Abstract
In recent years, electrospinning technology has sparked a revolution in the nanoengineering of gas-sensing materials. Nanofibers based on metal oxide semiconductors, carbon materials, or conductive polymers prepared by the electrospinning process have exhibited inspiring properties, including a large specific surface area, porous structure, and nice stability, with bright application prospects in advanced gas sensors. Meanwhile, the increasingly expanding applications of gas sensors, such as the Internet of Things (IoT), the food industry, disease diagnosis, etc., have raised higher sensor performance requirements. To further enhance the gas-sensing performance of nanofibers, the scheme of functionalized nanofiber strategies, either in electrospinning or post-treatment, has been proposed and verified. This review systematically summarized the nanostructures, gas-sensing properties, and functional mechanisms of modified nanofibers. Additionally, the perspectives and challenges regarding electrospun nanofibers for gas sensing were discussed.
1. Introduction
Gas sensors play a critical role in fields such as the Internet of Things (IoT), environmental monitoring, healthcare diagnostics, and food safety [1,2,3,4,5]. Among various types of gas sensors, chemiresistive gas sensors have attracted considerable attention due to their remarkable advantages, including high sensitivity, sub-ppm level detection limits, cost-effective fabrication processes, and easy integration [6,7,8,9,10]. With the widespread application of chemiresistive gas sensors, emerging application scenarios are imposing more demands on their performance, such as ultrahigh sensitivity, rapid response/recovery, excellent selectivity, and long-term stability [11,12]. Flexible gas sensors can be utilized in wearable human devices for sustained physiological monitoring, where the mechanical properties of the flexible sensors critically determine the reliability [13]. Gas-sensing materials serve as the fundamental determinant of sensor performance, where their strategic design and innovation constitute the cornerstone for developing next-generation high-performance gas sensors. Thereinto, metal oxides (SnO2, ZnO), carbon nanostructures (graphene, carbon nanotubes), and conductive polymers (polyaniline, polypyrrole) were widely applied [14,15]. The sensing properties of these materials rely on three critical factors: (1) receptor function, (2) transducer function, and (3) utility factor, which regulate the gas molecule adsorption, signal conversion, and gas diffusion, respectively [16].
Traditional chemiresistive gas sensors based on bulk materials widely suffer from limitations such as low specific surface area, limited sensitivity, poor reversibility, and sluggish adsorption/desorption kinetics [17]. In contrast, 1D nanofiber materials possess superior sensing performance due to unique advantages, including high porosity, restricted electronic transmission dimensions, tunable morphology, and scale fabrication [18]. Cho et al. investigated the NO2 sensing performance of SnO2 hollow nanofibers with diameters of 300–500 nm by electrospinning and SnO2 thin film with a thickness of 15–20 nm by RF-sputtering, respectively [19]. The SnO2 hollow nanofibers exhibited a promoted response of 81.4 to 2 ppm NO2 compared to that (19.9) of the SnO2 thin film. The enhancement resulted from the greater specific surface area and greater space charge modulation depth of the 1D hollow nanofibers. Conventional nanofiber fabrication techniques, such as chemical vapor deposition, sol–gel methods, and template-assisted synthesis, enable precise control over material composition and morphology, but suffer from limitations including high energy consumption and reliance on sophisticated equipment [20]. In contrast, electrospinning technology stands out due to its operational simplicity and low cost, allowing facile regulation of fiber morphology and composition [21]. Specifically, electrospun nanofibers possess a uniform 1D structure with a high surface area, which significantly enhances gas adsorption capacity and accelerates adsorption/desorption kinetics of the sensing layer. Furthermore, the physicochemical properties of nanofibers can be precisely tailored by optimizing electrospinning and post-treatment parameters (solution concentration, applied voltage, collector distance, calcination temperature), enabling highly sensitive and selective detection toward target gases. Morais et al. prepared a sensitive NO2 sensor based on WO3 nanofibers, which were synthesized by electrospinning and following calcination [22]. The thickness and grain size of WO3 nanofibers were dependent on the calcination heating rate and heating temperatures, and thus regulating the gas-sensing performance. The optimized WO3 nanofibers, calcined at 500 °C with a heating rate of 10 °C/min, presented a high response of 15,000 toward 25 ppm NO2.
Although the technology of electrospun nanofibers has significantly promoted the gas-sensing performance of sensitive materials, the intrinsic limitations of these materials themselves still frustrate their further application. For instance, metal oxides suffered from high operating temperatures and poor selectivity [23]; conductive polymers were limited by weak stability and poor reversibility [24]; carbon nanofibers lacked specific adsorption behavior to various gases and had insufficient sensitivity to target gas [25,26]. Despite photoirradiation [27] and plasma treatment [28] being effective strategies to overcome these obstacles, they still faced critical limitations such as material degradation under prolonged exposure, high operational costs, and insufficient selectivity due to non-specific surface activation, ultimately restricting their scalability and long-term stability in practical applications [29].
Utilizing surface and interface engineering strategies to functionalize the gas-sensitive materials is one facile method to promote the sensing performance of sensors. The functionalized gas-sensitive materials could overcome the limitations inherent in single-component materials, where the synergistic interaction between constituents significantly enhances gas-sensing performance [30,31]. Recently, composite nanofibers fabricated via co-electrospinning or post-treatment strategies have garnered significant attention [32,33]. For instance, doping with noble metal could improve the sensitivity and accelerate response speed by chemical sensitization and electronic sensitization [34]. Metal oxides could be constructed as heterojunctions with host nanofiber materials, thus regulating the interface potential energy barriers [35]. These composites synergistically integrate functional components into electrospun nanofibers, offering enhanced gas adsorption capacities, catalytic activity, and electrical properties compared to pristine nanofibers.
This review comprehensively summarized recent advancements of functionalized electrospun nanofibers in gas sensors. Initially, the gas-sensing materials derived by electrospinning technology were introduced, including the synthesis process and gas-sensing mechanism of metal oxides, carbon materials, and polymers. Then, the gas sensors based on functionalized nanofibers were discussed in detail, including the construction method, enhancement mechanism, and sensing performance. Furthermore, the emerging challenges and outlined future directions of composite nanofibers for gas sensing were presented to accelerate the practical application.
2. Overview of the Technology of Electrospinning Nanofibers
Electrospinning technology is a versatile and efficient technique for fabricating nanofibers characterized by remarkably high specific surface areas and precisely tunable morphologies. A standardized electrospinning apparatus consists of four functional modules [36]: (1) a high-voltage power supply, (2) a grounded collector designed for nascent fiber acquisition, (3) a precision syringe pump system with a metallic needle, and (4) a flow-control mechanism regulating polymer solution delivery, as shown in Figure 1a.
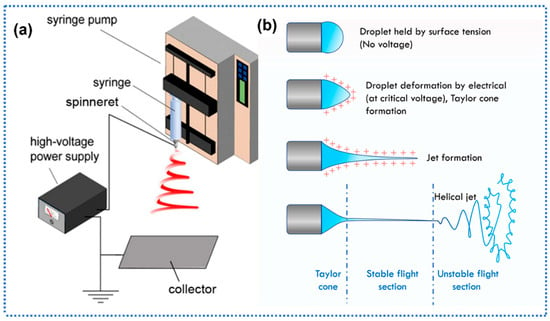
Figure 1.
(a) Schematic illustration of the setup for electrospinning, reprinted with permission from Ref. [37]. Copyright 2017, The American Chemical Society. (b) Mechanism of electrospinning, reprinted with permission from Ref. [38]. Copyright 2024, Cell Press.
The operation of electrospinning starts with the establishment of an intense electrostatic field between the needle tip and the collector. The syringe pump delivers polymer solution at controlled flow rates, generating charged droplets that remain suspended at the needle orifice. At critical voltage thresholds, the balance between electrostatic forces, surface tension, and gravitational pull induces cone-jetting phenomena, forming characteristic “Taylor cones” (Figure 1b).
Subsequent ejection of charged fluid filaments occurs as micro-jets directed toward the collector. During mid-air transport, non-uniform charge distribution within the jets induces dynamic instability under sustained electric fields, manifesting as whipping, bending, and progressive elongation. This morphological evolution is synergistically facilitated by concurrent solvent evaporation, leading to continuous jet thinning through successive bifurcations. The process culminates in electrostatic stretching and solidification of polymer matrices, ultimately depositing hierarchically structured micro/nanofibers with submicron-scale diameters onto the collector substrate.
3. Preparation and Sensing Mechanism of Electrospun Nanofiber Gas Sensors
3.1. Synthesis of Gas-Sensing Materials Using Electrospinning
Regarding the gas-sensitive materials synthesized by electrospinning, metal oxides, polymers, and carbon nanofibers were widely investigated [39,40]. As for the metal oxide nanofibers. Electrospinning technology, through optimizing the type of precursors and precise adjustment of process parameters supplemented by heat treatment, provides a versatile and scalable platform for producing metal oxide nanofibers (Figure 2a) [41]. Specifically, a polymer solution containing metal ion precursors (metal alkoxides or metal salts) was electrospun into nanofibers. Subsequently, these fibers undergo calcination at elevated temperatures, during which the polymer oxidatively decomposes and metal oxide crystals grows, ultimately forming metal oxide nanofibers with well-defined morphology, uniform diameter, and tunable porosity. By regulating precursor composition, solution properties, and calcination parameters, precise modulation of the crystallinity, specific surface area, and size of the metal oxide nanofibers can be achieved [42].
Electrospinning fabricates polymer nanofibers with uniform diameters and high aspect ratios, exhibiting exceptional structural consistency alongside scalable processability (Figure 2b). The critical advantages reside in facile tunability, where precise adjustments to key process parameters (polymer concentration, solution viscosity, applied voltage, and needle-collector distance) enable accurate control of mechanical characteristics. In addition, hollow or porous and cross-linked polymer nanofibers could be achieved by post-treatment [43,44]; core–shell nanofibers could be obtained via coaxial spinning [45]; composite nanofibers could be synthesized by incorporating multiple polymers or nanoparticles [46].
The carbon nanofiber electrospinning process begins with the polymer precursors, followed by two thermal treatment stages, involving stabilization and carbonization (Figure 2c). Stabilization is performed at 200–500 °C under an oxidative environment, where the polymer chains are chemically modified and strengthened [47]. The subsequent carbonization process occurs under an inert atmosphere at elevated temperatures (600–3000 °C), where the polymeric matrix transforms into nanofibers with either graphitic or amorphous microstructures, which is related to the thermal treatment temperature and annealing duration [48].
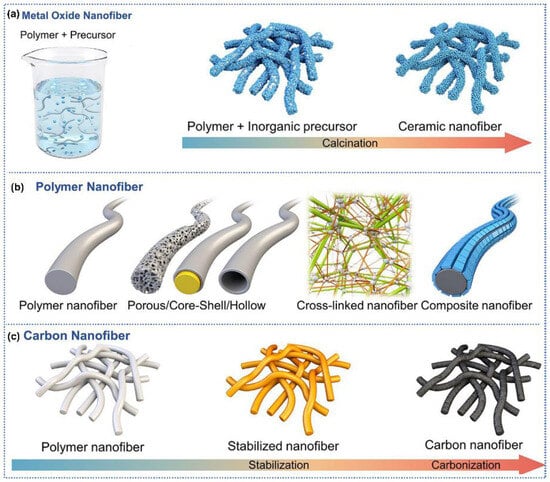
Figure 2.
Schematic illustration of gas-sensitive materials synthesized by electrospinning: (a) metal oxide nanofibers, (b) polymer nanofibers, (c) carbon nanofibers. Reprinted with permission from Ref. [49]. Copyright 2025, Wiely.
3.2. Electrospinning Parameters
The electrospinning process is critically regulated by a set of key operational parameters, which collectively dictate fiber morphology and resultant properties by modulating electrostatic interactions, solution rheology, and jet dynamics [50]. This section systematically elucidates the impact mechanisms of these principal variables:
(1) Applied voltage
The magnitude of the applied voltage directly governs the electric field gradient and charge density distribution. Within the critical voltage window (typically 10–30 kV), elevated voltage enhances electrostatic repulsion forces, thereby overcoming solution surface tension to induce significant jet stretching [51]. This process results in an exponential decrease in fiber diameter. However, exceeding critical thresholds induces corona discharge due to charge accumulation, disrupting jet stability and promoting bead formation through fragmentation. This transition is mechanistically attributed to an excessive charge density disrupting the dynamic charge migration equilibrium necessary for continuous jetting.
(2) Solution concentration and viscosity
Polymer concentration fundamentally dictates solution viscosity and rheological behavior, critically influencing fiber continuity [52]. Suboptimal concentrations yield insufficient entanglement, allowing surface tension to dominate and form discontinuous droplets or beaded fibers. Optimal concentrations establish a balance between viscosity and conductivity, facilitating the stable formation of a Taylor cone and enabling uniform solvent evaporation for the production of smooth, continuous fibers. Conversely, excessive concentrations engender high viscoelasticity within the solution, impeding jet elongation during flight and leading to the formation of fibers with irregular, rough surfaces. These concentration-dependent effects stem fundamentally from variations in polymer chain entanglement governed by the Huggins coefficient, coupled with interfacial Marangoni effects during phase separation. Zhang et al. prepared an ethanol sensor based on electrospun SnO2 nanofibers and investigated the effect of PVA concentration on the morphology of SnO2 fibers [53]. With the increase of the concentration of PVA from 6% to 8%, the average diameter of the SnO2 nanofibers increased. Moreover, the nanofibers were not continuous with the PVA concentration of 8% due to the large viscoelastic force. In addition, the fiber intensity decreased with the increase in PVA concentration. Hence, the solution with 6% PVA was utilized to prepare thin and uniform fibers.
(3) Solution flow rate
The flow rate, precisely controlled by a syringe pump, determines the mass flux of polymer solution to the Taylor cone and consequently influences jet residence time under the electric field [54]. Insufficient flow rates, while potentially promoting solvent evaporation, carry the risk of needle tip clogging. Conversely, excessively high flow rates perturb the electrostatic charge balance at the jet apex, inducing jet splaying or splitting. Moreover, empirical observations indicate a near-linear increase in average fiber diameter with increasing flow rate, which is attributed to the direct scaling of polymer mass deposition per unit time with the jet’s volumetric flow rate and consequently its cross-sectional area during solidification.
