, N.A.; Byzova, N.A.; Zaiko, V.V.; Starovoitova, T.A.; Vengerov, Y.Y.; Zherdev, A.V.; Dzantiev, B.B. Integration of lateral flow and microarray technologies for multiplex immunoassay: Application to the determination of drugs of abuse. Microchim. Acta 2013, 180, 1165–1172. [Google Scholar] [CrossRef]
- Ming, K.; Kim, J.; Biondi, M.J.; Syed, A.; Chen, K.; Lam, A.; Ostrowski, M.; Rebbapragada, A.; Feld, J.J.; Chan, W.C. Integrated Quantum Dot Barcode Smartphone Optical Device for Wireless Multiplexed Diagnosis of Infected Patients. ACS Nano 2025, 9, 3060–3074. [Google Scholar] [CrossRef]
- Bristow, C.C.; Severe, L.; Pape, J.W.; Javanbakht, M.; Lee, S.-J.; Comulada, W.S.; Klausner, J.D. Dual rapid lateral flow immunoassay fingerstick wholeblood testing for syphilis and HIV infections is acceptable and accurate, Port-au-Prince, Haiti. BMC Infect. Dis. 2016, 16, 302. [Google Scholar] [CrossRef]
- Sun, H.; Xia, L.; Li, J.; Zhang, Y.; Zhang, G.; Huang, P.; Wang, X.; Cui, Y.; Fang, T.; Fan, P.; et al. A novel bispecific antibody targeting two overlapping epitopes in RBD improves neutralizing potency and breadth against SARS-CoV-2. Emerg. Microbes Infect. 2024, 13, 2373307. [Google Scholar] [CrossRef]
- Adams, L.J.; VanBlargan, L.A.; Liu, Z.; Gilchuk, P.; Zhao, H.; Chen, R.E.; Raju, S.; Chong, Z.; Whitener, B.M.; Shrihari, S.; et al. A broadly reactive antibody targeting the N-terminal domain of SARS-CoV-2 spike confers Fc-mediated protection. Cell Rep. Med. 2023, 4, 101305. [Google Scholar] [CrossRef]
- Mao, R.; Ge, G.; Wang, Z.; Hao, R.; Zhang, G.; Yang, Z.; Lin, B.; Ma, Y.; Liu, H.; Du, Y. A multiplex microfluidic loop-mediated isothermal amplification array for detection of malaria-related parasites and vectors. Acta Trop. 2018, 178, 86–92. [Google Scholar] [CrossRef]
- Shirley, M. FebriDx®: A Rapid Diagnostic Test for Differentiating Bacterial and Viral Aetiologies in Acute Respiratory Infections. Mol. Diagn. Ther. 2019, 23, 803–809. [Google Scholar] [CrossRef]
- 5-in-1 Combo CoV, Flu A&B, RSV, ADV Test—BTNX Inc. Available online: https://www.btnx.com/product/5-in-1-combo-cov-flu-a-b-rsv-adv-test-10-test-kit (accessed on 14 August 2025).
- Sofia® 2 Flu + SARS Antigen FIA. Available online: https://www.quidelortho.com/global/en/products/sofia-platform/sofia-2-flu-sars-antigen-fia (accessed on 14 August 2025).
- Bioline HIV/Syphilis Duo | Abbott Point of Care. Available online: https://www.globalpointofcare.abbott/za/en/product-details/bioline-hiv-syphilis-duo.html (accessed on 18 August 2025).
