Determining Falls Risk in People with Parkinson’s Disease Using Wearable Sensors: A Systematic Review
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Search Strategy
- (“parkinson*” OR “PD”)
- AND
- ((“wearable (device OR sensor OR technology OR system)”) OR “body worn (sensor OR device OR technology)” OR “insoles” OR “accelerometer” OR “gyroscope” OR “smartwatch” OR “pressure sensor” OR “kinematic”)
- AND
- (“falls” OR “falls (risk OR prediction OR forecasting OR count OR detection OR modelling)” OR “falling”)
2.2. Eligibility Criteria
2.3. Screening and Data Extraction
2.4. Data Synthesis
2.5. Quality Assessment
3. Results
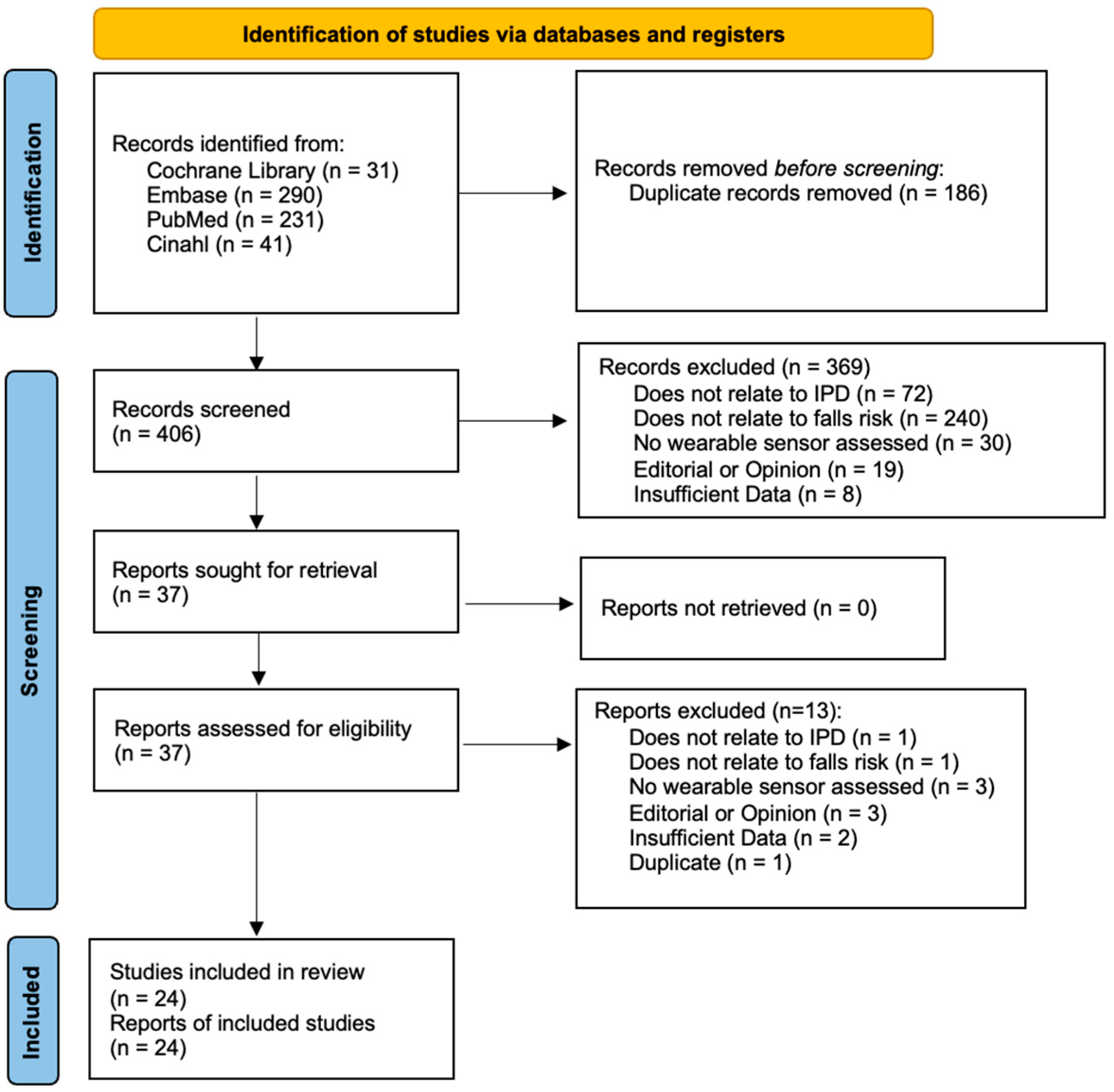
3.1. Article Selection
3.2. Risk of Bias
3.3. Study Design
3.4. Participant Characteristics
3.5. Task
3.6. Sensor Details
3.7. On/Off Designations
3.8. Falls Measurement
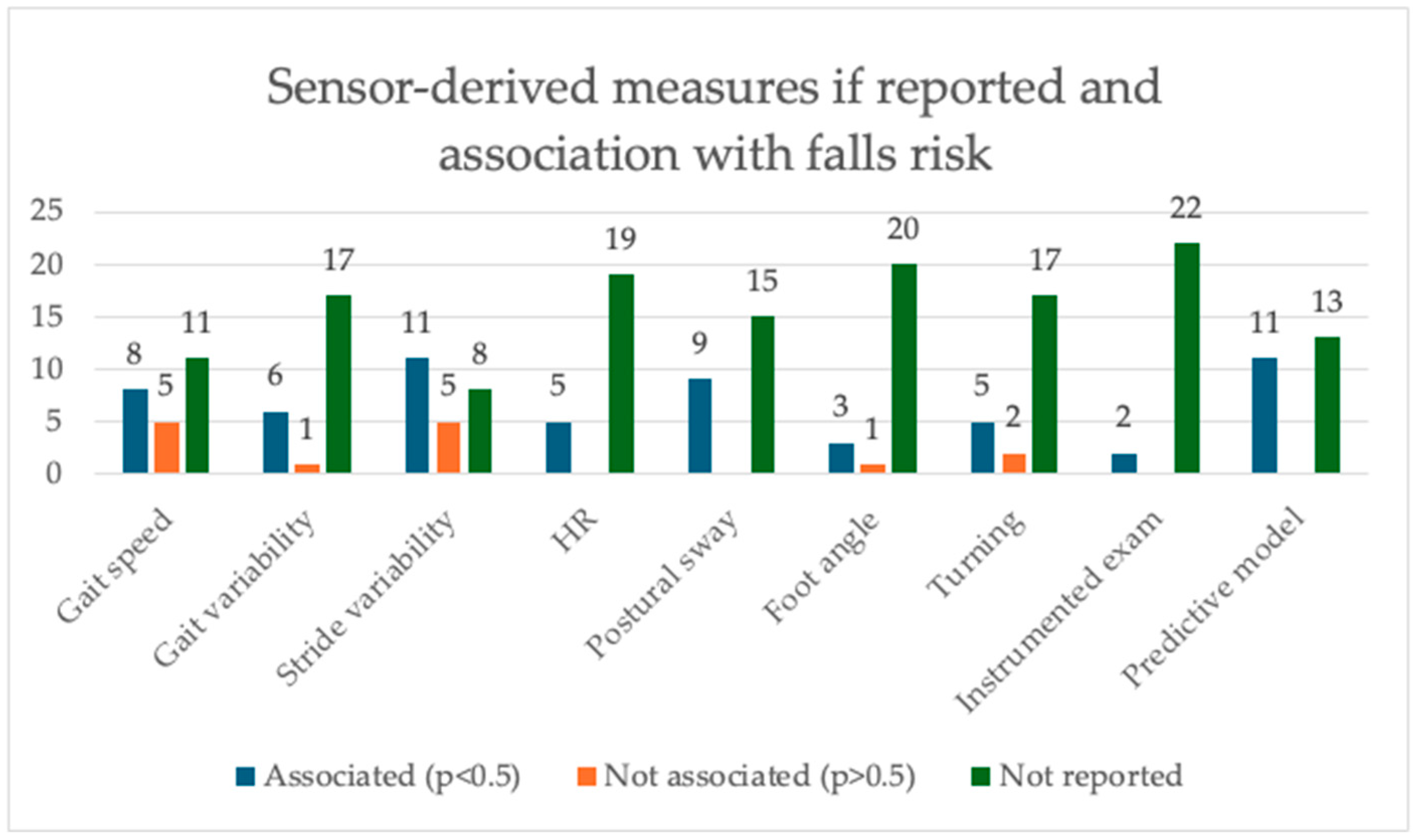
3.9. Sensor-Derived Parameters Most Strongly Associated with Falls
3.9.1. Walking Speed
3.9.2. Gait and Stride Variability
3.9.3. Gait Smoothness (Harmonic Ratio) and Postural Sway
3.9.4. Foot Angle
3.9.5. Turning
3.9.6. Instrumented Exams
3.9.7. Modeling
4. Discussion
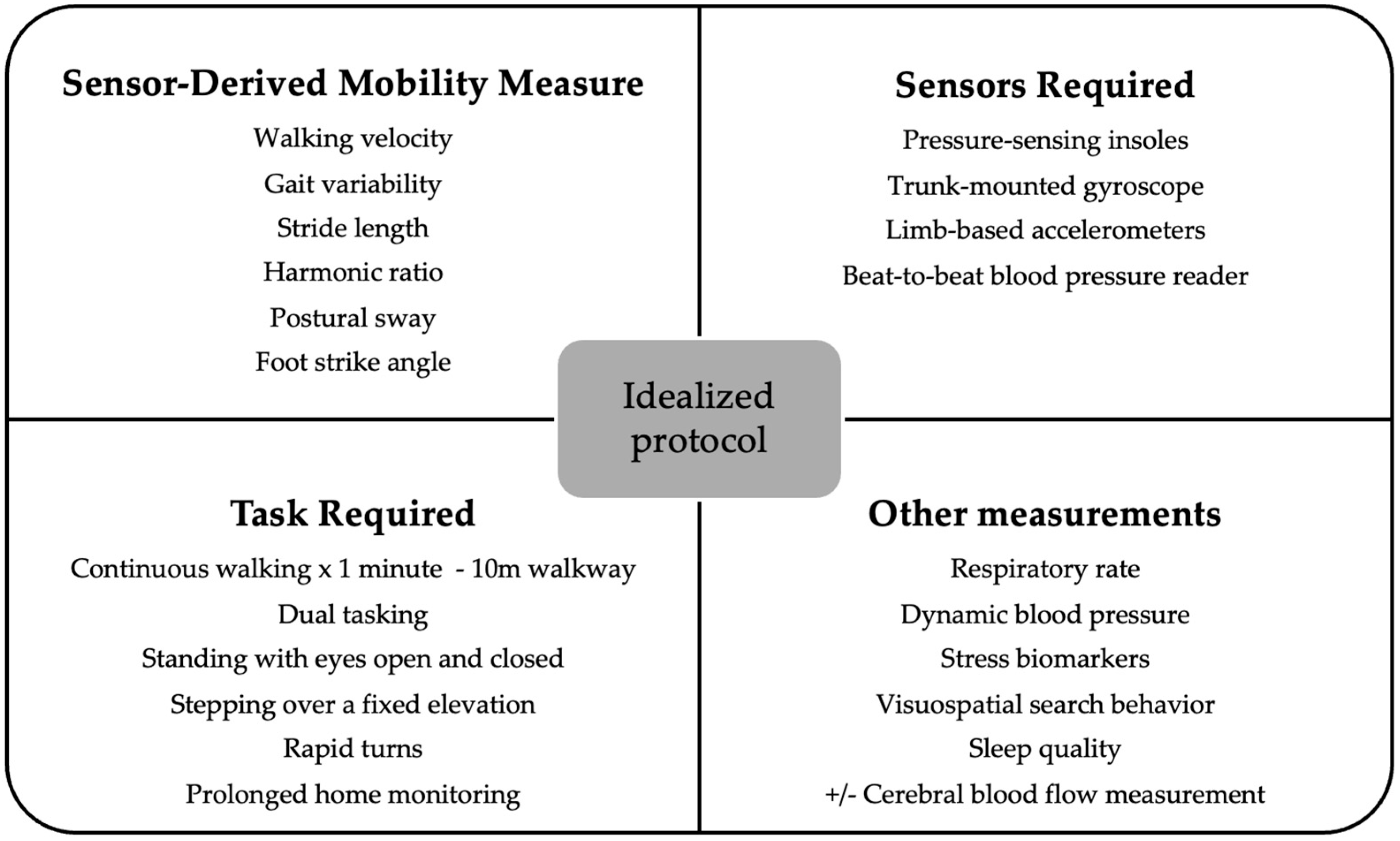
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| HR | Harmonic ratio |
| TUG | Timed up and go |
References
- Pickering, R.M.; Grimbergen, Y.A.; Rigney, U.; Ashburn, A.; Mazibrada, G.; Wood, B.; Gray, P.; Kerr, G.; Bloem, B.R. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov. Disord. 2007, 22, 1892–1900. [Google Scholar] [CrossRef]
- Genever, R.W.; Downes, T.W.; Medcalf, P. Fracture rates in Parkinson’s disease compared with age- and gender-matched controls: A retrospective cohort study. Age Ageing 2005, 34, 21–24. [Google Scholar] [CrossRef]
- Adkin, A.L.; Frank, J.S.; Jog, M.S. Fear of falling and postural control in Parkinson’s disease. Mov. Disord. 2003, 18, 496–502. [Google Scholar] [CrossRef]
- van Emde Boas, M.; Pongmala, C.; Biddix, A.M.; Griggs, A.; Luker, A.T.; Carli, G.; Marusic, U.; Bohnen, N.I. Post-Physical Therapy 4-Month In-Home Dynamic Standing Protocol Maintains Physical Therapy Gains and Improves Mobility, Balance Confidence, Fear of Falling and Quality of Life in Parkinson’s Disease: A Randomized Controlled Examiner-Blinded Feasibility Clinical Trial. J. Frailty Sarcopenia Falls 2024, 9, 267–280. [Google Scholar] [CrossRef]
- Voss, T.S.; Elm, J.J.; Wielinski, C.L.; Aminoff, M.J.; Bandyopadhyay, D.; Chou, K.L.; Sudarsky, L.R.; Tilley, B.C. Fall frequency and risk assessment in early Parkinson’s disease. Park. Relat. Disord. 2012, 18, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Parks. Dis. 2013, 2013, 906274. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Yarnall, A.J.; Coleman, S.; Burn, D.; Rochester, L. Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naive cohort. Mov. Disord. 2016, 31, 1829–1836. [Google Scholar] [CrossRef]
- Paul, S.S.; Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.; Fung, V.S. Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Mov. Disord. 2013, 28, 655–662. [Google Scholar] [CrossRef]
