Estimating Heart Rate from Inertial Sensors Embedded in Smart Eyewear: A Validation Study
Abstract
Highlights
- The “Essilor Connected Glasses”, using an IMU for heart rate estimation, showed high accuracy compared to ECG and smartwatch during controlled static activities.
- The quality index (QI) significantly impacted heart rate estimation accuracy, with higher QI values correlating with lower error rates.
- Smart eyewear with IMUs offers a viable, non-invasive alternative for opportunistic and unobtrusive heart rate monitoring during sedentary activities.
- QI thresholds can be used to optimize wearable HR monitoring systems for improved performance in different physiological states.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Population and Design
- A total of 5 min while standing;
- A total of 5 min in the sitting posture;
- A total of 3 min of paced breathing at a fixed breathing rate of 6 s per breath (10 breaths per minute);
- A total of 5 min whilst sitting, with the back of the seat tilted at 45°;
- A total of 5 min lying down in the supine position.
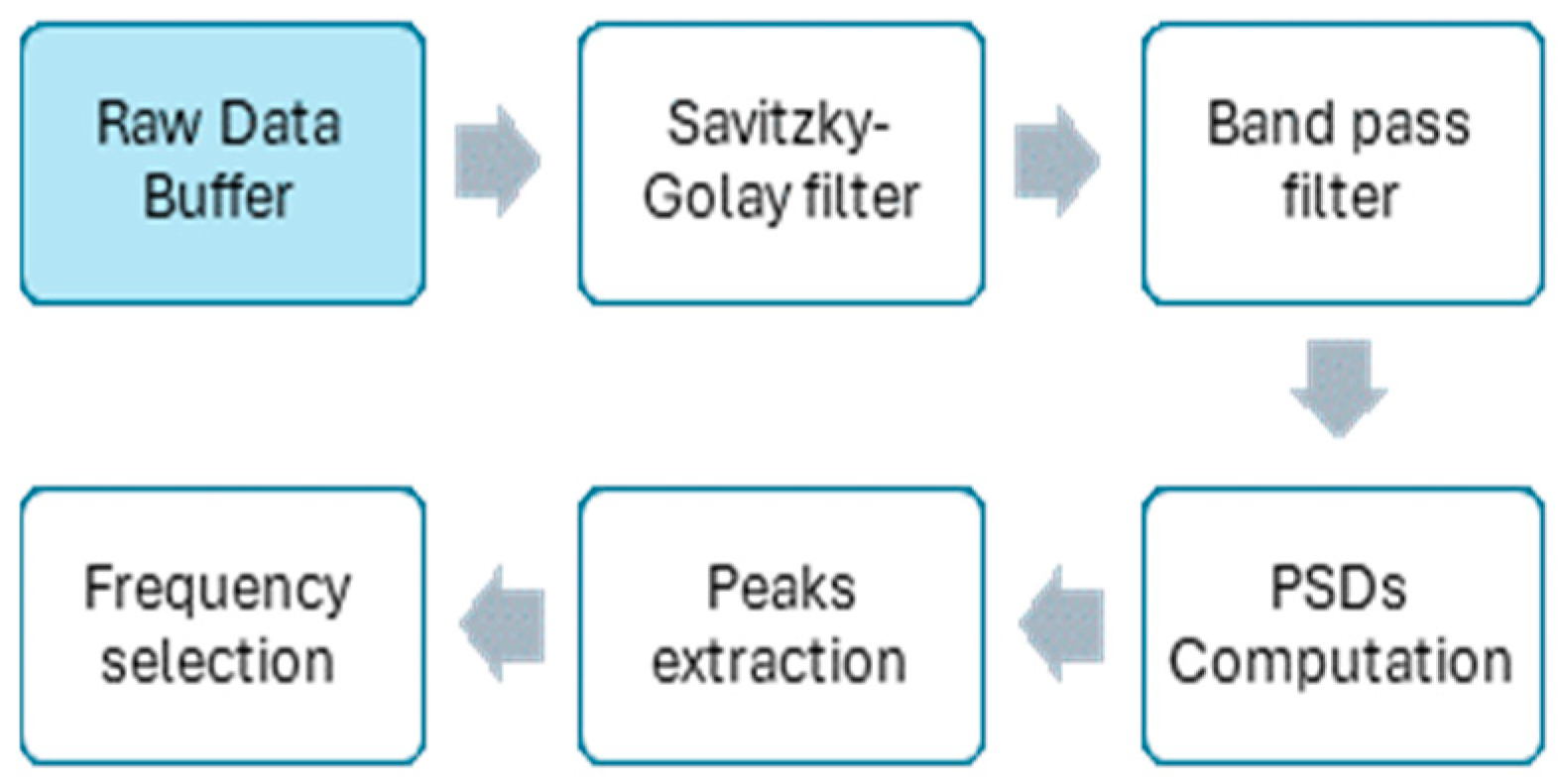
2.3. HR Estimation Algorithm with the SmartEW
2.4. Validation Procedure
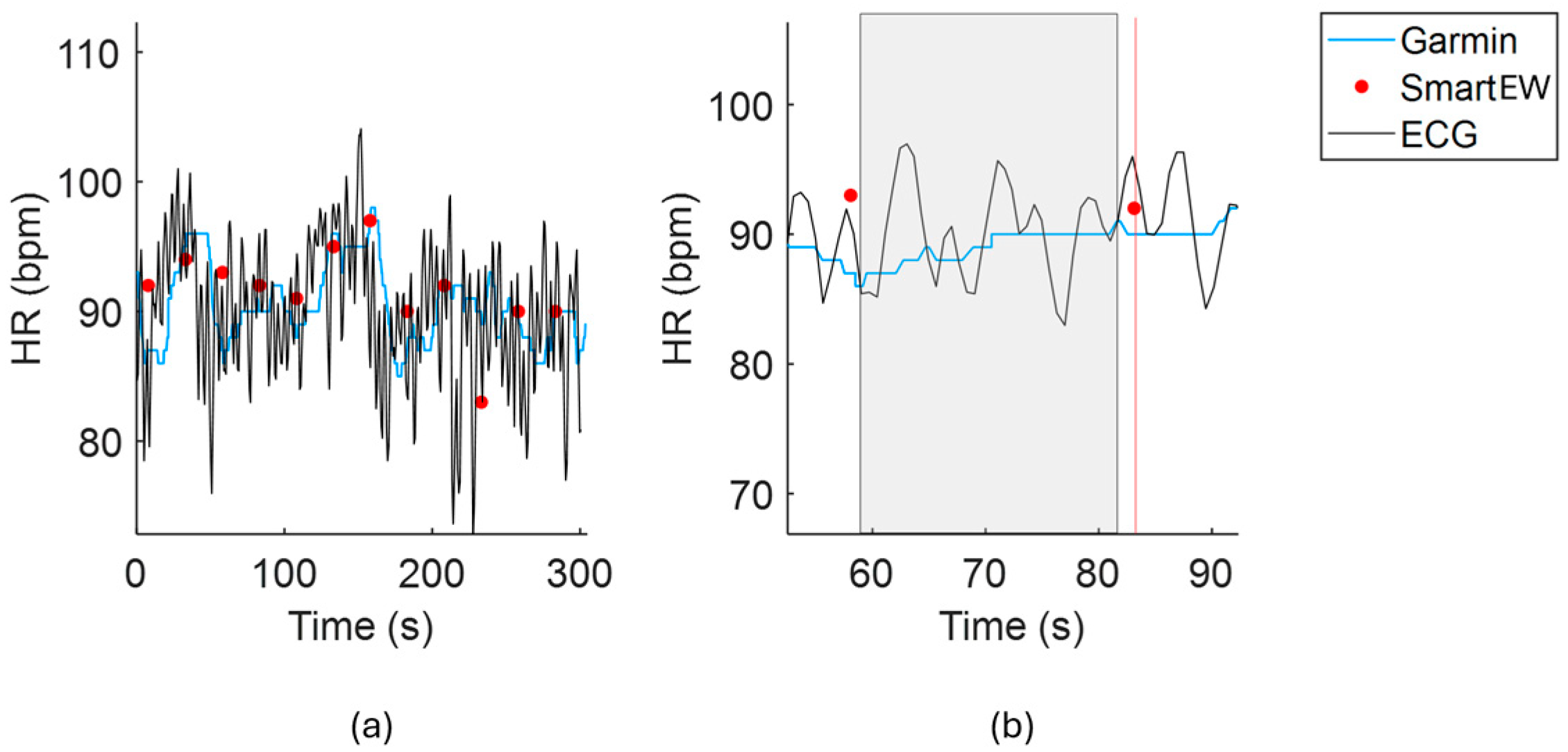