(4) Tip-to-collector distance
The distance separating the spinneret tip and the collector (typically 10–30 cm) is a key determinant of the jet flight time and solvent evaporation kinetics. Insufficient distances curtail flight time, leading to incomplete solvent evaporation and resulting in the deposition of wet fibers that fuse upon contact [51]. Increasing the distance prolongs jet whipping and extensional deformation, potentially generating finer fibers; however, it simultaneously enhances the likelihood of secondary jet instabilities and breakup. An overly extended distance also compromises fiber collection efficiency due to the wider spatial dispersion of the jet.
The above electrospinning parameters were critical for the morphology and structures of nanofibers, and further affected the gas-sensing property. Khomarloo et al. optimized the structural dimension of electrospinning ZnO nanofibers to enhance the NO sensing performance [54]. The effect of voltage, feed rate, and tip-to-collector distance on nanofibers was investigated initially. The nanofibers with optimized parameters (voltage: 18 kV, feed rate: 200 μL/h, distance: 20 cm) exhibited a more porous structure with a high specific surface area of 76.76 m2/g and a diameter of 0.16 μm. And the sensors based on optimized nanofibers showed the best response to NO of 1 ppm and 500 ppb. Moreover, the increase in ZnO nanofibers diameter resulted in response decrease toward NO, owing to the smaller diameter promoting the surface area and active adsorption sites.
3.3. Gas-Sensing Mechanism of Pristine Electrospinning Nanofibers
Figure 3a exhibited the typical structure of chemiresistive gas sensors, including the sensing layer, ceramic tubes, heating electrodes, and signal electrodes. As the critical part of gas sensors, currently, electrospinning gas-sensitive materials mainly include metal oxides, conductive oxides, and carbon materials [19,55,56]. For metal oxides, the widely accepted gas sensor mechanism involves surface redox reactions (Figure 3b) [57]. When exposed to air, oxygen molecules adsorb onto the metal oxide surface and extract electrons from its conduction band, forming oxygen species (O2−/O−), with the oxygen species types dependent on the operating temperature [58]. Upon exposure to reducing gases, n-type metal oxide semiconductors react with pre-adsorbed oxygen species, releasing electrons into the conduction band and increasing the electrical conductivity of the sensing layer. In contrast, p-type semiconductors exhibit opposite conductivity trends upon interacting with reducing gases. Unlike metal oxides requiring oxygen participation in gas–solid reactions, carbon-based materials and conductive polymers directly react with target gases through charge transfer mechanisms (Figure 3c,d) [40,41], thus altering the electrical conductivity of the sensitive layer without relying on atmospheric oxygen.
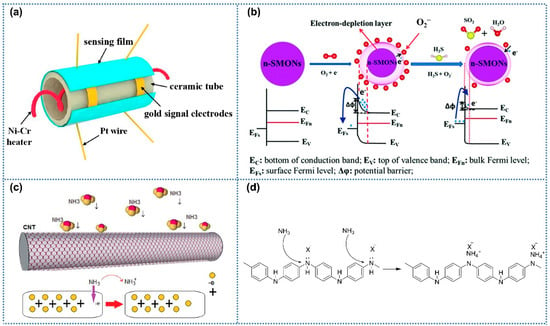
Figure 3.
(a) Schematic illustration of chemiresistive gas sensor, Reprinted with permission from Ref. [59] Copyright 2018, Elsevier. (b) Gas-sensing mechanism of n-type metal oxides semiconductors, Reprinted with permission from Ref. [60]. Copyright 2018, The Royal Society of Chemistry. (c) Gas-sensing mechanism of carbon nanotubes, Reprinted with permission from Ref. [41] Copyright 2014, MDPI. (d) Gas-sensing mechanism of PANI, Reprinted with permission from Ref. [61] Copyright 2007, MDPI.
To assess the performance of gas sensors, the essential indicators could be summarized as: response, response/recovery times, operation temperature, and limit of detection (LoD). The response (S) is defined as S = Rg/Ra or S = (Ra − Rg)/Ra for reducing gas and S = Ra/Rg or S = (Ra − Rg)/Ra for oxidizing gas, where Ra and Rg separately represent the stable resistance of the as-prepared sensor in air and target gas. The response time (tres) is defined as the duration required for the resistance of sensors to reach 90% of the total resistance change when exposed to the target gas. The recovery time (trec) refers to the time needed for the resistance to return to 90% of its baseline value after the target gas is removed. To assess the enhancement effects of the functionalization strategy on the gas-sensing performance of electrospun nanofibers, the ratio of the response (SF) of functionalized sensors to that (SN) of non-functionalized sensors (SF/SN) is employed as a performance enhancement metric in this study.
4. Gas Sensors Based on Metal Oxide (MO) Nanofibers
Metal oxides are currently the most widely used gas-sensitive materials due to their high sensitivity, fast response/recovery speed, and good stability [62,63]. Therefore, the preparation of metal oxide nanofibers for gas-sensitive materials via the electrospinning technique has attracted significant attention [64]. As for the synthesis of metal oxide nanofibers, a homogeneous precursor solution containing metal salts and polymers was prepared initially. Under a high-voltage electric field (10–30 kV), the solution is stretched into jets and collected as a fibrous membrane. Subsequently, high-temperature calcination (400–800 °C) was performed to remove organic components and convert metal salts into oxides, ultimately yielding uniform metal oxides nanofibers [65].
According to the difference in major carriers, the metal oxide semiconductors could be divided into n-type (ZnO, SnO2, TiO2, WO3, In2O3) and p-type (CuO, NiO, Co3O4, and Nb2O3) [66]. Kim et al. reported a highly sensitive NO2 sensor based on n-type SnO2 hollow nanofibers by electrospinning [19]. The SnO2 nanofibers exhibited a high response of 81.4 (Rg/Ra) toward 2 ppm NO2, surpassing that of planar SnO2 thin films (Rg/Ra = 19.9) at 300 °C. The enhanced NO2-performance could be attributed to the more accessible active sites and the larger space charge modulation depth with the 1D hollow nanofibers. Lee et al. prepared a highly sensitive and selective ethanol sensor based on p-type Co3O4 nanofibers [67]. The Co3O4 nanofibers prepared by heat treatment of as-spun precursor fibers at 500 °C showed a high response of 51.2 to 100 ppm ethanol and short response/recovery times of 7.9/22.7 s at 301 °C. It could be found that metal oxides always require operation at elevated temperatures to supply sufficient activation energy for surface redox reactions and overcome carrier transport barriers [68]. However, high operation temperatures lead to significant bottlenecks: (1) accelerating grain growth that severely degrades long-term stability of sensors, and (2) increasing energy consumption for sustained operation [69]. Furthermore, single-metal-oxide-based gas sensors demonstrated detection limits at ppm or sub-ppm levels, thereby failing to achieve highly sensitive detection for trace gases at ppb levels [70]. To overcome these barriers that metal oxide nanofibers faced, functionalization method on the metal oxide nanofibers has been verified as one facile strategy [29].
4.1. Gas Sensors Based on Noble Metal-Doped Metal Oxides
Noble metals (Pd, Pt, Au, etc.) are widely applied in surface modifications of gas-sensing materials to enhance sensor sensitivity, due to their chemical sensitization and electronic sensitization effects [71]. With the modification of noble metals, the adsorption energy of metal oxides would be reduced, and more target gas molecules would be adsorbed on the sensing layer [72]. In addition, electronic sensitization would result in the redistribution of charge carriers, accelerating the exchange of charge between the sensing layer and the target gas [73]. Moreover, chemical sensitization would reduce the activation energy of the redox reaction and thus decrease the operation temperature [73].
Cui et al. prepared Pt nanoparticles doped with ultrafine SnO2 mesopore nanofiber for acetone detection by an electrospinning technique and annealing process, as shown in Figure 4a [74]. The Pt-SnO2 sensors exhibited superior acetone-sensing performance than pure SnO2 sensors, including a high response of 31.2 to acetone of 2 ppm, rapid response/recovery times of 13/23 s, and a low detection limit of 100 ppb (Figure 4b). The enhanced performance could be attributed to the catalytic activity of Pt, which facilitated the conversion rate of adsorbed oxygen molecules to ions, speeding up the reaction between acetone and the sensitive layer (Figure 4c). Liu et al. prepared highly sensitive NO2 sensors based on Pt-loaded In2O3 mesoporous nanofibers, which were synthesized by electrospinning, followed by a facile reduction method [75]. The In2O3 nanofibers functionalized with small Pt nanoparticles demonstrated superior NO2 sensing properties at room temperature, achieving a low LoD down to 10 ppb (Rg/Ra = 2.8). In ambient conditions, the well-dispersed Pt nanoparticles within the composite fibers efficiently promoted oxygen molecular dissociation, generating abundant surface oxygen species. Upon NO2 exposure, these Pt nanoparticles actively accelerated the chemical interactions between NO2 molecules and adsorbed oxygen species. Through chemical sensitization effects, the optimized Pt distribution significantly lowered the activation energy required for NO2 adsorption. Additionally, NO2 molecules preferentially accumulated near Pt-In2O3 interfacial regions due to the reduced energy barrier for electron transfer from the conduction band of In2O3 to adsorbed NO2. However, the oxygen species induced by “spillover effect” were open acceptors for gases without selectivity. Furthermore, functionalization with noble metals could be coupled with morphostructural control to synergistically boost the performance of gas-sensing layers. Kim et al. synthesized both bi-modal pores and Pt catalyst-loaded thin-walled SnO2 nanofibers by Ostwald ripening-driven electrospinning combined with a sacrificial templating route using polystyrene (PS) colloid and bioinspired protein (Figure 4d) [76]. The Pt-decorated porous SnO2 nanofibers showed a high response of 192 to 5 ppm acetone, which was a 37-fold enhancement over that of pure SnO2. In addition, the Pt-PS-SnO2 sensors demonstrated negligible cross-response to interfering analytes, and a low detection limit of 10 ppb. The significant enhancements could be attributed to the synergistic effect between porous tubular structures and the superior sensitization effect of Pt/PtOx, as shown in Figure 4e. Choi et al. proposed microporous WO3 nanotubes functionalized with Pd nanoparticles for enhanced hydrogen sensing performance [77]. The microporous thin-walled nanotube structures were obtained by introducing colloidal polystyrene (PS) particles to a shell solution of W precursor and poly(vinylpyrrolidone). Pd nanoparticles, synthesized by bio-inspired protein cages, were uniformly dispersed within the shell solution and subsequently on the WO3 nanotubes. During the adsorption of H2S on the sensitive layer, Pd could dissociate H2 molecules into H atoms on the surface of Pd nanoparticles. The hydrogen atoms migrated across the WO3 surface through the spillover mechanism, where they interact with chemisorbed oxygen species (O−, O2−), thereby enhancing the receptor function of the sensitive layer. Pd-functionalized macroporous WO3 nanotubes exhibited a very high H2 response of 17.6 at 500 ppm and a low limit of detection (10 ppm) with a short response time of 25 s.
In addition to the catalytic effect of noble metals, the formation of Schottky junctions between noble metals and metal oxides would promote the sensing performance. Hung et al. utilized Au nanoparticles to dope SnO2/ZnO nanofibers to enhance the H2S sensing properties [78]. Due to the higher work function of Au (5.1 eV) than that of SnO2 (4.9 eV) and ZnO (4.45 eV), Schottky junctions were formed at the interface of Au and metal oxides. When exposed to H2S, the Schottky barrier height was reduced, leading to the resistance of Au-doped SnO2/ZnO nanofibers decreasing significantly. Hence, the Au-doped SnO2/ZnO sensor exhibited larger response toward H2S than undoped SnO2/ZnO (73.3 vs. 13.3 @1 ppm). To probe the selectivity mechanism of the noble metal modification on metal oxides, Liu et al. synthesized various noble metal nanoparticles modified ultrafine In2O3 nanofibers (Au-, Ag-, and Pt-In2O3) using a simple electrospinning method, followed by a controlled thermal treatment [79]. The Au-In2O3, Ag-In2O3, and Pt-In2O3 sensors exhibited improved selectivity toward H2S, HCHO, and acetone, respectively. Then, DFT calculations were implemented to reveal the adsorption energy and energy band structures of different noble metals doped In2O3. The most stable adsorption configurations occurred when H2S, HCHO, and acetone gas molecules adsorbed on the surface of Au-, Ag-, and Pt-In2O3, respectively. Therefore, the specific adsorption energies between the noble metal modified metal oxides and target gases, along with the “spillover effect” led to excellent specific selectivity.
In addition to conventional single noble metal modification for metal oxides, bimetallic modification strategies leveraging synergistic effects have recently garnered increasing research attention [80]. Sui et al. prepared 1D In2O3 nanofibers functionalized by the well-shaped and monodispersed AuPt nanocatalysts (diameter: ~9 nm) for highly sensitive gas sensors (Figure 4g) [81]. The introduction of Au into Pt-based nanocatalysts enabled precise modulation of noble metal electronic structures while optimizing structural parameters, including Pt-Pt interatomic distances and heterometallic coordination numbers. The atomic engineering resulted in ligand effects that lower Pt binding energies in the Au-Pt heterostructure, triggering a downshift in the d-band center position. Consequently, the AuPt bimetallic system exhibited superior electrocatalytic activity compared to monometallic Au or Pt counterparts, leading to enhanced sensitivity toward ozone and acetone (Figure 4h). Similarly, Zhao et al. reported a highly sensitive and selective chemiresistive sensor for acetone detection in exhaled breath, leveraging electrospun ZnFe2O4 nanofibers functionalized with Au@Pt core–shell nanospheres [82]. The Au@Pt-ZnFe2O4 achieved excellent sensing performance, including a low LoD of 30 ppb at a low operation temperature of 188 °C, and superior performance compared to pristine ZnFe2O4 nanofibers (response: 3.32 vs. 1.84 toward 0.5 ppm acetone). Enhanced sensing performance resulted from the synergistic effects: (1) oxygen vacancies introduced via compositional engineering created sufficient active sites and charge carriers; (2) the Au@Pt bimetallic structure induced Schottky junctions and catalytic activity, accelerating gas–solid interactions and lowering activation energy.
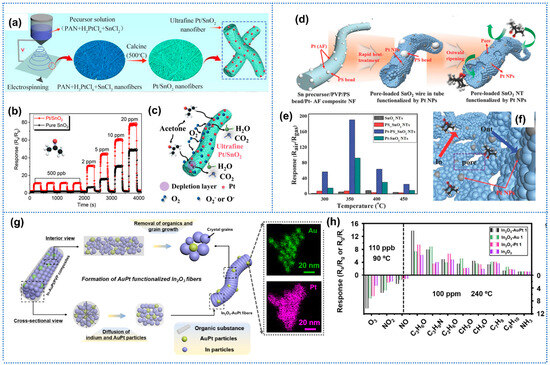
Figure 4.