- van der Marck, M.A.; Klok, M.P.C.; Okun, M.S.; Giladi, N.; Munneke, M.; Bloem, B.R.; Force, N.F.T. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Park. Relat. Disord. 2014, 20, 360–369. [Google Scholar] [CrossRef]
- Bloem, B.R.; Marinus, J.; Almeida, Q.; Dibble, L.; Nieuwboer, A.; Post, B.; Ruzicka, E.; Goetz, C.; Stebbins, G.; Martinez-Martin, P.; et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2016, 31, 1342–1355. [Google Scholar] [CrossRef]
- Cole, M.H.; Silburn, P.A.; Wood, J.M.; Worringham, C.J.; Kerr, G.K. Falls in Parkinson’s disease: Kinematic evidence for impaired head and trunk control. Mov. Disord. 2010, 25, 2369–2378. [Google Scholar] [CrossRef]
- Cappozzo, A. Gait analysis methodology. Hum. Mov. Sci. 1984, 3, 27–50. [Google Scholar] [CrossRef]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; Von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in Medical Wearable Biosensors: Design, Fabrication and Materials Strategies in Healthcare Monitoring. Molecules 2021, 27, 165. [Google Scholar] [CrossRef]
- Zanardi, A.P.J.; da Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; Dos Santos, I.O.; Kruel, L.F.M.; Peyré-Tartaruga, L.A. Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 752. [Google Scholar] [CrossRef]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.-R.; Drapier, S.; Mariani, L.-L.; Roze, E.; Devos, D.; et al. Overview on wearable sensors for the management of Parkinson’s disease. Npj Park’s Dis. 2023, 9, 153. [Google Scholar] [CrossRef]
- Reichmann, H.; Klingelhoefer, L.; Bendig, J. The use of wearables for the diagnosis and treatment of Parkinson’s disease. J. Neural Transm. 2023, 130, 783–791. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- di Biase, L.; Pecoraro, P.M.; Pecoraro, G.; Shah, S.A.; Di Lazzaro, V. Machine learning and wearable sensors for automated Parkinson’s disease diagnosis aid: A systematic review. J. Neurol. 2024, 271, 6452–6470. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2023. [Google Scholar] [CrossRef]
- Greene, B.R.; Premoli, I.; McManus, K.; McGrath, D.; Caulfield, B. Predicting Fall Counts Using Wearable Sensors: A Novel Digital Biomarker for Parkinson’s Disease. Sensors 2021, 22, 54. [Google Scholar] [CrossRef]
- Greene, B.R.; Caulfield, B.; Lamichhane, D.; Bond, W.; Svendsen, J.; Zurski, C.; Pratt, D. Longitudinal assessment of falls in patients with Parkinson’s disease using inertial sensors and the Timed Up and Go test. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668317750811. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.V.; McNames, J.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Sowalsky, K.; Mancini, M.; Horak, F.B. Effect of Levodopa and Environmental Setting on Gait and Turning Digital Markers Related to Falls in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2022, 10, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.V.; Jagodinsky, A.; McNames, J.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Sowalsky, K.; Harker, G.; Mancini, M.; Horak, F.B. Gait and turning characteristics from daily life increase ability to predict future falls in people with Parkinson’s disease. Front. Neurol. 2023, 14, 1096401. [Google Scholar] [CrossRef] [PubMed]
