SMART MAT: Fibre Optic Innovation for Bedside Monitoring and Validation of Continuous Vital Signs
Abstract
1. Introduction
- (1)
- HR: gold standard measurement using an ECG [32],
- (2)
- RR: clinical standard measurement by manual counting of RR over one minute at rest [33],
- (3)
- BP: gold standard measurement using the auscultatory technique with a manual sphygmomanometer [34], and
- (4)
- SpO2: clinical standard measurement via fingertip pulse oximeter [35].
2. Materials and Methods
2.1. Research Design and Ethics
2.2. Participants
- (1)
- (2)
- Presence of arteriovenous fistula or dialysis shunt, as BP measurement on the ipsilateral arm also poses elevated risks of trauma and clot formation [40],
- (3)
- A known allergy to the adhesive on the ECG, as a preventative measure against the onset of skin sensitivity or an allergic reaction [41], and
- (4)
2.3. Sample Size Calculation
2.4. Instrumentation
2.4.1. Anthropometric Measurements
2.4.2. Gold/Clinical Standard Devices
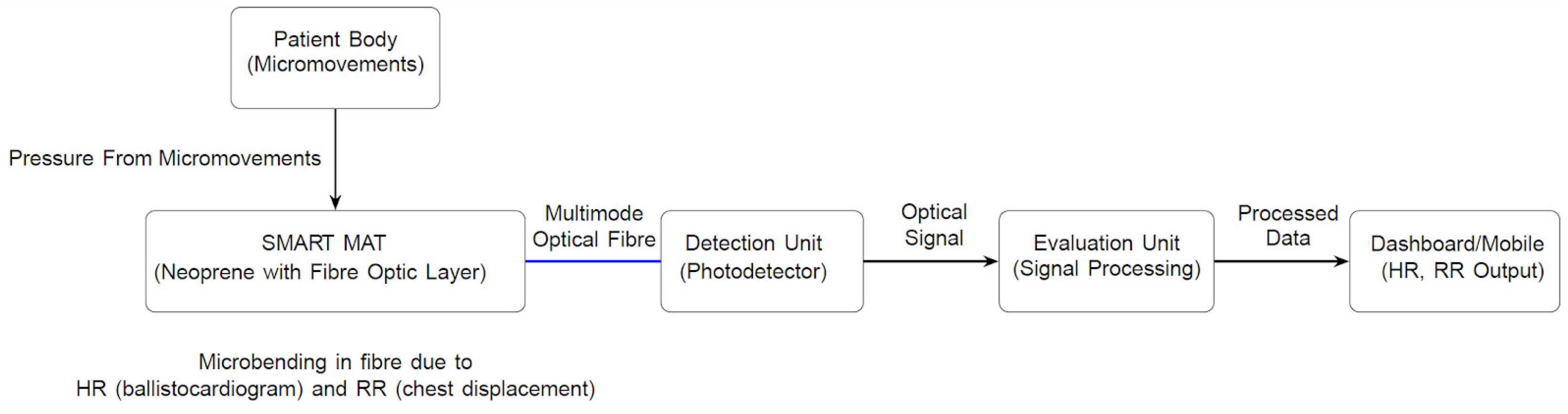
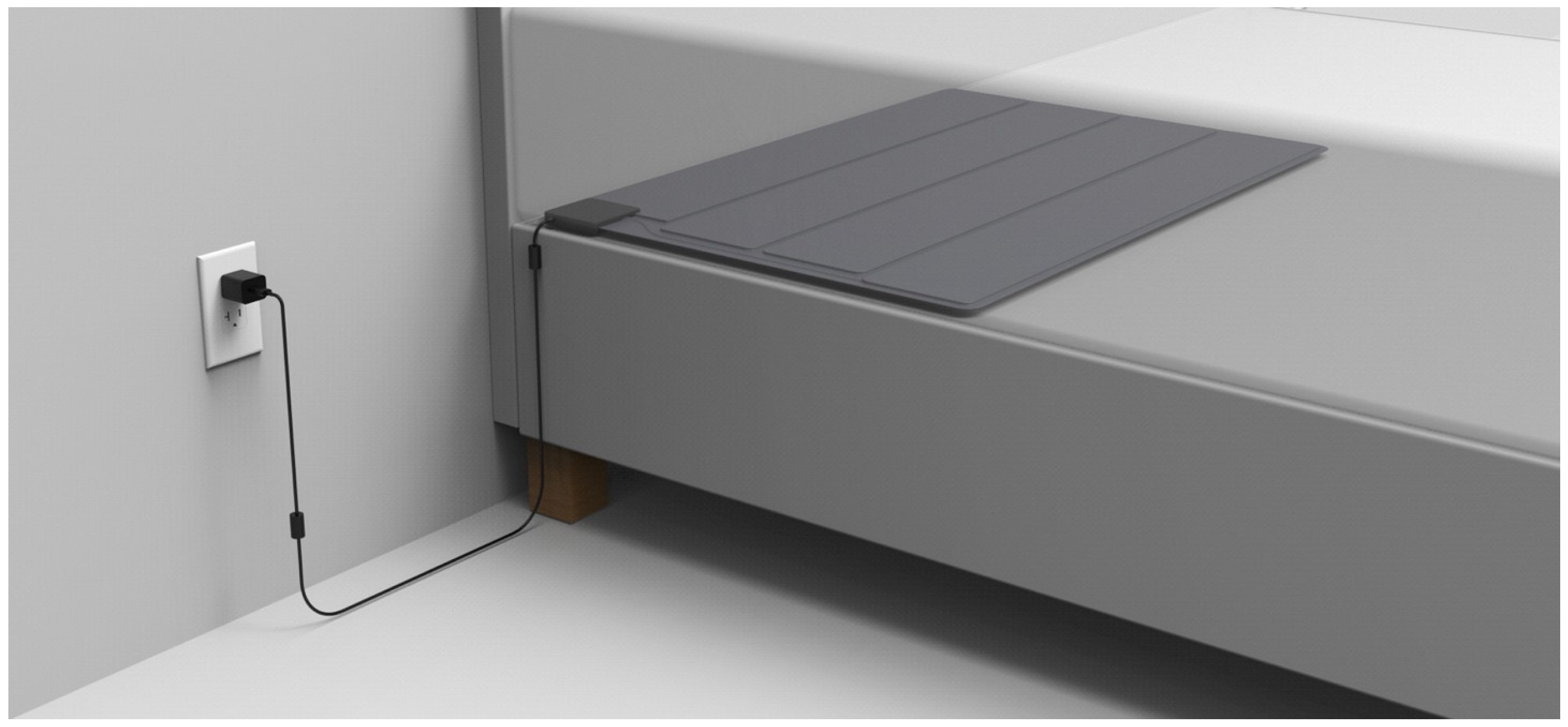
2.4.3. SMART MAT
2.4.4. Paired SMART MAT Devices
2.5. Research Protocol
- (1)
- Wear comfortable and loose clothing,
- (2)
- Empty bladder and bowel as needed,
- (3)
- Remove any nail polish,
- (4)
- Refrain from applying skincare products on the body,
- (5)
- Refrain from eating and drinking 30 min before the session,
- (6)
- Refrain from vigorous activity 1 h before the session,
- (7)
- Refrain from consuming caffeine and alcohol 24 h before the session,
- (8)
- Refrain from diuretics within seven days of the session, and
- (9)
- Inform co-investigators of the study if they are unwell or unable to attend the session.
