Future Designs of Clinical Trials in Nephrology: Integrating Methodological Innovation and Computational Power
Highlights
- Clinical research in nephrology faces persistent challenges that can be addressed by combining two key innovation streams: advanced trial methodologies (like adaptive and pragmatic designs) and powerful computational tools, including Artificial Intelligence (AI) and in silico clinical trials (ISCTs).
- Specific computational tools are emerging that may offer targeted solutions. For example, Augmented Reality (AR) shows promise for enhancing the precision of interventional procedures like biopsies, while Conditional Tabular Generative Adversarial Networks (CTGANs) are being investigated as a method to generate synthetic data to help address scarcity in rare disease research.
- The synergistic integration of advanced trial designs with AI-driven analytics and in silico simulations has the potential to provide a clear pathway toward conducting clinical trials that are faster, more precise, more cost-effective, and better tailored to individual patient needs.
- Realizing this potential is contingent upon the nephrology community proactively addressing significant implementation barriers related to data quality, model validation, evolving regulatory standards, and ethical oversight.
Abstract
1. Introduction
2. Persistent Challenges Shaping the Future of Trial Design
3. Methodological Innovations: Trial Frameworks
4. Computational Transformation
4.1. The Role of Artificial Intelligence
4.2. Sensor Technology and Digital Endpoints
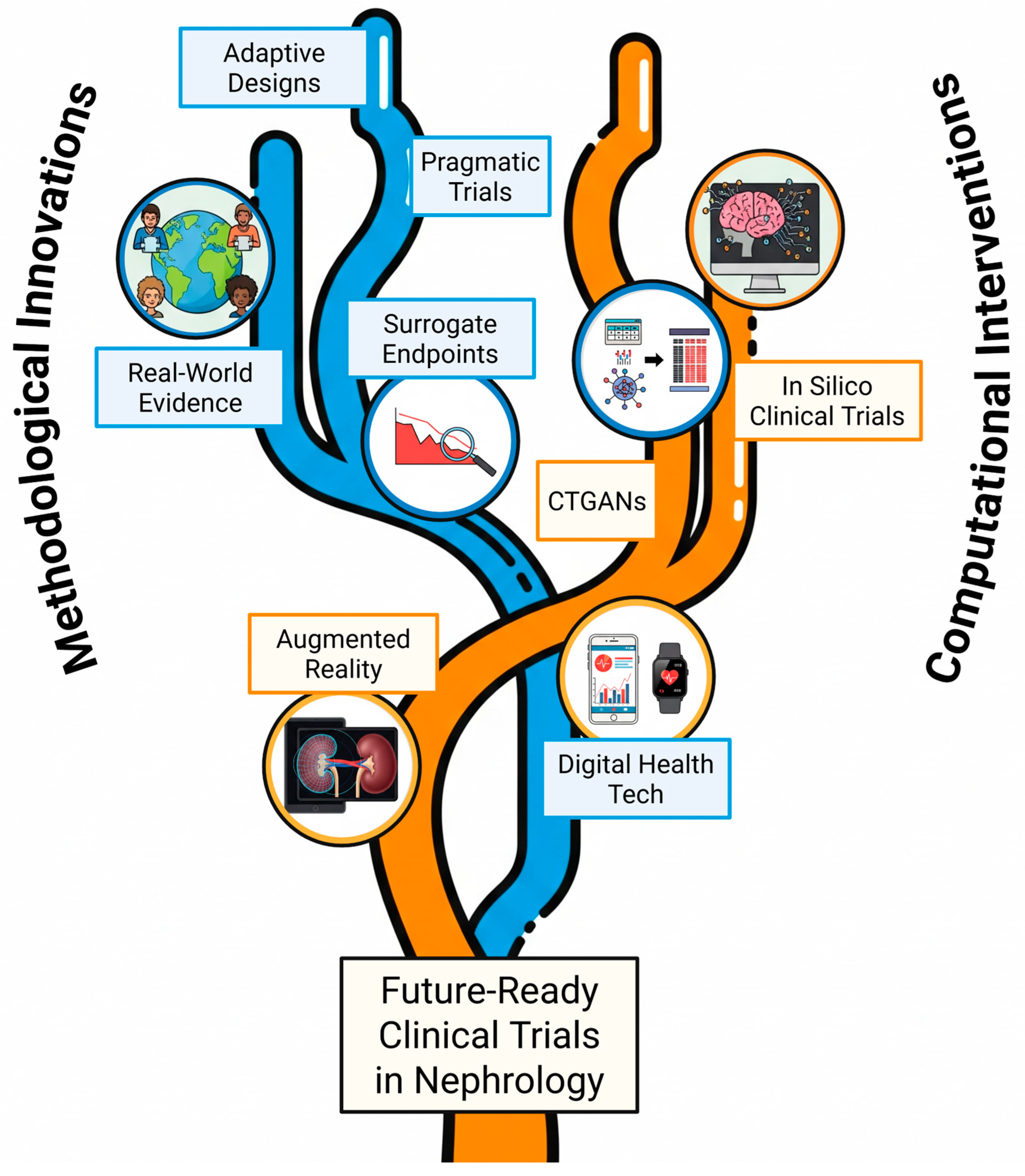
4.3. Leveraging In Silico Clinical Trials
4.4. Generative Adversarial Networks
4.5. Augmented Reality
4.6. Illustrating Synergy: AI-Enhanced Adaptive Trial in Action
5. Navigating Implementation: Overcoming Barriers in Innovation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baigent, C.; Herrington, W.G.; Coresh, J.; Landray, M.J.; Levin, A.; Perkovic, V.; Pfeffer, M.A.; Rossing, P.; Walsh, M.; Wanner, C.; et al. Challenges in conducting clinical trials in nephrology: Conclusions from a Kidney Disease—Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 92, 297–305. [Google Scholar] [CrossRef]
