Identification of Chicken Bone Paste in Starch-Based Sausages Using Laser-Induced Breakdown Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
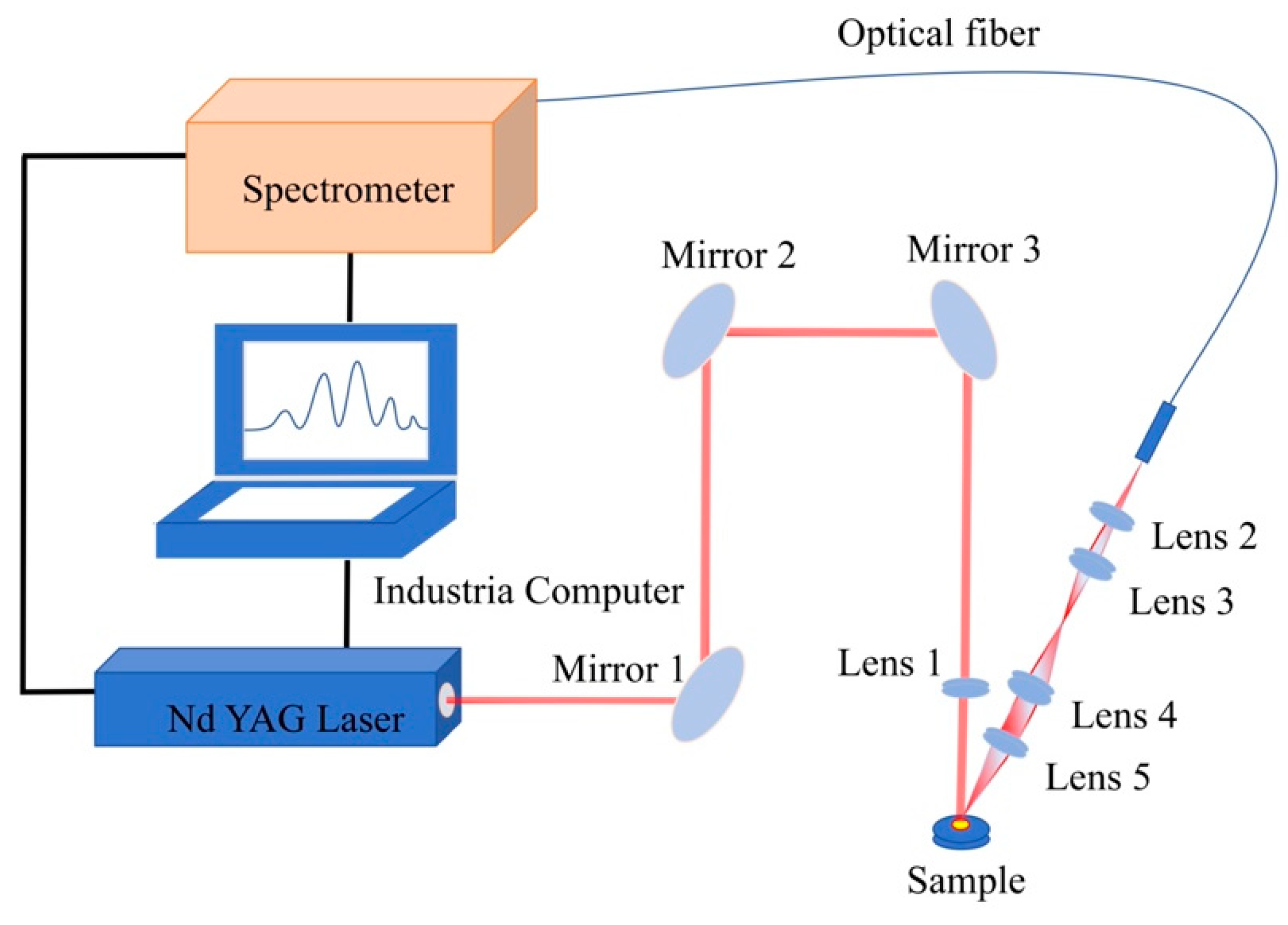
2.1. Experimental Setup
2.2. Sample Preparation
2.3. Methods
2.3.1. PCA Analysis
2.3.2. t-SNE Analysis
2.3.3. Electron Temperature Analysis
3. Results and Discussion
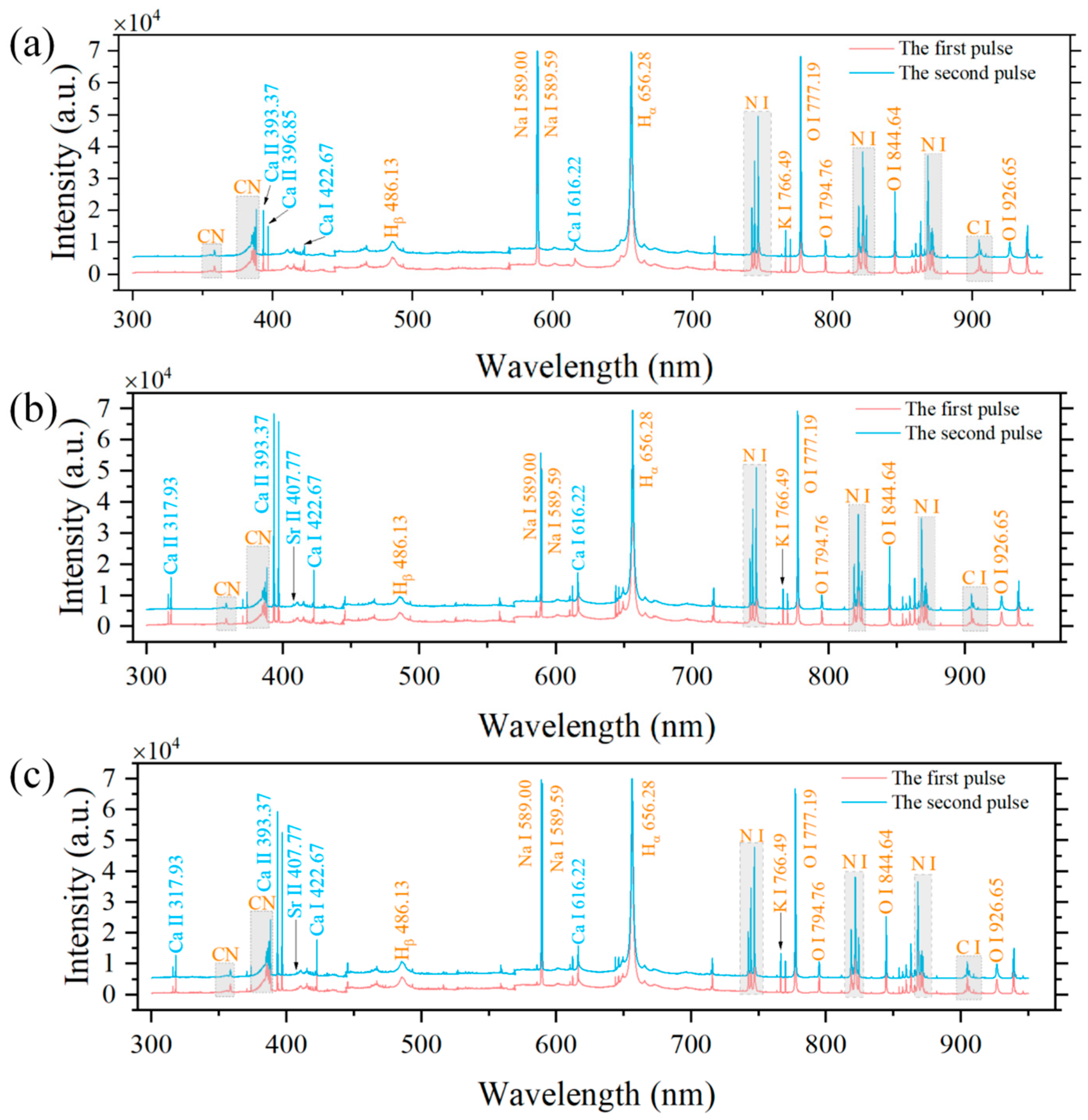
3.1. LIBS Spectral Analysis
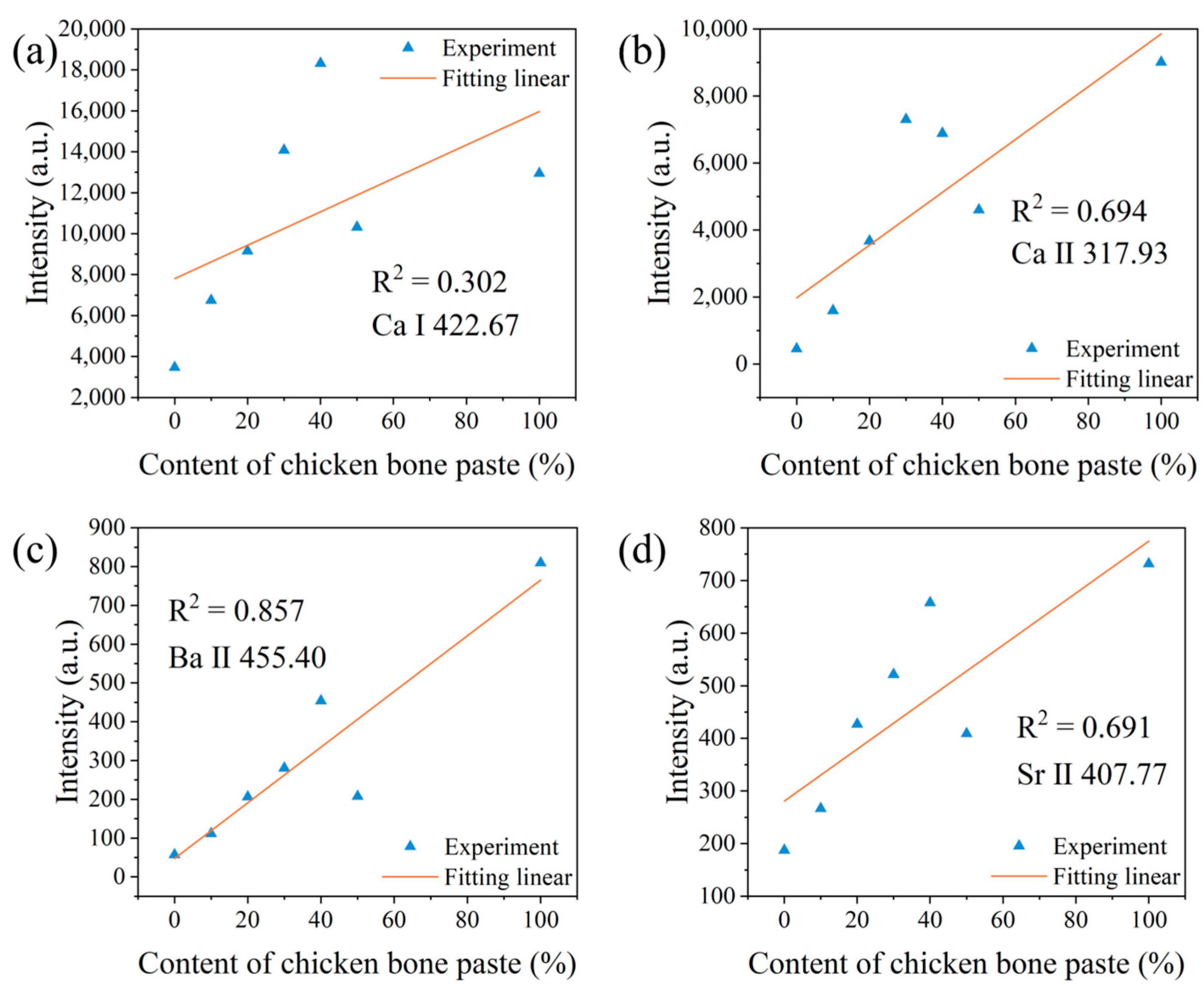
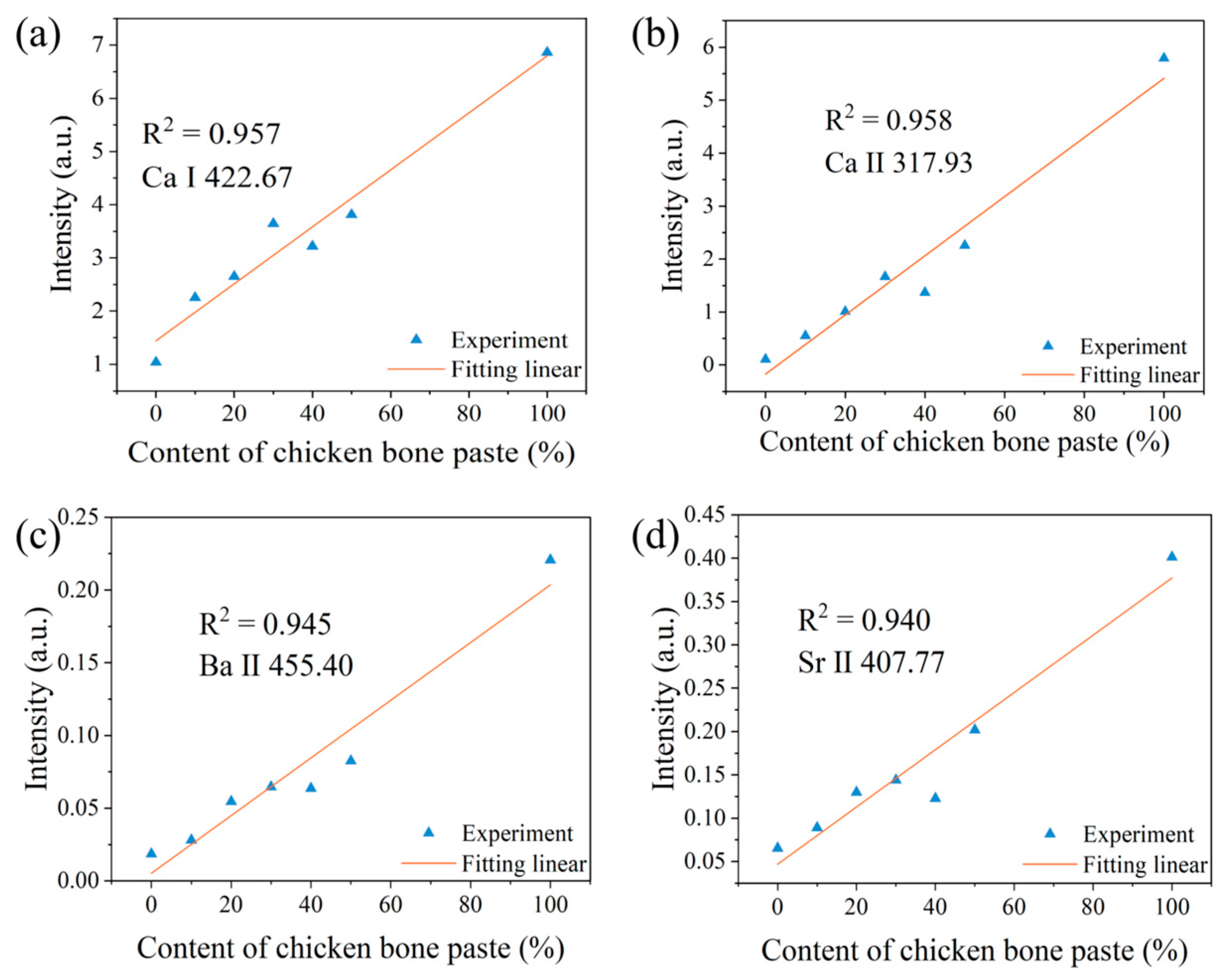
3.2. Variation of Spectral Peak Intensities of Different Elements
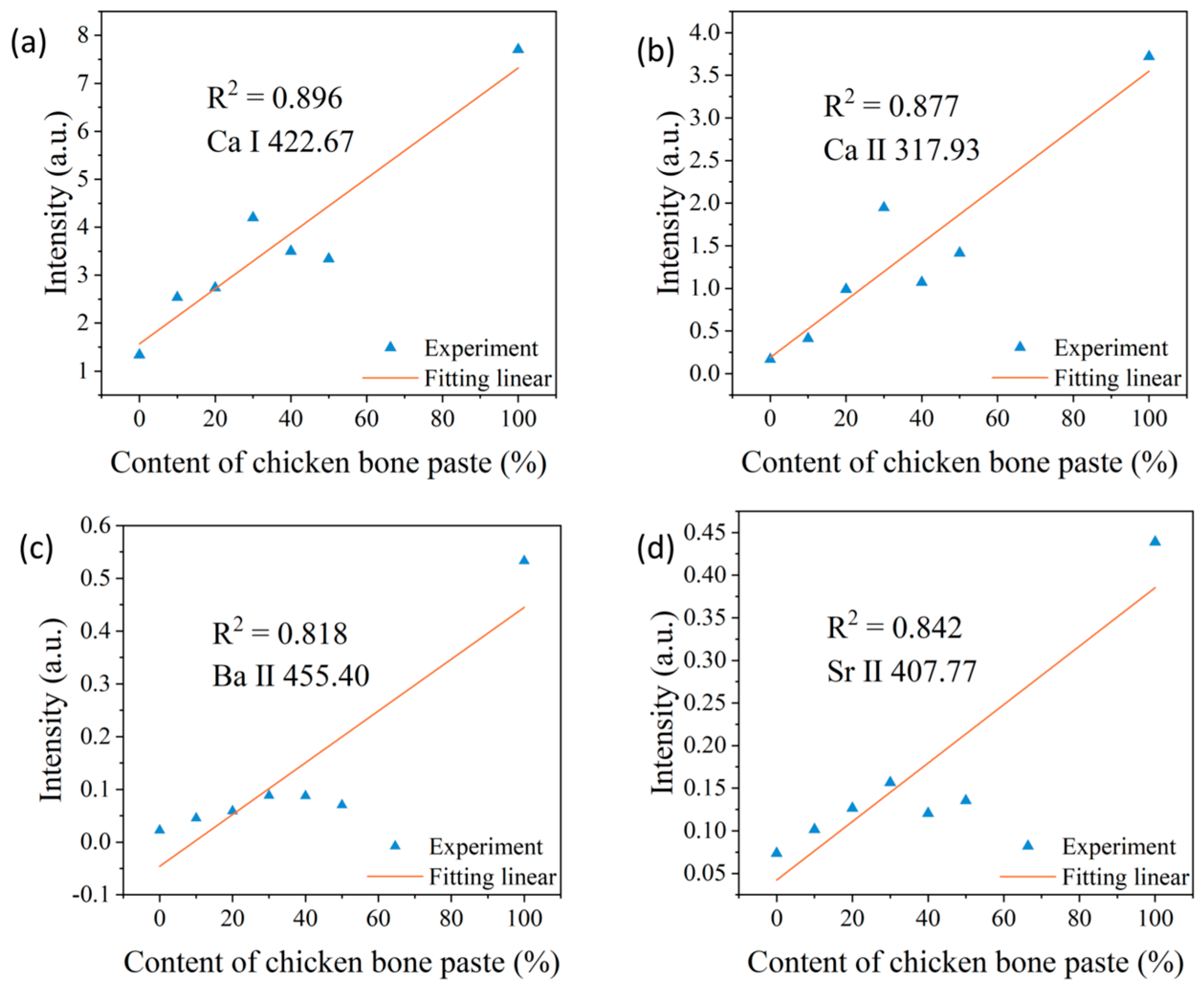
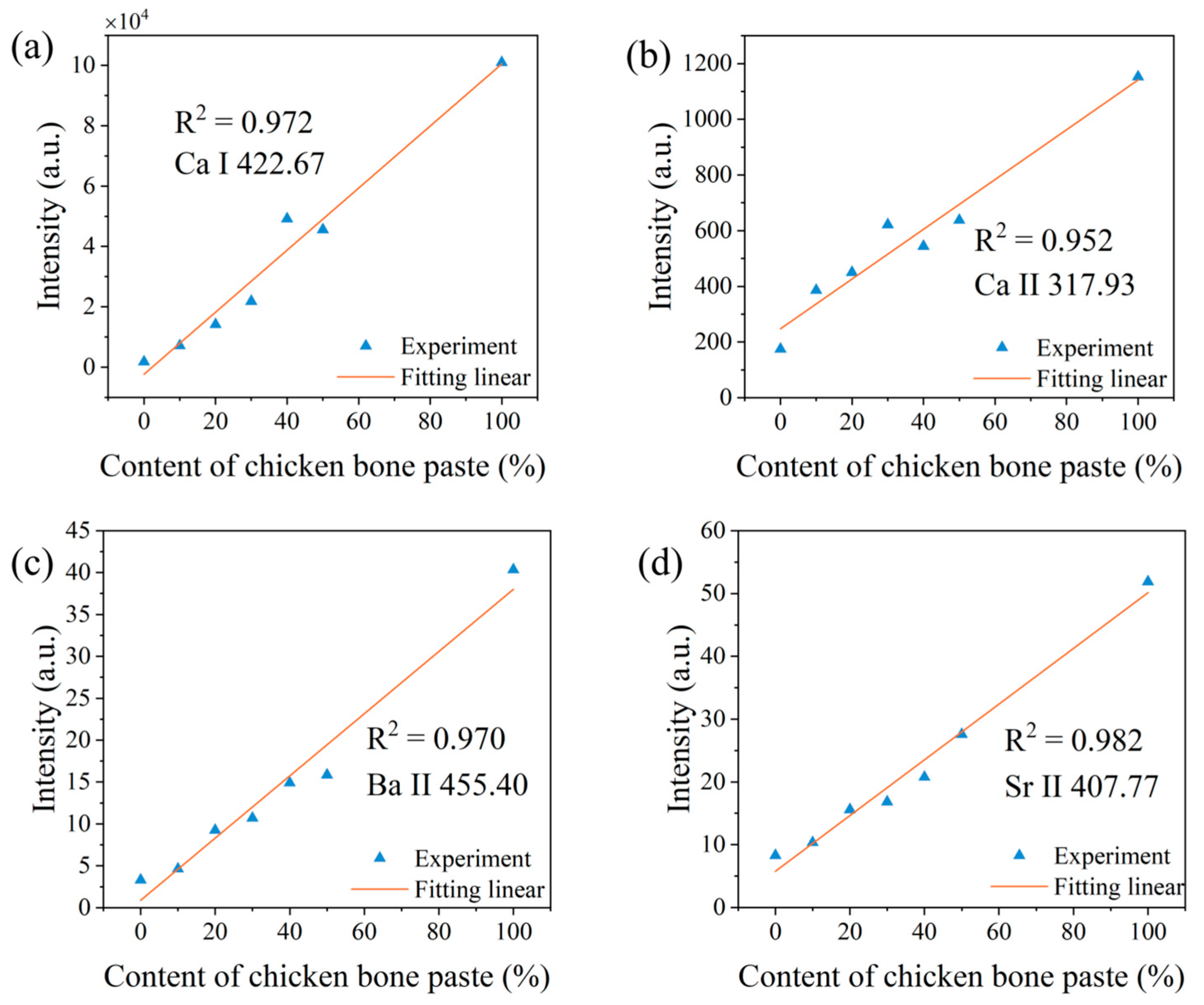
3.3. Correction with Electron Temperature
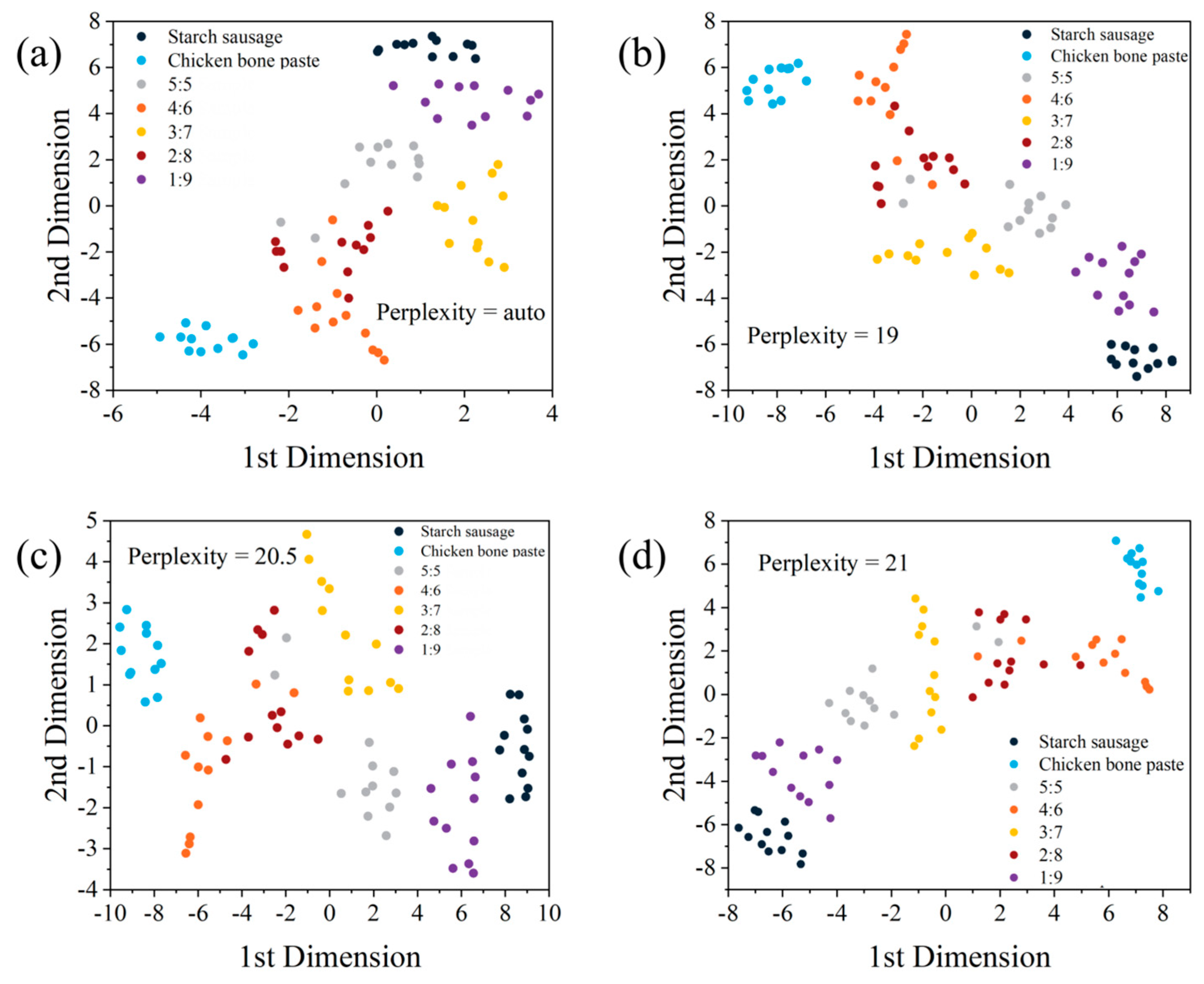
3.4. Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPNN | Backpropagation neural network |
| FWHM | Full width at half maximum |
| ICA | Independent component analysis |
| LAMP | Loop-mediated isothermal amplification |
| LIBS | Laser-induced breakdown spectroscopy |
| LoD | Limit of detection |
| LSA | Local semantic analysis |
| NIST | National Institute of Standards and Technology |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| PLS | Partial least squares |
| RSD | Relative standard deviation |
| t-SNE | t-distributed stochastic neighbor embedding |
Appendix A

| Sample | Electron Temperature (K) |
|---|---|
| Starch sausage | 9655 ± 745 |
| 1:9 | 9984 ± 1809 |
| 2:8 | 9863 ± 913 |
| 3:7 | 9961 ± 954 |
| 4:6 | 8759 ± 772 |
| 5:5 | 9402 ± 890 |
