Assessing Gross Motor and Gait Function Using Hip–Knee Cyclograms in Ambulatory Children with Spastic Cerebral Palsy
Abstract
Highlights
- The parameters of the hip–knee cyclogram showed moderately strong correlations with GMFM-66, gait speed, GDI, and the sagittal plane subscore of the GPS for the hip and knee, while the principal component ratio correlated with the SCALE.
- In particular, the swing phase area showed the strongest correlation and was used to build regression models to estimate the GMFM-66 and gait speed, while the principal component ratio was used to assess the SCALE.
- Cyclogram metrics showed potential as digital biomarkers for assessing gross motor, gait, and selective motor functions in children with cerebral palsy.
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | cerebral palsy |
| GMFCS | Gross Motor Function Classification System |
| GMFM-66 | Gross Motor Function Measure-66 |
| ROM | range of motion |
| SCALE | Selective Control Assessment of the Lower Extremity |
| GDI | gait deviation index |
| GPS | gait profile score |
| PCA | principal component analysis |
| SVMC | selective voluntary motor control |
References
- Crenna, P. Spasticity andspastic’gait in children with cerebral palsy. Neurosci. Biobehav. Rev. 1998, 22, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Deluca, P.A. Gait analysis in the treatment of the ambulatory child with cerebral palsy. Clin. Orthop. Relat. Res. 1991, 264, 65–75. [Google Scholar] [CrossRef]
- Shin, H.I.; Sung, K.H.; Chung, C.Y.; Lee, K.M.; Lee, S.Y.; Lee, I.H.; Park, M.S. Relationships between isometric muscle strength, gait parameters, and gross motor function measure in patients with cerebral palsy. Yonsei Med. J. 2016, 57, 217. [Google Scholar] [CrossRef] [PubMed]
- Armand, S.; Decoulon, G.; Bonnefoy-Mazure, A. Gait analysis in children with cerebral palsy. EFORT Open Rev. 2016, 1, 448. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jung, S.; Rha, D.W.; Park, E.S. Botulinum toxin type A injection for spastic equinovarus foot in children with spastic cerebral palsy: Effects on gait and foot pressure distribution. Yonsei Med. J. 2016, 57, 496. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.H.; Rozumalski, A. The Gait Deviation Index: A new comprehensive index of gait pathology. Gait Posture 2008, 28, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Massaad, A.; Assi, A.; Skalli, W.; Ghanem, I. Repeatability and validation of gait deviation index in children: Typically developing and cerebral palsy. Gait Posture 2014, 39, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Malt, M.A.; Aarli, Å.; Bogen, B.; Fevang, J.M. Correlation between the Gait Deviation Index and gross motor function (GMFCS level) in children with cerebral palsy. J. Child. Orthop. 2016, 10, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.C.; Signal, N.; Naude, Y.; Taylor, D.; Stott, N.S. Gait deviation index correlates with daily step activity in children with cerebral palsy. Arch. Phys. Med. Rehabil. 