Activity Type Effects Signal Quality in Electrocardiogram Devices
Abstract
Highlights
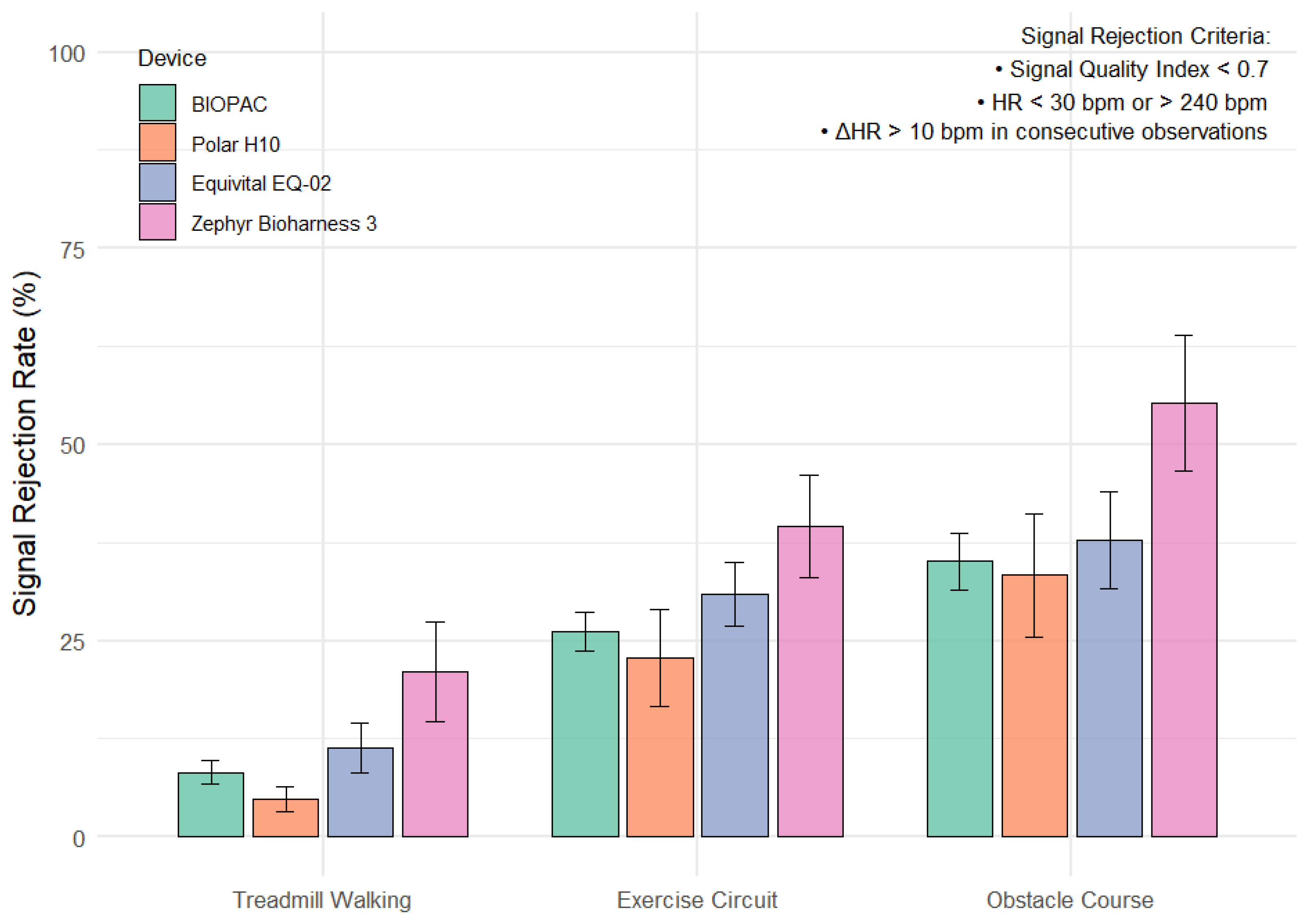
- ECG signal quality declined significantly during upper-body intensive tasks (e.g., ob-stacle course and circuit training) compared to treadmill walking.