2.4.1. Comparison of HR Estimates with the Gold Standard
2.4.2. Effect of the QI on HR Estimates
3. Results
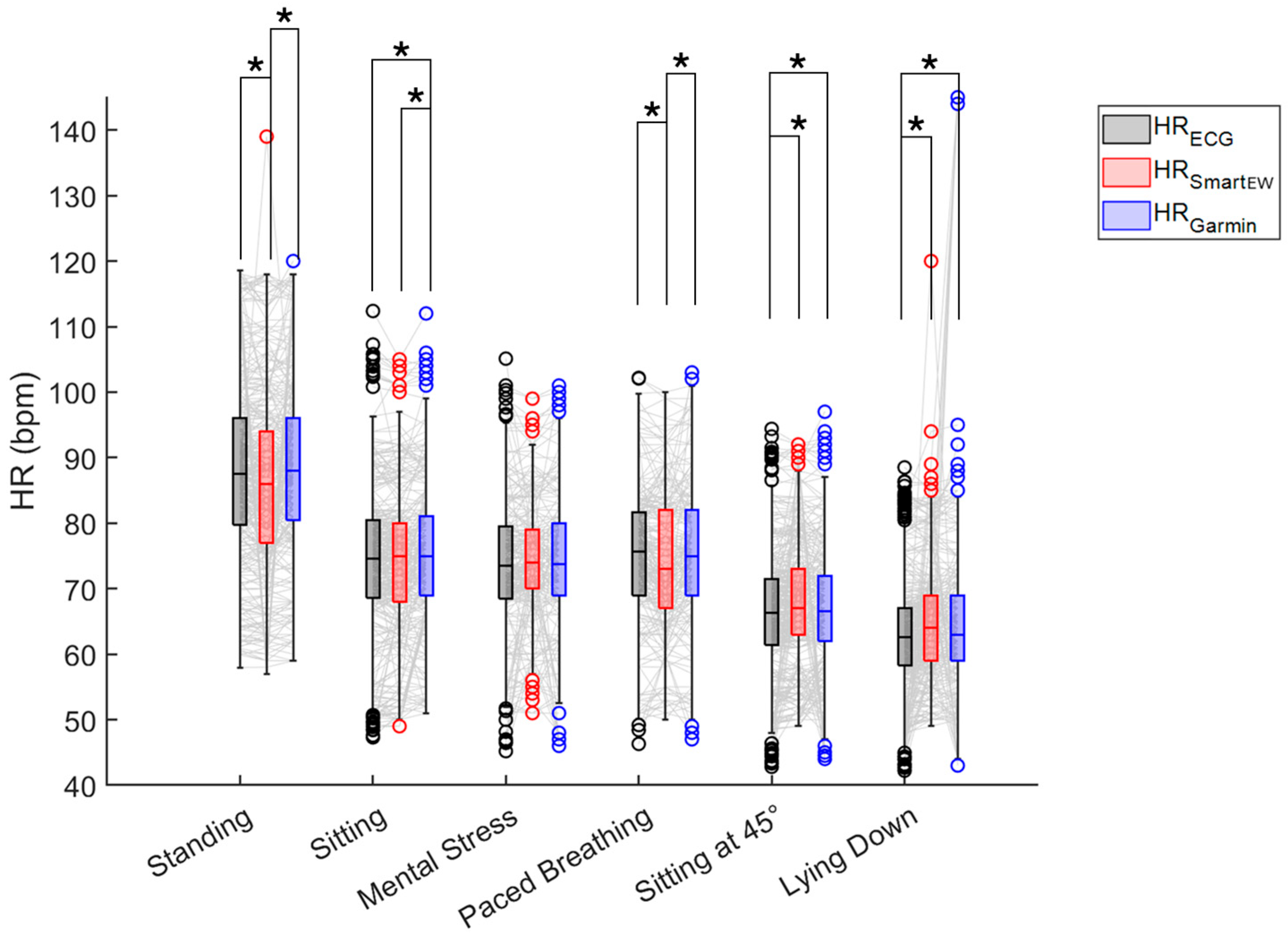
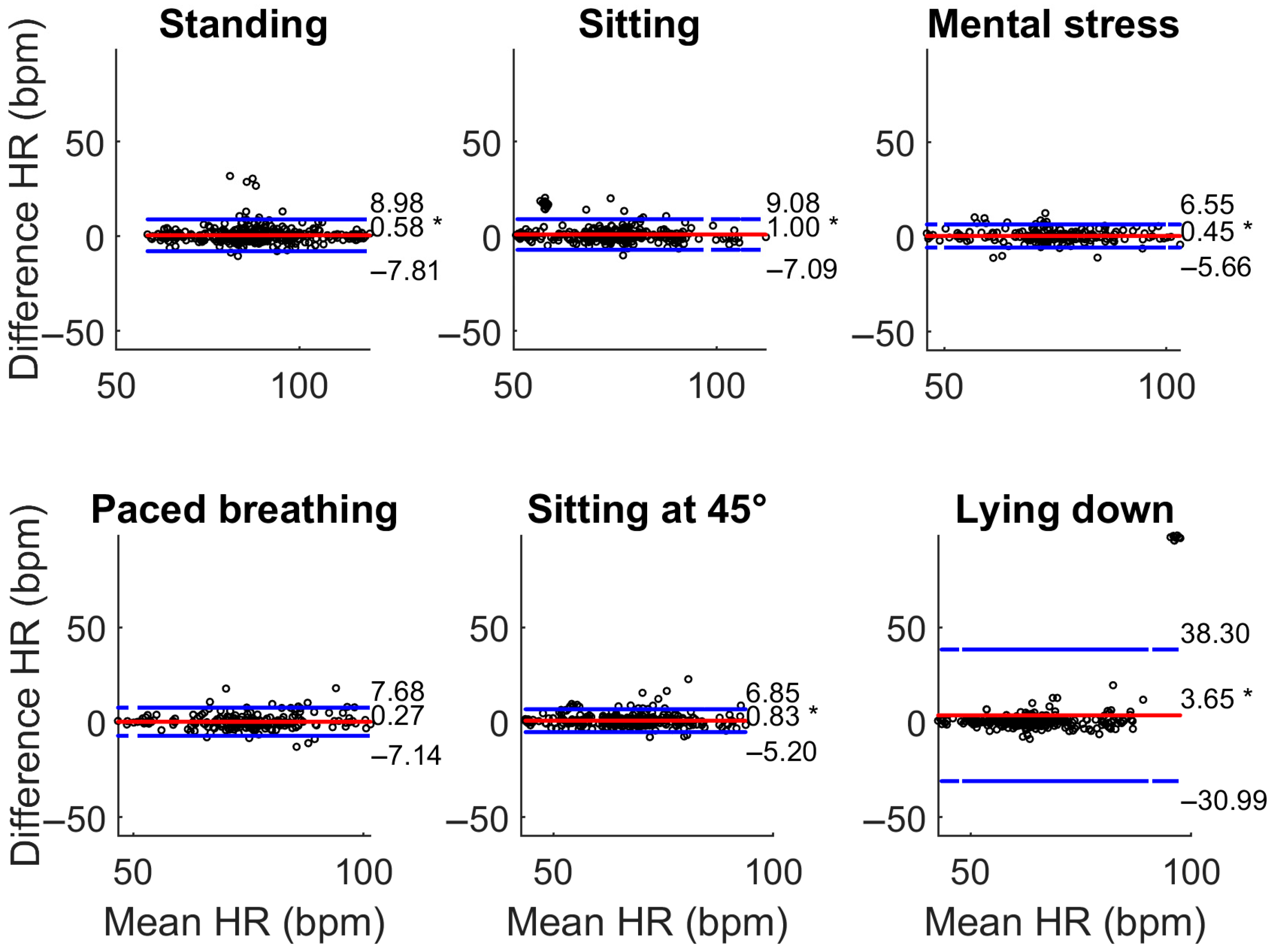
3.1. Comparison with the Gold Standard ECG
3.2. Selection of the Optimal QI
3.3. Impact of QI on SmartEW Accuracy Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| IMU | Inertial Measurement Unit |
| LoA | Limits of Agreement |
| MAE | Mean Absolute Error |
| MRE | Mean Relative Error |
| PPG | Photoplethysmography |
| PSD | Power Spectral Density |
| QI | Quality Index |
| SmartEW | Smart Eyewear “Essilor Connected Glasses” |
References
- Tröster, G. The agenda of wearable healthcare. Yearb. Med. Inform. 2005, 14, 125–138. [Google Scholar] [CrossRef]
- Wu, M.; Luo, J. Wearable technology applications in healthcare: A literature review. Online J. Nurs. Inform. 2019, 23, 3. [Google Scholar]
- Jensen, M.T.; Treskes, R.W.; Caiani, E.G.; Casado-Arroyo, R.; Cowie, M.R.; Dilaveris, P.; Duncker, D.; Di Rienzo, M.; Frederix, I.; De Groot, N.; et al. ESC working group on e-cardiology position paper: Use of commercially available wearable technology for heart rate and activity tracking in cardiovascular prevention. Eur. Heart. J. Digit. Health 2021, 2, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R. Worldwide survey of fitness trends for 2021. ACSM Health Fit. J. 2021, 25, 10–19. [Google Scholar] [CrossRef]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The rise of consumer health wearables: Promises and barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef]
- Doherty, C.; Baldwin, M.; Keogh, A. Keeping Pace with Wearables: A Living Umbrella Review of Systematic Reviews Evaluating the Accuracy of Consumer Wearable Technologies in Health Measurement. Sports Med. 2024, 54, 2907–2926. [Google Scholar] [CrossRef]
- Li, K.; Rüdiger, H.; Ziemssen, T. Spectral analysis of heart rate variability: Time window matters. Front. Neurol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Lee, J.; Sun, S.; Yang, S.M.; Sohn, J.J.; Park, J.; Lee, S.; Kim, H.C. Bidirectional recurrent auto-encoder for photoplethysmogram denoising. IEEE J. Biomed. Health Inform. 2019, 23, 2375–2385. [Google Scholar] [CrossRef]
- European Council of Optometry and Optics. Share of Individuals Who Wear Spectacles in Selected European Countries in 2020. In Statista. Available online: https://www.statista.com/statistics/711514/individuals-who-wear-spectacles-in-selected-european-countries/ (accessed on 9 December 2024).
