Variability of the Skin Temperature from Wrist-Worn Device for Definition of Novel Digital Biomarkers of Glycemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Description
2.2. Preprocessing
2.3. Analysis of Skin Temperature Signal: Standard Metrics and Definition of Novel Variability Metrics
2.3.1. Metrics for the Current Behavior
2.3.2. Metrics for the Retrospective Behavior
2.4. Stratification for Different Glycemic Levels
2.5. Statistical Analysis
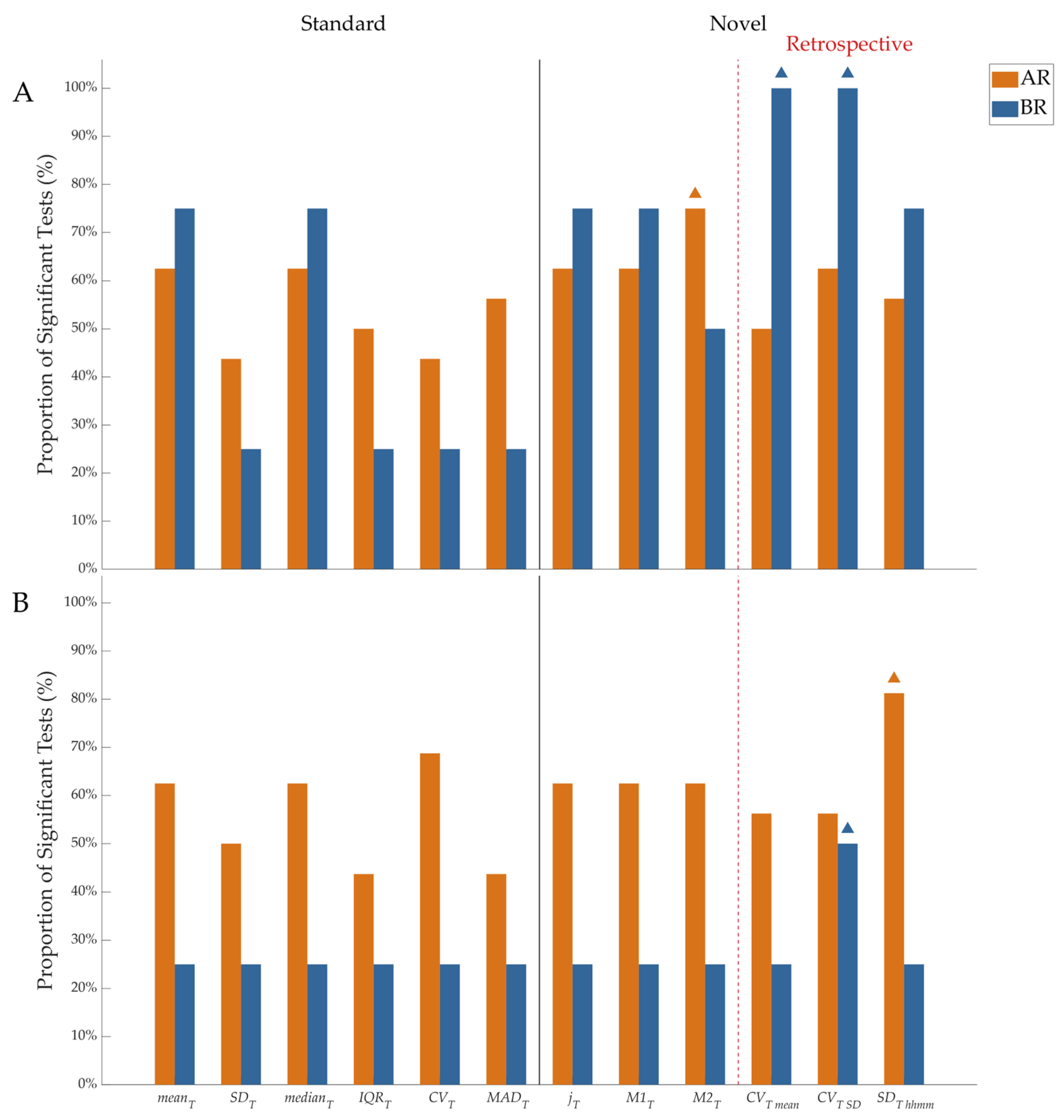
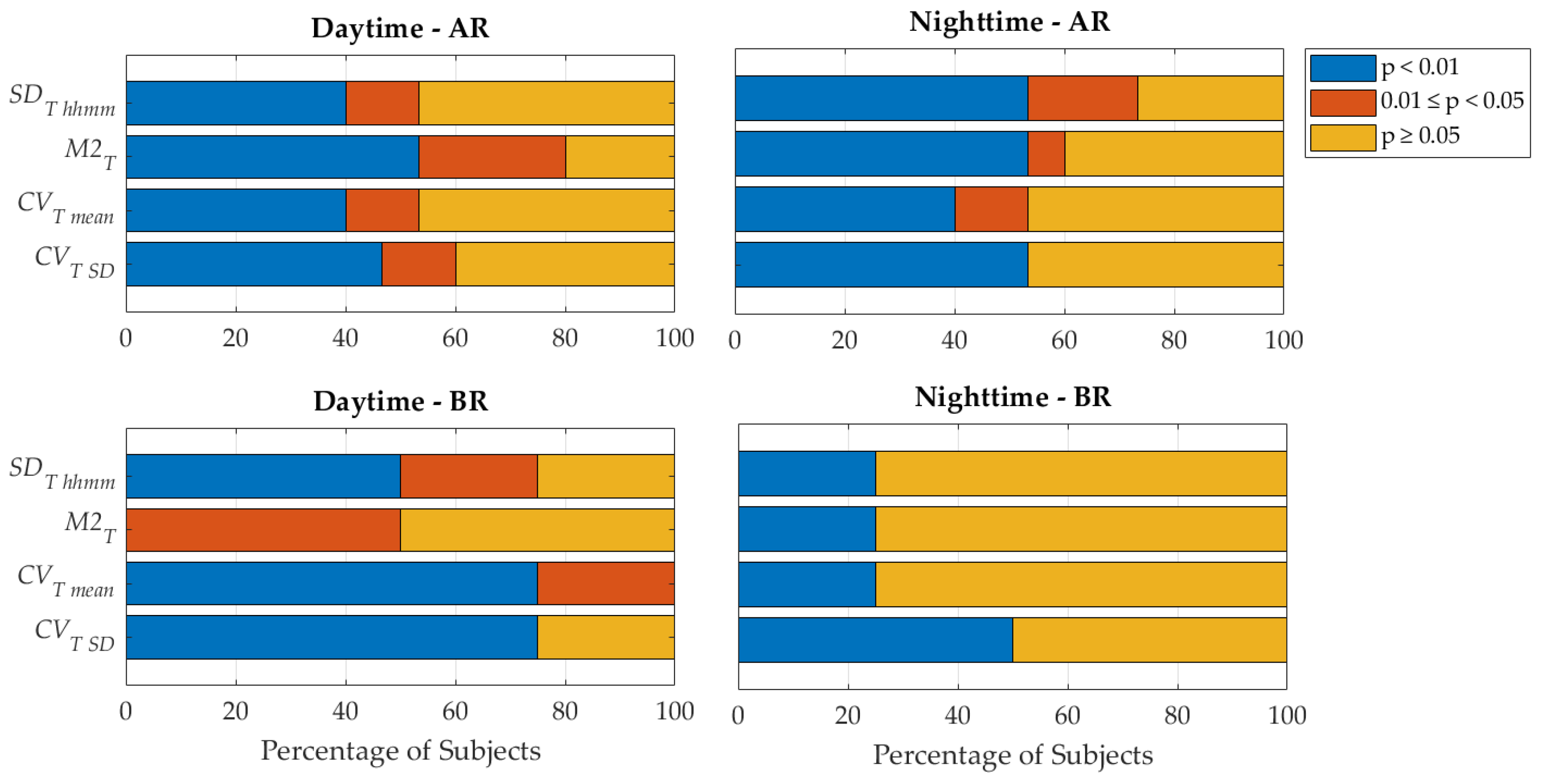
3. Results
4. Discussion
4.1. Novelty and Relevance
4.2. Advantages and Comments on the Methodology
4.3. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathew, T.K.; Zubair, M.; Tadi, P. Blood Glucose Monitoring; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Reddy, N.; Verma, N.; Dungan, K. Monitoring Technologies-Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Klonoff, D.C.; Nguyen, K.T.; Xu, N.Y.; Gutierrez, A.; Espinoza, J.C.; Vidmar, A.P. Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come? J. Diabetes Sci. Technol. 2023, 17, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Miller, K. Detection and Intervention: Use of Continuous Glucose Monitoring in the Early Stages of Type 2 Diabetes. Clin. Diabetes 2024, 42, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Zahalka, S.J.; Galindo, R.J.; Shah, V.N.; Low Wang, C.C. Continuous Glucose Monitoring for Prediabetes: What Are the Best Metrics? J. Diabetes Sci. Technol. 2024, 18, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Wu, J. Review of Non-Invasive Continuous Glucose Monitoring Based on Impedance Spectroscopy. Sens. Actuators A Phys. 2020, 311, 112103. [Google Scholar] [CrossRef]
- Tura, A.; Maran, A.; Pacini, G. Non-Invasive Glucose Monitoring: Assessment of Technologies and Devices According to Quantitative Criteria. Diabetes Res. Clin. Pract. 2007, 77, 16–40. [Google Scholar] [CrossRef]
