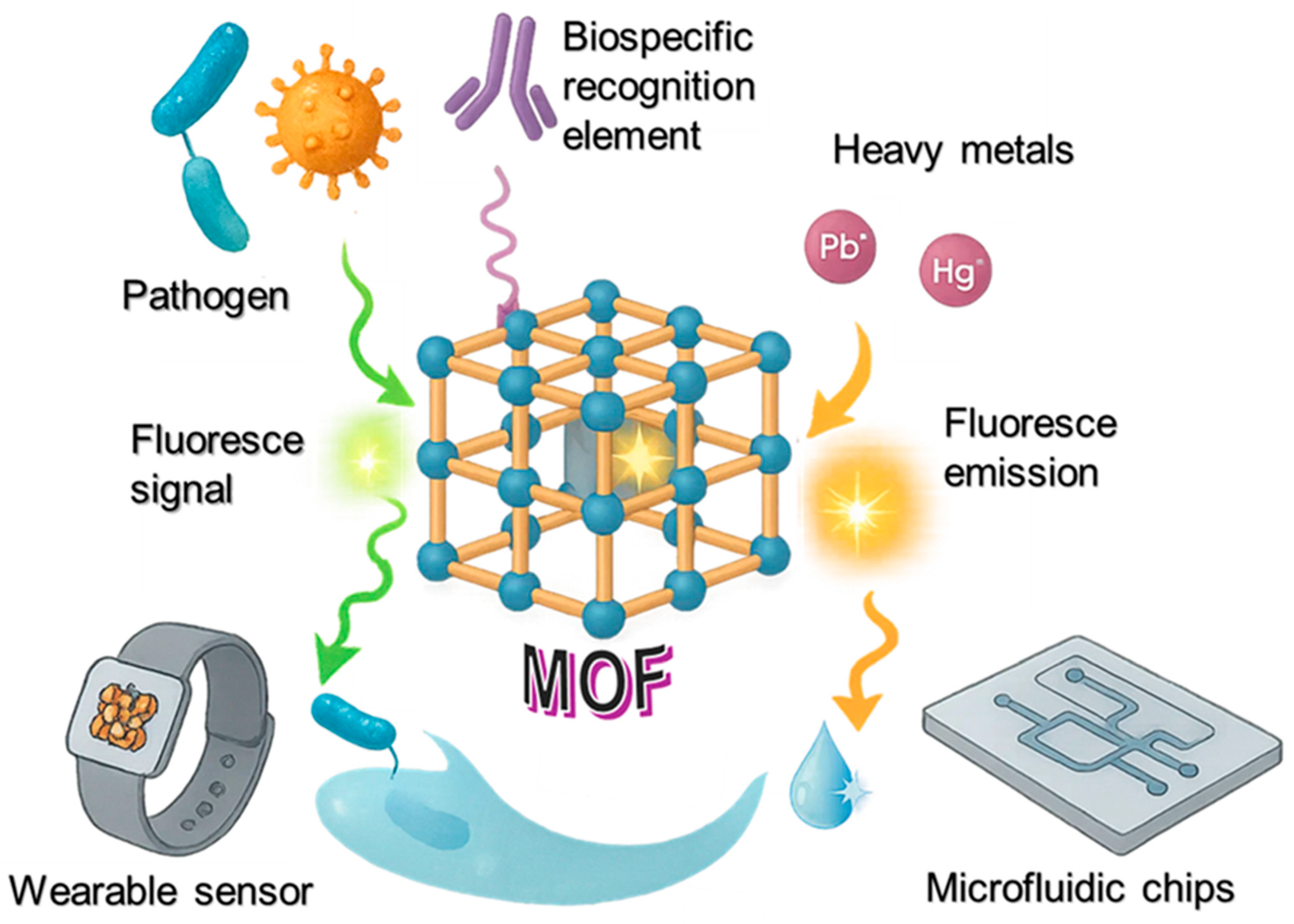
Metal–Organic-Framework-Based Optical Biosensors: Recent Advances in Pathogen Detection and Environmental Monitoring
Abstract
1. Introduction
2. Fundamental Mechanisms Underpinning MOF-Based Optical Biosensors
2.1. Structural and Chemical Tunability
2.2. Optical Biosensing Strategies Using MOFs (e.g., Fluorescence, Colorimetry, SERS)
2.3. Functionalization Strategies for Specificity and Sensitivity
3. MOF-Based Electrochemical and Optical Biosensors for Pathogen Detection
3.1. MOF-Based Biosensors for Microbial Detection
3.1.1. Optical Biosensing Platforms
3.1.2. Electrochemical Biosensing Platforms
3.1.3. Dual-Mode and Multiplex Biosensing Platforms
3.2. Detection Platforms (Label-Free vs. Labeled)
3.2.1. Label-Free Detection Platforms
3.2.2. Labeled Detection Platforms
3.3. Performance Metrics (Sensitivity, Selectivity, LOD)
4. MOF-Based Optical Biosensors for Environmental Monitoring
4.1. Detection of Pollutants (Heavy Metals, Pesticides, Toxins, etc.)
4.2. Detection of Pesticides and Herbicides
4.3. Detection of Microbial Pathogens
4.4. Detection of Biochemical and Volatile Environmental Contaminants
5. Integration with Advanced Technologies
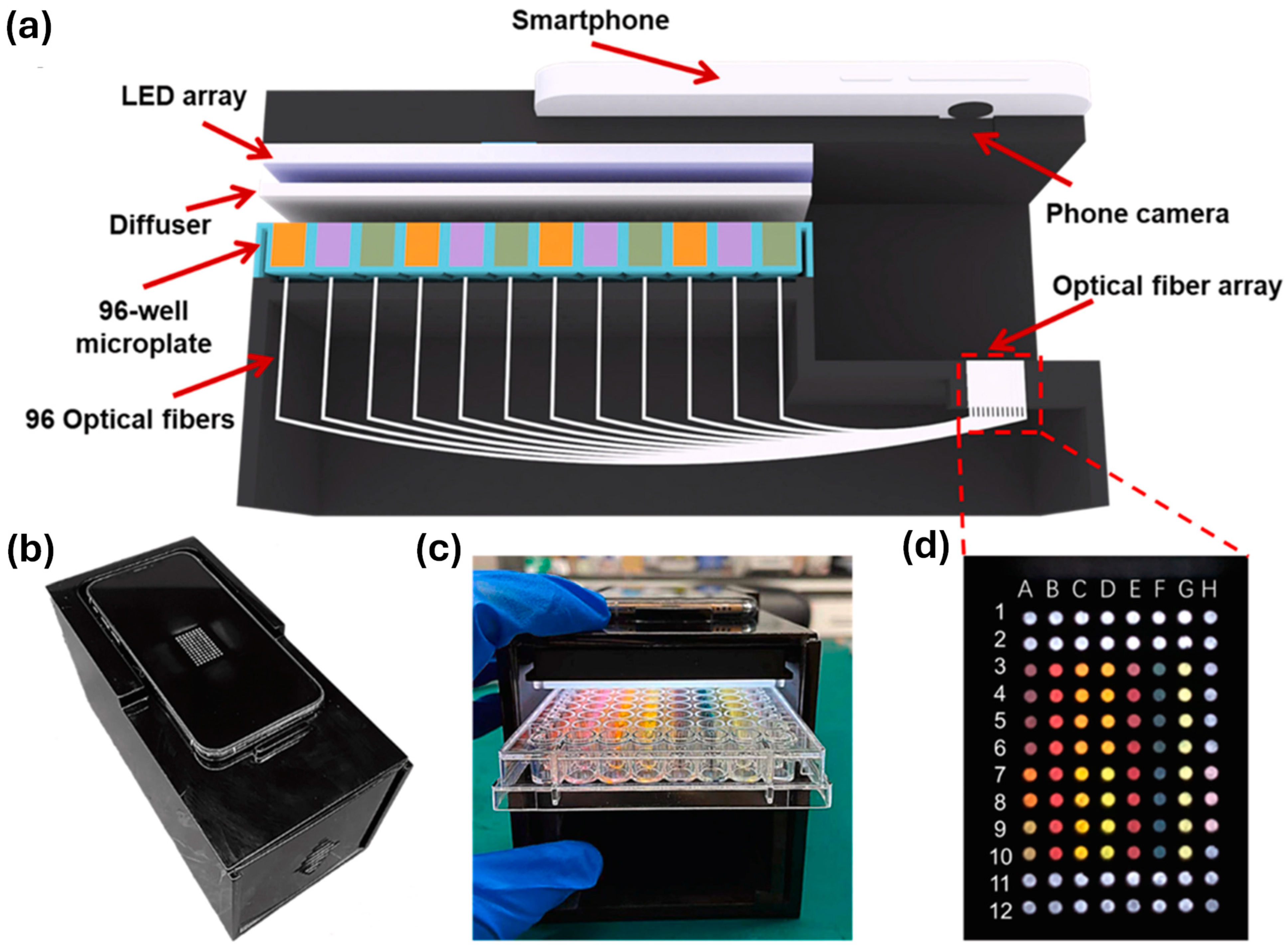
5.1. Portable-Based Sensing
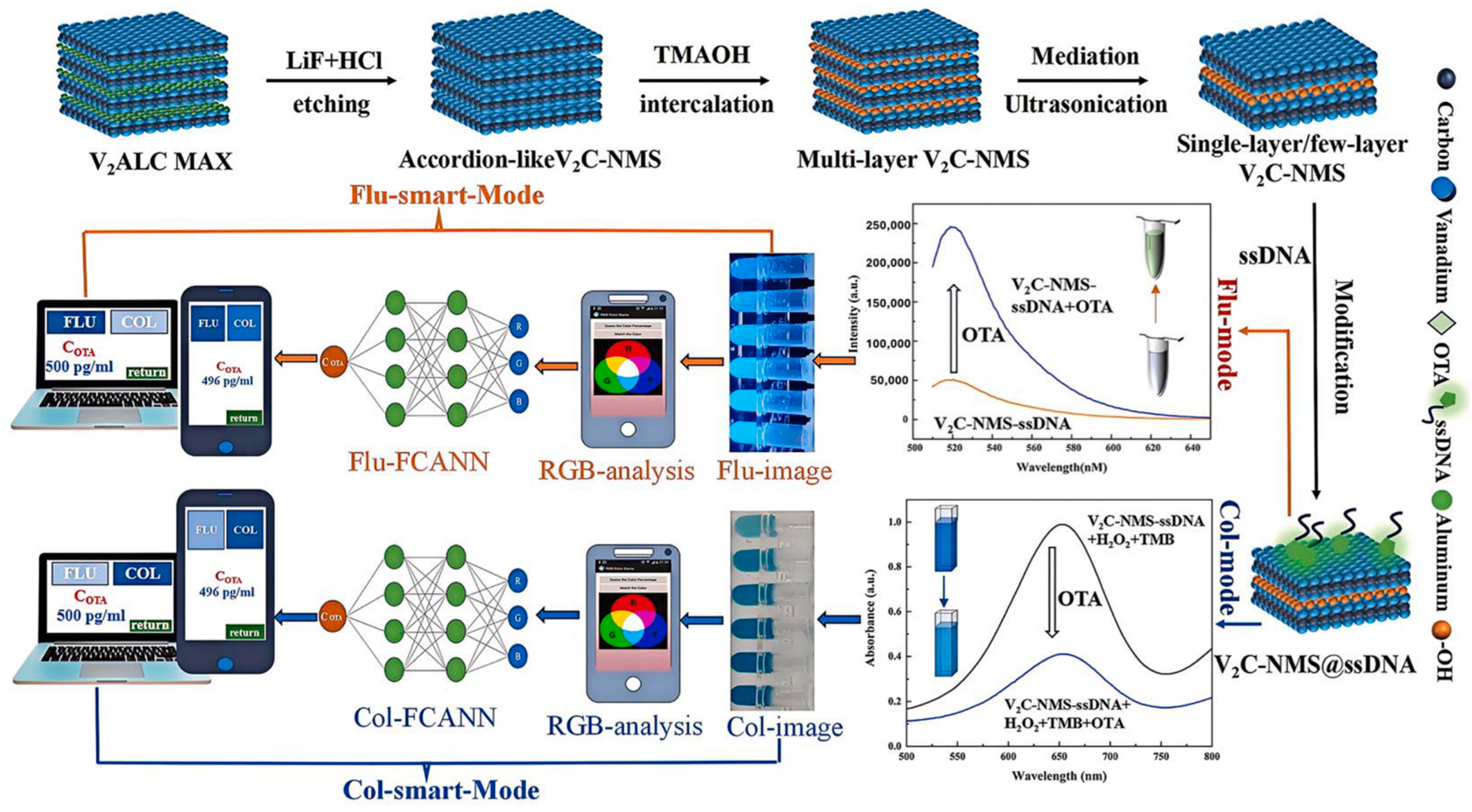
5.2. Data Processing with AI/ML for Signal Interpretation
6. Challenges and Opportunities
6.1. Stability, Reusability, Cost-Effectiveness
6.2. Bridging Lab-Scale and Real-World Application
6.3. Opportunities in Multimodal Sensing and MOF-Hybrids
7. Conclusions and Future Perspective
- Looking forward, a few directions seem particularly worth exploring though, admittedly, none of them are simple fixes. These are described as follows. Greener, scalable MOF synthesis: There is a real push toward eco-friendly methods that use less energy and fewer harsh solvents. If successful, this could make industrial-scale production not only more sustainable but also more practical for widespread deployment.