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Two-Way ANOVA Between SMART MAT I and II
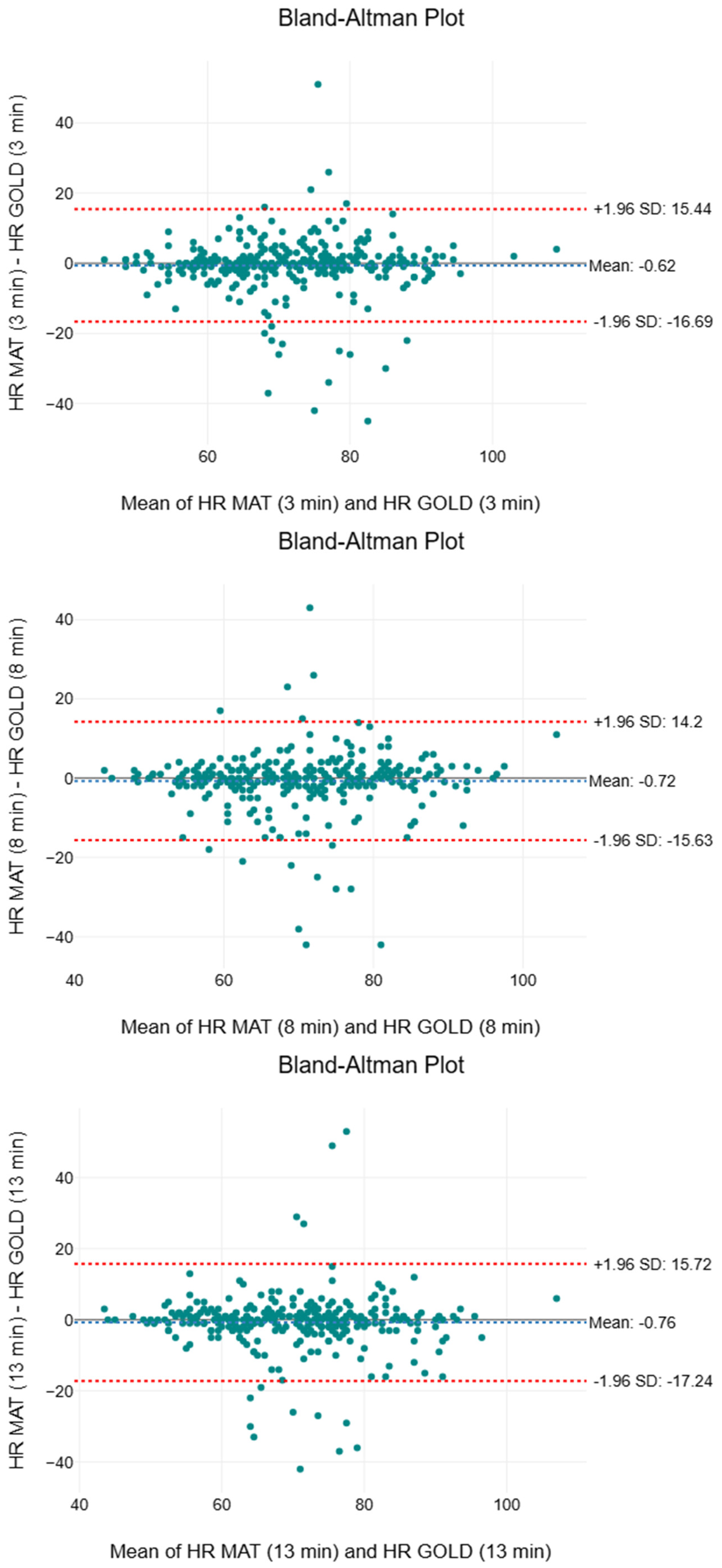
3.3. Heart Rate
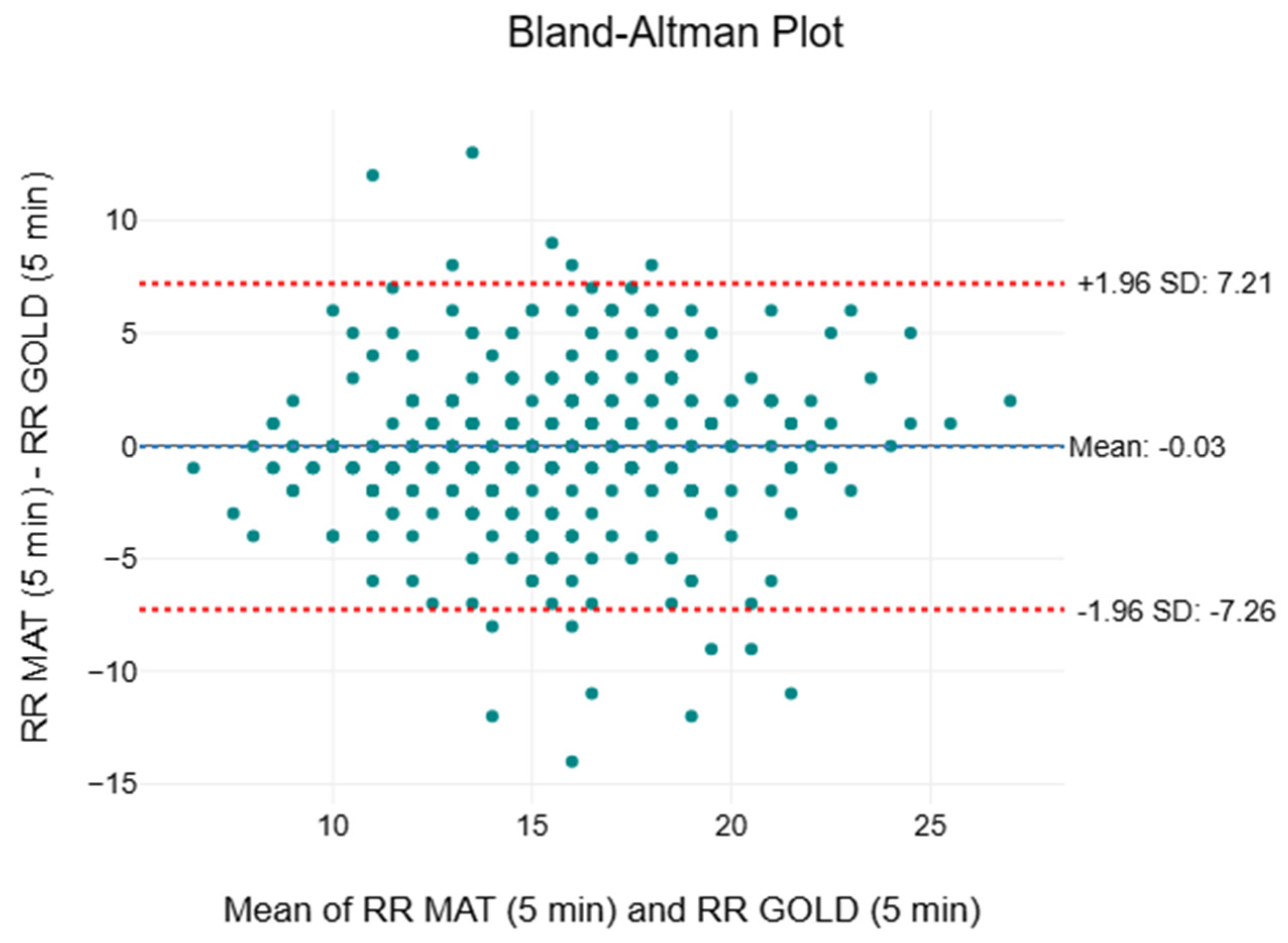
3.4. Respiratory Rate
3.5. Systolic Blood Pressure
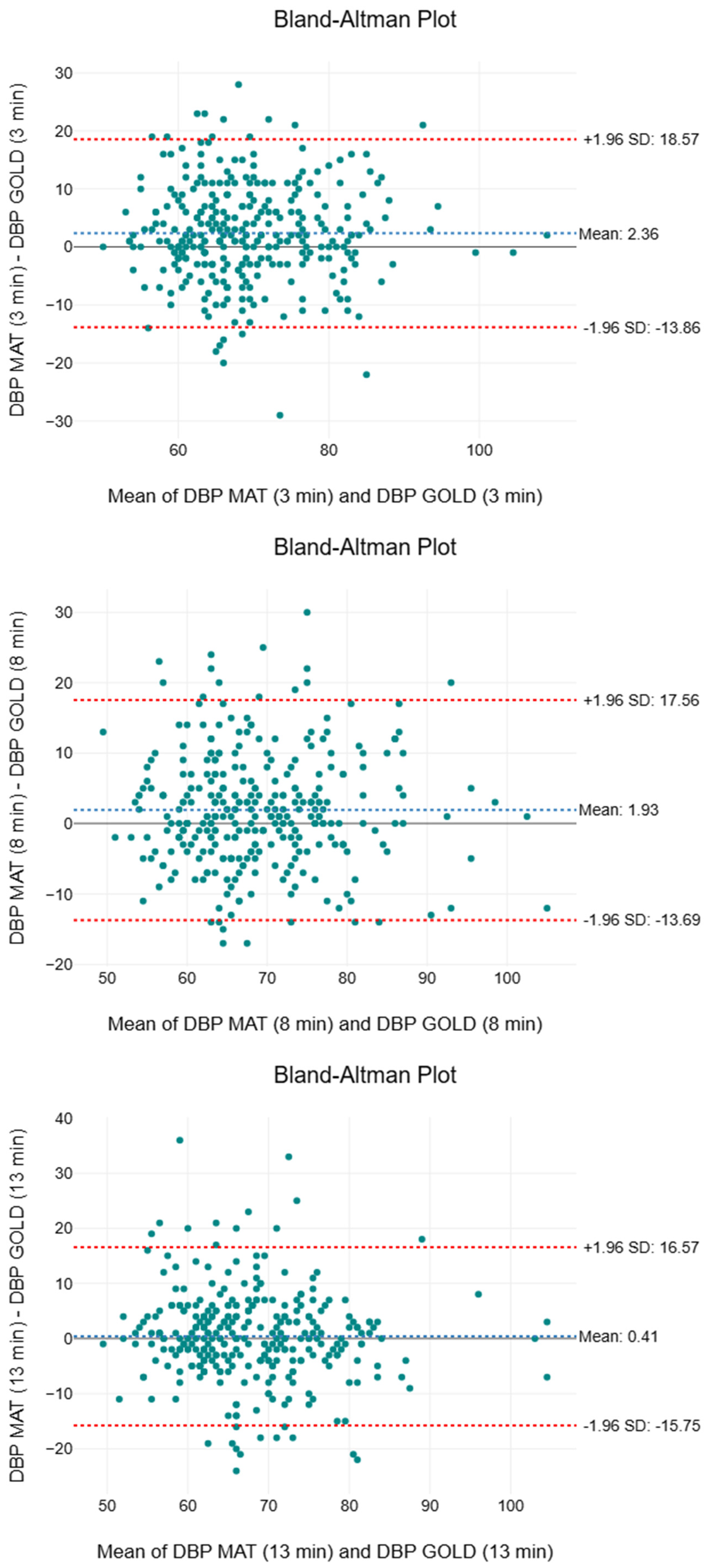
3.6. Diastolic Blood Pressure
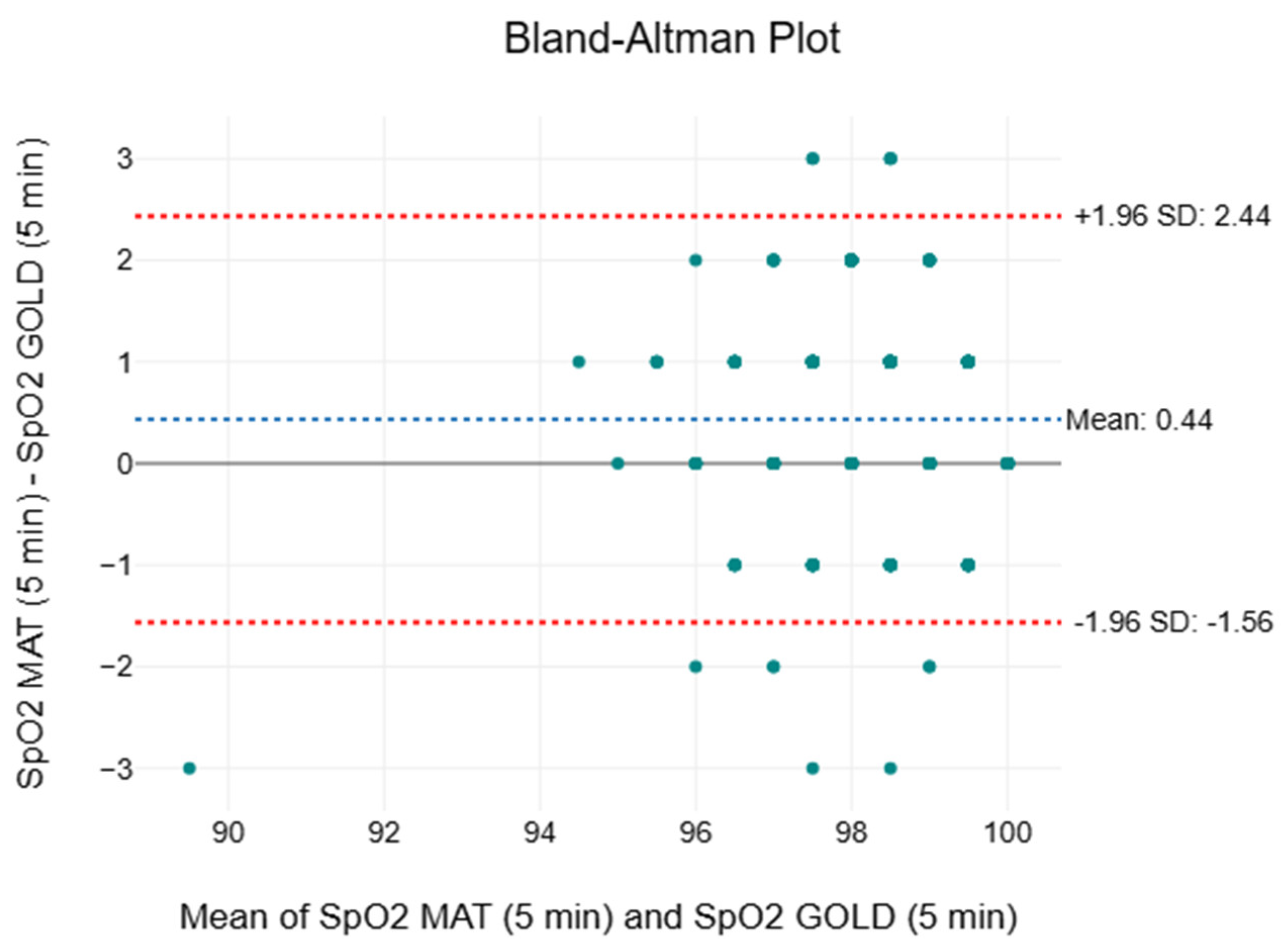
3.7. Oxygen Saturation
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HR | Heart rate |
| RR | Respiratory rate |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| SpO2 | Oxygen saturation |
| BP | Blood pressure |
| Effect size | |
| MAPE | Mean Absolute Percentage Error |
| IQR | Interquartile range |
| ANSI | American National Standards Institute |
| MAE | Mean Absolute Error |
References
- Brekke, I.J.; Puntervoll, L.H.; Pedersen, P.B.; Kellett, J.; Brabrand, M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS ONE 2019, 14, e0210875. [Google Scholar] [CrossRef]
- Kellett, J.; Sebat, F. Make vital signs great again—A call for action. Eur. J. Intern. Med. 2017, 45, 13–19. [Google Scholar] [CrossRef]
- Churpek, M.M.; Adhikari, R.; Edelson, D.P. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016, 102, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.J. Clinical Early Warning Scores: New Clinical Tools in Evolution. Open Anesth. J. 2018, 12, 26–33. Available online: https://openanesthesiajournal.com/VOLUME/12/PAGE/26/FULLTEXT/ (accessed on 21 June 2025). [CrossRef]
- Alam, N.; Hobbelink, E.L.; van Tienhoven, A.J.; van de Ven, P.M.; Jansma, E.P.; Nanayakkara, P.W.B. The impact of the use of the Early Warning Score (EWS) on patient outcomes: A systematic review. Resuscitation 2014, 85, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Redfern, O.C.; Griffiths, P.; Maruotti, A.; Saucedo, A.R.; Smith, G.B. The association between nurse staffing levels and the timeliness of vital signs monitoring: A retrospective observational study in the UK. BMJ Open 2019, 9, e032157. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Recio-Saucedo, A.; Dall’Ora, C.; Briggs, J.; Maruotti, A.; Meredith, P.; Smith, G.B.; Ball, J. Missed Care Study Group The association between nurse staffing and omissions in nursing care: A systematic review. J. Adv. Nurs. 2018, 74, 1474–1487. [Google Scholar] [CrossRef]
- Cardona-Morrell, M.; Prgomet, M.; Lake, R.; Nicholson, M.; Harrison, R.; Long, J.; Westbrook, J.; Braithwaite, J.; Hillman, K. Vital signs monitoring and nurse–patient interaction: A qualitative observational study of hospital practice. Int. J. Nurs. Stud. 2016, 56, 9–16. [Google Scholar] [CrossRef]
- Mok, W.; Wang, W.; Cooper, S.; Ang, E.N.K.; Liaw, S.Y. Attitudes towards vital signs monitoring in the detection of clinical deterioration: Scale development and survey of ward nurses. Int. J. Qual. Health Care 2015, 27, 207–213. [Google Scholar] [CrossRef]
- Chua, W.L.; Mackey, S.; Ng, E.K.C.; Liaw, S.Y. Front line nurses’ experiences with deteriorating ward patients: A qualitative study. Int. Nurs. Rev. 2013, 60, 501–509. [Google Scholar] [CrossRef]
- Wolfensberger, A.; Clack, L.; Kuster, S.P.; Passerini, S.; Mody, L.; Chopra, V.; Mann, J.; Sax, H. Transfer of pathogens to and from patients, healthcare providers, and medical devices during care activity-a systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 1093–1107. [Google Scholar] [CrossRef]
- Alugubelli, N.; Abuissa, H.; Roka, A. Wearable Devices for Remote Monitoring of Heart Rate and Heart Rate Variability—What We Know and What Is Coming. Sensors 2022, 22, 8903. [Google Scholar] [CrossRef]
- Jacobsen, M.; Dembek, T.A.; Kobbe, G.; Gaidzik, P.W.; Heinemann, L. Noninvasive Continuous Monitoring of Vital Signs With Wearables: Fit for Medical Use? J. Diabetes Sci. Technol. 