A Novel ML-Powered Nanomembrane Sensor for Smart Monitoring of Pollutants in Industrial Wastewater
Abstract
1. Introduction
- To secure and maintain discharge permits by evidencing adherence to environmental standards;
- To proactively assess effluent quality, reducing the risk of non-compliance penalties during unannounced regulatory inspections.
- Establish a new analysis methodology by exploiting the nanomembrane electrochemical properties;
- Evaluate performance in terms of sensitivity and selectivity via experimental comparison with commercial electrode sensors and conventional chemical analysis techniques;
- Evaluate performance in terms of sensitivity and selectivity under varying operational conditions for real-world field applications.
2. Sensors and Methods
2.1. Electrode Sensors and Conventional Methods
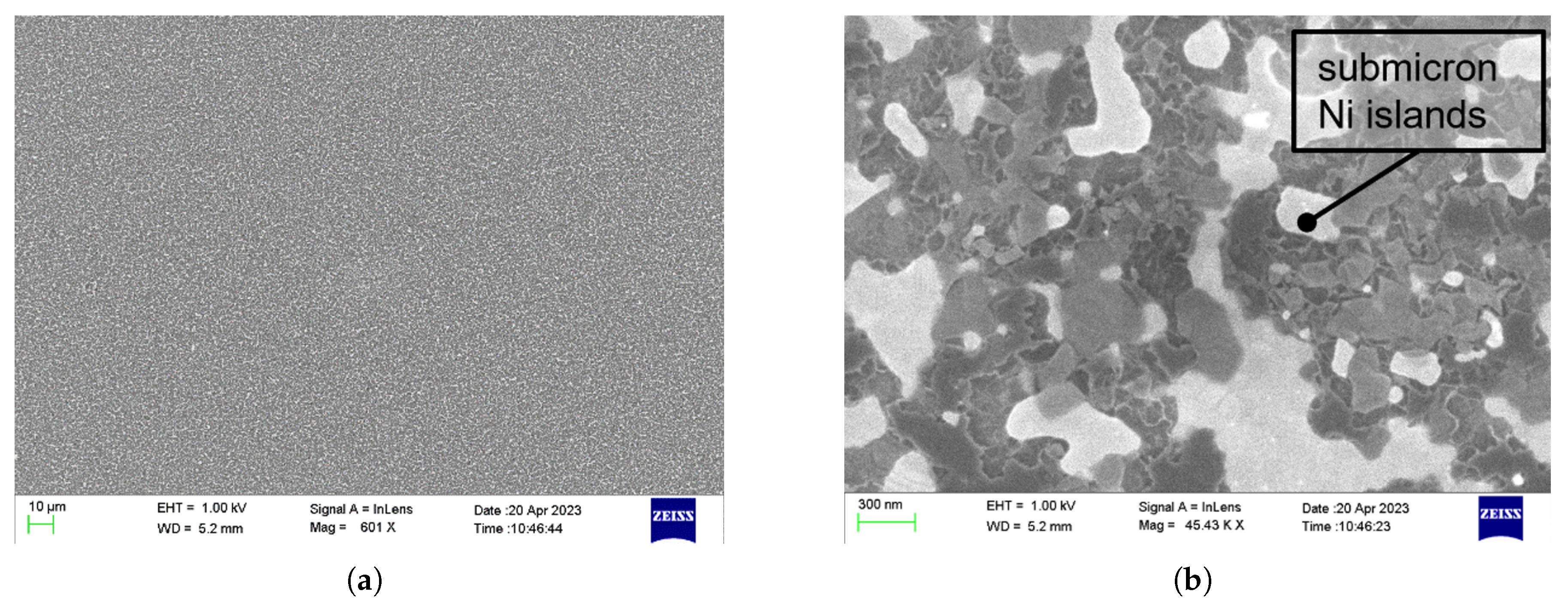
2.2. Nanosensor and Non-Conventional Method
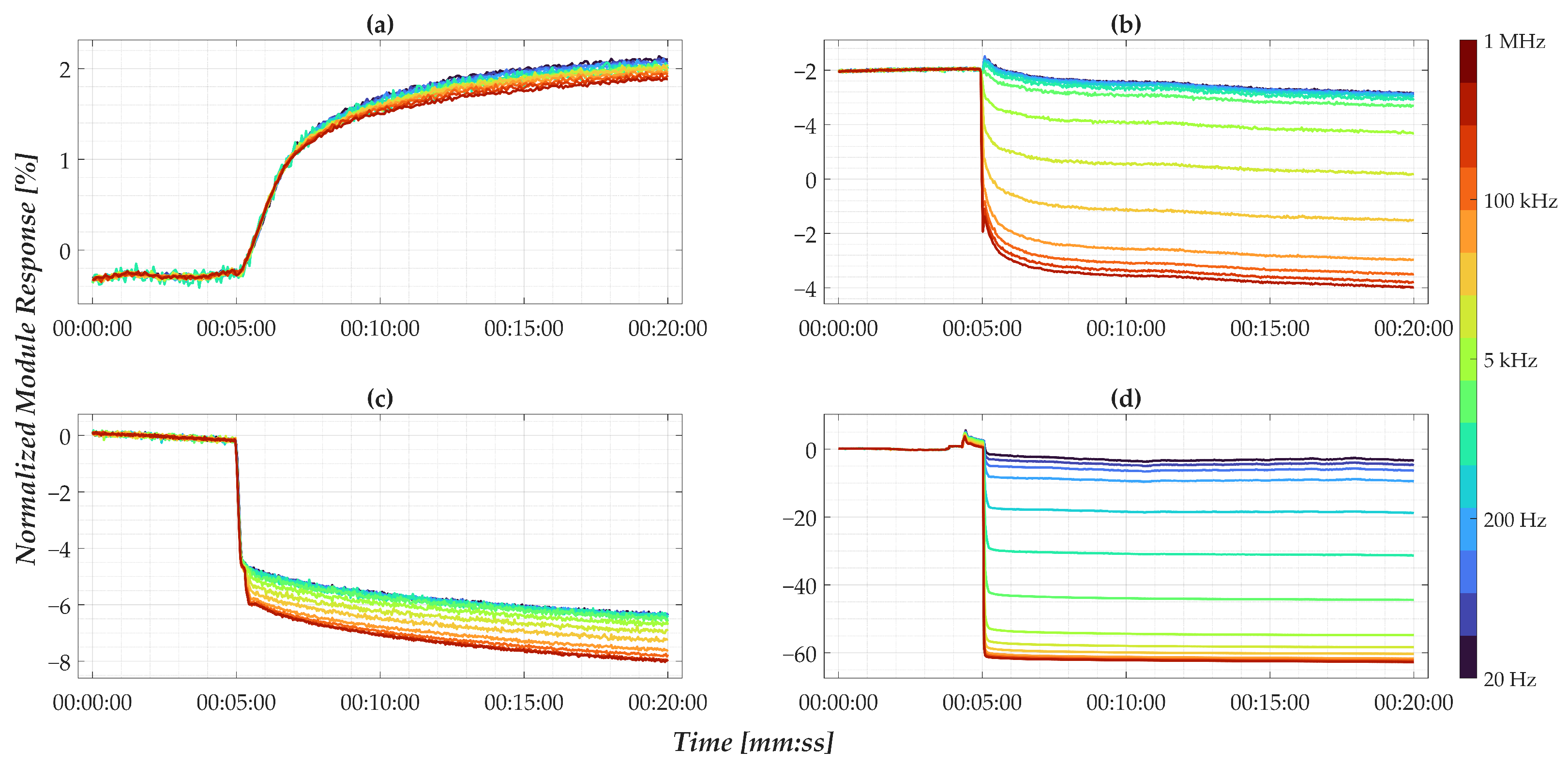
- is the normalized nanomembrane impedance module at a given frequency and at time , expressed in percentage;
- is the absolute nanomembrane impedance module at a given frequency and at time ;
- is the baseline nanomembrane impedance module at frequency , calculated as the average value in a specific time window before the drop.
