Abstract
A prior history of falls remains the strongest predictor of future falls in individuals with Parkinson’s disease (PD). There are limited biomarkers available to identify falls risk before falls begin to occur. The aim of this review is to investigate if features associated with falls risk may be detected by wearable sensors in patients with PD. A systematic search of the MEDLINE, EMBASE, Cochrane, and Cinahl databases was performed. Key quality criteria include sample size adequacy, data collection procedures, and the clarity of statistical analyses. The data from each included study were extracted into defined data extraction spreadsheets. Results were synthesized in a narrative manner. Twenty-four articles met the inclusion criteria. Of these, twelve measured falls prospectively, while the remaining relied on retrospective history. The definition of a “faller” varied across studies. Most assessments were conducted in a clinical setting (18/24). There was considerable variability in sensor placement and mobility tasks assessed. The most common sensor-derived measures that significantly differentiated “fallers” from “non-fallers” in Parkinson’s disease included gait variability, stride variability, trunk motion, walking speed, and stride length.
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by tremor, bradykinesia, postural instability, and rigidity. Falls occur frequently in patients with PD; incidence rates vary from 35 to 90% for falls and from 18 to 65% for recurrent falls [1]. The consequences of these falls are considerable and play a major role in diminished quality of life. These include injuries [2], fear of falling [3], and reduced activity levels [4]. Falls are not limited to those with advanced Parkinson’s disease; they also frequently occur in early, untreated PD [5].
Among all clinical predictors, a prior history of falls remains the strongest predictor of future falls in PD [6]. Additional clinical risk factors include freezing of gait, cognitive impairment, reduced walking speed, and an inability to stand on one leg [7,8]. The National Parkinson Foundation’s Falls Task Force provided a comprehensive overview of clinical fall risk factors, reinforcing the consensus that a history of previous falls is the strongest predictor of future falls [9].
However, there is a lack of reliable objective methods for prospectively identifying individuals at risk of falling. Most clinical rating scales for gait and posture either perform sub-optimally for falls prediction or have been evaluated insufficiently [10]. Camera-based motion analysis has allowed further biomechanical evidence for gait parameters and falls risk, identifying increased head motion and reduced toe clearance in PD “fallers” [11]. However, this method requires stationary and often costly laboratory equipment [12].
Wearable sensors have emerged as promising tools for falls prediction. These devices can be embedded into clothes, wristbands, patches, or insoles. Inertial measurement units (IMUs) typically consist of an accelerometer (measures acceleration), a magnetometer (orientation), and a gyroscope (angle and angular velocity) [13]. Recent advances in miniaturization, battery life, and materials have improved sensor comfort, wearability, and long-term usability [14]. Wearable sensors can quantitatively assess and differentiate movement problems in people with PD [15]. They can accurately differentiate between PD and healthy controls, track progression of motor symptoms and detect falls and freezing of gait [15,16,17,18].
However, challenges remain, including managing large volumes of variable data, ensuring device reliability, and optimizing usability for both patients and clinicians. Artificial intelligence (AI) has been adapted to analyze physiological movement using machine learning and neural network technologies to detect patterns within large datasets. In combination with wearable sensors, AI provides advanced noise filtering algorithms which can help to distinguish relevant signals from motion artefacts or environmental noise, thereby enhancing the reliability of clinical data [19].
It is still unknown whether there is an ideal protocol for using wearable sensors in PD to reliably distinguish between “fallers” and “non-fallers”, or if any specific sensor-derived measurements hold the highest predictive value. There is also no consistent interpretation of what defines a “faller” or “recurrent faller” in PD. A better understanding of PD kinematic risk factors for falls could help identify those at risk and inform early interventions to mitigate this risk.
Objective
Our objective is to determine the predictive accuracy of sensors in identifying PD “fallers” and to determine which specific sensor-derived parameters are most strongly associated with falls risk.
Furthermore, we intend to assess methodological differences across studies, including sensor type, placement, and analysis techniques.
2. Materials and Methods
A systematic review was conducted according to the PRISMA guidelines and in accordance with the Cochrane Collaboration Handbook for Systematic Review of Interventions [20]. The protocol was pre-registered (PROSPERO 2025 CRD420250640812).
2.1. Search Strategy
A systematic search of the MEDLINE, EMBASE, Cochrane, and Cinahl databases was performed with the search terms below. The last search was conducted on 31 March 2025. The search terms were as follows:
- (“parkinson*” OR “PD”)
- AND
- ((“wearable (device OR sensor OR technology OR system)”) OR “body worn (sensor OR device OR technology)” OR “insoles” OR “accelerometer” OR “gyroscope” OR “smartwatch” OR “pressure sensor” OR “kinematic”)
- AND
- (“falls” OR “falls (risk OR prediction OR forecasting OR count OR detection OR modelling)” OR “falling”)
In addition, the reference lists of all included articles were searched for additional relevant articles. No publication date restrictions were included. Articles that analyzed data from the same cohort were individually assessed to determine if outcome measures differed and were relevant to inclusion/exclusion criteria.