- Zhu, D.; Judge, P.K.; Staplin, N.; Haynes, R.; Herrington, W.G. Design considerations for future renoprotection trials in the era of multiple therapies for chronic kidney disease. Nephrol. Dial. Transplant. 2025, 40 (Suppl. 1), i70–i79. [Google Scholar] [CrossRef]
- Hartung, E.A. Biomarkers and surrogate endpoints in kidney disease. Pediatr. Nephrol. Berl. Ger. 2016, 31, 381–391. [Google Scholar] [CrossRef]
- Lazzareschi, D.; Mehta, R.L.; Dember, L.M.; Bernholz, J.; Turan, A.; Sharma, A.; Kheterpal, S.; Parikh, C.R.; Ali, O.; Schulman, I.H.; et al. Overcoming barriers in the design and implementation of clinical trials for acute kidney injury: A report from the 2020 Kidney Disease Clinical Trialists meeting. Nephrol. Dial. Transplant. 2023, 38, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Schnaper, H.W.; Furth, S.L.; Yao, L.P. Defining new surrogate markers for CKD progression. Pediatr. Nephrol. Berl. Ger. 2015, 30, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.; James, G.; Garcia Sanchez, J.J.; Carrero, J.J.; Arnold, M.; Lam, C.S.P.; Chen, H.; Nolan, S.; Pecoits-Filho, R.; Wheeler, D.C. Healthcare resource utilisation and related costs of patients with CKD from the UK: A report from the DISCOVER CKD retrospective cohort. Clin. Kidney J. 2022, 15, 2124–2134. [Google Scholar] [CrossRef] [PubMed]
- Natale, P.; Gutman, T.; Howell, M.; Dansie, K.; Hawley, C.M.; Cho, Y.; Viecelli, A.K.; Craig, J.C.; Jesudason, S.; Chapman, J.R.; et al. Recruitment and retention in clinical trials in chronic kidney disease: Report from national workshops with patients, caregivers and health professionals. Nephrol. Dial. Transplant. 2020, 35, 755–764. [Google Scholar] [CrossRef]
- Talbot, B.; Athavale, A.; Jha, V.; Gallagher, M. Data Challenges in Addressing Chronic Kidney Disease in Low- and Lower-Middle-Income Countries. Kidney Int. Rep. 2021, 6, 1503–1512. [Google Scholar] [CrossRef]
- Forbes, A.K.; Hinton, W.; Feher, M.D.; Elson, W.; Ordóñez-Mena, J.M.; Joy, M.; Fan, X.; Banerjee, D.; Cole, N.I.; Munro, N.; et al. A comparison of sodium-glucose co-transporter 2 inhibitor kidney outcome trial participants with a real-world chronic kidney disease primary care population. Nephrol. Dial. Transplant. 2024, 40, 71–82. [Google Scholar] [CrossRef]
