Advancements in Optical Fiber Sensors for pH Measurement: Technologies and Applications
Abstract
1. Introduction
2. Comparative Summary of Electrochemical and Optical pH Sensors
3. Optical Fiber Methods for pH Measurement
| Aspect | Fluorescence-Based Optical Fiber Sensors | Absorbance-Based Optical Fiber Sensors | SPR-Based Fiber Sensors | Interferometric Fiber Sensors | FBG Sensors | Luminescence Lifetime-Based Sensors | Optical Fiber Evanescent Wave Sensors |
|---|---|---|---|---|---|---|---|
| Principle | Fluorescence emission [53]. | Light absorption [54,55]. | Refractive index change [56,57]. | Light interference [58]. | Wavelength shift [59]. | Decay time measurement [60]. | Evanescent waves [61,62]. |
| Fiber Role | Guides excitation/collection [63]. | Light transmission [64]. | Guides and collects light [65]. | Interference medium [66]. | Sensing/light guide [67]. | Guides and collects light [68]. | Analyte interaction [69]. |
| Material | Fluorophores/dyes [70]. | Coated/uncoated fibers [71]. | Gold/silver [72]. | Silica/polymers [73]. | Refractive index-modulated silica [74]. | Luminescent dyes [75]. | Silica/polymers [76]. |
| Light Source | Lasers/LEDs [77]. | LEDs/lasers [78]. | Polarized lasers [79]. | Laser diode/LED [80]. | Narrowband lasers [81]. | Pulsed lasers/LEDs [82]. | Lasers/LEDs [83]. |
| Detection Mechanism | Intensity/wavelength shifts [84]. | Intensity changes [85]. | Resonance shifts [86]. | Phase/intensity changes [87]. | Wavelength shifts [88]. | Decay time analysis [89]. | Signal variation [90]. |
| Detectors | Photodiodes/spectrometers [91]. | Photodiodes/spectrometers [62]. | CCD/CMOS [92]. | Photodiodes [93]. | Spectrum analyzers [94]. | Photomultipliers [95]. | Photodiodes [96]. |
| Signal Processing | Fluorescence analysis [97]. | Absorbance quantification [98]. | Resonance analysis [99]. | Interference data [100]. | Wavelength conversion [101]. | Decay time correlation [102]. | Signal variation analysis [103]. |
| Applications | Biomedical, environment [104,105]. | Environment, industry [106]. | Diagnostics, monitoring [107,108]. | Structural, medical [109]. | Structural, aerospace [110]. | Diagnostics, control [111]. | Chemical, biosensing [48]. |
| Advantages | Sensitive, real-time [112]. | Compact, remote [113]. | Real-time, label-free [114]. | EMI immunity, sensitive [115]. | Multiplexing, sensitive [116]. | Intensity stability [117]. | Compact, real-time [118]. |
| Challenges | Photobleaching, cost [119]. | Cross-sensitivity [120]. | Noise, fouling [121]. | Noise, cost [122]. | Expensive systems [123]. | High cost, quenching [124]. | Noise, fabrication [125]. |
3.1. Fluorescence-Based Optical Fiber Sensors
3.1.1. Materials and Design Features
3.1.2. Measurement Range
3.1.3. Sensitivity
3.1.4. Advantages, Challenges, and Recent Developments
3.2. Absorbance-Based Optical Fiber Sensors: Materials, Design, and Performance
3.2.1. Materials and Design Features
3.2.2. Measurement Range
3.2.3. Sensitivity
3.2.4. Advantages, Challenges, and Recent Developments
3.3. Surface Plasmon Resonance Sensors
3.3.1. Materials and Design Features
3.3.2. Measurement Range
3.3.3. Sensitivity
3.3.4. Advantages, Challenges, and Recent Developments
3.4. Interferometric Fiber Sensors
3.4.1. Materials and Design Features
3.4.2. Measurement Range and Sensitivity
3.4.3. Advantages, Challenges, and Recent Developments
3.5. FBG-Based pH Sensing Technologies
3.5.1. Materials and Design Features
3.5.2. Measurement Range and Sensitivity
3.5.3. Advantages, Challenges, and Recent Developments
3.6. Luminescence Lifetime-Based Sensors
3.6.1. Materials and Design Features
3.6.2. Measurement Range and Sensitivity
3.6.3. Advantages, Challenges, and Recent Developments
3.7. Optical Fiber Evanescent Wave Sensors
3.7.1. Materials and Design Features
3.7.2. Measurement Range and Sensitivity
3.7.3. Advantages, Challenges, and Recent Developments
3.8. Comparative Summary of Sensor Performance
3.9. Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazaroff, W.W.; Weschler, C.J. Indoor acids and bases. Indoor Air 2020, 30, 559–644. [Google Scholar] [CrossRef]
- Agmon, N.; Bakker, H.J.; Campen, R.K.; Henchman, R.H.; Pohl, P.; Roke, S.; Thämer, M.; Hassanali, A. Protons and Hydroxide Ions in Aqueous Systems. Chem. Rev. 2016, 116, 7642–7672. [Google Scholar] [CrossRef]
- Becskereki, G.; Horvai, G.; Tóth, B. Adsorption of Hydrophobic Ions on Environmentally Relevant Sorbents. Polymers 2022, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Newsham, P. Acids and Bases. In Food Science and the Culinary Arts; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–109. ISBN 978-0-12-811816-0. [Google Scholar]
- Swindlehurst, B.R.; Narayanaswamy, R. Optical Sensing of pH in Low Ionic Strength Waters. In Optical Sensors; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2004; Volume 1, pp. 281–308. ISBN 978-3-642-07421-9. [Google Scholar]
- Villasana, Y.; Moradi, N.; Navas-Cárdenas, C.; Patience, G.S. Experimental methods in chemical engineering: pH. Can. J. Chem. Eng. 2022, 100, 1703–1717. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.T.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G.C.; Murzynowski, P.J.; Dagdeviren, C. Recent Progress in Electrochemical pH-Sensing Materials and Configurations for Biomedical Applications. Chem. Rev. 2019, 119, 5248–5297. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Jaselskis, B.; Moore, C.E. Development of the Glass Electrode and the pH Response. J. Chem. Educ. 2013, 90, 345–351. [Google Scholar] [CrossRef]
- Yong, F.; Zhu, Q.; Zhang, G.; Tao, G.; Qin, S. Simple and Economical Procedure to Assemble pH Glass Membrane Electrodes Used in Chemical Education. J. Chem. Educ. 2019, 96, 1773–1777. [Google Scholar] [CrossRef]
- Hershey, D.R. Measuring Growing Media pH with Metal-probe and Glass Electrode pH Meters. HortScience 1988, 23, 625. [Google Scholar] [CrossRef]
- Vonau, W.; Decker, M.; Enseleit, U.; Gerlach, F. Is there still a need for the antimony electrode 100 years after its introduction as a pH sensor? J. Solid State Electrochem. 2020, 24, 3269–3277. [Google Scholar] [CrossRef]
- Okubo, S.; Ozeki, Y.; Yamada, T.; Saito, K.; Ishihara, N.; Yanagida, Y.; Mayanagi, G.; Washio, J.; Takahashi, N. Facile Fabrication of All-solid-state Ion-selective Electrodes by Laminating and Drop-casting for Multi-sensing. Electrochemistry 2022, 90, 077001. [Google Scholar] [CrossRef]
- Mamilla, S.; Zahid Yousuf, S.; Mohan Avulapati, M.; Ahmad-Al-Mallahi; Narasimha Murty, N.V.L. A Low-Cost, Reusable All-Solid-State TiN-Based EGFET Soil pH Sensor. IEEE Sens. J. 2025, 25, 4184–4191. [Google Scholar] [CrossRef]
- Bagshaw, E.A.; Wadham, J.L.; Tranter, M.; Beaton, A.D.; Hawkings, J.R.; Lamarche-Gagnon, G.; Mowlem, M.C. Measuring pH in low ionic strength glacial meltwaters using ion selective field effect transistor (ISFET) technology. Limnol. Ocean. Methods 2021, 19, 222–233. [Google Scholar] [CrossRef]
- Takeshita, Y.; Martz, T.R.; Johnson, K.S.; Dickson, A.G. Characterization of an Ion Sensitive Field Effect Transistor and Chloride Ion Selective Electrodes for pH Measurements in Seawater. Anal. Chem. 2014, 86, 11189–11195. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Wu, C. Design and Implementation of a pH Sensor for Micro Solution Based on Nanostructured Ion-Sensitive Field-Effect Transistor. Sensors 2020, 20, 6921. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Disposable and Low-Cost Colorimetric Sensors for Environmental Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8331. [Google Scholar] [CrossRef]
- Luka, G.S.; Nowak, E.; Kawchuk, J.; Hoorfar, M.; Najjaran, H. Portable device for the detection of colorimetric assays. R. Soc. Open Sci. 2017, 4, 171025. [Google Scholar] [CrossRef]
- Li, G.; Su, H.; Ma, N.; Zheng, G.; Kuhn, U.; Li, M.; Klimach, T.; Pöschl, U.; Cheng, Y. Multifactor colorimetric analysis on pH-indicator papers: An optimized approach for direct determination of ambient aerosol pH. Atmos. Meas. Tech. 2020, 13, 6053–6065. [Google Scholar] [CrossRef]
- Chen, W.-H.; Dillon, W.D.N.; Armstrong, E.A.; Moratti, S.C.; McGraw, C.M. Self-referencing optical fiber pH sensor for marine microenvironments. Talanta 2021, 225, 121969. [Google Scholar] [CrossRef]
- Frankær, C.G.; Hussain, K.J.; Dörge, T.C.; Sørensen, T.J. Optical Chemical Sensor Using Intensity Ratiometric Fluorescence Signals for Fast and Reliable pH Determination. ACS Sens. 2019, 4, 26–31. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Venugopalan, T.; Sun, T.; Grattan, K.T.V. Intrinsic Fiber Optic pH Sensor for Measurement of pH Values in the Range of 0.5–6. IEEE Sens. J. 2016, 16, 881–887. [Google Scholar] [CrossRef]
- Leitão, C.; Pereira, S.O.; Marques, C.; Cennamo, N.; Zeni, L.; Shaimerdenova, M.; Ayupova, T.; Tosi, D. Cost-Effective Fiber Optic Solutions for Biosensing. Biosensors 2022, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Runcie, J.W.; Krause, C.; Torres Gabarda, S.A.; Byrne, M. Technical note: Continuous fluorescence-based monitoring of seawater pH in situ. Biogeosciences 2018, 15, 4291–4299. [Google Scholar] [CrossRef]
- Qi, J.; Liu, D.; Liu, X.; Guan, S.; Shi, F.; Chang, H.; He, H.; Yang, G. Fluorescent pH Sensors for Broad-Range pH Measurement Based on a Single Fluorophore. Anal. Chem. 2015, 87, 5897–5904. [Google Scholar] [CrossRef]
- Takahashi, S.; Kagami, Y.; Hanaoka, K.; Terai, T.; Komatsu, T.; Ueno, T.; Uchiyama, M.; Koyama-Honda, I.; Mizushima, N.; Taguchi, T.; et al. Development of a Series of Practical Fluorescent Chemical Tools to Measure pH Values in Living Samples. J. Am. Chem. Soc. 2018, 140, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Rovati, L.; Fabbri, P.; Pilati, F. Disposable Fluorescence Optical pH Sensor for Near Neutral Solutions. Sensors 2012, 13, 484–499. [Google Scholar] [CrossRef]
- Mahdi, K.A.S.; Al-Tahar, I.A.; Neamah, W.A.; Shakir, W.M.R. Realization of the importance of using fiber optic sensor technology in engineering and medical applications: A review. In Proceedings of the Fifth Scientific Conference for Electrical Engineering Techniques Research (EETR 2024), Baghdad, Iraq, 15–16 June 2024; p. 040034. [Google Scholar]
- Hassan Akhtar, M.; Azhar Hayat Nawaz, M.; Abbas, M.; Liu, N.; Han, W.; Lv, Y.; Yu, C. Advances in pH Sensing: From Traditional Approaches to Next-Generation Sensors in Biological Contexts. Chem. Rec. 2024, 24, e202300369. [Google Scholar] [CrossRef]