- Iluz, T.; Gazit, E.; Herman, T.; Sprecher, E.; Brozgol, M.; Giladi, N.; Mirelman, A.; Hausdorff, J.M. Automated detection of missteps during community ambulation in patients with Parkinson’s disease: A new approach for quantifying fall risk in the community setting. J. Neuroeng. Rehabil. 2014, 11, 48. [Google Scholar] [CrossRef]
- Smulders, K.; Esselink, R.A.J.; Weiss, A.; Kessels, R.P.C.; Geurts, A.C.H.; Bloem, B.R. Assessment of dual tasking has no clinical value for fall prediction in Parkinson’s disease. J. Neurol. 2012, 259, 1840–1847. [Google Scholar] [CrossRef]
- Latt, M.D.; Lord, S.R.; Morris, J.G.L.; Fung, V.S.C. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov. Disord. 2009, 24, 1280–1289. [Google Scholar] [CrossRef]
- Ullrich, M.; Roth, N.; Kuderle, A.; Richer, R.; Gladow, T.; Gasner, H.; Marxreiter, F.; Klucken, J.; Eskofier, B.M.; Kluge, F. Fall Risk Prediction in Parkinson’s Disease Using Real-World Inertial Sensor Gait Data. IEEE J. Biomed. Health Inform. 2023, 27, 319–328. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Lai, Y.-R.; Lien, C.-Y.; Huang, C.-C.; Chiu, W.-C.; Chen, Y.-S.; Yu, C.-C.; Cheng, B.-C.; Chiang, Y.-F.; Chang, H.-W.; et al. Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease. J. Clin. Med. 2022, 12, 27. [Google Scholar] [CrossRef]
- Ma, L.; Mi, T.-M.; Jia, Q.; Han, C.; Chhetri, J.K.; Chan, P. Gait variability is sensitive to detect Parkinson’s disease patients at high fall risk. Int. J. Neurosci. 2022, 132, 888–893. [Google Scholar] [CrossRef]
- Sturchio, A.; Dwivedi, A.K.; Marsili, L.; Hadley, A.; Sobrero, G.; Heldman, D.; Maule, S.; Lopiano, L.; Comi, C.; Versino, M.; et al. Kinematic but not clinical measures predict falls in Parkinson-related orthostatic hypotension. J. Neurol. 2021, 268, 1006–1015. [Google Scholar] [CrossRef]
- Cole, M.H.; Naughton, G.A.; Silburn, P.A. Neuromuscular Impairments Are Associated With Impaired Head and Trunk Stability During Gait in Parkinson Fallers. Neurorehabil. Neural Repair 2017, 31, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Herman, T.; Giladi, N.; Hausdorff, J.M. Objective Assessment of Fall Risk in Parkinson’s Disease Using a Body-Fixed Sensor Worn for 3 Days. PLoS ONE 2014, 9, e96675. [Google Scholar] [CrossRef]
- Hoskovcová, M.; Dušek, P.; Sieger, T.; Brožová, H.; Zárubová, K.; Bezdíček, O.; Šprdlík, O.; Jech, R.; Štochl, J.; Roth, J.; et al. Predicting Falls in Parkinson Disease: What Is the Value of Instrumented Testing in OFF Medication State? PLoS ONE 2015, 10, e0139849. [Google Scholar] [CrossRef]