- Fraga, M.; Vilarino, N.; Louzao, M.C.; Rodriguez, P.; Campbell, K.; Elliott, C.T.; Botana, L.M. Multidetection of Paralytic, Diarrheic, and Amnesic Shellfish Toxins by an Inhibition Immunoassay Using a Microsphere-Flow Cytometry System. Anal. Chem. 2013, 85, 7794–7802. [Google Scholar] [CrossRef]
- Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Dual lateral flow test for simple and rapid simultaneous immunodetection of bisphenol A and dimethyl phthalate, two priority plastic related environmental contaminants. Environ. Pollut. 2024, 363, 125171. [Google Scholar] [CrossRef]
- Hassan, Y.M.; Mohamed, A.S.; Hassan, Y.M.; El-Sayed, W.M. Recent developments and future directions in point-of-care next-generation CRISPR-based rapid diagnosis. Clin. Exp. Med. 2025, 25, 33. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, S.; Jin, Y.; Yang, C.; He, L.; Wang, J.; Chen, Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J. Hazard. Mater. 2020, 393, 122348. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef]
- Peng, T.; Wang, J.; Zhao, S.; Zeng, Y.; Zheng, P.; Liang, D.; Mari, G.M.; Jiang, H. Highly luminescent green-emitting Au nanocluster-based multiplex lateral flow immunoassay for ultrasensitive detection of clenbuterol and ractopamine. Anal. Chim. Acta 2018, 1040, 143–149. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, R.; Wang, S.; Yang, X.; Bai, Z.; Li, X.; Rong, Z.; Shen, B.; Wang, S. Magnetic quantum dot based lateral flow assay biosensor for multiplex and sensitive detection of protein toxins in food samples. Biosens. Bioelectron. 2019, 146, 111754. [Google Scholar] [CrossRef]
- Khelifa, L.; Hu, Y.; Jiang, N.; Yetisen, A.K. Lateral flow assays for hormone detection. Lab Chip 2022, 22, 2451–2475. [Google Scholar] [CrossRef]
- Su, G.; Zhu, M.; Li, D.; Xu, M.; Zhu, Y.; Zhang, Y.; Zhu, H.; Li, F.; Yu, Y. Multiplexed lateral flow assay integrated with orthogonal CRISPR-Cas system for SARS-CoV-2 detection. Sens. Actuators B Chem. 2022, 371, 132537. [Google Scholar] [CrossRef]
- Tilahun, A.; Yimer, M.; Gelaye, W.; Tegegne, B.; Endalamaw, D.; Estifanos, F.; Abebaw, A.; Abere, A. Comparison of malaria diagnostic methods for detection of asymptomatic Plasmodium infections among pregnant women in northwest Ethiopia. BMC Infect. Dis. 2024, 24, 492. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-chip or Chip-in-a-lab: Challenges of Commercialization Lost in Translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef]
- Chang, J.; He, J.; Mao, M.; Zhou, W.; Lei, Q.; Li, X.; Li, D.; Chua, C.-K.; Zhao, X. Advanced Material Strategies for Next-Generation Additive Manufacturing. Materials 2018, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Phung, N.L.; Walter, J.G.; Jonczyk, R.; Seiler, L.K.; Scheper, T.; Blume, C. Development of an Aptamer-Based Lateral Flow Assay for the Detection of C-Reactive Protein Using Microarray Technology as a Prescreening Platform. ACS Comb. Sci. 2020, 22, 617–629. [Google Scholar] [CrossRef]
- Bashir, A.; Yang, Q.; Wang, J.; Hoyer, S.; Chou, W.; McLean, C.; Davis, G.; Gong, Q.; Armstrong, Z.; Jang, J.; et al. Machine learning guided aptamer refinement and discovery. Nat. Commun. 2021, 12, 2366. [Google Scholar] [CrossRef]
- Majdinasab, M.; Badea, M.; Marty, J.L. Aptamer-Based Lateral Flow Assays: Current Trends in Clinical Diagnostic Rapid Tests. Pharmaceuticals 2022, 15, 90. [Google Scholar] [CrossRef]
- Cutts, Z.W.; Hong, J.M.; Shao, S.; Tran, A.; Dimon, M.; Berndl, M.; Wu, D.; Pawlosky, A. Target-switch SELEX: Screening with alternating targets to generate aptamers to conserved terminal dipeptides. STAR Protoc. 2022, 3, 101724. [Google Scholar] [CrossRef]
- Fallah, A.; Imani Fooladi, A.A.; Havaei, S.A.; Mahboobi, M.; Sedighian, H. Recent advances in aptamer discovery, modification and improving performance. Biochem. Biophys. Rep. 2024, 40, 101852. [Google Scholar] [CrossRef]
- Testing & Diagnostics. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/testing-diagnostics (accessed on 18 August 2025).