- Accastelli, E.; Scarbolo, P.; Ernst, T.; Palestri, P.; Selmi, L.; Guiducci, C. Multi-Wire Tri-Gate Silicon Nanowires Reaching Milli-pH Unit Resolution in One Micron Square Footprint. Biosensors 2016, 6, 9. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Kang, B.-C.; Ha, T.-J. Flexible pH sensors based on printed nanocomposites of single-wall carbon nanotubes and Nafion. Appl. Surf. Sci. 2020, 514, 145956. [Google Scholar] [CrossRef]
- Laffitte, Y.; Gray, B.L. Potentiometric pH Sensor Based on Flexible Screen-Printable Polyaniline Composite for Textile-Based Microfluidic Applications. Micromachines 2022, 13, 1376. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.; Ding, H.; Yi, D. Smartphone-Based Hand-Held Optical Fiber Fluorescence Sensor for On-Site pH Detection. IEEE Sens. J. 2019, 19, 9441–9446. [Google Scholar] [CrossRef]
- Rosenberg, M.; Laursen, B.W.; Frankær, C.G.; Sørensen, T.J. A Fluorescence Intensity Ratiometric Fiber Optics–Based Chemical Sensor for Monitoring pH. Adv. Mater. Technol. 2018, 3, 1800205. [Google Scholar] [CrossRef]
- Chauhan, M.; Singh, V.K. Fiber optic pH sensor using TiO2-SiO2 composite layer with a temperature cross-sensitivity feature. Optik 2020, 212, 164709. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Pathak, A.K.; Singh, V.K. No-core fiber-based highly sensitive optical fiber pH sensor. J. Biomed. Opt. 2017, 22, 057001. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, E.-B.; Li, P.; Cao, J.-W.; Lyu, G.-H. pH sensor based on tilted fiber Bragg grating surface plasmon resonance with a polyaniline reaction deposition film layer. Opt. Express 2024, 32, 10887. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-N. Fiber-Optic SPR pH Sensor Based on MMF–NCF–MMF Structure and Self-Assembled Nanofilm. IEEE Trans. Instrum. Meas. 2021, 70, 9502509. [Google Scholar] [CrossRef]
- Puppulin, L.; Hosogi, S.; Sun, H.; Matsuo, K.; Inui, T.; Kumamoto, Y.; Suzaki, T.; Tanaka, H.; Marunaka, Y. Bioconjugation strategy for cell surface labelling with gold nanostructures designed for highly localized pH measurement. Nat. Commun. 2018, 9, 5278. [Google Scholar] [CrossRef]
- Engholm, M.; Hammarling, K.; Andersson, H.; Sandberg, M.; Nilsson, H.-E. A Bio-Compatible Fiber Optic pH Sensor Based on a Thin Core Interferometric Technique. Photonics 2019, 6, 11. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, K.; Chen, K.P. Multiplexable Fiber pH Sensors Enabled by Intrinsic Fabry-Peìrot Interferometer Array. IEEE Sens. J. 2022, 22, 2167–2171. [Google Scholar] [CrossRef]
- Wang, Y.R.; Tou, Z.Q.; Ravikumar, R.; Lim, Y.Y.; Ding, Z.W.; Zhao, C.L.; So, P.L.; Chan, C.C. Reflection-Based Thin-Core Modal Interferometry Optical Fiber Functionalized With PAA-PBA/PVA for Glucose Detection Under Physiological pH. J. Light. Technol. 2019, 37, 2773–2777. [Google Scholar] [CrossRef]
- Ran, Y.; Xiao, P.; Zhang, Y.; Hu, D.; Xu, Z.; Liang, L.; Guan, B.-O. A Miniature pH Probe Using Functional Microfiber Bragg Grating. Optics 2020, 1, 202–212. [Google Scholar] [CrossRef]
- Saha, N.; Brunetti, G.; Armenise, M.N.; Ciminelli, C. A Compact, Highly Sensitive pH Sensor Based on Polymer Waveguide Bragg Grating. IEEE Photonics J. 2023, 15, 2200607. [Google Scholar] [CrossRef]
- Urriza-Arsuaga, I.; Ielasi, G.; Bedoya, M.; Orellana, G. Luminescence-Based Sensors for Bioprocess Applications. In Fluorescence in Industry; Pedras, B., Ed.; Springer Series on Fluorescence; Springer International Publishing: Cham, Switzerland, 2019; Volume 18, pp. 1–38. ISBN 978-3-030-20032-9. [Google Scholar]
- Chen, Z.; Meng, X.; Xie, M.; Shi, Y.; Zou, L.; Guo, S.; Jiang, J.; Liu, S.; Zhao, Q. A self-calibrating phosphorescent polymeric probe for measuring pH fluctuations in subcellular organelles and the zebrafish digestive tract. J. Mater. Chem. C 2020, 8, 2265–2271. [Google Scholar] [CrossRef]
- Gaston, A.; Lozano, I.; Perez, F.; Auza, F.; Sevilla, J. Evanescent wave optical-fiber sensing (temperature, relative humidity, and ph sensors). IEEE Sens. J. 2003, 3, 806–811. [Google Scholar] [CrossRef]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering-Gallery Sensors. Matter 2020, 3, 371–392. [Google Scholar] [CrossRef]
- Verma, S.; Pathak, A.K.; Rahman, B.M.A. Review of Biosensors Based on Plasmonic-Enhanced Processes in the Metallic and Meta-Material-Supported Nanostructures. Micromachines 2024, 15, 502. [Google Scholar] [CrossRef] [PubMed]
- Roriz, P.; Silva, S.; Frazão, O.; Novais, S. Optical Fiber Temperature Sensors and Their Biomedical Applications. Sensors 2020, 20, 2113. [Google Scholar] [CrossRef]
- Wang, C.; Su, X.; Brown, T.D.; Ohodnicki, P.R. Novel sensing materials for harsh environment subsurface pH sensing applications. In Fiber Optic Sensors and Applications XII; Pickrell, G., Udd, E., Du, H.H., Eds.; SPIE: Baltimore, MD, USA, 2015; p. 94800C. [Google Scholar]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Husain, I.; Choudhury, A.; Nath, P. Fiber-Optic Volumetric Sensor Based on Beer-Lambert Principle. IEEE Sens. J. 2013, 13, 3345–3346. [Google Scholar] [CrossRef]
- Olorounto, O.G.; Deniau, G.; Zekri, E.; Doizi, D.; Bertrand, J.; Corbas, V. Development of Optical Sensors Based on Neutral Red Absorbance for Real-Time pH Measurements. Sensors 2024, 24, 5610. [Google Scholar] [CrossRef]
- Wijaya, E.; Lenaerts, C.; Maricot, S.; Hastanin, J.; Habraken, S.; Vilcot, J.-P.; Boukherroub, R.; Szunerits, S. Surface plasmon resonance-based biosensors: From the development of different SPR structures to novel surface functionalization strategies. Curr. Opin. Solid State Mater. Sci. 2011, 15, 208–224. [Google Scholar] [CrossRef]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface Plasmon Resonance for Cell-Based Clinical Diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.H.; Park, K.S.; Eom, J.B.; Kim, M.J.; Rho, B.S.; Choi, H.Y. Interferometric Fiber Optic Sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [CrossRef] [PubMed]
- Majewska, K.; Opoka, S.; Kudela, P.; Ostachowicz, W. Novel FBG rosette for determining impact location in thin plate-like structure. J. Phys. Conf. Ser. 2015, 628, 012118. [Google Scholar] [CrossRef]
- Yan, T.; Weng, F.; Ming, Y.; Zhu, S.; Zhu, M.; Wang, C.; Guo, C.; Zhu, K. Luminescence Probes in Bio-Applications: From Principle to Practice. Biosensors 2024, 14, 333. [Google Scholar] [CrossRef]
- Messica, A.; Greenstein, A.; Katzir, A. Theory of fiber-optic, evanescent-wave spectroscopy and sensors. Appl. Opt. 1996, 35, 2274. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, A.; Pratiwi, A.C.; Suryanti, V. Evanescent Wave Absorption Based Fiber Sensor for Measuring Glucose Solution Concentration. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012016. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Cocker, E.D.; Piyawattanametha, W.; Jung, J.C.; Cheung, E.L.M.; Schnitzer, M.J. Fiber-optic fluorescence imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef]
- Puyol, M.; Villuendas, F.; Domínguez, C.; Cadarso, V.; Llobera, A.; Salinas, I.; Garcés, I.; Alonso, J. Absorbance-Based Integrated Optical Sensors. In Frontiers in Chemical Sensors; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–44. ISBN 978-3-540-27756-9. [Google Scholar]
- Liu, Y.; Peng, W. Fiber-Optic Surface Plasmon Resonance Sensors and Biochemical Applications: A Review. J. Light. Technol. 2021, 39, 3781–3791. [Google Scholar] [CrossRef]
- Meggitt, B.T. Fiber optic white-light interferometric sensors. In Optical Fiber Sensor Technology; Grattan, K.T.V., Meggitt, B.T., Eds.; Optical and Quantum Electronics Series; Springer: Dordrecht, The Netherlands, 1995; Volume 1, pp. 269–312. ISBN 978-94-010-4530-8. [Google Scholar]
- Kok, S.P.; Go, Y.I.; Wang, X.; Wong, M.L.D. Advances in Fiber Bragg Grating (FBG) Sensing: A Review of Conventional and New Approaches and Novel Sensing Materials in Harsh and Emerging Industrial Sensing. IEEE Sens. J. 2024, 24, 29485–29505. [Google Scholar] [CrossRef]
- McSherry, M.; Fitzpatrick, C.; Lewis, E. Review of luminescent based fibre optic temperature sensors. Sens. Rev. 2005, 25, 56–62. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pang, F.; Wang, T. Theoretical Analysis of Fiber-Optic Evanescent Wave Sensors. In Proceedings of the 2008 China-Japan Joint Microwave Conference, Shanghai, China, 10–12 September 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 583–587. [Google Scholar]
- Zacharioudaki, D.-E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef] [PubMed]
- Kundu, T.; Sai, V.V.R.; Dutta, R.; Titas, S.; Kumar, P.; Mukherjee, S. Development of evanescent wave absorbance-based fibre-optic biosensor. Pramana-J. Phys. 2010, 75, 1099–1113. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, Y.-W.; Kim, T.-H. Recent Advances in Surface Plasmon Resonance Sensors for Sensitive Optical Detection of Pathogens. Biosensors 2022, 12, 180. [Google Scholar] [CrossRef]
- Gallego, D.; Lamela, H. High sensitivity interferometric polymer optical fiber ultrasound sensors for optoacoustic imaging and biomedical application. In Proceedings of the 21st International Conference on Optical Fibre Sensors (OFS21), Ottawa, ON, Canada, 15–19 May 2011; p. 775370. [Google Scholar]
- Mishra, V.; Lohar, M.; Amphawan, A. Improvement in temperature sensitivity of FBG by coating of different materials. Optik 2016, 127, 825–828. [Google Scholar] [CrossRef]
- Lippitsch, M.E.; Draxler, S.; Kieslinger, D. Luminescence lifetime-based sensing: New materials, new devices. Sens. Actuators B Chem. 1997, 38, 96–102. [Google Scholar] [CrossRef]
- Romanova, E.; Korsakova, S.; Rozhnev, A.; Sukhanov, M.; Velmuzhov, A.; Kotereva, T.; Shiryaev, V. All—Fiber Evanescent Wave Sensors for the Mid-Infrared Spectroscopy of Liquids. In Proceedings of the 2019 Conference on Lasers and Electro-Optics Europe & European Quantum Electronics Conference (CLEO/Europe-EQEC), Munich, Germany, 23–27 June 2019; IEEE: Piscataway, NJ, USA, 2019; p. 1. [Google Scholar]
- Mukunda, D.C.; Joshi, V.K.; Mahato, K.K. Light emitting diodes (LEDs) in fluorescence-based analytical applications: A review. Appl. Spectrosc. Rev. 2022, 57, 1–38. [Google Scholar] [CrossRef]
- O’Toole, M.; Diamond, D. Absorbance Based Light Emitting Diode Optical Sensors and Sensing Devices. Sensors 2008, 8, 2453–2479. [Google Scholar] [CrossRef]
