Hemodynamic Response Asymmetry During Motor Imagery in Stroke Patients: A Novel NIRS-BCI Assessment Approach
Abstract
Highlights
- A novel task response asymmetry coefficient is introduced for assessing daily dynamics of interhemispheric hemodynamic response asymmetry in post-stroke patients and healthy individuals.
- The proposed task response asymmetry coefficient could be used for investigating interhemispheric dynamics even in heterogeneous groups of patients.
- For the patients, the proposed coefficient indicates significant difference between lesioned and intact hemisphere in terms of response to affected and intact hand movement imagery, as well as evident daily dynamics of the asymmetry for people with substantial recovery.
- Daily dynamics in functional asymmetry in post-stroke patients engaged in various rehabilitation procedures is required.
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Experimental Design
2.3. Data Acquisition
2.4. Data Processing
2.5. Hemodynamic Response Estimation
2.6. Interhemispheric Hemodynamic Response Asymmetry Metrics
2.7. Statistical Analysis
3. Results
3.1. LC and TRAC Numerical Stability
3.2. Laterality and Task Response Asymmetry in Patients
3.3. Laterality and Task Response Asymmetry in Healthy Participants
3.4. LC and TRAC Daily Dynamics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIRS | Near-infrared spectroscopy |
| fNIRS | Functional near-infrared spectroscopy |
| BCI | Brain–computer interface |
| HbO | Oxygenated hemoglobin |
| HbR | Deoxygenated hemoglobin |
| LC | Laterality coefficient |
| TRAC | Task response asymmetry coefficient |
| ARAT | Action Research Arm Test |
| ART ANOVA | Repeated-measures nonparametric factorial ANOVA with aligned rank transform |
References