| Chicken bone paste | 9583 ± 1613 |
References
- Abbasi, I.A.; Shamim, A.; Shad, M.K.; Ashari, H.; Yusuf, I. Circular economy-based integrated farming system for indigenous chicken: Fostering food security and sustainability. J. Clean. Prod. 2024, 436, 140368. [Google Scholar] [CrossRef]
- Connolly, G.; Campbell, W.W. Poultry consumption and human cardiometabolic health-related outcomes: A narrative review. Nutrients 2023, 15, 3550. [Google Scholar] [CrossRef]
- Verbeke, W.; Rutsaert, P.; Bonne, K.; Vermeir, I. Credence quality coordination and consumers’ willingness-to-pay for certified halal labelled meat. Meat Sci. 2013, 95, 790–797. [Google Scholar] [CrossRef]
- Farouk, M.M.; Al-Mazeedi, H.M.; Sabow, A.B.; Bekhit, A.E.; Adeyemi, K.D.; Sazili, A.Q.; Ghani, A. Halal and kosher slaughter methods and meat quality: A review. Meat Sci. 2014, 98, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, X.; Zhu, Z.; Huang, T.; Huang, J.; Huang, M. Study on the stability, functional activity and preservation effect of oregano essential oil Pickering emulsion with different proportions of chicken bone gelatin/bacterial cellulose during storage. Int. J. Biol. Macromol. 2024, 282, 137309. [Google Scholar] [CrossRef]
- Ananey-Obiri, D.; Matthews, L.; Tahergorabi, R. Chicken processing by-product: A source of protein for fat uptake reduction in deep-fried chicken. Food Hydrocoll. 2020, 101, 105500. [Google Scholar] [CrossRef]
- Malison, A.; Arpanutud, P.; Keeratipibul, S. Chicken foot broth byproduct: A new source for highly effective peptide-calcium chelate. Food Chem. 2021, 345, 128713. [Google Scholar] [CrossRef]