2020, 15, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.J.; Lee, G.W.; Moon, J.S.; Chang, M.C. Noncontact Sensors for Vital Signs Measurement: A Narrative Review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e944913-1–e944913-8. [Google Scholar] [CrossRef] [PubMed]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Vasinek, V.; Nazeran, H.; Siska, P. A Non-Invasive Multichannel Hybrid Fiber-Optic Sensor System for Vital Sign Monitoring. Sensors 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Perezcampos Mayoral, C.; Gutiérrez Gutiérrez, J.; Cano Pérez, J.L.; Vargas Treviño, M.; Gallegos Velasco, I.B.; Hernández Cruz, P.A.; Torres Rosas, R.; Tepech Carrillo, L.; Arnaud Ríos, J.; Apreza, E.L.; et al. Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field. Biosensors 2021, 11, 58. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Lyu, W.; Yu, C. DEMA: A Deep Learning-Enabled Model for Non-Invasive Human Vital Signs Monitoring Based on Optical Fiber Sensing. Sensors 2024, 24, 2672. [Google Scholar] [CrossRef]
- Bonefacino, J.; Tam, H.-Y.; Glen, T.S.; Cheng, X.; Pun, C.-F.J.; Wang, J.; Lee, P.-H.; Tse, M.-L.V.; Boles, S.T. Ultra-fast polymer optical fibre Bragg grating inscription for medical devices. Light Sci. Appl. 2018, 7, 17161. [Google Scholar] [CrossRef]
- Chethana, K.; Guru Prasad, A.S.; Omkar, S.N.; Asokan, S. Fiber bragg grating sensor based device for simultaneous measurement of respiratory and cardiac activities. J. Biophotonics 2017, 10, 278–285. [Google Scholar] [CrossRef]
- Manujło, A.; Osuch, T. Temperature fiber Bragg grating based sensor for respiration monitoring. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High Energy Physics Experiments, Wilga, Poland, 28 May–6 June 2017; SPIE: Bellingham, WA, USA, 2017; Volume 10445, pp. 362–367. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10445/104451A/Temperature-fiber-Bragg-grating-based-sensor-for-respiration-monitoring/10.1117/12.2281106.full (accessed on 4 August 2025).
