DFT Exploration of a Pd-Doped InSe Monolayer as a Novel Gas Sensing Candidate upon SF6 Decomposition: SO2, SOF2, and SO2F2
Abstract
1. Introduction
2. The Computational Details
3. Results and Discussion
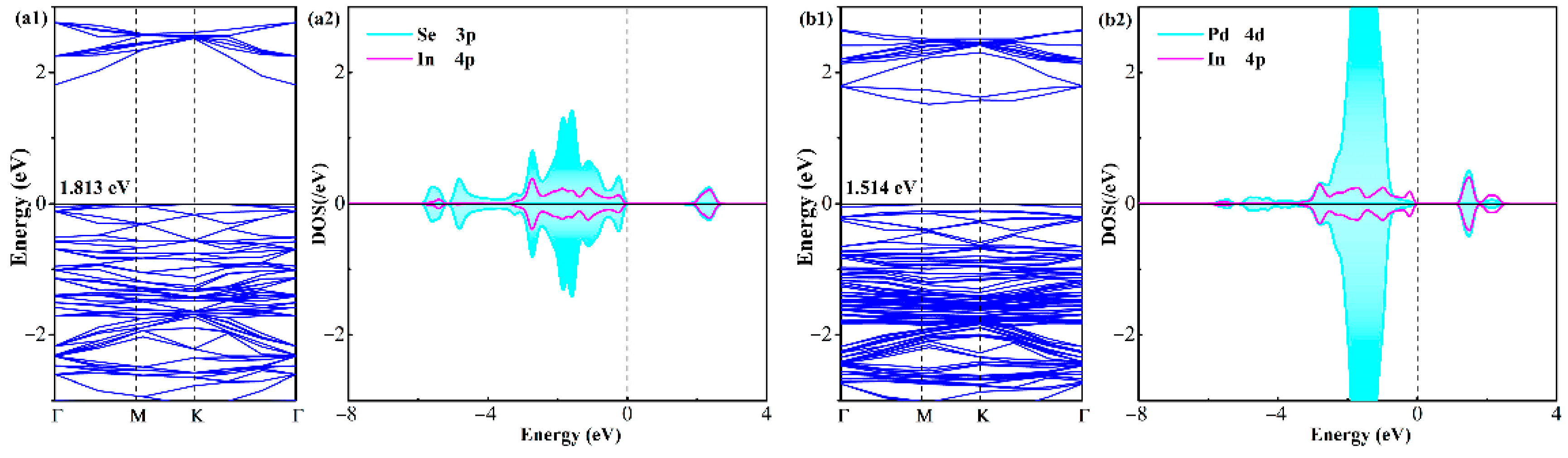
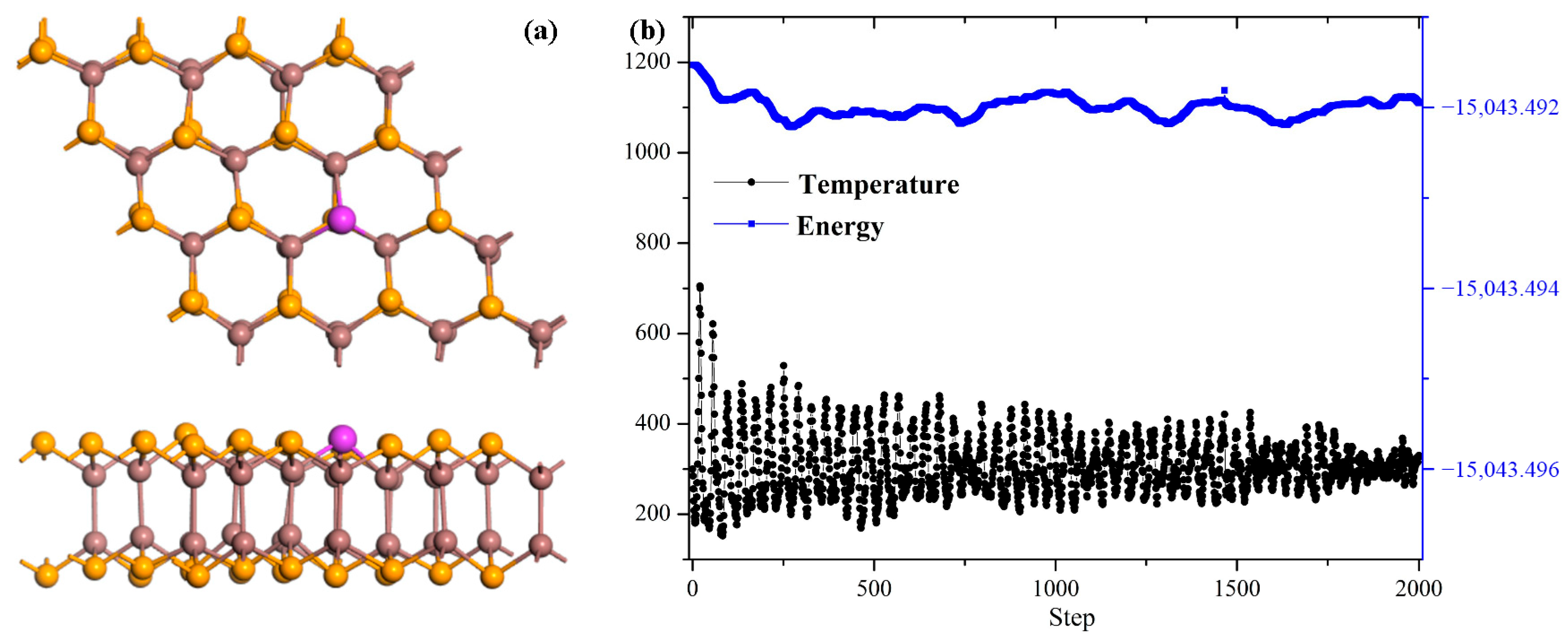
3.1. Pd Doping Properties Within the InSe Monolayer
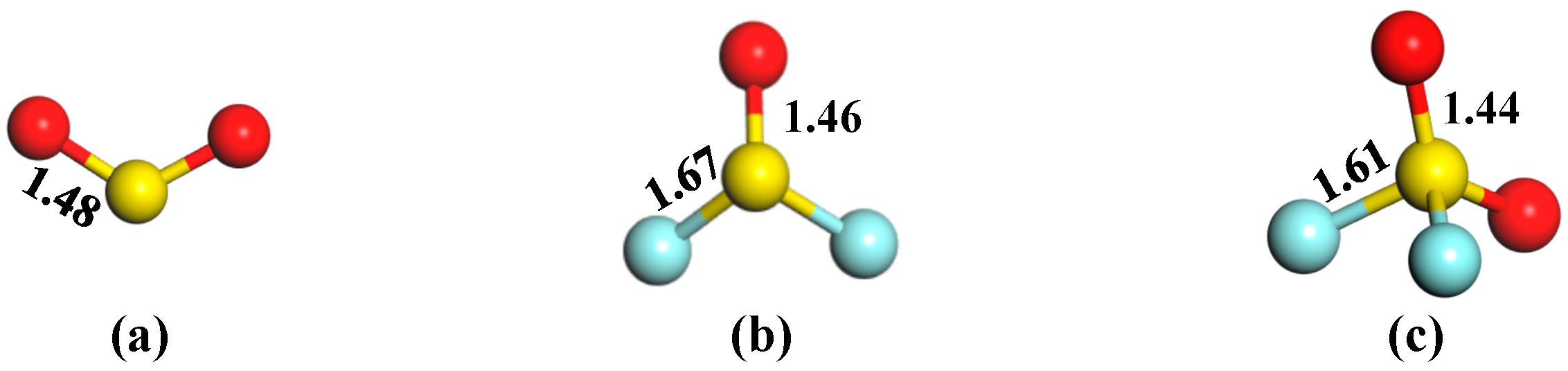
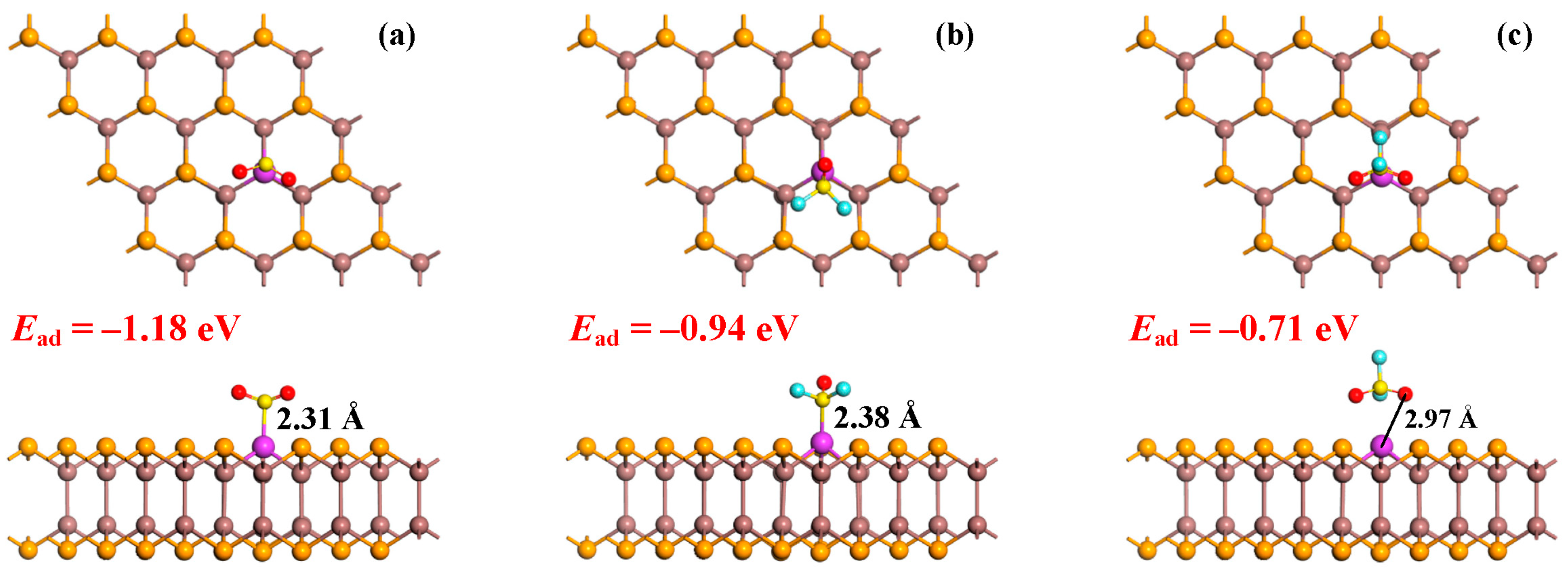
3.2. Gas Adsorption in the Pd-InSe Monolayer
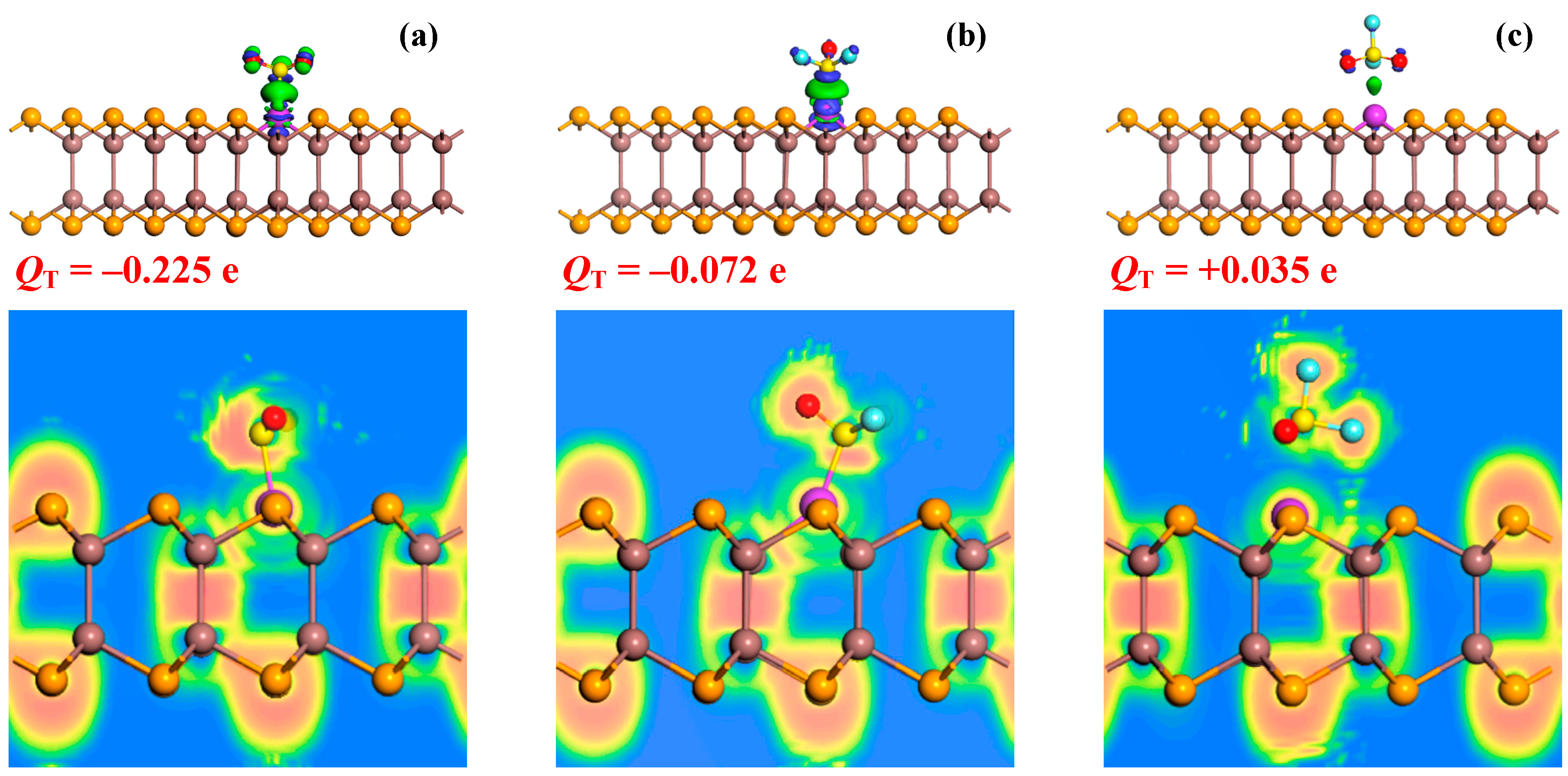
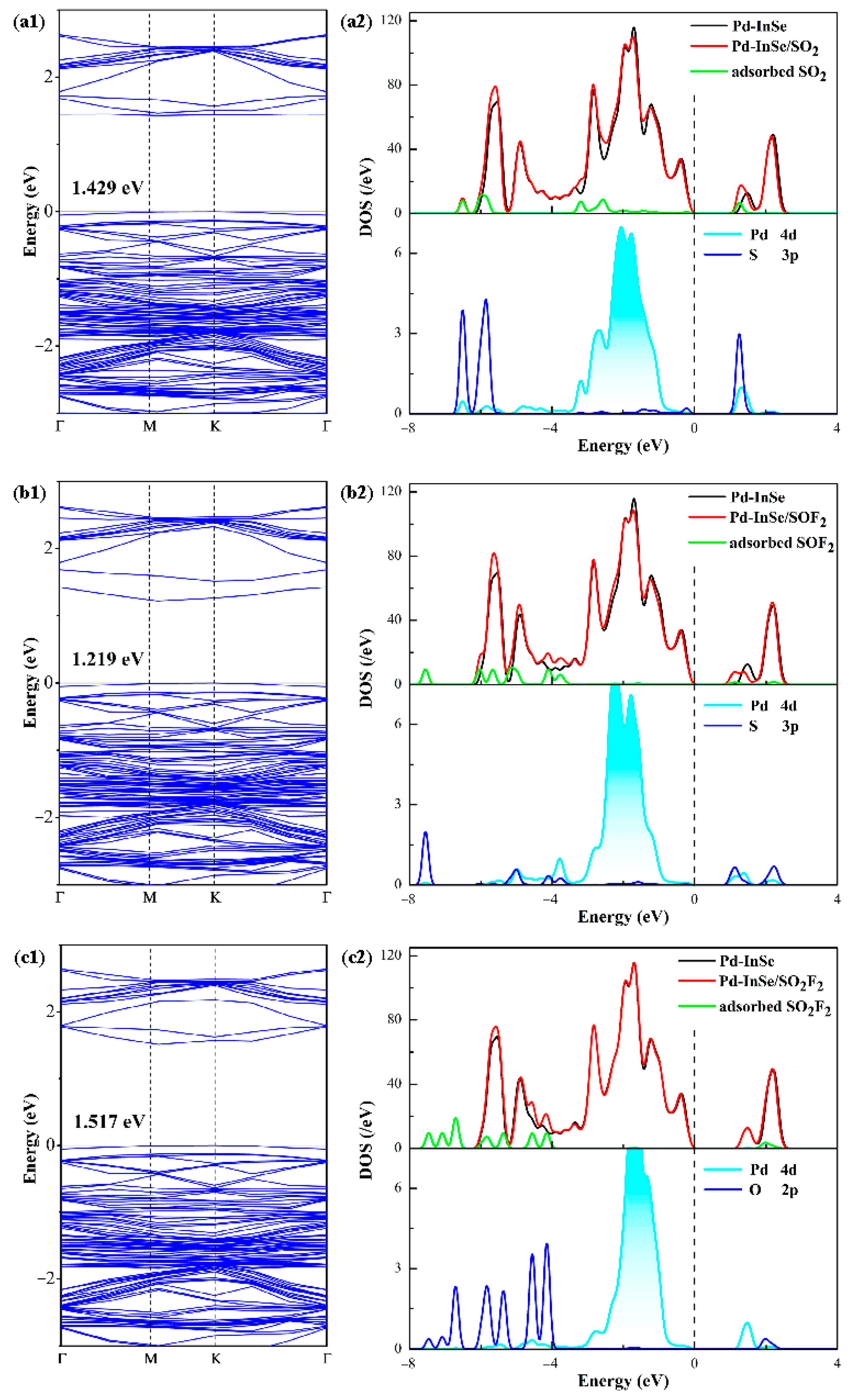
3.3. Modulated Electronic Properties in Gas Adsorption
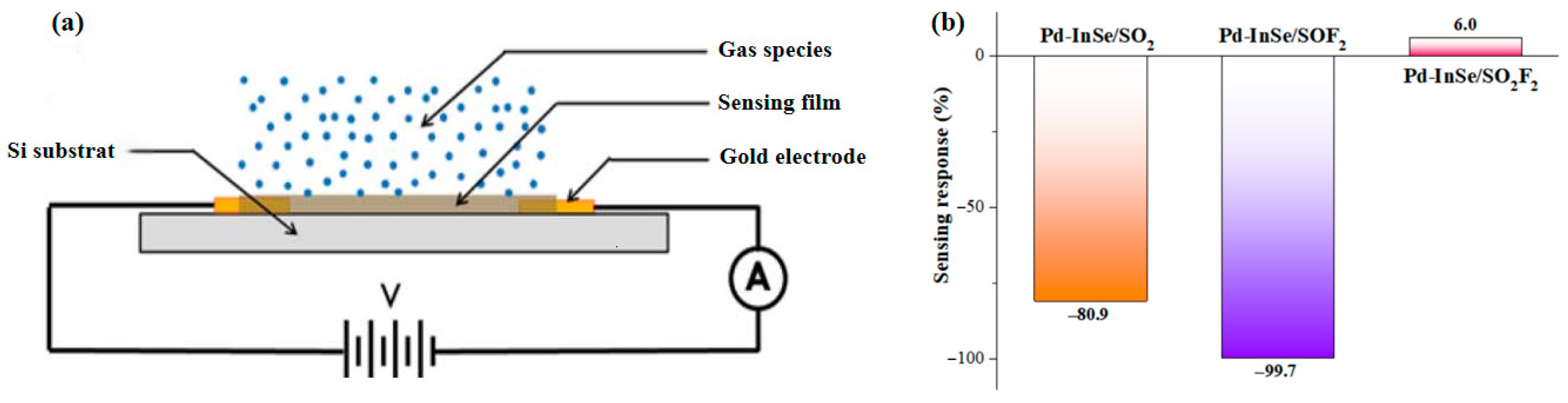
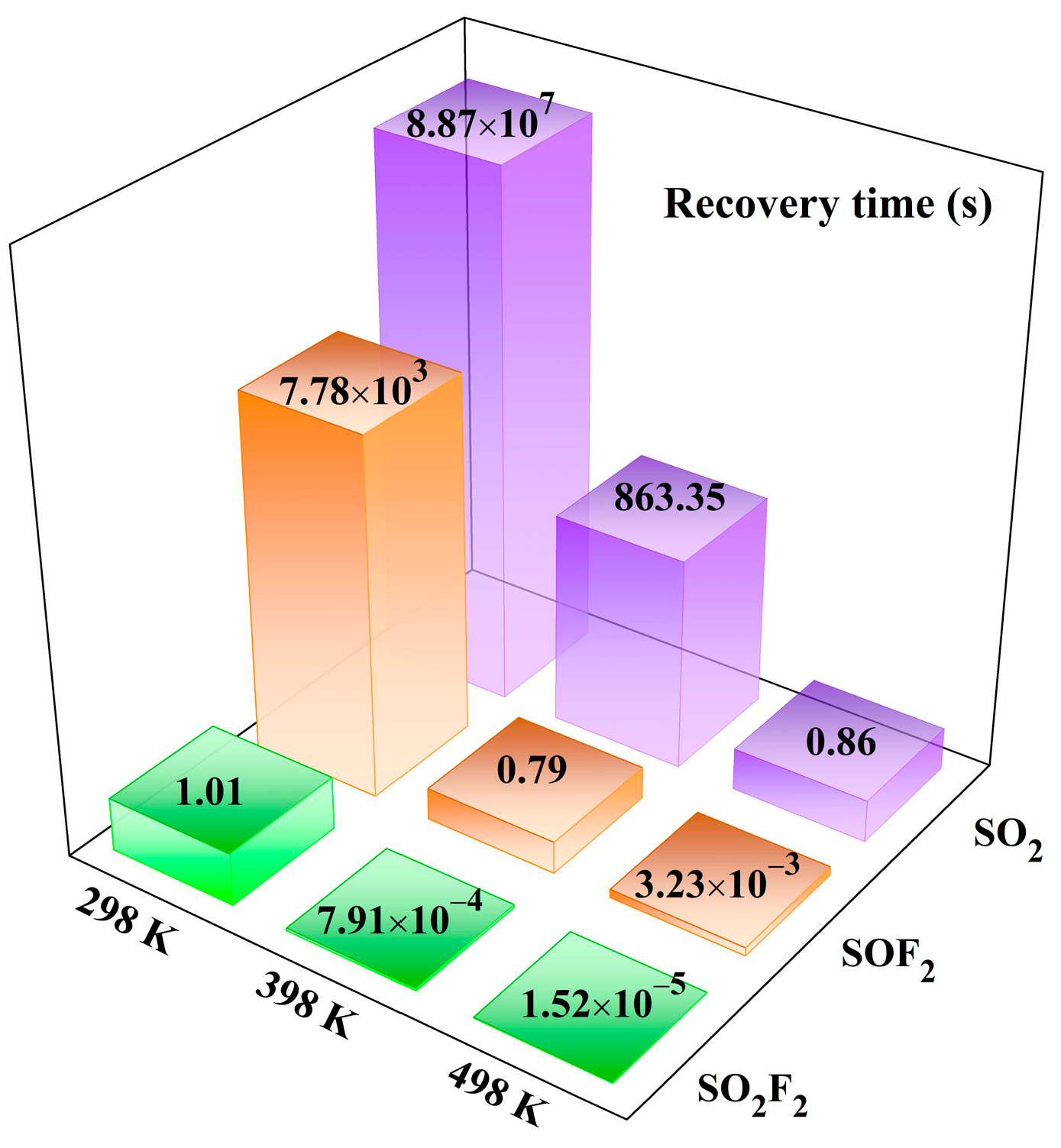
3.4. Gas Sensing Exploration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, H.; Yan, C.; Jia, P.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Gui, Y.; Hu, W. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Zhang, X.; Gui, Y.; Xiao, H.; Zhang, Y. Analysis of adsorption properties of typical partial discharge gases on Ni-SWCNTs using density functional theory. Appl. Surf. Sci. 2016, 379, 47–54. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, Q.; Chen, D.; Zhang, Y.; Liu, J.; Ma, C.; Jia, P. A machine learning feature descriptor approach: Revealing potential adsorption mechanisms for SF6 decomposition product gas-sensitive materials. J. Hazard. Mater. 2025, 481, 136567. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Zhang, X. Theoretical screening into Ru-doped MoS2 monolayer as a promising gas sensor upon SO2 and SOF2 in SF6 insulation devices. Mol. Phys. 2021, 120, e2018517. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Tie, J.; Dong, X. Gas Sensitivity and Sensing Mechanism Studies on Au-Doped TiO2 Nanotube Arrays for Detecting SF6 Decomposed Components. Sensors 2014, 14, 19517–19532. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Meng, Q.; Zhang, X.; Tao, J. Partial discharge recognition through an analysis of SF6 decomposition products part 2: Feature extraction and decision tree-based pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Fan, J.; Yan, R.; He, Y.; Zhang, J.; Zhao, W.; Liu, M.; An, S.; Ma, Q. Stochastic optimization of combined energy and computation task scheduling strategies of hybrid system with multi-energy storage system and data center. Renew. Energy 2025, 242, 122466. [Google Scholar] [CrossRef]
- Jha, R.K.; Bhat, N. Recent progress in chemiresistive gas sensing technology based on molybdenum and tungsten chalcogenide nanostructures. Adv. Mater. Interfaces 2020, 7, 1901992. [Google Scholar] [CrossRef]
- Marvan, P.; Mazánek, V.; Sofer, Z. Shear-force exfoliation of indium and gallium chalcogenides for selective gas sensing applications. Nanoscale 2019, 11, 4310–4317. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, S.; Zhao, X.-G.; Biswas, K.; Li, S.-L.; Zhang, L. InSe: A two-dimensional material with strong interlayer coupling. Nanoscale 2018, 10, 7991–7998. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Gao, C.; Nie, Q.; Wang, Q.-J.; Lin, Y.-F.; Chu, J.; Li, W. Properties, synthesis, and device applications of 2D layered InSe. Adv. Mater. Technol. 2022, 7, 2200321. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, Q.; Peng, R.; Zeng, W. Adsorption properties of InP3 monolayer toward SF6 decomposed gases: A DFT study. Phys. E Low-Dimens. Syst. Nanostructures 2021, 130, 114689. [Google Scholar] [CrossRef]
- Lu, D.; Huang, L.; Zhang, J.; Zhang, Y.; Feng, W.; Zeng, W.; Zhou, Q. Rh- and Ru-Modified InSe Monolayers for Detection of NH3, NO2, and SO2 in Agricultural Greenhouse: A DFT Study. ACS Appl. Nano Mater. 2023, 6, 14447–14458. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, X.; Huang, S.; Lin, S.; Liu, L.; Xiong, H. Adsorption and Sensing Properties of Ni-Modified InSe Monolayer Towards Toxic Gases: A DFT Study. Chemosensors 2024, 12, 219. [Google Scholar] [CrossRef]