- represents the nanomembrane Spread Factor at time after the drop;
- is the absolute nanomembrane impedance module at time after drop, where maximum, minimum and average values are evaluated over all frequencies.
3. Experimental Setup and Preliminary Results
3.1. Test Setup
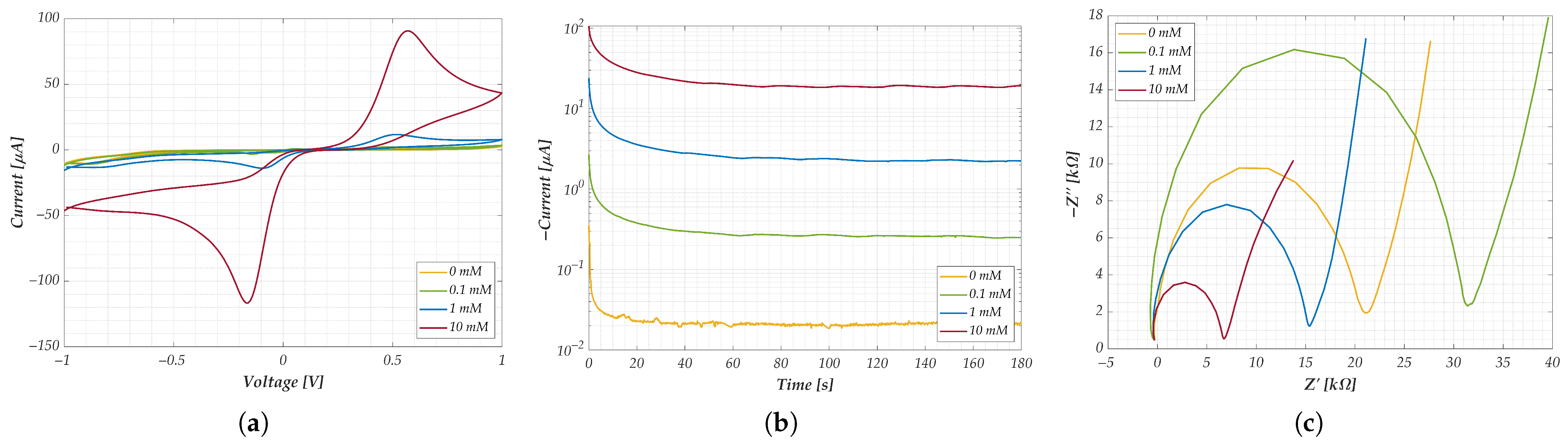
- CV: A potential sweep from −1 V to +1 V was applied twice using a triangular waveform. The potential increased by 0.01 V at each measurement, with a scan rate of 0.05 V/s. Voltage, anodic, and cathodic currents were extracted at the second sweep for analysis, for a total of 80 s each test.
- CA: A potential step of −0.05 V, consistent with the CV results, was applied. The resulting current was recorded over 3 min, yielding 1800 data points per SPE.
- EIS: A sinusoidal forcing potential of 0.01 V was applied, in “time scan” mode. Measurements were performed over a total duration of 15 min per SPE, with 20 complete frequency sweeps logarithmically spaced between 20 Hz and 1 MHz, for a total of 35 frequencies.
3.2. Experimental Setup
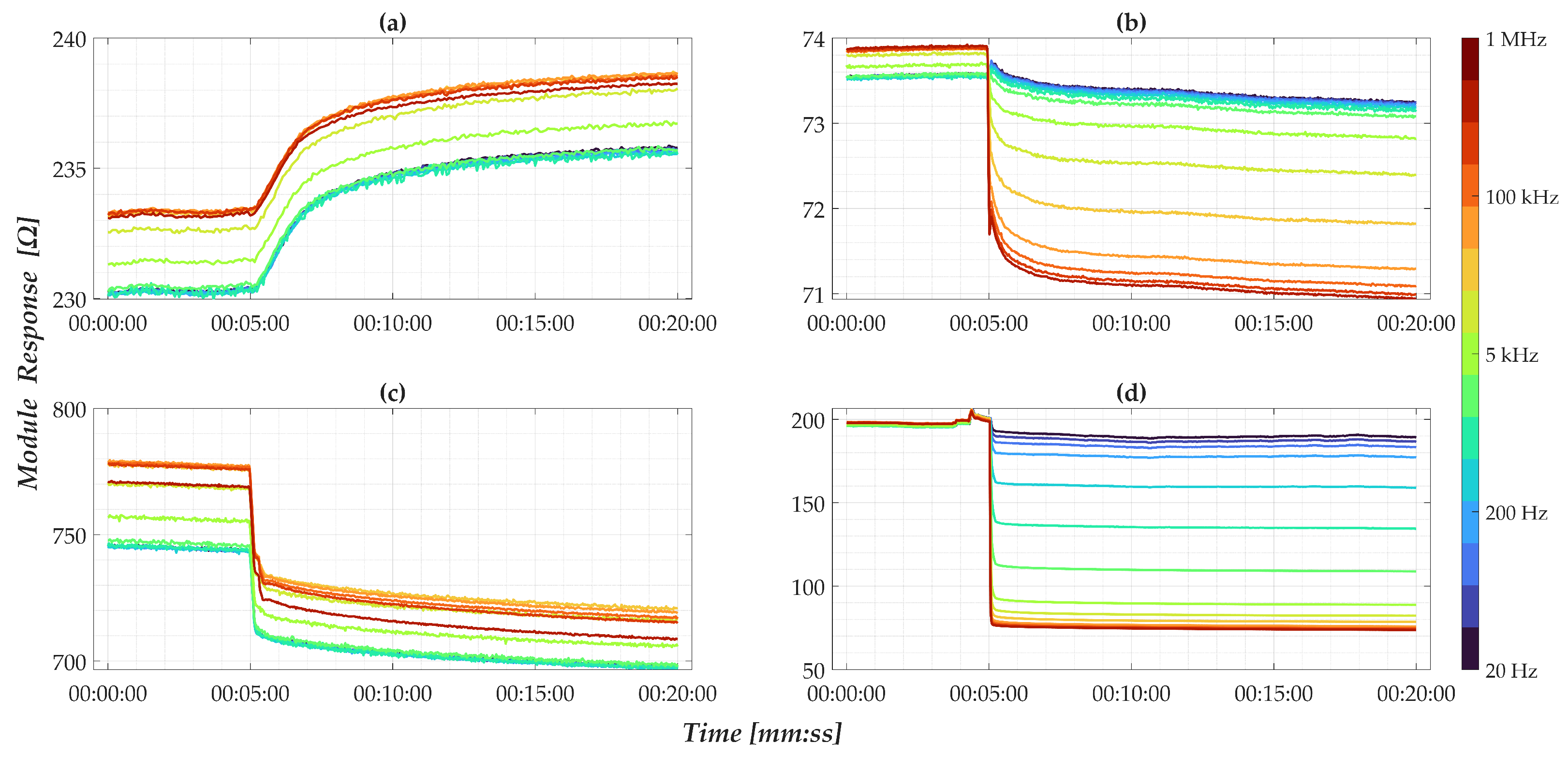
3.3. Preliminary Outputs
4. Linear Model and Machine-Learning Approach
4.1. Linear Model Approach
4.1.1. Model Configuration
- is the unknown concentration;
- and are the linear model coefficients;
- is the synthetic parameter.
- For CV, the area under the current-voltage curve was used. This was calculated in MATLAB by integrating the entire curve, representing the total charge transferred during the redox reaction, which correlates directly with the analyte concentration [53].