- Nedoma, J.; Fajkus, M.; Martinek, R.; Vasinek, V. Non-Invasive Fiber-Optic Biomedical Sensor for Basic Vital Sign Monitoring. Adv. Electr. Electron. Eng. 2017, 15, 336–342. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Martinek, R.; Nazeran, H. Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor. Sensors 2019, 19, 470. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Chen, S.; Lyu, W.; Liu, Z.; Yu, C.; Lu, C.; Tam, H.-Y. Non-invasive human vital signs monitoring based on twin-core optical fiber sensors. Biomed. Opt. Express 2019, 10, 5940–5951. [Google Scholar] [CrossRef]
- Tavares, C.; Leitão, C.; Lo Presti, D.; Domingues, M.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and heart rate monitoring using an FBG 3D-printed wearable system. Biomed. Opt. Express 2022, 13, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Sun, Q.; Ni, X.; Ai, F.; Wang, S.; Yan, Z.; Liu, D. Diaphragm-based optical fiber sensor for pulse wave monitoring and cardiovascular diseases diagnosis. J. Biophotonics 2019, 12, e201900084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Luo, B.; Zou, X.; Zou, C.; Wu, D.; Wang, Z.; Bai, Y.; Zhao, M.A. Wearable Sandwich Heterostructure Multimode Fiber Optic Microbend Sensor for Vital Signal Monitoring. Sensors 2024, 24, 2209. [Google Scholar] [CrossRef]
- Elsarnagawy, T.; Haueisen, J.; Farrag, M.; Ansari, S.G.; Fouad, H. Embedded Fiber Bragg Grating Based Strain Sensor as Smart Costume for Vital Signal Sensing. Sens. Lett. 2014, 12, 1669–1674. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Siska, P.; Martinek, R.; Vasinek, V. Non-Invasive Fiber Optic Probe Encapsulated Into PolyDiMethylSiloxane for Measuring Respiratory and Heart Rate of the Human Body. Adv. Electr. Electron. Eng. 2017, 15, 93–100. [Google Scholar] [CrossRef]
- Ogawa, K.; Koyama, S.; Ishizawa, H.; Fujiwara, S.; Fujimoto, K. Simultaneous Measurement of Heart Sound, Pulse Wave and Respiration with Single Fiber Bragg Grating Sensor. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–5. Available online: https://ieeexplore.ieee.org/document/8438629 (accessed on 4 August 2025).