(a) Illustration of the synthesis process of Pt-SnO2 nanofibers; (b) The dynamic response curves of Pt-SnO2 and SnO2 toward acetone; (c) Schematic illustration of the acetone sensing mechanism, Reprinted with permission from Ref. [74] Copyright 2023, Elsevier. (d) Schematic illustration of Pt load porous SnO2 nanofibers; (e) Temperature-dependent acetone response of different sensors; (f) schematic illustration of the sensing mechanism for Pt-PS-SnO2, Reprinted with permission from Ref. [76] Copyright 2016, Wiley. (g) Schematic diagram of In2O3 nanofibers functionalized with AtPt particles; (h) Response of sensors based on pristine In2O3, In2O3-AuPt, In2O3-Au, and In2O3-Pt toward different gases, Reprinted with permission from Ref. [81] Copyright 2022, The American Chemical Society.
4.2. Gas Sensor Based on MO-MO Heterojunctions
Constructing heterojunctions with other metal oxides is one facile strategy to improve the gas-sensing performance of pure metal oxide nanofibers [83]. According to the conductivity type of metal oxides, metal oxide heterojunctions can be classified into p-n heterojunctions, p-p heterojunctions, and n-n heterojunctions. Due to the difference in Fermi energy level of metal oxides, upon contact, electrons would transfer from the metal oxide with a high Fermi energy level to that with a low Fermi energy level until the equilibrium was established, with the formation of an electron depletion layer at their interface [83]. In addition, when the heterojunction is formed at the interface of two distinct metal oxide materials, the structural mismatch of their lattice constants and crystal structures generates interfacial stress. And the stress induced the dislodgement of oxygen atoms from the lattice framework, resulting in the formation of oxygen vacancies at the interface [84]. The oxygen vacancies would facilitate the adsorption of gas molecules on the sensitive layer. Moreover, abundant heterojunctions amplified the effect of the carrier-concentration change during the gas–solid interaction, eventually promoting the sensor response [63]. Therefore, metal oxide heterojunction nanofibers exhibited remarkable gas-sensing performance.
Park et al. prepared porous CuO-SnO2 nanotubes by electrospinning for highly sensitive detection toward H2S [85]. The composite nanotubes with homogeneously distributed CuO nanoparticles within a tubular SnO2 structure were synthesized by electrospinning with a mixed solution of Sn and Cu precursors, followed by thermal treatment (Figure 5a). The CuO-SnO2 sensor exhibited similar sensing behavior with n-type SnO2 sensor toward H2S, indicating the host character of SnO2 in composites. The heterojunction sensors exhibited excellent H2S sensing performance, including a high response of 1395 with a short response time of 5.27 s to 5 ppm H2S at 200 °C, and remarkable selectivity (Figure 5b). The noticeably high sensitivity could be attributed to the formation of CuO-SnO2 p-n heterojunctions (Figure 5c). And the formation of CuS and then conversion to CuO on the surface of the CuO-SnO2 during the H2S adsorption and desorption process enhanced the selectivity of the sensing layer toward H2S. Similarly, Li et al. synthesized nanofibers of p-type NiO/n-type ZnO heterojunction for trimethylamine (TMA) detection by a facile electrospinning technique [86]. The effect of NiO content in composites on TMA sensing performance was investigated in detail. And the NiO/ZnO nanofibers with optimized component ratios exhibited significantly improved TMA sensing performance, including a high response of 892 to 100 ppm TMA, a low LoD down to 0.5 ppm, and short response/recovery times of 30/35 s. Naderi et al. reported a highly selective and sensitive electrospun ZnO-CdO nanofiber-based sensor for trace NO2 detection at an optimal temperature of 215 °C (Figure 5d) [87]. Based on the necklace-like ZnO-CdO n-n type heterojunction with a straddling bandgap, the sensor exhibited superior sensitivity over interfering gases (NO2, H2S, CH4) and volatile organic compounds (VOCs), achieving a remarkable response of 22.6 toward 33 ppm NO (Figure 5e). Moreover, the ZnO-CdO sensor presented rapid response/recovery times (47/1249 s to 3 ppm; 35/630 s to 33 ppm), long-term stability, and negligible humidity interference. The n-n heterojunction facilitated efficient charge transfer and surface reactions, while the ZnO-CdO interface regulated oxygen vacancy density and band bending, thus promoting gas adsorption dynamics (Figure 5f).
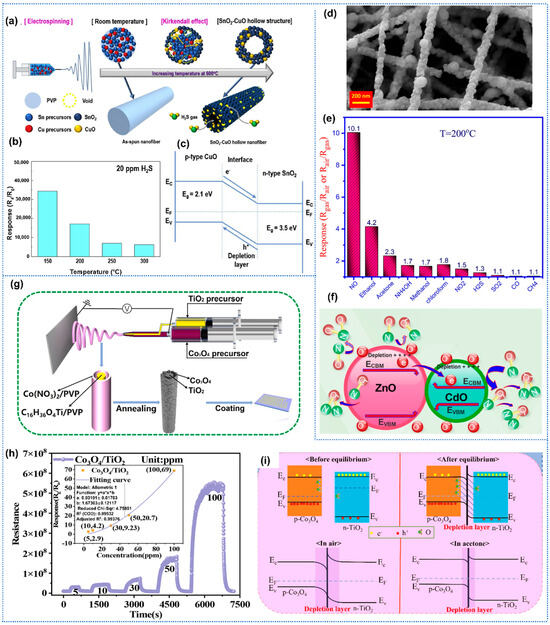
Figure 5.
(a) Schematic process of SnO2-CuO hollow nanofibers by electrospinning; (b) Response of SnO2-CuO hollow nanofibers sensor toward H2S under different operation temperatures; (c) Schematic illustration of the p-n junction of SnO2-CuO, Reprinted with permission from Ref. [85] Copyright 2020, Elsevier. (d) SEM images of ZnO/CdO nanofibers; (e) Response of the ZnO/CdO sensor toward various gases; (f) schematic illustration of the mechanism of ZnO/CdO, Reprinted with permission from Ref. [87] Copyright 2019, Elsevier. (g) Schematic diagram of the synthesis procedure of Co3O4/TiO2 core–shell nanofibers using coaxial electrospinning; (h) Co3O4/TiO2 sensors to acetone (5–100 ppm) at 300 °C; (i) Gas-sensing mechanism of the Co3O4/TiO2, Reprinted with permission from Ref. [88] Copyright 2024, Elsevier.
In addition to the above uniformly mixed metal oxides heterojunctions, the core–shell heterojunctions were also constructed to promote the gas-sensing performance of metal oxide nanofibers. Fu et al. reported an acetone sensor based on Co3O4-TiO2 core–shell nanofibers, which were fabricated easily by the coaxial electrospinning method (Figure 5g) [88]. In detail, they utilized a concentric spinneret to simultaneously process Co precursor Ti precursor, enabling the formation of core–shell nanostructured fibers with controlled interfacial architectures. The Co3O4-TiO2 sensors exhibited superior acetone sensing performance compared to pure TiO2 nanofibers and Co3O4 nanofibers sensors, including a high response of 20.7 toward 50 ppm acetone and a low detection limit of 0.5 ppm (Figure 5h). These enhancements could be attributed to the abundant adsorption and reaction sites provided by the core–shell nanostructures, the formation of p-n heterojunctions, and the good catalytic activity of Co3O4 (Figure 5i).
4.3. Gas Sensors Based on the Heterojunction of Metal Oxides-2D Materials
Recently, 2D materials have garnered considerable attention as potential candidates to functionalize metal oxides due to their large specific surface area, high carrier mobility, and strong gas adsorption ability [89]. Zhang et al. prepared conductive graphene oxide (GO)-WO3 composites nanofibers for acetone detection [90]. Thereinto, GO was added to the WCl6 precursor solution initially, then the GO-WCl6-PVP composites nanofibers were obtained by electrospinning, followed by calcination to synthesize GO-WO3 composites. Due to the modification of the GO, the GO-WO3 composites showed more porous structure compared to pure WO3, including higher specific surface area (17.01 vs. 11.40 m2/g), and larger pore sizes (29.36 vs. 7.95 nm). Porous structure would facilitate the adsorption and diffusion of gas molecules. Therefore, the GO-WO3 composites nanofibers showed a high response of 35.9 toward 100 ppm acetone at 375 °C, which was 4.3 times higher than that of pristine WO3 nanofibers. Meanwhile, the GO-WO3 composites exhibited short response/recovery times of 4/10 s toward acetone of 100 ppm. It is worth noting that with the increase in GO content in the composites, the response of GO-WO3 nanofibers would be decreased. This phenomenon resulted from the significant reduction in total sensor resistance due to the formation of conduction channel by interconnection of continuous GO layer, which led to the small magnitude of resistance change during the adsorption of gas molecules. Similarly, Wang et al. utilized the carbon materials including graphene, carbon nanotubes, and graphene oxide (GO) to sensitize SnO2 for enhanced formaldehyde sensing [91]. Due to the large specific surface area, rich functional groups, and the electric regulation effect of GO, GO-SnO2 composites nanofibers exhibited better sensing performance than pure SnO2, carbon nanotubes-SnO2, and graphene-SnO2 composites. The GO-SnO2 sensors showed a high response of 32 under exposure of 100 ppm HCHO at 120 °C, which was 4 times higher than that of pristine SnO2 nanofibers. In addition to graphene derivatives, transition metal chalcogenides (TMDs), such as MoS2 and WS2, as typical 2D materials, were widely used to functionalize metal oxides. Yang et al. developed hierarchical SnO2 nanofibers-MoS2 nanosheets core–shell nanocomposites for ultrasensitive xylene detection [92]. The synthesis process included two key steps: (1) electrospinning SnO2 nanofibers as the structural backbone, followed by (2) in situ hydrothermal growth of vertically aligned MoS2 nanosheets on the nanofiber surface to form heteroarchitected interfaces, as shown in Figure 6a. Benefiting from the high specific surface area of MoS2 nanosheets, the composites achieved a significantly enhanced specific surface area of 18.77 m2/g compared to 6.67 m2/g for pristine SnO2 nanofibers. The porous structure enabled the SnO2-MoS2 sensor to demonstrate remarkable performance at 220 °C, including a rapid response/recovery kinetics (21.5 s/60.4 s) and a high response of 2.35 to 100 ppm xylene, which was 4.7 times higher than that of pure SnO2 counterparts (Figure 6b). These enhancements originated from the synergistic effect of the following three aspects (Figure 6c): (1) abundant MoS2-SnO2 heterojunctions facilitating efficient charge transfer, (2) MoS2-induced oxygen vacancy defects providing more active adsorption sites, and (3) sufficient gas diffusion pathways through the hierarchical 3D nanostructure. Zhu et al. prepared hierarchical heterojunctions of WS2 nanosheets/ In2O3 nanofibers for an efficient detection of formaldehyde [93]. The In2O3 nanofibers were prepared by electrospinning, and then the WS2 nanosheets were grown on the In2O3 nanofibers by in situ hydrothermal reaction. The WS2-In2O3 composites possessed a high specific surface area of 84.9 m2/g, which significantly surpassed that of pure In2O3 (9.6 m2/g). Moreover, the WS2-In2O3 heterojunctions would enhance charge transfer and provide more active sites for gas adsorption. Therefore, the WS2-In2O3 composites demonstrated a high response of 12.6 to 100 ppm HCHO at 140 °C, which was two times higher than that of the pure In2O3 sensor.
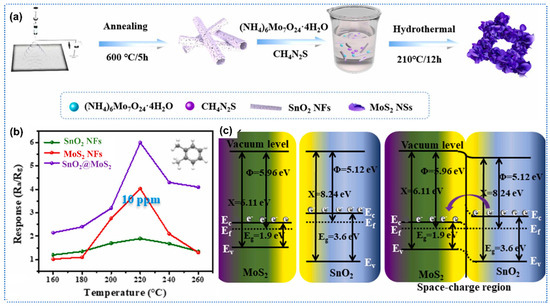
Figure 6.
(a) Schematic diagram of MoS2-SnO2 synthesis process; (b) Response of SnO2, MoS2, and MoS2-SnO2 nanofibers toward xylene at different temperatures, (c) Schematic illustration of the sensing mechanism of the MoS2-SnO2 nanocomposites, Reprinted with permission from Ref. [92] Copyright 2023, Elsevier.
4.4. Gas Sensors Based on Metal-Ion-Doped Metal Oxides
Metal ion doping serves as a dual-functional strategy to synergistically enhance surface lattice defects and oxygen vacancy concentrations, while doping sensitization introduces new donor/acceptor states through defect engineering, thereby elevating free carrier density and improving charge transport efficiency [94]. Electrospinning enables in situ metal ions doping within polymer-templated nanofibers, where spatial confinement effects and precursor solution viscosity regulate uniform metal distribution. This controllable doping approach enables precise stoichiometric engineering of metal-metal oxides, optimizing both gas adsorption sites and interfacial charge transfer kinetics.
