Time-Domain Analysis of Low- and High-Frequency Near-Infrared Spectroscopy Sensor Technologies for Characterization of Cerebral Pressure–Flow and Oxygen Delivery Physiology: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Human Population
2.2. Ethical Considerations
2.3. Data Collection
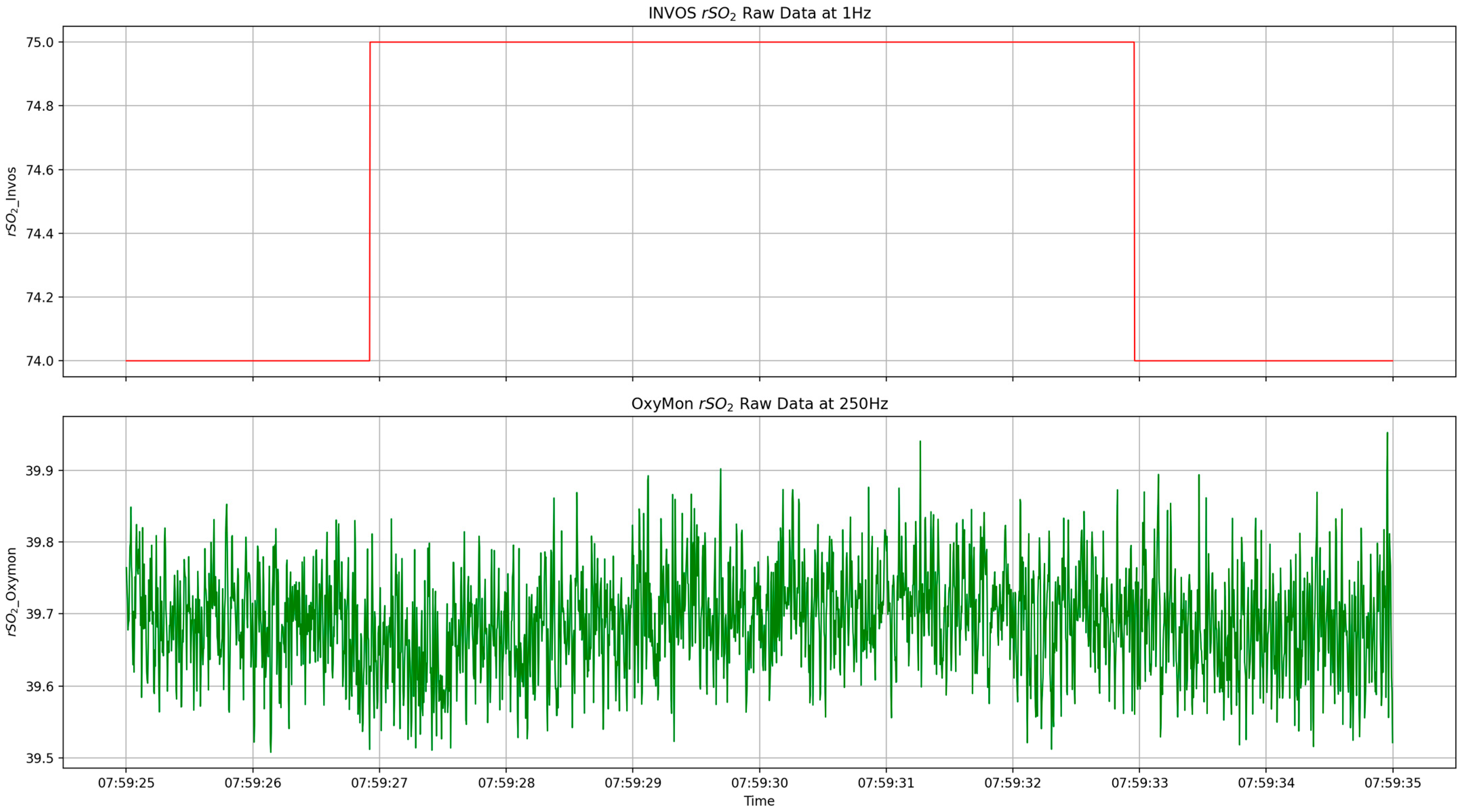
2.4. Physiologic Data Cleaning and Processing
2.5. Statistical Data Analysis
- Absolute differences between each of the rSO2 and COx-a signals obtained using OxyMon and INVOS systems (raw and 10-s decimated data);
- Pearson correlation, Bland–Altman agreement, and cross-correlation function comparison between each of the rSO2 and COx-a signals obtained using OxyMon and INVOS systems (raw and 10-s decimated data);
- Optimal time-series autocorrelative structure differences for rSO2 and COx-a signals between OxyMon and INVOS systems (1st order differenced data at 1 Hz and 250 Hz);
- Time-series impulse response function differences for ABP on rSO2 relationships between OxyMon and INVOS systems (1st order differenced at 1 Hz and 250 Hz);
- Difference in Granger causality relationships between ABP and rSO2 using OxyMon and INVOS systems (1st order differenced data at 1Hz and 250 Hz).