- Yang, N.; Mu, M.-Y.; Li, H.-K.; Long, M.; He, J.-B. Seroprevalence of Toxoplasma gondii infection in slaughtered chickens, ducks, and geese in Shenyang, northeastern China. Parasites Vectors 2012, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Evans, D.C.; Mizuno, K.I.; Sola, J.E. Diagnosis and management of gastrointestinal foreign bodies. Gastroenterol. Res. Pract. 2016, 2016, 5692650. [Google Scholar] [CrossRef]
- Macavei, M.G.; Gheorghe, V.-C.; Ionescu, G.; Volceanov, A.; Pătrașcu, R.; Mărculescu, C.; Magdziarz, A. Thermochemical conversion of animal-derived waste: A mini-review with a focus on chicken bone waste. Processes 2024, 12, 358. [Google Scholar] [CrossRef]
- Islam, M.R.; Wang, Q.; Guo, Y.; Wang, W.; Sharmin, S.; Ebere Enyoh, C. Physico-chemical characterization of food wastes for potential soil application. Processes 2023, 11, 250. [Google Scholar] [CrossRef]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, C.G.; Schwaiger, K.; Biagi, G.; Dobenecker, B. From nutritional adequacy to hygiene quality: A detailed assessment of commercial raw pet-food for dogs and cats. Animals 2022, 12, 2395. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Sentandreu, E. Authenticity of meat products: Tools against fraud. Food Res. Int. 2014, 60, 19–29. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, R.; Wang, N.; Zhang, J.; Sun, X.; Huang, F.; Chen, A. Integrating microneedle DNA extraction to hand-held microfluidic colorimetric LAMP chip system for meat adulteration detection. Food Chem. 2023, 411, 135508. [Google Scholar] [CrossRef]