- Daskalaki, E.; Parkinson, A.; Brew-Sam, N.; Hossain, M.Z.; O’Neal, D.; Nolan, C.J.; Suominen, H. The Potential of Current Noninvasive Wearable Technology for the Monitoring of Physiological Signals in the Management of Type 1 Diabetes: Literature Survey. J. Med. Internet Res. 2022, 24, e28901. [Google Scholar] [CrossRef]
- EN ISO 15197:2015; In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus (ISO 15197:2013). ISO: Geneva, Switzerland, 2015.
- 21CFR862.1355; Integrated Continuous Glucose Monitoring System. US Food and Drug Administration: New Hampshire, MD, USA, 2008.
- Li, T.; Wang, Q.; An, Y.; Guo, L.; Ren, L.; Lei, L.; Chen, X. Infrared Absorption Spectroscopy-Based Non-Invasive Blood Glucose Monitoring Technology: A Comprehensive Review. Biomed. Signal Process. Control 2025, 106, 107750. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Sun, C.; Huang, W.; Cui, X. A Review of Non-Invasive Blood Glucose Monitoring through Breath Acetone and Body Surface. Sens. Actuators A Phys. 2024, 374, 115500. [Google Scholar] [CrossRef]
- Alonso, A.K.M.; Hirt, J.; Woelfle, T.; Janiaud, P.; Hemkens, L.G. Definitions of Digital Biomarkers: A Systematic Mapping of the Biomedical Literature. BMJ Health Care Inform. 2024, 31, e100914. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Wang, K.; Grzesiak, E.; Jiang, C.; Qi, Y.; Jiang, Y.; Cho, P.; Zingler, K.; Ogbeide, F.I.; Zhao, A.; et al. The Digital Biomarker Discovery Pipeline: An Open-Source Software Platform for the Development of Digital Biomarkers Using MHealth and Wearables Data. J. Clin. Transl. Sci. 2021, 5, e19. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Vyas, K.; Branan, K.; McKay, S.; DeSalvo, D.J.; Gutierrez-Osuna, R.; Cote, G.L.; Erraguntla, M. Detection of Hypoglycemia and Hyperglycemia Using Noninvasive Wearable Sensors: Electrocardiograms and Accelerometry. J. Diabetes Sci. Technol. 2022, 18, 351–362. [Google Scholar] [CrossRef]