- Prabowo, B.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, L.; Jin, W.; Yang, J. Multiplexing of white light interferometric sensors using a fiber loop topology. In Proceedings of the 2002 15th Optical Fiber Sensors Conference Technical Digest, Portland, OR, USA, 10 May 2002; IEEE: Piscataway, NJ, USA, 2002; Volume 1, pp. 467–470. [Google Scholar]
- Werneck, M.M.; Allil, R.C.S.B.; Ribeiro, B.A.; De Nazar, F.V.B. A Guide to Fiber Bragg Grating Sensors. In Current Trends in Short- and Long-Period Fiber Gratings; Cuadrado-Laborde, C., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-1131-3. [Google Scholar]
- Campo, J.C.; Barragan, N.A.; Perez, M.A.; Alvarez, J.C. A comparison between different excitation/detection systems for luminescence lifetime based instrumentation. In Proceedings of the IMTC/2002—19th IEEE Instrumentation and Measurement Technology Conference, Anchorage, AK, USA, 21–23 May 2002; IEEE: Piscataway, NJ, USA, 2002; Volume 1, pp. 459–462. [Google Scholar]
- Willer, U.; Scheel, D.; Kostjucenko, I.; Bohling, C.; Schade, W.; Faber, E. Fiber-optic evanescent-field laser sensor for in-situ gas diagnostics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 2427–2432. [Google Scholar] [CrossRef]
- Matthews, D.R.; Summers, H.D.; Njoh, K.; Errington, R.J.; Smith, P.J.; Barber, P.; Ameer-Beg, S.; Vojnovic, B. Technique for measurement of fluorescence lifetime by use of stroboscopic excitation and continuous-wave detection. Appl. Opt. 2006, 45, 2115. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, J.; Sharma, I. Fiber optic evanescent wave absorption-based sensors: A detailed review of advancements in the last decade (2007–18). Optik 2019, 183, 1008–1025. [Google Scholar] [CrossRef]
- Nguyen, H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [PubMed]
- Spammer, S.J.; Swart, P.L.; Booysen, A. Interferometric distributed optical-fiber sensor. Appl. Opt. 1996, 35, 4522. [Google Scholar] [CrossRef]
- Shivananju, B.N.; Renilkumar, M.; Prashanth, G.R.; Asokan, S.; Varma, M.M. Detection Limit of Etched Fiber Bragg Grating Sensors. J. Light. Technol. 2013, 31, 2441–2447. [Google Scholar] [CrossRef]
- De Acha, N.; Elosua, C.; Matias, I.; Arregui, F. Luminescence-Based Optical Sensors Fabricated by Means of the Layer-by-Layer Nano-Assembly Technique. Sensors 2017, 17, 2826. [Google Scholar] [CrossRef]
- Okazaki, T.; Watanabe, T.; Kuramitz, H. Evanescent-Wave Fiber Optic Sensing of the Anionic Dye Uranine Based on Ion Association Extraction. Sensors 2020, 20, 2796. [Google Scholar] [CrossRef]
- Thompson, R.B.; Lakowicz, J.R. Fiber optic pH sensor based on phase fluorescence lifetimes. Anal. Chem. 1993, 65, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.S.; Kumar, A.; Kumar, R.; Srivastava, S.K. A study of surface plasmon resonance (SPR) based biosensor with improved sensitivity. Photonics Nanostruct.—Fundam. Appl. 2018, 31, 99–106. [Google Scholar] [CrossRef]
- Liu, K.; Ferguson, S.M.; Measures, R.M. Fiber-optic interferometric sensor for the detection of acoustic emission within composite materials. Opt. Lett. 1990, 15, 1255. [Google Scholar] [CrossRef]
- Hisham, H.K. Fiber Bragg Grating Sensors: Development and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-27513-5. [Google Scholar]
- Zhang, K.; Carrod, A.J.; Del Giorgio, E.; Hughes, J.; Rurack, K.; Bennet, F.; Hodoroaba, V.-D.; Harrad, S.; Pikramenou, Z. Luminescence Lifetime-Based Sensing Platform Based on Cyclometalated Iridium(III) Complexes for the Detection of Perfluorooctanoic Acid in Aqueous Samples. Anal. Chem. 2024, 96, 1565–1575. [Google Scholar] [CrossRef]
- Jiao, L.; Zhong, N.; Zhao, X.; Ma, S.; Fu, X.; Dong, D. Recent advances in fiber-optic evanescent wave sensors for monitoring organic and inorganic pollutants in water. TrAC Trends Anal. Chem. 2020, 127, 115892. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Y. Digital signal processing for fluorescence-based fiber optic temperature sensor. In Proceedings of the Photonics Asia, Shanghai, China, 14 October 2002; Rao, Y.-J., Jones, J.D.C., Naruse, H., Chen, R.I., Eds.; SPIE: Baltimore, MD, USA, 2002; p. 98. [Google Scholar]
- Silveira, J.P.; Grasdepot, F. New signal processing for absorption-based gas sensing. Sens. Actuators B Chem. 1995, 29, 363–367. [Google Scholar] [CrossRef]
- Rodríguez-Schwendtner, E.; González-Cano, A.; Díaz-Herrera, N.; Navarrete, M.C.; Esteban, Ó. Signal processing in SPR fiber sensors: Some remarks and a new method. Sens. Actuators B Chem. 2018, 268, 150–156. [Google Scholar] [CrossRef]
- Griffin, B.; Connelly, M.J. Digital signal processing of interferometric fiber optic sensors. In Proceedings of the Lightwave Technologies in Instrumentation and Measurement Conference, Palisades, NY, USA, 20 October 2004; IEEE: Piscataway, NJ, USA, 2004; pp. 153–156. [Google Scholar]
- Fernández-Ruiz, M.R.; Carballar, A. Fiber Bragg Grating-Based Optical Signal Processing: Review and Survey. Appl. Sci. 2021, 11, 8189. [Google Scholar] [CrossRef]
- López-Ruiz, N.; Hernández-Bélanger, D.; Carvajal, M.A.; Capitán-Vallvey, L.F.; Palma, A.J.; Martínez-Olmos, A. Fast lifetime and amplitude determination in luminescence exponential decays. Sens. Actuators B Chem. 2015, 216, 595–602. [Google Scholar] [CrossRef]
- Akashah, M.H.N.; Makhsin, S.R.; Rani, R.A.; Saad, N.H.; Razak, K.A.; Gardner, P.; Scully, P.J. Signal Improvement on Fibre Optic Evanescent Wave Sensor Based Polymeric Sensitive Coating of Chitosan-Agarose Hydrogel. In Proceeding of 5th International Conference on Advances in Manufacturing and Materials Engineering, Proceedings of the ICAMME 2022, Kuala Lumpur, Malaysia, 9–10 August 2022; Maleque, M.A., Ahmad Azhar, A.Z., Sarifuddin, N., Syed Shaharuddin, S.I., Mohd Ali, A., Abdul Halim, N.F.H., Eds.; Lecture Notes in Mechanical Engineering; Springer Nature Singapore: Singapore, 2023; pp. 109–115. ISBN 978-981-19-9508-8. [Google Scholar]
- Moeglen Paget, B.; Vinod Ram, K.; Zhang, S.; Perumal, J.; Vedraine, S.; Humbert, G.; Olivo, M.; Dinish, U.S. A review on photonic crystal fiber based fluorescence sensing for chemical and biomedical applications. Sens. Actuators B Chem. 2024, 400, 134828. [Google Scholar] [CrossRef]
- Ochoa, M.; Algorri, J.F.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Recent Advances in Biomedical Photonic Sensors: A Focus on Optical-Fibre-Based Sensing. Sensors 2021, 21, 6469. [Google Scholar] [CrossRef]
- Burgess, L.W. Absorption-based sensors. Sens. Actuators B Chem. 1995, 29, 10–15. [Google Scholar] [CrossRef]
- Rifat, A.A.; Rabiul Hasan, M.; Ahmed, R.; Miroshnichenko, A.E. Microstructured Optical Fiber-Based Plasmonic Sensors. In Computational Photonic Sensors; Springer International Publishing: Cham, Switzerland, 2019; pp. 203–232. ISBN 978-3-319-76555-6. [Google Scholar]
- Nguyen Thi Nhat, H.; Le, N.T.T.; Phuong Phong, N.T.; Nguyen, D.H.; Nguyen-Le, M.-T. Potential Application of Gold Nanospheres as a Surface Plasmon Resonance Based Sensor for In-Situ Detection of Residual Fungicides. Sensors 2020, 20, 2229. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Al-Hadad, M.; Han, X.; Xu, D.; Tan, Y.; Zhou, Z. Recent Progress in Fiber-Optic Acoustic Sensor and Its Applications: A Review. IEEE Sens. J. 2024, 24, 25249–25260. [Google Scholar] [CrossRef]
- Kori, T.; Kori, A.; Kori, A.; Nandi, S. A Study on Fiber Bragg Gratings and Its Recent Applications. In Innovative Mobile and Internet Services in Ubiquitous Computing; Barolli, L., Xhafa, F., Hussain, O.K., Eds.; Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2020; Volume 994, pp. 880–889. ISBN 978-3-030-22262-8. [Google Scholar]
- Pedras, B.; Orellana, G.; Berberan-Santos, M.N. Luminescence-Based Sensors for Aeronautical Applications. In Fluorescence in Industry; Pedras, B., Ed.; Springer Series on Fluorescence; Springer International Publishing: Cham, Switzerland, 2019; Volume 18, pp. 389–411. ISBN 978-3-030-20032-9. [Google Scholar]
- Khonina, S.N.; Kazanskiy, N.L.; Butt, M.A. Optical Fibre-Based Sensors—An Assessment of Current Innovations. Biosensors 2023, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Fouad, S. Fiber Optic Sensors: Short Review and Applications. In Recent Trends in Physics of Material Science and Technology; Gaol, F.L., Shrivastava, K., Akhtar, J., Eds.; Springer: Singapore, 2015; pp. 299–311. ISBN 978-981-287-128-2. [Google Scholar]
- Shpacovitch, V.; Hergenröder, R. Surface Plasmon Resonance (SPR)-Based Biosensors as Instruments with High Versatility and Sensitivity. Sensors 2020, 20, 3010. [Google Scholar] [CrossRef]
- Martincek, I.; Kacik, D.; Horak, J. Interferometric optical fiber sensor for monitoring of dynamic railway traffic. Opt. Laser Technol. 2021, 140, 107069. [Google Scholar] [CrossRef]
- Iadicicco, A.; Cutolo, A.; Cusano, A. Fiber Bragg Grating Sensors—Advancements and Industrial Applications. Adv. Sci. Technol. 2008, 55, 213–222. [Google Scholar]
- Knöbl, Y.J.; Johnston, L.M.; Quílez-Alburquerque, J.; Orellana, G. Luminescence Lifetime-Based Water Conductivity Sensing Using a Cationic Dextran-Supported Ru(II) Polypyridyl Complex. Sensors 2024, 25, 121. [Google Scholar] [CrossRef]
- Iadicicco, A.; Paladino, D.; Campopiano, S.; Bock, W.J.; Cutolo, A.; Cusano, A. Evanescent wave sensor based on permanently bent single mode optical fiber. Sens. Actuators B Chem. 2011, 155, 903–908. [Google Scholar] [CrossRef]
- Ehrlich, K.; Choudhary, T.R.; Ucuncu, M.; Megia-Fernandez, A.; Harrington, K.; Wood, H.A.C.; Yu, F.; Choudhury, D.; Dhaliwal, K.; Bradley, M.; et al. Time-Resolved Spectroscopy of Fluorescence Quenching in Optical Fibre-Based pH Sensors. Sensors 2020, 20, 6115. [Google Scholar] [CrossRef]
- Kumar, M.; Khamis, K.; Stevens, R.; Hannah, D.M.; Bradley, C. In-situ optical water quality monitoring sensors—Applications, challenges, and future opportunities. Front. Water 2024, 6, 1380133. [Google Scholar] [CrossRef]
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Lashari, G.A.; Mumtaz, F.; Ai, Z.; Dai, Y. Recent advancements and future challenges in hybrid optical fiber interferometers. Optik 2023, 282, 170860. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, H.; Kim, H.; Cho, J.; Jeong, M.; Kim, C.-S. A Fiber Bragg Grating Sensor Interrogation System Based on a Linearly Wavelength-Swept Thermo-Optic Laser Chip. Sensors 2014, 14, 16109–16116. [Google Scholar] [CrossRef] [PubMed]
- Lippitsch, M.E.; Draxler, S. Luminescence decay-time-based optical sensors: Principles and problems. Sens. Actuators B Chem. 1993, 11, 97–101. [Google Scholar] [CrossRef]