- Jauregui-Lopez, I.; Zulategi, F.; Beriain, M.J.; Sarries, M.V.; Beruete, M.; Insausti, K. Lipid and protein oxidation marker compounds in horse meat fetermined by MIR spectroscopy. Foods 2020, 9, 1828. [Google Scholar] [CrossRef]
- Mamani-Linares, L.W.; Gallo, C.; Alomar, D. Identification of cattle, llama and horse meat by near infrared reflectance or transflectance spectroscopy. Meat Sci. 2012, 90, 378–385. [Google Scholar] [CrossRef]
- Afseth, N.K.; Dankel, K.; Andersen, P.V.; Difford, G.F.; Horn, S.S.; Sonesson, A.; Hillestad, B.; Wold, J.P.; Tengstrand, E. Raman and near infrared spectroscopy for quantification of fatty acids in muscle tissue-A salmon case study. Foods 2022, 11, 962. [Google Scholar] [CrossRef]
- David, G.; Meslin, P.Y.; Dehouck, E.; Gasnault, O.; Cousin, A.; Forni, O.; Berger, G.; Lasue, J.; Pinet, P.; Wiens, R.C.; et al. Laser-induced breakdown spectroscopy (LIBS) characterization of granular soils: Implications for ChemCam analyses at Gale crater, Mars. Icarus 2021, 365, 114481. [Google Scholar] [CrossRef]
- Yang, G.; Chen, G.; Cai, Z.; Quan, X.; Zhu, Y. Laser-induced breakdown spectroscopy instrument and spectral analysis for deep-ocean Fe-Mn crusts. Front. Mar. Sci. 2023, 10, 1135058. [Google Scholar] [CrossRef]