2015, 96, 1924–1927. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.; Lennon, N.; Church, C.; Miller, F. Gait analysis parameters and walking activity pre-and postoperatively in children with cerebral palsy. Pediatr. Phys. Ther. 2018, 30, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Graham, H.K.; Tirosh, O. The gait profile score and movement analysis profile. Gait Posture 2009, 30, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Boulard, C.; Tavoosi, S.; Ravel, A.; Dohin, B. Quantification of gait impairment using the gait profile and variable score in children with cerebral palsy. Clin. Biomech. 2025, 125, 106528. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.M.; Nielsen, D.B.; Pedersen, N.W.; Overgaard, S.; Holsgaard-Larsen, A. Gait Deviation Index, Gait Profile Score and Gait Variable Score in children with spastic cerebral palsy: Intra-rater reliability and agreement across two repeated sessions. Gait Posture 2015, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Carcreff, L.; Gerber, C.N.; Paraschiv-Ionescu, A.; De Coulon, G.; Newman, C.J.; Aminian, K.; Armand, S. Comparison of gait characteristics between clinical and daily life settings in children with cerebral palsy. Sci. Rep. 2020, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Go, T.-H.; Hong, S.H.; Kim, S.H.; Han, J.H.; Kang, Y.; Kang, D.R. Digital biomarkers in living labs for vulnerable and susceptible individuals: An integrative literature review. Yonsei Med. J. 2022, 63, S43. [Google Scholar] [CrossRef] [PubMed]
- Grieve, D. The assessment of gait. Physiotherapy 1969, 55, 452–460. [Google Scholar] [PubMed]
- Goswami, A. A new gait parameterization technique by means of cyclogram moments: Application to human slope walking. Gait Posture 1998, 8, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Winters, T.; Gage, J.; Hicks, R. Gait patterns in spastic hemiplegia in children and young adults. J. Bone Jt. Surg. Am. 1987, 69, 437–441. [Google Scholar]
- Park, J.H.; Lee, H.; Cho, J.S.; Kim, I.; Lee, J.; Jang, S.H. Effects of knee osteoarthritis severity on inter-joint coordination and gait variability as measured by hip-knee cyclograms. Sci. Rep. 2021, 11, 1789. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Leban, B.; Porta, M.; Frau, J.; Coghe, G.; Cocco, E. Cyclograms reveal alteration of inter-joint coordination during gait in people with multiple sclerosis minimally disabled. Biomechanics 2022, 2, 331–341. [Google Scholar] [CrossRef]
- Lee, H.S.; Ryu, H.; Lee, S.U.; Cho, J.S.; You, S.; Park, J.H.; Jang, S.H. Analysis of gait characteristics using hip-knee cyclograms in patients with hemiplegic stroke. Sensors 2021, 21, 7685. [Google Scholar] [CrossRef] [PubMed]