- In the sub-sections to follow, the individual methods will be briefly covered.
2.5.1. Absolute Signal Difference—Raw and 10-s Decimated Data
2.5.2. Pearson Correlation Analysis—Raw and 10-s Decimated Data
2.5.3. Bland–Altman Agreement Analysis—Raw and 10-s Decimated Data
2.5.4. Cross-Correlation Function Analysis—Raw and 10-s Decimated Data
2.5.5. Optimal Time-Series Structures of rSO2 and COx-a Signals—1 Hz and 250 Hz Sampled Data
2.5.6. Time-Series Impulse Response Function (IRF) for ABP and rSO2—First-Order Differenced Data
2.5.7. Differences in Granger Causality Relationships Between ABP and rSO2—First-Order Differenced Data
2.5.8. Subgroup Analysis
3. Results
3.1. Population Demographics
3.2. Absolute Signal Differences (ASD)—Raw and 10-s Decimated Data
3.3. Pearson Correlation Analysis Results—Raw and 10-s Decimated Data
3.4. Bland–Altman Analysis Results—Raw and 10-s Decimated Data
3.5. Cross-Correlation Function Results—Raw and 10-s Decimated Data
3.6. Optimal ARIMA Structure Analysis Results—First-Order Differenced Data
3.7. Signal Difference in Impulse Response Function (IRF) of ABP on rSO2—First-Order Differenced Data
3.8. Signal Difference in Granger Causality Relationships between ABP and rSO2—First-Order Differenced Data
3.9. Subgroup Analysis Assessment
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α | Alpha |
| ABP | Arterial blood pressure |
| ADF | Augmented Dickey–Fuller |
| AIC | Akaike information criterion |
| ARIMA | Autoregressive integrative moving average |
| ASD | Absolute signal difference |
| CA | Cerebral autoregulation |
| CBF | Cerebral blood flow |
| CBv | Cerebral blood volume |
| COx | Cerebral oximetry index with CPP |
| COx-a | Cerebral oximetry index with ABP |
| COx-a_Invos | COx-a obtained with rSO2 from the INVOS NIRS system |
| COx-a_Oxymon | COx-a obtained with rSO2 from the OxyMon NIRS system |
| CPP | Cerebral perfusion pressure |
| CVR | Cerebrovascular reactivity |
| d-order | Moving average order |
| fNIRS | Functional near-infrared spectroscopy |
| HbDiff | Difference between oxyhemoglobin and deoxyhemoglobin |
| HbO | Oxyhemoglobin |
| HHb | Deoxyhemoglobin |
| HVA | Healthy volunteers |
| ICP | Intracranial pressure |
| IQR | Interquartile range |
| IRF | Impulse response function |
| KPSS | Kwiatkowski–Phillips–Schmidt–Shin |
| LoA | Limit of agreement |
| MAIN-HUB | Multi-Omic Analytics and Integrative Neuroinformatics in the HUman Brain |
| MAD | Median absolute deviation |
| NA | Represents missing value or undetermined value due to inadequate data |
| NIRS | Near-infrared spectroscopy |
| p-order | Autoregressive order |
| q-order | Moving average order |
| r | Pearson correlation coefficient |
| relative bias | Bias as a proportion of LoA spread |
| rSO2 | Regional cerebral oxygen saturation |
| rSO2_Invos | Frontal right rSO2 signal obtained using the INVOS NIRS system |
| rSO2_Oxymon | Frontal right rSO2 signal obtained using the OxyMon NIRS system |
| tHb | Total hemoglobin |
| VARIMA | Vector ARIMA |
References
- Fog, M. The Relationship Between the Blood Pressure and the Tonic Regulation of the Pial Arteries. J. Neurol. Psychiatry 1938, 1, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef] [PubMed]
- Lassen, N.A. Control of Cerebral Circulation in Health and Disease. Circ. Res. 1974, 34, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Laing, R.J.; Menon, D.; Pickard, J.D. Continuous Assessment of the Cerebral Vasomotor Reactivity in Head Injury. Neurosurgery 1997, 41, 11–19. [Google Scholar] [CrossRef]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical Thresholds for Cerebrovascular Reactivity After Traumatic Brain Injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef]