- Nisar, S.; Dastgeer, G.; Shafiq, M.; Usman, M. Qualitative and semi-quantitative analysis of health-care pharmaceutical products using laser-induced breakdown spectroscopy. J. Pharm. Anal. 2019, 9, 20–24. [Google Scholar] [CrossRef]
- Moncayo, S.; Manzoor, S.; Rosales, J.D.; Anzano, J.; Caceres, J.O. Qualitative and quantitative analysis of milk for the detection of adulteration by Laser Induced Breakdown Spectroscopy (LIBS). Food Chem. 2017, 232, 322–328. [Google Scholar] [CrossRef]
- Abbas, Q.; Israr, M.A.; Haq, S.U.; Nadeem, A. Exploiting calibration free laser-induced breakdown spectroscopy (CF-LIBS) for the analysis of food colors. Optik 2021, 236, 166531. [Google Scholar] [CrossRef]
- Mezoued, N.; Fabre, C.; Cauzid, J.; Kim, Y.; Jatteau, M. Analysis of minerals using handheld laser-induced breakdown spectroscopy technology. Data 2025, 10, 40. [Google Scholar] [CrossRef]
- Li, J.W.; Wang, Y.T.; Yao, L.X.; Wang, C.; Shen, L. Detection of lead chrome green in Tieguanyin tea by laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2023, 38, 1549–1555. [Google Scholar] [CrossRef]
- Bilge, G.; Velioglu, H.M.; Sezer, B.; Eseller, K.E.; Boyaci, I.H. Identification of meat species by using laser-induced breakdown spectroscopy. Meat Sci. 2016, 119, 118–122. [Google Scholar] [CrossRef]
- Velioglu, H.M.; Sezer, B.; Bilge, G.; Baytur, S.E.; Boyaci, I.H. Identification of offal adulteration in beef by laser induced breakdown spectroscopy (LIBS). Meat Sci. 2018, 138, 28–33. [Google Scholar] [CrossRef]
- Sezer, B.; Bjelak, A.; Velioglu, H.M.; Boyaci, I.H. Protein based evaluation of meat species by using laser induced breakdown spectroscopy. Meat Sci. 2021, 172, 8. [Google Scholar] [CrossRef]