- Xin, X.; Zhong, N.; Liao, Q.; Cen, Y.; Wu, R.; Wang, Z. High-sensitivity four-layer polymer fiber-optic evanescent wave sensor. Biosens. Bioelectron. 2017, 91, 623–628. [Google Scholar] [CrossRef]
- Werner, J.; Belz, M.; Klein, K.-F.; Sun, T.; Grattan, K.T.V. Fiber optic sensor designs and luminescence-based methods for the detection of oxygen and pH measurement. Measurement 2021, 178, 109323. [Google Scholar] [CrossRef]
- Cai, Y.; Li, M.; Wang, M.; Li, J.; Zhang, Y.; Zhao, Y. Optical Fiber Sensors for Metal Ions Detection Based on Novel Fluorescent Materials. Front. Phys. 2020, 8, 598209. [Google Scholar] [CrossRef]
- Sánchez-Escobar, S.; Hernández-Cordero, J. Fiber optic fluorescence temperature sensors using up-conversion from rare-earth polymer composites. Opt. Lett. 2019, 44, 1194. [Google Scholar] [CrossRef]
- Cennamo, N.; Mattiello, F.; Galatus, R.V.; Voiculescu, E.; Zeni, L. Plasmonic Sensing in D-Shaped POFs With Fluorescent Optical Fibers as Light Sources. IEEE Trans. Instrum. Meas. 2018, 67, 754–759. [Google Scholar] [CrossRef]
- Chu, C.-S.; Su, C.-J. Fluorescence Ratiometric Optical Broad Range pH Sensor Based on CdSe/ZnS Quantum Dots and O170 Embedded in Ethyl Cellulose Matrix. J. Light. Technol. 2018, 36, 857–862. [Google Scholar] [CrossRef]
- Moradi, V.; Akbari, M.; Wild, P. A fluorescence-based pH sensor with microfluidic mixing and fiber optic detection for wide range pH measurements. Sens. Actuators A Phys. 2019, 297, 111507. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Venugopala, T.; Chen, S.; Sun, T.; Grattan, K.T.V.; Taylor, S.E.; Basheer, P.A.M.; Long, A.E. Fluorescence based fibre optic pH sensor for the pH 10–13 range suitable for corrosion monitoring in concrete structures. Sens. Actuators B Chem. 2014, 191, 498–507. [Google Scholar] [CrossRef]
- Chen, W.-H.; Armstrong, E.; Dillingham, P.W.; Moratti, S.C.; Ennis, C.; McGraw, C.M. Dual-Lifetime Referencing (t-DLR) Optical Fiber Fluorescent pH Sensor for Microenvironments. Sensors 2023, 23, 8865. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Huang, Q.; Wu, X.; Nie, Y.; Yang, M. Optical fiber sensors for water and air quality monitoring: A review. Opt. Eng. 2023, 63, 031004. [Google Scholar] [CrossRef]
- Butt, M.A.; Voronkov, G.S.; Grakhova, E.P.; Kutluyarov, R.V.; Kazanskiy, N.L.; Khonina, S.N. Environmental Monitoring: A Comprehensive Review on Optical Waveguide and Fiber-Based Sensors. Biosensors 2022, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Qi, L.; Qin, W. Improving the Environmental Compatibility of Marine Sensors by Surface Functionalization with Graphene Oxide. Anal. Chem. 2019, 91, 13268–13274. [Google Scholar] [CrossRef]
- Murugan, K.; Jothi, V.K.; Rajaram, A.; Natarajan, A. Novel Metal-Free Fluorescent Sensor Based on Molecularly Imprinted Polymer N-CDs@MIP for Highly Selective Detection of TNP. ACS Omega 2022, 7, 1368–1379. [Google Scholar] [CrossRef]
- Panáček, D.; Zdražil, L.; Langer, M.; Šedajová, V.; Baďura, Z.; Zoppellaro, G.; Yang, Q.; Nguyen, E.P.; Álvarez-Diduk, R.; Hrubý, V.; et al. Graphene Nanobeacons with High-Affinity Pockets for Combined, Selective, and Effective Decontamination and Reagentless Detection of Heavy Metals. Small 2022, 18, 2201003. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, Y.; Nie, Y.; Jin, C.; Park, J.; Zhang, J.X.J. Dual fluorescent hollow silica nanofibers for in situ pH monitoring using an optical fiber. Nanoscale Adv. 2023, 5, 2180–2189. [Google Scholar] [CrossRef]
- Moulton, J.T.; Bruce, D.; Bunce, R.A.; Kim, M.; Snyder, L.O.; Seitz, W.R.; Lavine, B.K. Thermodynamic and Kinetic Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing. Molecules 2025, 30, 1416. [Google Scholar] [CrossRef]
- Bagchi, S.; Achla, R.; Mondal, S.K. Optical Fiber Sensor with ZnO Hierarchical Nano-Structures Grown on Electrospun ZnO Mat Base on the Sensor for Trace Level Detection of Volatile Amines. IEEE Sens. Lett. 2018, 2, 3500204. [Google Scholar] [CrossRef]
- De Acha, N.; Elía, V.; Delgado-Camón, A.; Arregui, F.J.; Elosúa, C. Straightforward nano patterning on optical fiber for sensors development. Opt. Lett. 2020, 45, 3877. [Google Scholar] [CrossRef]
- Mei, H.; Pan, J.; Zhang, Z.; Zhang, L.; Tong, L. Coiled Optical Nanofiber for Optofluidic Absorbance Detection. ACS Sens. 2019, 4, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef]
- Nivedha, S.; Ramesh Babu, P.; Senthilnathan, K. Surface Plasmon Resonance:Physics and Technology. Curr. Sci. 2018, 115, 56. [Google Scholar] [CrossRef]
- Lobry, M.; Loyez, M.; Chah, K.; Hassan, E.M.; Goormaghtigh, E.; DeRosa, M.C.; Wattiez, R.; Caucheteur, C. HER2 biosensing through SPR-envelope tracking in plasmonic optical fiber gratings. Biomed. Opt. Express 2020, 11, 4862. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, W.; Liu, Y.; Chen, X.; Qu, H.; Hu, X. Cladding Mode Fitting-Assisted Automatic Refractive Index Demodulation Optical Fiber Sensor Probe Based on Tilted Fiber Bragg Grating and SPR. Sensors 2022, 22, 3032. [Google Scholar] [CrossRef]
- Manuylovich, E.; Tomyshev, K.; Butov, O.V. Method for Determining the Plasmon Resonance Wavelength in Fiber Sensors Based on Tilted Fiber Bragg Gratings. Sensors 2019, 19, 4245. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Hassan, E.M.; Lobry, M.; Liu, F.; Caucheteur, C.; Wattiez, R.; DeRosa, M.C.; Willmore, W.G.; Albert, J. Rapid Detection of Circulating Breast Cancer Cells Using a Multiresonant Optical Fiber Aptasensor with Plasmonic Amplification. ACS Sens. 2020, 5, 454–463. [Google Scholar] [CrossRef]
- Leitao, C.; Pereira, S.O.; Alberto, N.; Lobry, M.; Loyez, M.; Costa, F.M.; Pinto, J.L.; Caucheteur, C.; Marques, C. Cortisol in-fiber ultrasensitive plasmonic immunosensing. IEEE Sens. J. 2021, 21, 3028–3034. [Google Scholar] [CrossRef]
- Hu, W.; Huang, Y.; Chen, C.; Liu, Y.; Guo, T.; Guan, B.-O. Highly sensitive detection of dopamine using a graphene functionalized plasmonic fiber-optic sensor with aptamer conformational amplification. Sens. Actuators B Chem. 2018, 264, 440–447. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Du, Y.; Wang, F.; Sun, Y.; Zhang, Y.; Jing, Z.; Peng, W. A tilted fiber Bragg grating pH sensor coated with polyaniline. In Proceedings of the 2021 19th International Conference on Optical Communications and Networks (ICOCN), Qufu, China, 23–27 August 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–3. [Google Scholar]
- Long, G.; Wan, L.; Xia, B.; Zhao, C.; Niu, K.; Hou, J.; Lyu, D.; Li, L.; Zhu, F.; Wang, N. Rapid-Response and Wide-Range pH Sensors Enabled by Self-Assembled Functional PAni/PAA Layer on No-Core Fiber. Materials 2022, 15, 7449. [Google Scholar] [CrossRef]
- Antohe, I.; Jinga, L.-I.; Antohe, V.-A.; Socol, G. Sensitive pH Monitoring Using a Polyaniline-Functionalized Fiber Optic—Surface Plasmon Resonance Detector. Sensors 2021, 21, 4218. [Google Scholar] [CrossRef] [PubMed]
- De Souza Filho, C.A.; Lima, A.M.N.; Neff, F.H. Modeling and Temperature Drift Compensation Method for Surface Plasmon Resonance-Based Sensors. IEEE Sens. J. 2017, 17, 6246–6257. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Liu, Q.; Peng, W. Temperature-Compensating Fiber-Optic Surface Plasmon Resonance Biosensor. IEEE Photon. Technol. Lett. 2016, 28, 213–216. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, L.; Qin, Y. Advancing Sensitivity in Guided-Wave Surface Plasmon Resonance Sensor through Integration of 2D BlueP/MoS2 Hybrid Layers. Biosensors 2023, 14, 25. [Google Scholar] [CrossRef]
- Tabasi, O.; Falamaki, C. Recent advancements in the methodologies applied for the sensitivity enhancement of surface plasmon resonance sensors. Anal. Methods 2018, 10, 3906–3925. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, R.; Wang, L.; Gu, D.; He, J.; Wu, S.-Y.; Ho, H.-P.; Li, X.; Qu, J.; Gao, B.Z.; et al. Recent advances in surface plasmon resonance imaging: Detection speed, sensitivity, and portability. Nanophotonics 2017, 6, 1017–1030. [Google Scholar] [CrossRef]
- Valle, A.; Ferreira, K.; Goulart, L.; Freire, C.; Medeiros, E.; Filho, C.S.; Cruz, R.; Rodrigues, L.; Moreira, C. Smartphone-based surface plasmon resonance sensor for glyphosate detection: Different pH and concentrations. Plasmonics 2023, 18, 821–830. [Google Scholar] [CrossRef]
- Capocefalo, A.; Mammucari, D.; Brasili, F.; Fasolato, C.; Bordi, F.; Postorino, P.; Domenici, F. Exploring the Potentiality of a SERS-Active pH Nano-Biosensor. Front. Chem. 2019, 7, 413. [Google Scholar] [CrossRef]
- Alberti, G.; Spina, S.; Arcadio, F.; Pesavento, M.; De Maria, L.; Cennamo, N.; Zeni, L.; Merli, D. MIP-Assisted 3-Hole POF Chip Faced with SPR-POF Sensor for Glyphosate Detection. Chemosensors 2023, 11, 414. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Telfah, A.D.; Shpacovitch, V.; Tavares, C.J.; Hergenröder, R. Switchable Polyacrylic Acid Polyelectrolyte Brushes for Surface Plasmon Resonance Applications. Sensors 2023, 23, 4283. [Google Scholar] [CrossRef]
- Miliou, A. In-Fiber Interferometric-Based Sensors: Overview and Recent Advances. Photonics 2021, 8, 265. [Google Scholar] [CrossRef]
- Aydin, D.; Barnes, J.A.; Loock, H.-P. In-fiber interferometry sensors for refractive index. Appl. Phys. Rev. 2023, 10, 011307. [Google Scholar] [CrossRef]
- Liao, S.; Wong, T. Optimal Design of Feedback-Interferometric Fiber Laser Sensors. IEEE Sens. J. 2019, 19, 12016–12023. [Google Scholar] [CrossRef]
- Al-Ithawi, S.; Hadi, A. Implementation of Pressure Sensor of Optical Fiber Using Optical Interferometer. Defect Diffus. Forum 2020, 398, 125–130. [Google Scholar]
- Liu, L.; Ren, Y.; Wang, M.; Zou, M. Optical Fiber Fabry-Perot Interferometer Sensor Fabricated by Femtosecond Laser-Induced Water Breakdown. Integr. Ferroelectr. 2020, 208, 55–59. [Google Scholar] [CrossRef]
- Bourdine, A.V.; Demidov, V.V.; Dukelskii, K.V.; Khokhlov, A.V.; Ter-Nersesyants, E.V.; Bureev, S.V.; Matrosova, A.S.; Pchelkin, G.A.; Kuznetsov, A.A.; Morozov, O.G.; et al. Six-Core GeO2-Doped Silica Microstructured Optical Fiber with Induced Chirality. Fibers 2023, 11, 28. [Google Scholar] [CrossRef]
- Bourdine, A.V.; Barashkin, A.Y.; Burdin, V.A.; Dashkov, M.V.; Demidov, V.V.; Dukelskii, K.V.; Evtushenko, A.S.; Ismail, Y.; Khokhlov, A.V.; Kuznetsov, A.A.; et al. Twisted Silica Microstructured Optical Fiber with Equiangular Spiral Six-Ray Geometry. Fibers 2021, 9, 27. [Google Scholar] [CrossRef]