2.2. Eligibility Criteria
Original research articles published in English were included. Case studies, review/opinion articles, and conference abstracts were not included.
The defined population was adults with idiopathic PD as diagnosed by a movement disorder specialist. Articles were excluded if idiopathic PD was not the primary disorder (i.e., atypical Parkinson syndromes or other causes of Parkinsonism not included). We included participants at all stages of PD including, patients who were levodopa naïve, on oral medications, and on device-assisted therapies.
We included articles that used wearable sensors deployed in gait, posture, or balance assessments including in normal daily activities. Pressure mats and motion capture cameras were not included as these did not qualify as “wearable”.
Articles were included that determined the predictive accuracy of sensors in identifying PD “fallers”. No standardized definition of “fallers” or defined length of follow up of falls measurement was applied. Both retrospective and prospective measurement of falls were included. We excluded articles that did not record relevant outcomes with falls risk. Articles that assessed only freezing of gait were not included. Articles that used sensors only for falls detection (recognizing a fall event) rather than falls risk detection (evaluating the likelihood of falling) were not included.
2.3. Screening and Data Extraction
Two review authors (MB and SOL) independently screened the abstracts. A third author (CF) arbitrated in case of disagreements. The data from each included study was extracted into defined data extraction spreadsheets.
The following data was extracted: number of participants, participant characteristics, number and type of devices used, the anatomical location they were worn, and the locations and procedures in which the device was deployed (i.e., remote or in a laboratory/clinic environment). The primary outcome was dichotomous—“faller” vs. “non faller”—and predictive measures for outcome were extracted and summarized. Specific sensor-derived measures and their validity in differentiating “fallers” from “non-fallers” were identified. In particular, gait and stride variability, cadence, stride length, postural sway, harmonic ratio, foot/toe angle, and turning characteristics were recorded.
2.4. Data Synthesis
For each included study, outcomes related to fall risk prediction were synthesized using descriptive statistics and effect measures appropriate to the data type. In a standardized extraction form, we collected study characteristics, including first author, year of publication, study design, geographic location, assessment task, participant selection criteria, follow-up duration, and reported outcomes. Participant-level data, such as age, gender, disease duration, levodopa dosage and timing, cognitive function, MDS-UPDRS score, and Hoehn and Yahr stage, as well as sensor type, position, and testing parameters were also extracted.
For continuous variables (e.g., age, disease duration, UPDRS scores), effect measures included means and standard deviations, allowing for comparison between fallers and non-fallers. For categorical variables (e.g., gender, sensor type, presence of comorbidities), results were summarized using frequencies and percentages.
Due to heterogeneity in outcome reporting and study design, a meta-analysis was not performed. Instead, a qualitative synthesis was conducted. Data were presented in tables, narrative summaries, and visual graphs to facilitate the interpretation of trends and variability across studies.
2.5. Quality Assessment
Key quality criteria included sample size adequacy, data collection procedures, and the clarity of statistical analyses. JBI version 2020 was used as a critical appraisal tool. Two reviewers independently assessed the risk of bias. Disagreements were resolved through discussion or arbitration by a third reviewer (CF). Articles considered to have a high risk of bias were excluded. All included studies were assessed for potential risk of bias due to missing results, particularly arising from selective reporting or attrition
3. Results
3.1. Article Selection
A total of 406 abstracts were screened against the inclusion/exclusion criteria. Twenty-five articles proceeded to quality assessment, while one study was excluded due to risk of bias (see below). The primary reasons for exclusion included a wearable sensor not being assessed, PD not being the primary disorder, and falls risk association not being properly assessed (Figure 1).

Figure 1.
PRISMA flow diagram article selection.
One article included analyses of two separate datasets, namely “PD1” and “PD2” [21]. The PD1 dataset had been previously reported in a 2018 article included in this review [22]. Therefore, for our analysis, we exclusively assessed the PD2 dataset from the 2021 article.
Additionally, two articles used the same dataset but were both included in the review, as each reported different outcome measures [23,24].
3.2. Risk of Bias
Overall, the quality of the included studies was moderate. One study was excluded due to high risk of bias, stemming from a short follow-up, reliance on missteps rather than actual falls, and an inability of the sensors to distinguish between near-misses and true events [25].
Controlling for confounders in Parkinson’s disease is challenging due to its multifactorial nature. Many studies excluded individuals with severe comorbidities, such as cognitive impairment or mobility limitations. While this improved internal validity, it may reduce generalizability to higher-risk, real-world populations.
Most included studies were judged to have mild to moderate risk of bias. Technical issues, like sensor malfunctions accounted for some missing data, but there was no evidence of systematic exclusion or selective reporting affecting the overall synthesis. A total of 24 studies were included in the final review.