- Onken, A.; Haanshuus, C.G.; Miraji, M.K.; Marijani, M.; Kibwana, K.O.; Abeid, K.A.; Mørch, K.; Reimers, M.; Langeland, N.; Müller, F.; et al. Malaria prevalence and performance of diagnostic tests among patients hospitalized with acute undifferentiated fever in Zanzibar. Malar. J. 2022, 21, 54. [Google Scholar] [CrossRef]
- hCG Levels: All You Need to Know—Clearblue. Available online: https://uk.clearblue.com/pregnancy-tests/hcg (accessed on 18 August 2025).
- Cardiac Troponin I Rapid Test: The Future of Heart Attack Diagnostics. JOYSBIO Biotechnology. Available online: http://en.joysbio.com/cardiac-troponin-i-rapid-test-the-future-of-heart-attack-diagnostics/ (accessed on 18 August 2025).
- Ahmad Najib, M.; Selvam, K.; Khalid, M.F.; Ozsoz, M.; Aziah, I. Quantum Dot-Based Lateral Flow Immunoassay as Point-of-Care Testing for Infectious Diseases: A Narrative Review of Its Principle and Performance. Diagnostics 2022, 12, 2158. [Google Scholar] [CrossRef]
- Liang, Z.Y.; Deng, Y.Q.; Tao, Z.Z. A quantum dot-based lateral flow immunoassay for the rapid, quantitative, and sensitive detection of specific IgE for mite allergens in sera from patients with allergic rhinitis. Anal. Bioanal. Chem. 2020, 412, 1785–1794. [Google Scholar] [CrossRef]
- UNICEF Supply Annual Report 2024 | UNICEF Supply Division. Available online: https://www.unicef.org/supply/reports/unicef-supply-annual-report-2024 (accessed on 18 August 2025).
- Risk Assessment of Food Allergens: Part 1: Review and Validation of Codex Alimentarius Priority Allergen List Through Risk Assessment: Meeting Report. Available online: https://www.who.int/publications/i/item/9789240042391 (accessed on 18 August 2025).
- Bazsefidpar, S.; Serrano-Pertierra, E.; Gutiérrez, G.; Calvo, A.S.; Matos, M.; Blanco-López, M.C. Rapid and Sensitive Detection of E. Coli O157:H7 by Lateral Flow Immunoassay and Silver Enhancement. Mikrochim. Acta. 2023, 190, 264. [Google Scholar] [CrossRef] [PubMed]
- Sundhoro, M.; Agnihotra, S.R.; Khan, N.D.; Barnes, A.; BelBruno, J.; Mendecki, L. Rapid and accurate electrochemical sensor for food allergen detection in complex foods. Sci. Rep. 2021, 11, 20831. [Google Scholar] [CrossRef] [PubMed]
- Bendhiab, I.; Dirtu, A.C.; Marchond, N.; Guérin, T.; Jitaru, P. A novel analytical approach for the determination of ethylene-thiourea and propylene-thiourea in vegetal foodstuffs by high-performance liquid chromatography hyphenated to inductively coupled plasma-tandem mass spectrometry. Anal. Bioanal. Chem. 2024, 416, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, H.; Glesener, H.; Su, B.; Bolsinger, B.; Krajmalnik-Brown, R.; Voth-Gaeddert, L.E. Evaluating Methods for Aflatoxin B1 Monitoring in Selected Food Crops Within Decentralized Agricultural Systems. Toxins 2025, 17, 37. [Google Scholar] [CrossRef]
- Xing, G.; Sun, X.; Li, N.; Li, X.; Wu, T.; Wang, F. New Advances in Lateral Flow Immunoassay (LFI) Technology for Food Safety Detection. Molecules 2022, 27, 6596. [Google Scholar] [CrossRef]
- Cvak, B.; Warth, B.; Atehnkeng, J.; Parich, A.; Moritz, A.; Sulyok, M.; Krska, R. Evaluating the Performance of Lateral Flow Devices for Total Aflatoxins with Special Emphasis on Their Robustness under Sub-Saharan Conditions. Toxins 2021, 13, 742. [Google Scholar] [CrossRef]
- Shahjahan, T.; Javed, B.; Sharma, V.; Tian, F. Overview of Various Components of Lateral-Flow Immunochromatography Assay for the Monitoring of Aflatoxin and Limit of Detection in Food Products: A Systematic Review. Chemosensors 2023, 11, 520. [Google Scholar] [CrossRef]
- Employer and Workplace Drug Testing Market Report, 2033. Available online: https://www.grandviewresearch.com/industry-analysis/employer-workplace-drug-testing-market-report (accessed on 18 August 2025).