- Cui, H.; Wu, H.; He, D.; Ma, S. Noble metal (Pd, Pt)-functionalized WSe2 monolayer for adsorbing and sensing thermal runaway gases in LIBs: A first-principles investigation. Environ. Res. 2025, 269, 120847. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Tan, S.; Bi, M.; Lei, S.; He, X.; Hu, X.; He, J.; Jiang, T. Adsorption of SF6 decomposition gases (H2S, SO2 and SOF2) on TM (Pd and Pt) modified monolayer ZrS2: A DFT study. Comput. Theor. Chem. 2024, 1236, 114586. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, D.; Dong, J.; Hou, D.; Xiong, H. Atomic-level insights into sensing performance of toxic gases on the InSe monolayer decorated with Pd and Pt under humid environment. Sens. Actuators A Phys. 2024, 378, 115846. [Google Scholar] [CrossRef]
- Yang, G.; Yan, P.; Zhu, C.; Gu, Y.; Fang, X. Selenium Vacancy–Enhanced Gas Adsorption of Monolayer Hafnium Diselenide (HfSe2) from a Theoretical Perspective. Adv. Theory Simul. 2019, 2, 1900052. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Y.; Zeng, W.; Zhou, Q. Theoretical screening into Ag-Embedded HfS2 monolayers as gas sensor for detecting SF6 decomposition gases. J. Mater. Res. Technol. 2022, 18, 1991–2000. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Chen, J.; Jia, L.; Cui, X.; Zeng, W.; Zhou, Q. Adsorption and gas-sensing properties of SF6 decomposition components (SO2, SOF2 and SO2F2) on Co or Cr modified GeSe monolayer: A DFT study. Mater. Today Chem. 2023, 28, 101382. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, J.; Yan, R.; He, Y.; Long, C.; Geng, X.; Zhang, Y.; Fan, J.; Liu, T. More flexibility and waste heat recovery of a combined heat and power system for renewable consumption and higher efficiency. Energy 2025, 315, 134392. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B Condens. Matter 2002, 66, 155125. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Di Stasio, R., Jr.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 171. [Google Scholar] [CrossRef]