- Yu, C.; Xu, W.; Shen, Y.; Bian, S.; Yu, C.; You, S. Non-invasive vital signs monitoring system based on smart sensor mat embedded with optical fiber interferometer. In Proceedings of the 2018 27th Wireless and Optical Communication Conference (WOCC), Taiwan, China, 30 April–1 May 2018; pp. 1–3. Available online: https://ieeexplore.ieee.org/document/8373787 (accessed on 4 August 2025).
- Chen, Z.; Lau, D.; Teo, J.T.; Ng, S.H.; Yang, X.; Kei, P.L. Simultaneous measurement of breathing rate and heart rate using a microbend multimode fiber optic sensor. J. Biomed. Opt. 2014, 19, 057001. [Google Scholar] [CrossRef]
- Sandau, K.E.; Funk, M.; Auerbach, A.; Barsness, G.W.; Blum, K.; Cvach, M.; Lampert, R.; May, J.L.; McDaniel, G.M.; Perez, M.V.; et al. Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e273–e344. [Google Scholar] [CrossRef]
- Hill, B.; Annesley, S.H. Monitoring respiratory rate in adults. Br. J. Nurs. 2020, 29, 12–16. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef]
- Plüddemann, A.; Thompson, M.; Heneghan, C.; Price, C. Pulse oximetry in primary care: Primary care diagnostic technology update. Br. J. Gen. Pract. 2011, 61, 358–359. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Strobe, I. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Allam, O.; Park, K.E.; Chandler, L.; Mozaffari, M.A.; Ahmad, M.; Lu, X.; Alperovich, M. The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020, 9, 59602. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Swaroop, M.N.; Horick, N.; Skolny, M.N.; Miller, C.L.; Jammallo, L.S.; Brunelle, C.; O’Toole, J.A.; Salama, L.; Specht, M.C.; et al. Impact of Ipsilateral Blood Draws, Injections, Blood Pressure Measurements, and Air Travel on the Risk of Lymphedema for Patients Treated for Breast Cancer. J. Clin. Oncol. 2016, 34, 691–698. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A.; Bagaria, S.; Gibson, T.; Arnold, M.; Diehl, N.; Crook, J.; Parker, A.; Nguyen, J. Trends in risk reduction practices for the prevention of lymphedema in the first 12 months after breast cancer surgery. J. Am. Coll. Surg. 2013, 216, 380–389. [Google Scholar] [CrossRef]
- Frese, E.M.; Fick, A.; Sadowsky, H.S. Blood Pressure Measurement Guidelines for Physical Therapists. Cardiopulm. Phys. Ther. J. 2011, 22, 5–12. [Google Scholar] [CrossRef]
- Sattar, Y.; Chhabra, L. Electrocardiogram. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK549803/ (accessed on 21 June 2025).
- Ahmed, N.; Zhu, Y. Early Detection of Atrial Fibrillation Based on ECG Signals. Bioengineering 2020, 7, 16. [Google Scholar] [CrossRef]
- Cosío, F.G. Atrial Flutter, Typical and Atypical: A Review. Arrhythmia Electrophysiol. Rev. 2017, 6, 55–62. [Google Scholar] [CrossRef]
- Tanita. MC980U Plus Multi-Frequency Segmental Body Composition Analyzer—Precision, Insights, and Transformation. Available online: https://tanita.com/products/mc-980uplus (accessed on 21 June 2025).
- Seca. Seca 213—Portable Stadiometer. Available online: https://www.seca.com/en_ae/products/all-products/product-details/seca213.html (accessed on 21 June 2025).
- Schiller. Cardiovit At-1 G2. Available online: https://www.schiller.ch/en/products/cardiovit-at-1-g2-p188 (accessed on 21 June 2025).
- Bokang. LCD Mercury Free Sphygmomanometer BK1016B. Available online: https://www.bokang.com/mercury-free-sphygmomanometer-5029499631059705.html (accessed on 21 June 2025).
- Medtronic. NellcorTM Portable SpO2 Patient Monitoring System, PM10N. Available online: https://www.medtronic.com/covidien/en-nz/products/pulse-oximetry/nellcor-portable-spo2-patient-monitoring-system.html (accessed on 21 June 2025).
- JUMPER. Blood Pressure Monitor JPD-HA121. Available online: https://www.jumper-medical.com/en/pros_d.aspx?CateId=328&sCateId=341&pid=311 (accessed on 21 June 2025).
- JUMPER. FIngertip Pulse Oximeter JPD-500F (OLED). Available online: https://www.jumper-medical.com/en/pros_d.aspx?CateId=328&sCateId=335&pid=415 (accessed on 21 June 2025).