- Donnelly, J.; Czosnyka, M.; Adams, H.; Cardim, D.; Kolias, A.G.; Zeiler, F.A.; Lavinio, A.; Aries, M.; Robba, C.; Smielewski, P.; et al. Twenty-Five Years of Intracranial Pressure Monitoring After Severe Traumatic Brain Injury: A Retrospective, Single-Center Analysis. Neurosurgery 2019, 85, E75–E82. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Ercole, A.; Beqiri, E.; Cabeleira, M.; Thelin, E.P.; Stocchetti, N.; Steyerberg, E.W.; Maas, A.I.R.; Menon, D.K.; Czosnyka, M.; et al. Association between Cerebrovascular Reactivity Monitoring and Mortality Is Preserved When Adjusting for Baseline Admission Characteristics in Adult Traumatic Brain Injury: A CENTER-TBI Study. J. Neurotrauma 2020, 37, 1233–1241. [Google Scholar] [CrossRef]
- Bennis, F.C.; Teeuwen, B.; Zeiler, F.A.; Elting, J.W.; van der Naalt, J.; Bonizzi, P.; Delhaas, T.; Aries, M.J. Improving Prediction of Favourable Outcome After 6 Months in Patients with Severe Traumatic Brain Injury Using Physiological Cerebral Parameters in a Multivariable Logistic Regression Model. Neurocrit. Care 2020, 33, 542–551. [Google Scholar] [CrossRef]
- Åkerlund, C.A.; Donnelly, J.; Zeiler, F.A.; Helbok, R.; Holst, A.; Cabeleira, M.; Güiza, F.; Meyfroidt, G.; Czosnyka, M.; Smielewski, P.; et al. Impact of Duration and Magnitude of Raised Intracranial Pressure on Outcome after Severe Traumatic Brain Injury: A CENTER-TBI High-Resolution Group Study. PLoS ONE 2020, 15, e0243427. [Google Scholar] [CrossRef]
- Depreitere, B.; Citerio, G.; Smith, M.; Adelson, P.D.; Aries, M.J.; Bleck, T.P.; Bouzat, P.; Chesnut, R.; De Sloovere, V.; Diringer, M.; et al. Cerebrovascular Autoregulation Monitoring in the Management of Adult Severe Traumatic Brain Injury: A Delphi Consensus of Clinicians. Neurocrit. Care 2021, 34, 731–738. [Google Scholar] [CrossRef]
- Güiza, F.; Depreitere, B.; Piper, I.; Citerio, G.; Chambers, I.; Jones, P.A.; Lo, T.-Y.M.; Enblad, P.; Nillson, P.; Feyen, B.; et al. Visualizing the Pressure and Time Burden of Intracranial Hypertension in Adult and Paediatric Traumatic Brain Injury. Intensive Care Med. 2015, 41, 1067–1076. [Google Scholar] [CrossRef]
- Güiza, F.; Meyfroidt, G.; Piper, I.; Citerio, G.; Chambers, I.; Enblad, P.; Nillson, P.; Feyen, B.; Jorens, P.; Maas, A.; et al. Cerebral Perfusion Pressure Insults and Associations with Outcome in Adult Traumatic Brain Injury. J. Neurotrauma 2017, 34, 2425–2431. [Google Scholar] [CrossRef]
- Donnelly, J.; Güiza, F.; Depreitere, B.; Meyfroidt, G.; Czosnyka, M.; Smielewski, P. Visualising the Pressure-Time Burden of Elevated Intracranial Pressure after Severe Traumatic Brain Injury: A Retrospective Confirmatory Study. Br. J. Anaesth. 2021, 126, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Ercole, A.; Czosnyka, M.; Smielewski, P.; Hawryluk, G.; Hutchinson, P.J.A.; Menon, D.K.; Aries, M. Continuous Cerebrovascular Reactivity Monitoring in Moderate/Severe Traumatic Brain Injury: A Narrative Review of Advances in Neurocritical Care. Br. J. Anaesth. 2020, 124, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Lee, J.K.; Smielewski, P.; Czosnyka, M.; Brady, K. Validation of Intracranial Pressure-Derived Cerebrovascular Reactivity Indices against the Lower Limit of Autoregulation, Part II: Experimental Model of Arterial Hypotension. J. Neurotrauma 2018, 35, 2812–2819. [Google Scholar] [CrossRef]
- Fraser, C.D.; Brady, K.M.; Rhee, C.J.; Easley, R.B.; Kibler, K.; Smielewski, P.; Czosnyka, M.; Kaczka, D.W.; Andropoulos, D.B.; Rusin, C. The Frequency Response of Cerebral Autoregulation. J. Appl. Physiol. 2013, 115, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Howells, T.; Johnson, U.; McKelvey, T.; Enblad, P. An Optimal Frequency Range for Assessing the Pressure Reactivity Index in Patients with Traumatic Brain Injury. J. Clin. Monit. Comput. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Lavinio, A.; Steiner, L.A.; Radolovich, D.; Smielewski, P.; Timofeev, I.; Hiler, M.; Balestreri, M.; Kirkpatrick, P.J.; Pickard, J.D.; et al. Continuous Monitoring of Cerebrovascular Pressure Reactivity in Patients with Head Injury. Neurosurg. Focus. 2008, 25, E2. [Google Scholar] [CrossRef]