- Rehme, A.K.; Fink, G.R.; von Cramon, D.Y.; Grefkes, C. The Role of the Contralesional Motor Cortex for Motor Recovery in the Early Days after Stroke Assessed with Longitudinal fMRI. Cereb. Cortex 2010, 21, 756–768. [Google Scholar] [CrossRef]
- Fedotova, I.R.; Bobrov, P.D.; Kondur, K.K. Measures of Neuroplastic and Functional Rearrangements During Recovery of Motor Function During Post-Stroke Rehabilitation. Neurosci. Behav. Physiol. 2023, 53, 1521–1533. [Google Scholar] [CrossRef]
- Dodd, K.C.; Nair, V.A.; Prabhakaran, V. Role of the Contralesional vs. Ipsilesional Hemisphere in Stroke Recovery. Front. Hum. Neurosci. 2017, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Perera, G.M.; Lazar, R.M.; Krakauer, J.W.; Constantine, R.C.; DeLaPaz, R.L. Evolution of Cortical Activation During Recovery from Corticospinal Tract Infarction. Stroke 2000, 31, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Ashe, J.; Hendrich, K.; Ellermann, J.M.; Merkle, H.; Uğurbil, K.; Georgopoulos, A.P. Functional Magnetic Resonance Imaging of Motor Cortex: Hemispheric Asymmetry and Handedness. Science 1993, 261, 615–617. [Google Scholar] [CrossRef]
- Stępień, M.; Conradi, J.; Waterstraat, G.; Hohlefeld, F.U.; Curio, G.; Nikulin, V.V. Event-related desynchronization of sensorimotor EEG rhythms in hemiparetic patients with acute stroke. Neurosci. Lett. 2011, 488, 17–21. [Google Scholar] [CrossRef]
- Calautti, C.; Naccarato, M.; Jones, P.S.; Sharma, N.; Day, D.D.; Carpenter, A.T.; Bullmore, E.T.; Warburton, E.A.; Baron, J.C. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. NeuroImage 2007, 34, 322–331. [Google Scholar] [CrossRef]
- Calautti, C.; Jones, P.S.; Naccarato, M.; Sharma, N.; Day, D.J.; Bullmore, E.T.; Warburton, E.A.; Baron, J.C. The relationship between motor deficit and primary motor cortex hemispheric activation balance after stroke: Longitudinal fMRI study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 788–792. [Google Scholar] [CrossRef]