- The Polar H10 exhibited lower average signal rejection rates across all activity types and may offer a practical reference point for wearable ECG validation in real-world conditions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Calculation of Signal Quality
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| SQI | Signal Quality Index |
| RR Interval | Time interval between successive R-peaks in the ECG waveform (often used interchangeably with NN interval, where NN = normal-to-normal) |
| EMG | Electromyography |
| bpm | Beats Per Minute |
References
- Mühlen, J.M.; Stang, J.; Skovgaard, E.L.; Judice, P.B.; Molina-Garcia, P.; Johnston, W.; Sardinha, L.B.; Ortega, F.B.; Caulfield, B.; Bloch, W. Recommendations for Determining the Validity of Consumer Wearable Heart Rate Devices: Expert Statement and Checklist of the INTERLIVE Network. Br. J. Sports Med. 2021, 55, 767–779. [Google Scholar] [CrossRef]
- Ruiz-Alias, S.A.; García-Pinillos, F.; Soto-Hermoso, V.M.; Ruiz-Malagón, E.J. Heart Rate Monitoring of the Endurance Runner During High Intensity Interval Training: Influence of Device Used on Training Functions. Proc. Inst. Mech. Eng. Part P 2023, 237, 166–172. [Google Scholar] [CrossRef]
- Harion, W.; Friedl, K.E.; Buller, M.J.; Arango, N.H.; Hoyt, R.W. Evolution of Physiological Status Monitoring for Ambulatory Military Applications. In Human Performance Optimization: The Science and Ethics of Enhancing Human Capabilities, 1st ed.; Matthews, M.D., Schneyer, D.M., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 142–164. [Google Scholar]
- Gancitano, G.; Baldassarre, A.; Lecca, L.I.; Mucci, N.; Petranelli, M.; Nicolia, M.; Brancazio, A.; Tessarolo, A.; Arcangeli, G. HRV in Active-Duty Special Forces and Public Order Military Personnel. Sustainability 2021, 13, 3867. [Google Scholar] [CrossRef]
- Wang, R.; Blackburn, G.; Desai, M.; Phelan, D.; Gillinov, L.; Houghtaling, P.; Gillinov, M. Accuracy of Wrist-Worn Heart Rate Monitors. Jama Cardiol. 2017, 2, 104–106. [Google Scholar] [CrossRef]
- Hinde, K.; White, G.; Armstrong, N. Wearable Devices Suitable for Monitoring Twenty Four Hour Heart Rate Variability in Military Populations. Sensors 2021, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in Recreational Men and Women. Sensors 2022, 22, 6536. [Google Scholar] [CrossRef]
- Hailstone, J.; Kilding, A.E. Reliability and Validity of the ZephyrTM BioHarnessTM to Measure Respiratory Responses to Exercise. Meas. Phys. Educ. Exerc. Sci. 2011, 15, 293–300. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, S.H.; Wang, G.H.; Ye, F.; Li, P.Z. Validity and Reliability of Multiparameter Physiological Measurements Recorded by the Equivital LifeMonitor During Activities of Various Intensities. J. Occup. Environ. Hyg. 2013, 10, 78–85. [Google Scholar] [CrossRef]
- Širaiy, B.; Trobec, R.; Ilić, V. Quality of One-Channel Telemetric ECG Sensor Signal in Maximum Exercise Stress Tests. Meas. Sci. Rev. 2019, 19, 79–85. [Google Scholar] [CrossRef]
- Takalokastari, T.; Alasaarela, E.; Kinnunen, M.; Jämsä, T. Quality of the Wireless Electrocardiogram Signal During Physical Exercise in Different Age Groups. IEEE J. Biomed. Health Inform. 2013, 18, 1058–1064. [Google Scholar] [PubMed]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. RR Interval Signal Quality of a Heart Rate Monitor and an ECG Holter at Rest and During Exercise. Eur. J. Appl. Physiol. 2019, 119, 1525–1532. [Google Scholar] [CrossRef]
- Chaudhary, S.; Ranamagar, R.; Shrestha, L.; Pun, D.; Karmacharya, P.; Mahotra, N. The Postural Effects on Electrical Activities of Heart in Apparently Healthy Young Adults. Kathmandu Univ. Med. J. 2021, 19, 499–502. [Google Scholar] [CrossRef]
- Apandi, Z.F.M.; Ikeura, R.; Hayakawa, S.; Tsutsumi, S. QRS Detection in Electrocardiogram Signal of Exercise Physical Activity; IOP Publishing: Bristol, UK, 2022; Volume 2319, p. 012021. [Google Scholar]
- Sharp, S.P. Validity of Heart Rate Variability Measured with Apple Watch Compared to Laboratory Measures. Ph.D. Thesis, University of California, San Diego, CA, USA, 2021. ISBN 979-8-4604-0974-7. [Google Scholar]
- Bonneval, L.; Wing, D.; Sharp, S.; Tristao Parra, M.; Moran, R.; LaCroix, A.; Godino, J. Validity of Heart Rate Variability Measured with Apple Watch Series 6 Compared to Laboratory Measures. Sensors 2025, 25, 2380. [Google Scholar] [CrossRef] [PubMed]
- Akintola, A.A.; Van de Pol, V.; Bimmel, D.; Maan, A.C.; Van Heemst, D. Comparative Analysis of the Equivital EQ02 Lifemonitor with Holter Ambulatory ECG Device for Continuous Measurement of ECG, Heart Rate, and Heart Rate Variability: A Validation Study for Precision and Accuracy. Front. Physiol. 2016, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lindsey, B.; Snyder, S.; Bell, E.; Reider, L.; Vignos, M.; Bar-Kochba, E.; Mousavi, A.; Parreira, J.; Hanley, C. Sampling Rate Requirement for Accurate Calculation of Heart Rate and Its Variability Based on the Electrocardiogram. Physiol. Meas. 2024, 45, 025007. [Google Scholar] [CrossRef]
- Lindsey, B.; Hanley, C.; Reider, L.; Snyder, S.; Zhou, Y.; Bell, E.; Shim, J.; Hahn, J.; Vignos, M.; Bar-Kochba, E. Accuracy of Heart Rate Measured by Military-Grade Wearable ECG Monitor Compared with Reference and Commercial Monitors. BMJ Mil. Health 2025, 171, 144–149. [Google Scholar] [CrossRef]
- Wilder, R.P.; Greene, J.A.; Winters, K.L.; Long, W.B., III; Gubler, K.D.; Edlich, R. Physical Fitness Assessment: An Update. J. Long-Term Eff. Med. Implant. 2006, 16, 193–204. [Google Scholar] [CrossRef]
- Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 9 December 2022).