- Peng, X.; Liu, D.; Zhao, F.; Tang, C. Gas sensing properties of Mg-doped graphene for HS2, SO2, SOF2, and SO2F2 based on DFT. Int. J. Quantum Chem. 2022, 122, e26989. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, X.; Gui, Y. First-Principles Insight into a Ru-Doped SnS2 Monolayer as a Promising Biosensor for Exhale Gas Analysis. ACS Omega 2020, 5, 8919–8926. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 612. [Google Scholar] [CrossRef]
- Xu, L.; Gui, Y.; Li, W.; Li, Q.; Chen, X. Gas-sensing properties of Ptn-doped WSe2 to SF6 decomposition products. J. Ind. Eng. Chem. 2021, 97, 452–459. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, S.; Bin, Y.; Jiang, X.; Cui, H. Ni-decorated WS2-WSe2 heterostructure as a novel sensing candidate upon C2H2 and C2H4 in oil-filled transformers: A first-principles investigation. Mol. Phys. 2025, e2492391. [Google Scholar] [CrossRef]
- Wu, P.; Yin, N.; Li, P.; Cheng, W.; Huang, M. The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS2 monolayer: A first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 20713–20722. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Mi, H.; Wang, J.; Zeng, W. Gas-sensing mechanism of Cr doped SnP3 monolayer to SF6 partial discharge decomposition components. Appl. Surf. Sci. 2021, 546, 149084. [Google Scholar] [CrossRef]
- Yong, Y.; Zhang, W.; Hou, Q.; Gao, R.; Yuan, X.; Hu, S.; Kuang, Y. Highly sensitive and selective gas sensors based on nanoporous CN monolayer for reusable detection of NO, H2S and NH3: A first-principles study. Appl. Surf. Sci. 2022, 606, 154806. [Google Scholar] [CrossRef]
- Tao, L.; Dastan, D.; Wang, W.; Poldorn, P.; Meng, X.; Wu, M.; Zhao, H.; Zhang, H.; Li, L.; An, B. Metal-decorated InN monolayer senses N2 against CO2. ACS Appl. Mater. Interfaces 2023, 15, 12534–12544. [Google Scholar] [CrossRef]