- Kaiser, V.; Daly, I.; Pichiorri, F.; Mattia, D.; Müller-Putz, G.R.; Neuper, C. Relationship between Electrical Brain Responses to Motor Imagery and Motor Impairment in Stroke. Stroke 2012, 43, 2735–2740. [Google Scholar] [CrossRef]
- Tang, Q.; Li, G.; Liu, T.; Wang, A.; Feng, S.; Liao, X.; Jin, Y.; Guo, Z.; He, B.; McClure, M.A.; et al. Modulation of interhemispheric activation balance in motor-related areas of stroke patients with motor recovery: Systematic review and meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2015, 57, 392–400. [Google Scholar] [CrossRef]
- Ramos–Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, Ö.; Brasil, F.L.; Liberati, G.; Curado, M.R.; Garcia–Cossio, E.; Vyziotis, A.; et al. Brain–machine interface in chronic stroke rehabilitation: A controlled study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Wang, X.; Chen, C.; Lau, C.C.Y.; Chu, W.C.W.; Tong, R.K.Y. Interhemispheric Functional Reorganization and its Structural Base After BCI-Guided Upper-Limb Training in Chronic Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2525–2536. [Google Scholar] [CrossRef]
- Riecker, A.; Gröschel, K.; Ackermann, H.; Schnaudigel, S.; Kassubek, J.; Kastrup, A. The role of the unaffected hemisphere in motor recovery after stroke. Hum. Brain Mapp. 2010, 31, 1017–1029. [Google Scholar] [CrossRef]
- Caria, A.; Weber, C.; Brötz, D.; Ramos, A.; Ticini, L.F.; Gharabaghi, A.; Braun, C.; Birbaumer, N. Chronic stroke recovery after combined BCI training and physiotherapy: A case report. Psychophysiology 2010, 48, 578–582. [Google Scholar] [CrossRef]
- Young, B.M.; Nigogosyan, Z.; Walton, L.M.; Song, J.; Nair, V.A.; Grogan, S.W.; Tyler, M.E.; Edwards, D.F.; Caldera, K.; Sattin, J.A.; et al. Changes in functional brain organization and behavioral correlations after rehabilitative therapy using a brain-computer interface. Front. Neuroeng. 2014, 7, 26. [Google Scholar] [CrossRef]