- AI and digital integration: Pairing MOF sensors with machine learning or AI systems could allow real-time signal analysis or even the ability to detect multiple analytes simultaneously. Still, these setups might be challenging to implement outside high-tech labs, so careful design and validation will be key.
- Regulatory and point-of-care readiness: Sensors need standardized testing and safety checks to meet the rules for clinical, food safety, or environmental applications. Without this, even the most sensitive device may never see actual use.
- Operational robustness: Developing MOFs that can reliably handle environmental stressors, humidity, heat, or light exposure would go a long way toward making these sensors field-ready.
- Miniaturized, multimodal devices: Compact, low-cost sensors that can measure several things at once could be game-changing, especially in remote or resource-limited locations. However, balancing sensitivity with simplicity is often easier said than done.
- Functional diversity and biocompatibility: Expanding the range of detectable targets, from microbes to volatile compounds, while keeping them compatible with biological samples, remains a delicate engineering challenge.
- Opportunities abound in developing multimodal sensing platforms that combine multiple optical transduction methods, enabling improved accuracy and reliability. Furthermore, coupling MOF sensors with digital technologies such as artificial intelligence and real-time data analytics offers exciting prospects for smart, autonomous monitoring systems. By addressing current limitations and leveraging emerging trends, MOF-based biosensors are poised to play a vital role in advancing global health, food safety, and environmental sustainability.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3-O-C10-HL | N-(3-oxodecanoyl)-L-homoserine lactone |
| AAS | Atomic Absorption Spectroscopy |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | Acetylcholinesterase |
| AI | Artificial Intelligence |
| AIA | 2-Aminoisonicotinic Acid |
| AIEgen | Aggregation-Induced Emission Luminogen |
| ALP | Alkaline Phosphatase |
| ATP | Adenosine Triphosphate |
| Au NPs | Gold Nanoparticles |
| CD | Carbon Nanodot |
| CFU | Colony-Forming Unit |
| mL | Milliliter |
| CMOS | Complementary Metal–Oxide–Semiconductor |
| COF | Covalent Organic Framework |
| Cr(VI) | Hexavalent Chromium |
| MOF | Metal–Organic Framework |
| Cu-MOF | Copper-Based Metal–Organic Framework |
| DNA | Deoxyribonucleic Acid |
| DPV | Differential Pulse Voltammetry |
| dsDNA | Double-Stranded Deoxyribonucleic Acid |
| E. coli | Escherichia coli |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FcMBL | Fragment Crystallizable Mannose-Binding Lectin |
| FRET | Fluorescence Resonance Energy Transfer |
| GBM | Glioblastoma Multiforme |
| GO | Graphene Oxide |
| HCR | Hybridization Chain Reaction |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| hCG | Human Chorionic Gonadotropin |
| HPLC | High-Performance Liquid Chromatography |
| HRP | Horseradish Peroxidase |
| IFE | Inner Filter Effect |
| IoT | Internet of Things |
| LOD | Limit of Detection |
| LSPR | Localized Surface Plasmon Resonance |
| miRNA | MicroRNA |
| ML | Machine Learning |
| MXene | Transition Metal Carbide |
| MWCNT | Multi-Walled Carbon Nanotube |
| NIR | Near-Infrared |
| NMOF | Nanoscale MOF |
| NS | Nanosheet |
| OTA | Ochratoxin A |
| PCR | Polymerase Chain Reaction |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) |
| PET | Photoinduced Electron Transfer |
| POCT | Point-of-Care Testing |
| PSA | Prostate-Specific Antigen |
| PtNP | Platinum Nanoparticle |
| PTS | Pregnancy Test Strip |
| RAA | Recombinase-Aided Amplification |
| RNA | Ribonucleic Acid |
| S. aureus | Staphylococcus aureus |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SERS | Surface-Enhanced Raman Scattering |
| SGI | SYBR Green I |
| SPR | Surface Plasmon Resonance |
| TcP | Tetraphenylporphyrin |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| UFD-DEC | Urease–Fe-cdDNA Dual-Enzyme Cascade |
| UV–vis | Ultraviolet–Visible Spectroscopy |
| VOC | Volatile Organic Compound |
| ZIF | Zeolitic Imidazolate Framework |
References
- Janikddfghx, E.; Ceremuga, M.; Niemcewicz, M.; Bijak, M. Dangerous pathogens as a potential problem for public health. Medicina 2020, 56, 591. [Google Scholar] [CrossRef] [PubMed]
- Buelow, E.; Ploy, M.C.; Dagot, C. Role of pollution on the selection of antibiotic resistance and bacterial pathogens in the environment. Curr. Opin. Microbiol. 2021, 64, 117–124. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Lawal, K.K.; Ekeleme, I.K.; Onuigbo, C.M.; Ikpeazu, V.O.; Obiekezie, S.O. A review on the public health implications of heavy metals. World J. Adv. Res. Rev. 2021, 10, 255–265. [Google Scholar] [CrossRef]
- Yang, A.M.; Lo, K.; Zheng, T.Z.; Yang, J.L.; Bai, Y.N.; Feng, Y.Q.; Cheng, N.; Liu, S.M. Environmental heavy metals and cardiovascular diseases: Status and future direction. Chronic Dis. Transl. Med. 2020, 6, 251–259. [Google Scholar] [CrossRef]
- Petrucci, S.; Costa, C.; Broyles, D.; Dikici, E.; Daunert, S.; Deo, S. On-site detection of food and waterborne bacteria—Current technologies, challenges, and future directions. Trends Food Sci. Technol. 2021, 115, 409–421. [Google Scholar] [CrossRef]
- Qiu, G.; Zhang, X.; deMello, A.J.; Yao, M.; Cao, J.; Wang, J. On-site airborne pathogen detection for infection risk mitigation. Chem. Soc. Rev. 2023, 52, 8531–8579. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Wu, X.; Hoffmann, M.R. Rapid detection methods for bacterial pathogens in ambient waters at the point of sample collection: A brief review. Clin. Infect. Dis. 2020, 71, S84–S90. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Biosensors—Recent Advances and Future Challenges; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Chen, Y.T.; Lee, Y.C.; Lai, Y.H.; Lim, J.C.; Huang, N.T.; Lin, C.T.; Huang, J.J. Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Trends, challenges, and advances in optical sensing for pathogenic bacteria detection (PathoBactD). Biosens. Bioelectron. 2023, 14, 100352. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, R.K.; Goud, K.Y.; Mohamed, M.A.; Kummari, S.; Tiwari, S.; Li, Z.; Narayan, R.; Stanciu, L.A.; Marty, J.L. Optical biosensors for diagnostics of infectious viral disease: A recent update. Diagnostics 2021, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical Biosensors for Virus Detection: Prospects for SARS-CoV-2/COVID-19. ChemBioChem 2021, 22, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, K.; Lin, J. Optical biosensors for the detection of foodborne pathogens: Recent development and future prospects. TrAC Trends Anal. Chem. 2024, 177, 117785. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Brenes-Acuña, M.; Castro-Rojas, A.; Cordero-Salmerón, R.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Biosensors for the detection of bacterial and viral clinical pathogens. Sensors 2020, 20, 6926. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, M.; Peng, T.; Zhang, P.; Shen, R.; Jia, Y. Detection and discrimination of pathogenic bacteria with nanomaterials-based optical biosensors: A review. Food Chem. 2023, 426, 136578. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A. Recent Progress on Optical Biosensors Developed for Nucleic Acid Detection Related to Infectious Viral Diseases. Micromachines 2023, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Kidanemariam, A.; Cho, S. Recent Advances in the Application of Metal–Organic Frameworks and Coordination Polymers in Electrochemical Biosensors. Chemosensors 2024, 12, 135. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Pham, D.T.T.; Muhammad, A.; Min, G.; Cho, S.; Park, J. Zn-H4dbp/GO for adsorptive removal and Zn-H4dbp/Au for electrochemical reduction of toxic Cr(VI) in aqueous solutions. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Lee, S.; Lee, G.; Oh, M. MOF-on-MOF Growth: Inducing Naturally Nonpreferred MOFs and Atypical MOF Growth. Acc. Chem. Res. 2024, 57, 3113–3125. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Cho, S. Recent Advances in Metal—Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications. Biosensors 2025, 15, 437. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Yuan, J.; Zheng, Q.; Hu, B.; Liu, R.; Deng, R.; Cao, J. Metal-organic frameworks-based photoelectrochemical Biosensors: From material design to intelligent detection. Trends Food Sci. Technol. 2025, 162, 105103. [Google Scholar] [CrossRef]