3.3. Study Design
Of the twenty-four included articles, twelve were prospective in design, and the remaining twelve were cross-sectional (summarized in Table 1). The average number of participants in the studies was 71 (standard deviation (STD) 59, median 48, range 15–260). Overall, the 24 articles consisted of 1724 participants, of which 43% were defined as “fallers” by the respective authors (749/1724) (Supplementary Table S1). Falls measurement details are summarized below.

Table 1.
Study design and outcome.
3.4. Participant Characteristics
“Fallers” as defined by the respective authors were older (average age 68.5 (STD 2.5) vs. 66.1 (STD 2.2)). On average, “fallers” had greater disease severity as measured by higher MDS-UPDRS part III scores (average 35.4 vs. 29.1) and H&Y scores (average 2.4 vs. 1.9). Thirteen articles provided details on levodopa equivalent doses (LEDD) with a range of 400–1335 mg. No participants were reported to be levodopa naïve or on advanced therapies (Supplementary Table S1)
3.5. Task
Five articles assessed some variation on the timed up and go test. Four articles used wearable sensors in the patient’s home environment with application between 3–14 days. Nine articles assessed patients walking in the clinic at a self-selected speed for varying durations and lengths. Dual tasking was assessed in two cohorts. The primary objective of two studies was to assess the utility of an instrumented version of a standard quantitative assessment tool, namely the QTUG (Quantitative TUG) and imCTSIB (instrumented Modified Clinical Test of Sensory Interaction in Balance).
3.6. Sensor Details
Accelerometers and gyroscopes were the most commonly used wearable sensors. Eleven articles used a single sensor application site and eleven used multiple sites (Supplementary Table S2).
3.7. On/Off Designations
Of the twenty-four articles, ten articles assessed patients in the “on” state and five in the “off” state. Three articles assessed patients in both “on” and “off” states, and in the reminder of articles “on/off” status was not specified. In the articles that assessed PD patients in both states, “off” parameters were more informative compared to “on” parameters. Tsai et al. found that dopaminergic therapy can improve clinical functional scores but worsens balance-related measures [29].
3.8. Falls Measurement
Falls were measured prospectively in eight articles, retrospectively in twelve, and both prospectively and retrospectively in four. The maximum duration of falls measurement was 60 months (mean 6 months, median 12 months). The prospective range of falls measurement was 3–60 months, and the retrospective range was 6–12 months. The definition of a “faller” was not consistent (range > 2 falls in 6 months—>/= 1 fall/12 months). Falls measurements were subjective in all articles, either self-reported via phone or interview or captured by a falls diar (Supplementary Table S3)
3.9. Sensor-Derived Parameters Most Strongly Associated with Falls
Findings varied regarding which sensor-derived measure was most strongly associated with fallers compared to non-fallers, and the measures reported were not consistent across studies (Figure 2, ST).

Figure 2.
Bar chart showing the number of articles reporting on specific sensor-derived measures and ability to distinguish between “fallers” and “non fallers”.
3.9.1. Walking Speed
Thirteen articles measured and reported on walking speed. Eight found reduced walking pace significantly distinguished “fallers” from “non-fallers” whereas five found no significant association.
3.9.2. Gait and Stride Variability
Seventeen articles measured and reported on gait or stride variability. Six articles reported that increased gait variability was positively associated with falls risk, while eleven found stride variability (either timing or length) to be positively associated with falls risk. Five articles found no association between increased stride variability and falling status and one article found no association with gait variability.
3.9.3. Gait Smoothness (Harmonic Ratio) and Postural Sway
The harmonic ratio (HR) is a measure of trunk acceleration commonly used as marker of gait smoothness. Five articles reported on this, and all five demonstrated that reduced HR was significantly associated with PD “fallers” (p < 0.001—p < 0.02). Nine articles reported on postural sway, and all nine demonstrated that increased or variability postural sway was significantly associated with the “faller” category; this included sway both anteroposteriorly and laterally.
3.9.4. Foot Angle
Four articles reported on foot strike angle, with three finding a significant association with PD “fallers”.
3.9.5. Turning
Seven articles reported on turning markers including velocity, duration, step count, and variability. Five found significant associations with PD “fallers”.
3.9.6. Instrumented Exams
Two articles found that standardized mobility assessments’ ability to predict falls risk was enhanced by using sensor-derived measurements with the clinical measurements (QTUG and imCTSIB).
3.9.7. Modeling
Eleven articles used clinical and sensor-derived mobility measures to develop falls prediction models for PD, demonstrating moderate to excellent predictive value (area under the curve 0.838—>0.988). In all models, a combination of clinical and sensor measurements outperformed clinical measurements alone.
4. Discussion
Falls risk in Parkinson’s disease is influenced by a complex interplay of motor, cognitive, and sensory impairments, making accurate prediction and prevention challenging [9,46,47]. While wearable technology shows promise in falls risk prediction, further validation and refinement are required to address variability. Key findings in this review suggest slower walking velocity, gait/stride variability, shorted stride length, reduced gait smoothness, and increased head and pelvis motion are associated with “fallers”. However, heterogeneity in studied population, sensor placement, and gait and posture assessment tasks complicate the generalization and comparison of results.