- Krishnasamy, R.; Jardine, M.J.; on behalf of the BEAT-Calci Trialists. Adaptive Designs for Clinical Trials in Nephrology. J. Am. Soc. Nephrol. 2025, 36, 147–149. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Shahidehpour, A.; Troy, A.M. Issues Regarding the Indexing of Adaptive Clinical Trial Articles. 2025. Available online: http://medrxiv.org/lookup/doi/10.1101/2025.03.10.25323694 (accessed on 12 April 2025).
- Adaptive Platform Trial Summaries—ACTA—Australian Clinical Trials Alliance. Available online: https://clinicaltrialsalliance.org.au/resource/adaptive-platform-trial-operations-special-interest-group-trial-summaries/ (accessed on 12 April 2025).
- Kotwal, S.S.; Perkovic, V.; Jardine, M.J.; Kim, D.; Shah, N.A.; Lin, E.; Coggan, S.; Billot, L.; Vart, P.; Wheeler, D.C.; et al. The Global Kidney Patient Trials Network and the CAPTIVATE Platform Clinical Trial Design: A Trial Protocol. JAMA Netw. Open 2024, 7, e2449998. [Google Scholar] [CrossRef]
- De Boer, I.H.; Kovesdy, C.P.; Navaneethan, S.D.; Peralta, C.A.; Tuot, D.S.; Vazquez, M.A.; Crews, D.C. Pragmatic Clinical Trials in CKD: Opportunities and Challenges. J. Am. Soc. Nephrol. 2016, 27, 2948–2954. [Google Scholar] [CrossRef]
- Thompson, A.M.; Southworth, M.R. Real World Data and Evidence: Support for Drug Approval: Applications to Kidney Diseases. Clin. J. Am. Soc. Nephrol. 2019, 14, 1531–1532. [Google Scholar] [CrossRef]
- Wilson, B.E.; Booth, C.M. Real-world data: Bridging the gap between clinical trials and practice. eClinicalMedicine 2024, 78, 102915. [Google Scholar] [CrossRef] [PubMed]
- PAR-25-170: Digital Health Technology Derived Biomarkers and Outcome Assessments for Remote Monitoring and Endpoint Development (UG3/UH3—Clinical Trial Optional). Available online: https://grants.nih.gov/grants/guide/pa-files/PAR-25-170.html (accessed on 12 April 2025).
- Digital Health Technology Derived Biomarkers and Outcome Assessments for Remote Monitoring and Endpoint Development (UG3/UH3—Clinical Trial Optional)|National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/news-events/events/digital-health-technology-derived-biomarkers-and-outcome-assessments-remote-monitoring-and-endpoint (accessed on 12 April 2025).