- Balakrishnan, G.; Durand, F.; Guttag, J. Detecting pulse from head motions in video. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Portland, OR, USA, 23–28 June 2013; pp. 3430–3437. [Google Scholar] [CrossRef]
- Hernandez, J.; Li, Y.; Rehg, J.M.; Picard, R.W. Cardiac and respiratory parameter estimation using head-mounted motion-sensitive sensors. EAI Endorsed Trans. Pervasive Health Technol. 2015, 1, e2. [Google Scholar] [CrossRef]
- Floris, C.; Solbiati, S.; Landreani, F.; Damato, G.; Lenzi, B.; Megale, V.; Caiani, E.G. Feasibility of heart rate and respiratory rate estimation by inertial sensors embedded in a virtual reality headset. Sensors 2020, 20, 7168. [Google Scholar] [CrossRef]
- Solbiati, S.; Buffoli, A.; Megale, V.; Damato, G.; Lenzi, B.; Langfelder, G.; Caiani, E.G. Monitoring cardiac activity by detecting subtle head movements using MEMS technology. In Proceedings of the 2022 IEEE International Symposium on Inertial Sensors and Systems (INERTIAL), Linz, Austria, 8–11 May 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Solbiati, S.; Charkas, S.; Mozzini, F.; LoMauro, A.; Bernasconi, S.; Angelucci, A.; Aliverti, A.; Trojaniello, D.; Caiani, E.G. Comparison of ECG-Free Algorithms for Heart Rate Computation from Head-BCG Signals Obtained with Smart Eyewear. In Proceedings of the 2024 IEEE International Conference on Metrology for eXtended Reality, Artificial Intelligence and Neural Engineering, St Albans, UK, 21–23 October 2024; pp. 213–218. [Google Scholar] [CrossRef]