This year, the Gait Advisors Leading Outcomes for Parkinson’s (GALOP) committee provided recommendations for a minimum set of gait measures to be adopted in projects evaluating PD [48]. These includes (1) one continuous gait task of at least 1 min in duration, (2) over a path of 10 m in length (allowing 1 m at each end for a safe turn), and (3) with participants performing 180 degrees turns at each end. Only two included articles fulfilled these criteria [40,49]. Both studies incorporated dual tasking, a recommendation also endorsed by the GALOP committee. Although 10 of the 25 articles did include measurements of walking at a self-selected pace, the path length varied.
An idealized study protocol utilizing wearable sensors in PD has yet to be clearly defined. Optimal protocols may vary depending on the specific primary outcomes of a given study. From this review, we would recommend ensuring walking velocity, gait variability, stride length, harmonic ratio, postural sway, and foot strike angle are evaluated (Figure 3). Thus, clinical assessments using wearable sensors should incorporate a comprehensive sensor suite, including pressure-sensing insoles, trunk-mounted gyroscopes, limb-based accelerometers, and device-based measurements of confounders e.g., orthostatic hypotension. Assessing patients in “on” and “off” states is also optimal but logistically challenging
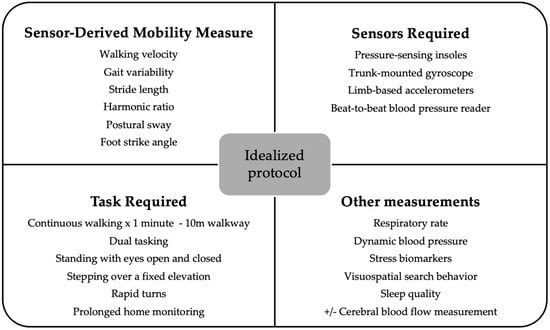
Figure 3.
Suggested idealized protocol for sensor-based evaluations of falls risk in PD.
Participants should also undergo a detailed clinical evaluation to identify and control for potential confounding factors, such as muscle weakness, musculoskeletal disorders, proprioceptive impairments, and visual deficits. To better distinguish PD-specific changes from those associated with aging, the inclusion of an age-matched healthy control group is strongly recommended. In addition to baseline clinical assessments, and the GALOP gait analysis [48], freezing of gait provocation protocols (rapid 360-degree turns in place [50]) and postural stability assessments (e.g., Mini-BESTest) should be considered.
For prolonged home monitoring, the selection of wearable sensors must be rationalized. Additional sensor modalities, such as those measuring respiratory rate, dynamic blood pressure, stress biomarkers, visuospatial search behavior, cerebral function/blood flow, and sleep quality, may provide complementary insights, but their inclusion should be guided by the specific aims and feasibility of the study.
This review was consistent with others in identifying slower walking speed and reduced stride length as markers of falls risk [9,51,52]. However, walking speed and its effect on fall risk is complex. Walking speed is determined by both the number of strides taken per unit of time (cadence) and the average stride length [53]. There is a suggestion that gait slowing in PD is primarily attributed to a reduction in stride length rather than a decrease in cadence [54,55], but they may coincide [15]. In this review, stride length was more frequently identified as a marker of falls risk than cadence. Additionally, individuals with PD, particularly those with postural instability, may consciously reduce their walking speed as a strategy to minimize fall risk.
In older adults, faster walking speeds have been associated with an increased risk of falls; however, the association is non-linear [42,53,56,57]. One study included in this review demonstrated that imposed faster walking speeds in individuals with PD led to decreased gait stability, while poorer stability was observed for all participant groups at walking speeds that were slower than preferred [58]. Further research is needed to determine whether reduced walking speed itself is an independent risk factor—separate from stride length—or if it primarily reflects a compensatory behavioral adaptation. Addressing this question would require sensor-based gait assessments. Regardless, reduced walking speed may serve as a valuable tool for identifying PD patients at risk of falls, as demonstrated in prior reviews [8,9,51,52,59].
In a recent review, gait variability, specifically stride-time variability, was identified as the only PD gait biomarker sensitive across four contexts of use (disease susceptibility/risk, progression, exercise response, and fall risk/prognosis), reflecting sensitivity but potentially poor specificity [60]. Gait variability in older adults is a well-documented risk factor for falls, with increased variability associated with a higher likelihood of falling [61,62,63,64]. However, gait variability was not identified as a strong predictor of falls risk in another meta-analysis of gait kinematics and falls risk in PD [65]. Levodopa can effectively improve stride length but does not fully restore gait variability and rhythmicity [64,66,67]. This underscores the need for multimodal approaches in gait rehabilitation, beyond just dopaminergic therapy.