- Ge, S.; Yang, Q.; Wang, R.; Lin, P.; Gao, J.; Leng, Y.; Yang, Y.; Wang, H. A Brain-Computer Interface Based on a Few-Channel EEG-fNIRS Bimodal System. IEEE Access 2017, 5, 208–218. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, K.S. Fnirs-Based Brain-Computer Interfaces: A Review. Front. Hum. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef]
- Mihara, M.; Fujimoto, H.; Hattori, N.; Otomune, H.; Kajiyama, Y.; Konaka, K.; Watanabe, Y.; Hiramatsu, Y.; Sunada, Y.; Miyai, I.; et al. Effect of Neurofeedback Facilitation on Poststroke Gait and Balance Recovery: A Randomized Controlled Trial. Neurology 2021, 96, e2587–e2598. [Google Scholar] [CrossRef]
- Mihara, M.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Hino, T.; Miyai, I. Near-infrared Spectroscopy–mediated Neurofeedback Enhances Efficacy of Motor Imagery–based Training in Poststroke Victims. Stroke 2013, 44, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Rieke, J.D.; Matarasso, A.K.; Yusufali, M.M.; Ravindran, A.; Alcantara, J.; White, K.D.; Daly, J.J. Development of a combined, sequential real-time fMRI and fNIRS neurofeedback system to enhance motor learning after stroke. J. Neurosci. Methods 2020, 341, 108719. [Google Scholar] [CrossRef]
- Mokienko, O.A.; Lyukmanov, R.K.; Bobrov, P.D.; Isaev, M.R.; Ikonnikova, E.S.; Cherkasova, A.N.; Suponeva, N.A.; Piradov, M.A. Brain-computer interfaces based on near-infrared spectroscopy and electroencephalography registration in post-stroke rehabilitation: A comparative study. Neurol. Neuropsychiatry Psychosom. 2024, 16, 17–23. [Google Scholar] [CrossRef]