- Amir, B.; Gil, P.; Sahler, J. Embedding an Accelerometer-Based Heart Rate Evaluation Algorithm in a Smart Eyewear. In Proceedings of the 2024 IEEE Sensors Applications Symposium (SAS), Washington, DC, USA, 23–25 July 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.; Altenburg, T.M.; Chinapaw, M.J. Sedentary Behavior Research Network (SBRN)–Terminology Consensus Project Process and Outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Stoet, G. PsyToolkit: A software package for programming psychological experiments using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef]
- Stoet, G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 2017, 44, 24–31. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fischler, M.A.; Bolles, R.C. Random sample consensus: A paradigm for model fitting with applications to image analysis and automated cartography. Commun. ACM 1981, 24, 381–395. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- ANSI/AAMI EC13-2002; Cardiac Monitors, Heart Rate Meters, and Alarms. American National Standard: Piscataway, NJ, USA, 2008. Available online: https://webstore.ansi.org/standards/aami/ansiaamiec132002 (accessed on 29 May 2025).
- Harvey, J.A.; Chastin, S.F.; Skelton, D.A. How Sedentary Are Older People? A Systematic Review of the Amount of Sedentary Behavior. J. Aging Phys. Act. 2015, 23, 471–487. [Google Scholar] [CrossRef]
- Puranen, A.; Halkola, T.; Kirkeby, O.; Vehkaoja, A. Effect of Skin Tone and Activity on the Performance of Wrist-Worn Optical Beat-to-Beat Heart Rate Monitoring. In Proceedings of the IEEE Sensors Conference, Rotterdam, The Netherlands, 25–28 October 2020; pp. 1–4. [Google Scholar] [CrossRef]
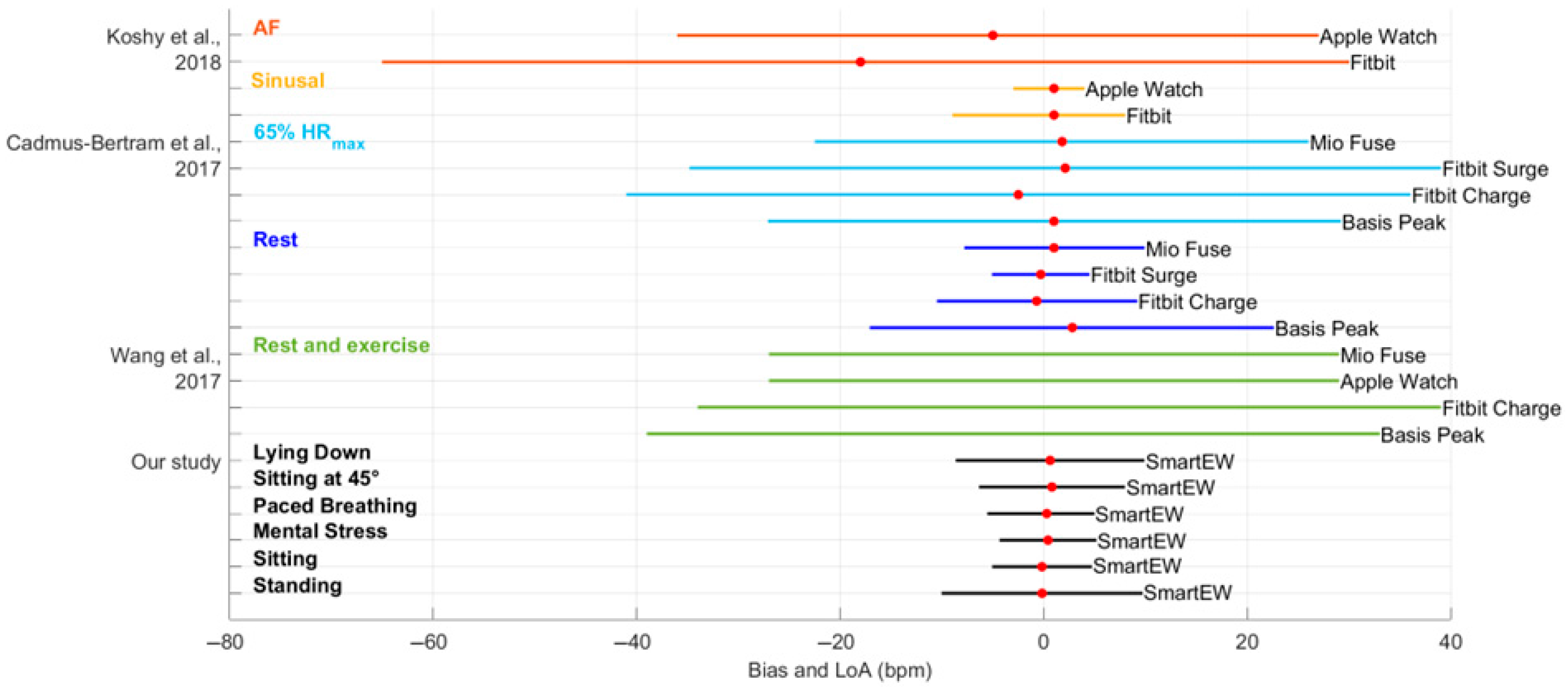
- Koshy, A.N.; Sajeev, J.K.; Nerlekar, N.; Brown, A.J.; Rajakariar, K.; Zureik, M.; Wong, M.C.; Roberts, L.; Street, M.; Cooke, J.; et al. Smart watches for heart rate assessment in atrial arrhythmias. Int. J. Cardiol. 2018, 266, 124–127. [Google Scholar] [CrossRef]