- Gjerde, H.; Clausen, G.B.; Andreassen, E.; Furuhaugen, H. Evaluation of Dräger DrugTest 5000 in a Naturalistic Setting. J Anal Toxicol 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Angelini, D.J.; Biggs, T.D.; Maughan, M.N.; Feasel, M.G.; Sisco, E.; Sekowski, J.W. Evaluation of a Lateral Flow Immunoassay for the Detection of the Synthetic Opioid Fentanyl. Forensic Sci. Int. 2019, 300, 75–81. [Google Scholar] [CrossRef]
- Martinez, J.; Gonyea, J.; Zaney, M.E.; Kahl, J.; Moore, D.M. The Evolution of Fentanyl-Related Substances: Prevalence and Drug Concentrations in Postmortem Biological Specimens at the Miami-Dade Medical Examiner Department. J. Anal. Toxicol 2024, 48, 104–110. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Miyakawa, K.; Jeremiah, S.S.; Funabashi, R.; Okudela, K.; Kikuchi, S.; Katada, J.; Wada, A.; Takei, T.; Nishi, M.; et al. Highly specific monoclonal antibodies and epitope identification against SARS-CoV-2 nucleocapsid protein for antigen detection tests. Cell Rep. Med. 2021, 2, 100311. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Walter-Weingärtner, J.; Bergmann, M.; Weber, K.; Truyen, U.; Muresan, C.; Hartmann, K. Comparison of Eight Commercially Available Faecal Point-of-Care Tests for Detection of Canine Parvovirus Antigen. Viruses 2021, 13, 2080. [Google Scholar] [CrossRef]
- Sensitivity of Lateral Flow Technique for Diagnosis of Canine Parvovirus | Scientific Reports. Available online: https://www.nature.com/articles/s41598-024-55548-x (accessed on 18 August 2025).
- Veterinary Diagnostics Market Size Worth $21.94 Billion by 2032 | CAGR: 9.9%. Polaris. Available online: https://www.polarismarketresearch.com/press-releases/veterinary-diagnostic-market (accessed on 18 August 2025).
- Global AIV with Zoonotic Potential. AnimalHealth. Available online: https://www.fao.org/animal-health/situation-updates/global-aiv-with-zoonotic-potential/en (accessed on 18 August 2025).
- Picardeau, M.; Bertherat, E.; Jancloes, M.; Skouloudis, A.N.; Durski, K.; Hartskeerl, R.A. Rapid Tests for Diagnosis of Leptospirosis: Current Tools and Emerging Technologies. Diagn. Microbiol. Infect. Dis. 2014, 78, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Reagan, K.L.; Nally, J.E.; Galloway, R.L.; Haake, D.A. Role of Diagnostics in Epidemiology, Management, Surveillance, and Control of Leptospirosis. Pathogens 2022, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Lyme Disease Multiplex Testing for Dogs | Cornell University College of Veterinary Medicine. Available online: https://www.vet.cornell.edu/animal-health-diagnostic-center/testing/testing-protocols-interpretations/lyme-disease-multiplex-testing-dogs (accessed on 18 August 2025).
- Public Health Consequences of a False-Positive Laboratory Test Result for Brucella—Florida, Georgia, and Michigan. 2005. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5722a3.htm (accessed on 18 August 2025).
- Varshochi, M.; Majidi, J.; Amini, M.; Ghabili, K.; Shoja, M.M. False Positive Seroreactivity to Brucellosis in Tuberculosis Patients: A Prevalence Study. Int. J. Gen. Med. 2011, 4, 207–210. [Google Scholar] [CrossRef]
- Test Portfolio. Available online: https://www.zoetis.co.uk/diagnostics/vetscan-rapid-tests/rapid-tests.aspx (accessed on 18 August 2025).