- Zhang, W.; Yong, Y.; Li, Z.; Li, Z.; Tao, J.; Kuang, Y. First-principles study of highly sensitive and selective gas-sensing properties of the C3N2 monolayers towards SO2 gas. Surf. Interfaces 2022, 33, 102254. [Google Scholar] [CrossRef]
- Ma, D.; Li, T.; Yuan, D.; He, C.; Lu, Z.; Lu, Z.; Yang, Z.; Wang, Y. The role of the intrinsic Se and In vacancies in the interaction of O2 and H2O molecules with the InSe monolayer. Appl. Surf. Sci. 2018, 434, 215–227. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, B.; Zhang, N.; Lu, Z.; Yang, Z.; Ma, D. Tuning the Physical and Chemical Properties of 2D InSe with Interstitial Boron Doping: A First-Principles Study. J. Phys. Chem. C 2017, 121, 28312–28316. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Liu, L.; Jia, Y. Electrocatalytic nitrogen reduction on the transition-metal dimer anchored N-doped graphene: Performance prediction and synergetic effect. Phys. Chem. Chem. Phys. 2021, 23, 4018–4029. [Google Scholar] [CrossRef]
- Rahm, M.; Hoffmann, R.; Ashcroft, N.W. Atomic and ionic radii of elements 1–96. Chemistry—A Eur. J. 2016, 22, 14625–14632. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; He, B.; Wang, Y.; Lv, P.; Ma, D.; Jia, Y. Double-atom catalysts for energy-related electrocatalysis applications: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 203001. [Google Scholar] [CrossRef]
- Yong, Y.; Gao, R.; Yuan, X.; Zhao, Z.; Hu, S.; Kuang, Y. Gas sensing and capturing based on the C7N6 monolayer with and without metal decoration: A first-principles investigation. Appl. Surf. Sci. 2022, 591, 153129. [Google Scholar] [CrossRef]
- Gu, M.; Tao, L.; Dastan, D.; Dang, J.; Fang, T.; An, B. Metal-Modified C3N1 Monolayer Sensors for Battery Instability Monitoring. J. Mater. Chem. A 2024, 12, 15254–15264. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, X.; Xiao, F.; Liu, Y.; Ming, X. First-Principles Study on the Selective Detection of Toxic Gases by 2D Monolayer PdS2: Insight into Charge Transfer Dynamics and Alignment of Frontier Molecular Orbitals. ACS Appl. Nano Mater. 2023, 6, 12470–12478. [Google Scholar] [CrossRef]