Wu et al. reported a systematic investigation of Cu-doped α-Fe2O3 semiconductor nanofibers synthesized via electrospinning for selective NO2 gas sensing at ppm levels [95]. By varying the Cu molar ratio in precursor solutions, the study revealed a unique phenomenon of p-to-n-type semiconducting transition in α-Fe2O3 nanofibers when Cu molar ratios exceeded 0.23, driven by the formation of CuFe2O4 spinel phases via cation substitution. The Cu-doped α-Fe2O3 fibers exhibited significantly shortened response time (up to 40% reduction at 33 ppm NO2) compared to undoped α-Fe2O3. Wang et al. prepared Ni-doped ZnO electrospun nanofibers for enhanced C2H2 gas sensing [96]. Through electrospinning followed by calcination, Ni doping (up to 5 at%) preserved the hexagonal wurtzite structure of ZnO nanofibers while introducing lattice distortions and intrinsic defects. Notably, 5 at% Ni-doped ZnO exhibited a sixfold increase in sensitivity (16.9 vs. 2.6 for undoped) toward 2000 ppm C2H2 at 250 °C, attributed to Ni2+-induced oxygen vacancy modulation and facilitated charge transfer. The hierarchical structure of nanofibers (diameters ~100 nm) displayed rapid response/recovery times (~5/10 s). Wu et al. utilized Al-doping to induce the formation of oxygen vacancy for promoting the gas-sensing performance of SnO2 nanofibers [97]. The hierarchical mesoporous Al-doped SnO2 nanofibers were prepared by a simple electrospinning method and annealing. Due to the substitution of Sn4+ by Al3+ in SnO2 lattice, large amount of oxygen vacancies generated with the doping of Al. The oxygen vacancy of SnO2 changed as the function of Al/(Al + Sn) ratio and the oxygen vacancy exhibited a maximum value with the ratio of 8%. Meanwhile, the sensor showed the maximum response of 7.82 toward formaldehyde (1 ppm) when the Al/(Al + Sn) ratio was 8%. Kou et al. reported a highly sensitive acetone sensor based on rhodium (Rh)-doped SnO2 electrospun nanofibers [98]. Utilizing electrospinning to fabricate uniform SnO2 nanofibers (~150 nm diameter) and subsequent calcination (Figure 7a), 0.5 mol% Rh doping SnO2 achieved a 9.6-fold enhancement in response (Ra/Rg = 60.6) to 50 ppm acetone at 200 °C compared to undoped SnO2 (Ra/Rg = 6.3) (Figure 7b). Rh doping induced lattice contraction, and thus reduced the crystallite size of SnO2 (from 9.85 nm to 6.56 nm). In addition, the Rh doping induced more oxygen vacancies (Ov) than pure SnO2, which could supply more active sites for the gas reaction and adsorption, thereby enhancing the response (Figure 7c). Furthermore, the Rh-doped SnO2 sensor exhibited exceptional selectivity towards acetone over ethanol and other interfering gases, with short response/recovery times of 2/64 s.
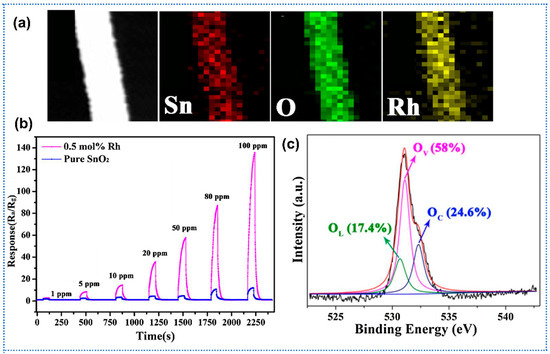
Figure 7.
(a) EDS elemental mapping images of Rh-doped SnO2 nanofibers; (b) Response of sensors based on pure SnO2 and Rh-doped SnO2 nanofibers toward acetone of 1–100 ppm at 200 °C; (c) O 1s XPS of Rh-doped SnO2 nanofibers, Reprinted with permission from Ref. [98] Copyright 2018, Elsevier.
Above reports exhibited the effective enhancement strategies for the metal oxide nanofibers, as shown in Table 1. However, current research primarily focused on enhancing the sensitivity of sensitive materials, and the proposed metal oxide functionalization method still suffered from high operation temperatures, which limits the further utilization of metal oxide nanocomposite fibers. In addition, the introduction of hydrophilic materials, such as CuO and NiO, would facilitate the adsorption of water molecules, thus reducing the response of metal oxide nanofibers [99].

Table 1.
Gas-sensing performance based on metal oxide composites.
Table 1.
Gas-sensing performance based on metal oxide composites.
| Material | Gas | S | OT | tres/trec | LOD | SF/SN | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Noble meta-doped | Pt-SnO2 | acetone (2 ppm) | 31.2 a | 150 °C | 13/25 s | 100 ppb | 10 | [74] |
| Pd-WO3 | H2 (500 ppm) | 17.6 a | 450 °C | 25 s/- | 10 ppm | 3.6 | [77] | |
| Pt-PS-SnO2 | Acetone (5 ppm) | 192 a | 350 °C | 10 ppb | 37 | [76] | ||
| Au-ZnO/SnO2 | H2S (1ppm) | 73.3 a | 350 °C | 36/786 s | 0.1 ppm | 7 | [78] | |
| Au-In2O3 | H2S (1 ppm) | 13.6 a | 300 °C | 35/108 s | 50 ppb | 3.5 | [79] | |
| Ag-In2O3 | HCHO (1 ppm) | 12.6 a | 300 °C | 19/24 s | 8 ppb | 4.1 | [79] | |
| Pt-In2O3 | acetone (1 ppm) | 17.9 a | 300 °C | 22/28 s | 20 ppb | 4.0 | [79] | |
| Pt-In2O3 | NO2 (1 ppm) | 23.9 a | RT | -/- | 20 ppb | 2 | [75] | |
| AuPt-In2O3 | acetone (50 ppm) | 7.1 a | 240 °C | -/- | 10 ppm | 3.1 | [81] | |
| Pd-Co3O4/ZnO | ethanol (200 ppm) | 59 a | 240 °C | 6/12 s | 1 ppm | 2 | [100] | |
| Ag-SnO2 | acetone (50 ppm) | 40 a | 160 °C | 6/10 s | 5 ppm | - | [101] | |
| AuPt-ZnFe2O4 | acetone (0.5 ppm) | 3.32 a | 188 °C | 33/28 s | 30 ppb | 1.8 | [82] | |
| MO-MO heterojunction | Co3O4-TiO2 | acetone (50 ppm) | 20.7 a | 300 °C | 68/18 s | 500 ppb | 7 | [88] |
| NiO-ZnO | TMA (100 ppm) | 892 a | 260 °C | 18/20 s | 0.5 ppm | 9 | [86] | |
| CuO-CdO | NO (33 ppm) | 22.6 a | 215 °C | 35/630 s | 1.2 ppm | 2.4 | [87] | |
| SnO2-CuO | H2S (5 ppm) | 1395 a | 150 °C | 5.27 s/- | 2 ppm | - | [85] | |
| ZnO-SnO2 | NH3 (100 ppm) | 60.41% b | RT | 70/23 s | 10 ppm | - | [102] | |
| ZnO-In2O3 | ethanol (100 ppm) | 31.9 a | 225 °C | 3/21 s | 0.2 ppm | 2 | [103] | |
| ZnO-CuO | H2S (10 ppm) | 4489.9 a | 150 °C | -/- | 1 ppm | - | [104] | |
| NiO-SnO2 | toluene (50 ppm) | 11 a | 330 °C | 11.2/4 s | 50 ppm | 10 | [105] | |
| GO-SnO2 | HCHO (100 ppm) | 32 a | 120 °C | 66/10 s | 0.5 ppm | 4 | [91] | |
| MoS2-SnO2 | xylene (100 ppm) | 23.5 a | 220 °C | 21.5/60.4 s | 0.5 ppm | 4.7 | [92] | |
| MO-2D material heterojunciton | GO-WO3 | acetone (100 ppm) | 35.9 a | 375 | 4/10 s | 20 ppm | 4.3 | [90] |
| WS2-In2O3 | HCHO (100 ppm) | 12.6 a | 140 | 30 s/43 s | 1 ppm | 2.0 | [93] | |
| rGO-ZnO | H2 (0.1 ppm) | 866 a | 400 | 210/234 s | 0.1 ppm | - | [106] | |
| rGO-In2O3 | NH3 (15 ppm) | 23.37 a | RT | 17/241s | 44 ppb | 10 | [107] | |
| rGO-ZnFe2O4 | H2S (1 ppm) | 147 a | 350 | 10 s/- | 0.14 ppb | 1.5 | [108] | |
| Ions-doping | Rh-SnO2 | acetone (50 ppm) | 60.6 a | 200 | 2/64 s | 1 ppm | 9.6 | [98] |
| Ni-ZnO | C2H2 (2000 ppm) | 16.9 a | 250 | 5/10 s | 100 ppm | 6.5 | [96] | |
| Cu-Fe2O3 | NO2 (50 ppm) | 2.0 a | 300 | 118/258 s | 5 ppm | - | [95] | |
| Al-SnO2 | H2 (100 ppm) | 7.7 a | 340 | 3/2 s | 10 ppm | 2.5 | [109] | |
| Al-SnO2 | formaldehyde (1000 ppb) | 7.82 a | 240 | -/- | 100 ppb | 1.33 | [97] | |
| Ni-ZnO | H2S (50 ppm) | 474 a | 215 | 50/124 s | 1 ppm | 23.7 | [110] | |
| Ce-ZnO | acetone (100 ppm) | 20.3 a | 300 | 10/9 s | 10 ppm | 2 | [111] | |
a S = Ra/Rg or Rg/Ra, b S = (Ra − Rg)/Ra × 100% or (Rg − Ra)/Rg × 100%.
5. Gas Sensors Based on Carbon Nanofibers (CNFs)
Carbon materials (carbon nanotubes, graphene, carbon nanofibers), serving as novel gas-sensitive materials, have earned significant attention in gas detection [112]. Thereinto, carbon nanofibers, as one weak p-type semiconductor, possess a high aspect ratio, sufficient adsorption sites, and high electrical conductivity [113]. Hence, carbon nanofibers were seen as the potential gas-sensitive materials to realize the high sensitivity at room temperature. Generally, carbon materials exhibited similar gas-sensing performance with p-type semiconductors, where their resistance decreased upon exposure to oxidizing gases and increased upon exposure to reducing gases. Compared to metal oxides electrospinning nanofibers, electrospun CNFs were less investigated due to their weak response, sluggish adsorption/desorption process, and poor selectivity, which frustrated their further application [114].
Recent advancements in carbon-based gas sensors have demonstrated significant improvements in sensitivity and performance through structural and chemical modifications. Regarding noble metal modification, G. Nair et al. proposed bimetallic functionalized CNFs for the highly sensitive detection of H2 [115]. The composite material was synthesized via electrospinning followed by chemical reduction, resulting in uniformly distributed Au–Pt nanoislands (NIs) on acid-functionalized CNFs (Figure 8a). Figure 8b presented the TEM images of CNFs doped with Au-Pt nanoislands. The Au-Pt-CNFs exhibited superior hydrogen sensing performance, detecting trace-level H2 (0.01–4%) with a rapid response time (6.6 s) and recovery time (18 s), outperforming pure CNFs (Figure 8c). As for the enhanced gas-sensing mechanism, the adsorption kinetics studies aligned with the Langmuir model, demonstrating the dissociative H2 adsorption at Au-Pt active sites. In situ Raman spectroscopy revealed reduced structural disorder during H2 exposure, indicating the transfer of electrons from Pt to Au and CNFs, which enhanced the response of sensors (Figure 8d). In addition, the integration of Au mitigated irreversible Pt hydride formation, enhancing desorption and stability. Similarly, Nair et al. proposed one highly sensitive, flexible H2 gas sensor based on Ni/Pt-functionalized carbon fibers [116]. The Ni/Pt-CNFs sensors exhibited a superior response of 50% to H2 of 2%. Furthermore, the flexible sensor demonstrated a negligible decrease in response with mechanical stress under flat (21%) and bent (17%) states. Additionally, the baseline resistance of the sensor remains virtually unchanged after 100 successive bending cycles, due to the high aspect ratio of the carbon nanofiber networks, which allowed for a long bending path, indicating excellent mechanical robustness.
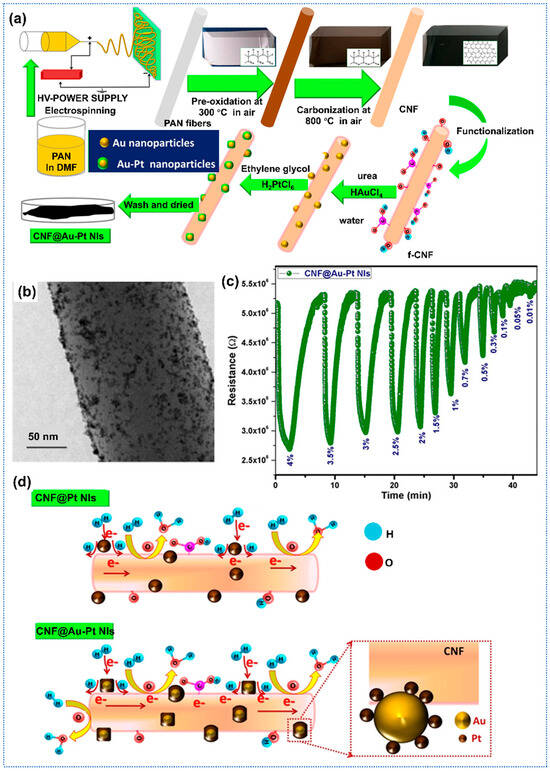
Figure 8.
(a) Synthesis process of the Au-Pt doped carbon nanofibers; (b) SEM images of Au-Pt doped carbon nanofibers; (c) Dynamic response of Au-Pt doped carbon nanofibers toward H2; (d) Gas-sensing mechanism of noble metal doped carbon nanofibers to H2, Reprinted with permission from Ref. [115] Copyright 2020, The American Chemical Society.
In addition to the noble metal modification, constructing heterojunctions with 2D materials, metal oxides, and other carbon materials was one facile strategy to improve the sensing performance of carbon nanofibers. Cha et al. utilized one 2D WS2 to functionalize CNFs for highly sensitive detection of NO2 at room temperature [117]. The WS2-CNFs were prepared via copolymer-electrospinning using two immiscible polymers, thereinto poly(styrene-acrylonitrile) (SAN) serving as sacrificial templates and poly(acrylonitrile) (PAN) serving as the carbon matrix, which enabled the creation of hierarchically porous CNFs with axial tubular channels, offering a high specific surface area (41.4 m2/g) and enhanced gas permeability. Moreover, monolayered WS2 nanoflakes (thickness ~0.3 nm) were uniformly embedded with edge-rich configurations on the CNF surface, leveraging their high d-orbital electron density to strengthen NO2 adsorption. The WS2-CNFs exhibited a notable response (15% at 1 ppm NO2) and excellent selectivity, attributed to synergistic effects between the WS2 edge sites and the porous conductive network. Wang et al. prepared an MoS2-carbon nanofibers network sensor by electrospinning and followed thermal treatment [118]. The MoS2-CNFs sensors exhibited a high response of 5.82% toward methane, which was 16 times higher than that of the pure CNFs. The introduction of n-type MoS2 nanoparticles not only increased the specific surface of the sensing layer and active sites, but also formed the heterojunction between carbon nanofibers, facilitating charge transfer between MoS2 and carbon nanofibers.