- Kumar, R.; Shafique, M.S.; Chapa, S.O.M.; Madou, M.J. Recent Advances in MOF-Based Materials for Biosensing Applications. Sensors 2025, 25, 2473. [Google Scholar] [CrossRef]
- Englich, F.V.; Foo, T.C.; Richardson, A.C.; Ebendorff-Heidepriem, H.; Sumby, C.J.; Monro, T.M. Photoinduced electron transfer based ion sensing within an optical fiber. Sensors 2011, 11, 9560–9572. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, Z.; Huang, L.; Zeng, Y.; Liu, X.; Tang, D. Photoinduced Electron Transfer Modulated Photoelectric Signal: Toward an Organic Small Molecule-Based Photoelectrochemical Platform for Formaldehyde Detection. Anal. Chem. 2023, 95, 9130–9137. [Google Scholar] [CrossRef] [PubMed]
- Jyoti; Dutta, T.; Kumar, P.; Jangra, R.; Sharma, A.K.; Singh, M.; Chaturvedi, P.; Sharma, S.; Garita, M.R.; Sharma, J.; et al. Recent advances in Metal-Organic Framework-Based fiber optic sensors and Photodetectors: Synthesis, Properties, and applications. Chem. Eng. J. 2025, 507, 160543. [Google Scholar] [CrossRef]
- Shen, Y.; Tissot, A.; Serre, C. Recent progress on MOF-based optical sensors for VOC sensing. Chem. Sci. 2022, 13, 13978–14007. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Boakye, A.; Chai, H.; Qu, L.; Zhang, X. Recent Advances in Metal-Organic Framework-Based Electrochemical Biosensing Applications. Front. Bioeng. Biotechnol. 2021, 9, 797067. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Yin, Y.; Fang, W.; Xue, H. Metal-organic framework-based sensors for the detection of toxins and foodborne pathogens. Food Control 2022, 133, 108684. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Cho, S. Recent Advancements in Metal–Organic Framework-Based Microfluidic Chips for Biomedical Applications. Micromachines 2025, 16, 736. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Kim, K.H.; Deep, A. MOF-bacteriophage biosensor for highly sensitive and specific detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 33589–33598. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Girigoswami, K.; Pallavi, P.; Girigoswami, A. Crafting porous nanoscaled architecture as a potential frontier for drug delivery. Mol. Syst. Des. Eng. 2024, 9, 1085–1106. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhou, K.; Ullah, S.; Wang, H.; Zou, J.; Thonhauser, T.; Li, J. Tuning Metal-Organic Framework (MOF) Topology by Regulating Ligand and Secondary Building Unit (SBU) Geometry: Structures Built on 8-Connected M6(M = Zr, Y) Clusters and a Flexible Tetracarboxylate for Propane-Selective Propane/Propylene Separation. J. Am. Chem. Soc. 2022, 144, 21702–21709. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, H.; Pang, J.; Lyu, Q.; Wang, Y.; Fan, W.; Lu, X.; Sun, D. Tunable rare-earth metal−organic frameworks for ultra-high selenite capture. J. Hazard. Mater. 2022, 436, 129094. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, J.; Ling, G.; Zhang, P. Tunable metal-organic frameworks assist in catalyzing DNAzymes with amplification platforms for biomedical applications. Chem. Soc. Rev. 2023, 52, 7549–7578. [Google Scholar] [CrossRef]
- He, W.; Lv, D.; Guan, Y.; Yu, S. Post-synthesis modification of metal-organic frameworks: Synthesis, characteristics, and applications. J. Mater. Chem. A 2023, 11, 24519–24550. [Google Scholar] [CrossRef]
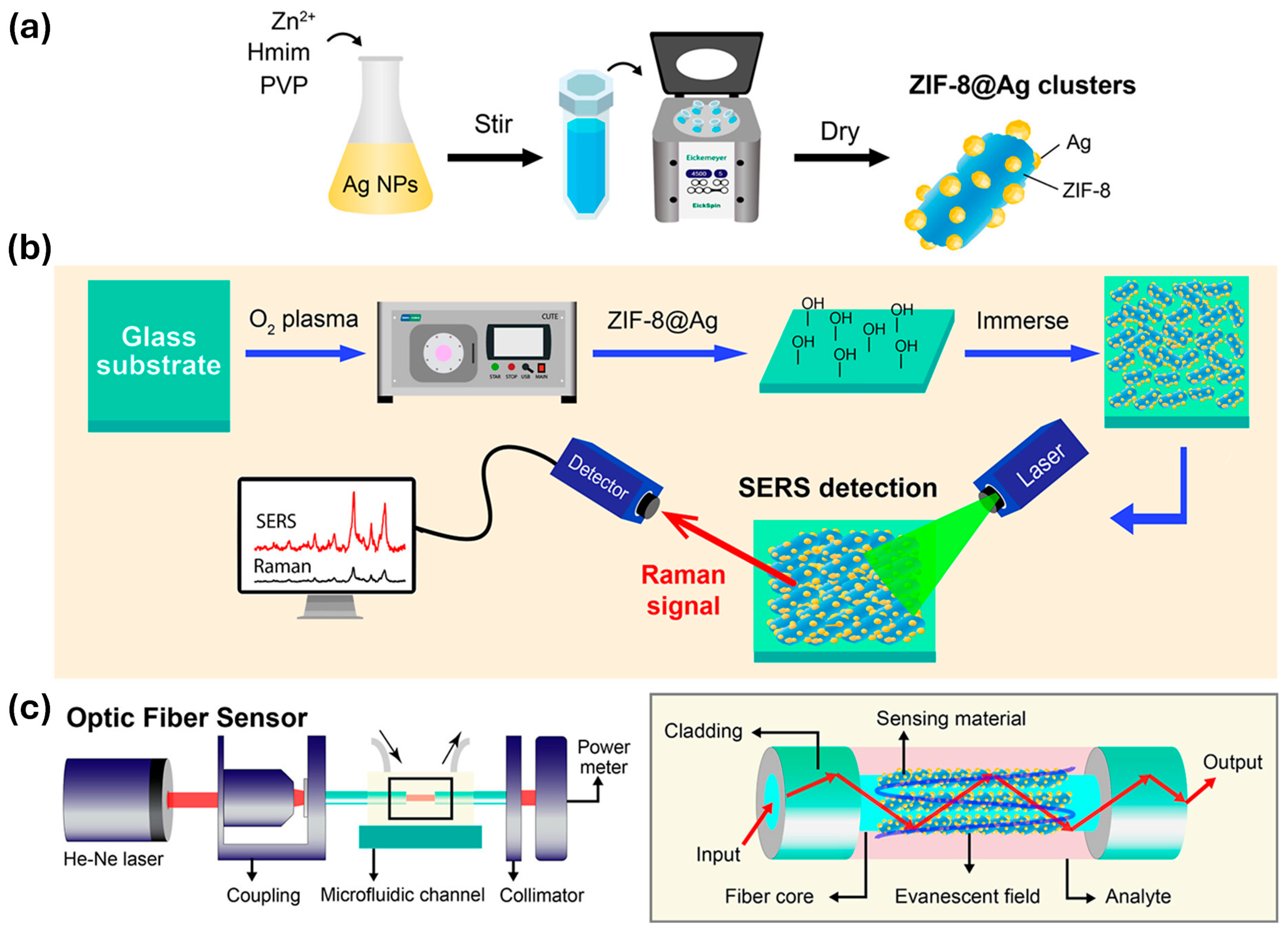
- La Ngoc Tran, N.; Anh, D.T.; Tran, N.Q.; Tho, L.H.; Ta, H.K.T.; Tran, N.H.T. MOF-Integrated Plasmonic Nanostructures for Ultrasensitive SERS and Fiber-Optic LSPR Sensing: A ZIF-8@Ag Hybrid Platform for Trace-Level Detection. ACS Appl. Nano Mater. 2025, 8, 13047–13059. [Google Scholar] [CrossRef]
- Li, D.; Ren, S.; Wang, X.; Chen, L.; You, S.; Tang, Y.; Chen, L. Gated nanoprobe utilizing metal-organic frameworks for identifying and distinguishing between the wild strains and the vaccine strains of brucella. Analyst 2024, 149, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; Sheta, S.M.; Salem, S.R.; Abd-Elzaher, M.M.; Basaleh, A.S.; Labib, A.A. Prostate-Specific Antigen Monitoring Using Nano Zinc(II) Metal–Organic Framework-Based Optical Biosensor. Biosensors 2022, 12, 931. [Google Scholar] [CrossRef]
- Rehman, T.U.; Agnello, S.; Gelardi, F.M.; Calvino, M.M.; Lazzara, G.; Buscarino, G.; Cannas, M. Unveiling the MIL-53(Al) MOF: Tuning Photoluminescence and Structural Properties via Volatile Organic Compounds Interactions. Nanomaterials 2024, 14, 388. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Li, X.; Gong, P.; Zhang, Y.; Zhao, Y. Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects. Biosensors 2023, 13, 405. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhang, Z.; Wang, Z.; Gong, Z.; Fan, M. Functionalized fluorescent Zr-MOF based on photoinduced electron transfer for highly sensitive detection of nitroaromatic explosives. Dye. Pigment. 2023, 210, 111035. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lin, Y.; Chu, W.; Luo, Z.; Zhao, M.; Hu, J.; Miao, X.; He, F. A catalytic hairpin assembly–based Förster resonance energy transfer sensor for ratiometric detection of ochratoxin A in food samples. Anal. Bioanal. Chem. 2023, 415, 867–874. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Wu, Y.; Qin, D.; Deng, B. Novel immunosensor based on electrochemiluminescence inner filter effect and static quenching between fibrillary Ag-MOGs and SiO2@PANI@AuNPs for enabling the sensitive detection of neuron-specific enolase. Microchim. Acta 2024, 191, 204. [Google Scholar] [CrossRef]
- Ding, X.; Ahmad, W.; Zareef, M.; Rong, Y.; Zhang, Y.; Wu, J.; Ouyang, Q.; Chen, Q. MIL-101(Cr)-induced nano-optical sensor for ultra-sensitive detection of enrofloxacin in aquatic products using a fluorescence turn-on mechanism via upconversion nanoparticles. Sens. Actuators B Chem. 2022, 365, 131915. [Google Scholar] [CrossRef]
- Fu, L.; Du, Y.; Zhou, J.; Li, H.; Wang, M.; Wang, Y.B. A novel AgNPs/MOF substrate-based SERS sensor for high-sensitive on-site detection of wheat gluten. Food Sci. Hum. Wellness 2024, 13, 681–687. [Google Scholar] [CrossRef]
- Hong, F.; Zhao, Y.; Pan, S.; Ren, L.; Jiang, F.; Wu, L.; Chen, Y. Click Reaction-Mediated Fluorescent Immunosensor Based on Cu-MOF Nanoparticles for Ultrasensitive and High-Throughput Detection of Aflatoxin B1 in Food Samples. J. Agric. Food Chem. 2024, 72, 5975–5982. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, W.; Yu, S.; Xia, S.L.; Liu, Y.N.; Yang, G.J. Application of MOF-based nanotherapeutics in light-mediated cancer diagnosis and therapy. J. Nanobiotech. 2022, 20, 421. [Google Scholar] [CrossRef]