- Delorme, M.; Vergotte, G.; Perrey, S.; Froger, J.; Laffont, I. Time course of sensorimotor cortex reorganization during upper extremity task accompanying motor recovery early after stroke: An fNIRS study. Restor. Neurol. Neurosci. 2019, 37, 207–218. [Google Scholar] [CrossRef]
- He, X.; Lei, L.; Yu, G.; Lin, X.; Sun, Q.; Chen, S. Asymmetric cortical activation in healthy and hemiplegic individuals during walking: A functional near-infrared spectroscopy neuroimaging study. Front. Neurol. 2023, 13, 1044982. [Google Scholar] [CrossRef]
- Takeda, K.; Gomi, Y.; Imai, I.; Shimoda, N.; Hiwatari, M.; Kato, H. Shift of motor activation areas during recovery from hemiparesis after cerebral infarction: A longitudinal study with near-infrared spectroscopy. Neurosci. Res. 2007, 59, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Tao, L.; Gong, S.; Ma, Y.; Wu, J.; Li, W.; Kang, J.; Tang, M.; Zuo, G.; Shi, C. Upper limb motor assessment for stroke with force, muscle activation and interhemispheric balance indices based on sEMG and fNIRS. Front. Neurol. 2024, 15, 1337230. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Gomi, Y.; Kato, H. Near-Infrared Spectroscopy and Motor Lateralization after Stroke: A Case Series Study. Int. J. Phys. Med. Rehabil. 2014, 2, 192. [Google Scholar] [CrossRef]
- Borrell, J.A.; Fraser, K.; Manattu, A.K.; Zuniga, J.M. Laterality Index Calculations in a Control Study of Functional Near Infrared Spectroscopy. Brain Topogr. 2023, 36, 210–222. [Google Scholar] [CrossRef]
- Isaev, M.R.; Mokienko, O.A.; Lyukmanov, R.K.; Ikonnikova, E.S.; Cherkasova, A.N.; Suponeva, N.A.; Piradov, M.A.; Bobrov, P.D. A multiple session dataset of NIRS recordings from stroke patients controlling brain–computer interface. Sci. Data 2024, 11, 1168. [Google Scholar] [CrossRef]
- Isaev, M.R.; Bobrov, P.D. Effects of Selection of the Learning Set Formation Strategy and Filtration Method on the Effectiveness of a BCI Based on Near Infrared Spectrometry. Neurosci. Behav. Physiol. 2023, 53, 373–380. [Google Scholar] [CrossRef]