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Setiawan, I.N.; Kurniawan, R.; Yuniarto, B.; Caraka, R.E.; Pardamean, B. Parameter Optimization of Support Vector Regression Using Harris Hawks Optimization. Procedia Comput. Sci. 2021, 179, 17–24. [Google Scholar] [CrossRef]
- Zaki, R.; Bulgiba, A.; Ismail, R.; Ismail, N.A. Statistical methods used to test for agreement of medical instruments measuring continuous variables in method comparison studies: A systematic review. PLoS ONE 2012, 7, e37908. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.Y.; Ryan, N.P.; Chen, D.; McNeil, J.; Hopper, I. Novel wearable and contactless heart rate, respiratory rate, and oxygen saturation monitoring devices: A systematic review and meta-analysis. Anaesthesia 2022, 77, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Marinari, S.; Volpe, P.; Simoni, M.; Aventaggiato, M.; De Benedetto, F.; Nardini, S.; Sanguinetti, C.M.; Palange, P. Accuracy of a New Pulse Oximetry in Detection of Arterial Oxygen Saturation and Heart Rate Measurements: The SOMBRERO Study. Sensors 2022, 22, 5031. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lilot, M.; Sidhu, K.S.; Rinehart, J.; Yu, Z.; Canales, C.; Cannesson, M. Accuracy and precision of continuous noninvasive arterial pressure monitoring compared with invasive arterial pressure: A systematic review and meta-analysis. Anesthesiology 2014, 120, 1080–1097. [Google Scholar] [CrossRef]
- Torp, K.D.; Modi, P.; Pollard, E.J.; Simon, L.V. Pulse Oximetry. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470348/ (accessed on 22 June 2025).
- Shuzan, M.N.I.; Chowdhury, M.H.; Chowdhury, M.E.H.; Murugappan, M.; Hoque Bhuiyan, E.; Arslane Ayari, M.; Khandakar, A. Machine Learning-Based Respiration Rate and Blood Oxygen Saturation Estimation Using Photoplethysmogram Signals. Bioengineering 2023, 10, 167. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. FDA Executive Summary Review of Pulse Oximeters and Factors That Can Impact Their Accuracy. 2022. Available online: https://www.fda.gov/media/162709/download (accessed on 21 June 2025).
- Harskamp, R.E.; Bekker, L.; Himmelreich, J.C.L.; De Clercq, L.; Karregat, E.P.M.; Sleeswijk, M.E.; Lucassen, W.A.M. Performance of popular pulse oximeters compared with simultaneous arterial oxygen saturation or clinical-grade pulse oximetry: A cross-sectional validation study in intensive care patients. BMJ Open Respir. Res. 2021, 8, e000939. [Google Scholar] [CrossRef]
- Smith, R.N.; Hofmeyr, R. Perioperative comparison of the agreement between a portable fingertip pulse oximeter v. a conventional bedside pulse oximeter in adult patients (COMFORT trial). S. Afr. Med. J. 2019, 109, 154–158. [Google Scholar] [CrossRef]
- Mason, J.W.; Ramseth, D.J.; Chanter, D.O.; Moon, T.E.; Goodman, D.B.; Mendzelevski, B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J. Electrocardiol. 2007, 40, 228–234. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Chester, J.G.; Rudolph, J.L. Vital signs in older patients: Age-related changes. J. Am. Med. Dir. Assoc. 2011, 12, 337–343. [Google Scholar] [CrossRef]
- ISO 80601-2-61:2017; Medical Electrical Equipment Part 2–61: Particular Requirements for Basic Safety and Essential Performance of Pulse Oximeter Equipment. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/67963.html (accessed on 22 June 2025).
- United States Food and Drug Administration. Pulse Oximeters-Premarket Notification Submissions [510(k)s] Guidance for Industry and Food and Drug Administration Staff. 2013. Available online: https://www.fda.gov/media/72470/download (accessed on 4 March 2013).
- Jung, M.-H.; Kim, G.-H.; Kim, J.-H.; Moon, K.-W.; Yoo, K.-D.; Rho, T.-H.; Kim, C.-M. Reliability of home blood pressure monitoring: In the context of validation and accuracy. Blood Press. Monit. 2015, 20, 215–220. [Google Scholar] [CrossRef]
- Ministry of Health Singapore. Ministry of Health. Hospital Admission Rates by Age and Sex 2020. 2021. Available online: https://www.moh.gov.sg/others/resources-and-statistics/healthcare-institution-statistics-hospital-admission-rates-by-age-and-sex-hospital-admission-rates-by-age-and-sex-2020/ (accessed on 21 June 2025).
- Singapore Department of Statistics. Recommendations on Definition and Classification of Age. 2020. Available online: https://www.singstat.gov.sg/-/media/files/standards_and_classifications/nsa.ashx (accessed on 21 June 2025).