- Sotirakis, C.; Brzezicki, M.A.; Patel, S.; Conway, N.; FitzGerald, J.J.; Antoniades, C.A. Predicting future fallers in Parkinson’s disease using kinematic data over a period of 5 years. Npj Digit. Med. 2024, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, S.F.; Tatarelli, A.; Trabassi, D.; Icco, R.D.; Grillo, V.; Ranavolo, A.; Varrecchia, T.; Magnifica, F.; Di Lenola, D.; Coppola, G.; et al. Ability of a Set of Trunk Inertial Indexes of Gait to Identify Gait Instability and Recurrent Fallers in Parkinson’s Disease. Sensors 2021, 21, 3449. [Google Scholar] [CrossRef] [PubMed]
- Latt, M.D.; Menz, H.B.; Fung, V.S.; Lord, S.R. Acceleration Patterns of the Head and Pelvis During Gait in Older People With Parkinson’s Disease: A Comparison of Fallers and Nonfallers. J. Gerontol. Ser. A 2009, 64A, 700–706. [Google Scholar] [CrossRef]
- Din, S.D.; Galna, B.; Godfrey, A.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Mirelman, A.; Hausdorff, J.M.; Rochester, L. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’s Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J. Gerontol. Ser. A 2019, 74, 500–506. [Google Scholar] [CrossRef]
- Hubble, R.P.; Silburn, P.A.; Naughton, G.A.; Cole, M.H. Assessing stability in mild and moderate Parkinson’s disease: Can clinical measures provide insight? Gait Posture 2016, 49, 7–13. [Google Scholar] [CrossRef]
- Araújo HAG de, O.; Vitório, R.; Smaili, S.M. COMBINATION OF CLINICAL AND GAIT MEASURES TO CLASSIFY FALLERS AND NON-FALLERS IN PARKINSON’S DISEASE. Braz. J. Phys. Ther. 2024, 28, 100757. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J. Neurol. Sci. 2003, 212, 47–53. [Google Scholar] [CrossRef]
- Cole, M.H.; Sweeney, M.; Conway, Z.J.; Blackmore, T.; Silburn, P.A. Imposed Faster and Slower Walking Speeds Influence Gait Stability Differently in Parkinson Fallers. Arch. Phys. Med. Rehabil. 2017, 98, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.; Gera, G.; Horak, F.B.; Blackinton, M.T.; Besch, M.; King, L. Instrumented Test of Sensory Integration for Balance: A Validation Study. J. Geriatr. Phys. Ther. 2018, 41, 77–84. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Dagan, Y.; Hausdorff, J.M. Postural instability and fall risk in Parkinson’s disease: Impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp. Brain Res. 2011, 210, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Mancini, M.; Carlson-Kuhta, P.; Horak, F.B.; Shah, V.V. Should we use both clinical and mobility measures to identify fallers in Parkinson’s disease? Park. Relat. Disord. 2023, 106, 105235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yan, Y.; Kong, X.; Su, S. Risk factors for falls in Parkinson’s disease: A cross-sectional observational and Mendelian randomization study. Front. Aging Neurosci. 2024, 16, 1420885. [Google Scholar] [CrossRef] [PubMed]
- Camicioli, R.; Morris, M.E.; Pieruccini-Faria, F.; Montero-Odasso, M.; Son, S.; Buzaglo, D.; Hausdorff, J.M.; Nieuwboer, A. Prevention of Falls in Parkinson’s Disease: Guidelines and Gaps. Mov. Disord. Clin. Pract. 2023, 10, 1459–1469. [Google Scholar] [CrossRef]