- Bermejo-Peláez, D.; Alastruey-Izquierdo, A.; Medina, N.; Capellán-Martín, D.; Bonilla, O.; Luengo-Oroz, M.; Rodríguez-Tudela, J.L. Artificial Intelligence-Driven Mobile Interpretation of a Semi-Quantitative Cryptococcal Antigen Lateral Flow Assay. IMA Fungus 2024, 15, 27. [Google Scholar] [CrossRef]
- Davis, A.M.; Tomitaka, A. Machine Learning-Based Quantification of Lateral Flow Assay Using Smartphone-Captured Images. Biosensors 2025, 15, 19. [Google Scholar] [CrossRef]
- Han, G.-R.; Goncharov, A.; Eryilmaz, M.; Joung, H.-A.; Ghosh, R.; Yim, G.; Chang, N.; Kim, M.; Ngo, K.; Veszpremi, M.; et al. Deep Learning-Enhanced Paper-Based Vertical Flow Assay for High-Sensitivity Troponin Detection Using Nanoparticle Amplification. ACS Nano 2024, 18, 27933–27948. [Google Scholar] [CrossRef]
- Prevedello, L.M.; Halabi, S.S.; Shih, G.; Wu, C.C.; Kohli, M.D.; Chokshi, F.H.; Erickson, B.J.; Kalpathy-Cramer, J.; Andriole, K.P.; Flanders, A.E. Challenges Related to Artificial Intelligence Research in Medical Imaging and the Importance of Image Analysis Competitions. Radiol. Artif. Intell. 2019, 1, e180031. [Google Scholar] [CrossRef] [PubMed]
- Perkonigg, M.; Hofmanninger, J.; Herold, C.J.; Brink, J.A.; Pianykh, O.; Prosch, H.; Langs, G. Dynamic Memory to Alleviate Catastrophic Forgetting in Continual Learning with Medical Imaging. Nat. Commun. 2021, 12, 5678. [Google Scholar] [CrossRef] [PubMed]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.S.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018, 172, 1122–1131.e9. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cui, Y. Deep Transfer Learning for Reducing Health Care Disparities Arising from Biomedical Data Inequality. Nat. Commun. 2020, 11, 5131. [Google Scholar] [CrossRef] [PubMed]
- Ballard, Z.S.; Joung, H.A.; Goncharov, A.; Liang, J.; Nugroho, K.; Di Carlo, D.; Garner, O.B.; Ozcan, A. Deep Learning-Enabled Point-of-Care Sensing Using Multiplexed Paper-Based Sensors. Npj Digit. Med. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Chen, K.; Ma, X.; Guo, J. Artificial Intelligence Reinforced Upconversion Nanoparticle-Based Lateral Flow Assay via Transfer Learning. Fundam. Res. 2023, 3, 544–556. [Google Scholar] [CrossRef]
- León, D.; Amez, I.; Radojević, M.; Manić, N.; Stojiljković, D.; Milivojević, A.; García-Torrent, J.; Castells, B. Emissions and Fire Risk Assessment of Nitrocellulose as a Sustainable Alternative in Pyrotechnic Compositions. Fire 2024, 7, 265. [Google Scholar] [CrossRef]
- Kamtsikakis, A.; McBride, S.; Zoppe, J.O.; Weder, C. Cellulose Nanofiber Nanocomposite Pervaporation Membranes for Ethanol Recovery. ACS Appl. Nano Mater. 2021, 4, 568–579. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green Synthesis, Biomedical and Biotechnological Applications of Carbon and Graphene Quantum Dots. A Review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Circular Economy: New Rules on Single-Use Plastics. European Commission—European Commission. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_19_2631 (accessed on 18 August 2025).
- Elter, A.; Bock, T.; Spiehl, D.; Russo, G.; Hinz, S.C.; Bitsch, S.; Baum, E.; Langhans, M.; Meckel, T.; Dörsam, E.; et al. Carbohydrate Binding Module-Fused Antibodies Improve the Performance of Cellulose-Based Lateral Flow Immunoassays. Sci. Rep. 2021, 11, 7880. [Google Scholar] [CrossRef]
- Okos | Biodegradable Diagnostics. Okos Diagnostics. Available online: https://www.okosdiagnostics.com (accessed on 14 August 2025).