Limitations
There was limited consistency across the studies in terms of the task and sensor protocol. Differences in sensor types, placement, and the nature of the tasks assessed limited the comparability of results across studies. Upper body and foot measures were often excluded. Similarly, there was a lack of participant-level data to ensure that selected PD populations were similar for comparison, while outcome measures (including definition of a “faller”) varied. Furthermore, all articles included relied on some method of self-reporting falls, which is subject to recall bias and underreporting [68,69]. No study employed automated fall detection, although they also have false positives and negatives [70].
PD symptoms and, therefore, falls risk fluctuate diurnally and with medication cycles, so a single assessment as deployed by many studies may not capture an accurate falls risk profile. Although some studies utilized home-based monitoring, many relied on structured clinical assessments. Real-world gait and balance assessments may provide more ecologically valid indicators of fall risk. Many studies did not clearly specify whether participants were in the “on” or “off” medication state during the assessment.
5. Conclusions
Wearable sensors hold strong potential for early fall risk detection in PD. In this review, PD patients at risk of falling showed a profile of abnormalities detectable by wearable sensors, e.g., slower and more variable gait, increased sway, and often overall greater motor impairment. This review included twenty-four articles exploring wearable sensors in predicting falls risk in PD. We have highlighted significant variability in protocols and measurement standards across studies. Nonetheless, sensor-augmented assessments consistently outperformed traditional clinical evaluations in predicting fall risk, but models combining both performed superiorly again. Further work is needed to determine the minimum sensor setup and duration of application that still yields high predictive accuracy. Furthermore, there is a need for a unified definition of “faller” to standardize outcome measures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s25134071/s1, Supplementary Table S1: Summary of participant characteristics; Supplementary Table S2: Sensor details; Supplementary Table S3: Falls measurement; Supplementary Table S4: Summary of sensor derived measures and association with “fallers”.
Author Contributions
M.B. and S.O.: Article screening, data extraction and analysis, manuscript preparation. Both authors contributed equally. E.D., A.G., C.O., J.I., F.R., R.B.R., R.W., T.L. and D.G.D.L.: Provided a review and editing of manuscript. C.F.: Project conception and methodology, supervision, arbitration, manuscript review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PD | Parkinson’s disease |
| HR | Harmonic ratio |
| TUG | Timed up and go |
References
- Pickering, R.M.; Grimbergen, Y.A.; Rigney, U.; Ashburn, A.; Mazibrada, G.; Wood, B.; Gray, P.; Kerr, G.; Bloem, B.R. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov. Disord. 2007, 22, 1892–1900. [Google Scholar] [CrossRef]
- Genever, R.W.; Downes, T.W.; Medcalf, P. Fracture rates in Parkinson’s disease compared with age- and gender-matched controls: A retrospective cohort study. Age Ageing 2005, 34, 21–24. [Google Scholar] [CrossRef]
- Adkin, A.L.; Frank, J.S.; Jog, M.S. Fear of falling and postural control in Parkinson’s disease. Mov. Disord. 2003, 18, 496–502. [Google Scholar] [CrossRef]
- van Emde Boas, M.; Pongmala, C.; Biddix, A.M.; Griggs, A.; Luker, A.T.; Carli, G.; Marusic, U.; Bohnen, N.I. Post-Physical Therapy 4-Month In-Home Dynamic Standing Protocol Maintains Physical Therapy Gains and Improves Mobility, Balance Confidence, Fear of Falling and Quality of Life in Parkinson’s Disease: A Randomized Controlled Examiner-Blinded Feasibility Clinical Trial. J. Frailty Sarcopenia Falls 2024, 9, 267–280. [Google Scholar] [CrossRef]
- Voss, T.S.; Elm, J.J.; Wielinski, C.L.; Aminoff, M.J.; Bandyopadhyay, D.; Chou, K.L.; Sudarsky, L.R.; Tilley, B.C. Fall frequency and risk assessment in early Parkinson’s disease. Park. Relat. Disord. 2012, 18, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Parks. Dis. 2013, 2013, 906274. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Yarnall, A.J.; Coleman, S.; Burn, D.; Rochester, L. Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naive cohort. Mov. Disord. 2016, 31, 1829–1836. [Google Scholar] [CrossRef]