- Mancini, M.; Hausdorff, J.M.; Pelosin, E.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Klucken, J.; Gifford, L.; Fasano, A.; Nieuwboer, A.; et al. A framework to standardize gait study protocols in Parkinson’s disease. J. Parks. Dis. 2025, 15, 1877718X241305626. [Google Scholar] [CrossRef]
- de Lima, A.L.S.; Smits, T.; Darweesh, S.K.L.; Valenti, G.; Milosevic, M.; Pijl, M.; Baldus, H.; de Vries, N.M.; Meinders, M.J.; Bloem, B.R. Home-based monitoring of falls using wearable sensors in Parkinson’s disease. Mov. Disord. 2020, 35, 109–115. [Google Scholar] [CrossRef]
- Snijders, A.H.; Haaxma, C.A.; Hagen, Y.J.; Munneke, M.; Bloem, B.R. Freezer or non-freezer: Clinical assessment of freezing of gait. Park. Relat. Disord. 2012, 18, 149–154. [Google Scholar] [CrossRef]
- Fasano, A.; Canning, C.G.; Hausdorff, J.M.; Lord, S.; Rochester, L. Falls in Parkinson’s disease: A complex and evolving picture. Mov. Disord. 2017, 32, 1524–1536. [Google Scholar] [CrossRef]
- Fukuchi, C.A.; Fukuchi, R.K.; Duarte, M. Effects of walking speed on gait biomechanics in healthy participants: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 153. [Google Scholar] [CrossRef]
- Morris, M.; Iansek, R.; Matyas, T.; Summers, J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov. Disord. 1998, 13, 61–69. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1532. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef]
- Huijben, B.; Van Schooten, K.S.; Van Dieën, J.H.; Pijnappels, M. The effect of walking speed on quality of gait in older adults. Gait Posture 2018, 65, 112–116. [Google Scholar] [CrossRef]
- Quach, L.; Galica, A.M.; Jones, R.N.; Procter-Gray, E.; Manor, B.; Hannan, M.T.; Lipsitz, L.A. The Nonlinear Relationship Between Gait Speed and Falls: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J. Am. Geriatr. Soc. 2011, 59, 1069–1073. [Google Scholar] [CrossRef]
- Almeida, L.R.S.; Piemonte, M.E.P.; Cavalcanti, H.M.; Canning, C.G.; Paul, S.S. A Self-Reported Clinical Tool Predicts Falls in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 427–434. [Google Scholar] [CrossRef]
- Mancini, M.; Afshari, M.; Almeida, Q.; Amundsen-Huffmaster, S.; Balfany, K.; Camicioli, R.; Christiansen, C.; Dale, M.L.; Dibble, L.E.; Earhart, G.M.; et al. Digital gait biomarkers in Parkinson’s disease: Susceptibility/risk, progression, response to exercise, and prognosis. Npj Park’s Dis. 2025, 11, 51. [Google Scholar] [CrossRef]
- Lord, S.; Howe, T.; Greenland, J.; Simpson, L.; Rochester, L. Gait variability in older adults: A structured review of testing protocol and clinimetric properties. Gait Posture 2011, 34, 443–450. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Annweiler, C.; Bridenbaugh, S.; Assal, F.; Kressig, R.W.; Herrmann, F.R. Gait Variability among Healthy Adults: Low and High Stride-to-Stride Variability Are Both a Reflection of Gait Stability. Gerontology 2009, 55, 702–706. [Google Scholar] [CrossRef]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Blin, O.; Ferrandez, A.M.; Pailhous, J.; Serratrice, G. Dopa-sensitive and Dopa-resistant gait parameters in Parkinson’s disease. J. Neurol. Sci. 1991, 103, 51–54. [Google Scholar] [CrossRef]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Bryant, M.S.; Rintala, D.H.; Hou, J.G.; Charness, A.L.; Fernandez, A.L.; Collins, R.L.; Baker, J.; Lai, E.C.; Protas, E.J. Gait variability in Parkinson’s disease: Influence of walking speed and dopaminergic treatment. Neurol. Res. 2011, 33, 959–964. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng. Rehabil. 2005, 2, 23. [Google Scholar] [CrossRef]
- Perry, L.; Kendrick, D.; Morris, R.; Dinan, S.; Masud, T.; Skelton, D.; Iliffe, S.; ProAct65+ Study Team. Completion and Return of Fall Diaries Varies With Participants’ Level of Education, First Language, and Baseline Fall Risk. J. Gerontol. Ser. A Biomed. Sci. Méd. Sci. 2012, 67A, 210–214. [Google Scholar] [CrossRef]
- Hiorth, Y.H.; Larsen, J.P.; Lode, K.; Pedersen, K.F. Natural history of falls in a population-based cohort of patients with Parkinson’s disease: An 8-year prospective study. Park. Relat. Disord. 2014, 20, 1059–1064. [Google Scholar] [CrossRef]
- Silva de Lima, A.L.; Evers, L.J.; Hahn, T.; Bataille, L.; Hamilton, J.L.; Little, M.A.; Okuma, Y.; Bloem, B.R.; Faber, M.J. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: A systematic review. J. Neurol. 2017, 264, 1642–1654. [Google Scholar] [CrossRef]
| First Author | Year | Study Design | Study Location | Total PD | HC | Task | Outcome |
|---|---|---|---|---|---|---|---|
| Smulders, Katrijn [26] | 2012 | Prospective cohort | Clinic | 260 | Walk along a 10 m walkway with and without a dual task | Walking speed (p = 0.041) and stride length (0.012) associated with “fallers”. Deterioration of gait under dual task conditions not associated with falls risk. | |
| Latt, Mark D [27] | 2009 | Prospective cohort | Clinic | 113 | Physiological Profile Assessment (PPA). Stand as still as possible on the floor and a foam rubber mat with eyes open and eyes closed. Leaning balance | Abnormal axial posture (p = 0.05) and poor coordinated stability (p = 0.015) associated with “fallers”. Model accurately identified “fallers”. | |
| Ullrich, Martin [28] | 2023 | Prospective cohort | Both | 35 | Usual activites (home) × 2 weeks + unsupervised 4 × 10 MWT three times a day | Gait variability (p < 0.05), stride length, IC angle, max foot lift, and walking speed (p < 0.001) associated with fallers. Falls risk better predicted with real world gait data. | |
| Tsai, Chang-Lin [29] | 2022 | Prospective cohort | Clinic | 95 | Standing with eyes open in the off- and on-medication states | Length and velocity of postural sway (p = 0.013 (cluster) was the highest predictor. Dopaminergic therapy improved clinical scores but worsened balance. | |
| Shah, Vrutangkumar [24] | 2023 | Prospective cohort | Home | 34 | Usual activities (home) × 1 week | Stride time variability (p = −0.004), toe out angle variability (p < 0.001), angle foot midswing (p = 0.002), and turn velocity (p = 0.003) were the most selected discrimaters. | |