- He, B.; Lv, P.; Wu, D.; Li, X.; Zhu, R.; Chu, K.; Ma, D.; Jia, Y. Confinement catalysis of a single atomic vacancy assisted by aliovalent ion doping enabled efficient NO electroreduction to NH3. J. Mater. Chem. A 2022, 10, 18690–18700. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Lv, P.; He, B.; Li, X.; Ma, D.; Jia, Y. Theoretical insights into the electroreduction of nitrate to ammonia on graphene-based single-atom catalysts. Nanoscale 2022, 14, 10862–10872. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, D.; Wang, D.; Deng, J.; Kong, D.; Zhang, H. Performance prediction of 2D vertically stacked MoS2-WS2 heterostructures base on first-principles theory and Pearson correlation coefficient. Appl. Surf. Sci. 2022, 596, 153498. [Google Scholar] [CrossRef]
- Pan, Q.; Li, T.; Zhang, D. Ammonia gas sensing properties and density functional theory investigation of coral-like Au-SnSe2 Schottky junction. Sens. Actuators B Chem. 2021, 332, 129440. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Wu, W.; Liu, Y.; Zhou, Z. Transition metal (Pd, Pt, Ag, Au) decorated InN monolayer and their adsorption properties towards NO2: Density functional theory study. Appl. Surf. Sci. 2018, 455, 106–114. [Google Scholar] [CrossRef]
- Ma, D.; Zeng, Z.; Liu, L.; Jia, Y. Theoretical screening of the transition metal heteronuclear dimer anchored graphdiyne for electrocatalytic nitrogen reduction. J. Energy Chem. 2021, 54, 501–509. [Google Scholar] [CrossRef]
- Chu, J.; Wang, Q.; Yang, A.; Pan, J.; Liu, Y.; Yuan, H.; Rong, M.; Wang, X. Method of sieving the optimal NO2 sensitive material. Sens. Actuators B Chem. 2023, 375, 132929. [Google Scholar] [CrossRef]