- Zhao, Z.; Dang, Y.; Tang, M. Advances in Multicore Fiber Grating Sensors. Photonics 2022, 9, 381. [Google Scholar] [CrossRef]
- Guzmán-Sepúlveda, J.; Guzmán-Cabrera, R.; Torres-Cisneros, M.; Sánchez-Mondragón, J.; May-Arrioja, D. A Highly Sensitive Fiber Optic Sensor Based on Two-Core Fiber for Refractive Index Measurement. Sensors 2013, 13, 14200–14213. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Z.; Tang, M. Advances in Multicore Fiber Interferometric Sensors. Sensors 2023, 23, 3436. [Google Scholar] [CrossRef]
- Nagar, M.A.; Janner, D. Polymer-Based Optical Guided-Wave Biomedical Sensing: From Principles to Applications. Photonics 2024, 11, 972. [Google Scholar] [CrossRef]
- Morozov, O.; Agliullin, T.; Sakhabutdinov, A.; Kuznetsov, A.; Valeev, B.; Qaid, M.; Ponomarev, R.; Nurmuhametov, D.; Shmyrova, A. Development and Application of a High-sensitivity Acoustic Sensor Based on Open Cavity Fabry-Perot Interferometer. In Science and Technology—Recent Updates and Future Prospects; Jakóbczak, D.J., Ed.; B P International: London, UK, 2024; Volume 6, pp. 90–118. ISBN 978-81-974774-5-4. [Google Scholar]
- Chandrasekharan, H.K.; Wlodarczyk, K.L.; MacPherson, W.N.; Maroto-Valer, M.M. In-situ multicore fibre-based pH mapping through obstacles in integrated microfluidic devices. Sci. Rep. 2023, 14, 2839. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, J.; Arrizabalaga, O.; Antonio-Lopez, E.; Zubia, J.; De Ocáriz, I.S. Multicore Fiber Sensors. In Proceedings of the Optical Fiber Communication Conference, Los Angeles, CA, USA, 19–23 March 2017; OSA: Washington, DC, USA, 2017; p. Th3H.1. [Google Scholar]
- Morozov, O.; Tunakova, Y.; Hussein, S.M.R.H.; Shagidullin, A.; Agliullin, T.; Kuznetsov, A.; Valeev, B.; Lipatnikov, K.; Anfinogentov, V.; Sakhabutdinov, A. Addressed Combined Fiber-Optic Sensors as Key Element of Multisensor Greenhouse Gas Monitoring Systems. Sensors 2022, 22, 4827. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, L.; Xiao, D.; Zhao, K.; Lin, C.; Si, L. An Accurate, Flexible and Small Optical Fiber Sensor: A Novel Technological Breakthrough for Real-Time Analysis of Dynamic Blood Flow Data In Vivo. PLoS ONE 2014, 9, e114794. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Xiao, K.; Ushakov, N.; Kumar, S.; Li, X.; Min, R. Portable optical fiber biosensors integrated with smartphone: Technologies, applications, and challenges [Invited]. Biomed. Opt. Express 2024, 15, 1630–1650. [Google Scholar] [CrossRef]
- Butt, M.A. Topic Editorial on Fiber-Optic Sensors. Micromachines 2024, 15, 1452. [Google Scholar] [CrossRef] [PubMed]
- Lenner, M.; Yang, L.; Frank, A.; Bohnert, K. Long-term reliability of fiber-optic sensor components in harsh industrial environments. In Proceedings of the Advanced Photonics 2017 (IPR, NOMA, Sensors, Networks, SPPCom, PS), New Orleans, LA, USA, 24–27 July 2017; OSA: Washington, DC, USA, 2017; p. SeW1E.3. [Google Scholar]
- Mihailov, S.J. Fiber Bragg Grating Sensors for Harsh Environments. Sensors 2012, 12, 1898–1918. [Google Scholar] [CrossRef]
- Li, C.; Yang, W.; Wang, M.; Yu, X.; Fan, J.; Xiong, Y.; Yang, Y.; Li, L. A Review of Coating Materials Used to Improve the Performance of Optical Fiber Sensors. Sensors 2020, 20, 4215. [Google Scholar] [CrossRef]
- Stolov, A.A.; Wrubel, J.A.; Li, J.; Hines, M.J. Coatings for Harsh Environment Applications of Optical Fibers. In Proceedings of the Workshop on Specialty Optical Fibers and Their Applications, Hong Kong, China, 4–6 November 2015; OSA: Washington, DC, USA, 2015; p. WF4A.3. [Google Scholar]
- Lu, Q.; Liu, Z.; Zhang, H.; Xue, L.; Chen, Z. Optical pH sensor based on fiber interferometer coated with intelligent hydrogel. In Proceedings of the Advanced Sensor Systems and Applications XIII, Beijing, China, 14–16 October 2023; Yang, M., Fan, X., Zhang, J., Kim, C.-S., Eds.; SPIE: Baltimore, MD, USA, 2023; p. 23. [Google Scholar]
- Feng, C.; Niu, H.; Wang, H.; Wang, D.; Wei, L.; Ju, T.; Yuan, L. Probe-Type Multi-Core Fiber Optic Sensor for Simultaneous Measurement of Seawater Salinity, Pressure, and Temperature. Sensors 2024, 24, 1766. [Google Scholar] [CrossRef]
- Bharathan, G.; Fernandez, T.T.; Ams, M.; Carrée, J.-Y.; Poulain, S.; Poulain, M.; Fuerbach, A. Femtosecond laser direct-written fiber Bragg gratings with high reflectivity and low loss at wavelengths beyond 4 μm. Opt. Lett. 2020, 45, 4316. [Google Scholar] [CrossRef]
- Roberge, A.; Loranger, S.; Boisvert, J.-S.; Monet, F.; Kashyap, R. Femtosecond laser direct-writing of high quality first-order Bragg gratings with arbitrary complex apodization by phase modulation. Opt. Express 2022, 30, 30405. [Google Scholar] [CrossRef] [PubMed]
- Sahota, J.K.; Gupta, N.; Dhawan, D. Fiber Bragg grating sensors for monitoring of physical parameters: A comprehensive review. Opt. Eng. 2020, 59, 060901. [Google Scholar] [CrossRef]
- Nadeem, M.D.; Raghuwanshi, S.K.; Kumar, S. Recent Advancement of Phase Shifted Fiber Bragg Grating Sensor for Ultrasonic Wave Application: A Review. IEEE Sens. J. 2022, 22, 7463–7474. [Google Scholar] [CrossRef]
- Pabbisetti, V.N.K.; Madhuvarasu, S.S. Hydrogel-coated fiber Bragg grating sensor for pH monitoring. Opt. Eng. 2016, 55, 066112. [Google Scholar] [CrossRef]
- Lopez Aldaba, A.; González-Vila, Á.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018, 254, 1087–1093. [Google Scholar] [CrossRef]
- Gu, B.; Yin, M.; Zhang, A.P.; Qian, J.; He, S. Optical fiber relative humidity sensor based on FBG incorporated thin-core fiber modal interferometer. Opt. Express 2011, 19, 4140. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Zourab, S.M.; Kodeh, F.S.; Abd El-Salam, F.H.; Baker, S.A. Sol–gel entrapment of bromothymol blue (BTB) indicator in the presence of cationic 16E1Q and 16E1QS surfactants. J. Sol-Gel Sci. Technol. 2016, 79, 628–636. [Google Scholar] [CrossRef]
- Legett, S.A.; Stockdale, J.R.; Torres, X.; Yeager, C.M.; Pacheco, A.; Labouriau, A. Functional Filaments: Creating and Degrading pH-Indicating PLA Filaments for 3D Printing. Polymers 2023, 15, 436. [Google Scholar] [CrossRef]
- Cheng, X.; Bonefacino, J.; Guan, B.O.; Tam, H.Y. All-polymer fiber-optic pH sensor. Opt. Express 2018, 26, 14610. [Google Scholar] [CrossRef]
- Yulianti, I.; Sahmah, A.; Supa’at, M.; Idrus, M.; Kassim, N.M.; Al-hetar, A.M.; Hashim, A.M.; Arora, V.K. Fiber Bragg Grating Based pH Sensor. AIP Conf. Proc. 2011, 1341, 424–428. [Google Scholar]
- Lin, Y.; Chen, S.; Wang, M.; Liu, W. Fiber-optic fast response pH sensor in fiber Bragg gating using intelligent hydrogel coatings. Opt. Eng. 2015, 54, 057107. [Google Scholar] [CrossRef]
- Janting, J.; Pedersen, J.K.M.; Woyessa, G.; Nielsen, K.; Bang, O. Small and Robust All-Polymer Fiber Bragg Grating Based pH Sensor. J. Light. Technol. 2019, 37, 4480–4486. [Google Scholar] [CrossRef]
- Lei, X.; Dong, B.; Gong, J.; Wang, A.; Chen, W. PH sensor using fiber Bragg grating based on swelling of hydrogel. In Proceedings of the Photonics Asia, Beijing, China, 14 October 2002; Culshaw, B., Liao, Y., Wang, A., Bao, X., Fan, X., Eds.; SPIE: Baltimore, MD, USA, 2012; p. 856111. [Google Scholar]
- Shao, L.Y.; Yin, M.J.; Tam, H.Y.; Albert, J. Fiber optic pH sensor with self-assembled multilayer nanocoatings on tilted FBG. In Proceedings of the OFS2012 22nd International Conference on Optical Fiber Sensors, Beijing, China, 15–19 October 2012; Liao, Y., Jin, W., Sampson, D.D., Yamauchi, R., Chung, Y., Nakamura, K., Rao, Y., Eds.; SPIE: Baltimore, MD, USA, 2012; p. 84216A. [Google Scholar]
- Dhara, P.; Singh, V.K. Improved mesostructure by incorporating surfactant on thin film to develop an advanced optical fiber pH sensor with a temperature cross sensitivity feature. Laser Phys. 2017, 27, 035101. [Google Scholar] [CrossRef]
- Vivaldi, F.; Salvo, P.; Poma, N.; Bonini, A.; Biagini, D.; Del Noce, L.; Melai, B.; Lisi, F.; Francesco, F.D. Recent Advances in Optical, Electrochemical and Field Effect pH Sensors. Chemosensors 2021, 9, 33. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Z.; Zheng, Y.; Du, Y.; Wang, Y.; Yue, X.; Ding, Y.; Ran, Y.; Guan, B.-O. Dual-modal fiber-optic sensor for simultaneous pH and temperature monitoring in tumor microenvironment. Sens. Actuators A Phys. 2024, 377, 115729. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, H.; Yang, Q.; Xu, Z.; Wang, W.; Yuan, Z.; Li, Z.; Cao, S.; Guan, B.-O.; Qiu, L.; et al. A capillary-aided microfiber Bragg grating pH sensor for hydrovoltaic technology. Talanta 2024, 274, 125958. [Google Scholar] [CrossRef]
- Yan, R.; Sang, G.; Yin, B.; Wu, S.; Wang, M.; Hou, B.; Gao, M.; Chen, R.; Yu, H. Temperature self-calibrated pH sensor based on GO/PVA-coated MZI cascading FBG. Opt. Express 2021, 29, 13530. [Google Scholar] [CrossRef]
- Binetti, L.; Stankiewicz, A.; Alwis, L.S.M. Graphene-Oxide and Hydrogel Coated FBG-Based pH Sensor for Biomedical Applications. Proceedings 2018, 2, 789. [Google Scholar]
- Azhari, A.; Yilman, D.; Chan, B.; Alemohammad, H.; Liang, R.; Pope, M.; Mathers, K.E.; Chang, C. Development of fiber Bragg grating pH sensors for harsh environments. In Proceedings of the Fiber Optic Sensors and Applications XV, Orlando, FL, USA, 17–18 April 2018; Du, H.H., Mendez, A., Baldwin, C.S., Eds.; SPIE: Baltimore, MD, USA, 2018; p. 25. [Google Scholar]
- Aruna, N. Monitoring of pH by using stimulus responsive hydrogel and Fiber Bragg grating for bioreactor application. In Proceedings of the Biomedical Imaging and Sensing Conference 2021, Online, Japan, 20–22 April 2021; Matoba, O., Awatsuji, Y., Luo, Y., Yatagai, T., Aizu, Y., Eds.; SPIE: Baltimore, MD, USA, 2021; p. 47. [Google Scholar]
- Lei, M.; Zhang, Y.-N.; Han, B.; Zhao, Q.; Zhang, A.; Fu, D. In-Line Mach–Zehnder Interferometer and FBG With Smart Hydrogel for Simultaneous pH and Temperature Detection. IEEE Sens. J. 2018, 18, 7499–7504. [Google Scholar] [CrossRef]
- Hartings, M.R.; Castro, N.J.; Gill, K.; Ahmed, Z. A photonic pH sensor based on photothermal spectroscopy. Sens. Actuators B Chem. 2019, 301, 127076. [Google Scholar] [CrossRef]
- Knöbl, Y.J.; Bedoya, M.; Farquharson, A.; Courtney, P.; Orellana, G. Wide range luminescence lifetime-based pH sensing with covalently immobilized multi-protonatable Ru(II) complexes. Sens. Actuators B Chem. 2025, 425, 136960. [Google Scholar] [CrossRef]
- New Luminescent Europium Complexes as Indicators and in Sensors for pH in Biosamples and Water Samples. Available online: https://inis.iaea.org/records/gfxkn-zpn88 (accessed on 7 February 2025).