| Ma, Lin [30] | 2022 | Prospective cohort | Clinic | 51 | TUG extended to 7 m | Gait variability (p = 0.010). RoMtrunk sagittal (p = 0.002), stride length variability (p = 0.039), and swing phase variability (0.023) were risk factors for falls. | |
| Sturchio, Andrea [31] | 2021 | Prospective cohort | Both | 26 | Usual activities (home) + lying to standing + TUG + 2MWT + sway eyes open/closed | Waist sway (p < 0.01), jerkiness, and centroidal frequency | |
| Greene, Barry R [22] | 2018 | Prospective cohort | Clinic | 15 | TUG monthly × 6 months | QTUG (73.3% accuracy predicting falls at 90 days) | |
| Cole, Michael H [32] | 2017 | Prospective cohort | Clinic | 79 | 82 | 4 self-paced and barefoot walking trials along a 9 m-long firm walkway. | Increased trunk flexion (p = 0.01), lateral head (p = 0.009) and trunk motion (p = 0.008), and increased trunk muscle activiation on EMG (p < 0.05) associated with fallers. |
| Weiss, Aner [33] | 2014 | Prospective cohort | Home | 107 | Usual activities (home) × 3 days | Gait variability (AP) (p = 0.012), lower stride regularity (p = 0.018), and less smooth gait pattern (lower HR) (p = 0.011) associated with fallers. | |
| Hoskovcová, Martina [34] | 2015 | Prospective cohort | Clinic | 45 | 22 | TUG extended to 7 m | Stride variability (off) (p < 0.01) and cadences (off) (p < 0.01) and gait speed (p < 0.01) were the most significant predictors. |
| Sotirakis, Charalampos [35] | 2024 | Prospective cohort | Clinic | 104 * | Walk for 2 min on 15 m corridor. Stand still with eyes closed. | Gait variability, postural sway acceleration variability, and stride length (p < 0.01 each) were the most significant predictors. | |
| Castiglia, Stefano Filippo [36] | 2021 | Cross-sectional | Clinic | 55 | 30 | Walk at a self-selected speed | Reduced harmonic ratio AP (p = 0.004), increased pelvic obliquity (p = 0.024), and increased pelvic rotation (p = 0.040) associated with fallers. |
| Latt, M. D. [37] | 2009 | Cross-sectional | Clinic | 66 | 33 | Walk at a self-selected speed along a 20 m corridor | Walking speed (p < 0.01), step timing variability (p < 0.01), and reduced HR (vertical p < 0.001) were the most significant predictors. |
| Del Din, Silvia [38] | 2019 | Cross-sectional | Home | 170 | 172 | Usual activities (home) × 1 week | Lower step velocity and length (p < 0.05), and stride length variabilty (p = 0.004) were the most significant predictors. |
| Hubble, Ryan P [39] | 2016 | Cross-sectional | Clinic | 29 | TUG × 5 + 6MWT + retropulsion | Reduced HR (rhymicity) of the head and trunk (p < 0.02) was the most significant predictor. | |
| Araújo, Hayslenne A G O [40] | 2023 | Cross-sectional | Clinic | 127 | Walking at self-selected pace along a 10 m corridor for 2 min with and without dual task. | Foot strike angle, variability of trunk tranverse ROM, stride variability, and turn duration/steps (p < 0.05 for each) were the most significant predictors. | |