- Shookster, D.; Lindsey, B.; Cortes, N.; Martin, J.R. Accuracy of Commonly Used Age-Predicted Maximal Heart Rate Equations. Int. J. Exerc. Sci. 2020, 13, 1242. [Google Scholar] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Behar, J.; Oster, J.; Clifford, G.D. Combining and Benchmarking Methods of Foetal ECG Extraction Without Maternal or Scalp Electrode Data. Physiol. Meas. 2014, 35, 1569–1589. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mark, R.G.; Clifford, G.D. Robust Heart Rate Estimation from Multiple Asynchronous Noisy Sources Using Signal Quality Indices and a Kalman Filter. Physiol Meas. 2008, 29, 15–32. [Google Scholar] [CrossRef]
- Oster, J.; Clifford, G.D. Impact of the Presence of Noise on RR Interval-Based Atrial Fibrillation Detection. J. Electrocardiol. 2015, 48, 947–951. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zhao, L.; Jiang, X.; Zhang, Z.; Li, J.; Wei, S.; Zhang, Y. Dynamic ECG Signal Quality Evaluation Based on the Generalized bSQI Index. IEEE Access 2018, 6, 41892–41902. [Google Scholar] [CrossRef]
- Castro Miller, I.D.; Varon, C.; Torfs, T.; Van Huffel, S.; Puers, B.; Van Hoof, C. Evaluation of a Multichannel Non-Contact ECG System and Signal Quality Algorithms Towards Sleep Apnea Detection and Monitoring. Sensors 2018, 18, 577. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Behar, J.; Li, Q.; Rezek, I. Signal Quality Indices and Data Fusion for Determining Clinical Acceptability of Electrocardiograms. Physiol. Meas. 2012, 33, 1419. [Google Scholar] [CrossRef]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable Devices Accurately Measure Heart Rate Variability? A Systematic Review. Folia Medica 2018, 60, 7–20. [Google Scholar] [CrossRef]
- Nepi, D.; Sbrollini, A.; Agostinelli, A.; Maranesi, E.; Morettini, M.; Di Nardo, F.; Fioretti, S.; Pierleoni, P.; Pernini, L.; Valenti, S. Validation of the Heart-Rate Signal Provided by the Zephyr Bioharness 3.0.; IEEE: New York, NY, USA, 2016; pp. 361–364. [Google Scholar]
- Dobbs, W.C.; Fedewa, M.V.; MacDonald, H.V.; Holmes, C.J.; Cicone, Z.S.; Plews, D.J.; Esco, M.R. The Accuracy of Acquiring Heart Rate Variability from Portable Devices: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 417–435. [Google Scholar] [CrossRef]
- Hostettler, R.; Lumikari, T.; Palva, L.; Nieminen, T.; Särkkä, S. Motion Artifact Reduction in Ambulatory Electrocardiography Using Inertial Measurement Units and Kalman Filtering; IEEE: New York, NY, USA, 2018; pp. 1–8. [Google Scholar]
- Oo, T.; Phukpattaranont, P. Signal-to-Noise Ratio Estimation in Electromyography Signals Contaminated with Electrocardiography Signals. Fluct. Noise Lett. 2020, 19, 2050027. [Google Scholar] [CrossRef]
- Teferra, M.N.; Hobbs, D.A.; Clark, R.A.; Reynolds, K.J. Preliminary Analysis of a Wireless and Wearable Electronic-Textile EASI-Based Electrocardiogram. Front. Cardiovasc. Med. 2021, 8, 806726. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Zhu, M.; He, Y.; Wei, Z.; Wang, Y.; Xu, Y.; Pan, H.; Huang, W.; Chen, S. Flexible Non-Contact Electrodes for Wearable Biosensors System on Electrocardiogram Monitoring in Motion. Front. Neurosci. 2022, 16, 900146. [Google Scholar] [CrossRef] [PubMed]