- Yao, L.; He, S.; Chen, Y.; Lian, H.; Liu, B.; Lai, C.; Wei, X. Carbon dot/Co-MOF nanocoral mediated fluorescence-scattering ratiometric sensor for highly sensitive detection of alkaline phosphatase. Talanta 2023, 265, 124863. [Google Scholar] [CrossRef]
- Pal, T.K. Metal-organic framework (MOF)-based fluorescence “turn-on” sensors. Mater. Chem. Front. 2022, 7, 405–441. [Google Scholar] [CrossRef]
- Ibrahim, M.R.; Greish, Y.E. MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review. Molecules 2023, 28, 5970. [Google Scholar] [CrossRef]
- Liao, X.; Fu, H.; Yan, T.; Lei, J. Electroactive metal–organic framework composites: Design and biosensing application. Biosens. Bioelectron. 2019, 146, 111743. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal-Organic Framework-Functionalized Paper-Based Electrochemical Biosensor for Ultrasensitive Exosome Assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Teng, M.; Hao, M.; Feng, B.; Yang, F.; Shen, H.; Yu, S.; Wang, L. Dual recognition strategy for the rapid and precise detection of Bacillus cereus using post-modified nano-MOF and aptamer. Sens. Actuators B Chem. 2023, 386, 133745. [Google Scholar] [CrossRef]
- Shan, X.; Xie, H.; Zhou, T.; Wu, M.; Yang, J. Dual DNA recycling amplifications coupled with Au NPs@ZIF-MOF accelerator for enhanced electrochemical ratiometric sensing of pathogenic bacteria. Talanta 2023, 263, 124751. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, X.T.; Li, Q.L.; Loh, X.J.; Su, X.; Zhao, S. Antibody conjugated Au/Ir@Cu/Zn-MOF probe for bacterial lateral flow immunoassay and precise synergistic antibacterial treatment. Biosens. Bioelectron. 2023, 224, 115033. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Li, S.; Li, Y.; Tan, H. Portable foodborne pathogen detection via ratiometric fluorescence nanoprobe for adenosine triphosphate quantification based on DNA-functionalized metal-organic framework. Int. J. Biol. Macromol. 2025, 286, 138410. [Google Scholar] [CrossRef]
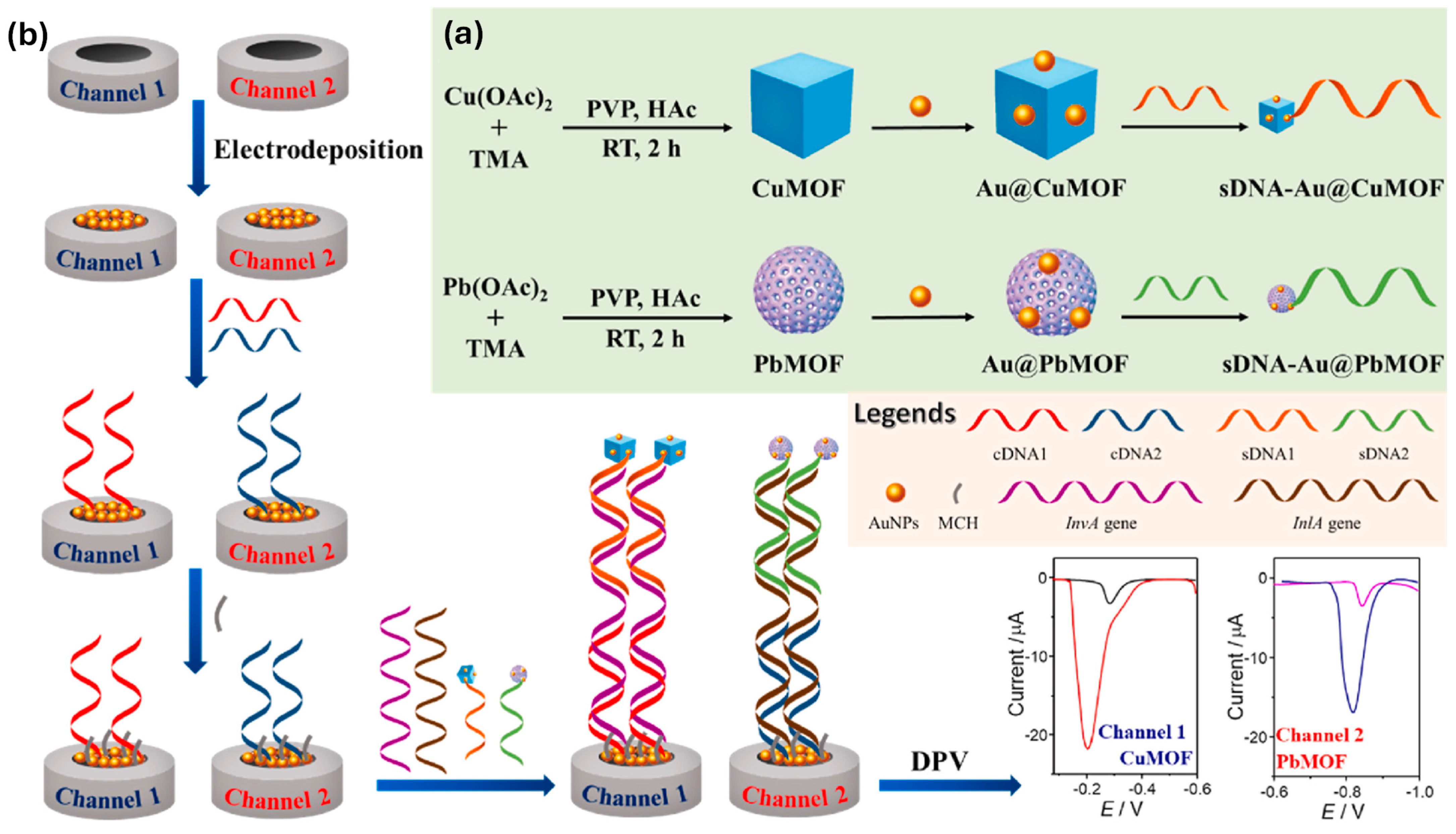
- Ye, Y.; Yan, W.; Wang, T.; Zhang, C.; Wang, K.; Lu, Y.; Zheng, H.; Tao, Y.; Cao, X.; He, S.; et al. Dual-channel biosensor for simultaneous detection of S. typhimurium and L. monocytogenes using nanotags of gold nanoparticles loaded metal-organic frameworks. Anal. Chim. Acta 2023, 1279, 341816. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Saleh, R.O.; Pallathadka, H.; Kumar, A.; Mansouri, S.; Bhupathi, P.; Ali, S.H.J.; Al-Mashhadani, Z.I.; Alzubaidi, L.H.; Hizam, M.M. Advances in electrochemical-optical dual-mode biosensors for detection of environmental pathogens. Anal. Methods 2024, 16, 1306–1322. [Google Scholar] [CrossRef]
- Shahrashoob, M.; Dehshiri, M.; Yousefi, V.; Moassesfar, M.; Saberi, H. Optical and Electrochemical Biosensors for Detection of Pathogens Using Metal Nanoclusters: A Systematic Review. Biosensors 2025, 15, 460. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Peng, Y.; Wang, M.; Lin, Y.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Yang, J.; Li, G. Electrochemical deposition of Cu metal-organic framework films for the dual analysis of pathogens. Anal. Chem. 2020, 93, 8994–9001. [Google Scholar] [CrossRef]
- Jebakumari, K.A.E.; Murugasenapathi, N.K.; Palanisamy, T. Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review. Biosensors 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, G.S.; El-Khawaga, A.M.; Rashdan, H.R.M. Gamma-irradiated copper-based metal organic framework nanocomposites for photocatalytic degradation of water pollutants and disinfection of some pathogenic bacteria and fungi. BMC Microbiol. 2024, 24, 453. [Google Scholar] [CrossRef]
- Bian, J.; Liu, M.; Liu, X.; Bian, X.; Gu, C.; Ma, J.; Jiang, T. SERS detection of pelvic infection-related pathogenic bacteria based on flexible PDMS-MXene@MOF@Ag ternary substrate. Microchem. J. 2025, 210, 113021. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.Z.; Luo, X.; Gu, H.W.; Yin, X.L.; Han, W.L.; Yi, H.C.; Chen, Y. Molecularly imprinted polymer combined with MOF-assisted redox recycling amplification: A powerful electrochemical sensing strategy for pathogenic bacteria. Sens. Actuators B Chem. 2024, 410, 135682. [Google Scholar] [CrossRef]
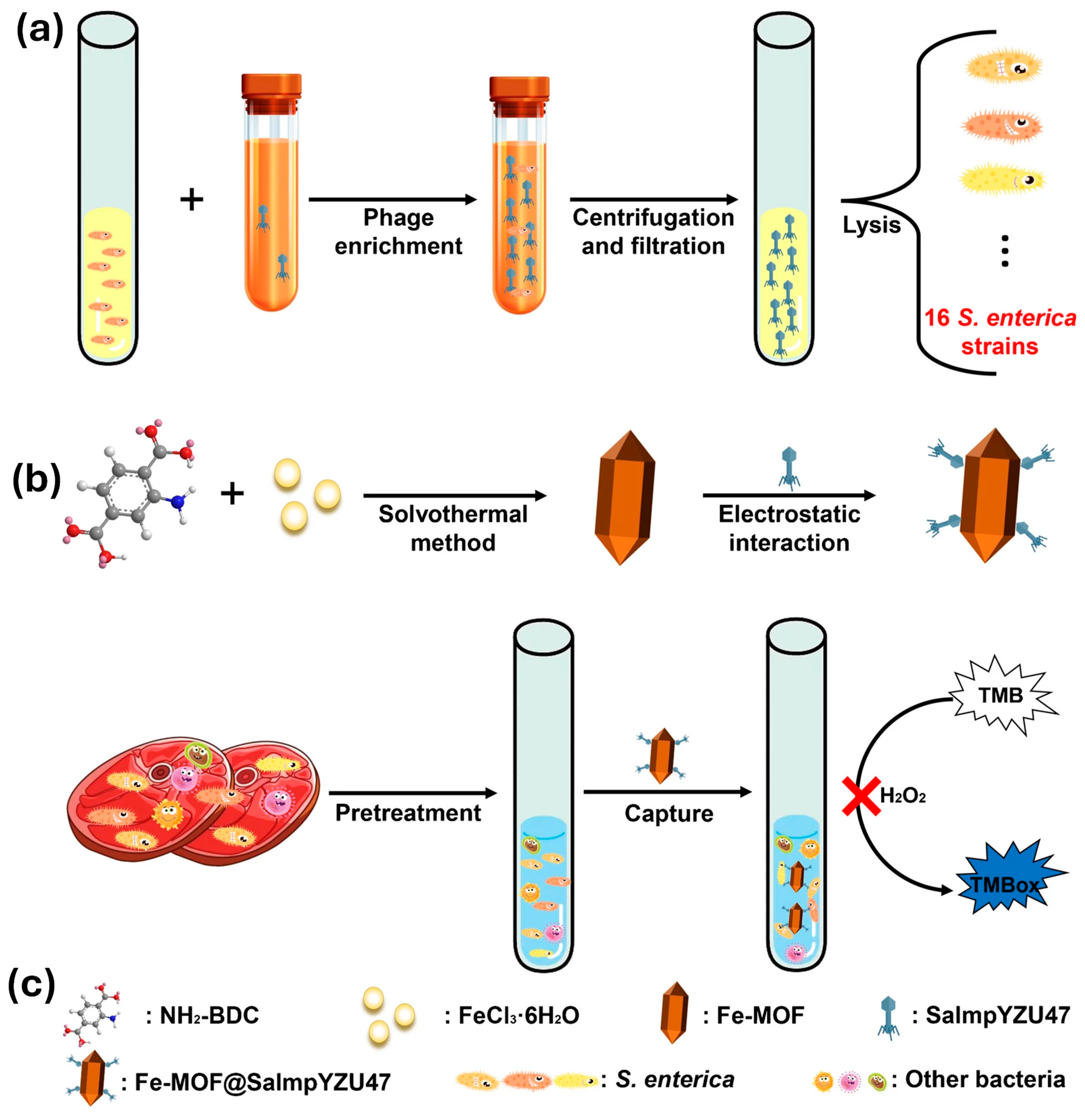
- Gao, L.; Zhang, L.; Yang, J.; Ma, T.; Wang, B.; Yang, H.; Lin, F.; Xu, X.; Yang, Z.Q. Immobilization of a broad host range phage on the peroxidase-like Fe-MOF for colorimetric determination of multiple Salmonella enterica strains in food. Microchim. Acta 2024, 191, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bu, S.; Zhang, J.; Ma, L.; Liu, X.; Wang, X.; Li, Z.; Hao, Z.; Li, Z.; Wan, J. Point-of-care detection of pathogenic bacteria based on pregnancy test strips and metal–organic frameworks. Microchem. J. 2022, 175, 107142. [Google Scholar] [CrossRef]
- Pandiyaraj, K.; Elkaffas, R.A.; Mohideen, M.I.H.; Eissa, S. Graphene oxide/Cu–MOF-based electrochemical immunosensor for the simultaneous detection of Mycoplasma pneumoniae and Legionella pneumophila antigens in water. Sci. Rep. 2024, 14, 17172. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, Y.; Gao, X.; Li, S.; Li, K.; Xiong, C.; Huang, G.; Zhang, J. Sensitive electrochemical immunosensor for rapid detection of Salmonella in milk using polydopamine/CoFe-MOFs@Nafion modified gold electrode. Int. J. Food Microbiol. 2024, 425, 110870. [Google Scholar] [CrossRef]
- Li, H.; Huang, S.; Ling, T.; Li, S.; Zhang, Y.; Ying, Y.; Huang, G.; Zhang, J. Rapid detection of Salmonella in milk by a label-free electrochemical immunosensor based on CoFe-MOFs@MWCNTs modified electrode. Int. Dairy J. 2025, 166, 106242. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Yao, S.; Wei, S.; Shi, X.; Zhao, C.; Li, J.; Wang, J. Colorimetry/fluorescence dual-mode detection of Salmonella typhimurium based on self-assembly of MCOF with Au NPs nanozyme coupled AIEgen. Talanta 2024, 270, 125505. [Google Scholar] [CrossRef]
- Ali, G.K.; Omer, K.M. Ultrasensitive aptamer-functionalized Cu-MOF fluorescent nanozyme as an optical biosensor for detection of C-reactive protein. Anal. Biochem. 2022, 658, 114928. [Google Scholar] [CrossRef]
- Ibrahim, M.R.; Alneyadi, S.; Truong, K.-N.; Maghraby, H.E.; Abdellah, M.; El-Zohry, A.M.; Wuttke, S.; Greish, Y. Design of a Zn-based porphyrin MOF biosensor for fluorometric detection of HER2 as a breast cancer biomarker. RSC Adv. 2025, 15, 21479–21492. [Google Scholar] [CrossRef]
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic Acid-Functionalized Metal-Organic Framework-Based Homogeneous Electrochemical Biosensor for Simultaneous Detection of Multiple Tumor Biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chi, K.N.; Li, D.L.; Deng, Y.; Ma, Y.C.; Xu, Q.Q.; Hu, R.; Yang, Y.H. 2D-porphrinic covalent organic framework-based aptasensor with enhanced photoelectrochemical response for the detection of C-reactive protein. Biosens. Bioelectron. 2019, 129, 64–71. [Google Scholar] [CrossRef]
- Yoo, S.M.; Lee, S.Y. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef]
- Liu, S.; Huo, Y.; Bai, J.; Ning, B.; Peng, Y.; Li, S.; Han, D.; Kang, W.; Gao, Z. Rapid and sensitive detection of prostate-specific antigen via label-free frequency shift Raman of sensing graphene. Biosens. Bioelectron. 2020, 158, 112184. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.; Bai, M.; Gu, H.; Li, X. Metal-organic frameworks as a therapeutic strategy for lung diseases. J. Mater. Chem. B 2022, 10, 5666–5695. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; You, S.; Wang, X.; Li, D.; Ren, S.; Chen, L. Dual carminic acid/hemin-marked DNA probes for simultaneously detecting CV-A16 and EV-A71 based on the mechanism of dimer to monomer transition. Talanta 2023, 265, 124884. [Google Scholar] [CrossRef] [PubMed]