- Cadmus-Bertram, L.; Gangnon, R.; Wirkus, E.J.; Thraen-Borowski, K.M.; Gorzelitz-Liebhauser, J. Accuracy of heart rate monitoring by some wrist-worn activity trackers. Ann. Intern. Med. 2017, 167, 607–608. [Google Scholar] [CrossRef]
- Wang, R.; Blackburn, G.; Desai, M.; Phelan, D.; Gillinov, L.; Houghtaling, P.; Gillinov, M. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol. 2017, 2, 104–106. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Fox, K.; Borer, J.S.; Camm, A.J.; Danchin, N.; Ferrari, R.; Sendon, J.L.L.; Steg, P.G. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007, 50, 823–830. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhang, W. Heart rate, lifespan, and mortality risk. Ageing Res. Rev. 2016, 8, 52–60. [Google Scholar] [CrossRef]


| SmartEW | Garmin Venu 3 | Movesense Flash | |
|---|---|---|---|
| Position on the body | Head | Wrist | Chest |
| HR sensor | Accelerometer | PPG based on Garmin Elevate™ technology | 1-lead ECG |
| Sampling rate | 200 Hz | Unknown | 512 Hz |
| Measurement interval | ~22.5 s | 1 s | Beat-by-beat |
| Data logging | RasPad tablet | RasPad tablet | Movesense Showcase app (© Suunto Oy 2019) |
| Phase | r2 | ρ | RANSAC | |||
|---|---|---|---|---|---|---|
| SmartEW | Garmin | SmartEW | Garmin | SmartEW | Garmin | |
| Standing | 0.58 | 0.91 | 0.82 | 0.93 | 0.97 | 0.97 |
| Sitting | 0.92 | 0.81 | 0.95 | 0.93 | 0.97 | 0.96 |
| Mental stress | 0.67 | 0.93 | 0.85 | 0.94 | 0.93 | 0.96 |
| Paced breathing | 0.85 | 0.89 | 0.92 | 0.94 | 0.91 | 0.93 |
| Sitting at 45° | 0.54 | 0.90 | 0.80 | 0.94 | 0.97 | 0.96 |
| Lying down | 0.33 | 0.05 | 0.70 | 0.77 | 0.96 | 0.99 |
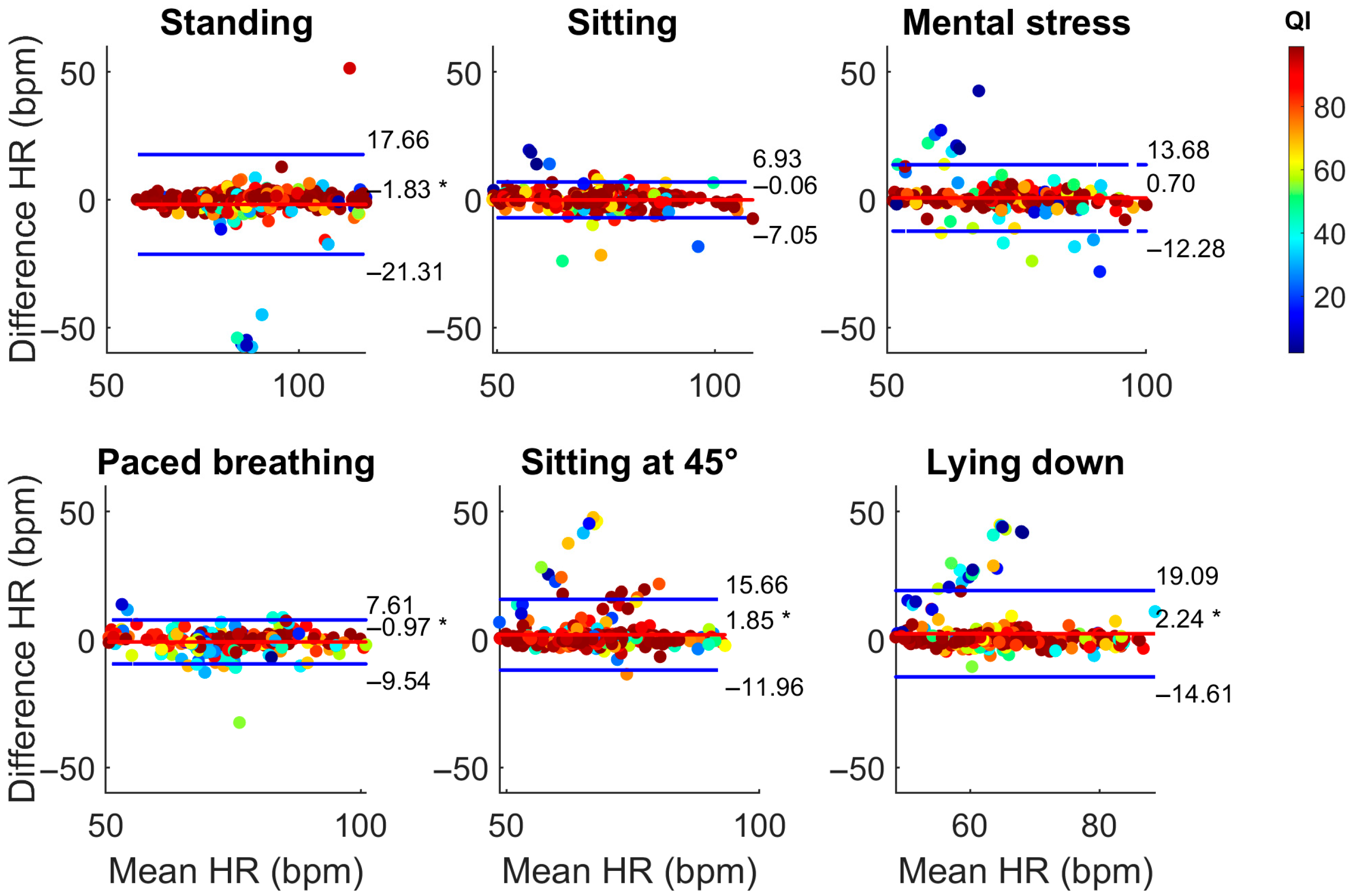
| Phase | Bias (bpm) | LoA (bpm) | Accuracy (±5 bpm) | |||