- Skála, T.; Vícha, M.; Rada, M.; Vácha, J.; Flašík, J.; Táborský, M. Feasibility of Evaluation of Polar H10 Chest-Belt ECG in Patients with a Broad Range of Heart Conditions. Cor Vasa 2022, 64, 411–422. [Google Scholar] [CrossRef]
- Clifford, G.D.; Lopez, D.; Li, Q.; Rezek, I. Signal Quality Indices and Data Fusion for Determining Acceptability of Electrocardiograms Collected in Noisy Ambulatory Environments. In Proceedings of the IEEE Computing in Cardiology Conference (CinC 2011), Hangzhou, China, 18–21 September 2011; IEEE: New York, NY, USA, 2011; pp. 285–288. [Google Scholar]
| Device | n | Male/Female | Age (Years) | Height (cm) | Mass (kg) | BF% |
|---|---|---|---|---|---|---|
| BIOPAC | 39 | 27/12 | 22.4 ± 3.9 | 173.7 ± 8.1 | 70.1 ± 11.9 | 17.6 ± 7.9 |
| Polar | 12 | 8/4 | 22.4 ± 2.6 | 173.0 ± 8.2 | 67.3 ± 10.6 | 17.2 ± 6.6 |
| Equivital | 13 | 10/3 | 24.2 ± 5.7 | 175.4 ± 6.6 | 71.8 ± 10.6 | 17.8 ± 9.1 |
| Zephyr | 14 | 10/4 | 20.6 ± 1.2 | 172.2 ± 9.5 | 70.9 ± 14.3 | 17.8 ± 9.4 |
| Factor | Comparison | Mean Difference | 95% CI | Effect Size (g) | p Adj |
|---|---|---|---|---|---|
| Device | BIOPAC—Polar H10 | −2.86 | −14.2 to 8.5 | 0.13 | 0.908 |
| BIOPAC—Equivital EQ-02 | 3.54 | −6.07 to 13.1 | −0.17 | 0.766 | |
| BIOPAC—Zephyr Bioharness 3.0 | 15.50 | 2.17 to 28.80 | −0.59 | 0.017 * | |
| Polar H10—Equivital EQ-02 | 6.39 | −19.60 to 6.83 | −0.30 | 0.582 | |
| Polar H10—Zephyr Bioharness 3.0 | 18.30 | 2.33 to 34.30 | −0.70 | 0.018 * | |
| Equivital EQ-02—Zephyr Bioharness 3.0 | 11.9 | −2.98 to 26.8 | −0.47 | 0.161 | |
| Task | Treadmill Walking—Exercise Circuit | 18.3 | 12.5 to 24.2 | −1.1 | <0.001* |
| Treadmill Walking—Obstacle Course | 28.1 | 20.6 to 35.6 | −1.32 | <0.001 * | |
| Exercise Circuit—Obstacle Course | 9.79 | 1.54 to 18.0 | −0.42 | 0.003 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindsey, B.; Snyder, S.; Zhou, Y.; Shim, J.K.; Hahn, J.-O.; Evans, W.; Martin, J. Activity Type Effects Signal Quality in Electrocardiogram Devices. Sensors 2025, 25, 5186. https://doi.org/10.3390/s25165186
Lindsey B, Snyder S, Zhou Y, Shim JK, Hahn J-O, Evans W, Martin J. Activity Type Effects Signal Quality in Electrocardiogram Devices. Sensors. 2025; 25(16):5186. https://doi.org/10.3390/s25165186
Chicago/Turabian StyleLindsey, Bryndan, Samantha Snyder, Yuanyuan Zhou, Jae Kun Shim, Jin-Oh Hahn, William Evans, and Joel Martin. 2025. "Activity Type Effects Signal Quality in Electrocardiogram Devices" Sensors 25, no. 16: 5186. https://doi.org/10.3390/s25165186
APA StyleLindsey, B., Snyder, S., Zhou, Y., Shim, J. K., Hahn, J.-O., Evans, W., & Martin, J. (2025). Activity Type Effects Signal Quality in Electrocardiogram Devices. Sensors, 25(16), 5186. https://doi.org/10.3390/s25165186