- Sheta, S.M.; El-Sheikh, S.M.; Osman, D.I.; Salem, A.M.; Ali, O.I.; Harraz, F.A.; Shousha, W.G.; Shoeib, M.A.; Shawky, S.M.; Dionysiou, D.D. A novel HCV electrochemical biosensor based on a polyaniline@Ni-MOF nanocomposite. Dalt. Trans. 2020, 49, 8918–8926. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Wu, S.; Pan, Y.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.; Li, G. An Electrochemical Biosensor Designed by Using Zr-Based Metal-Organic Frameworks for the Detection of Glioblastoma-Derived Exosomes with Practical Application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, H.; Di, W.; Gao, X. Imprinted membrane-covalent organic framework platform for efficient label-free visual detection of Listeria monocytogenes and Salmonella typhimurium in food samples. Anal. Chim. Acta 2024, 1320, 343002. [Google Scholar] [CrossRef]
- Feng, K.; Li, T.; Ye, C.; Gao, X.; Yang, T.; Liang, X.; Yue, X.; Ding, S.; Dong, Q.; Yang, M.; et al. A label-free electrochemical immunosensor for rapid detection of Salmonella in milk by using CoFe-MOFs-graphene modified electrode. Food Control 2021, 130, 108357. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, H.; Ying, S.; Sharif, S.; You, Z.; Wang, Y.; Ying, Y. Simultaneous fluorometric determination of the DNAs of Salmonella enterica, Listeria monocytogenes and Vibrio parahemolyticus by using an ultrathin metal-organic framework (type Cu-TCPP). Microchim. Acta 2019, 186, 93. [Google Scholar] [CrossRef]
- Guo, R.; Xue, L.; Cai, G.; Qi, W.; Liu, Y.; Lin, J. Fe-MIL-88NH2Metal-Organic Framework Nanocubes Decorated with Pt Nanoparticles for the Detection of Salmonella. ACS Appl. Nano Mater. 2021, 4, 5115–5122. [Google Scholar] [CrossRef]
- Duan, X.; Shi, X.; He, Z.; Chen, H.; Shi, Z.; Zhao, Z.; Chen, H.; Yu, M.; Guo, C. Conducting polymer functionalized Cu-metal organic framework–based electrochemical immunosensor for rapid and sensitive quantitation of Escherichia coli O157:H7. Microchim. Acta 2024, 191, 740. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Z.; Wang, K.; Zhang, L.; Wan, Y.; Yang, T.; Zeng, H. Ultrasensitive visual detection of the food-borne pathogen via MOF encapsulated enzyme. Talanta 2023, 259, 124503. [Google Scholar] [CrossRef]
- Duan, H.; Li, D.; Wang, J.; Shen, Y.; Zheng, L.; Huang, X. A cocatalytic nanozyme based on metal-organic framework-embedded iron porphyrin for the sensitive detection of Salmonella typhimurium in milk. Talanta 2024, 280, 126765. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Q.; Duan, M.; Zhang, Q.; Li, Z.; Zhang, Y.; Liu, Y.; Wang, H.; Li, X.; Dai, R.; et al. Locking-Fluorescence Signals Regulated CRISPR/Cas12a Biosensor Based on Metal-Organic Framework for Sensitive Detection of Salmonella typhimurium. J. Agric. Food Chem. 2024, 72, 25987–25996. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Yang, W.; Wu, S.; Zou, Y.; Wang, Z. A Visual and Sensitive Detection of Escherichia coli Based on Aptamer and Peroxidase-like Mimics of Copper-Metal Organic Framework Nanoparticles. Food Anal. Methods 2020, 13, 1433–1441. [Google Scholar] [CrossRef]
- Wang, L.; Huo, X.; Zheng, L.; Cai, G.; Wang, Y.; Liu, N.; Wang, M.; Lin, J. An ultrasensitive biosensor for colorimetric detection of Salmonella in large-volume sample using magnetic grid separation and platinum loaded zeolitic imidazolate Framework-8 nanocatalysts. Biosens. Bioelectron. 2020, 150, 111862. [Google Scholar] [CrossRef]
- Yuan, J.; Jia, K.; Li, Y.; Li, Y.; Lin, J. Dual-functionalized defective metal–organic frameworks for fast and sensitive biosensing of Salmonella. Food Chem. 2025, 492, 145350. [Google Scholar] [CrossRef]
- Zhan, K.; Chen, L.; Li, S.; Yu, Q.; Zhao, Z.; Li, J.; Xing, Y.; Ren, H.; Wang, N.; Zhang, G. A novel metal–organic framework based electrochemical immunosensor for the rapid detection of Salmonella typhimurium detection in milk. Food Chem. 2024, 444, 138672. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Peng, Y.; Wang, Y.; Xu, D.; Chen, W.; Wang, W.; Yan, X.; Ma, X. Intrinsic Properties Enabled Metal Organic Framework Micromotors for Highly Efficient Self-Propulsion and Enhanced Antibacterial Therapy. ACS Nano 2022, 16, 14666–14678. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, C.; Nguyen, M.K.; Hussain, A.; Bui, X.T.; Ngo, H.H. A review of biosensor for environmental monitoring: Principle, application, and corresponding achievement of sustainable development goals. Bioengineered 2023, 14, 58–80. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, M. Optical biosensors for environmental monitoring: Recent advances and future perspectives in bacterial detection. Environ. Res. 2023, 236, 116826. [Google Scholar] [CrossRef]
- El-Sewify, I.M.; Radwan, A.; Elghazawy, N.H.; Fritzsche, W.; Azzazy, H.M.E. Optical chemosensors for environmental monitoring of toxic metals related to Alzheimer’s disease. RSC Adv. 2022, 12, 32744–32755. [Google Scholar] [CrossRef]
- Kashem, M.A.; Suzuki, M.; Kimoto, K.; Iribe, Y. An optical biochemical oxygen demand biosensor chip for environmental monitoring. Sens. Actuators B Chem. 2015, 221, 1594–1600. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Shakib, S.; Majidi, M.R.; Razmjou, A.; Yoon, Y.; Khataee, A. Metal-Organic Framework-Based Biosensing Platforms for the Sensitive Determination of Trace Elements and Heavy Metals: A Comprehensive Review. Ind. Eng. Chem. Res. 2023, 62, 4611–4627. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H.; Wang, H.; Liu, J. Rapid on-site/in-situ detection of heavy metal ions in environmental water using a structure-switching DNA optical biosensor. Sci. Rep. 2013, 3, 2308. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, S.A.; Pournara, A.D.; Koutsouroubi, E.D.; Moularas, C.; Deligiannakis, Y.; Armatas, G.S.; Hatzidimitriou, A.G.; Manos, M.J.; Lazarides, T. Detection and Sorption of Heavy Metal Ions in Aqueous Media by a Fluorescent Zr(IV) Metal−Organic Framework Functionalized with 2-Picolylamine Receptor Groups. Inorg. Chem. 2022, 61, 7847–7858. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhao, J. Loading fluorescent dyes into MOF pores for the optical sensing and adsorption of heavy metal ions: Synthesis, characterization, and performance. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 340, 126323. [Google Scholar] [CrossRef]
- Du, T.; Wang, J.; Zhang, L.; Wang, S.C.; Yang, C.; Xie, L.; Liu, Z.; Ni, Y.; Xie, X.H.; Sun, J.; et al. Missing-linker engineering of Eu (III)-doped UiO-MOF for enhanced detection of heavy metal ions. Chem. Eng. J. 2022, 431, 134050. [Google Scholar] [CrossRef]
- Menon, S.; Usha, S.P.; Manoharan, H.; Kishore, P.V.N.; Sai, V.V.R. Metal-Organic Framework-Based Fiber Optic Sensor for Chromium(VI) Detection. ACS Sens. 2023, 8, 684–693. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. Amine- and Imine-Functionalized Mn-Based MOF as an Unusual Turn-On and Turn-Off Sensor for d10Heavy Metal Ions and an Efficient Adsorbent to Capture Iodine. Cryst. Growth Des. 2022, 22, 3277–3294. [Google Scholar] [CrossRef]
- Menon, S.; Dutta, S.; Madaboosi, N.; Sai, V.V.R. MOF-5 fortified fiber optic plasmonic absorption-based Pb(ii) ion sensor for rapid water quality monitoring. Environ. Sci. Nano 2024, 11, 4007–4019. [Google Scholar] [CrossRef]