- For EIS on electrode sensors, the chosen parameter was the difference between the mean values of the minimum peaks in the Nyquist diagrams for each concentration. This metric represents the charge transfer resistance (), which is inversely proportional to analyte concentration, as widely demonstrated in previous studies [70,73,74].
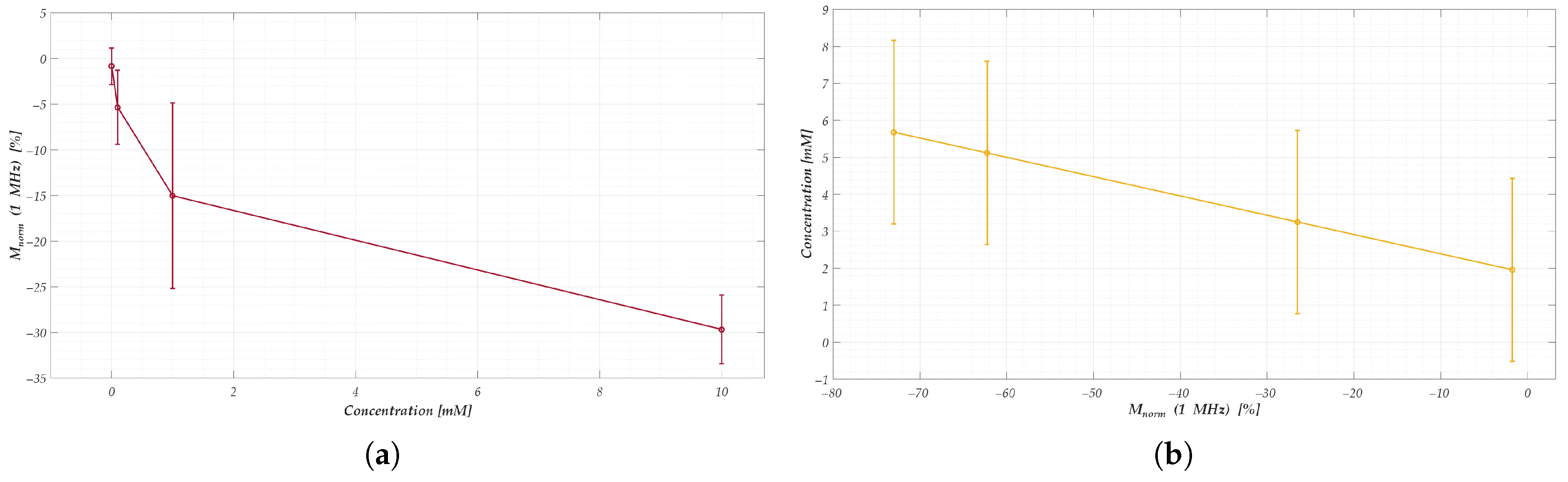
- For nanomembranes, EIS time-domain analysis, the response at 1 MHz was selected as the representative synthetic parameter. This value was computed as the average of the normalized signal over a 180-s window, starting 10 s after the initial impedance drop. The choice of this high-frequency component is justified by the observations discussed in Figure 6, which highlighted its high sensitivity to concentration changes. Additionally, the selected empirical times are entirely consistent with the considerations regarding signal drift and post-drop stabilization presented in Section 3.
4.1.2. Model Test
4.2. Machine-Learning Approach
- CV features consisted of both voltage and current at each excitation level;
- CA included the full current–time profile;
- EIS for nanomembranes in the time domain included 15 normalized frequency responses and a spread factor (see Equations (6) and (7)), extracted from the first 180 s following pollutant addition (excluding the initial 10 s to ensure a minimum settling time). The baseline was defined as the average of the first 5 min to guarantee sensor stability. The choice of the 3-min window reflects the fact that the most informative signal dynamics occur immediately after pollutant addition. At the same time, longer acquisition periods may introduce drift caused by external disturbances (e.g., evaporation or environmental contaminants) not directly related to the interaction with the target molecules. If one had trained the model with all available data, including the drift, it would have been trained on potentially erroneous data, so 3 min is an optimal balance between volume and stability of data.
- Cubic SVM. A support vector machine with a third-degree polynomial kernel. The normalization of the data was enabled, and the kernel scaling together with the box constraint from which the support vectors derive were automatically optimized by the toolbox (the software uses a heuristic procedure to select the parameters).
- Subspace k-NN. An ensemble of k-nearest neighbors learners trained on selected feature subspaces. The number of learners and the subspace dimensions were determined by the toolbox optimization procedure, while Euclidean distance was used as the similarity metric.
- Efficient Logistic Regression. The regularization strength was adjusted automatically, ensuring an appropriate trade-off between bias and variance. The Auto setting sets lambda equal to 1/n, where n is the number of observations in the training sample. The solver used was SGD for efficient logistic regression. While the regularization strength (lambda) (or the number of in-fold observations, if using cross-validation).
- Kernel Naive Bayes. A probabilistic classifier using Gaussian kernel density estimation. Bandwidth parameters for the kernel were optimized through the toolbox’s built-in procedure.
- SVM is a supervised max-margin model with associated learning algorithms that analyze data by finding the optimal hyperplane that maximizes the margin between classes;
- KNN assigns classes based on the closest training samples, using different metrics;
- Logistic Regression models class probabilities using a sigmoid function; ELR is a computationally efficient variant implemented in MATLAB;
- KNB combines kernel density estimation with Bayes’ theorem, offering robustness in small datasets and good classification accuracy.
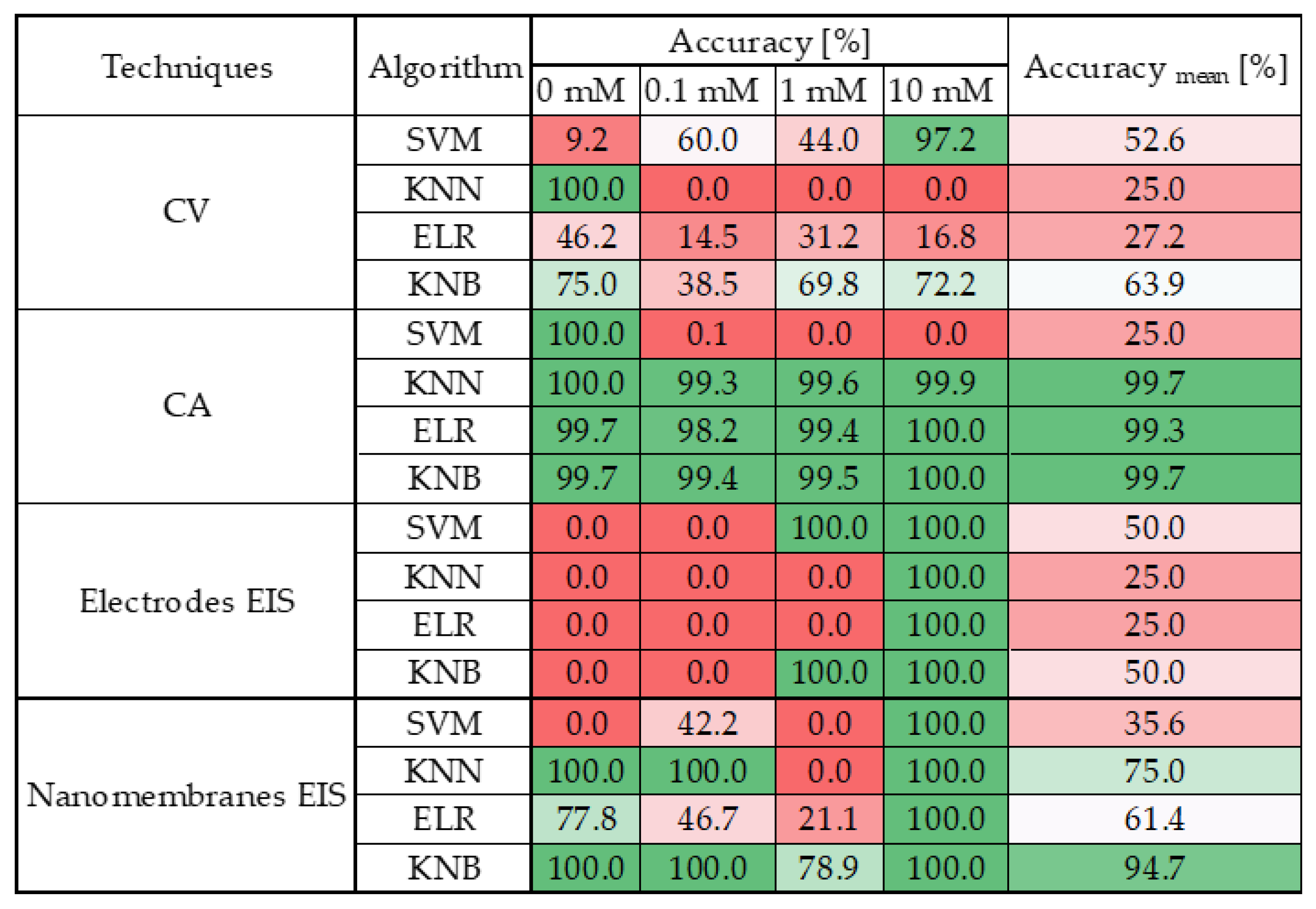
- CV does not lend itself well to classification, as all tested algorithms yielded poor performance. Only the k-Nearest Bayesian (KNB) classifier achieved a moderate average accuracy level; however, even in this case, the classification accuracy for the 0.1 mM class remained below 50%.