- Guzman, L.; Cazares, A.M.G.; Martinez-Torteya, A. Model for Glycemic Level Detection Using Heart Rate Variability in a Mexican Sample. In Proceedings of the 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Langkawi Island, Malaysia, 1–3 March 2021; pp. 505–510. [Google Scholar]
- Gu, W.; Zhou, Y.; Zhou, Z.; Liu, X.; Zou, H.; Zhang, P.; Spanos, C.J.; Zhang, L. SugarMate: Non-Intrusive Blood Glucose Monitoring with Smartphones. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 1–27. [Google Scholar] [CrossRef]
- Bent, B.; Cho, P.J.; Henriquez, M.; Wittmann, A.; Thacker, C.; Feinglos, M.; Crowley, M.J.; Dunn, J.P. Engineering Digital Biomarkers of Interstitial Glucose from Noninvasive Smartwatches. npj Digit. Med. 2021, 4, 89. [Google Scholar] [CrossRef]
- Ahmed, A.; Aziz, S.; Qidwai, U.; Abd-Alrazaq, A.; Sheikh, J. Performance of Artificial Intelligence Models in Estimating Blood Glucose Level among Diabetic Patients Using Non-Invasive Wearable Device Data. Comput. Methods Programs Biomed. Update 2023, 3, 100094. [Google Scholar] [CrossRef]
- Bent, B.; Cho, P.J.; Wittmann, A.; Thacker, C.; Muppidi, S.; Snyder, M.; Crowley, M.J.; Feinglos, M.; Dunn, J.P. Non-Invasive Wearables for Remote Monitoring of HbA1c and Glucose Variability: Proof of Concept. BMJ Open Diabetes Res. Care 2021, 9, e002027. [Google Scholar] [CrossRef]
- Jabara, M.; Kose, O.; Perlman, G.; Corcos, S.; Pelletier, M.-A.; Possik, E.; Tsoukas, M.; Sharma, A. Artificial Intelligence-Based Digital Biomarkers for Type 2 Diabetes: A Review. Can. J. Cardiol. 2024, 40, 1922–1933. [Google Scholar] [CrossRef]
- Tatli, D.; Papapanagiotou, V.; Liakos, A.; Tsapas, A.; Delopoulos, A. Prediabetes Detection in Unconstrained Conditions Using Wearable Sensors. Clin. Nutr. Open Sci. 2024, 58, 163–174. [Google Scholar] [CrossRef]
- Site, A.; Nurmi, J.; Lohan, E.S. Machine-Learning-Based Diabetes Prediction Using Multisensor Data. IEEE Sens. J. 2023, 23, 28370–28377. [Google Scholar] [CrossRef]