- FineEngineering Magazine. Okos Diagnostics, Leading the Way in Sustainable Healthcare. Available online: https://fineeng.eu/okos-diagnostics-leading-the-way-in-sustainable-healthcare/ (accessed on 14 August 2025).
- Tian, Y.; Du, L.; Zhu, P.; Chen, Y.; Chen, W.; Wu, C.; Wang, P. Recent Progress in Micro/Nano Biosensors for Shellfish Toxin Detection. Biosens. Bioelectron. 2021, 176, 112899. [Google Scholar] [CrossRef] [PubMed]
- Ansaryan, S.; Liu, Y.C.; Li, X.; Economou, A.M.; Eberhardt, C.S.; Jandus, C.; Altug, H. High-Throughput Spatiotemporal Monitoring of Single-Cell Secretions via Plasmonic Microwell Arrays. Nat. Biomed. Eng. 2023, 7, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Dilbaghi, N.; Yadav, N. CRISPR Integrated Biosensors: A New Paradigm for Cancer Detection. Clin. Chim. Acta 2025, 569, 120179. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Maragh, S.; Ghiran, I.C.; Jones, J.C.; DeRose, P.C.; Elsheikh, E.; Vreeland, W.N.; Wang, L. Measurement and Standardization Challenges for Extracellular Vesicle Therapeutic Delivery Vectors. Nanomedicine 2020, 15, 2149–2170. [Google Scholar] [CrossRef]
- Liquid Biopsy Market Size & Share | Industry Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/liquid-biopsy-market (accessed on 18 August 2025).
- Dalirirad, S.; Steckl, A.J. Aptamer-Based Lateral Flow Assay for Point of Care Cortisol Detection in Sweat. Sens. Actuators B Chem. 2019, 283, 79–86. [Google Scholar] [CrossRef]
- Sivakumar, J.; Yang, J.H.; Kelly, M.S.; Koh, A.; Won, D. An Automated Lateral Flow Assay Identification Framework: Exploring the Challenges of a Wearable Lateral Flow Assay in Mobile Application. Expert Syst. Appl. 2022, 210, 118471. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Gao, Z.; Zhang, J.; Ou, H.; Gao, H.; Kwok, R.T.K.; Ding, D.; Tang, B.Z. A Wearable AIEgen-Based Lateral Flow Test Strip for Rapid Detection of SARS-CoV-2 RBD Protein and N Protein. CR-PHYS-SC 2022, 3. [Google Scholar] [CrossRef]
- Wilkirson, E.C.; Li, D.; Lillehoj, P.B. Lateral Flow-Based Skin Patch for Rapid Detection of Protein Biomarkers in Human Dermal Interstitial Fluid. ACS Sens. 2024, 9, 5792–5801. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G.; et al. Wearable Sweat Biosensors Refresh Personalized Health/Medical Diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef]
- Wang, C.; Sani, E.S.; Gao, W. Wearable Bioelectronics for Chronic Wound Management. Adv. Funct. Mater. 2022, 32, 2111022. [Google Scholar] [CrossRef]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e01991-21. [Google Scholar] [CrossRef]
- Tin, D.; Sabeti, P.; Ciottone, G.R. Bioterrorism: An Analysis of Biological Agents Used in Terrorist Events. Am. J. Emerg. Med. 2022, 54, 117–121. [Google Scholar] [CrossRef]
- Batra, R.; Blandford, E.; Kulasegaran-Shylini, R.; Futschik, M.E.; Bown, A.; Catton, M.; Conti-Frith, H.; Alexandridou, A.; Gill, R.; Milroy, C.; et al. Multiplex Lateral Flow Test Sensitivity and Specificity in Detecting Influenza A, B and SARS-CoV-2 in Adult Patients in a UK Emergency Department. Emerg. Med. J. 2025, 42, 98–104. [Google Scholar] [CrossRef]
- Osborn, M.J.; Bhardwaj, A.; Bingea, S.P.; Knipping, F.; Feser, C.J.; Lees, C.J.; Collins, D.P.; Steer, C.J.; Blazar, B.R.; Tolar, J. CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering 2021, 8, 23. [Google Scholar] [CrossRef]