- CA clearly emerges as the most robust technique, consistently delivering outstanding results across nearly all algorithms. With the sole exception of the Support Vector Machine (SVM), all models achieved an average accuracy exceeding 99%, with class-wise accuracies surpassing 98% across all valid algorithms.
- EIS on electrode sensors proves to be the most challenging technique, as anticipated from the preliminary analysis in Section 3. None of the tested algorithms demonstrated satisfactory performance in this context.
- EIS on nanomembranes, by contrast, exhibited good average classification performance—above 60% accuracy—with all algorithms except SVM. Nonetheless, certain classes failed to exceed 50% accuracy when classified using KNN and ELR. However, KNB yielded exceptional results: each class was accurately identified, achieving 100% accuracy in all but the 1 mM class, which still reached over 75% accuracy. The overall average KNB accuracy exceeded 90%.
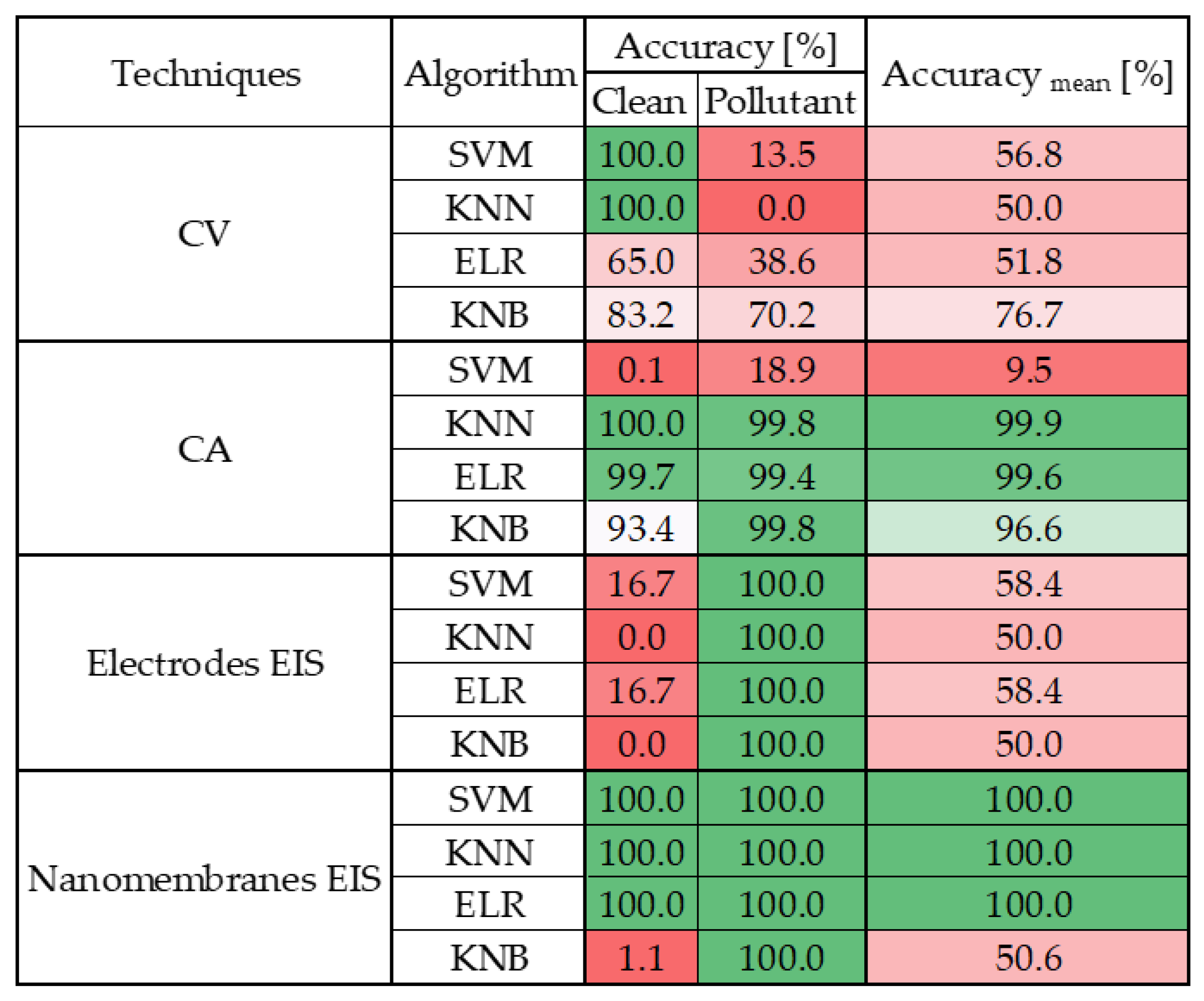
4.3. Sensitivity Performance Evaluation over Selectivity
- ML significantly improves the performance of nanomembrane-based EIS over linear modeling;
- Only the KNB algorithm provides acceptable classification accuracy for nanomembranes.
5. Extensive Nanomembranes Performance Analysis with ML Approach
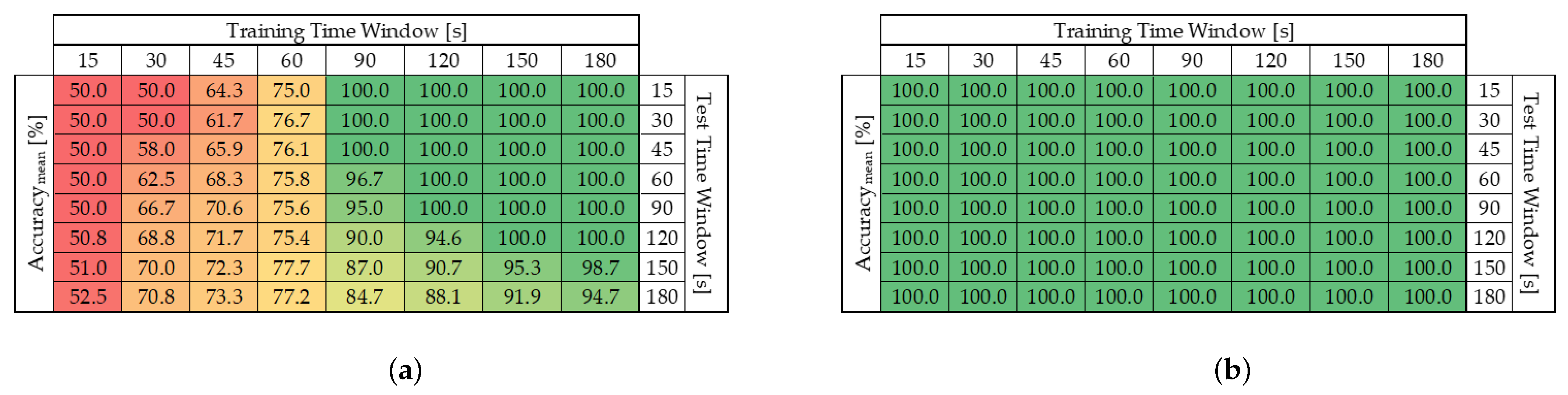
5.1. Test Duration: Dataset Size
- A rapid, real-time response for pollutant detection, feasible within a few seconds;
- A more detailed, offline quantification analysis, with cloud-based training performed within minutes after detection.