- Kosch, U.; Klimant, I.; Werner, T.; Wolfbeis, O.S. Strategies to Design pH Optodes with Luminescence Decay Times in the Microsecond Time Regime. Anal. Chem. 1998, 70, 3892–3897. [Google Scholar] [CrossRef]
- Belokrylov, M.E.; Kambur, D.A.; Konstantinov, Y.A.; Claude, D.; Barkov, F.L. An Optical Frequency Domain Reflectometer’s (OFDR) Performance Improvement via Empirical Mode Decomposition (EMD) and Frequency Filtration for Smart Sensing. Sensors 2024, 24, 1253. [Google Scholar] [CrossRef]
- Botewad, S.N.; Pahurkar, V.G.; Muley, G.G.; Gaikwad, D.K.; Bodkhe, G.A.; Shirsat, M.D.; Pawar, P.P. PANI-ZnO Cladding-Modified Optical Fiber Biosensor for Urea Sensing Based on Evanescent Wave Absorption. Front. Mater. 2020, 7, 184. [Google Scholar] [CrossRef]
- Tosi, D. Advanced Interrogation of Fiber-Optic Bragg Grating and Fabry-Perot Sensors with KLT Analysis. Sensors 2015, 15, 27470–27492. [Google Scholar] [CrossRef] [PubMed]
- Sahabutdinov, A.Z.; Morozov, O.G.; Agliullin, T.A.; Gubaidullin, R.R.; Ivanov, V. Modeling of Spectrum Response of Addressed FBG-Structures in Load Sensing Bearings. In Proceedings of the 2020 Systems of Signals Generating and Processing in the Field of on Board Communications, Moscow, Russia, 19–20 March 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Morozov, O.G.; Sakhabutdinov, A.J. Addressed fiber bragg structures in quasi-distributed microwave-photonic sensor systems. Comput. Opt. 2019, 43, 535–543. [Google Scholar] [CrossRef]
- Jin, L.; Shi, L.; Shi, W.; Meng, Z.; Shang, L.; Shen, Y. Fluorescence lifetime-based pH sensing by platinum nanoclusters. Analyst 2019, 144, 3533–3538. [Google Scholar] [CrossRef]
- Koren, K.; Moßhammer, M.; Scholz, V.V.; Borisov, S.M.; Holst, G.; Kühl, M. Luminescence Lifetime Imaging of Chemical Sensors—A Comparison between Time-Domain and Frequency-Domain Based Camera Systems. Anal. Chem. 2019, 91, 3233–3238. [Google Scholar] [CrossRef]
- Kocincová, A.S. Coating Materials for Optical Fiber Sensors. Ph.D. Thesis, Faculty of Chemistry and Pharmacy, University of Regensburg, Regensburg, Germany, 2011. Available online: https://core.ac.uk/download/pdf/11550213.pdf (accessed on 5 February 2025).
- Avolio, R.; Grozdanov, A.; Avella, M.; Barton, J.; Cocca, M.; De Falco, F.; Dimitrov, A.T.; Errico, M.E.; Fanjul-Bolado, P.; Gentile, G.; et al. Review of pH sensing materials from macro- to nano-scale: Recent developments and examples of seawater applications. Crit. Rev. Environ. Sci. Technol. 2022, 52, 979–1021. [Google Scholar] [CrossRef]
- Su, X.; Wen, Y.; Yuan, W.; Xu, M.; Liu, Q.; Huang, C.; Li, F. Lifetime-based nanothermometry in vivo with ultra-long-lived luminescence. Chem. Commun. 2020, 56, 10694–10697. [Google Scholar] [CrossRef]
- Al-Qaysi Wafaa _Thesis on 15-11-2018.pdf. n.d. Available online: https://epub.uni-regensburg.de/38035/1/Al-Qaysi%20Wafaa%20_Thesis%20on%2015-11-2018.pdf (accessed on 5 February 2025).
- Kong, M.; Gu, Y.; Liu, Y.; Shi, Y.; Wu, N.; Feng, W.; Li, F. Luminescence Lifetime–Based In Vivo Detection with Responsive Rare Earth–Dye Nanocomposite. Small 2019, 15, 1904487. [Google Scholar] [CrossRef]
- Memon, S.F.; Ali, M.M.; Pembroke, J.T.; Chowdhry, B.S.; Lewis, E. Measurement of Ultralow Level Bioethanol Concentration for Production Using Evanescent Wave Based Optical Fiber Sensor. IEEE Trans. Instrum. Meas. 2018, 67, 780–788. [Google Scholar] [CrossRef]
- Lopes, G.; Cennamo, N.; Zeni, L.; Singh, R.; Kumar, S.; Fernandes, A.J.S.; Costa, F.; Pereira, S.O.; Marques, C. Innovative optical pH sensors for the aquaculture sector: Comprehensive characterization of a cost-effective solution. Opt. Laser Technol. 2024, 171, 110355. [Google Scholar] [CrossRef]
- Botewad, S.N.; Pahurkar, V.G.; Muley, G.G. Fabrication and evaluation of evanescent wave absorption based polyaniline-cladding modified fiber optic urea biosensor. Opt. Fiber Technol. 2018, 40, 8–12. [Google Scholar] [CrossRef]
- Swain, S.K.; Phaomei, G.; Swain, S.K.; Sahoo, N.K.; Tripathy, S.K. A new configuration of fiber optic sensor based on evanescent field absorption utilizing the emission properties of Fe3O4 @BaMoO4: Eu nanocomposite probe. Opt. Commun. 2020, 471, 125842. [Google Scholar] [CrossRef]
- Potdar, R.P.; Khollam, Y.B.; Shaikh, S.F.; Raut, R.W.; Pandit, B.; More, P.S. Evanescent wave sensor for potassium ion detection with special reference to agricultural application. J. Photochem. Photobiol. A Chem. 2023, 441, 114707. [Google Scholar] [CrossRef]
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors: Advances of the last decade. Biosens. Bioelectron. 2016, 76, 103–112. [Google Scholar] [CrossRef]
- Jali, M.H.; Rahim, H.R.A.; Johari, M.A.M.; Thokchom, S.; Harun, S.W. Applied microfiber evanescent wave on ZnO nanorods coated glass surface towards temperature sensing. Sens. Actuators A Phys. 2018, 277, 103–111. [Google Scholar] [CrossRef]
- Zahra, S.; Furka, S.; Campopiano, S.; Iadicicco, A.; Dueñas Santana, J.A.; Neogrády, P.; Tóth, A.; Furka, D. Advanced novel asymmetric notched plastic optic fibers for sensing applications. Opt. Laser Technol. 2025, 183, 112283. [Google Scholar] [CrossRef]
- Sabek, J.; Díaz-Fernández, F.J.; Torrijos-Morán, L.; Díaz-Betancor, Z.; Maquieira, Á.; Bañuls, M.-J.; Pinilla-Cienfuegos, E.; García-Rupérez, J. Experimental study of an evanescent-field biosensor based on 1D photonic bandgap structures. Beilstein J. Nanotechnol. 2019, 10, 967–974. [Google Scholar] [CrossRef]
- Azargoshasb, T.; Navid, H.A.; Parvizi, R.; Heidari, H. Evanescent Wave Optical Trapping and Sensing on Polymer Optical Fibers for Ultra-Trace Detection of Glucose. ACS Omega 2020, 5, 22046–22056. [Google Scholar] [CrossRef] [PubMed]
- Nag, P.; Sadani, K.; Mohapatra, S.; Mukherji, S.; Mukherji, S. Evanescent Wave Optical Fiber Sensors Using Enzymatic Hydrolysis on Nanostructured Polyaniline for Detection of β-Lactam Antibiotics in Food and Environment. Anal. Chem. 2021, 93, 2299–2308. [Google Scholar] [CrossRef]