- Kay, M.; Wobbrock, J. ARTool: Aligned Rank Transform for Nonparametric Factorial ANOVAs, R Package Version 0.11.2. 2025. Available online: https://github.com/mjskay/ARTool (accessed on 4 June 2025).
- Friesen, C.L.; Lawrence, M.; Ingram, T.G.J.; Boe, S.G. Home-based portable fNIRS-derived cortical laterality correlates with impairment and function in chronic stroke. Front. Hum. Neurosci. 2022, 16, 1023246. [Google Scholar] [CrossRef] [PubMed]
- Delorme, M.; Froger, J.; Perrey, S.; Vergotte, G.; Laffont, I. Changes in hemodynamic responses during movements of the upper extremities in the acute phase after stroke: A fNIRS study. Ann. Phys. Rehabil. Med. 2017, 60, e1. [Google Scholar] [CrossRef]
- Batula, A.M.; Mark, J.A.; Kim, Y.E.; Ayaz, H. Comparison of Brain Activation during Motor Imagery and Motor Movement Using fNIRS. Comput. Intell. Neurosci. 2017, 2017, 5491296. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.P.; Eugène, F.; Michon, P.E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Kokotilo, K.J.; Eng, J.J.; Boyd, L.A. Reorganization of Brain Function During Force Production After Stroke. J. Neurol. Phys. Ther. 2009, 33, 45–54. [Google Scholar] [CrossRef]
- Barany, D.A.; Revill, K.P.; Caliban, A.; Vernon, I.; Shukla, A.; Sathian, K.; Buetefisch, C.M. Primary motor cortical activity during unimanual movements with increasing demand on precision. J. Neurophysiol. 2020, 124, 728–739. [Google Scholar] [CrossRef]
- Verstynen, T.; Diedrichsen, J.; Albert, N.; Aparicio, P.; Ivry, R.B. Ipsilateral Motor Cortex Activity During Unimanual Hand Movements Relates to Task Complexity. J. Neurophysiol. 2005, 93, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
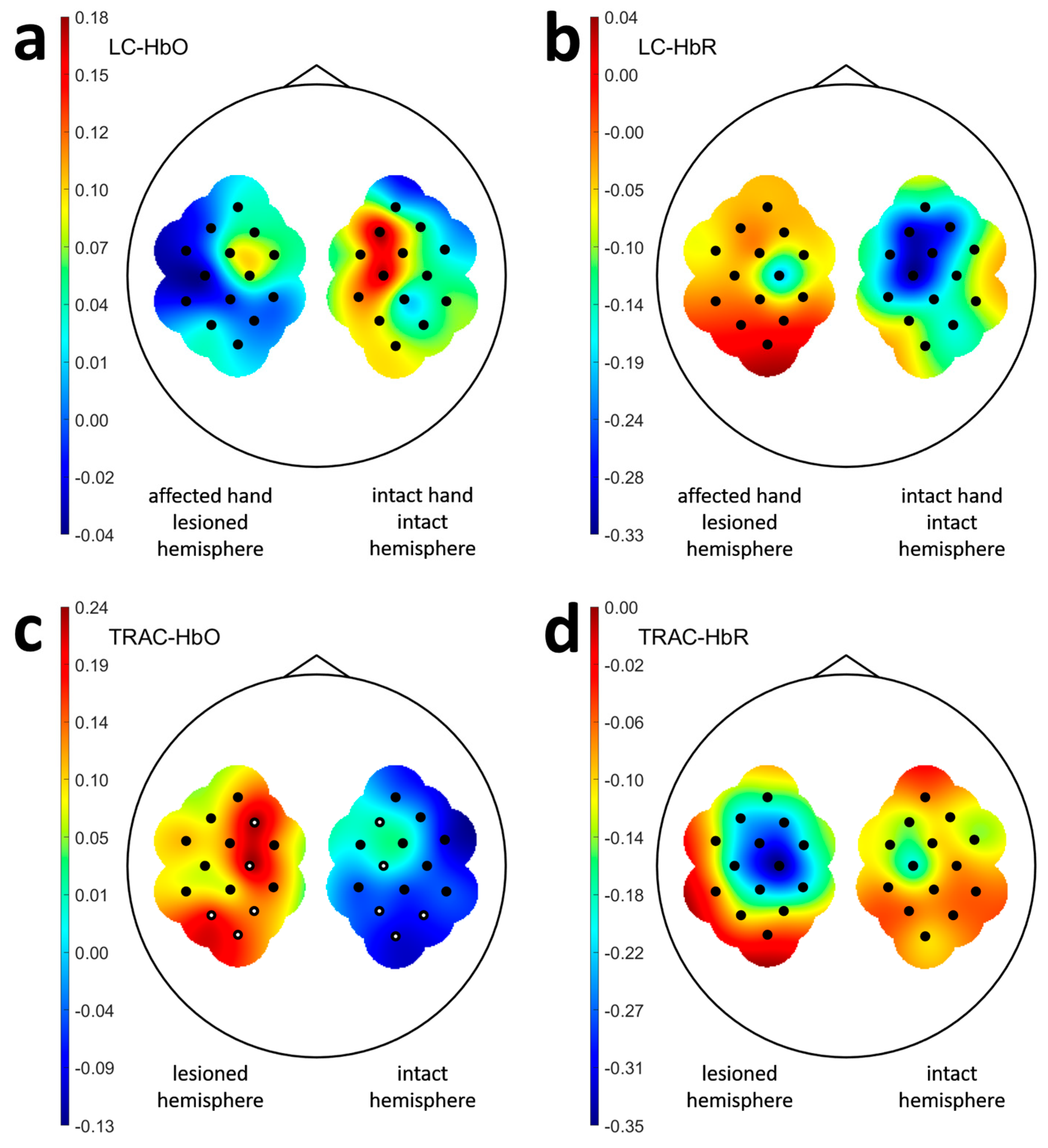
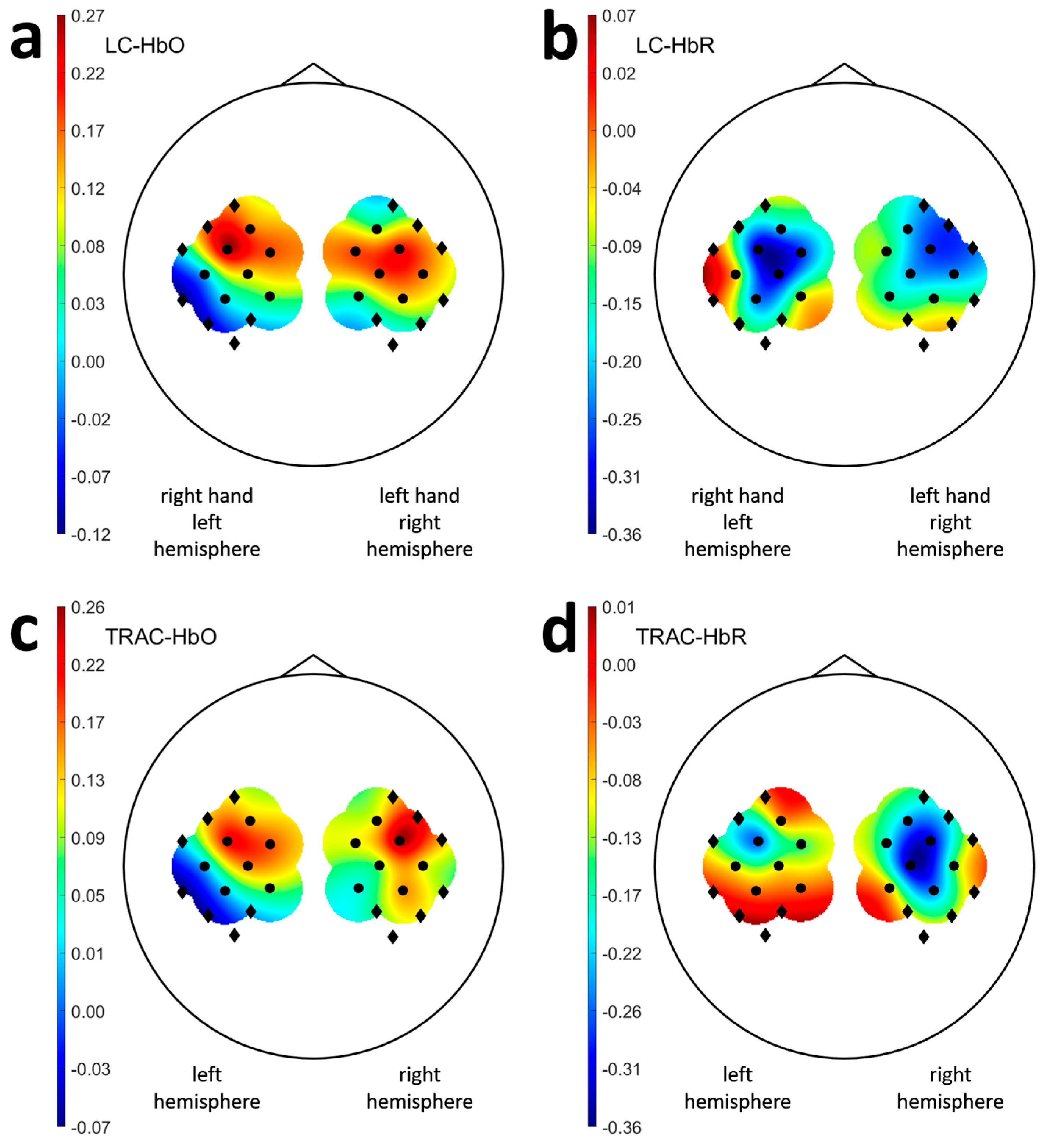