- Singh, S.; Bhatt, D.; Deep, A.; Tiwari, U.K. An antibody conjugated NH2-MIL-101(Fe) metal-organic framework based optical biosensor for sensitive detection of lead ions. Microchem. J. 2024, 199, 110122. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, K.; Zhu, L.; Xu, M.; Song, Y.; Zhang, Z.; Du, M. Direct growth of two-dimensional phthalocyanine-based COF on Cu-MOF to construct a photoelectrochemical-electrochemical dual-mode biosensing platform for high-efficiency determination of Cr(iii). Dalt. Trans. 2021, 50, 14285–14295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Ma, Y.; Luo, H.; Hou, J.; Hou, C.; Huo, D. Ultra-sensitive electrochemical sensors through self-assembled MOF composites for the simultaneous detection of multiple heavy metal ions in food samples. Anal. Chim. Acta 2024, 1289, 342155. [Google Scholar] [CrossRef]
- Tu, X.; Yuan, J.; Xu, S.; Zhang, X. Low background dual-ligand Cu-MOF nanoprobe for plant tissue imaging and fast screening as well as sensitive detection of glyphosate in environmental samples. J. Hazard. Mater. 2025, 482, 136519. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Wang, H.; Xu, Y.; Yi, C.; Dai, Z.; Liu, S.Y. MOF-Integrated Single-Enzymatic Colorimetric Paper-Based Biosensor for Point-of-Care Testing of Organophosphorus Pesticides. Anal. Chem. 2025, 97, 15402–15409. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, M.; Singh, S.; Deep, A.; Sharma, A.L. Highly sensitive optical detection of Escherichia coli using terbium-based metal-organic framework. ACS Appl. Mater. Interfaces 2020, 12, 48198–48205. [Google Scholar] [CrossRef] [PubMed]
- Mirsadoughi, E.; Pebdeni, A.B.; Hosseini, M. Sensitive colorimetric aptasensor based on peroxidase-like activity of ZrPr-MOF to detect Salmonella typhimurium in water and milk. Food Control 2023, 146, 109500. [Google Scholar] [CrossRef]
- Ju, P.; Wang, S.; Wen, S.; Liu, W.; Wang, J.; Xiao, L.; Wang, S.; Ma, F.; Chi, Z. A dual-inhibition aptamer gated OPECT biosensor based on a MOF-derived CAU-17/Bi2S3 Z-scheme heterojunction for rapid detection of bacterial quorum sensing signal molecules. J. Mater. Chem. C 2024, 13, 3382–3391. [Google Scholar] [CrossRef]
- Yan, Y.; Ni, M.; Wang, F.; Yu, Y.; Gong, X.; Huang, Y.; Tao, W.; Li, C.; Wang, F. Metal-Organic Framework-Based Biosensor for Detecting Hydrogen Peroxide in Plants through Color-to-Thermal Signal Conversion. ACS Nano 2022, 16, 15175–15187. [Google Scholar] [CrossRef]
- He, C.; Liu, L.; Korposh, S.; Correia, R.; Morgan, S.P. Volatile organic compound vapour measurements using a localised surface plasmon resonance optical fibre sensor decorated with a metal-organic framework. Sensors 2021, 21, 1420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, L.; Qi, R.; Zhang, M.; Zhao, J.; Zhu, L.; Dong, M. A metal-organic zeolitic framework with immobilized urease for use in a tapered optical fiber urea biosensor. Microchim. Acta 2020, 187, 72. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Li, H.; Shi, J.; Sun, C.; Lu, G.; Yan, X. Dual-enhanced enzyme cascade hybrid hydrogel for the construction of optical biosensor. Biosens. Bioelectron. 2024, 263, 116613. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Yu, J.; Ding, W.; Ghosh, S.; Brumels, D.; Tan, S.; Jaishi, L.R.; Amjad, A.; Xian, X. A Metal-Organic Framework-Based Colorimetric Sensor Array for Transcutaneous CO2 Monitoring via Lensless Imaging. Biosensors 2024, 14, 516. [Google Scholar] [CrossRef] [PubMed]
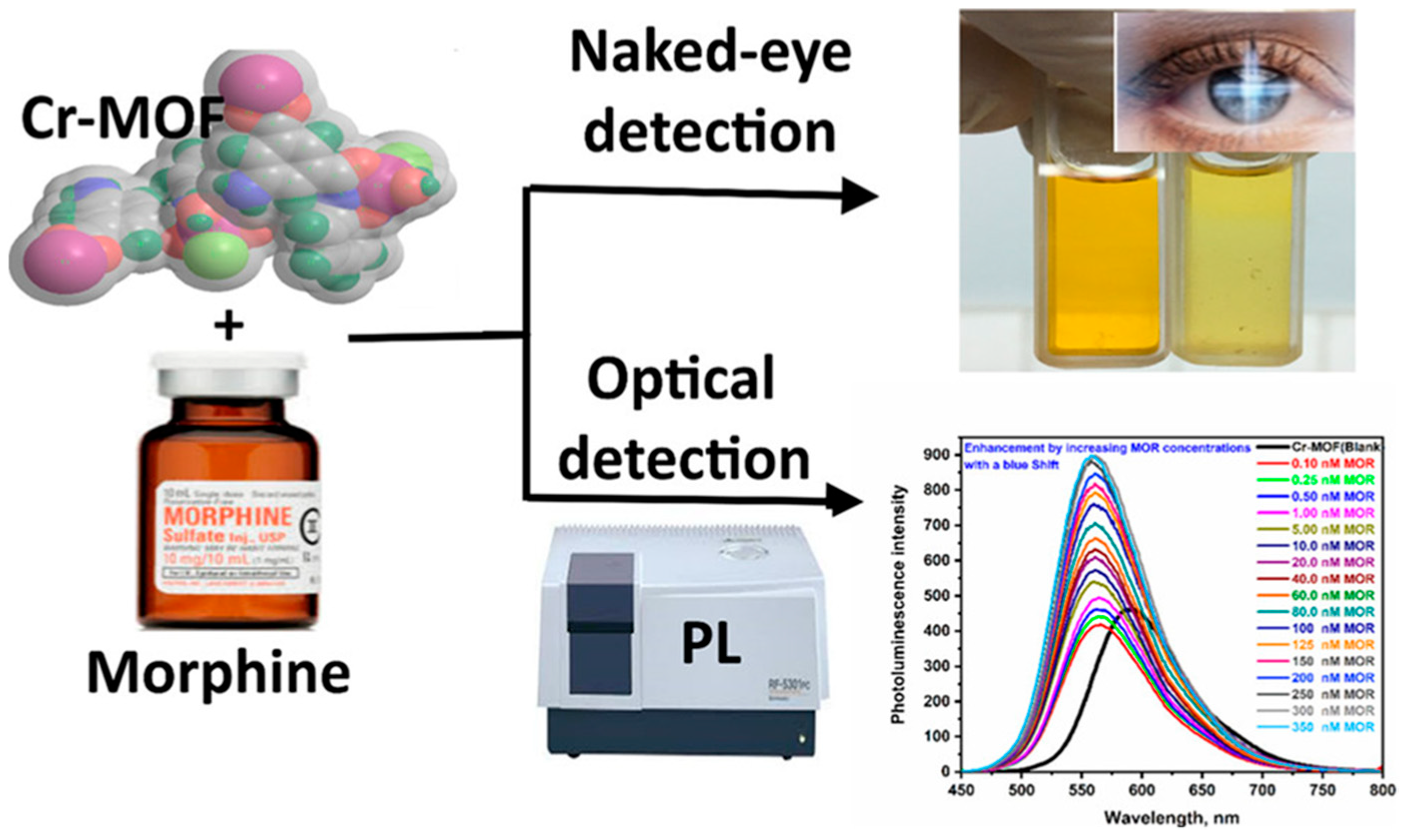
- Alhaddad, M.; Sheta, S.M. Dual naked-eye and optical chemosensor for morphine detection in biological real samples based on Cr(III) metal-organic framework nanoparticles. ACS Omega 2020, 5, 28296–28304. [Google Scholar] [CrossRef]
- Thawany, P.; Khanna, A.; Tiwari, U.K.; Deep, A. A gold/MXene/MOF composite based optical fiber biosensor for haemoglobin detection. In Proceedings of the European Workshop on Optical Fibre Sensors (EWOFS 2023), Mons, Belgium, 23–26 May 2023; p. 126431M. [Google Scholar] [CrossRef]
- Liu, S.; Huo, Y.; Yin, S.; Chen, C.; Shi, T.; Mi, W.; Hu, Z.; Gao, Z. A smartphone-based fluorescent biosensor with metal-organic framework biocomposites and cotton swabs for the rapid determination of tetrodotoxin in seafood. Anal. Chim. Acta 2024, 1311, 342738. [Google Scholar] [CrossRef]
- Huang, X.; Xu, D.; Chen, J.; Liu, J.; Li, Y.; Song, J.; Ma, X.; Guo, J. Smartphone-based analytical biosensors. Analyst 2018, 143, 5339–5351. [Google Scholar] [CrossRef]
- Hou, L.; Qin, Y.; Li, J.; Qin, S.; Huang, Y.; Lin, T.; Guo, L.; Ye, F.; Zhao, S. A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens. Bioelectron. 2019, 143, 111605. [Google Scholar] [CrossRef]
- Zhong, N.; Gao, R.; Shen, Y.; Kou, X.; Wu, J.; Huang, S.; Chen, G.; Ouyang, G. Enzymes-Encapsulated Defective Metal-Organic Framework Hydrogel Coupling with a Smartphone for a Portable Glucose Biosensor. Anal. Chem. 2022, 94, 14385–14393. [Google Scholar] [CrossRef]
- Jiang, D.; Sheng, K.; Gui, G.; Jiang, H.; Liu, X.; Wang, L. A novel smartphone-based electrochemical cell sensor for evaluating the toxicity of heavy metal ions Cd2+, Hg2+, and Pb2+ in rice. Anal. Bioanal. Chem. 2021, 413, 4277–4287. [Google Scholar] [CrossRef]
- Xiong, H.; Li, P.; Cun, F.; Chen, H.; Kong, J. Methylene-Blue-Encapsulated Metal-Organic-Framework-Based Electrochemical POCT Platform for Multiple Detection of Heavy Metal Ions in Milk. Biosensors 2023, 13, 783. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Tu, H.; Yang, Z.; Zhao, D.; Feng, H.; Yang, J. A self-designed versatile and portable sensing device based on smart phone for colorimetric detection. Anal. Bioanal. Chem. 2021, 413, 533–541. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Xiao, M.; Jiang, L.; Yi, C. A smartphone-based quantitative point-of-care testing (POCT) system for simultaneous detection of multiple heavy metal ions. Chem. Eng. J. 2020, 394, 124966. [Google Scholar] [CrossRef]
- Mohanty, S.; Chowdary, G.; Singh, S.G. Smartphone-powered portable chemiresistive sensing system for label free detection of lead ions in water. Microchem. J. 2023, 194, 109239. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Qian, L.; Cui, Y.; Zheng, X.; Kang, Y.; Fu, X.; Wang, S.; Wang, P.; Wang, D. A hand-held optoelectronic tongue for the identification of heavy-metal ions. Sens. Actuators B Chem. 2022, 352, 130971. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, Z.; Xu, N.; Jiang, L.; Yang, M.; Yi, C. A Smartphone-Based Sensing System for On-Site Quantitation of Multiple Heavy Metal Ions Using Fluorescent Carbon Nanodots-Based Microarrays. ACS Sens. 2020, 5, 870–878. [Google Scholar] [CrossRef]
- Rao, K.T.; Gangwar, R.; Bhagavathi, A.; Khatun, S.; Sahu, P.K.; Putta, C.L.; Rengan, A.K.; Subrahmanyam, C.; Garlapati, S.K.; Vanjari, S.R.K. Silk-polyurethane composite based flexible electrochemical biosensing platform for pathogen detection. Biosens. Bioelectron. 2025, 271, 117024. [Google Scholar] [CrossRef]
- Li, C.; Tang, Q.; Wei, H.; Liu, J.; Wang, Q.; Wang, Y.; Du, Z.; Wang, J.; Xu, R.; Bi, Y.; et al. Smart Wearable Fluorescence Sensing of Bacterial Pathogens and Toxic Contaminants by Eu3+-Induced Sodium Alginate/Ag Nanoparticle Aggregates. ACS Appl. Nano Mater. 2022, 5, 8393–8403. [Google Scholar] [CrossRef]
- Taha, B.A.; Ahmed, N.M.; Talreja, R.K.; Haider, A.J.; Al Mashhadany, Y.; Al-Jubouri, Q.; Huddin, A.B.; Mokhtar, M.H.H.; Rustagi, S.; Kaushik, A.; et al. Synergizing Nanomaterials and Artificial Intelligence in Advanced Optical Biosensors for Precision Antimicrobial Resistance Diagnosis. ACS Synth. Biol. 2024, 13, 1600–1620. [Google Scholar] [CrossRef]
- Das, S.; Mandal, B.; Rao, V.R.; Kundu, T. Detection of tomato leaf curl New Delhi virus DNA using U-bent optical fiber-based LSPR probes. Opt. Fiber Technol. 2022, 74, 103108. [Google Scholar] [CrossRef]