- Hussein, T.A.; Alaarage, W.K.; Abdulhussein, H.A.; Seriani, N.; Nasria, A.H.A. Ga-doped AlN monolayer nano-sheets as promising materials for environmental sensing applications. Comput. Theor. Chem. 2023, 1223, 114086. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular single-bond covalent radii for elements 1–118. Chemistry 2009, 15, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ju, W.; Yong, Y.; Zhang, Q.; Liu, Y.; Li, J. Effect of the N/P/S and transition-metal co-doping on the quantum capacitance of supercapacitor electrodes based on mono-and multilayer graphene. Carbon 2020, 170, 368–379. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Cui, H.; Ran, M.; Peng, X.; Zhang, G. First-principles design of noble metal (Rh and Pd) dispersed Janus WSTe monolayer for toxic gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112047. [Google Scholar] [CrossRef]
- Cui, H.; Hu, J.; Jiang, X.; Zhang, X. A first-principles study of SOF2 and SO2F2 adsorption onto PdSe2-based monolayers: Favorable sensitivity and selectivity by doping single Cu or Rh atom. Environ. Res. 2025, 269, 120843. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, F.; Zou, J.; Liu, H.; Zhang, C.; Huang, Y.; Jiang, T. Comparison of gas-sensitive properties of Pb, Pd and Pt metal-doped Ti3C2X2 for aqueous zinc ion battery H2 gas. Appl. Surf. Sci. 2025, 681, 161517. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Jiang, X.; Zhang, Z.; Li, T.; Ma, L.; Niu, S.; Chen, Z.; Xiao, S.; Dan, M.; et al. Ir-doped MoSe2: A promising candidate for C4F7N decomposed species detection and scavenging. Surf. Interfaces 2024, 51, 104634. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Liu, Y.; Li, J.; Zhang, Q. Influence of defects and dopants on the sensitivity of arsenene towards HCN. Appl. Surf. Sci. 2020, 506, 144936. [Google Scholar] [CrossRef]