As for the modification of metal oxides, Lee et al. prepared carbon nanofibers decorated with WO3 using a single co-electrospinning process [119]. By leveraging phase-separated polymer solutions (PAN as the carbon matrix and PVP as the sacrificial template), the diameter of carbon nanofibers was controlled precisely over 40–130 nm, exhibiting a high specific surface area of 147–276 m2/g. The WO3 nanonodules (12–20 nm) were uniformly distributed on the CNF surface, forming p-n junctions which facilitated charge transfer upon NO2 adsorption, leading to a high response of 36% to 1 ppm of NO2. In addition, UV irradiation (75 mW cm−2) was employed to reduce recovery time from a few days to just 7 min. Similarly, Tang et al. prepared one flexible NH3 sensor based on ZnO nanoparticles decorated carbon nanofibers by electrospinning, peroxidation, oxidation, and carbonization [120]. The response of ZnO-CNFs toward NH3 of 50 ppm was 12.3%, which was ten times higher than that of pure CNFs. However, the NH3-sensing performance of ZnO-CNFs dropped significantly when the humidity exceeded 50%RH, indicating the poor humidity-resistant property.
Lee et al. report one synergistic strategy to improve the gas-sensing performance of carbon fibers in the aspect of pore structure, conductivity, and surface modification [55]. In this study, electrospun carbon nanofibers derived from polyacrylonitrile (PAN) were integrated with carbon black (CB) additives to enhance electrical conductivity, forming an internal conductive network critical for efficient electron transfer. Subsequent chemical activation using potassium hydroxide (KOH) generated a hierarchical porous structure, amplifying the specific surface area by approximately 100-fold (up to 2200 m2/g) and optimizing gas adsorption capacity. Surface fluorination further introduced electronegative functional groups (C-F bonds), which enhanced the selective attraction of polar gas molecules such as NO and CO. The synergistic effects of the modifications, including CB-enhanced conductivity, KOH-induced micro/mesopores, and fluorination-driven surface reactivity, synergistically improved sensor sensitivity by fivefold. Notably, the prepared sensors exhibited twice the sensitivity to NO compared to CO, attributed to its stronger electron localization and reactivity. This work underscored the potential of combining conductive additives, pore engineering, and surface functionalization to develop high-performance carbon-based sensors for environmental monitoring. To achieve a balance between high specific surface area and robust mechanical stability in carbon nanofibers, the mechanical stability can be effectively enhanced by optimizing stabilization and carbonization temperatures [121,122], as well as incorporating nanomaterials (such as carbon nanotubes and graphene nanosheets [123,124]) for precursor optimization.
The above modification strategy for the carbon nanofibers promoted the response significantly at room temperature, as shown in Table 2. It is worth noting that the carbon nanofiber-based sensors still suffered from the sluggish adsorption/desorption kinetics at room temperature, always taking considerable minutes to reach a steady state, which was significantly inferior to that of metal oxide gas sensors. Hence, developing more advanced research methodologies is critical to shorten response/recovery times of carbon nanofibers. Meanwhile, carbon nanofibers as hydrophilic materials, humidity would affect the gas-sensing performance of carbon nanofibers [125]. Shooshtari et al. reported the effect of humidity on the organic gases sensing performance of carbon nanotubes at room temperature [126]. The response to target gas decreased with the humidity increasing from 10% to 80%RH, indicating the unreliability of carbon nanofibers sensors under various humid environments.

Table 2.
Gas-sensing performance of electrospinning carbon nanofibers gas sensors.
Table 2.
Gas-sensing performance of electrospinning carbon nanofibers gas sensors.
| Material | Gas | S | OT | tres/trec | LOD | SF/SN | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Metal doped | Au/Pt-CNFs | H2 (4%) | 47% b | RT | 6.6/18 s | 100 ppm | - | [115] |
| Fe-CNFs | H2 (500 ppm) | 0.35% b | RT | 193/97 s | 50 ppm | - | [127] | |
| Ni/Pt-CNFs | H2 (100 ppm) | 13% b | RT | 32/72 s | 100 ppm | - | [116] | |
| Heterojunction | ZnO-CNFs | NH3 (50 ppm) | 12.3% b | RT | 5/18 s | 10 ppm | 10 | [120] |
| WO3-CNFs | NO2 (20 ppm) | 7.5% b | RT | -/600 s | 1 ppm | - | [119] | |
| WS2-CNFs | NO2 (1 ppm) | 15% b | RT | 223.8 s/- | 0.2 ppm | 1.5 | [117] | |
| MoS2-CNFs | CH4 (250 ppm) | 5.82% b | RT | -/- | 10 ppm | 16 | [118] | |
| CB -CNFs | NO (50 ppm) | 10% b | RT | -/- | - | 5 | [55] | |
| fluorinated MWCNTs-CNFs | NO (50 ppm) | 8% b | RT | -/- | - | 8 | [128] | |
b S = (Ra − Rg)/Ra × 100% or (Rg − Ra)/Rg × 100%.
6. Gas Sensors Based on Conductive Polymer (CP) Nanofibers
The tendency for agglomeration and non-uniform distribution during the synthesis of conductive polymers significantly compromises the accessibility of adsorption sites, thereby diminishing gas-sensing capabilities [129]. Moreover, their susceptibility to ambient degradation, unsatisfactory repeatability, and weak selectivity pose significant challenges for practical application [130]. To address limitations, advanced electrospinning engineering strategies have been pursued.
Recently, composites of conducting polymers (CPs)-metal oxide for gas sensing have attracted considerable attention because the synergic effects of the CPs and metal oxides could significantly improve the gas-sensing performance compared with the pure CPs. Sharma et al. reported a highly sensitive H2 gas sensor based on SnO2-PANI composite nanofibers [131]. SnO2 nanofibers were initially synthesized by the electrospinning method as a framework. And then the SnO2-PANI nanocomposites were prepared by chemical oxidative polymerization of aniline on the substrate bearing SnO2 fibers. The SnO2-PANI sensor showed similar sensing behavior to pure PANI sensor, with a high response of 180 toward 5000 ppm H2 and a fast response time (<30 s) at room temperature. When the composites are exposed to H2, gas molecules would react with nitrogen atoms at imine sites of doped PANI, and these hydrogen molecules might form a bridge between nitrogen atoms of two polymer chains, which could enhance the conductivity of the sensing layer and thus improve the response. Beniwal et al. proposed SnO2-PPy nanocomposite for ultra-low ammonia concentration detection at room temperature [132]. Porous SnO2 nanofibers synthesized by electrospinning have a diameter in 70–150 nm, followed by vapor phase polymerization of pyrrole to develop SnO2-PPy composites The SnO2-PPy sensor exhibited a high response of 57% toward 100 ppb NH3 with fast response/recovery speed of 18/30 s at room temperature. Under ammonia exposure, the NH3 molecules would interact with the PPy and deprotonate the PPy sheath by decreasing the conducting charge carriers, leading to a decrease in conductance and thus an enhancement in response. Hong et al. reported a highly sensitive room-temperature ammonia (NH3) gas sensor based on a ternary composite of polyaniline (PANI), nitrogen-doped graphene quantum dots (N-GQDs), and hollow indium oxide (In2O3) nanofibers [133]. In their work, In2O3 nanofibers prepared by electrospinning and high-temperature calcination were coated with N-GQDs by hydrothermal reaction. Then, the N-GQD-coated hollow In2O3 nanofibers served as a core for the synthesis of polyaniline (PANI)/NGQD/ hollow In2O3 nanofiber ternary composites using in situ chemical oxidative polymerization (Figure 9a). The composite leverages the synergistic effects of high conductivity of PANI, catalytic activity of N-GQD, and the hollow nanostructure of In2O3 to achieve a remarkable response (Rg/Ra = 15.2 @ 1 ppm NH3), and 4.4 times higher than that of pristine PANI (Figure 9b). The p-n heterojunction between PANI and N-GQD/In2O3 enhanced electron depletion layer dynamics (Figure 9c,d), while the hollow structure and N-doping increased specific surface area (102.1 m2/g) and active adsorption sites. Moreover, the composite sensor exhibited excellent selectivity to NH3 over interferents (methanol, ethanol), repeatability, fast response speed (<90 s), and a linear response across 0.6–2.0 ppm with a sensitivity of 23.95%/ppm (R2 = 0.994).
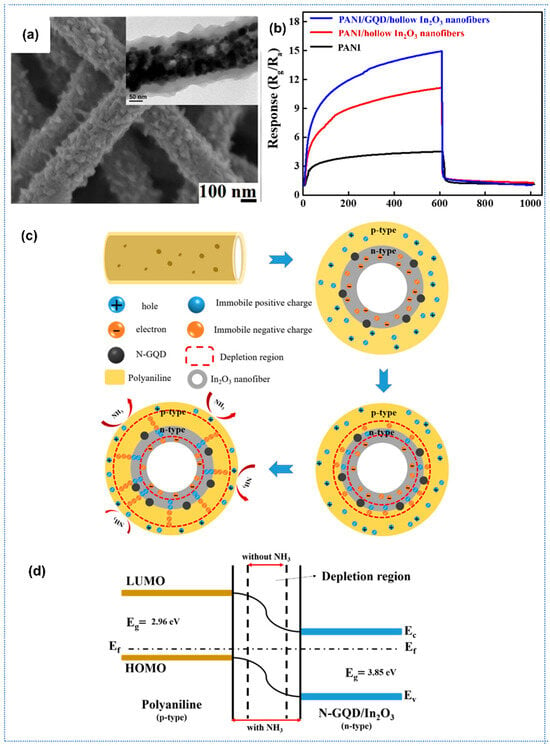
Figure 9.
(a) SEM and TEM images of In2O3-PANI nanofibers, (b) response curves of PANI, In2O3-PANI, and N-GQD/In2O3-PANI composites, (c) schematic illustrations of the sensing mechanism and (d) energy band structure diagram of N-GQD/In2O3-PANI composites, Reprinted with permission from Ref. [133] Copyright 2021, MDPI.
In addition to metal oxides, the introduction of noble metals, carbon-based materials, or ion doping is also an effective method to enhance the performance of polymer gas sensors. Han et al. proposed a methanol gas sensor based on the electrospun polymer/carbon nanotubes, which were prepared by mixing the poly (methyl methacrylate) and SWCNTs in solution with DMF and then using electrospinning technology [134]. The diameter of composite nanofibers ranged from 200 nm to 500 nm. The incorporation of carbon nanotubes improved the electrical percolation conductivity of polymers. When the polymer swelled due to methanol adsorption, the carbon nanotubes separated from each other and thus increased the resistance of the sensing layer, leading to an enhanced response. Huang et al. prepared magnesium-ion linked binder-less PEDOT:PSS nanofibers for highly sensitive detection toward organic gases dimethyl-formamide (DMF), dimethyl sulfoxide (DMSO), and propylene carbonate (PC) [135]. The binder-free PEDOT:PSS nanofibers were synthesized via electrospinning by introducing magnesium nitrate as a physical cross-linker, enabling controlled entanglement of rigid PEDOT:PSS chains without relying on traditional carrier polymers. The optimized Mg2+/PEDOT:PSS ratio (0.1) facilitated uniform nanofiber formation with diameters of 70–100 nm. XPS analysis demonstrated preferential migration of PEDOT to the fiber surface during electrospinning, creating a higher active surface area and enhanced porosity compared to bulk films. Impressively, the electrospun nanofibers exhibited superior sensing performance for DMF, DMSO, and propylene carbonate vapors at room temperature, achieving response magnitudes 2–3 orders of magnitude greater than pristine PEDOT:PSS films. The nice selectivity to DMF was attributed to its close solubility parameter match with PEDOT (23.54 vs. 24.7 MPa1/2), enabling efficient analyte-polymer interactions. The facile strategy for fabricating high-performance organic gas sensors through synergistic optimization of material morphology and interfacial chemistry offered promising avenues for non-invasive environmental monitoring and wearable biomedical applications. Saeb Mousavi et al. developed a flexible hydrogen sulfide (H2S) gas sensor based on electrospun polyaniline-polyethylene oxide (PANI-PEO) nanofibers doped with camphorsulfonic acid (HCSA), achieving room-temperature detection with high sensitivity, rapid response/recovery, and excellent stability [56]. The sensor, fabricated via a scalable electrospinning technique directly onto paper and polyimide substrates, exhibited a detection limit of 1 ppm H2S with response/recovery times of 120/250 s. The HCSA-doped PANI-PEO composite demonstrated superior selectivity toward H2S against interfering gases (NO2, acetone), while long-term stability tests revealed 4.7% sensitivity degradation over 45 days. In addition, mechanical bending tests confirmed the excellent robustness, with <1% performance loss after 1000 cycles. Pang et al. proposed an NH3 gas sensors based on Ag-PAN-PANI nanofibers [136]. The PAN nanofibers were prepared by electrospinning, serving as templates, and then Ag/APNI were synthesized by in situ polymerization method assisted with light irradiation and grew on the surface of PAN nanofibers. The Ag-PAN-PANI sensor showed a low limit of detection of 200 ppb NH3 at room temperature with high response, short response/recovery time, and excellent repeatability. It should be noted that lots of Ag nanoparticles in composites would form a separate current channel, and the charge transferred to Ag would not affect the resistance of PANI of sensors significantly, leading to a decrease in sensors. In addition, it should be noted that the response of Ag-PAN-PANI sensor toward NH3 decreased with the increase of humidity from 11 to 97%. Moreover, the Ag-PAN-PANI sensor exhibited significant response degradation of 36% in 15 days, indicating that the long-term stability should be enhanced.
It could be found that the above method could promote the sensing performance of polymers nanofibers (Table 3), including ultralow limit detection, enhanced response, and selectivity. However, the polymer nanofibers were vulnerable to ambient humidity due to the existence of the proton effect, swelling effects, and the competitive target gas and H2O molecules, leading to a reduction in response [137]. Moreover, the sensitivity shift in the sensor based on polymers during long-term applications should be considered. Regarding calibration of sensors, calibrated transfer strategy and classifier integration could offset drift efficiently [69]. In addition, accelerating life test, including increasing operation temperature and humidity, promoting the adsorption/desorption cycle frequency, would evaluate the sensor’s reliability [138]. Moreover, novel encapsulation strategy, such as utilizing polyvinylidene difluoride (PVDF) [139] or MOF-membrane as gas barrier layers [140], would improve the long-term stability of sensors.

Table 3.
Gas-sensing performance of electrospinning polymer nanofibers gas sensors.
Table 3.