- Imaizumi, T.; Komaba, H.; Hamano, T.; Nangaku, M.; Murotani, K.; Hasegawa, T.; Fujii, N.; Nitta, K.; Isaka, Y.; Wada, T.; et al. Clinically meaningful eGFR slope as a surrogate endpoint differs across CKD stages and slope evaluation periods: The CKD-JAC study. Clin Kidney J. 2025, 18, sfae398. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.W.; Fullem, R.; Cheng, C.; Amos, C.I. Artificial intelligence, machine learning, and deep learning for clinical outcome prediction. Emerg Top Life Sci. 2021, 5, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Shickel, B.; Ozrazgat-Baslanti, T.; Ren, Y.; Glicksberg, B.S.; Cao, J.; Singh, K.; Chan, L.; Nadkarni, G.N.; Bihorac, A. Artificial intelligence-enabled decision support in nephrology. Nat. Rev. Nephrol. 2022, 18, 452–465. [Google Scholar] [CrossRef]
- Teodoro, D.; Naderi, N.; Yazdani, A.; Zhang, B.; Bornet, A. A Scoping Review of Artificial Intelligence Applications in Clinical Trial Risk Assessment. 2025. Available online: http://medrxiv.org/lookup/doi/10.1101/2025.01.21.25320310 (accessed on 12 April 2025).
- Ball, R.; Dal Pan, G. “Artificial Intelligence” for Pharmacovigilance: Ready for Prime Time? Drug Saf. 2022, 45, 429–438. [Google Scholar] [CrossRef]
- Daryaee, F.; Tonge, P.J. Pharmacokinetic–pharmacodynamic models that incorporate drug–target binding kinetics. Curr. Opin. Chem. Biol. 2019, 50, 120–127. [Google Scholar] [CrossRef]
- Kuemmel, C.; Yang, Y.; Zhang, X.; Florian, J.; Zhu, H.; Tegenge, M.; Huang, S.; Wang, Y.; Morrison, T.; Zineh, I. Consideration of a Credibility Assessment Framework in Model-Informed Drug Development: Potential Application to Physiologically-Based Pharmacokinetic Modeling and Simulation. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 21–28. [Google Scholar] [CrossRef]
- Yang, S.; Varghese, P.; Stephenson, E.; Tu, K.; Gronsbell, J. Machine learning approaches for electronic health records phenotyping: A methodical review. J. Am. Med. Inform. Assoc. 2023, 30, 367–381. [Google Scholar] [CrossRef]
- Renuka Balireddi, B.N. Exploring the Impact of Natural Language Processing in Clinical Trials, Regulatory, Healthcare Efficiency, and Drug Discovery Processes. 5 December 2024. Available online: https://zenodo.org/doi/10.5281/zenodo.14280626 (accessed on 12 April 2025).
- Gulamali, F.F.; Sawant, A.S.; Nadkarni, G.N. Machine learning for risk stratification in kidney disease. Curr. Opin. Nephrol. Hypertens. 2022, 31, 548–552. [Google Scholar] [CrossRef]
- Nadkarni, G.N. Introduction to Artificial Intelligence and Machine Learning in Nephrology. Clin. J. Am. Soc. Nephrol. CJASN 2023, 18, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Muhanna, D.A.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Pedarzani, E.; Fogangolo, A.; Baldi, I.; Berchialla, P.; Panzini, I.; Khan, M.R.; Valpiani, G.; Spadaro, S.; Gregori, D.; Azzolina, D. Prioritizing Patient Selection in Clinical Trials: A Machine Learning Algorithm for Dynamic Prediction of In-Hospital Mortality for ICU Admitted Patients Using Repeated Measurement Data. J. Clin. Med. 2025, 14, 612. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.S.; Chow, C.K.; Daryabeygikhotbehsara, R.; Subedi, N.; Rawstorn, J.; Tegegne, T.; Karmakar, C.; Siddiqui, M.U.; Lambert, G.; Maddison, R. Wearable cuffless blood pressure monitoring devices: A systematic review and meta-analysis. Eur. Heart J. Digit. Health 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Mansour, M.; Darweesh, M.; Soltan, A. Wearable devices for glucose monitoring: A review of state-of-the-art technologies and emerging trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Bremnes, F.; Øien, C.; Kværness, J.; Jaatun, E.; Nyvik, S.; Sæther, T.; Lund, H.; Romundstad, S. Measuring fluid balance in end-stage renal disease with a wearable bioimpedance sensor. BMC Nephrol. 2025, 26, 14. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhou, H.; Liu, Y.; Chen, R.; Zhang, X.; Nie, S.; Hou, F.F.; Zhao, Y.; Xu, X.; Zhao, L. A multi-modal fusion model with enhanced feature representation for chronic kidney disease progression prediction. Brief. Bioinform. 2024, 26, bbaf003. [Google Scholar] [CrossRef]