- Niemi, J.K. 1—The economic cost of bacterial infections. In Advancements and Technologies in Pig and Poultry Bacterial Disease Control; Foster, N., Kyriazakis, I., Barrow, P., Eds.; Academic Press: Cambridge, UK, 2021; pp. 1–23. [Google Scholar] [CrossRef]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I.; et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef]
- Mazur, F.; Tjandra, A.D.; Zhou, Y.; Gao, Y.; Chandrawati, R. Paper-based sensors for bacteria detection. Nat. Rev. Bioeng. 2023, 1, 180–192. [Google Scholar] [CrossRef]
- Wang, R.; Kim, K.; Choi, N.; Wang, X.; Lee, J.; Jeon, J.H.; Rhie, G.; Choo, J. Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips. Sens. Actuators B Chem. 2018, 270, 72–79. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Zhang, X.; Zeng, H.; Cui, L.; He, S. Biotinylation-based lateral flow assays for pathogenic and total bacteria detection. Anal. Chim. Acta 2025, 1338, 343607. [Google Scholar] [CrossRef]
- Nan, X.; Yao, X.; Yang, L.; Cui, Y. Lateral flow assay of pathogenic viruses and bacteria in healthcare. Analyst 2023, 148, 4573–4590. [Google Scholar] [CrossRef]
- Dhaked, R.K.; Singh, M.K.; Singh, P.; Gupta, P. Botulinum toxin: Bioweapon & magic drug. Indian J. Med. Res. 2010, 132, 489–503. [Google Scholar] [PubMed]
- CDC. Bioterrorism and Botulism: The Threat. Botulism. Available online: https://www.cdc.gov/botulism/bioterrorism/index.html (accessed on 13 April 2025).
- Liu, J.; Gao, S.; Kang, L.; Ji, B.; Xin, W.; Kang, J.; Li, P.; Gao, J.; Wang, H.; Wang, J.; et al. An Ultrasensitive Gold Nanoparticle-based Lateral Flow Test for the Detection of Active Botulinum Neurotoxin Type A. Nanoscale Res. Lett. 2017, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Thomas, C.A.; Halliwell, J.; Gwenin, C.D. Rapid Detection of Botulinum Neurotoxins—A Review. Toxins 2019, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Multiplex LFA Strips-Lateral Flow Assay (LFA) Strips and Readers for Biological Threat Detection-Southern Scientific. Available online: https://www.southernscientific.co.uk/products-by-manufacturer/tetracore/biothreat-alert (accessed on 13 April 2025).
- Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.T. Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid. Nanofluid. 2020, 24, 17. [Google Scholar] [CrossRef]
- Fischer, C.; Wessels, H.; Paschke-Kratzin, A.; Fischer, M. Aptamers: Universal capture units for lateral flow applications. Anal. Biochem. 2017, 522, 53–60. [Google Scholar] [CrossRef]
- Zhao, A.P.; Li, S.; Cao, Z.; Hu, P.J.-H.; Wang, J.; Xiang, Y.; Xie, D.; Lu, X. AI for science: Predicting infectious diseases. J. Saf. Sci. Resil. 2024, 5, 130–146. [Google Scholar] [CrossRef]
- Joung, H.-A.; Ballard, Z.S.; Wu, J.; Tseng, D.K.; Teshome, H.; Zhang, L.; Horn, E.J.; Arnaboldi, P.M.; Dattwyler, R.J.; Garner, O.B.; et al. Point-of-Care Serodiagnostic Test for Early-Stage Lyme Disease Using a Multiplexed Paper-Based Immunoassay and Machine Learning. ACS Nano 2020, 14, 229–240. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Koydemir, H.C.; Zhang, Y.; Yang, E.; Eryilmaz, M.; Wang, H.; Li, J.; Bai, B.; Ma, G.; et al. Rapid and stain-free quantification of viral plaque via lens-free holography and deep learning. Nat. Biomed. Eng. 2023, 7, 1040–1052. [Google Scholar] [CrossRef]
- Biothreat Detection Market Forecast to 2030. MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/biothreat-detection-market-48023215.html (accessed on 13 April 2025).