- Paul, S.S.; Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.; Fung, V.S. Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Mov. Disord. 2013, 28, 655–662. [Google Scholar] [CrossRef]
- van der Marck, M.A.; Klok, M.P.C.; Okun, M.S.; Giladi, N.; Munneke, M.; Bloem, B.R.; Force, N.F.T. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Park. Relat. Disord. 2014, 20, 360–369. [Google Scholar] [CrossRef]
- Bloem, B.R.; Marinus, J.; Almeida, Q.; Dibble, L.; Nieuwboer, A.; Post, B.; Ruzicka, E.; Goetz, C.; Stebbins, G.; Martinez-Martin, P.; et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2016, 31, 1342–1355. [Google Scholar] [CrossRef]
- Cole, M.H.; Silburn, P.A.; Wood, J.M.; Worringham, C.J.; Kerr, G.K. Falls in Parkinson’s disease: Kinematic evidence for impaired head and trunk control. Mov. Disord. 2010, 25, 2369–2378. [Google Scholar] [CrossRef]
- Cappozzo, A. Gait analysis methodology. Hum. Mov. Sci. 1984, 3, 27–50. [Google Scholar] [CrossRef]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; Von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in Medical Wearable Biosensors: Design, Fabrication and Materials Strategies in Healthcare Monitoring. Molecules 2021, 27, 165. [Google Scholar] [CrossRef]
- Zanardi, A.P.J.; da Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; Dos Santos, I.O.; Kruel, L.F.M.; Peyré-Tartaruga, L.A. Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 752. [Google Scholar] [CrossRef]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.-R.; Drapier, S.; Mariani, L.-L.; Roze, E.; Devos, D.; et al. Overview on wearable sensors for the management of Parkinson’s disease. Npj Park’s Dis. 2023, 9, 153. [Google Scholar] [CrossRef]
- Reichmann, H.; Klingelhoefer, L.; Bendig, J. The use of wearables for the diagnosis and treatment of Parkinson’s disease. J. Neural Transm. 2023, 130, 783–791. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- di Biase, L.; Pecoraro, P.M.; Pecoraro, G.; Shah, S.A.; Di Lazzaro, V. Machine learning and wearable sensors for automated Parkinson’s disease diagnosis aid: A systematic review. J. Neurol. 2024, 271, 6452–6470. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2023. [Google Scholar] [CrossRef]
- Greene, B.R.; Premoli, I.; McManus, K.; McGrath, D.; Caulfield, B. Predicting Fall Counts Using Wearable Sensors: A Novel Digital Biomarker for Parkinson’s Disease. Sensors 2021, 22, 54. [Google Scholar] [CrossRef]
- Greene, B.R.; Caulfield, B.; Lamichhane, D.; Bond, W.; Svendsen, J.; Zurski, C.; Pratt, D. Longitudinal assessment of falls in patients with Parkinson’s disease using inertial sensors and the Timed Up and Go test. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668317750811. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.V.; McNames, J.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Sowalsky, K.; Mancini, M.; Horak, F.B. Effect of Levodopa and Environmental Setting on Gait and Turning Digital Markers Related to Falls in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2022, 10, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.V.; Jagodinsky, A.; McNames, J.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Sowalsky, K.; Harker, G.; Mancini, M.; Horak, F.B. Gait and turning characteristics from daily life increase ability to predict future falls in people with Parkinson’s disease. Front. Neurol. 2023, 14, 1096401. [Google Scholar] [CrossRef] [PubMed]
- Iluz, T.; Gazit, E.; Herman, T.; Sprecher, E.; Brozgol, M.; Giladi, N.; Mirelman, A.; Hausdorff, J.M. Automated detection of missteps during community ambulation in patients with Parkinson’s disease: A new approach for quantifying fall risk in the community setting. J. Neuroeng. Rehabil. 2014, 11, 48. [Google Scholar] [CrossRef]
- Smulders, K.; Esselink, R.A.J.; Weiss, A.; Kessels, R.P.C.; Geurts, A.C.H.; Bloem, B.R. Assessment of dual tasking has no clinical value for fall prediction in Parkinson’s disease. J. Neurol. 2012, 259, 1840–1847. [Google Scholar] [CrossRef]
- Latt, M.D.; Lord, S.R.; Morris, J.G.L.; Fung, V.S.C. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov. Disord. 2009, 24, 1280–1289. [Google Scholar] [CrossRef]
- Ullrich, M.; Roth, N.; Kuderle, A.; Richer, R.; Gladow, T.; Gasner, H.; Marxreiter, F.; Klucken, J.; Eskofier, B.M.; Kluge, F. Fall Risk Prediction in Parkinson’s Disease Using Real-World Inertial Sensor Gait Data. IEEE J. Biomed. Health Inform. 2023, 27, 319–328. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Lai, Y.-R.; Lien, C.-Y.; Huang, C.-C.; Chiu, W.-C.; Chen, Y.-S.; Yu, C.-C.; Cheng, B.-C.; Chiang, Y.-F.; Chang, H.-W.; et al. Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease. J. Clin. Med. 2022, 12, 27. [Google Scholar] [CrossRef]