- Devendiran, S.; Priya, A.K.; Sastikumar, D. Design of aluminium oxide (Al2O3) fiber optic gas sensor based on detection of refracted light in evanescent mode from the side-polished modified clad region. Sens. Actuators B Chem. 2022, 361, 131738. [Google Scholar] [CrossRef]
- Ren, S.; Chen, S.; Wang, J.; Xu, H.; Hou, X.; Huang, M.; Liu, J.; Wang, G. A distributed photonic crystal fiber reverse design framework based on multi-source knowledge fusion. Opt. Fiber Technol. 2024, 84, 103718. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, Y.; Ye, Z.; Guan, Y. Flow Injection Small-volume Fiber-optic pH Sensor Based on Evanescent Wave Excitation and Fluorescence Determination. J. Fluoresc. 2011, 21, 1137–1142. [Google Scholar] [CrossRef]
- Rico-Mendez, M.A.; Selvas, R.; Kharissova, O.V.; Toral-Acosta, D.; Puente-Ramirez, N.P.; Chapa-Garcia, R.; Gonzalez-Roque, A.A. An Efficient pH Detector for Water Contamination Based on Mach–Zehnder Interferometer Application. Sci 2024, 6, 80. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Sheng, J.-W.; He, M.; Shi, H.-C. Matrix Effects on the Microcystin-LR Fluorescent Immunoassay Based on Optical Biosensor. Sensors 2009, 9, 3000–3010. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, X.; An, D. Design of an intelligent pH sensor based on IEEE1451.2. IFAC-PapersOnLine 2018, 51, 191–198. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, X.; Wang, Z.; Li, C.; Hu, Q.; Gao, J.; Feng, L. Polyaniline-Reduced Graphene Oxide Nanosheets for Room Temperature NH3 Detection. ACS Appl. Nano Mater. 2021, 4, 5263–5272. [Google Scholar] [CrossRef]
- Li, L.; Xue, M.; Ma, Q.; Liu, X.; Gu, X.; Xiu, W.; Yang, X.; Lv, M. Graphene/Polyaniline Film Optical Fiber Ammonia Gas Sensor with Excellent Sensing Performance. IEEE Sens. J. 2023, 23, 15652–15659. [Google Scholar] [CrossRef]
- Hernaez, M.; Zamarreño, C.; Melendi-Espina, S.; Bird, L.; Mayes, A.; Arregui, F. Optical Fibre Sensors Using Graphene-Based Materials: A Review. Sensors 2017, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Wang, Z.; Chen, M.; Xin, X.; Wu, R.; Cen, Y.; Li, Y. Three-layer-structure polymer optical fiber with a rough inter-layer surface as a highly sensitive evanescent wave sensor. Sens. Actuators B Chem. 2018, 254, 133–142. [Google Scholar] [CrossRef]
- Gubaidullin, R.R.; Agliullin, T.A.; Morozov, O.G.; Sahabutdinov, A.Z. Mathematical modeling of optical response of address fiber Bragg structure using Gauss function. In Proceedings of the Optical Technologies for Telecommunications 2019, Samara, Russia, 19–21 November 2019; SPIE: Baltimore, MD, USA, 2020; Volume 11516. [Google Scholar]
- Sakhabutdinov, A.Z.; Morozov, O.G.; Agliullin, T.A.; Gubaidullin, R.R.; Karimov, K.G. Radiophotonic sensor system based on multi-addressed fiber Bragg structures. In Proceedings of the Optical Technologies for Telecommunications 2021, Samara, Russia, 23–26 November 2021; SPIE: Baltimore, MD, USA, 2022; Volume 12295, pp. 32–37. [Google Scholar]
- Morozov, O.G.; Sakhabutdinov, A.Z. Ontology of Addressed Fiber Bragg Structures as a New Type of Sensor Elements. J. Phys. Conf. Ser. 2024, 2894, 012019. [Google Scholar] [CrossRef]
- Agliullin, T.; Il’In, G.; Kuznetsov, A.; Misbakhov, R.; Misbakhov, R.; Morozov, G.; Morozov, O.; Nureev, I.; Sakhabutdinov, A. Overview of Addressed Fiber Bragg Structures’ Development. Photonics 2023, 10, 175. [Google Scholar] [CrossRef]
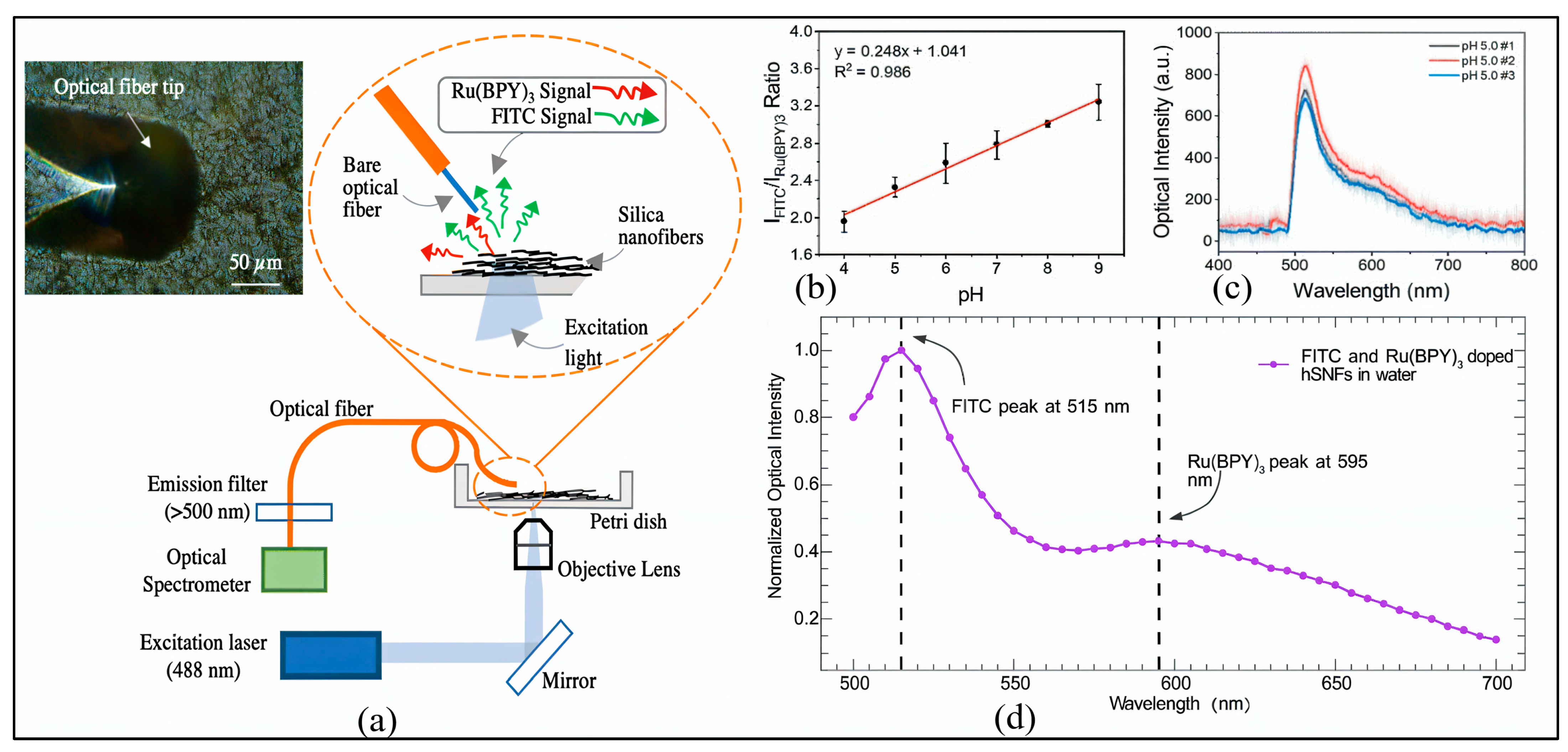
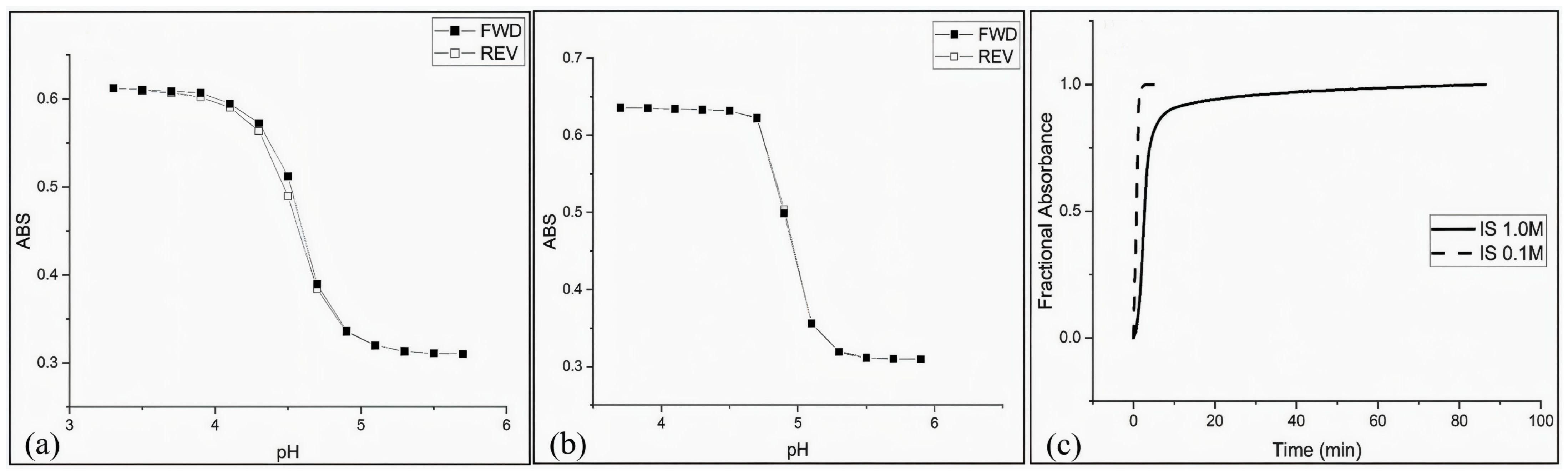
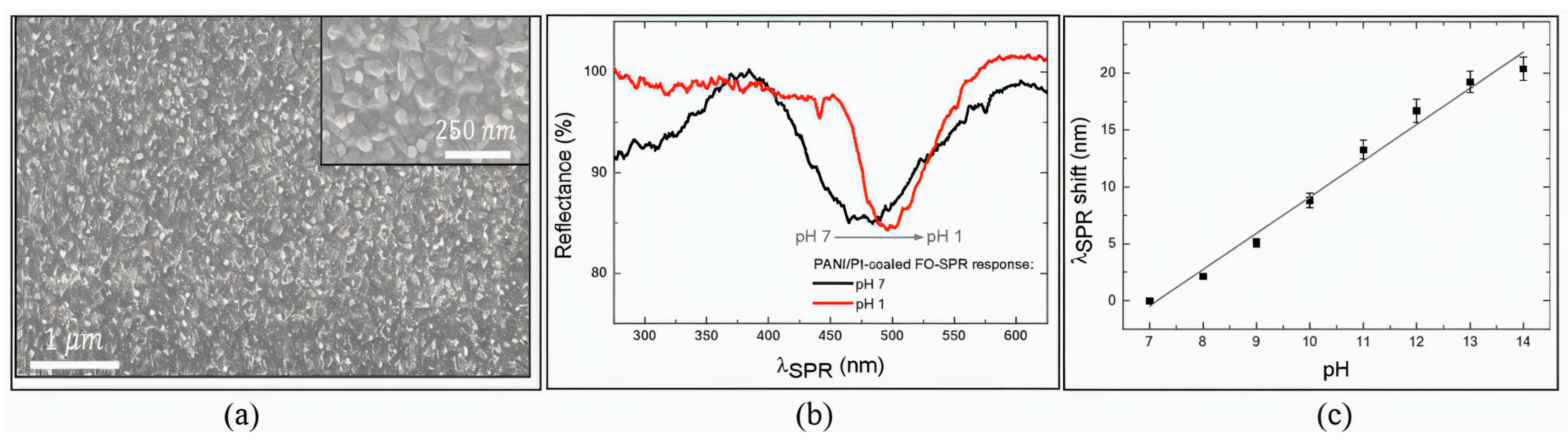

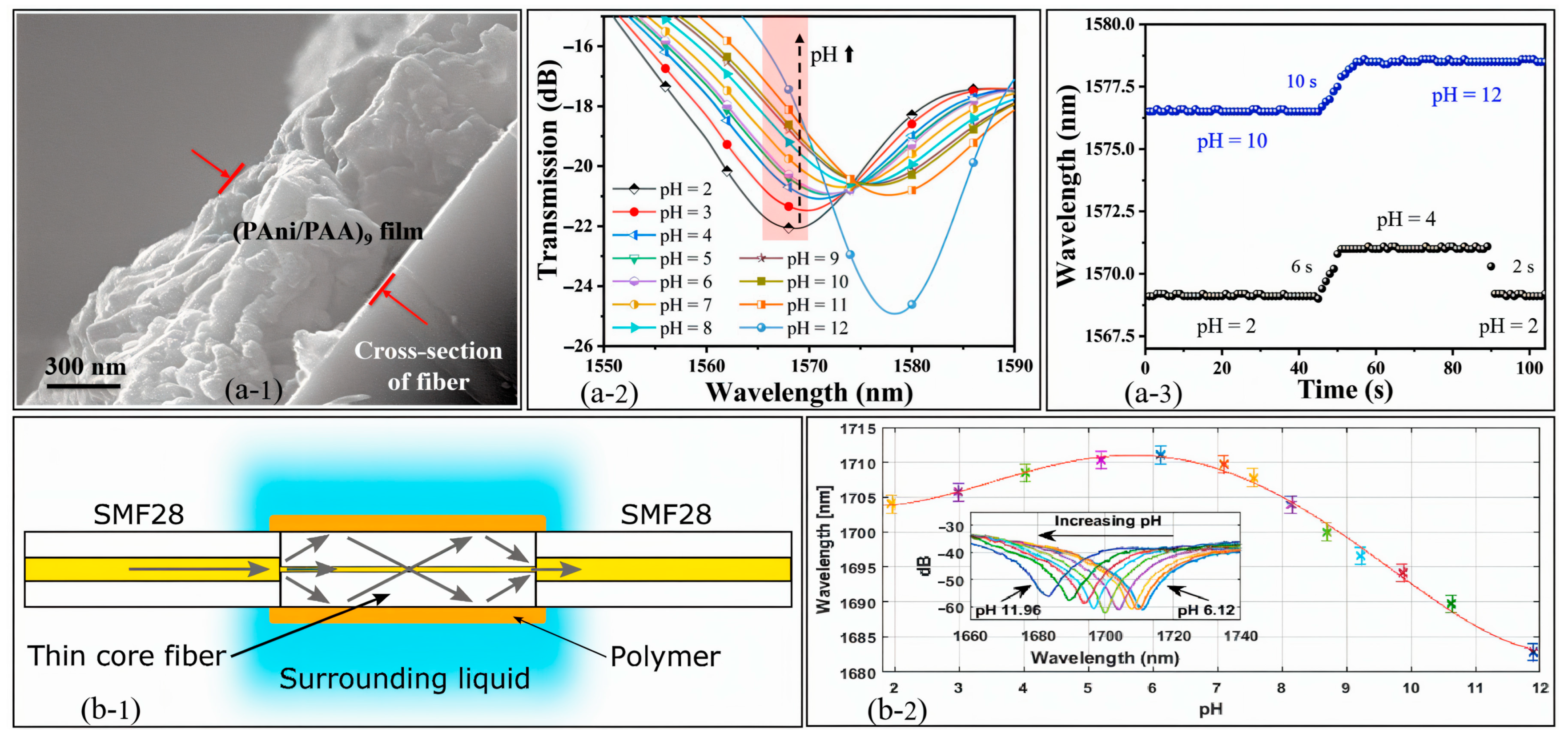
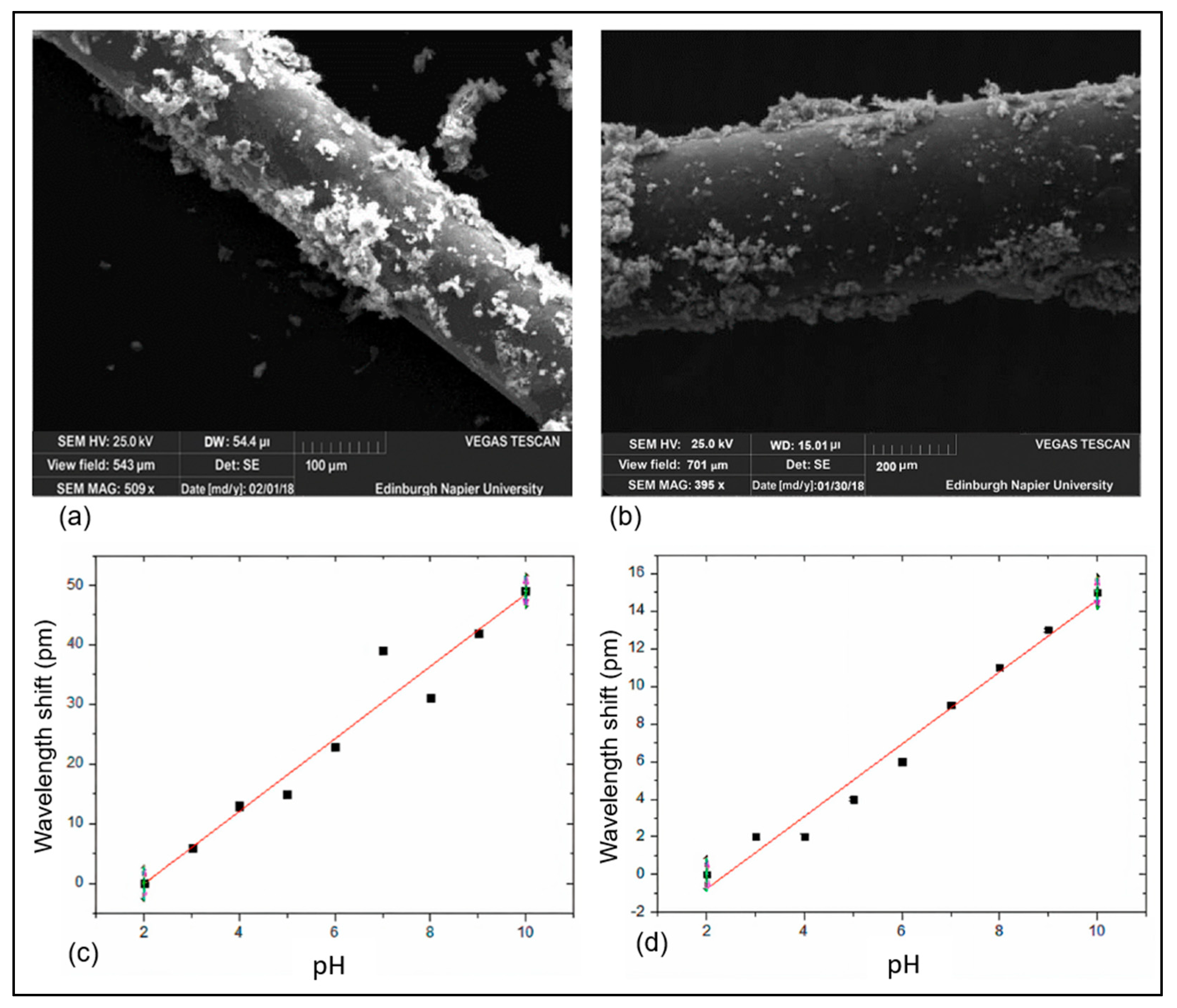

| Sensing Method | Key Advantages | Key Limitations | Estimated Cost | Ref. |
|---|---|---|---|---|
| Glass Electrodes | High accuracy and reliability; sensitive to hydrogen ion concentration; broad pH measurement range (0–14); simple operation; and wide availability. | Fragile and prone to damage; requires frequent calibration and maintenance; and not suitable for harsh or arid environments. | USD 5–21 | [9,10,11,30] |
| Solid-State Electrodes | Robust and resistant to harsh environments; requires minimal maintenance; and suitable for dry or extreme conditions. | Lower sensitivity than glass electrodes; may exhibit reduced accuracy in specific applications. | Low Cost | [12,13,14] |
| ISFET Electrodes | Durable and breakage-resistant; well suited for portable and compact devices; and performs reliably across diverse environments. | Higher cost than traditional electrodes; requires more complex electronic systems. | High Cost | [15,16,17] |
| Colorimetric Indicators | Simple to use and inexpensive; does not require complex instrumentation; and suitable for rapid, low-precision measurements. | Limited accuracy; relies on visual estimation or auxiliary analytical tools; and unsuitable for turbid or opaque samples. | Very Low Cost | [18,19,20] |
| Optical Fiber Sensors | Highly accurate and responsive to subtle pH fluctuations; suitable for harsh and hazardous environments; and ideal for continuous monitoring applications. | Relatively high cost; requires complex setup and routine maintenance. | High Cost | [21,22,23,24] |
| Fluorescence-Based Techniques | Superior accuracy and high sensitivity; ideal for biomedical and life science applications; and enables fast and continuous measurements. | Requires specialized reagents or sensitive materials; high cost; and susceptible to ambient light and signal interference. | Very High Cost | [25,26,27,28] |
| Nano-Based Sensors | Very high accuracy and compact size; easily integrated into modern electronic platforms; and suitable for medical and portable applications. | Relatively new technology requiring further validation; high cost; and sensitive to environmental fluctuations and external conditions. | High Cost | [31,32,33] |
| Sensor Configuration and Mechanism | Key Features | Ref. |
|---|---|---|
| FBG coated with hydrogel that expands in response to pH changes, inducing axial strain and shifting the Bragg wavelength. | Good linearity (pH 3–7), cost-effective, biodegradable, and repeatable. | [192] |