- Yao, L.X.; Li, J.W.; Liu, Y.; Shen, L.; Wang, C. Traceability of cold medications with similar ingredients based on laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2024, 39, 735–742. [Google Scholar] [CrossRef]
- Porizka, P.; Klus, J.; Prochazka, D.; Vitkova, G.; Brada, M.; Novotny, J.; Novotny, K.; Kaiser, J. Assessment of the most effective part of echelle laser-induced plasma spectra for further classification using Czerny-Turner spectrometer. Spectroc. Acta Part B-Atom. Spectr. 2016, 124, 116–123. [Google Scholar] [CrossRef]
- Porizka, P.; Demidov, A.; Kaiser, J.; Keivanian, J.; Gornushkin, I.; Panne, U.; Riedel, J. Laser-induced breakdown spectroscopy for in situ qualitative and quantitative analysis of mineral ores. Spectroc. Acta Part B-Atom. Spectr. 2014, 101, 155–163. [Google Scholar] [CrossRef]
- Gottfried, J.L.; Harmon, R.S.; De Lucia, F.C.; Miziolek, A.W. Multivariate analysis of laser-induced breakdown spectroscopy chemical signatures for geomaterial classification. Spectroc. Acta Part B-Atom. Spectr. 2009, 64, 1009–1019. [Google Scholar] [CrossRef]
- van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A.; Tognoni, E. New procedure for quantitative elemental analysis by laser-induced plasma spectroscopy. Appl. Spectrosc. 1999, 53, 960–964. [Google Scholar] [CrossRef]
- El Sherbini, A.M.; Hegazy, H.; El Sherbini, T.M. Measurement of electron density utilizing the H alpha-line from laser produced plasma in air. Spectrochim. Acta Part B-At. Spectrosc. 2006, 61, 532–539. [Google Scholar] [CrossRef]