Gas-sensing performance of electrospinning polymer nanofibers gas sensors.
| Material | Gas | S | OT | tres/trec | LOD | SF/SN | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Heterojunction | SnO2-PPy | NH3 (0.1 ppm) | 57% b | RT | 18/30 s | 0.1 ppm | - | [132] |
| SnO2-PANI | H2 (5000 ppm) | 1.35 a | RT | 30 s/- | 1000 ppm | - | [131] | |
| TiO2-PANI | NH3 (0.2 ppb) | 1.8% b | RT | -/- | 50 ppt | 1000 | [141] | |
| TiO2-PANI | NH3 (50 ppb) | 0.52 b | 22 °C | -/- | 25 ppb | - | [142] | |
| NiFe2O4-PANI | NH3 (100 ppm) | 30.8 b | RT | 15/21 s | 250 ppb | 3 | [143] | |
| N-GQD/In2O3-PANI | NH3 (1 ppm) | 15.6% b | RT | -/- | 0.6 ppm | 4.4 | [133] | |
| MoS2/SnO2-PANI | NH3 (100 ppm) | 10.9 a | RT | 21/130 s | 200 ppb | 3.1 | [144] | |
| rGO/SnO2-PANI | H2S (0.1 ppm) | 9.1 a | RT | 81/78 s | 50 ppb | 2 | [145] | |
| Ag/SnO2-PANI | H2 (1000 ppm) | 2.2 a | 40 °C | 16/24 s | 500 ppm | [146] | ||
| Ions-doping | Mg-PEDOT:PSS | DMF (200 ppm) | 1.12 a | RT | -/- | 50 ppm | - | [135] |
| HCSA-doped PANI-PEO | H2S (1 ppm) | 5% b | RT | -/- | 1 ppm | - | [56] | |
| Noble metal doped | Ag-PAN-PANI | NH3 (100 ppm) | 15 a | RT | -/- | 0.4 ppm | 4.8 | [136] |
a S = Ra/Rg or Rg/Ra, b S = (Ra − Rg)/Ra × 100% or (Rg − Ra)/Rg × 100%.
7. Summary and Future Perspectives
Electrospinning technology offers a transformative method in the synthesis of gas-sensitive materials. This review initially introduced the prepared method, gas-sensing mechanism of electrospinning materials, including metal oxides, carbon, and polymer nanofibers. This review subsequently addressed the inherent limitations of pure nanofiber-based gas sensors, and summarized recent advancements in enhancing sensing performance through strategic structural modifications of nanofibers, including noble metal functionalization, heterojunction construction, and controlled ion doping. The functional strategies exhibited significant enhancement effects on the gas-sensing performance of electrospinning nanofibers. Corresponding enhancement mechanisms of composite nanofibers were also discussed in this review. The electrospinning composite nanofibers exhibited considerable promise in the development of advanced gas sensors with highly sensitive, low-energy consumption, and rapid response. Furthermore, the metal oxides nanofibers presented high sensitivity and rapid response/recovery speed, and nice stability. However, gas–solid reactions demand substantial activation energy and mandate oxygen involvement. Carbon and conductive polymers nanofibers gas sensors could react with the target gas without the involvement of oxygen at room temperature, leading to the great potential of application. Whereas, the reaction kinetics were weaker than that of metal oxides, resulting in long response/recovery times. In addition, the hydrophilicity of carbon nanofibers and the proton effect and swelling effects of conductive polymers would compromise their gas-sensing performance. Following sections would discuss some critical challenges frustrated their further applications in detail:
(1) Precise synthesis and scale-up production of electrospinning nanofibers remain inevitable obstacles. On one hand, electrospinning is susceptible to voltage, temperature, and humidity fluctuations, resulting in excessively broad fiber diameter distributions that compromise gas diffusion path consistency. On the other hand, the functional additives strategy, particularly for noble metal, suffers from particle agglomeration within fibers, impairing the exposure of active sites. To mitigate batch-to-batch variability in electrospun nanofibers, characterized by fluctuations in fiber diameter, morphology, and porosity that critically compromise sensor consistency, a systematic approach integrating solution standardization, closed-loop process control, scalability management, and performance correlation is essential [147,148]. Solution properties must be rigorously standardized by controlling viscosity and conductivity via real-time monitoring, optimizing solvent evaporation through binary systems, and adjusting surface tension to ensure stable Taylor cone formation. In addition, closed-loop process control requires dynamic voltage compensation scaled to needle density in multi-needle systems, strict environmental stabilization to balance solvent retention and polymer integrity, and adaptive collector distance adjustments based on real-time fiber diameter feedback to maintain optimal jet stretching. Moreover, scalability challenges could be addressed by adopting needleless methods or asymmetric needle layouts to minimize field interference, alongside in-line quality control using laser diffractometry and machine learning for predictive adjustment [147].
(2) The operation temperature is still too high for the metal oxide-based nanofibers. Metal oxides were the most widely investigated electrospinning gas-sensitive materials. And the operation temperature of sensors was successfully reduced to a certain extent by noble metal doping, constructing heterojunctions with 2D materials or metal oxides, and element doping. However, the operation temperatures of metal oxide-based sensors typically exceed 200 °C, as shown in Table 1. Hence, it is necessary to further lower the operating temperatures of metal oxide nanofibers gas sensors for excellent stability and less power consumption. To address this challenge, in addition to the surface engineering on gas sensitive materials, introducing external light irradiation or plasmonic heating on materials are effective strategy [149,150]. Nikfarjam et al. utilized UV irradiation to promote the H2 sensing performance of TiO2 nanofibers [151]. Under UV irradiation, the response was enhanced 10 times, reducing the response and recovery times by a factor of 3–6. Furthermore, the operation temperature was reduced from 290 °C to 170 °C. The promotion of gas-sensing performance under UV could be attributed to the reduction in activation energy between the TiO2 surface and H2. Moreover, the synergistic effect of light irradiation and Au plasmonic nanostructure could promote the sensing performance of sensors. Gogurla et al. proposed Au-ZnO plasmonic nanostructure for highly sensitive detection toward NO at room temperature [152]. The Au-ZnO exhibited a localized surface plasma resonance (LSPR), leading to strong absorption, scattering, and local field enhancement. Hence, the sensitivity was significantly enhanced due to the LSPR effect of Au nanoparticles under visible light illumination at room temperature.
(3) An anti-humidity strategy should be considered in the electrospinning nanofibers preparation. For metal oxide nanofibers, the doping of Ni and Cu would facilitate the adsorption of H2O on the sensitive layer, and thus decrease the active sites for target gases with reduced sensitivity. Moreover, carbon nanofibers as hydrophilic materials and humidity would affect the gas-sensing performance of carbon nanofibers. Regarding polymer nanofibers, such as PANI and PPy, proton effect and swelling effects would affect the reaction between the sensitive layer and target gases, thus resulting in the degradation of sensitivity. Recent reports proposed effective anti-humidity methods for gas-sensitive materials, including surface engineering (noble metal doping [153], element doping [154], modification with hydrophobic materials [155]), physical isolation [156], working parameters modulation [157], humidity compensation [158]. Hence, introducing a novel anti-humidity strategy in electrospinning nanofibers is one bright way for further application.
(4) The selectivity of electrospinning nanofibers merits further intensive investigation. The sensitivity enhancement has been achieved by the introduction of additives and adjusting the architectures in the above reports. However, the discrimination capability for target gases continues to demand enhancement, particularly in multi-component systems where competitive adsorption effects significantly compromise detection specificity. And more selectivity enhancement mechanisms need to be proposed. Moreover, integrating a sensor array with artificial intelligence algorithms is a promising strategy for detecting the target gas in mixed gases [159]. Pattern recognition methods including principal component analysis (PCA) [160], linear discriminant analysis (LDA) [161], fast Fourier transform (FFT) [162], discrete wavelet transform (DWT) [160], and artificial neural networks (ANN) [163] have been employed to detect target gas in mixture gases. Hence, the integration of functionalization strategies for sensitive materials with pattern recognition algorithms represents a promising strategy to enhance the selectivity of electrospun fiber-based sensors.
(5) The wide application of gas sensors based on electrospun fibers still faced the challenge of cost, regulatory hurdles, and compatibility with IoT platforms. Continuous optimization of functionalization strategies for sensing materials can effectively reduce sensor costs. For instance, when using noble metal-modified nanofibers, the adoption of single-atom catalysts significantly reduces noble metal loading, thereby lowering material costs [164]. Additionally, prioritizing environmentally benign solvents and non-toxic reagents during material synthesis ensures compliance with health and environmental regulations. Furthermore, implementing self-calibration models in IoT deployments counteracts temperature drift during field operation.
Author Contributions
Conceptualization, Y.W. and Y.Z.; investigation, Y.W.; writing—original draft, preparation, Y.W.; writing—review and editing, Z.L. and Y.W.; visualization, K.Z., J.W. and J.H.; supervision, Y.Z.; project administration, X.C.; funding acquisition, Y.W. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental and Frontier Research Project of Chongqing (Grant No. cstc2019jcyj-msxmX0037), the National Natural Science Foundation of China (Grant No. 61704014), and the China Postdoctoral Science Foundation (Grant No. 2025M773617).
Conflicts of Interest
The authors declare no competing financial interest.
References
- Kim, K.; Park, J.K.; Lee, J.; Kwon, Y.J.; Choi, H.; Yang, S.-M.; Lee, J.-H.; Jeong, Y.K. Synergistic approach to simultaneously improve response and humidity-independence of metal-oxide gas sensors. J. Hazard. Mater. 2022, 424, 127524. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Dong, D.; Yao, G.; Wei, G.; He, A.; Wu, H.; Zhu, H.; Huang, Z.; Tang, Z. E-nose based on a high-integrated and low-power metal oxide gas sensor array. Sens. Actuators B Chem. 2023, 380, 133289. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, C.; Qu, G.; Pan, K.; Qin, J.; Wei, K.; Liang, Y. Modification strategies for semiconductor metal oxide nanomaterials applied to chemiresistive NOx gas sensors: A review. Talanta 2024, 273, 125853. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.-X.; Liu, M.-Y.; Zhu, L.-Y.; Zhang, D.W.; Lu, H.-L. Recent progress on flexible room-temperature gas sensors based on metal oxide semiconductor. Nano-Micro Lett. 2022, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, Y.; Liu, H. Room-temperature semiconductor gas sensors: Challenges and opportunities. ACS Sens. 2022, 7, 3582–3597. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Yu, J. Semiconductor gas sensor for triethylamine detection. Small 2021, 18, 2104984. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Singh, A.; Sikarwar, S.; Verma, A.; Chandra Yadav, B. The recent development of metal oxide heterostructures based gas sensor, their future opportunities and challenges: A review. Sens. Actuators A Phys. 2021, 332, 113127. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Amini, A. Metal oxide-based gas sensors for the detection of exhaled breath markers. Med. Devices Sens. 2021, 4, e10161. [Google Scholar] [CrossRef]
- Yang, D.; Gopal, R.A.; Lkhagvaa, T.; Choi, D. Metal-oxide gas sensors for exhaled-breath analysis: A review. Meas. Sci. Technol. 2021, 32, 102004. [Google Scholar] [CrossRef]
- Lawaniya, S.D.; Kumar, S.; Yu, Y.; Rubahn, H.-G.; Mishra, Y.K.; Awasthi, K. Functional nanomaterials in flexible gas sensors: Recent progress and future prospects. Mater. Today Chem. 2023, 29, 101428. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting polymers as chemiresistive gas sensing materials: A review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K.; Penza, M. Receptor function and response of semiconductor gas sensor. J. Sens. 2009, 2009, 875704. [Google Scholar] [CrossRef]
- Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Correa, D.S. Electrospun ceramic nanofibers and hybrid-nanofiber composites for gas sensing. ACS Appl. Nano Mater. 2019, 2, 4026–4042. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T.T. 1D metal oxide semiconductor materials for chemiresistive gas sensors: A review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Cho, N.G.; Yang, D.J.; Jin, M.-J.; Kim, H.-G.; Tuller, H.L.; Kim, I.-D. Highly sensitive SnO2 hollow nanofiber-based NO2 gas sensors. Sens. Actuators B Chem. 2011, 160, 1468–1472. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Choong, T.S.Y.; Rashid, U. Recent progress in the design and synthesis of nanofibers with diverse synthetic methodologies: Characterization and potential applications. New J. Chem. 2020, 44, 9581–9606. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Morais, P.V.; Suman, P.H.; Silva, R.A.; Orlandi, M.O. High gas sensor performance of WO3 nanofibers prepared by electrospinning. J. Alloys Compd. 2021, 864, 158745. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. MGas sensors based on one dimensional nanostructured metal-oxides: A review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.; Verma, A.S.; Kumar, T. Review—Recent advances and challenges of conducting polymer-metal nanocomposites for the detection of industrial waste gases. ECS J. Solid State Sci. Technol. 2023, 12, 047002. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef]