- Marrone, F.; Pau, M.; Vismara, L.; Porta, M.; Bigoni, M.; Leban, B.; Cerfoglio, S.; Galli, M.; Mauro, A.; Cimolin, V. Synchronized cyclograms to assess inter-limb symmetry during gait in post-stroke patients. Symmetry 2022, 14, 1560. [Google Scholar] [CrossRef]
- Pilkar, R.; Ramanujam, A.; Chervin, K.; Forrest, G.F.; Nolan, K.J. Cyclogram-based joint symmetry assessment after utilization of a foot drop stimulator during post-stroke hemiplegic gait. J. Biomech. Eng. 2018, 140, 121005. [Google Scholar] [CrossRef] [PubMed]
- Chruscikowski, E.; Fry, N.R.; Noble, J.J.; Gough, M.; Shortland, A.P. Selective motor control correlates with gait abnormality in children with cerebral palsy. Gait Posture 2017, 52, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.G.; Staudt, L.A.; Greenberg, M.B.; Oppenheim, W.L. Selective Control Assessment of the Lower Extremity (SCALE): Development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Kang, L.J.; Chiarello, L.A.; Orlin, M.; Oeffinger, D.; Maggs, J. Social and community participation of children and youth with cerebral palsy is associated with age and gross motor function classification. Phys. Ther. 2009, 89, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Drouin, L.M.; Malouin, F.; Richards, C.L.; Marcoux, S. Correction between the gross motor function measure scores and gait spatiotemporal measures in children with neurological impairments. Dev. Med. Child Neurol. 1996, 38, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- McDowell, B.C.; Salazar-Torres, J.J.; Kerr, C.; Cosgrove, A.P. Passive range of motion in a population-based sample of children with spastic cerebral palsy who walk. Phys. Occup. Ther. Pediatr. 2012, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Engsberg, J.R.; Ross, S.A.; Hollander, K.W.; Park, T. Hip spasticity and strength in children with spastic diplegia cerebral palsy. J. Appl. Biomech. 2000, 16, 221–233. [Google Scholar] [CrossRef]
- Damiano, D.L.; Laws, E.; Carmines, D.V.; Abel, M.F. Relationship of spasticity to knee angular velocity and motion during gait in cerebral palsy. Gait Posture 2006, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lowes, L.P.; Westcott, S.L.; Palisano, R.J.; Effgen, S.K.; Orlin, M.N. Muscle force and range of motion as predictors of standing balance in children with cerebral palsy. Phys. Occup. Ther. Pediatr. 2004, 24, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Andriacchi, T.; Ogle, J.; Galante, J. Walking speed as a basis for normal and abnormal gait measurements. J. Biomech. 1977, 10, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.; Simon-Martinez, C.; Molenaers, G.; Ortibus, E.; Van Campenhout, A.; Desloovere, K. Are spasticity, weakness, selectivity, and passive range of motion related to gait deviations in children with spastic cerebral palsy? A statistical parametric mapping study. PLoS ONE 2019, 14, e0223363. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.J.; Mudge, A.J.; Wojciechowski, E.A.; Axt, M.W.; Burns, J. Impact of multilevel joint contractures of the hips, knees and ankles on the Gait Profile score in children with cerebral palsy. Clin. Biomech. 2018, 59, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.; McDowell, B.C.; Parkes, J.; Stevenson, M.; Cosgrove, A.P. Age-related changes in energy efficiency of gait, activity, and participation in children with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.J.; Schwartz, M.H. Low gait efficiency is the primary reason for the increased metabolic demand during gait in children with cerebral palsy. Hum. Mov. Sci. 2018, 57, 426–433. [Google Scholar] [CrossRef] [PubMed]
- McMulkin, M.L.; Gordon, A.B.; Caskey, P.M.; Tompkins, B.J.; Baird, G.O. Outcomes of orthopaedic surgery with and without an external femoral derotational osteotomy in children with cerebral palsy. J. Pediatr. Orthop. 2016, 36, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.G.; Goldberg, E.J. The effect of lower extremity selective voluntary motor control on interjoint coordination during gait in children with spastic diplegic cerebral palsy. Gait Posture 2009, 29, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.J.; Requejo, P.S.; Fowler, E.G. Joint moment contributions to swing knee extension acceleration during gait in individuals with spastic diplegic cerebral palsy. Gait Posture 2011, 33, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Švehlík, M.; Zwick, E.B.; Steinwender, G.; Saraph, V.; Linhart, W.E. Genu recurvatum in cerebral palsy–part A: Influence of dynamic and fixed equinus deformity on the timing of knee recurvatum in children with cerebral palsy. J. Pediatr. Orthop. B 2010, 19, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Deutsch, S.; Nuzzo, R.; Mansour, M.; Jackson, J.; Koskinen, M.; Rosenthal, R. Genu recurvatum in spastic cerebral palsy. Report on findings by gait analysis. JBJS 1978, 60, 882–894. [Google Scholar] [CrossRef]
- Vos, R.C.; Becher, J.G.; Voorman, J.M.; Gorter, J.W.; van Eck, M.; van Meeteren, J.; Smits, D.-W.; Twisk, J.W.; Dallmeijer, A.J.; van Schie, P. Longitudinal association between gross motor capacity and neuromusculoskeletal function in children and youth with cerebral palsy. Arch. Phys. Med. Rehabil. 2016, 97, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; Rozumalski, A.; Schwartz, M.H. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev. Med. Child Neurol. 2015, 57, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, X.; Cao, S.; Wu, D.; Zhang, X.; Chen, X. Gait synergetic neuromuscular control in children with cerebral palsy at different gross motor function classification system levels. J. Neurophysiol. 2019, 121, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Son, E.J.; Kim, J.H.; Noh, H.E.; Kim, I.; Lim, J.A.; Han, S.H. Comparison of Gait Parameters during Forward Walking under Different Visual Conditions Using Inertial Motion Sensors. Yonsei Med. J. 2022, 63, 82. [Google Scholar] [CrossRef] [PubMed]