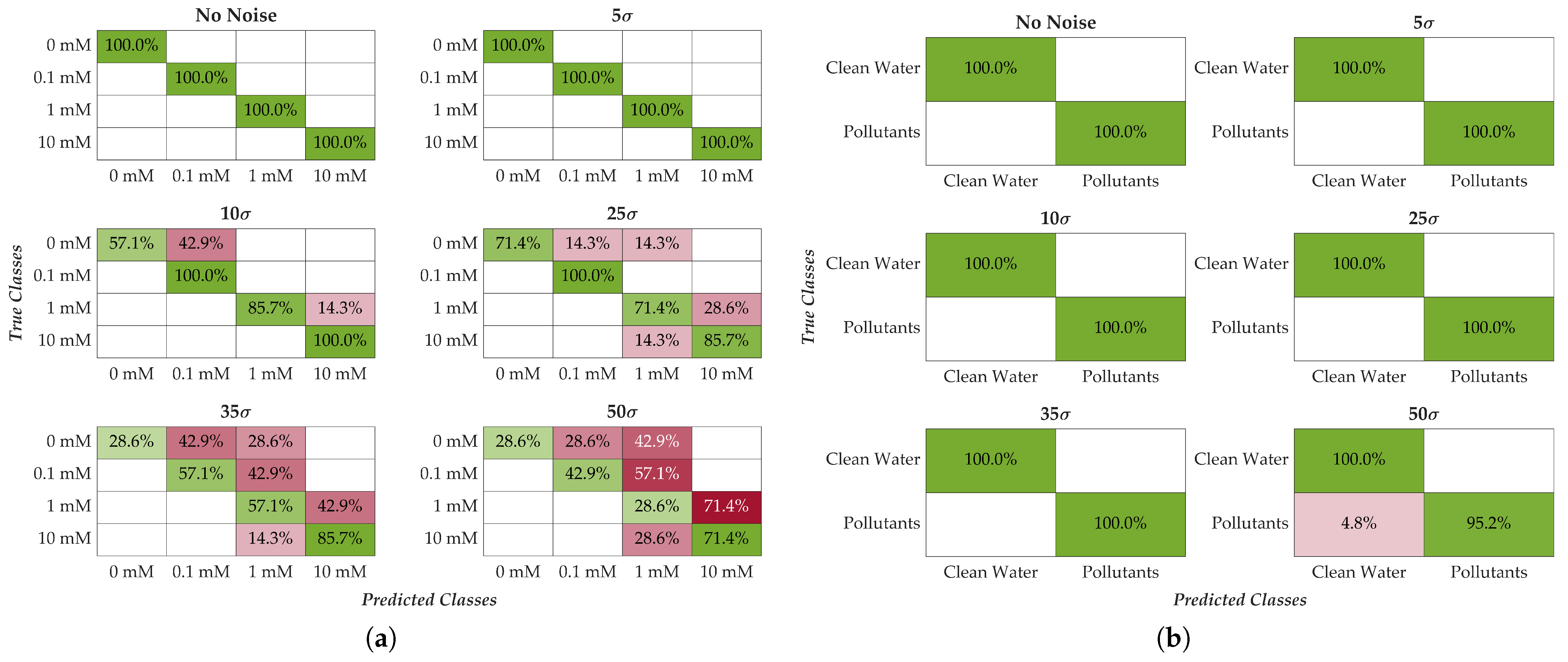
5.2. Noise Analysis
6. Conclusions
- Validation on real-world water samples, including complex industrial and environmental matrices;
- Chemical functionalization of nanomembranes to enhance selectivity toward specific analytes;
- Extension of the study to additional pollutants and concentration ranges, with a more refined investigation between 0.1–1 mM and 1–10 mM;
- Adoption of ML-based regression algorithms, rather than classifiers, to evaluate intermediate values within the newly tested concentration ranges;
- Cost estimation and a large-scale integration plan.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| WWTP | Wastewater Treatment Plant |
| EIS | Electrochemical Impedance Spectroscopy |
| ML | Machine Learning |
| SVM | Support Vector Machine |
| KNN | k-Nearest Neighbors |
| LR | Logistic Regression |
| BQ | Benzoquinone |
| CV | Cyclic Voltammetry |
| CA | Chronoamperometry |
| SPE | Screen Printed Electrode |
| PPF | Pyrolysed Photoresist Films |
| SEM | Scanning Electron Microscopy |
References
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Ghasemi, N.; Zare, F.; Hosano, H. A review of pulsed power systems for degrading water pollutants ranging from microorganisms to organic compounds. IEEE Access 2019, 7, 150863–150891. [Google Scholar] [CrossRef]
- Blaisdell, J.; Turyk, M.E.; Almberg, K.S.; Jones, R.M.; Stayner, L.T. Prenatal exposure to nitrate in drinking water and the risk of congenital anomalies. Environ. Res. 2019, 176, 108553. [Google Scholar] [CrossRef]
- Shankar, R.; Kumar, S.; Prasad, A.K.; Khare, P.; Varma, A.K.; Yadav, V.K. Biological wastewater treatment plants (WWTPs) for industrial wastewater. In Microbial Ecology of Wastewater Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–216. [Google Scholar]
- Pisa, I.; Santin, I.; Morell, A.; Vicario, J.L.; Vilanova, R. LSTM-based wastewater treatment plants operation strategies for effluent quality improvement. IEEE Access 2019, 7, 159773–159786. [Google Scholar] [CrossRef]
- Systems, L. Industrial Wastewater Discharge Limits and Requirements. 2024. Available online: https://liqtech.com/systems/industrial-wastewater/industrial-wastewater-discharge-limits-and-requirements/ (accessed on 28 March 2025).