- Bondavalli, P.; Legagneux, P.; Pribat, D. Carbon nanotubes based transistors as gas sensors: State of the art and critical review. Sens. Actuators B Chem. 2009, 140, 304–318. [Google Scholar] [CrossRef]
- Li, G.; Sun, Z.; Zhang, D.; Xu, Q.; Meng, L.; Qin, Y. Mechanism of Sensitivity Enhancement of a ZnO Nanofilm Gas Sensor by UV Light Illumination. ACS Sens. 2019, 4, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Bannov, A.G.; Jašek, O.; Manakhov, A.; Márik, M.; Nečas, D.; Zajíčková, L. High-performance ammonia gas sensors based on plasma treated carbon nanostructures. IEEE Sens. J. 2017, 17, 1964–1970. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Wang, J.; Zhuang, R.; Mu, P.; Wang, J.; Yan, W. Advances in functional guest materials for resistive gas sensors. RSC Adv. 2022, 12, 24614–24632. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yang, Z.; Hu, N. Review of recent progress on graphene-based composite gas sensors. Ceram. Int. 2021, 47, 16367–16384. [Google Scholar] [CrossRef]
- Gong, C.; Chen, M.; Song, F.; Yin, P.; Zhao, X.; You, X.; Fu, H.; Yu, S.; Liu, X.; Zhang, K.; et al. A highly sensitive toluene gas sensor based on Pd/PdO decorated SnO2 prepared by electrospinning. ACS Appl. Electron. Mater. 2024, 6, 6036–6048. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, C.; Hu, Y.; Zhang, H.; Shao, X.; Ding, Z.; Zhang, D. High-performance SO2 gas sensor based on MXene/LaFeO3 nanotubes by electrospinning technology. J. Mater. Sci. Mater. Electron. 2024, 35, 1309. [Google Scholar] [CrossRef]
- Lai, T.-Y.; Fang, T.-H.; Hsiao, Y.-J.; Chan, C.-A. Characteristics of Au-doped SnO2–ZnO heteronanostructures for gas sensing applications. Vacuum 2019, 166, 155–161. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal Oxide Based Heterojunctions for Gas Sensors: A Review. Nanomaterials 2021, 11, 1026. [Google Scholar] [CrossRef]
- Khomarloo, N.; Mohsenzadeh, E.; Gidik, H.; Bagherzadeh, R.; Latifi, M. Overall perspective of electrospun semiconductor metal oxides as high-performance gas sensor materials for NOx detection. RSC Adv. 2024, 14, 7806–7824. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Si, Y.; Shi, S.; Hu, J. Electrospinning and electrospraying synergism: Twins-tech collaboration across dimensions. Matter 2024, 7, 1373–1405. [Google Scholar] [CrossRef]
- Kang, X.; Yu, H.; Ma, X.; Shang, E.; Chen, H.; Zhao, H.; Zhang, J.; Tan, R.; Wang, D.; Fang, H. Fast detection of NO2 by In2O3@ZnO nanofibers synthesized by coaxial electrospinning: Real-time monitoring application of smart mask. Chem. Eng. J. 2025, 504, 158872. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on the metal oxide based gas sensing mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z.; Ahmad, M.; Enzevaee, A.; Yousof, R.; Iqbal, S.; Ahmadi, M.; Sidik, M.; Karimi, H. Analytical calculation of sensing parameters on carbon nanotube based gas sensors. Sensors 2014, 14, 5502–5515. [Google Scholar] [CrossRef]
- Khomarloo, N.; Mohsenzadeh, E.; Bagherzadeh, R.; Latifi, M.; Lahem, D.; Hakgor, A.; Ahmadou, L.; Gidik, H. Development of NOx Gas Sensor Based on Electrospun ZnO Nanofibers for Diagnosing Asthma Disease. Proceedings 2024, 97, 30. [Google Scholar] [CrossRef]
- Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and hollow carbon nanofibers by coaxial electrospinning of alcell lignin without binder polymers. Adv. Mater. 2007, 19, 4292–4296. [Google Scholar] [CrossRef]
- Pawłowska, S.; Rinoldi, C.; Nakielski, P.; Ziai, Y.; Urbanek, O.; Li, X.; Kowalewski, T.A.; Ding, B.; Pierini, F. Ultraviolet light-assisted electrospinning of core–shell fully cross-linked P(NIPAAm-co-NIPMAAm) hydrogel-based nanofibers for thermally induced drug delivery self-regulation. Adv. Mater. Interfaces 2020, 7, 2000247. [Google Scholar] [CrossRef]
- Li, T.; Qu, M.; Carlos, C.; Gu, L.; Jin, F.; Yuan, T.; Wu, X.; Xiao, J.; Wang, T.; Dong, W.; et al. High-performance poly(vinylidene difluoride)/dopamine core/shell piezoelectric nanofiber and its application for biomedical sensors. Adv. Mater. 2021, 33, 2006093. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, X.; Xu, C.; Kotov, N.A. Water-rich biomimetic composites with abiotic self-organizing nanofiber network. Adv. Mater. 2017, 30, 1703343. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, T.-J.; Zhou, J.-H.; Sui, Z.-J.; Dai, Y.-C.; Yuan, W.-K. Characterization of carbon nanofiber composites synthesized by shaping process. Carbon 2005, 43, 2701–2710. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Cho, Y.; Beak, J.W.; Sagong, M.; Ahn, S.; Nam, J.S.; Kim, I.D. Electrospinning and nanofiber technology: Fundamentals, innovations, and applications. Adv. Mater. 2025, 37, 2500162. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research progress, models and simulation of electrospinning technology: A review. J. Mater. Sci. 2021, 57, 58–104. [Google Scholar] [CrossRef]
- Imash, A.; Smagulova, G.; Kaidar, B.; Keneshbekova, A.; Kazhdanbekov, R.; Velasco, L.F.; Mansurov, Z. Chemoresistive gas sensors based on electrospun 1D nanostructures: Synergizing morphology and performance optimization. Sensors 2024, 24, 6797. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Kim, J.J.; Tuller, H.L.; Rutledge, G.C.; Hashaikeh, R. Gas sensing behavior of electrospun nickel oxide nanofibers: Effect of morphology and microstructure. Sens. Actuators B Chem. 2016, 227, 54–64. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Li, J.; Miao, Z.; Huang, F. Fabrication and ethanol-sensing properties of micro gas sensor based on electrospun SnO2 nanofibers. Sens. Actuators B Chem. 2008, 132, 67–73. [Google Scholar] [CrossRef]
- Khomarloo, N.; Gidik, H.; Bagherzadeh, R.; Latifi, M.; Debliquy, M.; Ly, A.; Lahem, D.; Mohsenzadeh, E. Enhancing nitric oxide gas detection by tuning the structural dimension of electrospun ZnO nanofibers fibers and polymers. Fibers Polym. 2024, 26, 197–209. [Google Scholar] [CrossRef]
- Im, J.S.; Kang, S.C.; Lee, S.-H.; Lee, Y.-S. Improved gas sensing of electrospun carbon fibers based on pore structure, conductivity and surface modification. Carbon 2010, 48, 2573–2581. [Google Scholar] [CrossRef]
- Mousavi, S.; Kang, K.; Park, J.; Park, I. A room temperature hydrogen sulfide gas sensor based on electrospun polyaniline–polyethylene oxide nanofibers directly written on flexible substrates. RSC Adv. 2016, 6, 104131–104138. [Google Scholar] [CrossRef]
- Goel, N.; Kunal, K.; Kushwaha, A.; Kumar, M. Metal oxide semiconductors for gas sensing. Eng. Rep. 2022, 5, e12604. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Akbar, S.; Mulla, I.S.; Suryavanshi, S.S.; Bhandari, N.L.; Chaskar, M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023, 58, 559–582. [Google Scholar] [CrossRef]
- Gao, X.; Li, F.; Wang, R.; Zhang, T. A formaldehyde sensor: Significant role of p-n heterojunction in gas-sensitive core-shell nanofibers. Sens. Actuators B Chem. 2018, 258, 1230–1241. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Aydas, B.; Atılgan, A.; Ajjaq, A.; Acar, S.; Öktem, M.F.; Yildiz, A. Flexible NH3 gas sensors based on ZnO nanostructures deposited on kevlar substrates via hydrothermal method. Ceram. Int. 2024, 50 Pt A, 32477–32489. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Li, J.; Zhao, H.; Zhang, R.; Ou, Y.; Xie, L.; Yang, J.; Zou, C.; Zhou, Y. Black phosphorus nanosheet/tin oxide quantum dot heterostructures for highly sensitive and selective trace hydrogen sulfide sensing. ACS Appl. Nano Mater. 2023, 6, 4034–4045. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Jiang, X.; She, X.; Chen, Y.; Long, Y.; Zhou, Y. Deciphering the humidity resistance and oxygen-content independence of conductometrichydrogen sulfide sensors based on electrospun CeO2/CuO nanotubes. ACS Sens. 2025, 10, 4262–4275. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Li, W.; Zhang, J.; Xie, X. Chemiresistive gas sensor based on electrospun hollow SnO2 nanotubes for detecting NO at the ppb level. Vacuum 2022, 199, 110961. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Choi, J.-K.; Lee, J.-H. Design of a highly sensitive and selective C2H5OH sensor using p-type Co3O4 nanofibers. Sens. Actuators B Chem. 2012, 161, 570–577. [Google Scholar] [CrossRef]
- Acharyya, S.; Nag, S.; Kimbahune, S.; Ghose, A.; Pal, A.; Guha, P.K. Selective discrimination of VOCs applying gas sensing kinetic analysis over a metal oxide-based chemiresistive gas sensor. ACS Sens. 2021, 6, 2218–2224. [Google Scholar] [CrossRef]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of metal oxide semiconductor gas sensors: A review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar] [CrossRef]
- Isaac, N.A.; Pikaar, I.; Biskos, G. Metal oxide semiconducting nanomaterials for air quality gas sensors: Operating principles, performance, and synthesis techniques. Microchim. Acta 2022, 189, 196. [Google Scholar] [CrossRef]
- Shooshtari, M.; Vollebregt, S.; Vaseghi, Y.; Rajati, M.; Pahlavan, S. The sensitivity enhancement of TiO2-based VOCs sensor decorated by gold at room temperature. Nanotechnology 2023, 34, 255501. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrog. Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Qin, J.; Liu, W. Ultrafine Pt-doped SnO2 mesopore nanofibers-based gas sensor for enhanced acetone sensing. Chin. J. Anal. Chem. 2023, 51, 100188. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Li, F.; Lu, G.; Zhang, T.; Barsan, N. Pt-In2O3 mesoporous nanofibers with enhanced gas sensing performance towards ppb-level NO2 at room temperature. Sens. Actuators B Chem. 2018, 260, 927–936. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.J.; Kim, S.J.; Hakim, M.; Kim, I.D. Rational design of highly porous SnO2 nanotubes functionalized with biomimetic nanocatalysts for direct observation of simulated diabetes. Adv. Funct. Mater. 2016, 26, 4740–4748. [Google Scholar] [CrossRef]
- Choi, S.-J.; Chattopadhyay, S.; Kim, J.J.; Kim, S.-J.; Tuller, H.L.; Rutledge, G.C.; Kim, I.-D. Coaxial electrospinning of WO3 nanotubes functionalized with bio-inspired Pd catalysts and their superior hydrogen sensing performance. Nanoscale 2016, 8, 9159–9166. [Google Scholar] [CrossRef]
- Hung, C.M.; Phuong, H.V.; Van Thinh, V.; Hong, L.T.; Thang, N.T.; Hanh, N.H.; Dich, N.Q.; Van Duy, N.; Van Hieu, N.; Hoa, N.D. Au doped ZnO/SnO2 composite nanofibers for enhanced H2S gas sensing performance. Sens. Actuators A Phys. 2021, 317, 112454. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Xu, L.; Zhu, S.; Zhou, X.; Yang, S.; Dong, B.; Bai, X.; Lu, G.; Song, H. Understanding the noble metal modifying effect on In2O3 nanowires: Highly sensitive and selective gas sensors for potential early screening of multiple diseases. Nanoscale Horiz. 2019, 4, 1361–1371. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.-H.; Shin, H.; Cho, H.-J.; Koo, W.-T.; Choi, S.-J.; Park, C.; Ahn, J.; Güntner, A.T.; Penner, R.M.; et al. Selectivity in chemiresistive gas sensors: Strategies and challenges. Chem. Rev. 2025, 125, 4111–4183. [Google Scholar] [CrossRef]
- Sui, N.; Wei, X.; Cao, S.; Zhang, P.; Zhou, T.; Zhang, T. Nanoscale bimetallic AuPt-functionalized metal oxide chemiresistors: Ppb-level and selective detection for ozone and acetone. ACS Sens. 2022, 7, 2178–2187. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; She, X.; Chen, Y.; Wang, M.; Wang, Y.; Du, A.; Tang, C.; Zou, C.; Zhou, Y. Oxygen vacancy-rich bimetallic Au@Pt core–shell nanosphere-functionalized electrospun ZnFe2O4 nanofibers for chemiresistive breath acetone detection. ACS Sens. 2024, 9, 2183–2193. [Google Scholar] [CrossRef]
- Ma, S.; Xu, J. Nanostructured metal oxide heterojunctions for chemiresistive gas sensors. J. Mater. Chem. A 2023, 11, 23742–23771. [Google Scholar] [CrossRef]
- Dharanya, C.; Dharmalingam, G. Oxygen vacancies in nanostructured hetero-interfacial oxides: A review. J. Nanoparticle Res. 2022, 24, 60. [Google Scholar] [CrossRef]
- Park, K.-R.; Cho, H.-B.; Lee, J.; Song, Y.; Kim, W.-B.; Choa, Y.-H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Li, C.; Feng, C.; Qu, F.; Liu, J.; Zhu, L.; Lin, Y.; Wang, Y.; Li, F.; Zhou, J.; Ruan, S. Electrospun nanofibers of p-type NiO/n-type ZnO heterojunction with different NiO content and its influence on trimethylamine sensing properties. Sens. Actuators B Chem. 2015, 207, 90–96. [Google Scholar] [CrossRef]
- Naderi, H.; Hajati, S.; Ghaedi, M.; Espinos, J.P. Highly selective few-ppm NO gas-sensing based on necklace-like nanofibers of ZnO/CdO n-n type I heterojunction. Sens. Actuators B Chem. 2019, 297, 126774. [Google Scholar] [CrossRef]
- Fu, L.; Xu, J.; Liu, Q.; Liu, C.; Fan, S.; Ramakrishna, S.; Tang, W. Gas sensors based on Co3O4/TiO2 core-shell nanofibers prepared by coaxial electrospinning for breath marker acetone detection. Ceram. Int. 2024, 50, 3443–3452. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent advances in emerging 2D material-based gas sensors: Potential in disease diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, H.; Yan, C.; Yang, Z.; Zhu, G.; Gao, J.; Yin, F.; Wang, C. Fabrication of conductive graphene oxide-WO3 composite nanofibers by electrospinning and their enhanced acetone gas sensing properties. Sens. Actuators B Chem. 2018, 264, 128–138. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Chen, Z.; Li, H.; Chen, A.; Wang, X.; Yang, J. Enhanced formaldehyde sensing properties of hollow SnO2 nanofibers by graphene oxide. Sens. Actuators B Chem. 2017, 250, 533–542. [Google Scholar] [CrossRef]
- Yang, G.; Cao, C.; Zhong, H.; Cheng, Y.; Zhang, W.; Wang, D. Construction of SnO2 nanofibers @ MoS2 nanosheets core-shell nanocomposites for high efficiency xylene detection. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130813. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Wang, J.; Liu, J.; Yan, W. Hierarchical heterojunctions of metal sulfide WS2 nanosheets/metal oxide In2O3 nanofibers for an efficient detection of formaldehyde. Nanomaterials 2024, 14, 1702. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef]