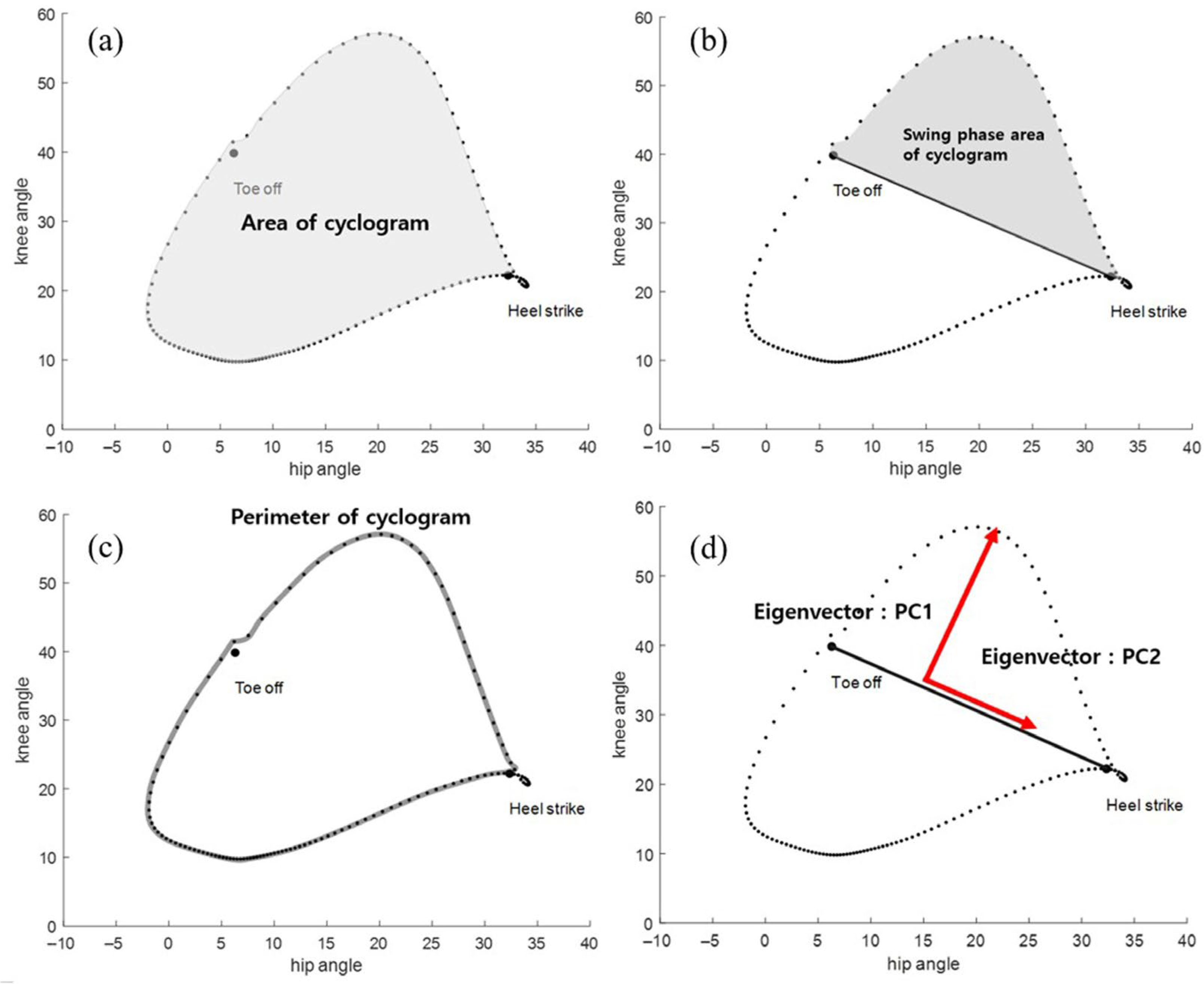

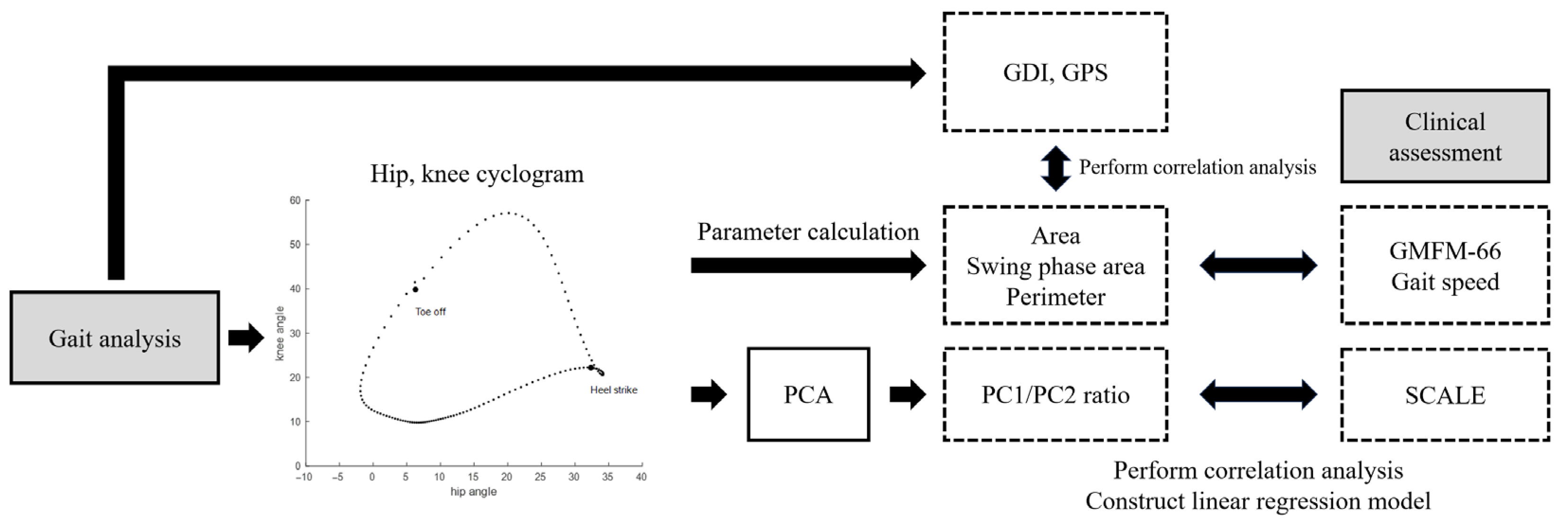
| Demographic Variable | No. (%) |
|---|---|
| Sex | |
| Male | 82 (56.94%) |
| Female | 62 (43.06%) |
| GMFCS | |
| I | 36 (25.00%) |
| II | 91 (63.19%) |
| III | 17 (11.80%) |
| Age (years) | |
| Range | 6–20 |
| Average | 13 years |
| Mean | 13 years 8 months |
| Standard deviation | 3 years 6 months |
| Area | Swing Phase Area | Perimeter | PC1/PC2 | |
|---|---|---|---|---|
| GMFM-66 | 0.48 * | 0.55 * | 0.41 * | |
| Normalized gait speed | 0.60 * | 0.70 * | 0.66 * | |
| GDI | 0.48 * | 0.41 * | 0.34 * | |
| GPS (hip fl/ex, knee fl/ex) | −0.51 * | −0.48 * | −0.41 * | |
| SCALE | −0.57 * |
| GMFM-66 | B* | β (95% CI) | p | R2 |
|---|---|---|---|---|
| Constant | 54.779 | 0.301 | ||
| Swing phase area | 0.010 | 0.549 | <0.001 | |
| Normalized gait speed | B* | β (95% CI) | p | R2 |
| Constant | 0.672 | 0.484 | ||
| Swing phase area | 0.0004 | 0.695 | <0.001 | |
| SCALE | B* | β (95% CI) | p | R2 |
| Constant | 6.413 | 0.320 | ||
| PC1/PC2 | −0.211 | −0.566 | <0.001 |
| (a) | ||||||
| GMFCS I (n = 36) | GMFCS II (n = 91) | GMFCS III (n = 17) | Multiple comparisons | |||
| I vs. II | I vs. III | II vs. III | ||||
| Area | 1270.7 ± 334.4 | 1002.6 ± 409.4 | 557.9 ± 174.1 | <0.001 | <0.001 | <0.001 |
| Swing phase area | 762.5 ± 247.9 | 530.2 ± 282.5 | 238.7 ± 115.8 | <0.001 | <0.001 | <0.001 |
| Perimeter | 158.5 ± 18.4 | 142.9 ± 29.3 | 118.4 ± 21.8 | <0.001 | <0.001 | <0.001 |
| (b) | ||||||
| GMFCS I | GMFCS II | GMFCS III | ||||
| GMFM-66 | Gait speed | GMFM-66 | Gait speed | GMFM-66 | Gait speed | |
| Area | 0.20 | 0.49 * | 0.33 * | 0.56 * | 0.38 | 0.01 |
| Swing phase area | 0.29 | 0.56 * | 0.39 * | 0.67 * | 0.49 * | 0.61 * |
| Perimeter | 0.30 | 0.60 * | 0.23 * | 0.63 * | 0.42 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Hong, J.; Lee, J.; Cho, Y.; Lee, T.; Rha, D.-w. Assessing Gross Motor and Gait Function Using Hip–Knee Cyclograms in Ambulatory Children with Spastic Cerebral Palsy. Sensors 2025, 25, 4485. https://doi.org/10.3390/s25144485
Yoo J, Hong J, Lee J, Cho Y, Lee T, Rha D-w. Assessing Gross Motor and Gait Function Using Hip–Knee Cyclograms in Ambulatory Children with Spastic Cerebral Palsy. Sensors. 2025; 25(14):4485. https://doi.org/10.3390/s25144485
Chicago/Turabian StyleYoo, Jehyun, Juntaek Hong, Jeuhee Lee, Yebin Cho, Taekyung Lee, and Dong-wook Rha. 2025. "Assessing Gross Motor and Gait Function Using Hip–Knee Cyclograms in Ambulatory Children with Spastic Cerebral Palsy" Sensors 25, no. 14: 4485. https://doi.org/10.3390/s25144485
APA StyleYoo, J., Hong, J., Lee, J., Cho, Y., Lee, T., & Rha, D.-w. (2025). Assessing Gross Motor and Gait Function Using Hip–Knee Cyclograms in Ambulatory Children with Spastic Cerebral Palsy. Sensors, 25(14), 4485. https://doi.org/10.3390/s25144485