- Czosnyka, M.; Brady, K.; Reinhard, M.; Smielewski, P.; Steiner, L.A. Monitoring of Cerebrovascular Autoregulation: Facts, Myths, and Missing Links. Neurocrit Care 2009, 10, 373–386. [Google Scholar] [CrossRef]
- Copplestone, S.; Welbourne, J. A Narrative Review of the Clinical Application of Pressure Reactiviy Indices in the Neurocritical Care Unit. Br. J. Neurosurg. 2018, 32, 4–12. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of Optical Pathlength through Tissue from Direct Time of Flight Measurement. Phys. Med. Biol. 1988, 33, 1433. [Google Scholar] [CrossRef] [PubMed]
- Sainbhi, A.S.; Gomez, A.; Froese, L.; Slack, T.; Batson, C.; Stein, K.Y.; Cordingley, D.M.; Alizadeh, A.; Zeiler, F.A. Non-Invasive and Minimally-Invasive Cerebral Autoregulation Assessment: A Narrative Review of Techniques and Implications for Clinical Research. Front. Neurol. 2022, 13, 872731. [Google Scholar] [CrossRef]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous Time-Domain Analysis of Cerebrovascular Autoregulation Using Near-Infrared Spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous Measurement of Autoregulation by Spontaneous Fluctuations in Cerebral Perfusion Pressure. Stroke 2008, 39, 2531–2537. [Google Scholar] [CrossRef]
- Lee, J.K.; Kibler, K.K.; Benni, P.B.; Easley, R.B.; Czosnyka, M.; Smielewski, P.; Koehler, R.C.; Shaffner, D.H.; Brady, K.M. Cerebrovascular Reactivity Measured by Near-Infrared Spectroscopy. Stroke 2009, 40, 1820–1826. [Google Scholar] [CrossRef]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Helmy, A.; Manktelow, A.; Carrera, E.; Brady, K.M.; Hutchinson, P.J.A.; Menon, D.K.; Pickard, J.D.; et al. Noninvasive Monitoring of Cerebrovascular Reactivity with Near Infrared Spectroscopy in Head-Injured Patients. J. Neurotrauma 2010, 27, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Smielewski, P.; Czosnyka, M.; Zweifel, C.; Brady, K.; Hogue, C.; Steiner, L.; Hutchinson, P.; Mennon, D.; Pickard, J. Multicentre Experience of Using ICM+ for Investigations of Cerebrovascular Dynamics with Near-Infrared Spectroscopy. Crit. Care 2010, 14, P348. [Google Scholar] [CrossRef]
- Brady, K.M.; Mytar, J.O.; Kibler, K.K.; Hogue, C.W.; Lee, J.K.; Czosnyka, M.; Smielewski, P.; Easley, R.B. Noninvasive Autoregulation Monitoring with and without Intracranial Pressure in the Naïve Piglet Brain. Anesth. Analg. 2010, 111, 191–195. [Google Scholar] [CrossRef]
- Weerakkody, R.A.; Czosnyka, M.; Zweifel, C.; Castellani, G.; Smielewski, P.; Keong, N.; Haubrich, C.; Pickard, J.; Czosnyka, Z. Slow Vasogenic Fluctuations of Intracranial Pressure and Cerebral near Infrared Spectroscopy—An Observational Study. Acta Neurochir. 2010, 152, 1763–1769. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Donnelly, J.; Calviello, L.; Smielewski, P.; Menon, D.K.; Czosnyka, M. Pressure Autoregulation Measurement Techniques in Adult Traumatic Brain Injury, Part II: A Scoping Review of Continuous Methods. J. Neurotrauma 2017, 34, 3224–3237. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Donnelly, J.; Menon, D.K.; Smielewski, P.; Zweifel, C.; Brady, K.; Czosnyka, M. Continuous Autoregulatory Indices Derived from Multi-Modal Monitoring: Each One Is Not Like the Other. J. Neurotrauma 2017, 34, 3070–3080. [Google Scholar] [CrossRef]