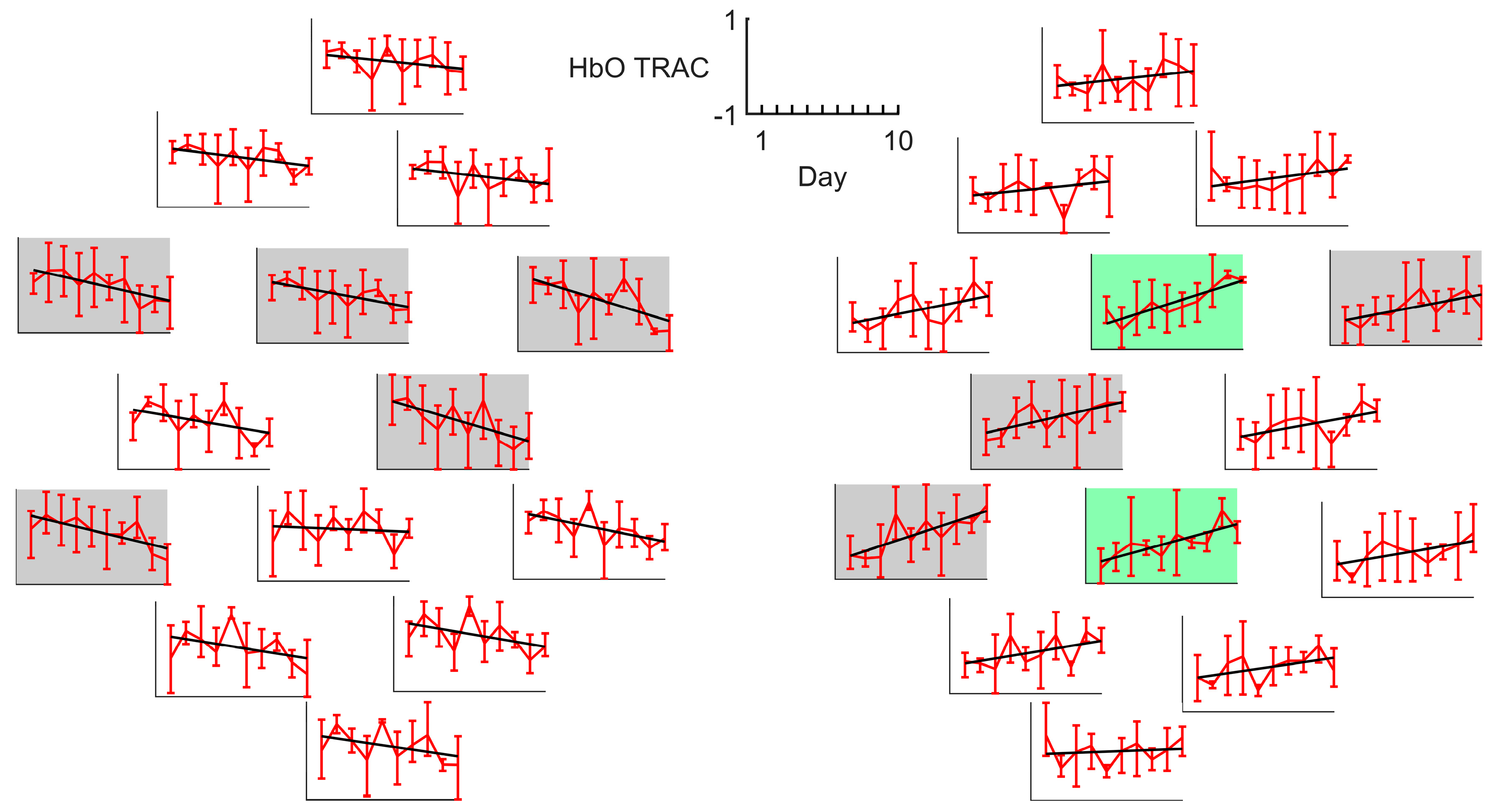
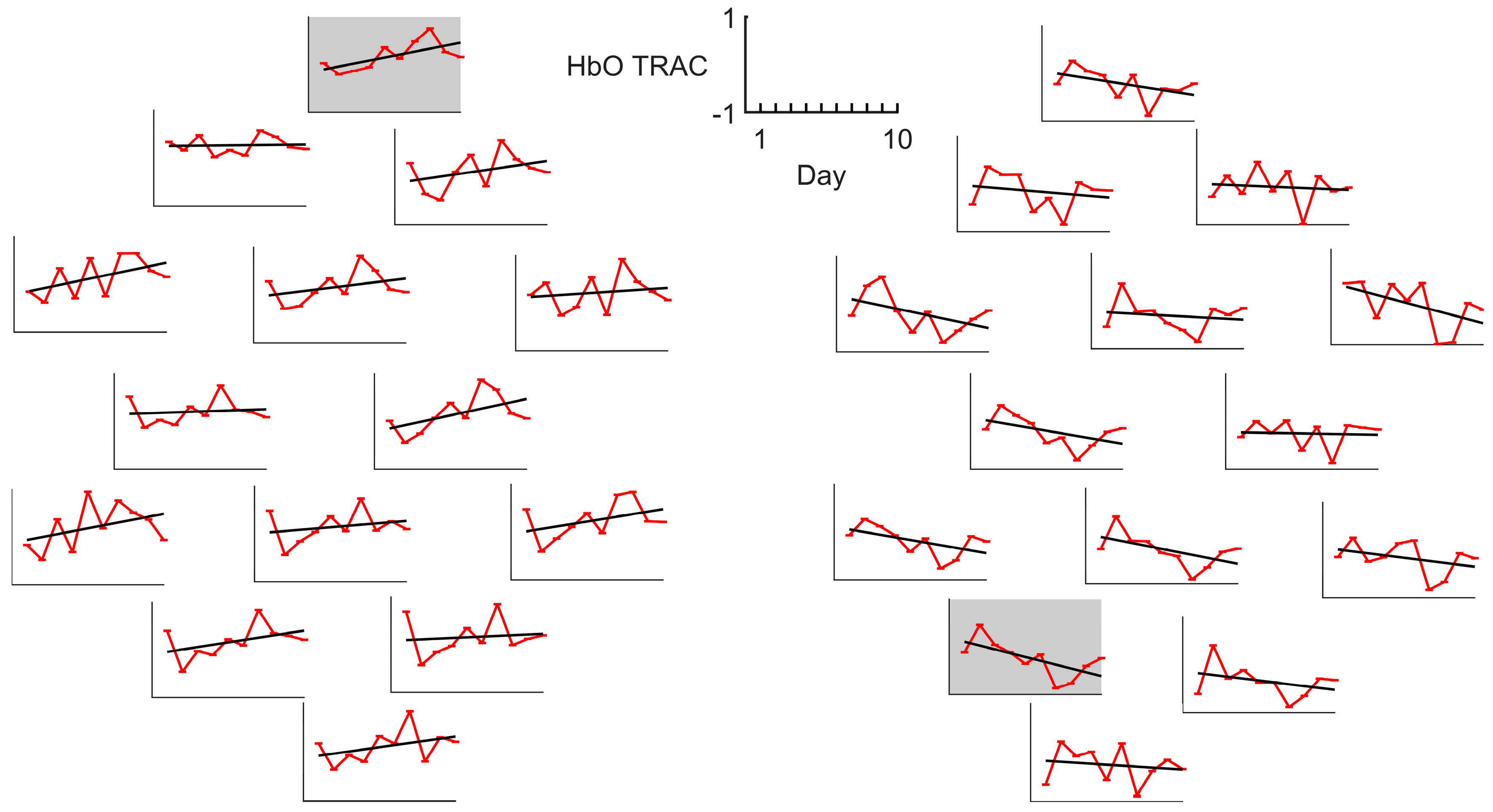
| ID | Sex | Age Range, y.o. | Stroke Time, Months | Lesioned Hemisphere | Baseline ARAT | ARAT Outcome | ARAT Improvement | TRAC Dynamics † |
|---|---|---|---|---|---|---|---|---|
| S1 | M | 46–50 | ≤3 | Right | 35 | 55 | 57% | ↘ ** |
| S2 | M | 71–75 | >6, ≤12 | Left | 44 | 50 | 14% | ↘ |
| S3 | M | 56–60 | >6, ≤12 | Right | 35 | 41 | 17% | ↗ |
| S4 | M | 56–60 | >6, ≤12 | Left | 39 | 43 | 10% | ↘ |
| S5 | F | 41–45 | ≤3 | Left | 52 | 57 | 10% | ↗ |
| S6 | M | 66–70 | >6, ≤12 | Left | 1 | 1 | 0% | ↗ |
| S7 | M | 56–60 | ≤3 | Right | 49 | 57 | 16% | ↘ |
| S8 | F | 56–60 | ≤3 | Left | 38 | 45 | 18% | ↗ |
| S9 | M | 76–80 | >12 | Left | 42 | 46 | 10% | ↗ |
| S10 | F | 56–60 | >12 | Left | 10 | 10 | 0% | ↘ |
| S11 | M | 66–70 | >3, ≤6 | Right | 6 | 16 | 167% | ↗ * |
| S12 | F | 46–50 | >3, ≤6 | Left | 24 | 29 | 21% | ↗ |
| S13 | M | 66–70 | >6, ≤12 | Right | 46 | 50 | 9% | ↗ |
| S14 | F | 66–70 | >6, ≤12 | Right | 4 | 9 | 125% | ↘ ** |
| S15 | F | 31–35 | ≤3 | Right | 19 | 28 | 47% | ↘ ** |
| Patients | Healthy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HbO LC | F | df | df.res | p-value | HbO LC | F | df | df.res | p-value |
| hand | 1.81 | 1 | 392 | 0.18 | hand | 0.55 | 1 | 104 | 0.46 |
| channel | 0.11 | 13 | 392 | 0.99 | channel | 1.91 | 6 | 104 | 0.08 |
| hand/channel | 0.82 | 13 | 392 | 0.61 | hand/channel | 1.68 | 6 | 104 | 0.13 |
| HbR LC | F | df | df.res | p-value | HbR LC | F | df | df.res | p-value |
| hand | 28.49 | 1 | 392 | <10−6 | hand | 0.38 | 1 | 104 | 0.54 |
| channel | 0.11 | 13 | 392 | 0.99 | channel | 1.73 | 6 | 104 | 0.12 |
| hand/channel | 1.13 | 13 | 392 | 0.32 | hand/channel | 1.14 | 6 | 104 | 0.35 |
| HbO TRAC | F | df | df.res | p-value | HbO TRAC | F | df | df.res | p-value |
| hemisphere | 34.03 | 1 | 392 | <10−6 | hemisphere | 0.85 | 1 | 104 | 0.36 |