|---|---|---|---|---|---|---|
| SmartEW | Garmin | SmartEW | Garmin | SmartEW | Garmin | |
| Standing | −1.83 * | 0.58 * | [−21.31; 17.66] | [−7.81; 8.98] | 0.89 | 0.90 |
| Sitting | −0.06 | 1.00 * | [−7.05; 6.93] | [−7.09; 9.08] | 0.92 | 0.90 |
| Mental stress | 0.70 | 0.45 * | [−12.28; 13.68] | [−5.66; 6.55] | 0.84 | 0.90 |
| Paced breathing | −0.97 * | 0.27 | [−9.54; 7.61] | [−5.20; 6.85] | 0.82 | 0.87 |
| Sitting at 45° | 1.85 * | 0.83 * | [−11.96; 15.66] | [−5.20; 6.85] | 0.90 | 0.92 |
| Lying down | 2.24 * | 3.65 * | [−14.61; 19.09] | [−30.99; 38.30] | 0.90 | 0.91 |
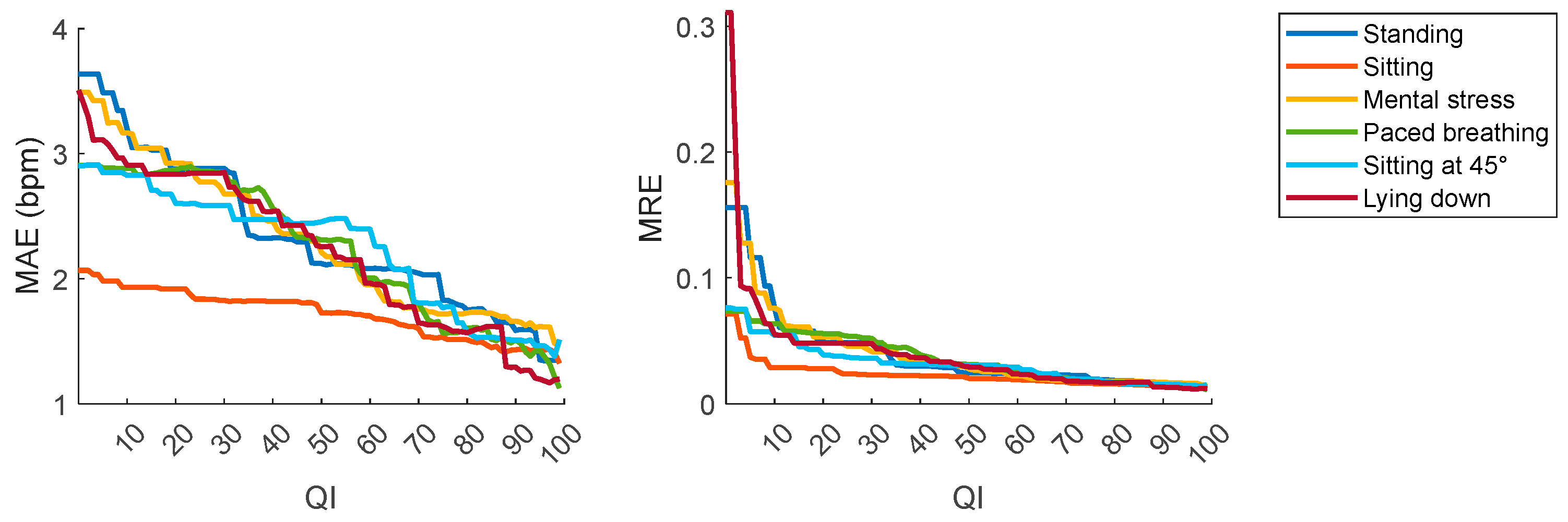
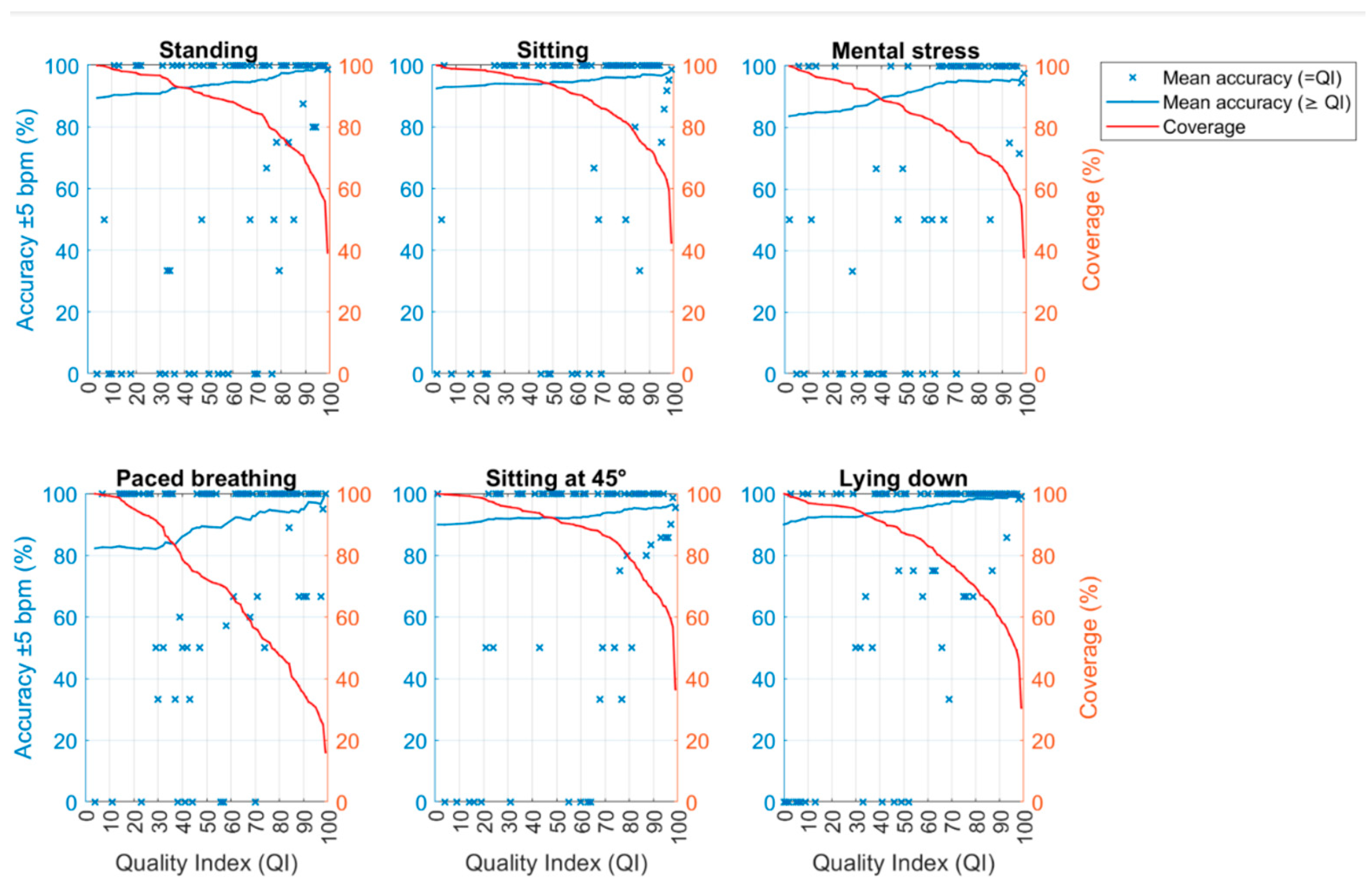
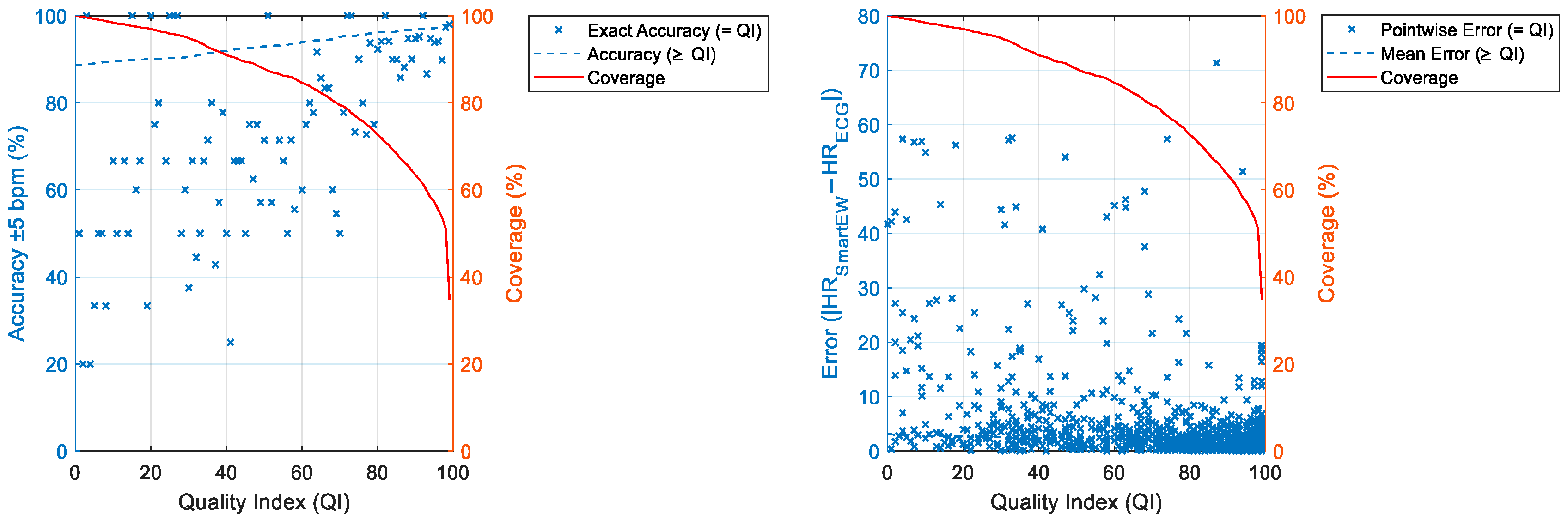
| QI ≥ 50 | QI ≥ 60 | QI ≥ 70 | QI ≥ 80 | QI ≥ 90 | |
|---|---|---|---|---|---|
| Avg. accuracy (%) | 93 | 94 | 95 | 96 | 97 |
| Pnt. accuracy (%) | >50 | >50 | >50 (mainly > 73) | >79 | >93 |
| Avg. error (bpm) | ±2.16 | ±2.02 | ±1.75 | ±1.62 | ±1.50 |
| Pnt. error (bpm) | Mainly < ±10 | Mainly < ±10 | Mainly < ±10 | Mainly < ±10 | Mainly < ±10 |
| Coverage (%) | 88 | 85 | 80 | 73 | 63 |
| Phase | r2 | ρ | RANSAC | |||
|---|---|---|---|---|---|---|
| All QI | QI ≥ 70 | All QI | QI ≥ 70 | All QI | QI ≥ 70 | |
| Standing | 0.58 | 0.87 ↑ | 0.82 | 0.96 ↑ | 0.97 | 0.98 ↑ |
| Sitting | 0.92 | 0.96 ↑ | 0.95 | 0.96 ↑ | 0.97 | 0.98 ↑ |
| Mental stress | 0.67 | 0.94 ↑ | 0.85 | 0.95 ↑ | 0.93 | 0.95 ↑ |
| Paced breathing | 0.85 | 0.95 ↑ | 0.92 | 0.97 ↑ | 0.91 | 0.97 ↑ |
| Sitting at 45° | 0.54 | 0.84 ↑ | 0.80 | 0.92 ↑ | 0.97 | 0.97 ↑ |
| Lying down | 0.33 | 0.70 ↑ | 0.70 | 0.93 ↑ | 0.96 | 0.96 ↑ |
| Phase | Bias (bpm) | LoA (bpm) | Accuracy (±5 bpm) | ||||
|---|---|---|---|---|---|---|---|