- Pi, W.; Chen, X.; Fu, Q.; Lu, Z.; Li, H.; Tang, Z.; Luo, W. The gas-sensing performance of a core–shell SnO2-based chemiresistive MEMS sensor for H2S detection under vacuum. J. Mater. Chem. C 2023, 11, 12517–12524. [Google Scholar] [CrossRef]
- Rawat, S.; Bamola, P.; Rani, C.; Kaushik, V.; Kumar, U.; Dwivedi, C.; Rattan, R.; Sharma, M.; Kumar, R.; Sharma, H. Interdigitated electrodes-based Au-MoS2 hybrid gas sensor for sensing toxic CO and NH3 gases at room temperature. Nanotechnology 2023, 34, 305601. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, X.; Xiao, F.; Liu, Y.; Ming, X. First-Principles Calculations of Two-Dimensional Monolayer PdSe2 for Selective Sensing of Nitrogen-Containing Gases. ACS Appl. Nano Mater. 2022, 5, 11519–11528. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, K.; Zhang, J.; Yang, Y.; Li, X.; Yang, S.; Yang, J. Adsorption and sensing properties of transition metal (Ni, Rh) modified Janus ReSSe monolayers for lithium-ion battery thermal runaway gases: A first principle study. Chin. J. Phys. 2025, 95, 231–247. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Cui, H.; Liu, Z.; Liu, Y. DFT Exploration of a Pd-Doped InSe Monolayer as a Novel Gas Sensing Candidate upon SF6 Decomposition: SO2, SOF2, and SO2F2. Sensors 2025, 25, 4156. https://doi.org/10.3390/s25134156
Yang X, Cui H, Liu Z, Liu Y. DFT Exploration of a Pd-Doped InSe Monolayer as a Novel Gas Sensing Candidate upon SF6 Decomposition: SO2, SOF2, and SO2F2. Sensors. 2025; 25(13):4156. https://doi.org/10.3390/s25134156
Chicago/Turabian StyleYang, Xu, Hao Cui, Zhongchao Liu, and Yun Liu. 2025. "DFT Exploration of a Pd-Doped InSe Monolayer as a Novel Gas Sensing Candidate upon SF6 Decomposition: SO2, SOF2, and SO2F2" Sensors 25, no. 13: 4156. https://doi.org/10.3390/s25134156
APA StyleYang, X., Cui, H., Liu, Z., & Liu, Y. (2025). DFT Exploration of a Pd-Doped InSe Monolayer as a Novel Gas Sensing Candidate upon SF6 Decomposition: SO2, SOF2, and SO2F2. Sensors, 25(13), 4156. https://doi.org/10.3390/s25134156