- Gu, K.; Liu, Y.; Liu, H.; Liu, B.; Qiao, J.; Lin, W.; Zhang, W. Air pollution monitoring by integrating local and global information in self-adaptive multiscale transform domain. IEEE Trans. Multimed. 2025, 27, 3716–3728. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zeng, G.; Yang, Z.; Lin, Q.; Wang, Y.; Wang, X.; Pu, S. Two-dimensional Na-Bentonite@ MXene composite membrane with switchable wettability for selective oil/water separation. Sep. Purif. Technol. 2023, 306, 122677. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, H.; Huang, Z.; Zhang, W.; Gao, H.; Liang, L.; Dong, L.; Meng, H. Membranes based on covalent organic frameworks through green and scalable interfacial polymerization using ionic liquids for antibiotic desalination. Angew. Chem. 2024, 136, e202316315. [Google Scholar] [CrossRef]
- Fan, W.; Xin, Q.; Dai, Y.; Chen, Y.; Liu, S.; Zhang, X.; Yang, Y.; Gao, X. Competitive transport and adsorption of CO2/H2O in the graphene nano-slit pore: A molecular dynamics simulation study. Sep. Purif. Technol. 2025, 353, 128394. [Google Scholar] [CrossRef]
- Qiao, Y.; Lü, J.; Wang, T.; Liu, K.; Zhang, B.; Snoussi, H. A multihead attention self-supervised representation model for industrial sensors anomaly detection. IEEE Trans. Ind. Inform. 2023, 20, 2190–2199. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, C.; Shao, H.; Deng, J.; Wang, Y. TMFF: Trustworthy multi-focus fusion framework for multi-label sewer defect classification in sewer inspection videos. IEEE Trans. Circuits Syst. Video Technol. 2024, 34, 12274–12287. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Yuan, S.; Yang, X.; Lu, J.; Jiang, B. A centrifugal microfluidic system for automated detection of multiple heavy metal ions by aptamer-based colorimetric assay. Sens. Actuators B Chem. 2024, 403, 135210. [Google Scholar] [CrossRef]
- La Cognata, R.; Piazza, S.; Freni, G. Pollutant Monitoring Solutions in Water and Sewerage Networks: A Scoping Review. Water 2025, 17, 1423. [Google Scholar] [CrossRef]
- Ibarlucea Cantón, B. Monolithically Integrated Polymeric Lab-on-(Bio) Chips with Photonic/Electrochemical Detection. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2014. [Google Scholar]
- Bonyar, A.; Nagy, P.; Mayer, V.; Vitéz, A.; Gerecs, A.; Sántha, H.; Harsányi, G. A colorimetry based, semi-automated portable sensor device for the detection of arsenic in drinking water. Sens. Actuators B Chem. 2017, 251, 1042–1049. [Google Scholar] [CrossRef]
- Sajed, S.; Arefi, F.; Kolahdouz, M.; Sadeghi, M. Improving sensitivity of mercury detection using learning based smartphone colorimetry. Sens. Actuators B Chem. 2019, 298, 126942. [Google Scholar] [CrossRef]
- da Silva, V.A.O.P.; de Freitas, R.C.; de Oliveira, P.R.; Moreira, R.C.; Marcolino-Junior, L.H.; Bergamini, M.F.; Coltro, W.K.; Janegitz, B.C. Microfluidic paper-based device integrated with smartphone for point-of-use colorimetric monitoring of water quality index. Measurement 2020, 164, 108085. [Google Scholar] [CrossRef]
- Nguyen, H.; Misbah, I.; Shih, W.C. Smartphone nano-colorimetry for on-demand multiplex lead and mercury detection and quantitation in drinking water. IEEE Sens. J. 2020, 20, 6685–6691. [Google Scholar] [CrossRef]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low cost lab on chip for the colorimetric detection of nitrate in mineral water products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, K.; Hwang, Y. Arduino and IoT-based direct filter observation method monitoring the color change of water filter for safe drinking water. J. Water Process Eng. 2022, 49, 103158. [Google Scholar] [CrossRef]
- Kaviya, K.; Rajamanikandan, R.; Santhamoorthy, M.; Farah, M.A.; Mani, K.S. A rhodamine-conjugated fluorescent and colorimetric receptor for the detection of Cu2+ ions: Environmental utility and smartphone integration. Anal. Methods 2025, 17, 1389–1400. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, A.; Zhang, D.; Xie, W.; Peng, H. Fluorescence and colorimetric analysis of β-estradiol based on aptamer assembled spherical nucleic acids. Anal. Methods 2024, 16, 6356–6363. [Google Scholar] [CrossRef]
- Goyal, C.; Malkurthi, S.; Yellakonda, K.V.R.; Hussain, A.M. Machine Learning Based Low-Cost Colorimetric Sensor for Ph and Free-Chlorine Measurement. IEEE Sens. Lett. 2024, 8, 4503904. [Google Scholar] [CrossRef]
- Shang, Z.; Tian, S.; Wang, Y.; Zhang, C.; Meng, Q.; Zhang, R.; Zhang, Z. 1,8-naphthalimide-triphenylamine-based red-emitting fluorescence probes for the detection of hydrazine in real water samples and applications in bioimaging in vivo. Sens. Actuators B Chem. 2024, 398, 134725. [Google Scholar] [CrossRef]
- Fan, X.; Lv, J.; Li, R.; Chen, Y.; Zhang, S.; Liu, T.; Zhou, S.; Shao, X.; Wang, S.; Hu, G.; et al. Paper test strip for silver ions detection in drinking water samples based on combined fluorometric and colorimetric methods. Arab. J. Chem. 2023, 16, 104492. [Google Scholar] [CrossRef]
- Doǧan, V.; Isık, T.; Kılıç, V.; Horzum, N. A field-deployable water quality monitoring with machine learning-based smartphone colorimetry. Anal. Methods 2022, 14, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Mannaerts, C.M.; Shang, Z. A low-cost digital colorimetry setup to investigate the relationship between water color and its chemical composition. Sensors 2021, 21, 6699. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T.; Yang, Z.; Pires, N.; Høivik, N. Compatible immuno-NASBA LOC device for quantitative detection of waterborne pathogens: Design and validation. Lab Chip 2012, 12, 602–612. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T. A household LOC device for online monitoring bacterial pathogens in drinking water with green design concept. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1708–1711. [Google Scholar]
- Peng, G.; Chen, Y.; Deng, R.; He, Q.; Liu, D.; Lu, Y.; Lin, J.M. Highly sensitive and selective determination of Hg (II) based on microfluidic chip with on-line fluorescent derivatization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 1–6. [Google Scholar] [CrossRef]
- Giménez-Gómez, P.; Baldi, A.; Ayora, C.; Fernandez-Sanchez, C. Automated determination of As (III) in waters with an electrochemical sensor integrated into a modular microfluidic system. ACS Sens. 2019, 4, 3156–3165. [Google Scholar] [CrossRef]
- Kremers, T.; Thelen, S.; Bosbach, N.; Schnakenberg, U. PortaDrop: A portable digital microfluidic platform providing versatile opportunities for Lab-On-A-Chip applications. PLoS ONE 2020, 15, e0238581. [Google Scholar] [CrossRef]
- Gagliardi, M.; Agostini, M.; Lunardelli, F.; Lamanna, L.; Miranda, A.; Bazzichi, A.; Luminare, A.G.; Cervelli, F.; Gambineri, F.; Totaro, M.; et al. Surface acoustic wave-based lab-on-a-chip for the fast detection of Legionella pneumophila in water. Sens. Actuators B Chem. 2023, 379, 133299. [Google Scholar] [CrossRef]
- Catini, A.; Capuano, R.; Tancredi, G.; Dionisi, G.; Di Giuseppe, D.; Filippi, J.; Martinelli, E.; Di Natale, C. A lab-on-a-chip based automatic platform for continuous nitrites sensing in aquaculture. Sensors 2022, 22, 444. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, L.; Zhong, G.; Wu, Q.; Liu, J.; Liu, X. A portable analytical system for rapid on-site determination of total nitrogen in water. Water Res. 2021, 202, 117410. [Google Scholar] [CrossRef] [PubMed]
- Thio, S.K.; Bae, S.W.; Park, S.Y. Lab on a smartphone (LOS): A smartphone-integrated, plasmonic-enhanced optoelectrowetting (OEW) platform for on-chip water quality monitoring through LAMP assays. Sens. Actuators B Chem. 2022, 358, 131543. [Google Scholar] [CrossRef]