- Kepple, P.; Griem, H.R. Improved Stark profile calculations for the Hydrogen lines Hɑ, Hβ, Hγ, and Hδ. Phys. Rev. 1968, 173, 317–325. [Google Scholar] [CrossRef]
- Griem, H.R. Spectral Line Broadening by Plasmas; Academic Press: New York NY, USA, 1974. [Google Scholar]
- Borodich, F.M. Analytical studies of contact problems for fractal surfaces. In Tribology and Interface Engineering Series; Dowson, D., Priest, M., Dalmaz, G., Lubrecht, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 48, pp. 537–545. [Google Scholar]
- Klus, J.; Pořízka, P.; Prochazka, D.; Novotný, J.; Novotný, K.; Kaiser, J. Effect of experimental parameters and resulting analytical signal statistics in laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 126, 6–10. [Google Scholar] [CrossRef]
- van der Maaten, L. Accelerating t-SNE using tree-based algorithms. J. Mach. Learn. Res. 2014, 15, 3221–3245. [Google Scholar]


| Possible Elements | Wavelength (nm) |
|---|---|
| Ca I | 422.67, 430.25, 443.57, 445.48, 458.14, 458.59, 534.95, 558.88, 585.75, 610.27, 612.22, 616.22, 643.91, 646.26, 649.38, 671.77, 714.82, 720.22, 732.61 |
| Ca Ⅱ | 315.89, 317.93, 370.60, 373.69, 393.37, 396.85, 849.80, 854.21, 866.21 |
| K I | 766.49, 769.90 |
| Mg II | 279.55, 280.27 |
| Na I | 568.26, 568.82, 589.00, 589.59 |
| Ba II | 455.40, 493.41 |
| Sr II | 407.77 |
| N I | 742.36, 744.32, 746.83, 818.49, 821.63, 856.77, 859.40, 862.92, 868.34, 939.28 |