- Taha, B.A.; Al-Jubouri, Q.; Al Mashhadany, Y.; Zan, M.S.D.B.; Bakar, A.A.A.; Fadhel, M.M.; Arsad, N. Photonics enabled intelligence system to identify SARS-CoV 2 mutations. Appl. Microbiol. Biotechnol. 2022, 106, 3321–3336. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Gehani, M.; Kammili, N.; Bhardwaj, P.; Nag, V.; Devara, S.M.; Sharad, S. Clinical validation of innovative optical-sensor-based, low-cost, rapid diagnostic test to reduce antimicrobial resistance. J. Clin. Med. 2019, 8, 2098. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tharani, L. Optical sensing for real-time detection of food-borne pathogens in fresh produce using machine learning. Sci. Prog. 2024, 107, 368504231223029. [Google Scholar] [CrossRef]
- Khan, H.; Jan, Z.; Ullah, I.; Alwabli, A.; Alharbi, F.; Habib, S.; Islam, M.; Shin, B.J.; Lee, M.Y.; Koo, J.K. A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring. Nanotechnol. Rev. 2024, 13, 20240056. [Google Scholar] [CrossRef]
- Periyasamy, R.; Sasi, S.; Malagi, V.P.; Shivaswamy, R.; Chikkaiah, J.; Pathak, R.K. Artificial intelligence assisted photonic bio sensing for rapid bacterial diseases. Z. Fur Naturforschung Sect. A-A J. Phys. Sci. 2025, 80, 1–7. [Google Scholar] [CrossRef]
- Yi, J.; Wisuthiphaet, N.; Raja, P.; Nitin, N.; Earles, J.M. AI-enabled biosensing for rapid pathogen detection: From liquid food to agricultural water. Water Res. 2023, 242, 120258. [Google Scholar] [CrossRef]
- Feng, N.; Wang, S.; Wei, L.; Wang, Q.; Cheng, X.; Lu, P.; Peng, X.; Wang, X.; Zhan, C.; Dong, Y.; et al. Artificial Intelligence-Based Imaging Transcoding System for Multiplex Screening of Viable Foodborne Pathogens. Anal. Chem. 2023, 95, 8649–8659. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Q.; Zhu, Z. The Application of Multi-Parameter Multi-Modal Technology Integrating Biological Sensors and Artificial Intelligence in the Rapid Detection of Food Contaminants. Foods 2024, 13, 1936. [Google Scholar] [CrossRef]
- Liu, Q.; Li, S.; Li, Z.; Zou, C.; Feng, S.; Song, J.; Zhang, J.; Li, X. A novel multimodal nano-sensor detection system based on artificial intelligence and two-dimensional Mxenes for Ochratoxin A in food. Food Control 2025, 170, 111055. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Zhu, Y.; Wan, J.; Wang, X.; Zhou, X.; Li, X.; Zhou, W. Research Progress on Multiplexed Pathogen Detection Using Optical Biosensors. Biosensors 2025, 15, 378. [Google Scholar] [CrossRef]
- Jafrasteh, F.; Farmani, A.; Mohamadi, J. Meticulous research for design of plasmonics sensors for cancer detection and food contaminants analysis via machine learning and artificial intelligence. Sci. Rep. 2023, 13, 15349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.; Li, X.; Fan, Y.; Li, J.; Lu, K.; Wen, H.; Ren, J. Recent advances of fluorescent sensors for bacteria detection-A review. Talanta 2023, 254, 124133. [Google Scholar] [CrossRef]
- Afzalinia, A.; Mirzaee, M. Ultrasensitive Fluorescent miRNA Biosensor Based on a “sandwich” Oligonucleotide Hybridization and Fluorescence Resonance Energy Transfer Process Using an Ln(III)-MOF and Ag Nanoparticles for Early Cancer Diagnosis: Application of Central Composite Design. ACS Appl. Mater. Interfaces 2020, 12, 16076–16087. [Google Scholar] [CrossRef]
- Liu, A.Q.; Huang, H.J.; Chin, L.K.; Yu, Y.F.; Li, X.C. Label-free detection with micro optical fluidic systems (MOFS): A review. Anal. Bioanal. Chem. 2008, 391, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, Y.; Song, W.J. Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: A review. Microchim. Acta 2020, 187, 234. [Google Scholar] [CrossRef]
- Basaleh, A.S.; Sheta, S.M. Manganese Metal–Organic Framework: Chemical Stability, Photoluminescence Studies, and Biosensing Application. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1726–1737. [Google Scholar] [CrossRef]
- Can, M.; Demirci, S.; Sunol, A.K.; Sahiner, N. An amino acid, L-Glutamic acid-based metal-organic frameworks and their antibacterial, blood compatibility, biocompatibility, and sensor properties. Microporous Mesoporous Mater. 2020, 309, 110533. [Google Scholar] [CrossRef]
- Yadav, S.; Parihar, A.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Khan, R. Emerging Point-of-Care Optical Biosensing Technologies for Diagnostics of Microbial Infections. ACS Appl. Opt. Mater. 2023, 1, 1245–1262. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef]
- Huo, D.Q.; Liu, Z.; Hou, C.J.; Yang, J.; Luo, X.G.; Fa, H.B.; Le Dong, J.; Zhang, Y.C.; Zhang, G.P.; Li, J.J. Recent advances on optical detection methods and techniques for cell-based microfluidic systems. Chin. J. Anal. Chem. 2010, 38, 1357–1365. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, H.; Wang, H.; Sun, L.; Zhang, N.; Zhang, X.; Liang, J. Antibiotic quantitative fluorescence chemical sensor based on Zn-MOF aggregation-induced emission characteristics. Microchem. J. 2023, 190, 108626. [Google Scholar] [CrossRef]
- Ayyanar, N.; Konnova, S.S.; Zanishevskaya, A.A.; Lepilin, P.A.; Shuvalov, A.A.; Skibina, J.S.; Alzahrani, F.A. Protein Detection Using Hollow Core Microstructured Optical Fiber. IEEE Sens. J. 2024, 24, 32172–32178. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and Methods of Biosensor Interfaces with Stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Choi, J.R.; Song, H.; Sung, J.H.; Kim, D.; Kim, K. Microfluidic assay-based optical measurement techniques for cell analysis: A review of recent progress. Biosens. Bioelectron. 2016, 77, 227–236. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, E.; Rather, M.D.; Sundararajan, A.; Sharma, A.; Choudhary, F.S.; Sundramoorthy, A.K.; Dixit, S.; Vatin, N.I.; Arya, S. Marketing Strategies in Nanomaterials for Sensor Applications: Bridging Lab to Market. Glob. Chall. 2025, 9, 2400294. [Google Scholar] [CrossRef]
- Choi, H.K.; Yoon, J. Nanotechnology-Assisted Biosensors for the Detection of Viral Nucleic Acids: An Overview. Biosensors 2023, 13, 208. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M. Frontiers in nanoparticles redefining enzyme immobilization: A review addressing challenges, innovations, and unlocking sustainable future potentials. Micro Nano Syst. Lett. 2025, 13, 7. [Google Scholar] [CrossRef]
- Ali, A.; Majhi, S.M.; Siddig, L.A.; Deshmukh, A.H.; Wen, H.; Qamhieh, N.N.; Greish, Y.E.; Mahmoud, S.T. Recent Advancements in MXene-Based Biosensors for Health and Environmental Applications—A Review. Biosensors 2024, 14, 497. [Google Scholar] [CrossRef]
- Khan, S.T.; Moosavi, S.M. Connecting metal-organic framework synthesis to applications with a self-supervised multimodal model. Mater. Chem. 2024, 1, 15–17. [Google Scholar]
- Lu, X.; Jayakumar, K.; Wen, Y.; Hojjati-Najafabadi, A.; Duan, X.; Xu, J. Recent advances in metal-organic framework (MOF)-based agricultural sensors for metal ions: A review. Microchim. Acta 2024, 191, 58. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.B.; Shang, X.; Sun, M.; Bo, X.; Bai, J.; Du, Y.; Zhou, M. Emerging Multifunctional Wearable Sensors: Integrating Multimodal Sweat Analysis and Advanced Material Technologies for Next-Generation Health Monitoring. ACS Sens. 2025, 10, 2388–2408. [Google Scholar] [CrossRef] [PubMed]
- Turasan, H.; Kokini, J.; Turasan, H. Novel Nondestructive Biosensors for the Food Industry. Annu. Rev. Food Sci. Technol. 2021, 12, 539–566. [Google Scholar] [CrossRef]
- Mondal, P.P.; Neem, M.; Chand, R.; Pandit, A.; Neogi, S. Luminescent Metal-Organic Frameworks as Multimodal Platforms for Advanced Anticounterfeiting and Security Applications. Chem. Mater. 2024, 36, 10451–10473. [Google Scholar] [CrossRef]