| All QI | QI ≥ 70 | All QI | QI ≥ 70 | All QI | QI ≥ 70 | Garmin | |
| Standing | −1.83 * | −0.19 ↓ | [−21.31; 17.66] | [−10.16; 9.77] ↓ | 0.89 | 0.95 ↑ | 0.89 |
| Sitting | −0.06 | −0.15 | [−7.05; 6.93] | [−4.98; 4.68] ↓ | 0.92 | 0.96 ↑ | 0.90 |
| Mental stress | 0.70 | 0.43 * ↓ | [−12.28; 13.68] | [−4.40; 5.26] ↓ | 0.84 | 0.95 ↑ | 0.90 |
| Paced breathing | −0.97 * | −0.27 ↓ | [−9.54; 7.61] | [−5.45; 4.91] ↓ | 0.82 | 0.93 ↑ | 0.87 |
| Sitting at 45° | 1.85 * | 0.84 * ↓ | [−11.96; 15.66] | [−6.26; 7.93] ↓ | 0.90 | 0.94 ↑ | 0.92 |
| Lying down | 2.24 * | 0.60 * ↓ | [−14.61; 19.09] | [−8.84; 10.34] ↓ | 0.90 | 0.97 ↑ | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solbiati, S.; Mozzini, F.; Sahler, J.; Gil, P.; Amir, B.; Antonello, N.; Trojaniello, D.; Caiani, E.G. Estimating Heart Rate from Inertial Sensors Embedded in Smart Eyewear: A Validation Study. Sensors 2025, 25, 4531. https://doi.org/10.3390/s25154531
Solbiati S, Mozzini F, Sahler J, Gil P, Amir B, Antonello N, Trojaniello D, Caiani EG. Estimating Heart Rate from Inertial Sensors Embedded in Smart Eyewear: A Validation Study. Sensors. 2025; 25(15):4531. https://doi.org/10.3390/s25154531
Chicago/Turabian StyleSolbiati, Sarah, Federica Mozzini, Jean Sahler, Paul Gil, Bruno Amir, Niccolò Antonello, Diana Trojaniello, and Enrico Gianluca Caiani. 2025. "Estimating Heart Rate from Inertial Sensors Embedded in Smart Eyewear: A Validation Study" Sensors 25, no. 15: 4531. https://doi.org/10.3390/s25154531
APA StyleSolbiati, S., Mozzini, F., Sahler, J., Gil, P., Amir, B., Antonello, N., Trojaniello, D., & Caiani, E. G. (2025). Estimating Heart Rate from Inertial Sensors Embedded in Smart Eyewear: A Validation Study. Sensors, 25(15), 4531. https://doi.org/10.3390/s25154531