- Budd, J.; Miller, B.S.; Weckman, N.E.; Cherkaoui, D.; Huang, D.; Decruz, A.T.; Fongwen, N.; Han, G.-R.; Broto, M.; Estcourt, C.S.; et al. Lateral flow test engineering and lessons learned from COVID-19. Nat. Rev. Bioeng. 2023, 1, 13–31. [Google Scholar] [CrossRef]
- Martin, C.; Zhao, Q.; Patel, A.; Velasquez, E.; Chen, D.; Li, W. A Review of Advanced Roll-to-Roll Manufacturing: System Modeling and Control. J. Manuf. Sci. Eng. 2025, 147, 041004. [Google Scholar] [CrossRef]
- Pick and Place with Robotic Handling. Huxley Bertram. Available online: https://www.huxleybertram.com/portfolio-item/pick-and-place-robotic-handling-machine/ (accessed on 18 August 2025).
- Rabiee, N.; Sharma, R.; Foorginezhad, S.; Jouyandeh, M.; Asadnia, M.; Rabiee, M.; Akhavan, O.; Lima, E.C.; Formela, K.; Ashrafizadeh, M.; et al. Green and Sustainable Membranes: A review. Environ. Res. 2023, 231, 116133. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Proll, G.; Builes-Münden, E.; Dietzel, A.; Wagner, S.; Gauglitz, G. Tools to compare antibody gold nanoparticle conjugates for a small molecule immunoassay. Microchim. Acta 2023, 190, 62. [Google Scholar] [CrossRef]
- Ventura, B.D.; Banchelli, M.; Funari, R.; Illiano, A.; Angelis, M.D.; Taroni, P.; Amoresano, A.; Matteini, P.; Velotta, R. Biosensor surface functionalization by a simple photochemical immobilization of antibodies: Experimental characterization by mass spectrometry and surface enhanced Raman spectroscopy. Analyst 2019, 144, 6871–6880. [Google Scholar] [CrossRef]
- Ventura, B.D.; Cennamo, M.; Minopoli, A.; Campanile, R.; Censi, S.B.; Terracciano, D.; Portella, G.; Velotta, R. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs. ACS Sens. 2020, 5, 3043–3048. [Google Scholar] [CrossRef]
- Anzevino, M.; Marra, D.; Fulgione, A.; Giarra, A.; Nava, D.; Biondi, L.; Capuano, F.; Iannotti, V.; Della Ventura, B.; Velotta, R. Antibody-Functionalized Gold Nanoparticles as a Highly Sensitive Two-Step Colorimetric Biosensor for Detecting Salmonella Typhimurium in Food. ACS Appl. Nano Mater. 2024, 7, 21048–21056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinyua, D.M.; Memeu, D.M.; Mugo Mwenda, C.N.; Ventura, B.D.; Velotta, R. Advancements and Applications of Lateral Flow Assays (LFAs): A Comprehensive Review. Sensors 2025, 25, 5414. https://doi.org/10.3390/s25175414
Kinyua DM, Memeu DM, Mugo Mwenda CN, Ventura BD, Velotta R. Advancements and Applications of Lateral Flow Assays (LFAs): A Comprehensive Review. Sensors. 2025; 25(17):5414. https://doi.org/10.3390/s25175414
Chicago/Turabian StyleKinyua, Dickson Mwenda, Daniel Maitethia Memeu, Cynthia Nyambura Mugo Mwenda, Bartolomeo Della Ventura, and Raffaele Velotta. 2025. "Advancements and Applications of Lateral Flow Assays (LFAs): A Comprehensive Review" Sensors 25, no. 17: 5414. https://doi.org/10.3390/s25175414
APA StyleKinyua, D. M., Memeu, D. M., Mugo Mwenda, C. N., Ventura, B. D., & Velotta, R. (2025). Advancements and Applications of Lateral Flow Assays (LFAs): A Comprehensive Review. Sensors, 25(17), 5414. https://doi.org/10.3390/s25175414