- Mathieu, F.; Khellaf, A.; Ku, J.C.; Donnelly, J.; Thelin, E.P.; Zeiler, F.A. Continuous Near-Infrared Spectroscopy Monitoring in Adult Traumatic Brain Injury: A Systematic Review. J. Neurosurg. Anesthesiol. 2020, 32, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Dian, J.; Zeiler, F.A. Continuous and Entirely Non-Invasive Method for Cerebrovascular Reactivity Assessment: Technique and Implications. J. Clin. Monit. Comput. 2021, 35, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Zeiler, F.A. Non-Invasive Continuous Cerebrovascular Monitoring for Subacute Bedside and Outpatient Settings: An Important Advancement. Neurotrauma Rep. 2021, 2, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A. Point-of-Care Noninvasive Assessments of Cerebrovascular Reactivity in Traumatic Brain Injury: Integrating the Physiome with Clinical Phenotype. Ann. Neurol. 2021, 90, 19–21. [Google Scholar] [CrossRef]
- Bindra, J.; Pham, P.; Aneman, A.; Chuan, A.; Jaeger, M. Non-Invasive Monitoring of Dynamic Cerebrovascular Autoregulation Using Near Infrared Spectroscopy and the Finometer Photoplethysmograph. Neurocrit. Care 2016, 24, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Adatia, K.; Geocadin, R.G.; Healy, R.; Ziai, W.; Ponce-Mejia, L.; Anderson-White, M.; Shah, D.; Radzik, B.R.; Palmisano, C.; Hogue, C.W.; et al. Lateral Brain Displacement and Cerebral Autoregulation in Acutely Comatose Patients. Crit. Care Med. 2020, 48, 1018. [Google Scholar] [CrossRef]
- Zipfel, J.; Bantle, S.J.; Magunia, H.; Schlensak, C.; Neunhoeffer, F.; Schuhmann, M.U.; Lescan, M. Non-Invasive Cerebral Autoregulation Monitoring During Awake Carotid Endarterectomy Identifies Clinically Significant Brain Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 647–654. [Google Scholar] [CrossRef]
- Burma, J.S.; Lapointe, A.P.; Soroush, A.; Oni, I.K.; Smirl, J.D.; Dunn, J.F. Insufficient Sampling Frequencies Skew Heart Rate Variability Estimates: Implications for Extracting Heart Rate Metrics from Neuroimaging and Physiological Data. J. Biomed. Inform. 2021, 123, 103934. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Marquez, I.; Gomez, A.; Stein, K.Y.; Amenta, F.; Vakitbilir, N.; Froese, L.; Zeiler, F.A. Regional Disparity in Continuously Measured Time-Domain Cerebrovascular Reactivity Indices: A Scoping Review of Human Literature. Physiol. Meas. 2023, 44, 07TR02. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Froese, L.; Stein, K.Y.; Vakitbilir, N.; Gomez, A.; Islam, A.; Bergmann, T.; Silvaggio, N.; Hayat, M.; Zeiler, F.A. Commercial NIRS May Not Detect Hemispheric Regional Disparity in Continuously Measured COx/COx-a: An Exploratory Healthy and Cranial Trauma Time-Series Analysis. Bioengineering 2025, 12, 247. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Vakitbilir, N.; Gomez, A.; Stein, K.Y.; Froese, L.; Zeiler, F.A. Non-Invasive Mapping of Cerebral Autoregulation Using Near-Infrared Spectroscopy: A Study Protocol. Methods Protoc. 2023, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Sainbhi, A.S.; Froese, L.; Gomez, A.; Marquez, I.; Amenta, F.; Batson, C.; Stein, K.Y.; Zeiler, F.A. High Spatial and Temporal Resolution Cerebrovascular Reactivity for Humans and Large Mammals: A Technological Description of Integrated fNIRS and niABP Mapping System. Front. Physiol. 2023, 14, 1124268. [Google Scholar] [CrossRef]
- Gomez, A.; Sainbhi, A.S.; Stein, K.Y.; Vakitbilir, N.; Froese, L.; Zeiler, F.A. Statistical Properties of Cerebral near Infrared and Intracranial Pressure-Based Cerebrovascular Reactivity Metrics in Moderate and Severe Neural Injury: A Machine Learning and Time-Series Analysis. Intensive Care Med. Exp. 2023, 11, 57. [Google Scholar] [CrossRef]
- Froese, L.; Gomez, A.; Sainbhi, A.S.; Vakitbilir, N.; Marquez, I.; Amenta, F.; Park, K.; Stein, K.Y.; Berrington, N.; Dhaliwal, P.; et al. Optimal Bispectral Index Exists in Healthy Patients Undergoing General Anesthesia: A Validation Study. J. Clin. Monit. Comput. 2024, 38, 791–802. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Vakitbilir, N.; Gomez, A.; Stein, K.Y.; Froese, L.; Zeiler, F.A. Time-Series Autocorrelative Structure of Cerebrovascular Reactivity Metrics in Severe Neural Injury: An Evaluation of the Impact of Data Resolution. Biomed. Signal Process. Control 2024, 95, 106403. [Google Scholar] [CrossRef]