- Ma, L.; Mi, T.-M.; Jia, Q.; Han, C.; Chhetri, J.K.; Chan, P. Gait variability is sensitive to detect Parkinson’s disease patients at high fall risk. Int. J. Neurosci. 2022, 132, 888–893. [Google Scholar] [CrossRef]
- Sturchio, A.; Dwivedi, A.K.; Marsili, L.; Hadley, A.; Sobrero, G.; Heldman, D.; Maule, S.; Lopiano, L.; Comi, C.; Versino, M.; et al. Kinematic but not clinical measures predict falls in Parkinson-related orthostatic hypotension. J. Neurol. 2021, 268, 1006–1015. [Google Scholar] [CrossRef]
- Cole, M.H.; Naughton, G.A.; Silburn, P.A. Neuromuscular Impairments Are Associated With Impaired Head and Trunk Stability During Gait in Parkinson Fallers. Neurorehabil. Neural Repair 2017, 31, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Herman, T.; Giladi, N.; Hausdorff, J.M. Objective Assessment of Fall Risk in Parkinson’s Disease Using a Body-Fixed Sensor Worn for 3 Days. PLoS ONE 2014, 9, e96675. [Google Scholar] [CrossRef]
- Hoskovcová, M.; Dušek, P.; Sieger, T.; Brožová, H.; Zárubová, K.; Bezdíček, O.; Šprdlík, O.; Jech, R.; Štochl, J.; Roth, J.; et al. Predicting Falls in Parkinson Disease: What Is the Value of Instrumented Testing in OFF Medication State? PLoS ONE 2015, 10, e0139849. [Google Scholar] [CrossRef]
- Sotirakis, C.; Brzezicki, M.A.; Patel, S.; Conway, N.; FitzGerald, J.J.; Antoniades, C.A. Predicting future fallers in Parkinson’s disease using kinematic data over a period of 5 years. Npj Digit. Med. 2024, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, S.F.; Tatarelli, A.; Trabassi, D.; Icco, R.D.; Grillo, V.; Ranavolo, A.; Varrecchia, T.; Magnifica, F.; Di Lenola, D.; Coppola, G.; et al. Ability of a Set of Trunk Inertial Indexes of Gait to Identify Gait Instability and Recurrent Fallers in Parkinson’s Disease. Sensors 2021, 21, 3449. [Google Scholar] [CrossRef] [PubMed]
- Latt, M.D.; Menz, H.B.; Fung, V.S.; Lord, S.R. Acceleration Patterns of the Head and Pelvis During Gait in Older People With Parkinson’s Disease: A Comparison of Fallers and Nonfallers. J. Gerontol. Ser. A 2009, 64A, 700–706. [Google Scholar] [CrossRef]
- Din, S.D.; Galna, B.; Godfrey, A.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Mirelman, A.; Hausdorff, J.M.; Rochester, L. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’s Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J. Gerontol. Ser. A 2019, 74, 500–506. [Google Scholar] [CrossRef]
- Hubble, R.P.; Silburn, P.A.; Naughton, G.A.; Cole, M.H. Assessing stability in mild and moderate Parkinson’s disease: Can clinical measures provide insight? Gait Posture 2016, 49, 7–13. [Google Scholar] [CrossRef]
- Araújo HAG de, O.; Vitório, R.; Smaili, S.M. COMBINATION OF CLINICAL AND GAIT MEASURES TO CLASSIFY FALLERS AND NON-FALLERS IN PARKINSON’S DISEASE. Braz. J. Phys. Ther. 2024, 28, 100757. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J. Neurol. Sci. 2003, 212, 47–53. [Google Scholar] [CrossRef]
- Cole, M.H.; Sweeney, M.; Conway, Z.J.; Blackmore, T.; Silburn, P.A. Imposed Faster and Slower Walking Speeds Influence Gait Stability Differently in Parkinson Fallers. Arch. Phys. Med. Rehabil. 2017, 98, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.; Gera, G.; Horak, F.B.; Blackinton, M.T.; Besch, M.; King, L. Instrumented Test of Sensory Integration for Balance: A Validation Study. J. Geriatr. Phys. Ther. 2018, 41, 77–84. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Dagan, Y.; Hausdorff, J.M. Postural instability and fall risk in Parkinson’s disease: Impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp. Brain Res. 2011, 210, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Mancini, M.; Carlson-Kuhta, P.; Horak, F.B.; Shah, V.V. Should we use both clinical and mobility measures to identify fallers in Parkinson’s disease? Park. Relat. Disord. 2023, 106, 105235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yan, Y.; Kong, X.; Su, S. Risk factors for falls in Parkinson’s disease: A cross-sectional observational and Mendelian randomization study. Front. Aging Neurosci. 2024, 16, 1420885. [Google Scholar] [CrossRef] [PubMed]
- Camicioli, R.; Morris, M.E.; Pieruccini-Faria, F.; Montero-Odasso, M.; Son, S.; Buzaglo, D.; Hausdorff, J.M.; Nieuwboer, A. Prevention of Falls in Parkinson’s Disease: Guidelines and Gaps. Mov. Disord. Clin. Pract. 2023, 10, 1459–1469. [Google Scholar] [CrossRef]