- Furuya, N.; Matsuyuki, A.; Higuchi, S.; Tanaka, S. Determination of nitrite ion in waste and treated waters by resonance Raman spectrometry. Water Res. 1980, 14, 747–752. [Google Scholar] [CrossRef]
- Guo, Y.; Li, D.; Zheng, S.; Xu, N.; Deng, W. Utilizing Ag–Au core-satellite structures for colorimetric and surface-enhanced Raman scattering dual-sensing of Cu (II). Biosens. Bioelectron. 2020, 159, 112192. [Google Scholar] [CrossRef]
- Franca, R.D.; Carvalho, V.C.; Fradinho, J.C.; Reis, M.A.; Lourenço, N.D. Raman spectrometry as a tool for an online control of a phototrophic biological nutrient removal process. Appl. Sci. 2021, 11, 6600. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Z.; Huang, Y.; Wang, T.; Zheng, S.; DePasquale, A.; Brüeckner, C.; Lei, Y.; Li, B. Long-term continuous and real-time in situ monitoring of Pb (II) toxic contaminants in wastewater using solid-state ion selective membrane (S-ISM) Pb and pH auto-correction assembly. J. Hazard. Mater. 2020, 400, 123299. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, T.; Xu, Z.; Hughes, E.; Qian, F.; Lee, M.; Fan, Y.; Lei, Y.; Brückner, C.; Li, B. Real-time in situ monitoring of nitrogen dynamics in wastewater treatment processes using wireless, solid-state, and ion-selective membrane sensors. Environ. Sci. Technol. 2019, 53, 3140–3148. [Google Scholar] [CrossRef]
- Marino, L.; Gagliano, E.; Santoro, D.; Roccaro, P. Fluorescence sensor enabled control of contaminants of emerging concern in reclaimed wastewater using ozone-based treatment processes. Water Res. 2025, 268, 122616. [Google Scholar] [CrossRef]
- Behmel, S.; Damour, M.; Ludwig, R.; Rodriguez, M. Water quality monitoring strategies—A review and future perspectives. Sci. Total Environ. 2016, 571, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, E.T.; Spanhol, F.A.; Slongo, J.S.; da Silva, M.V.R.; Pazinato, J.; de Lima Lobo, A.V.; Coutinho, F.R.; Pfrimer, F.W.D.; Lindino, C.A.; Oyamada, M.S.; et al. Low-cost water quality sensors for IoT: A systematic review. Sensors 2023, 23, 4424. [Google Scholar] [CrossRef]
- Baghel, L.K.; Gautam, S.; Malav, V.K.; Kumar, S. TEMPSENSE: LoRa enabled integrated sensing and localization solution for water quality monitoring. IEEE Trans. Instrum. Meas. 2022, 71, 3000311. [Google Scholar] [CrossRef]
- Baah, M.; Rahman, A.; Sibilia, S.; Trezza, G.; Ferrigno, L.; Micheli, L.; Maffucci, A.; Soboleva, E.; Svirko, Y.; Kuzhir, P. Electrical impedance sensing of organic pollutants with ultrathin graphitic membranes. Nanotechnology 2021, 33, 075207. [Google Scholar] [CrossRef] [PubMed]
- Baah, M.; Obraztsov, P.; Paddubskaya, A.; Biciunas, A.; Suvanto, S.; Svirko, Y.; Kuzhir, P.; Kaplas, T. Electrical, transport, and optical properties of multifunctional graphitic films synthesized on dielectric surfaces by nickel nanolayer-assisted pyrolysis. ACS Appl. Mater. Interfaces 2020, 12, 6226–6233. [Google Scholar] [CrossRef]
- Kaplas, T.; Matikainen, A.; Nuutinen, T.; Suvanto, S.; Vahimaa, P.; Svirko, Y. Scalable fabrication of the graphitic substrates for graphene-enhanced Raman spectroscopy. Sci. Rep. 2017, 7, 8561. [Google Scholar] [CrossRef]
- PalmSens. ItalSens IS-C. 2024. Available online: https://www.palmsens.com/product/italsens-is-c/ (accessed on 22 June 2025).
- Yamada, H.; Yoshii, K.; Asahi, M.; Chiku, M.; Kitazumi, Y. Cyclic voltammetry part 1: Fundamentals. Electrochemistry 2022, 90, 102005. [Google Scholar] [CrossRef]
- Rafiee, M.; Abrams, D.J.; Cardinale, L.; Goss, Z.; Romero-Arenas, A.; Stahl, S.S. Cyclic voltammetry and chronoamperometry: Mechanistic tools for organic electrosynthesis. Chem. Soc. Rev. 2024, 53, 566–585. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Maffucci, A. Carbon interconnects. Nanopackaging: Nanotechnologies and Electronics Packaging; Springer: Cham, Switzerland, 2018; pp. 725–774. [Google Scholar]
- Singh, R.; Samuel, M.S.; Ravikumar, M.; Ethiraj, S.; Kumar, M. Graphene materials in pollution trace detection and environmental improvement. Environ. Res. 2024, 243, 117830. [Google Scholar] [CrossRef]
- Hwang, E.; Adam, S.; Das Sarma, S. Transport in chemically doped graphene in the presence of adsorbed molecules. Phys. Rev. B—Condens. Matter Mater. Phys. 2007, 76, 195421. [Google Scholar] [CrossRef]
- Forestiere, C.; Maffucci, A.; Maksimenko, S.A.; Miano, G.; Slepyan, G.Y. Transmission-line model for multiwall carbon nanotubes with intershell tunneling. IEEE Trans. Nanotechnol. 2012, 11, 554–564. [Google Scholar] [CrossRef]
- Forestiere, C.; Maffucci, A.; Miano, G. Hydrodynamic model for the signal propagation along carbon nanotubes. J. Nanophotonics 2010, 4, 041695. [Google Scholar] [CrossRef]
- Al-Nu’airat, J.; Dlugogorski, B.Z.; Gao, X.; Zeinali, N.; Skut, J.; Westmoreland, P.R.; Oluwoye, I.; Altarawneh, M. Reaction of phenol with singlet oxygen. Phys. Chem. Chem. Phys. 2019, 21, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Terzian, R.; Colarusso, P.; Minero, C.; Pelizzetti, E.; Hidaka, H. Sonochemical oxidation of phenol and three of its intermediate products in aqueous media: Catechol, hydroquinone, and benzoquinone. Kinetic and mechanistic aspects. Res. Chem. Intermed. 1993, 18, 183–202. [Google Scholar] [CrossRef]
- Agrahari, S.; Singh, A.K.; Gautam, R.K.; Tiwari, I. Electrochemical oxidation and sensing of para benzoquinone using a novel SPE based disposable sensor. Chemosphere 2023, 342, 140078. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Fierro, S.; Karafyllis, I.; Mantzavinos, D.; Kalogerakis, N.; Katsaounis, A. Anodic oxidation of phenol on Ti/IrO2 electrode: Experimental studies. Catal. Today 2010, 151, 185–189. [Google Scholar] [CrossRef]
- Pulgarin, C.; Adler, N.; Peringer, P.; Comninellis, C. Electrochemical detoxification of a 1,4-benzoquinone solution in wastewater treatment. Water Res. 1994, 28, 887–893. [Google Scholar] [CrossRef]
- PalmSens. PalmSens4. 2024. Available online: https://www.palmsens.com/product/palmsens4/ (accessed on 18 July 2025).
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Fletcher, S. Screen-printed carbon electrodes. In Electrochem. Carbon Electrodes; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 425–444. [Google Scholar]
- Monk, P.M. Fundamentals of Electroanalytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Vanhove, E.; Ben-Amor, S.; Charlot, S.; Colin, D.; Devin, A.; Rigoulet, M.; Sojic, N.; Belaïdi, F.S.; Launay, J.; Temple-Boyer, P.; et al. Development of electrochemical microsensors for the monitoring of mitochondrial activities. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1135–1138. [Google Scholar]
- Tonello, S.; Fapanni, T.; Bonaldo, S.; Giorgi, G.; Narduzzi, C.; Paccagnella, A.; Serpelloni, M.; Sardini, E.; Carrara, S. Amperometric measurements by a novel aerosol jet printed flexible sensor for wearable applications. IEEE Trans. Instrum. Meas. 2022, 72, 7500512. [Google Scholar] [CrossRef]
- Alfonta, L.; Katz, E.; Willner, I. Sensing of acetylcholine by a tricomponent-enzyme layered electrode using faradaic impedance spectroscopy, cyclic voltammetry, and microgravimetric quartz crystal microbalance transduction methods. Anal. Chem. 2000, 72, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef] [PubMed]
- Classification Learner—Train Models to Classify Data Using Supervised Machine Learning—MATLAB. Available online: https://www.mathworks.com/help/stats/classificationlearner-app.html (accessed on 22 August 2025).