| O I | 777.19, 794.76, 844.64, 926.60 |
| Hα | 656.28 |
| Hβ | 486.13 |
| CN | 358.40, 358.64, 359.05, 385.10, 385.48, 386.18, 387.18, 388.33, 415.79, 416.73, 418.09, 419.67, 421.59 |
| C2 | 471.45, 473.65, 516.49 |
| Elements | The First Laser Pulse Non-Normalized | The First Laser Pulse Normalized | The Second Laser Pulse Normalized | Correction with Electron Temperature |
|---|---|---|---|---|
| Ca I | 0.302 | 0.896 | 0.957 | 0.972 |
| Ca Ⅱ | 0.694 | 0.877 | 0.958 | 0.952 |
| Ba II | 0.857 | 0.818 | 0.945 | 0.97 |
| Sr II | 0.691 | 0.842 | 0.940 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Shen, L.; Han, X.; Liu, Y.; Wang, Y. Identification of Chicken Bone Paste in Starch-Based Sausages Using Laser-Induced Breakdown Spectroscopy. Sensors 2025, 25, 4226. https://doi.org/10.3390/s25134226
Li H, Shen L, Han X, Liu Y, Wang Y. Identification of Chicken Bone Paste in Starch-Based Sausages Using Laser-Induced Breakdown Spectroscopy. Sensors. 2025; 25(13):4226. https://doi.org/10.3390/s25134226
Chicago/Turabian StyleLi, Haoyu, Li Shen, Xiang Han, Yu Liu, and Yutong Wang. 2025. "Identification of Chicken Bone Paste in Starch-Based Sausages Using Laser-Induced Breakdown Spectroscopy" Sensors 25, no. 13: 4226. https://doi.org/10.3390/s25134226
APA StyleLi, H., Shen, L., Han, X., Liu, Y., & Wang, Y. (2025). Identification of Chicken Bone Paste in Starch-Based Sausages Using Laser-Induced Breakdown Spectroscopy. Sensors, 25(13), 4226. https://doi.org/10.3390/s25134226