| Shah, Vrutangkumar V [23] | 2022 | Cross-sectional | Both | 34 | 3 min walk test at natural pace in on and off state + usual activities (home) × 1 week | Turn velocity, number of steps in turn, and variability in gait speed (in “off” state) (p < 0.03) were the most significant predictors. No sig difference in “on” state. | |
| Schaafsma, Joanna D [41] | 2003 | Cross-sectional | Clinic | 32 | 80 m walking test in the “off” and “on” state | Stride time variability (p < 0.009) was the most significant predictor. | |
| Cole, Michael H [42] | 2017 | Cross-sectional | Clinic | 20 | 10 | Walking on a treadmill at 70%, 100%, and 130% of preferred speed. | Reduced HR, reduced speed, and stride length were the most significant predictors. |
| Freeman, Lynn [43] | 2018 | Cross-sectional | Clinic | 26 | Sensory organisation test and modified clinical test of sensory integration | I-mCTSIB (instrumented exam) may distinguish between fallers and nonfallers (p = 0.04) | |
| Plotnik [44] | 2011 | Cross-sectional | Clinic | 30 | Walking along 20 m walkway with and without dual tasking | Walking speed, gait variability, and asymmetry. Larger DT effect in fallers. | |
| Greene, Barry R [21] | 2021 | Cross-sectional | Clinic | 27 | 1015 | TUG | Predictive model: mean R2 value 0.43, mean error 0.42, mean correlation 30% across 2 data sets. PD2: mean stride length and no. strides in turn. |
| Vitorio, Rodrigo [45] | 2023 | Cross-sectional | Clinic | 144 | Walk at comfortable pace for 2 min. Stand for 30 s in 3 different condition; firm surface, eyes either open or closed; foam surface, eyes open. | Turn variability (p = 0.04), step duration (p = 0.007) and stride Length variability (p = 0.002) and trunk transverse ROM (p = 0.11) were the most significant predictors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradley, M.; O’Loughlin, S.; Donlon, E.; Gallagher, A.; O’Keeffe, C.; Inocentes, J.; Ruggieri, F.; Reilly, R.B.; Walsh, R.; Lynch, T.; et al. Determining Falls Risk in People with Parkinson’s Disease Using Wearable Sensors: A Systematic Review. Sensors 2025, 25, 4071. https://doi.org/10.3390/s25134071
Bradley M, O’Loughlin S, Donlon E, Gallagher A, O’Keeffe C, Inocentes J, Ruggieri F, Reilly RB, Walsh R, Lynch T, et al. Determining Falls Risk in People with Parkinson’s Disease Using Wearable Sensors: A Systematic Review. Sensors. 2025; 25(13):4071. https://doi.org/10.3390/s25134071
Chicago/Turabian StyleBradley, Maeve, Sarah O’Loughlin, Eoghan Donlon, Amy Gallagher, Clodagh O’Keeffe, John Inocentes, Federica Ruggieri, Richard B. Reilly, Richard Walsh, Tim Lynch, and et al. 2025. "Determining Falls Risk in People with Parkinson’s Disease Using Wearable Sensors: A Systematic Review" Sensors 25, no. 13: 4071. https://doi.org/10.3390/s25134071
APA StyleBradley, M., O’Loughlin, S., Donlon, E., Gallagher, A., O’Keeffe, C., Inocentes, J., Ruggieri, F., Reilly, R. B., Walsh, R., Lynch, T., Di Luca, D. G., & Fearon, C. (2025). Determining Falls Risk in People with Parkinson’s Disease Using Wearable Sensors: A Systematic Review. Sensors, 25(13), 4071. https://doi.org/10.3390/s25134071