- Wu, R.-A.; Wei Lin, C.; Tseng, W.J. Preparation of electrospun Cu-doped α-Fe2O3 semiconductor nanofibers for NO2 gas sensor. Ceram. Int. 2017, 43, S535–S540. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Liu, F.; Jia, J.; Li, X.; Cao, L. C2H2 gas sensor based on Ni-doped ZnO electrospun nanofibers. Ceram. Int. 2013, 39, 2883–2887. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Q.; Zeng, D.; Zhang, S.; Yang, L.; Xia, D.; Xiong, Z.; Xie, C. Al-doping induced formation of oxygen-vacancy for enhancing gas-sensing properties of SnO2 NTs by electrospinning. Sens. Actuators B Chem. 2014, 198, 62–69. [Google Scholar] [CrossRef]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B Chem. 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y. Recent progress on anti-humidity strategies of chemiresistive gas sensors. Materials 2022, 15, 8728. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Wang, W.; Li, G.; Li, P.; Lian, K.; Zhang, W.; Zhuiykov, S.; Hu, J.; Chen, L. Electrospinning preparation of Pd@Co3O4-ZnO composite nanofibers and their highly enhanced VOC sensing properties. Mater. Res. Bull. 2019, 109, 255–264. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhang, G.; Ma, S.; Lu, Y.; Bian, H.; Chen, Q. Highly sensitive VOCs-acetone sensor based on Ag-decorated SnO2 hollow nanofibers. J. Alloys Compd. 2017, 703, 572–579. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, X.; Shiu, B.-C.; Lou, C.-W.; Lin, J.-H.; Li, T.-T. Wearable smart yarn sensor based on ZnO/SnO2 heterojunction for ammonia detecting. J. Mater. Sci. 2022, 57, 21946–21959. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Z.; Zhao, C.; Cairang, L.; Bai, J.; Zhang, Y.; Mu, X.; Du, J.; Wang, H.; Pan, X.; et al. Enhanced gas-sensing performance of ZnO@In2O3 core@shell nanofibers prepared by coaxial electrospinning. Sens. Actuators B Chem. 2018, 255, 2248–2257. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Kim, J.-H.; Lee, J.H.; Lee, J.-S.; Kim, S.S. Importance of the nanograin size on the H2S-sensing properties of ZnO–CuO composite nanofibers. Sens. Actuators B Chem. 2015, 214, 111–116. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, G.; Li, S.; Wang, L.; Han, Y.; Jiang, X.; Wei, A. High toluene sensing properties of NiO–SnO2 composite nanofiber sensors operating at 330 °C. Sens. Actuators B Chem. 2011, 160, 448–454. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, H.W.; Kim, S.S. An ultra-sensitive hydrogen gas sensor using reduced graphene oxide-loaded ZnO nanofibers. Chem. Commun. 2015, 51, 15418–15421. [Google Scholar] [CrossRef]
- Andre, R.S.; Mercante, L.A.; Facure, M.H.M.; Mattoso, L.H.C.; Correa, D.S. Enhanced and selective ammonia detection using In2O3/reduced graphene oxide hybrid nanofibers. Appl. Surf. Sci. 2019, 473, 133–140. [Google Scholar] [CrossRef]
- Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Park, I.; Van Hieu, N. Excellent detection of H2S gas at ppb concentrations using ZnFe2O4 nanofibers loaded with reduced graphene oxide. Sens. Actuators B Chem. 2019, 282, 876–884. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Zhang, H.; Wang, Z.; Dong, B.; Jiang, T.; Wang, W.; Li, Z.; Wang, C. Effects of Al doping on SnO2 nanofibers in hydrogen sensor. Sens. Actuators B Chem. 2011, 160, 858–863. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.; Liu, S.; Song, J.; Chen, J.; Dong, B.; Song, H. NiO@ZnO heterostructured nanotubes: Coelectrospinning fabrication, characterization, and highly enhanced gas sensing properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef]
- Li, F.M.; Li, X.B.; Ma, S.Y.; Chen, L.; Li, W.Q.; Zhu, C.T.; Xu, X.L.; Chen, Y.; Li, Y.F.; Lawson, G. Influence of Ce doping on microstructure of ZnO nanoparticles and their acetone sensing properties. J. Alloys Compd. 2015, 649, 1136–1144. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Hou, P.-X.; Zhang, F.; Liu, C.; Cheng, H.-M. Gas sensors based on single-wall carbon nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef] [PubMed]
- Shooshtari, M. Ammonia gas sensors based on multi-wall carbon nanofiber field effect transistors by using gate modulation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135563. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Nair, K.G.; Ramakrishnan, V.; Unnathpadi, R.; Karuppanan, K.K.; Pullithadathil, B. Unraveling hydrogen adsorption kinetics of bimetallic Au–Pt nanoisland-functionalized carbon nanofibers for room-temperature gas sensor applications. J. Phys. Chem. C 2020, 124, 7144–7155. [Google Scholar] [CrossRef]
- Nair, K.G.; Vishnuraj, R.; Pullithadathil, B. Highly sensitive, flexible H2 gas sensors based on less platinum bimetallic Ni–Pt nanocatalyst-functionalized carbon nanofibers. ACS Appl. Electron. Mater. 2021, 3, 1621–1633. [Google Scholar] [CrossRef]
- Cha, J.-H.; Choi, S.-J.; Yu, S.; Kim, I.-D. 2D WS2-edge functionalized multi-channel carbon nanofibers: Effect of WS2 edge-abundant structure on room temperature NO2 sensing. J. Mater. Chem. A 2017, 5, 8725–8732. [Google Scholar] [CrossRef]
- Xu, C.; Du, X.; Yang, J.; Long, Y.; Wang, Y. A 3D MoS2/carbon nanofiber network for room temperature methane sensing. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October 2021–3 November 2021; pp. 1–4. [Google Scholar]
- Lee, J.S.; Kwon, O.S.; Shin, D.H.; Jang, J. WO3 nanonodule-decorated hybrid carbon nanofibers for NO2 gas sensor application. J. Mater. Chem. A 2013, 1, 9099. [Google Scholar] [CrossRef]
- Fan, S.X.; Tang, W. Synthesis, characterization and mechanism of electrospun carbon nanofibers decorated with ZnO nanoparticles for flexible ammonia gas sensors at room temperature. Sens. Actuators B Chem. 2022, 362, 131789. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Liu, X.; Yu, J.; Ding, B. Strategies in precursors and post treatments to strengthen carbon nanofibers. Adv. Fiber Mater. 2020, 2, 46–63. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.I.; Cho, A.E.; Kumar, S.; Jang, S.S.; Kim, Y.H. Origin and control of polyacrylonitrile alignments on carbon nanotubes and graphene nanoribbons. Adv. Funct. Mater. 2018, 28, 1706970. [Google Scholar] [CrossRef]
- Maitra, T.; Sharma, S.; Srivastava, A.; Cho, Y.-K.; Madou, M.; Sharma, A. Improved graphitization and electrical conductivity of suspended carbon nanofibers derived from carbon nanotube/polyacrylonitrile composites by directed electrospinning. Carbon 2012, 50, 1753–1761. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, T.; McCarthy, S.; Kozbial, A.; Tan, S.; Pekker, D.; Li, L.; Leu, P.W. Parahydrophobicity and stick-slip wetting dynamics of vertically aligned carbon nanotube forests. Carbon 2019, 152, 474–481. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of humidity on gas sensing performance of carbon nanotube gas sensors operated at room temperature. IEEE Sens. J. 2021, 21, 5763–5770. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.-S.; Wang, Q.; Yu, Y.; Chen, Z.; Cao, C.-Y.; Song, W.-G. Low-cost synthesis of graphitic carbon nanofibers as excellent room temperature sensors for explosive gases. J. Mater. Chem. 2012, 22, 15342–15347. [Google Scholar] [CrossRef]
- Kang, S.C.; Im, J.S.; Lee, S.-H.; Bae, T.-S.; Lee, Y.-S. High-sensitivity gas sensor using electrically conductive and porosity-developed carbon nanofiber. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 297–303. [Google Scholar] [CrossRef]
- Dong, R.; Yang, M.; Zuo, Y.; Liang, L.; Xing, H.; Duan, X.; Chen, S. Conducting polymers-based gas sensors: Principles, materials, and applications. Sensors 2025, 25, 2724. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Wang, Y.; Zhou, Y. Approaches for selectivity improvement of conductometric gas sensors: An overview. Sens. Diagn. 2024, 3, 336–353. [Google Scholar] [CrossRef]
- Sharma, H.J. H2 and CO gas sensor from SnO2/polyaniline composite nanofibers fabricated by electrospinning. Adv. Mater. Proc. 2017, 2, 61–66. [Google Scholar] [CrossRef]
- Beniwal, A.; Sunny. Electrospun SnO2/PPy nanocomposite for ultra-low ammonia concentration detection at room temperature. Sens. Actuators B Chem. 2019, 296, 126660. [Google Scholar] [CrossRef]
- Hong, S.-Z.; Huang, Q.-Y.; Wu, T.-M. The room temperature highly sensitive ammonia gas sensor based on polyaniline and nitrogen-doped graphene quantum dot-coated hollow indium oxide nanofiber composite. Polymers 2021, 13, 3676. [Google Scholar] [CrossRef]
- Han, L.; Andrady, A.L.; Ensor, D.S. Chemical sensing using electrospun polymer/carbon nanotube composite nanofibers with printed-on electrodes. Sens. Actuators B Chem. 2013, 186, 52–55. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lo, T.-Y.; Chen, C.-H.; Wu, K.-H.; Lin, C.-M.; Whang, W.-T. Electrospinning of magnesium-ion linked binder-less PEDOT:PSS nanofibers for sensing organic gases. Sens. Actuators B Chem. 2015, 216, 603–607. [Google Scholar] [CrossRef]
- Pang, Z.; Zhu, Y.; Li, X.; Chen, M.; Ge, M. Room temperature ammonia gas sensor based on polyacrylonitrile/silver@Polyaniline nanofibers. IEEE Sens. J. 2019, 19, 11021–11026. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Bose, J.M.; Yifan, G.; Sarihan, V.; Lee, T. Accelerated life testing for micro-machined chemical sensors. IEEE Trans. Reliab. 1998, 47, 135–141. [Google Scholar] [CrossRef]
- Lee, H.-K.; Yang, W.S.; Choi, N.-J.; Moon, S.E. Encapsulation of semiconductor gas sensors with gas barrier films for USN application. ETRI J. 2012, 34, 713–718. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Hu, Z.; Zhou, Z.; Deng, Y. Ultrasensitive NH3 gas sensor from polyaniline nanograin enchased TiO2 Fibers. J. Phys. Chem. C 2010, 114, 9970–9974. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; He, G.; Deng, Y. Fabrication of polyaniline/titanium dioxide composite nanofibers for gas sensing application. Mater. Chem. Phys. 2011, 129, 477–482. [Google Scholar] [CrossRef]
- Sonwane, N.D.; Kondawar, S.B. Electrospun nickel ferrite nanofibers reinforced polyaniline composite for high-performance room temperature ammonia sensing. Synth. Met. 2022, 284, 117004. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C.; et al. The gas sensor utilizing polyaniline/MoS2 nanosheets/SnO2 nanotubes for the room temperature detection of ammonia. Sens. Actuators B Chem. 2021, 332, 129444. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Kondawar, S.B.; More, A.M.; Sharma, H.J.; Dongre, S.P. Ag-SnO2/Polyaniline composite nanofibers for low operating temperature hydrogen gas sensor. Mater. Nanosci. 2017, 4, 13–18. [Google Scholar]
- Kannan, B.; Cha, H.; Hosie, I.C. Electrospinning—Commercial applications, challenges and opportunities. In Nano-Size Polymers: Preparation, Properties, Applications; Fakirov, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 309–342. [Google Scholar]
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Mishra, S.; Ghanshyam, C.; Ram, N.; Bajpai, R.P.; Bedi, R.K. Detection mechanism of metal oxide gas sensor under UV radiation. Sens. Actuators B Chem. 2004, 97, 387–390. [Google Scholar] [CrossRef]
- Proença, M.; Rodrigues, M.S.; Borges, J.; Vaz, F. Optimization of Au:CuO nanocomposite thin films for gas sensing with high-resolution localized surface plasmon resonance spectroscopy. Anal. Chem. 2020, 92, 4349–4356. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Salehifar, N. Improvement in gas-sensing properties of TiO2 nanofiber sensor by UV irradiation. Sens. Actuators B Chem. 2015, 211, 146–156. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of water vapor on Pd-loaded SnO2 nanoparticles gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Kim, J.S.; Kim, T.H.; Hong, Y.J.; Kang, Y.C.; Lee, J.H. A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, L.; Sheng, K.; Chen, C.; Zhou, X.; Dong, B.; Bai, X.; Zhang, S.; Lu, G.; Song, H. APTES-functionalized thin-walled porous WO3 nanotubes for highly selective sensing of NO2 in a polluted environment. J. Mater. Chem. A 2018, 6, 10976–10989. [Google Scholar] [CrossRef]
- Rathi, K.; Pal, K. Wireless hand-held device based on polylactic acid-protected, highly stable, CTAB-functionalized phosphorene for CO2 gas sensing. ACS Appl. Mater. Interfaces 2020, 12, 38365–38375. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Three-dimensional graphene hydrogel decorated with SnO2 for high-performance NO2 sensing with enhanced immunity to humidity. ACS Appl. Mater. Interfaces 2020, 12, 2634–2643. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Hua, Z.; Lu, N.; Sun, W.; Zhang, J.; Fan, S. Humidity compensation based on power-law response for MOS sensors to VOCs. Sens. Actuators B Chem. 2021, 334, 129601. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Distante, C.; Leo, M.; Persaud, K.C. Wavelet transform for electronic nose signal analysis. In Discrete Wavelet Transforms Biomedical Applications; Intech Open: Rijeka, Croatia, 2011; pp. 177–200. [Google Scholar]
- Itoh, T.; Koyama, Y.; Sakumura, Y.; Akamatsu, T.; Tsuruta, A.; Masuda, Y.; Shin, W. Discrimination of volatile organic compounds using a sensor array via a rapid method based on linear discriminant analysis. Sens. Actuators B Chem. 2023, 387, 133803. [Google Scholar] [CrossRef]
- Sears, W.M.; Colbow, K.; Slamka, R.; Consadori, F. Selective thermally cycled gas sensing using fast Fourier-transform techniques. Sens. Actuators B Chem. 1990, 2, 283–289. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, Y.; Hu, J.; Sun, Z.; Zhang, T.; Wang, M. Exploration for a BP-ANN model for gas identification and concentration measurement with an ultrasonically radiated catalytic combustion gas sensor. Sens. Actuators B Chem. 2022, 362, 131733. [Google Scholar] [CrossRef]
- Yuan, T.; Xue, Z.; Chen, Y.; Xu, J. Single Pt atom-based gas sensor: Break the detection limit and selectivity of acetone. Sens. Actuators B Chem. 2023, 397, 134139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).