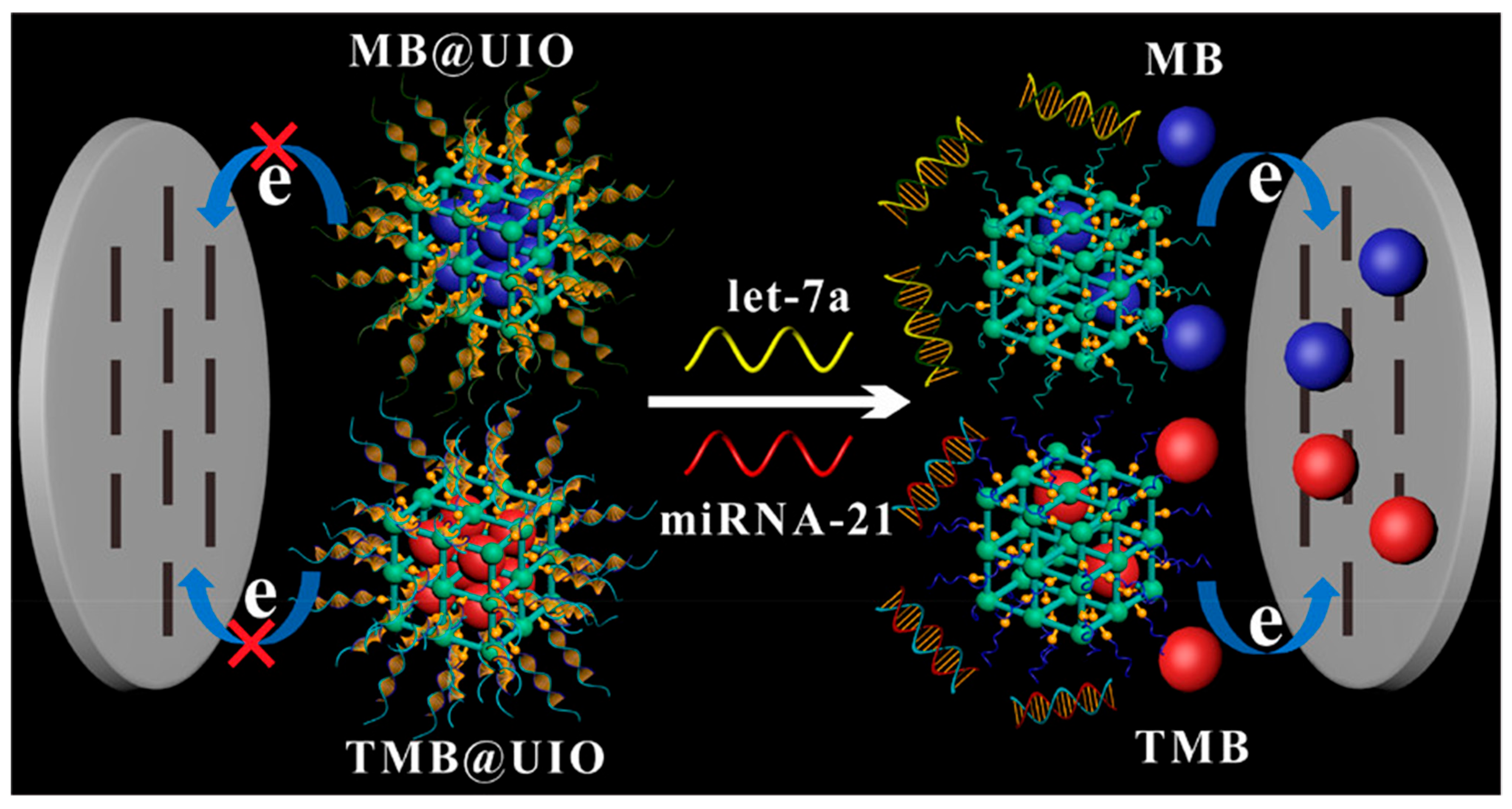
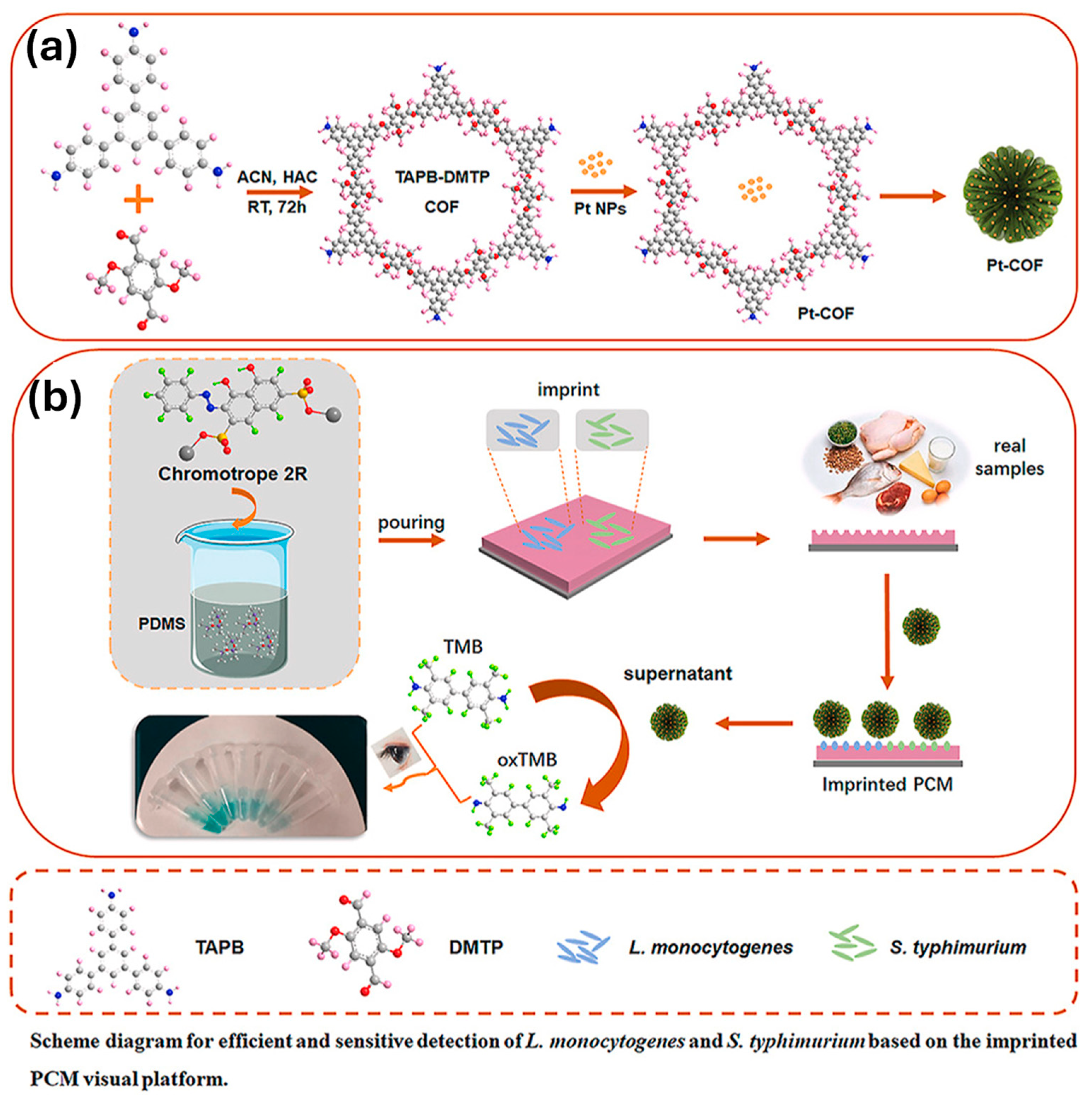
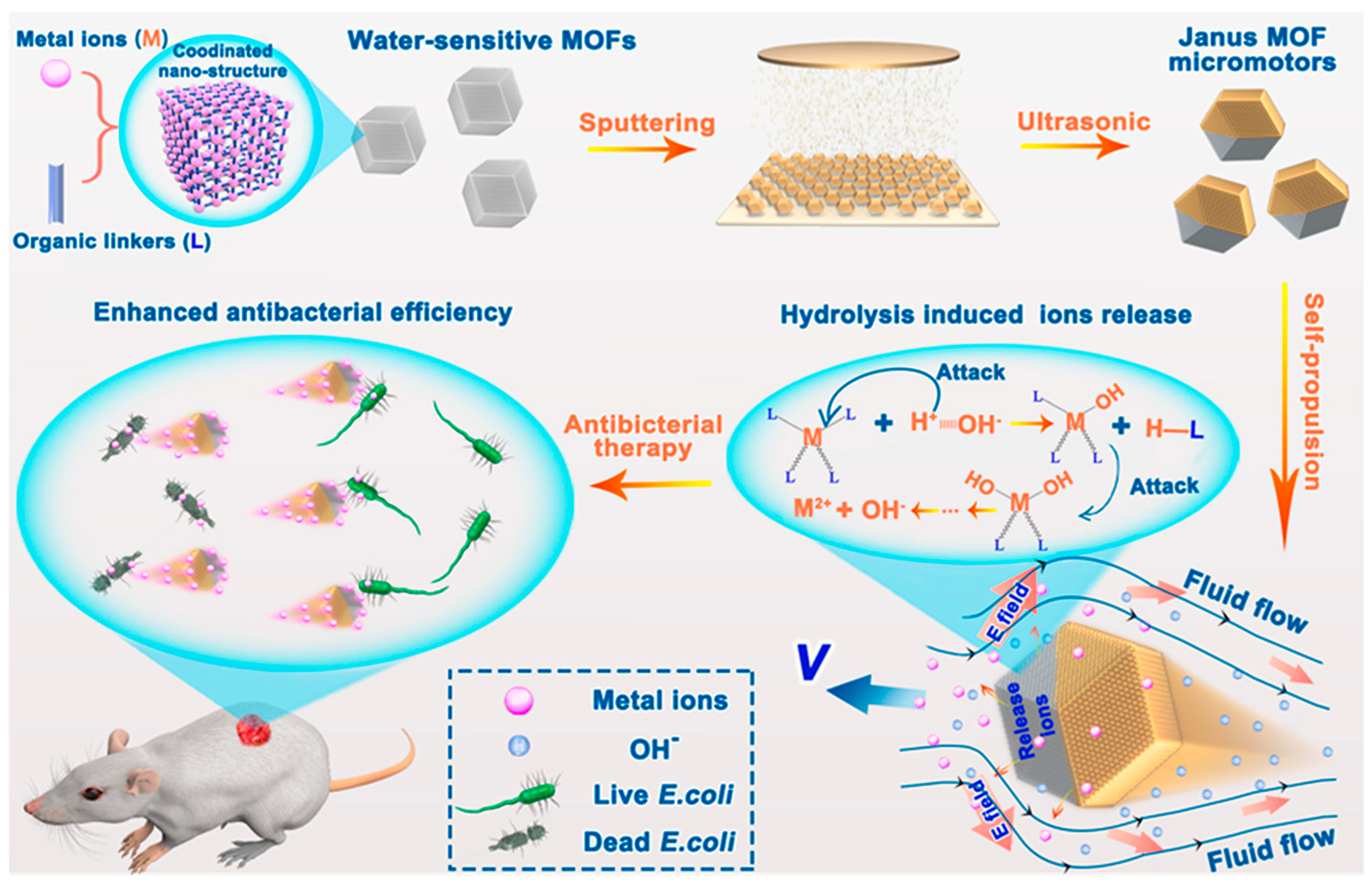

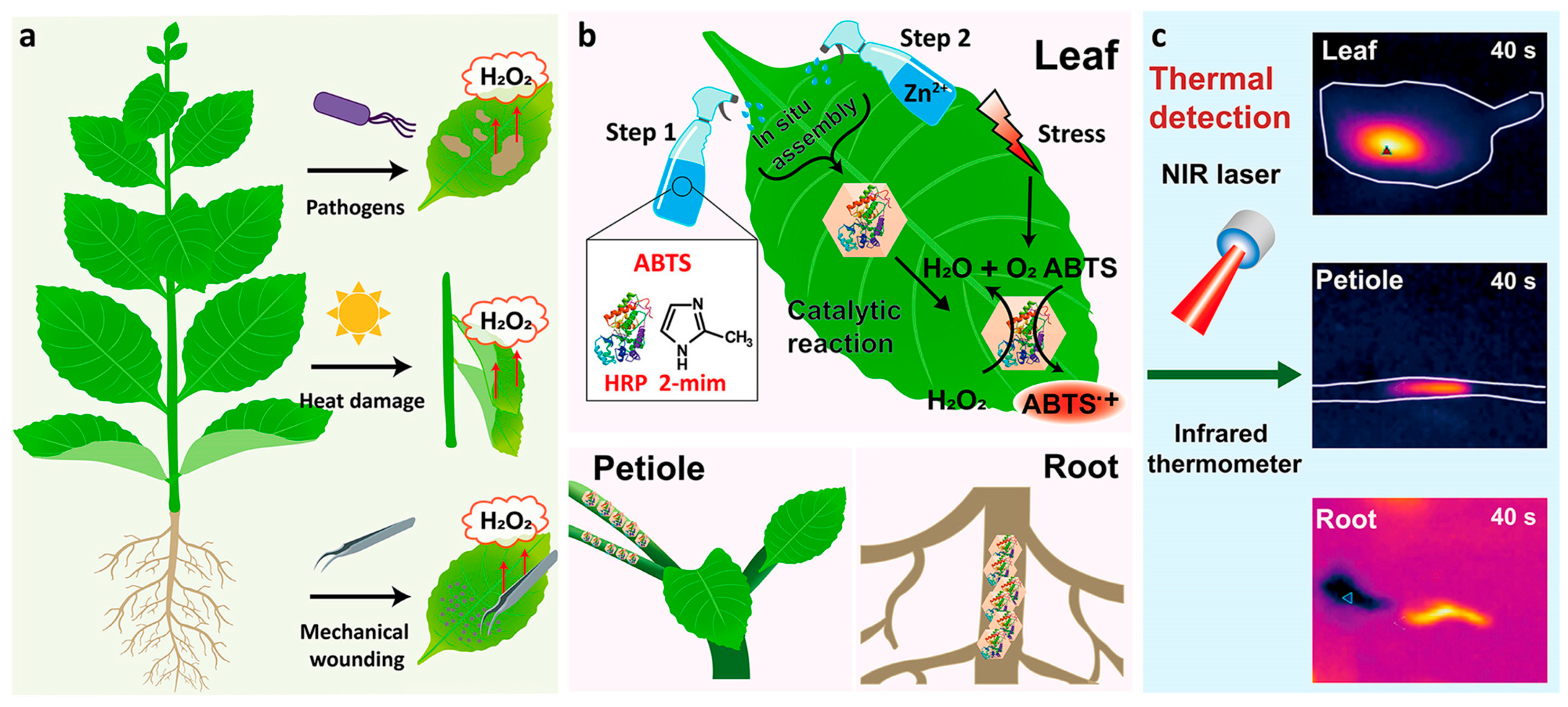
| Design Strategy | Target Microorganism/Analyte | Detection Method | Advantages | Ref. |
|---|---|---|---|---|
| MOF with hairpin DNA for gated dye release | Brucella (wild vs. vaccine strain) | Fluorescence | DNA-specific release; low cross-reactivity | [45] |
| Zn-MOFs with modifiable chemistry | PSA | Fluorescence | Enhanced stability and selectivity | [46] |
| Cu-MOF with antigen and click-reaction signal amplification | Aflatoxin B1 | Fluorescence | Strong proximity-triggered signal amplification | [54] |
| CD/Co-MOF nanocoral (ratiometric probe) | Alkaline Phosphatase (ALP) | Fluorescence | Dual-signal output; low background noise | [56] |
| MIL-53(Al)-NH2 with FcMBL and aptamer-functionalized beads | Bacillus cereus | Fluorescence | Dual recognition; fast detection | [61] |
| Au NPs@ZIF-MOF functionalized with aptamers | S. aureus | Fluorescence | Wide dynamic range; built-in signal calibration | [62] |
| Au/Ir@Cu/Zn-MOF with anti-S. aureus antibodies | S. aureus | Immunoassay | High sensitivity; multifunctional | [63] |
| Ru(bpy)32+@Zr-MOF with dsDNA and SGI | ATP (viable bacterial marker) | Fluorescence | Real-time detection; smartphone compatible | [64] |
| Au@CuMOF and Au@PbMOF functionalized with DNA probes | S. typhimurium and L. monocytogenes | Electrochemical | Dual-pathogen detection; high selectivity; broad range | [65] |
| Materials | Target Microorganisms | Method | LOD (µg/L) | Linear Range | Ref. |
|---|---|---|---|---|---|
| Zr-MOF + DNA aptamer | E. coli O157:H7 | Fluorescence | – | – | [70] |
| MOFs + AuNPs + antibodies | SARS-CoV-2 | Colorimetric | – | – | [71] |
| MOFs + lectins | Candida albicans | SERS | – | – | [72] |
| Fe-MOF@SalmpYZU47 | S. enterica | Colorimetric | 5.5 | 1.0 × 102 to 1.0 × 108 CFU/mL | [73] |
| MOF + PTS | E. coli O157:H7 | Visual | 265 | – | [74] |
| GO + Cu–MOF | M. pneumoniae, L. pneumophila | Electrochemical | 0.001 | 1 pg/mL to 100 ng/mL | [75] |
| CoFe-MOFs@Nafion | Salmonella | Electrochemical | 69 | 1.38 × 102 to 1.38 × 108 CFU/mL | [76] |
| CoFe-MOFs + MWCNTs + AuNPs | Salmonella | Electrochemical | 1445 | 1.04 × 104 to 1.04 × 108 CFU/mL | [77] |
| MCOF + AuNPs + AIEgens | S. typhimurium | Colorimetric/fluorescent | 500/5 | – | [78] |
| Cu-MOF | CRP | Colorimetric/fluorescent | 0.04/0.240 | – | [79] |
| Zn-MOF | HER2 (non-microbial) | Fluorescence | 0.12 | – | [80] |
| UiO-66-NH2 MOF + MB + TMB | let-7a, miRNA-21 | Electrochemical | 0.0022, 0.005 | – | [81] |
| Porphyrinic COFs + AgNPs | CRP | Photoelectrochemical | – | – | [82] |
| Polyaniline@Ni-MOF | HCV RNA | Electrochemical | 0.0048 | 1 fM to 100 nM | [87] |
| Zr-MOF + methylene blue | GBM exosomes | Electrochemical | 0.0041 | 9.5 × 103 to 1.9 × 107 particles/μL | [87] |
| Pt-COF nanozyme + Chromotrope 2R | L. monocytogenes, S. typhimurium | Colorimetric | 0.655, 0.805 | – | [89] |
| CoFe-MOFs + graphene + Au–NH2 | Salmonella | Electrochemical | 60 | 2.4 × 102 to 2.4 × 108 CFU/mL | [90] |
| Ultrathin MOF-NSs | Multiple bacterial DNAs | Fluorescence | 0.009 | – | [91] |
| Fe-MIL-88NH2 + PtNPs | Salmonella | Colorimetric | 46.5 | – | [92] |
| Cu-MOF/PEDOT:PSS | E. coli O157:H7 | Electrochemical | 3.7 | 3 × 102 to 3 × 108 CFU/mL | [93] |
| Enzyme-loaded ZIF-8 | E. coli O157:H7 | Colorimetric | 0.5 | – | [94] |
| MIL-88@TcP | S. typhimurium | Colorimetric | 84 | – | [95] |
| ZIF-8 + FLS + CRISPR/Cas12a + RAA | S. typhimurium | Fluorescence | 65 | – | [96] |
| Cu-MOF + streptavidin | E. coli | Colorimetric | 1 | 16 to 1.6 × 106 CFU/mL | [97] |
| Pt@ZIF-8 | S. typhimurium | Colorimetric | 5.5 | 101–104 CFU/mL | [98] |
| MOF@B(OH)2 + Ni mesh | Salmonella | Fluorescence | 9.4 | – | [99] |
| PtNPs + Co/Zn-MOF + MWCNTs | S. typhimurium | Electrochemical | 47 | 1.3 × 102 to 1.3 × 108 CFU/mL | [100] |
| MOF-based micromotors | E. coli | Theranostic | – | – | [101] |
| Materials | Target Analytes | Methods | LOD (µg/L) | Real-World Application Challenges | Ref. |
|---|---|---|---|---|---|
| Zr-based MOF | Cu2+, Pb2+, Hg2+ | Fluorescence | <2 | Humic substances, turbidity quenching fluorescence; fouling of sensing sites in natural waters | [108] |
| P1@BMOF | Cu2+ | Fluorescence | 12.71 | UV/temperature induced degradation of azobenzene; limited stability outdoors | [109] |
| Eu@UiO-MOFs | Cd2+ | Fluorescence | 114 | Defect-rich sites prone to hydrolysis at high humidity or pH extremes | [110] |
| ZIF-67 | Cr(VI) | Evanescent | 1 | Biofilm growth on fiber tip reduces light throughput; requires cleaning | [111] |
| SM-1 | Ag+, Cd2+, Hg2+ | Fluorescence | - | Unclear durability under repeated adsorption desorption in field use | [112] |
| MOF-5 | Pb2+ | Plasmonic | 0.5 | Ionic strength variations affect plasmonic shifts; turbidity interference | [113] |
| NH2-MIL-101(Fe)-mAb | Pb2+ | Fluorescence | 9.51 | Antibody denaturation at high temperatures; cold-chain requirements | [114] |
| CoPc-PT-COF@Cu-MOF | Cr3+ | PEC | 7.54 × 10−7 | High background ion interference; PEC components sensitive to fouling | [115] |
| Bi2CuO4 | Cd2+ | Electrochemical | 2.25 × 10−6 | Cross-contamination between analytes; electrode surface fouling | [116] |
| Cu-MOF | Glyphosate | Fluorescence | 5.58 | Suspended particles scatter light; binding site blockage in field runoff | [117] |
| ZIF-8@Cellulose | Dichlorvos | Colorimetric | 0.29 | Enzyme leaching, reduced activity in humid storage | [118] |
| Tb-BTC MOF | E. coli | Fluorescence | 0.003 | Antibody degradation with temperature/UV; matrix background fluorescence | [119] |
| ZrPr-MOF | S. typhimurium | Colorimetric | 0.037 | Paper background color interference; aptamer storage stability | [120] |
| CAU-17/Bi2S3 | 3-O-C10-HL | Optoelectronic | 1.12 × 10−4 | Salinity changes affect aptamer binding; marine biofouling | [121] |
| ZIF-8 | H2O2 | NIR-induced | - | Dust, waxes affect coating uniformity; outdoor weather effects | [122] |
| AuNPs/HKUST-1 | VOCs | LSPR | - | VOC mixture overlap; humidity impact on refractive index | [123] |
| Urease@ZIF-8 | Urea | Refractive index | 6006 | Enzyme instability in heat; long-term wet storage challenges | [124] |
| UFD-DEC | Urea | Colorimetric | 7207.2 | Hydrogel dehydration or overhydration alters response | [125] |
| MOF-CMOS | CO2 | Colorimetric | 26,000 | Skin oils/sweat contamination; long-term adhesion to skin | [126] |
| Cr(III)-MOF-NPs | Morphine | Fluorescence | 4.76 × 10−2 | Autofluorescent backgrounds in biological samples | [127] |
| Tb-BTC MOF | Hemoglobin | Optical | - | MXene oxidation over time; interference from complex fluids | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidanemariam, A.; Cho, S. Metal–Organic-Framework-Based Optical Biosensors: Recent Advances in Pathogen Detection and Environmental Monitoring. Sensors 2025, 25, 5081. https://doi.org/10.3390/s25165081
Kidanemariam A, Cho S. Metal–Organic-Framework-Based Optical Biosensors: Recent Advances in Pathogen Detection and Environmental Monitoring. Sensors. 2025; 25(16):5081. https://doi.org/10.3390/s25165081
Chicago/Turabian StyleKidanemariam, Alemayehu, and Sungbo Cho. 2025. "Metal–Organic-Framework-Based Optical Biosensors: Recent Advances in Pathogen Detection and Environmental Monitoring" Sensors 25, no. 16: 5081. https://doi.org/10.3390/s25165081
APA StyleKidanemariam, A., & Cho, S. (2025). Metal–Organic-Framework-Based Optical Biosensors: Recent Advances in Pathogen Detection and Environmental Monitoring. Sensors, 25(16), 5081. https://doi.org/10.3390/s25165081