- Brady, K.; Joshi, B.; Zweifel, C.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Hogue, C.W. Real Time Continuous Monitoring of Cerebral Blood Flow Autoregulation Using Near-Infrared Spectroscopy in Patients Undergoing Cardiopulmonary Bypass. Stroke 2010, 41, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Carrera, E.; Brady, K.M.; Kirkpatrick, P.J.; Pickard, J.D.; Smielewski, P. Continuous Assessment of Cerebral Autoregulation With Near-Infrared Spectroscopy in Adults After Subarachnoid Hemorrhage. Stroke 2010, 41, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Laurikkala, J.; Aneman, A.; Peng, A.; Reinikainen, M.; Pham, P.; Jakkula, P.; Hästbacka, J.; Wilkman, E.; Loisa, P.; Toppila, J.; et al. Association of Deranged Cerebrovascular Reactivity with Brain Injury Following Cardiac Arrest: A Post-Hoc Analysis of the COMACARE Trial. Crit. Care 2021, 25, 350. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Froese, L.; Stein, K.Y.; Vakitbilir, N.; Hasan, R.; Gomez, A.; Bergmann, T.; Silvaggio, N.; Hayat, M.; Moon, J.; et al. Time-Series Autoregressive Models for Point and Interval Forecasting of Raw and Derived Commercial Near-Infrared Spectroscopy Measures: An Exploratory Cranial Trauma and Healthy Control Analysis. Bioengineering 2025, 12, 682. [Google Scholar] [CrossRef]
- Chatfield, C. The Analysis of Time Series: An Introduction, 6th ed.; Chapman and Hall/CRC: New York, NY, USA, 2003; ISBN 978-0-429-20870-6. [Google Scholar]
- Lütkepohl, H. New Introduction to Multiple Time Series Analysis; Springer: Berlin, Germany; New York, NY, USA, 2005; ISBN 978-3-540-40172-8. [Google Scholar]
- Chatfield, C.; Xing, H. The Analysis of Time Series: An Introduction with R, 7th ed.; Chapman and Hall/CRC: New York, NY, USA, 2019; ISBN 978-1-138-06613-7. [Google Scholar]
- Granger, C.W.J. Investigating Causal Relations by Econometric Models and Cross-Spectral Methods. Econometrica 1969, 37, 424–438. [Google Scholar] [CrossRef]
- Thudium, M.; Kornilov, E.; Moestl, S.; Hoffmann, F.; Hoff, A.; Kulapatana, S.; Urechie, V.; Oremek, M.; Rigo, S.; Heusser, K.; et al. Continuous Wavelet Based Transfer Function Analysis of Cerebral Autoregulation Dynamics for Neuromonitoring Using Near-Infrared Spectroscopy. Front. Physiol. 2025, 16, 1616125. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Shahbakhti, M.; Horschig, J.M.; Alderliesten, T.; Van Bel, F.; Colier, W.N.J.M.; Dudink, J. Respiratory Rate Extraction from Neonatal Near-Infrared Spectroscopy Signals. Sensors 2023, 23, 4487. [Google Scholar] [CrossRef] [PubMed]
- Sappia, M.S.; Hakimi, N.; Colier, W.N.J.M.; Horschig, J.M. Signal Quality Index: An Algorithm for Quantitative Assessment of Functional near Infrared Spectroscopy Signal Quality. Biomed. Opt. Express BOE 2020, 11, 6732–6754. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A Motion Correction Method for fNIRS. NeuroImage 2019, 184, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Li, H.; Li, N.; Zhang, S.; Liu, C.; Zhang, Q.; Cai, W. Functional Near-Infrared Spectroscopy as a Potential Objective Evaluation Technique in Neurocognitive Disorders after Traumatic Brain Injury. Front. Psychiatry 2022, 13, 903756. [Google Scholar] [CrossRef] [PubMed]
- Cabella, B.; Donnelly, J.; Cardim, D.; Liu, X.; Cabeleira, M.; Smielewski, P.; Haubrich, C.; Hutchinson, P.J.A.; Kim, D.-J.; Czosnyka, M. An Association Between ICP-Derived Data and Outcome in TBI Patients: The Role of Sample Size. Neurocrit Care 2017, 27, 103–107. [Google Scholar] [CrossRef] [PubMed]