| Characteristics | Female (Mean ± SD) | Male (Mean ± SD) |
|---|---|---|
| Number | 131 | 190 |
| Age (years) | 28.6 ± 11.9 | 28.2 ± 9.11 |
| Height (cm) | 161 ± 13.3 | 172 ± 6.72 |
| Weight (kg) | 55.9 ± 10.9 | 72.0 ± 12.9 |
| Body mass index (kg/) | 21.3 ± 4.00 | 24.2 ± 3.55 |
| Muscle mass (kg) | 37.1 ± 3.56 | 54.7 ± 7.99 |
| Body fat (%) | 28.2 ± 7.87 | 20.0 ± 13.3 |
| Total body water (%) | 50.2 ± 4.76 | 56.1 ± 6.82 |
| Vital Sign | F | p | |
|---|---|---|---|
| HR | 0.07 | 0.791 | <0.01 |
| RR | 3.79 | 0.052 | 0.01 |
| SBP | 1.60 | 0.207 | <0.01 |
| DBP | 3.01 | 0.084 | 0.01 |
| SpO2 | 0.74 | 0.392 | <0.01 |
| Time Interval | Mean ± SD (bpm) | Median (bpm) | IQR (bpm) | F | p | MAPE (%) | |
|---|---|---|---|---|---|---|---|
| 3rd minute | |||||||
| GOLD | 71 ± 12 | 71 | 64 to 79 | 1.95 | 0.163 | <0.01 | 6.45 |
| SMART MAT | 71 ± 12 | 71 | 62 to 79 | ||||
| 8th minute | |||||||
| GOLD | 71 ± 11 | 70 | 62 to 78 | 3.02 | 0.083 | <0.01 | 6.24 |
| SMART MAT | 70 ± 12 | 71 | 61 to 78 | ||||
| 13th minute | |||||||
| GOLD | 70 ± 12 | 70 | 62 to 78 | 2.75 | 0.098 | <0.01 | 6.44 |
| SMART MAT | 70 ± 12 | 70 | 61 to 77 |
| Time Interval | Mean ± SD (bpm) | Median (bpm) | IQR (bpm) | F | p | MAPE (%) | |
|---|---|---|---|---|---|---|---|
| 5th minute | |||||||
| CLINICAL | 15 ± 4 | 15 | 13 to 18 | 0.02 | 0.892 | <0.01 | 18.3 |
| SMART MAT | 15 ± 4 | 15 | 13 to 18 | ||||
| 10th minute | |||||||
| CLINICAL | 15 ± 4 | 15 | 13 to 18 | 3.70 | 0.055 | <0.01 | 17.9 |
| SMART MAT | 16 ± 4 | 16 | 13 to 18 | ||||
| 15th minute | |||||||
| CLINICAL | 15 ± 4 | 15 | 13 to 18 | 5.47 | 0.020 * | <0.01 | 15.3 |
| SMART MAT | 16 ± 4 | 16 | 13 to 18 |
| Time Interval | Mean ± SD (mmHg) | Median (mmHg) | IQR (mmHg) | F | p | MAPE (%) | |
|---|---|---|---|---|---|---|---|
| 3rd minute | |||||||
| GOLD | 115 ± 15 | 113 | 106 to 123 | 87.7 | <0.001 * | 0.04 | 7.16 |
| SMART MAT | 118 ± 17 | 120 | 110 to 131 | ||||
| 8th minute | |||||||
| GOLD | 114 ± 14 | 112 | 104 to 122 | 72.9 | <0.001 * | 0.02 | 5.67 |
| SMART MAT | 118 ± 14 | 117 | 108 to 127 | ||||
| 13th minute | |||||||
| GOLD | 114 ± 14 | 111 | 103 to 121 | 74.5 | <0.001 * | 0.02 | 5.39 |
| SMART MAT | 118 ± 14 | 117 | 107 to 125 |
| Time Interval | Mean ± SD (mmHg) | Median (mmHg) | IQR (mmHg) | F | p | MAPE (%) | |
|---|---|---|---|---|---|---|---|
| 3rd minute | |||||||
| GOLD | 68 ± 10 | 67 | 61 to 74 | 26.7 | <0.001 * | 0.01 | 9.22 |
| SMART MAT | 70 ± 10 | 69 | 63 to 77 | ||||
| 8th minute | |||||||
| GOLD | 68 ± 10 | 67 | 60 to 74 | 19.7 | <0.001 * | 0.01 | 8.76 |
| SMART MAT | 70 ± 10 | 69 | 63 to 76 | ||||
| 13th minute | |||||||
| GOLD | 68 ± 10 | 66 | 61 to 74 | 0.80 | 0.370 | <0.01 | 8.78 |
| SMART MAT | 68 ± 9 | 67 | 62 to 74 |
| Time Interval | Mean ± SD (%) | Median (%) | IQR (%) | F | p | MAPE (%) | |
|---|---|---|---|---|---|---|---|
| 5th minute | |||||||
| CLINICAL | 98 ± 1 | 98 | 97 to 99 | 58.7 | <0.001 * | 0.03 | 0.83 |
| SMART MAT | 98 ± 1 | 99 | 98 to 99 | ||||
| 10th minute | |||||||
| CLINICAL | 98 ± 1 | 98 | 97 to 99 | 51.0 | <0.001 * | 0.03 | 0.87 |
| SMART MAT | 98 ± 1 | 99 | 98 to 99 | ||||
| 15th minute | |||||||
| CLINICAL | 98 ± 1 | 98 | 97 to 99 | 28.5 | <0.001 * | 0.02 | 0.89 |
| SMART MAT | 98 ± 1 | 99 | 98 to 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, J.L.A.; Noordin, A.A.B.; Chan, C.W.M.; Chew, J.; Lim, C.W.Q.; Sanghavi, N.T.; Murat, O.A.B.M.; Tan, H.W.; Yeo, L.S.; Yow, E.M.Y.; et al. SMART MAT: Fibre Optic Innovation for Bedside Monitoring and Validation of Continuous Vital Signs. Sensors 2025, 25, 5321. https://doi.org/10.3390/s25175321
Ng JLA, Noordin AAB, Chan CWM, Chew J, Lim CWQ, Sanghavi NT, Murat OABM, Tan HW, Yeo LS, Yow EMY, et al. SMART MAT: Fibre Optic Innovation for Bedside Monitoring and Validation of Continuous Vital Signs. Sensors. 2025; 25(17):5321. https://doi.org/10.3390/s25175321
Chicago/Turabian StyleNg, Jace L. A., Ahmad Azeem Bin Noordin, Clare W. M. Chan, Jael Chew, Clarissa W. Q. Lim, Nikhil T. Sanghavi, Omar Arif Bin Mohamed Murat, Hao Wen Tan, Lyn S. Yeo, Esther M. Y. Yow, and et al. 2025. "SMART MAT: Fibre Optic Innovation for Bedside Monitoring and Validation of Continuous Vital Signs" Sensors 25, no. 17: 5321. https://doi.org/10.3390/s25175321
APA StyleNg, J. L. A., Noordin, A. A. B., Chan, C. W. M., Chew, J., Lim, C. W. Q., Sanghavi, N. T., Murat, O. A. B. M., Tan, H. W., Yeo, L. S., Yow, E. M. Y., & Yeung, M. T. (2025). SMART MAT: Fibre Optic Innovation for Bedside Monitoring and Validation of Continuous Vital Signs. Sensors, 25(17), 5321. https://doi.org/10.3390/s25175321