- Yammouri, G.; Lahcen, A. Ai-reinforced wearable sensors and intelligent point-of-care tests. J. Pers. Med. 2024, 14, 1088. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Filippidis, F. Accelerometer-measured physical activity, frailty, and all-cause mortality and life expectancy among middle-aged and older adults: A uk biobank longitudinal study. BMC Med. 2025, 23, 125. [Google Scholar] [CrossRef]
- Gelis, L.; Stoeckert, I.; Podhaisky, H. Digital tools—Regulatory considerations for application in clinical trials. Ther. Innov. Regul. Sci. 2023, 57, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, J.; Laken, S.; Langer, R.; Traverso, G. Clinical opportunities for continuous biosensing and closed-loop therapies. Trends Chem. 2020, 2, 319–340. [Google Scholar] [CrossRef]
- Singh, P.; Goyal, L.; Mallick, D.C.; Surani, S.R.; Kaushik, N.; Chandramohan, D.; Simhadri, P.K. Artificial Intelligence in Nephrology: Clinical Applications and Challenges. Kidney Med. 2025, 7, 100927. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Musuamba Tshinanu, F. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Karanasiou, G.; Edelman, E.; Boissel, F.H.; Byrne, R.; Emili, L.; Fawdry, M.; Filipovic, N.; Flynn, D.; Geris, L.; Hoelstra, A.; et al. Advancing in Silico Clinical Trials for Regulatory Adoption and Innovation. IEEE J. Biomed. Health Inform. 2025, 29, 2654–2668. [Google Scholar] [CrossRef]
- Arsène, S.; Parès, Y.; Tixier, E.; Granjeon-Noriot, S.; Martin, B.; Bruezière, L.; Couty, C.; Courcelles, E.; Kahoul, R.; Pitrat, J.; et al. In Silico Clinical Trials: Is It Possible? In High Performance Computing for Drug Discovery and Biomedicine; Heifetz, A., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2024; Volume 2716, pp. 51–99. Available online: https://link.springer.com/10.1007/978-1-0716-3449-3_4 (accessed on 12 April 2025).
- Chen, B.; Schneider, L.C.; Roever, C.; Comets, E.; Elze, M.C.; Hooker, A.; IntHout, J.; Jannot, A.-S.; Julkowska, D.; Mimouni, Y.; et al. In silico clinical trials in drug development: A systematic review. arXiv 2025, arXiv:2503.08746. [Google Scholar]
- Sayama, H.; Takubo, H.; Komura, H.; Kogayu, M.; Iwaki, M. Application of a Physiologically Based Pharmacokinetic Model Informed by a Top-Down Approach for the Prediction of Pharmacokinetics in Chronic Kidney Disease Patients. AAPS J. 2014, 16, 1018–1028. [Google Scholar] [CrossRef]
- Zamir, A.; Alqahtani, F.; Rasool, M.F. Chronic kidney disease and physiologically based pharmacokinetic modeling: A critical review of existing models. Expert. Opin. Drug Metab. Toxicol. 2024, 20, 95–105. [Google Scholar] [CrossRef]
- Scotcher, D.; Galetin, A. PBPK Simulation-Based Evaluation of Ganciclovir Crystalluria Risk Factors: Effect of Renal Impairment, Old Age, and Low Fluid Intake. AAPS J. 2022, 24, 13. [Google Scholar] [CrossRef]
- Su, M.; Liu, X.; Zhao, Y.; Zhu, Y.; Wu, M.; Liu, K.; Yang, G.; Liu, W.; Wang, L. In Silico and In Vivo Pharmacokinetic Evaluation of 84-B10, a Novel Drug Candidate against Acute Kidney Injury and Chronic Kidney Disease. Molecules 2023, 29, 159. [Google Scholar] [CrossRef]
- Viceconti, M.; Emili, L. (Eds.) Toward Good Simulation Practice: Best Practices for the Use of Computational Modelling and Simulation in the Regulatory Process of Biomedical Products; Synthesis Lectures on Biomedical Engineering; Springer Nature: Cham, Switzerland, 2024; Available online: https://link.springer.com/10.1007/978-3-031-48284-7 (accessed on 12 April 2025).