| Technology | Real-Time Capability | Portability | Limitations |
|---|---|---|---|
| Colorimetric [17,18,19,20,21,22,23,24,25,26,27,28,29,30] | Limited | Very high | Color, turbidity and lighting interferences; long-term stability. |
| Lab-on-a-Chip [31,32,33,34,35,36,37,38,39] | High | High | Fabrication process complexity, costs |
| Raman/SERS [40,41,42] | Moderate | Moderate | Fluorescence interference; costly instrumentation. |
| Technology | Real-Time Capability | Portability | Limitations |
|---|---|---|---|
| Electrochemical ISM [43] | High | High | pH/O2 interferences, fouling |
| Thin-film ISM [44] | High | High | T/pH corrections, limited lifetime |
| Tryptophan-like fluorescence probe [45] | High | High | Calibration, particles interferences, cost |
| Optical fluorescence/UV sensors [46] | Moderate | Moderate | Turbidity, fouling |
| Electrochemical sensors [46] | High | High | Fouling |
| Spectroscopy & chromatography [46] | Low | Low | Cost, slow response |
| IoT wireless multiparameter systems [47] | High | High | Energy consumption, reliability |
| Enzymatic biosensors [47] | High | High | Biological stability, regeneration |
| Integrated multisensor with LoRa transmission [48] | High | High | Calibration, network dependency |
| Technique | [mM] | [mM] | |
|---|---|---|---|
| CV | 2.77 | 4.36 [mM/C] | 0.14 |
| CA | 2.77 | −4.15 [mM/A] | 0.30 |
| Classical EIS | 3.70 | −1.87 [mM/] | 3.16 |
| 2.75 | −2.09 [mM/%] | 2.48 |
| Concentration [mM] | CV Error [mM] | CA Error [mM] | EIS Error [mM] | (1 MHz) Error [mM] |
|---|---|---|---|---|
| 0 | −0.09 | −0.08 | 4.62 | 0.83 |
| 0.1 | 0.01 | −0.03 | 3.13 | 0.76 |
| 1 | 0.11 | 0.26 | 4.14 | −0.34 |
| 10 | −0.02 | −0.07 | −2.16 | −8.01 |
| SPE Index | Nanomembrane Index | Class |
|---|---|---|
| 1, 2, 3 | 1, 2, 3 | 0 mM |
| 4, 5, 6 | 4, 5, 6 | 0.1 mM |
| 7, 8, 9 | 7, 8, 9 | 1 mM |
| 10, 11, 12 | 10, 11, 12 | 10 mM |
| SPE Index | Nanomembrane Index | Class |
|---|---|---|
| 13 | 13 | 0 mM |
| 14 | 14 | 0.1 mM |
| 15 | 15 | 1 mM |
| 16 | 16 | 10 mM |
| Technique | Data Points |
|---|---|
| CV | 400 × 2/SPE |
| CA | 1800 × 1/SPE |
| Electrode Sensors EIS | 20 × 140/SPE |
| Nanomembranes EIS | 90 × 16/Nanomembrane |
| Dataset | CV | CA | Electrode Sensors EIS | Nanomembranes EIS |
|---|---|---|---|---|
| Training Data | 4800 × 2 1200 × 2/class | 21600 × 1 5400 × 1/class | 240 × 140 60 × 140/class | 1080 × 16 270 × 16/class |
| Test Data | 1600 × 2 400 × 2/class | 7200 × 1 1800 × 1/class | 80 × 140 20 × 140/class | 360 × 16 90 × 16/class |
| Technique | Data Points |
|---|---|
| CV | 400 × 2/SPE for clean water class 133 × 2/SPE for polluted water class |
| CA | 1800 × 1/SPE for clean water class 600 × 1/SPE for polluted water class |
| Electrode Sensors EIS | 20 × 40/SPE for clean water class 7 × 40/SPE for polluted water class |
| Nanomembranes EIS | 90 × 16/SPE for clean water class 30 × 16/SPE for polluted water class |
| Dataset | CV | CA | Electrode Sensors EIS | Nanomembranes EIS |
|---|---|---|---|---|
| Training Data | 2400 × 2 1200 × 2/class | 10800 × 1 5400 × 1/class | 120 × 140 60 × 140/class | 540 × 16 270 × 16/class |
| Test Data | 800 × 2 400 × 2/class | 3600 × 1 1800 × 1/class | 40 × 140 20 × 140/class | 180 × 16 90 × 16/class |
| Dataset | 180 s | 150 s | 120 s | 90 s | 60 s | 45 s | 30 s | 15 s |
|---|---|---|---|---|---|---|---|---|
| Training | 1080 × 16 | 900 × 16 | 720 × 16 | 540 × 16 | 360 × 16 | 270 × 16 | 180 × 16 | 90 × 16 |
| Test | 360 × 16 | 300 × 16 | 240 × 16 | 180 × 16 | 120 × 16 | 90 × 16 | 60 × 16 | 30 × 16 |
| Dataset | 180 s | 150 s | 120 s | 90 s | 60 s | 45 s | 30 s | 15 s |
|---|---|---|---|---|---|---|---|---|
| Training | 540 × 16 | 450 × 16 | 360 × 16 | 270 × 16 | 180 × 16 | 135 × 16 | 90 × 16 | 45 × 16 |
| Test | 180 × 16 | 150 × 16 | 120 × 16 | 90 × 16 | 60 × 16 | 45 × 16 | 30 × 16 | 15 × 16 |
| Noise Level | Accuracy (4 Classes) [%] | Accuracy (2 Classes) [%] |
|---|---|---|
| 0 | 100 | 100 |
| 100 | 100 | |
| 85 | 100 | |
| 82 | 100 | |
| 57 | 100 | |
| 42 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaliere, G.; Tari, L.; Siconolfi, F.; Rehman, H.; Kuzhir, P.; Maffucci, A.; Ferrigno, L. A Novel ML-Powered Nanomembrane Sensor for Smart Monitoring of Pollutants in Industrial Wastewater. Sensors 2025, 25, 5390. https://doi.org/10.3390/s25175390
Cavaliere G, Tari L, Siconolfi F, Rehman H, Kuzhir P, Maffucci A, Ferrigno L. A Novel ML-Powered Nanomembrane Sensor for Smart Monitoring of Pollutants in Industrial Wastewater. Sensors. 2025; 25(17):5390. https://doi.org/10.3390/s25175390
Chicago/Turabian StyleCavaliere, Gabriele, Luca Tari, Francesco Siconolfi, Hamza Rehman, Polina Kuzhir, Antonio Maffucci, and Luigi Ferrigno. 2025. "A Novel ML-Powered Nanomembrane Sensor for Smart Monitoring of Pollutants in Industrial Wastewater" Sensors 25, no. 17: 5390. https://doi.org/10.3390/s25175390
APA StyleCavaliere, G., Tari, L., Siconolfi, F., Rehman, H., Kuzhir, P., Maffucci, A., & Ferrigno, L. (2025). A Novel ML-Powered Nanomembrane Sensor for Smart Monitoring of Pollutants in Industrial Wastewater. Sensors, 25(17), 5390. https://doi.org/10.3390/s25175390