| Variable | Median [IQR] or Count (%) |
|---|---|
| Duration of Recording (min) | 89.5 (85.7–95.3) |
| Number of Subjects | 50 |
| Age (years) | 28 (23.3–35) |
| Biological Sex (Male) | 28 (56%) |
| Hand Dominance (Right) | 49 (98%) |
| Physiologic Variable | Median (IQR) | |
|---|---|---|
| Raw Data | 10-s Decimated Data | |
| 1 Hz Sampled Data | ||
| ASD of rSO2 (%) | 31.85 (28.52–34.34) | 31.56 (28.65–34.43) |
| ASD of COx-a (au) | – | 0.26 (0.12–0.47) |
| MAD of ASD rSO2 (%) | 2.2 (1.88–2.73) | 2.16 (1.75–2.7) |
| MAD of ASD COx-a (au) | – | 0.16 (0.13–0.19) |
| 250 Hz Sampled Data | ||
| ASD of rSO2 (%) | 31.36 (28.08–34.64) | 31.48 (28.71–34.39) |
| ASD of COx-a (au) | – | 0.26 (0.12–0.46) |
| MAD of ASD rSO2 (%) | 2.26 (1.94–3.01) | 2.18 (1.74–2.67) |
| MAD of ASD COx-a (au) | – | 0.16 (0.14–0.19) |
| Physiologic Variable | Value | Median (IQR) | |
|---|---|---|---|
| Raw Data | 10-s Decimated Data | ||
| 1 Hz Sampled Data | |||
| rSO2 | r | 0.14 (−0.03–0.27) | 0.14 (−0.05–0.33) |
| p | 4.0 × 10−50 (3.2 × 10−231–2.5 × 10−10) | 1.6 × 10−9 (1.0 × 10−16–0.01) | |
| COx-a | r | – | 0.08 (−0.07–0.19) |
| p | – | 1.20 × 10−3 (4.8 × 10−9–0.07) | |
| 250 Hz Sampled Data | |||
| rSO2 | r | 0.07 (−0.02–0.26) | 0.14 (−0.05–0.33) |
| p | 0 (0–0) | 2.5 × 10−7 (9.1 × 10−16–0.01) | |
| COx-a | r | – | 0.08 (−0.07–0.18) |
| p | – | 3.0 × 10−3 (2.9 × 10−9–0.08) | |
| Physiologic Variable | Value | Median (IQR) | |
|---|---|---|---|
| Raw Data | 10-s Decimated Data | ||
| 1 Hz Sampled Data | |||
| rSO2 | Bias | 31.57 (21.31–35.79) | 31.53 (21.45–35.24) |
| Lower LoA | 23.89 (12.1–27.93) | 24.53 (12.68–28.66) | |
| Upper LoA | 38.96 (27.48–43.9) | 38.87 (27.26–43.14) | |
| LoA Spread | 12.47 (11.47–16.43) | 12.33 (10.96–15.82) | |
| Relative Bias | 208.05 (154.14–287.69) | 230.51 (161.51–310.5) | |
| Regression Slope | 0.96 (0.66–1.57) | 1.1 (0.72–1.63) | |
| Regression Intercept | −29.56 (−58.07–−3.86) | −31.08 (−63.86–−8.67) | |
| COx-a | Bias | – | −0.05 (−0.16–0.01) |
| Lower LoA | – | −0.78 (−1.03–−0.7) | |
| Upper LoA | – | 0.74 (0.65–0.84) | |
| LoA Spread | – | 1.55 (1.4–1.8) | |
| Relative Bias | – | −3.03 (−10.04–0.85) | |
| Regression Slope | – | 0.05 (−0.17–0.18) | |
| Regression Intercept | – | −0.04 (−0.14–0.02) | |
| 250 Hz Sampled Data | |||
| rSO2 | Bias | 31.49 (21.31–35.73) | 31.51 (21.44–35.17) |
| Lower LoA | 22.11 (10.04–27.39) | 23.69 (12.68–28.06) | |
| Upper LoA | 39.1 (28.61–44.42) | 38.86 (27.25–43.64) | |
| LoA Spread | 13.2 (11.69–21.09) | 12.32 (10.91–15.92) | |
| Relative Bias | 191.27 (107.95–278.3) | 211.72 (154.9–310.2) | |
| Regression Slope | 0.86 (−0.33–1.49) | 1.06 (0.69–1.59) | |
| Regression Intercept | −21.73 (−53.7–54.47) | −29.38 (−63.67–−4.91) | |
| COx-a | Bias | – | −0.05 (−0.17–0.02) |
| Lower LoA | – | −0.78 (−1.03–−0.7) | |
| Upper LoA | – | 0.74 (0.65–0.85) | |
| LoA Spread | – | 1.57 (1.4–1.8) | |
| Relative Bias | – | −3.37 (−9.62–1.08) | |
| Regression Slope | – | 0.05 (−0.15–0.21) | |
| Regression Intercept | – | −0.04 (−0.16–0.02) | |
| Physiologic Variable | Best Lag [Median (IQR)] | |
|---|---|---|
| Raw Data | 10-s Decimated Data | |
| 1 Hz Sampled Data | ||
| rSO2 | 0 (0–0) | 0 (0–0) |
| COx-a | – | 10.5 (−76.5–87) |
| 250 Hz Sampled Data | ||
| rSO2 | 0 (0–0) | 0 (0–0) |
| COx-a | – | 10.5 (−83–105) |
| ADF results for non-differenced data | |||||||||||||||
| Frequency | ABP | rSO2_Invos | COx-a_Invos | rSO2_OxyMon | COx-a_OxyMon | ||||||||||
| S | NS | NA | S | NS | NA | S | NS | NA | S | NS | NA | S | NS | NA | |
| 1 Hz | 49 | 1 | 0 | 36 | 14 | 0 | 48 | 2 | 0 | 38 | 12 | 0 | 50 | 0 | 0 |
| 250 Hz | 49 | 1 | 0 | 37 | 13 | 0 | 48 | 2 | 0 | 39 | 11 | 0 | 48 | 2 | 0 |
| ADF results for first-order differenced data | |||||||||||||||
| Frequency | ABP | rSO2_Invos | COx-a_Invos | rSO2_OxyMon | COx-a_OxyMon | ||||||||||