- Aycock, K.I.; Battisti, T.; Peterson, A.; Yao, J.; Kreuzer, S.; Capelli, C.; Pant, S.; Pathmanathan, P.; Hoganson, D.M.; Levine, S.M.; et al. Toward trustworthy medical device in silico clinical trials: A hierarchical framework for establishing credibility and strategies for overcoming key challenges. Front. Med. 2024, 11, 1433372. [Google Scholar] [CrossRef] [PubMed]
- Spanakis, M. In Silico Pharmacology for Evidence-Based and Precision Medicine. Pharmaceutics 2023, 15, 1014. [Google Scholar] [CrossRef] [PubMed]
- Checa-Ros, A.; Locascio, A.; Steib, N.; Okojie, O.J.; Malte-Weier, T.; Bermúdez, V.; D’mArco, L. In silico medicine and -omics strategies in nephrology: Contributions and relevance to the diagnosis and prevention of chronic kidney disease. Kidney Res. Clin. Pract. 2025, 44, 49–57. [Google Scholar] [CrossRef] [PubMed]
- odeling & Simulation at FDA. 9 August 2024. Available online: https://www.fda.gov/science-research/about-science-research-fda/modeling-simulation-fda (accessed on 12 April 2025).
- Mendes, J.M.; Barbar, A.; Refaie, M. Synthetic data generation: A privacy-preserving approach to accelerate rare disease research. Front. Digit. Health 2025, 7, 1573991. [Google Scholar] [CrossRef]
- Sun, C.; Dumontier, M. Generating unseen diseases patient data using ontology enhanced generative adversarial networks. NPJ Digit. Med. 2025, 8, 4. [Google Scholar] [CrossRef]
- Trabassi, D.; Castiglia, S.F.; Bini, F.; Marinozzi, F.; Ajoudani, A.; Lorenzini, M.; Chini, G.; Varrecchia, T.; Ranavolo, A.; De Icco, R.; et al. Optimizing Rare Disease Gait Classification through Data Balancing and Generative AI: Insights from Hereditary Cerebellar Ataxia. Sensors 2024, 24, 3613. [Google Scholar] [CrossRef]
- Smolyak, D.; Bjarnadóttir, M.V.; Crowley, K.; Agarwal, R. Large language models and synthetic health data: Progress and prospects. JAMIA Open 2024, 7, ooae114. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F. Exploring the metaverse: Opportunities for nephrology in patient care and education. Nephrol. Dial. Transplant. 2025, 40, 865–873. [Google Scholar] [CrossRef]
- Saruwatari, M.S.; Nguyen, T.N.; Talari, H.F.; Matisoff, A.J.; Sharma, K.V.; Donoho, K.G.; Basu, S.; Dwivedi, P.; Bost, J.E.; Shekhar, R. Assessing the Effect of Augmented Reality on Procedural Outcomes During Ultrasound-Guided Vascular Access. Ultrasound Med. Biol. 2023, 49, 2346–2353. [Google Scholar] [CrossRef]
- Pfefferle, M.; Shahub, S.; Shahedi, M.; Gahan, J.; Johnson, B.; Le, P.; Vargas, J.; Judson, B.O.; Alshara, Y.; Li, Q.; et al. Renal biopsy under augmented reality guidance. Proc. SPIE Int. Soc. Opt. Eng. 2020, 11315, 113152W. [Google Scholar]
- Eikstadt, R.N.; Desmond, H.E.; Lindner, C.; Chen, L.Y.; Courtlandt, C.D.; Massengill, S.F.; Kamil, E.S.; Lafayette, R.; Pesenson, A.; Elliott, M.; et al. The Development and Use of an EHR-Linked Database for Glomerular Disease Research and Quality Initiatives. Glomerular Dis. 2021, 1, 173–179. [Google Scholar] [CrossRef]
- Yu, S.; Lee, S.S.; Hwang, H. The ethics of using artificial intelligence in medical research. Kosin Med. J. 2024, 39, 229–237. [Google Scholar] [CrossRef]
- Strippoli, G.F.M.; Green, S.C. Actioning the findings of hard endpoint clinical trials as they emerge in the realm of chronic kidney disease care: A review and a call to action. Clin Kidney J. 2024, 17, sfae035. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, M.; Lin, L.; Zhou, Z.; Li, H.; Tian, X.; Huang, W.-J. Artificial intelligence alphafold model for molecular biology and drug discovery: A machine-learning-driven informatics investigation. Mol. Cancer 2024, 23, 223. [Google Scholar] [CrossRef]