- Mancini, M.; Hausdorff, J.M.; Pelosin, E.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Klucken, J.; Gifford, L.; Fasano, A.; Nieuwboer, A.; et al. A framework to standardize gait study protocols in Parkinson’s disease. J. Parks. Dis. 2025, 15, 1877718X241305626. [Google Scholar] [CrossRef]
- de Lima, A.L.S.; Smits, T.; Darweesh, S.K.L.; Valenti, G.; Milosevic, M.; Pijl, M.; Baldus, H.; de Vries, N.M.; Meinders, M.J.; Bloem, B.R. Home-based monitoring of falls using wearable sensors in Parkinson’s disease. Mov. Disord. 2020, 35, 109–115. [Google Scholar] [CrossRef]
- Snijders, A.H.; Haaxma, C.A.; Hagen, Y.J.; Munneke, M.; Bloem, B.R. Freezer or non-freezer: Clinical assessment of freezing of gait. Park. Relat. Disord. 2012, 18, 149–154. [Google Scholar] [CrossRef]
- Fasano, A.; Canning, C.G.; Hausdorff, J.M.; Lord, S.; Rochester, L. Falls in Parkinson’s disease: A complex and evolving picture. Mov. Disord. 2017, 32, 1524–1536. [Google Scholar] [CrossRef]
- Fukuchi, C.A.; Fukuchi, R.K.; Duarte, M. Effects of walking speed on gait biomechanics in healthy participants: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 153. [Google Scholar] [CrossRef]
- Morris, M.; Iansek, R.; Matyas, T.; Summers, J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov. Disord. 1998, 13, 61–69. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1532. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef]
- Huijben, B.; Van Schooten, K.S.; Van Dieën, J.H.; Pijnappels, M. The effect of walking speed on quality of gait in older adults. Gait Posture 2018, 65, 112–116. [Google Scholar] [CrossRef]
- Quach, L.; Galica, A.M.; Jones, R.N.; Procter-Gray, E.; Manor, B.; Hannan, M.T.; Lipsitz, L.A. The Nonlinear Relationship Between Gait Speed and Falls: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J. Am. Geriatr. Soc. 2011, 59, 1069–1073. [Google Scholar] [CrossRef]
- Almeida, L.R.S.; Piemonte, M.E.P.; Cavalcanti, H.M.; Canning, C.G.; Paul, S.S. A Self-Reported Clinical Tool Predicts Falls in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 427–434. [Google Scholar] [CrossRef]
- Mancini, M.; Afshari, M.; Almeida, Q.; Amundsen-Huffmaster, S.; Balfany, K.; Camicioli, R.; Christiansen, C.; Dale, M.L.; Dibble, L.E.; Earhart, G.M.; et al. Digital gait biomarkers in Parkinson’s disease: Susceptibility/risk, progression, response to exercise, and prognosis. Npj Park’s Dis. 2025, 11, 51. [Google Scholar] [CrossRef]
- Lord, S.; Howe, T.; Greenland, J.; Simpson, L.; Rochester, L. Gait variability in older adults: A structured review of testing protocol and clinimetric properties. Gait Posture 2011, 34, 443–450. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Annweiler, C.; Bridenbaugh, S.; Assal, F.; Kressig, R.W.; Herrmann, F.R. Gait Variability among Healthy Adults: Low and High Stride-to-Stride Variability Are Both a Reflection of Gait Stability. Gerontology 2009, 55, 702–706. [Google Scholar] [CrossRef]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Blin, O.; Ferrandez, A.M.; Pailhous, J.; Serratrice, G. Dopa-sensitive and Dopa-resistant gait parameters in Parkinson’s disease. J. Neurol. Sci. 1991, 103, 51–54. [Google Scholar] [CrossRef]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Bryant, M.S.; Rintala, D.H.; Hou, J.G.; Charness, A.L.; Fernandez, A.L.; Collins, R.L.; Baker, J.; Lai, E.C.; Protas, E.J. Gait variability in Parkinson’s disease: Influence of walking speed and dopaminergic treatment. Neurol. Res. 2011, 33, 959–964. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng. Rehabil. 2005, 2, 23. [Google Scholar] [CrossRef]
- Perry, L.; Kendrick, D.; Morris, R.; Dinan, S.; Masud, T.; Skelton, D.; Iliffe, S.; ProAct65+ Study Team. Completion and Return of Fall Diaries Varies With Participants’ Level of Education, First Language, and Baseline Fall Risk. J. Gerontol. Ser. A Biomed. Sci. Méd. Sci. 2012, 67A, 210–214. [Google Scholar] [CrossRef]
- Hiorth, Y.H.; Larsen, J.P.; Lode, K.; Pedersen, K.F. Natural history of falls in a population-based cohort of patients with Parkinson’s disease: An 8-year prospective study. Park. Relat. Disord. 2014, 20, 1059–1064. [Google Scholar] [CrossRef]
- Silva de Lima, A.L.; Evers, L.J.; Hahn, T.; Bataille, L.; Hamilton, J.L.; Little, M.A.; Okuma, Y.; Bloem, B.R.; Faber, M.J. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: A systematic review. J. Neurol. 2017, 264, 1642–1654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).