| S | NS | NA | S | NS | S | NS | NA | S | NS | S | NS | NA | S | NS | |
| 1 Hz | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 |
| 250 Hz | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 |
| KPSS results for non-differenced data | |||||||||||||||
| Frequency | ABP | rSO2_Invos | COx-a_Invos | rSO2_OxyMon | COx-a_OxyMon | ||||||||||
| S | NS | NA | S | NS | S | NS | NA | S | NS | NA | S | NS | NA | ||
| 1 Hz | 22 | 28 | 0 | 9 | 41 | 0 | 31 | 19 | 0 | 8 | 42 | 0 | 40 | 10 | 0 |
| 250 Hz | 21 | 29 | 0 | 9 | 41 | 0 | 31 | 19 | 0 | 8 | 42 | 0 | 40 | 10 | 0 |
| KPSS results for first-order differenced data | |||||||||||||||
| Frequency | ABP | rSO2_Invos | COx-a_Invos | rSO2_OxyMon | COx-a_OxyMon | ||||||||||
| S | NS | NA | S | NS | S | NS | NA | S | NS | NA | S | NS | NA | ||
| 1 Hz | 49 | 1 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 |
| 250 Hz | 48 | 2 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 | 50 | 0 | 0 |
| Physiologic Variable | Optimal ARIMA Models (Median [IQR]) | |
|---|---|---|
| 1 Hz | 250 Hz | |
| ABP | (5, 1, 3) [(3, 1, 3)–(6, 1, 7)] | (5, 1, 1) [(3, 1, 3)–(7, 1, 3)] |
| rSO2_Invos | (4, 1, 7) [(3, 1, 3)–(6, 1, 7)] | (4, 1, 4) [(2, 1, 2)–(6, 1, 7)] |
| COx-a_Invos | (3, 1, 6) [(1, 1, 10)–(6, 1, 5)] | (3, 1, 0) [(1, 1, 8)–(6, 1, 3)] |
| rSO2_OxyMon | (2, 1, 3) [(1, 1, 10)–(7, 1, 1)] | (3, 1, 3) [(2, 1, 1)–(5, 1, 1)] |
| COx-a_ OxyMon | (2, 1, 9) [(2, 1, 0)–(4, 1, 8)] | (2, 1, 9) [(2, 1, 1)–(4, 1, 2)] |
| Direction | 1 Hz [% (Count)] | 250 Hz [% (Count)] | ||
|---|---|---|---|---|
| >0.1% | NA | >0.1% | NA | |
| ABP → rSO2_Invos | 84% (42) | 4% (2) | 94% (47) | 2% (1) |
| rSO2_Invos → ABP | 80% (40) | 4% (2) | 94% (47) | 2% (1) |
| ABP → rSO2_OxyMon | 90% (45) | 2% (1) | 96% (48) | 2% (1) |
| rSO2_OxyMon → ABP | 90% (45) | 2% (1) | 98% (49) | 2% (1) |
| Signal | Direction | 1 Hz [% (Count)] | 250 Hz [% (Count)] |
|---|---|---|---|
| ABP and rSO2_Invos | ABP → rSO2_Invos | 54% (27) | 52% (26) |
| rSO2_Invos → ABP | 46% (23) | 48% (24) | |
| ABP and rSO2_OxyMon | ABP → rSO2_OxyMon | 50% (25) | 52% (26) |
| rSO2_OxyMon → ABP | 50% (25) | 48% (24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainbhi, A.S.; Vakitbilir, N.; Bergmann, T.; Stein, K.Y.; Hasan, R.; Silvaggio, N.; Hayat, M.; Moon, J.; Zeiler, F.A. Time-Domain Analysis of Low- and High-Frequency Near-Infrared Spectroscopy Sensor Technologies for Characterization of Cerebral Pressure–Flow and Oxygen Delivery Physiology: A Prospective Observational Study. Sensors 2025, 25, 5391. https://doi.org/10.3390/s25175391
Sainbhi AS, Vakitbilir N, Bergmann T, Stein KY, Hasan R, Silvaggio N, Hayat M, Moon J, Zeiler FA. Time-Domain Analysis of Low- and High-Frequency Near-Infrared Spectroscopy Sensor Technologies for Characterization of Cerebral Pressure–Flow and Oxygen Delivery Physiology: A Prospective Observational Study. Sensors. 2025; 25(17):5391. https://doi.org/10.3390/s25175391
Chicago/Turabian StyleSainbhi, Amanjyot Singh, Nuray Vakitbilir, Tobias Bergmann, Kevin Y. Stein, Rakibul Hasan, Noah Silvaggio, Mansoor Hayat, Jaewoong Moon, and Frederick A. Zeiler. 2025. "Time-Domain Analysis of Low- and High-Frequency Near-Infrared Spectroscopy Sensor Technologies for Characterization of Cerebral Pressure–Flow and Oxygen Delivery Physiology: A Prospective Observational Study" Sensors 25, no. 17: 5391. https://doi.org/10.3390/s25175391
APA StyleSainbhi, A. S., Vakitbilir, N., Bergmann, T., Stein, K. Y., Hasan, R., Silvaggio, N., Hayat, M., Moon, J., & Zeiler, F. A. (2025). Time-Domain Analysis of Low- and High-Frequency Near-Infrared Spectroscopy Sensor Technologies for Characterization of Cerebral Pressure–Flow and Oxygen Delivery Physiology: A Prospective Observational Study. Sensors, 25(17), 5391. https://doi.org/10.3390/s25175391