| channel | 0.32 | 13 | 392 | 0.99 | channel | 1.01 | 6 | 104 | 0.41 |
| hemi/channel | 0.33 | 13 | 392 | 0.99 | hemi/channel | 0.27 | 6 | 104 | 0.96 |
| HbR TRAC | F | df | df.res | p-value | HbR TRAC | F | df | df.res | p-value |
| hemisphere | 9.88 | 1 | 392 | 0.0018 | hemisphere | 0.82 | 1 | 104 | 0.06 |
| channel | 0.38 | 13 | 392 | 0.97 | channel | 0.98 | 6 | 104 | 0.44 |
| hemi/channel | 0.89 | 13 | 392 | 0.56 | hemi/channel | 0.26 | 6 | 104 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaev, M.; Bobrov, P.; Mokienko, O.; Fedotova, I.; Lyukmanov, R.; Ikonnikova, E.; Cherkasova, A.; Suponeva, N.; Piradov, M.; Ustinova, K. Hemodynamic Response Asymmetry During Motor Imagery in Stroke Patients: A Novel NIRS-BCI Assessment Approach. Sensors 2025, 25, 5040. https://doi.org/10.3390/s25165040
Isaev M, Bobrov P, Mokienko O, Fedotova I, Lyukmanov R, Ikonnikova E, Cherkasova A, Suponeva N, Piradov M, Ustinova K. Hemodynamic Response Asymmetry During Motor Imagery in Stroke Patients: A Novel NIRS-BCI Assessment Approach. Sensors. 2025; 25(16):5040. https://doi.org/10.3390/s25165040
Chicago/Turabian StyleIsaev, Mikhail, Pavel Bobrov, Olesya Mokienko, Irina Fedotova, Roman Lyukmanov, Ekaterina Ikonnikova, Anastasiia Cherkasova, Natalia Suponeva, Michael Piradov, and Ksenia Ustinova. 2025. "Hemodynamic Response Asymmetry During Motor Imagery in Stroke Patients: A Novel NIRS-BCI Assessment Approach" Sensors 25, no. 16: 5040. https://doi.org/10.3390/s25165040
APA StyleIsaev, M., Bobrov, P., Mokienko, O., Fedotova, I., Lyukmanov, R., Ikonnikova, E., Cherkasova, A., Suponeva, N., Piradov, M., & Ustinova, K. (2025). Hemodynamic Response Asymmetry During Motor Imagery in Stroke Patients: A Novel NIRS-BCI Assessment Approach. Sensors, 25(16), 5040. https://doi.org/10.3390/s25165040