| All-polymer FBG with a hydrogel coating that swells under pH variation, producing lateral strain in the polymer structure. | High sensitivity (−0.41 nm/pH) and fast response (30 s). | [197] |
| Tilted FBG coated with polyaniline (PANI), which alters its optical properties with pH, causing wavelength shifts. | Wide detection range (pH 2–12), temperature-independent, and long-term stability. | [193] |
| FBG with modeled hydrogel swelling, where a mathematical model estimates the strain induced by hydrogel expansion for accurate pH detection. | Mathematical approach with validated strain analysis for accuracy. | [198] |
| FBG coated with smart hydrogel materials that respond rapidly to pH fluctuations by changing their volume and inducing strain. | Full pH range (0–14) coverage with enhanced sensitivity and fast response. | [199] |
| Miniature microfiber FBG fabricated via electrostatic self-assembly technique; pH changes affect its structure and optical response. | Ultra-compact size (~10−14 m3) with high sensitivity (−72 pm/pH). | [44] |
| All-polymer FBG with 5–10 μm hydrogel coating that expands/contracts due to pH variations, modulating the Bragg wavelength. | High sensitivity (73 pm/pH) with fast response (<4.5 min). | [200] |
| FBG with multi-point hydrogel detection, where multiple sensing regions detect pH-induced hydrogel expansion through wavelength shifts. | Experimental validation with ~100 pm shift for pH 4–10 range. | [201] |
| pH Range | Sensitivity | Materials Used | Applications | Key Features | Ref. |
|---|---|---|---|---|---|
| 3–7 | 12.16 pm/pH | Hydrogel (PVA/PAA) | Acidic pH monitoring | Good repeatability, oscillator behavior | [192] |
| 2–12 | 46 pm/pH | Polyaniline (PAni) | Biochemical applications, corrosion monitoring | Fast response, biocompatibility | [193] |
| 5–7 | 73 pm/pH | Hydrogel (PMMA) | Small-scale pH measurement | Fast response (<4.5 min), robust design | [200] |
| 4.66–6.02 | 117 a.u./pH | Polymers (PDDA/PAA) | Aqueous pH measurement | Fast dynamic response (10 s rise time) | [202] |
| 3–12 | 79.96 nW/pH | Bromothymol Blue (BTB) | Chemical pH measurement | High sensitivity, temperature compensation | [203] |
| pH Measurement Method | Key Features | Ref. |
|---|---|---|
| Luminescence lifetime-based pH sensing using emission lifetime monitoring rather than intensity-based detection. | Demonstrates the advantages of lifetime-based sensors over intensity-based ones, particularly for bioprocess monitoring. | [46] |
| Ru(II) polypyridyl complexes used as lifetime-responsive luminophores, covalently immobilized for stable, wide-range pH sensing. | Provides enhanced photostability, tunable pKa ranges, and robust performance across the pH range 3.5–8.5. | [213] |
| FRET mechanisms integrated with luminescence lifetime changes for pH detection. | Offers high accuracy, environmental stability, and minimal interference due to self-referencing decay time signals. | [214] |
| Microsecond-range luminescence decay pH sensors developed using Ru(II) complexes and pH-sensitive acceptors. | Ensures long-term stability, high sensitivity, and compatibility with frequency–domain detection systems. | [215] |
| Description | Key Topics | Ref. |
|---|---|---|
| Study on evanescent wave optical fiber sensing for temperature, humidity, and pH measurement. | Optical fibers with porous silica for stable and sensitive pH detection under temperature variations. | [48] |
| Evanescent wave-based optical fiber sensors detect changes in the refractive index of the surrounding medium, enabling accurate pH measurement. | High-sensitivity pH measurement based on refractive index changes in the surrounding medium detected through optical fiber transmission. | [229] |
| Development of an evanescent wave absorption-based fiber-optic biosensor with polyaniline cladding. | Evanescent wave absorption, chemical sensing. | [230] |
| Investigation of a new fiber-optic sensor configuration utilizing evanescent field absorption for chemical sensing. | Enhanced fiber-optic pH sensor through cladding removal and Fe3O4@BaMoO4: Eu nanocoating to improve measurement accuracy. | [231] |
| Implementation of a coil-shaped plastic optical fiber sensor for pH and concentration response analysis. | Coil-shaped optical fiber, pH, and concentration response. | [85] |
| Sensor Type | pH Range | Sensitivity | Response Time | Ref. |
|---|---|---|---|---|
| Fluorescence-based | 1.6–13.2 | ~0.02 pH units | ~5–10 s | [133] |
| Absorbance-based | 4–10 (up to 3–11) | ~0.44 nm/pH | ~10 s | [37] |
| SPR-based | 2–12 (up to 14) | ~0.01 pH resolution | Seconds to minutes | [157] |
| Interferometric | 1.95–11.89 | ~11 nm/pH [41] | ~1.6–15.7 s | [41] |
| FBG-based | 2–12 | 12–117 pm/pH | 10–30 s | [192,193] |
| Lifetime-based | 3–10 | ~0.01–0.05 pH | A few seconds—3 min | [221] |
| Evanescent wave-based (OFEWSs) | 2–12 | Variable (e.g., ~−27.8 pm/ppm) | Seconds | [85,233,236] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhussein, A.N.D.; Qaid, M.R.T.M.; Agliullin, T.; Valeev, B.; Morozov, O.; Sakhabutdinov, A.; Konstantinov, Y.A. Advancements in Optical Fiber Sensors for pH Measurement: Technologies and Applications. Sensors 2025, 25, 4275. https://doi.org/10.3390/s25144275
Alhussein AND, Qaid MRTM, Agliullin T, Valeev B, Morozov O, Sakhabutdinov A, Konstantinov YA. Advancements in Optical Fiber Sensors for pH Measurement: Technologies and Applications. Sensors. 2025; 25(14):4275. https://doi.org/10.3390/s25144275
Chicago/Turabian StyleAlhussein, Alaa N. D., Mohammed R. T. M. Qaid, Timur Agliullin, Bulat Valeev, Oleg Morozov, Airat Sakhabutdinov, and Yuri A. Konstantinov. 2025. "Advancements in Optical Fiber Sensors for pH Measurement: Technologies and Applications" Sensors 25, no. 14: 4275. https://doi.org/10.3390/s25144275
APA StyleAlhussein, A. N. D., Qaid, M. R. T. M., Agliullin, T., Valeev, B., Morozov, O., Sakhabutdinov, A., & Konstantinov, Y. A. (2025). Advancements in Optical Fiber Sensors for pH Measurement: Technologies and Applications. Sensors, 25(14), 4275. https://doi.org/10.3390/s25144275