- Guo, S.; Cai, X.; Yuan, M.; Huang, W.; Tian, X. Ai model using clinical images for genomic prediction and tailored treatment in patients with cancer. Lancet Oncol. 2025, 26, e126. [Google Scholar] [CrossRef]
- Canbay, Y.; Adsiz, S.; Canbay, P. Privacy-preserving transfer learning framework for kidney disease detection. Appl. Sci. 2024, 14, 8629. [Google Scholar] [CrossRef]

| Challenge | Description | Impact on Clinical Trials |
|---|---|---|
| Slow Disease Progression | Many kidney diseases, especially CKD, progress slowly, leading to low event rates for hard clinical endpoints (e.g., ESKD, mortality). | Requires very large sample sizes and long follow-up durations, increasing costs and time. |
| Treatment Success Paradox | Effective new therapies (like SGLT2i) lower baseline risk in participants, making it harder to show incremental benefits of new agents. | Decreases the statistical power of traditionally designed trials, necessitating the enrollment of larger populations, extended trial durations, or focusing on high-risk cohorts. |
| Recruitment and Retention | Difficulty finding and keeping participants due to complex protocols, participant burden (time, cost, travel), lack of awareness, mistrust. | Jeopardizes trial feasibility, statistical power, timelines, and generalizability of results. |
| Lack of Diversity | Underrepresentation of minority ethnic and socioeconomically disadvantaged groups who often have a higher disease burden. | Limits generalizability and equity of research findings. |
| Disease Heterogeneity | Kidney diseases encompass diverse etiologies (DKD, GN, ADPKD, AKI) and varying progression rates. | Complicates trial design, patient selection, endpoint definition, and interpretation; a “one-size-fits-all” approach is often ineffective. |
| Endpoint Selection | Balancing the clinical relevance of hard endpoints against the feasibility challenges of low event rates. | Drives the need for validated surrogate endpoints (e.g., eGFR slope), but validation is rigorous and ongoing. |
| Innovation | Description | Potential Benefit(s) |
|---|---|---|
| Adaptive Designs | Allow pre-planned modifications (e.g., sample size, arm dropping) based on interim data, often using Bayesian methods. | Increased efficiency, flexibility, shorter duration, smaller sample size, ethical advantages (stopping early). |
| Platform Trials | Test multiple interventions against a common control group using a master protocol. | Dramatically increased efficiency for evaluating numerous therapies, especially for heterogeneous diseases. |
| Pragmatic Clinical Trials (PCTs) | Evaluate interventions in real-world settings with broad eligibility, often using routine data collection (EHRs). | Increased generalizability, relevance to routine care, potentially lower cost. |
| Real-World Evidence (RWE) | Leverage data from EHRs, registries, claims databases to understand long-term effectiveness/safety in diverse populations. | Complements RCT data, provides insights into routine practice, supports regulatory decisions in some contexts. |
| Digital Health Technologies (DHTs) | Use wearables, sensors, apps for remote monitoring (e.g., BP, weight, ePROs). | Reduced participant burden, wider participation, frequent data collection, potential for novel/sensitive digital endpoints. |
| Surrogate Endpoints (Validated) | Use markers (e.g., % eGFR decline, eGFR slope) reliably predicting clinical outcomes to shorten trials. | Allows for smaller/shorter trials, feasible when hard endpoints are rare, accelerates development. |
| Conditional Tabular Generative Adversarial Networks (CTGANs) | AI-driven technique engineered to learn the distributions within real-world tabular data and generate high-fidelity, synthetic patient records. | Augments scarce datasets in rare diseases, can improve the robustness of predictive models, helps preserve data privacy, and enables more reliable subgroup analyses. |
| Augmented Reality (AR) | Overlays real-time, 3D imaging data (e.g., CT scans) onto a clinician’s view of the operative field to provide intuitive guidance during procedures. | Improves procedural precision and efficiency for interventions like renal biopsies and vascular access cannulation, reduces inter-operator variability, and contributes to higher quality endpoint data. |
| Barrier Category | Description | Implications |
|---|---|---|
| Data | Quality, fragmentation, lack of standardization, access issues, privacy. | Hinders reliable AI model training, RWE generation, PCT integration; requires robust governance and interoperability efforts. |
| Algorithmic bias. | AI models trained on biased data may perpetuate/amplify health disparities; requires mitigation techniques. | |
| Validation | Establishing AI model credibility (transparency, explainability, external validation). | Crucial for clinical trust and regulatory acceptance; often lacking rigorous external validation. |
| Establishing ISCT model credibility (VVUQ—Verification, Validation, Uncertainty Quantification). | Central challenge requiring meticulous processes and standards (e.g., Good Simulation Practices) for regulatory trust. | |
| Regulatory | Evolving landscape, lack of harmonized pathways for AI/ISCT evidence. | Creates uncertainty; requires early engagement with regulators and transparent reporting. |
| Ethical | Informed consent complexity (for adaptive designs, AI use). | Requires clear communication to ensure participants understand the trial processes. |
| Bias, privacy, accountability, equity, maintaining patient trust. | Foundational concerns requiring careful oversight, ethical frameworks, and focus on equitable benefit distribution. | |
| Workforce | Need for specialized expertise (data science, computation) and interdisciplinary collaboration. | Requires investment in training and fostering collaboration between clinical and computational experts. |
| Integration | Seamless integration into clinical workflows and EHRs. | Essential for practical adoption; tools should not unduly burden clinicians. |
| Adoption | Bridging the implementation gap for proven innovations. | Requires addressing system-level factors, resource constraints, and clinician behavior beyond just evidence generation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strizzi, C.T.; Pesce, F. Future Designs of Clinical Trials in Nephrology: Integrating Methodological Innovation and Computational Power. Sensors 2025, 25, 4909. https://doi.org/10.3390/s25164909
Strizzi CT, Pesce F. Future Designs of Clinical Trials in Nephrology: Integrating Methodological Innovation and Computational Power. Sensors. 2025; 25(16):4909. https://doi.org/10.3390/s25164909
Chicago/Turabian StyleStrizzi, Camillo Tancredi, and Francesco Pesce. 2025. "Future Designs of Clinical Trials in Nephrology: Integrating Methodological Innovation and Computational Power" Sensors 25, no. 16: 4909. https://doi.org/10.3390/s25164909
APA StyleStrizzi, C. T., & Pesce, F. (2025). Future Designs of Clinical Trials in Nephrology: Integrating Methodological Innovation and Computational Power. Sensors, 25(16), 4909. https://doi.org/10.3390/s25164909