- Ali, H.; Niazi, I.; White, D.; Akhter, M.; Madanian, S. Comparison of Machine Learning Models for Predicting Interstitial Glucose Using Smart Watch and Food Log. Electronics 2024, 13, 3192. [Google Scholar] [CrossRef]
- Föll, S.; Maritsch, M.; Spinola, F.; Mishra, V.; Barata, F.; Kowatsch, T.; Fleisch, E.; Wortmann, F. FLIRT: A Feature Generation Toolkit for Wearable Data. Comput. Methods Programs Biomed. 2021, 212, 106461. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, V.; Föll, S.; Maritsch, M.; van Weenen, E.; Kraus, M.; Lagger, S.; Odermatt, K.; Albrecht, C.; Fleisch, E.; Zueger, T.; et al. Noninvasive Hypoglycemia Detection in People with Diabetes Using Smartwatch Data. Diabetes Care 2023, 46, 993–997. [Google Scholar] [CrossRef]
- Mendez, C.; Kaykayoglu, C.A.; Bähler, T.; Künzler, J.; Lizoain, A.; Rothenbühler, M.; Schmidt, M.H.; Laimer, M.; Witthauer, L. Toward Detection of Nocturnal Hypoglycemia in People with Diabetes Using Consumer-Grade Smartwatches and a Machine Learning Approach. J. Diabetes Sci. Technol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Maggs, D.G.; Scott, A.R.; MacDonald, I.A. Thermoregulatory Responses to Hyperinsulinemic Hypoglycemia and Euglycemia in Humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 267, R1266–R1272. [Google Scholar] [CrossRef]
- Molnar, G.W.; Read, R.C. Hypoglycemia and Body Temperature. JAMA J. Am. Med. Assoc. 1974, 227, 916–921. [Google Scholar] [CrossRef]
- Sejling, A.-S.; Lange, K.H.W.; Frandsen, C.S.; Diemar, S.S.; Tarnow, L.; Faber, J.; Holst, J.J.; Hartmann, B.; Hilsted, L.; Kjaer, T.W.; et al. Infrared Thermographic Assessment of Changes in Skin Temperature during Hypoglycaemia in Patients with Type 1 Diabetes. Diabetologia 2015, 58, 1898–1906. [Google Scholar] [CrossRef][Green Version]
- Armstrong, D.G.; Holtz-Neiderer, K.; Wendel, C.; Mohler, M.J.; Kimbriel, H.R.; Lavery, L.A. Skin Temperature Monitoring Reduces the Risk for Diabetic Foot Ulceration in High-Risk Patients. Am. J. Med. 2007, 120, 1042–1046. [Google Scholar] [CrossRef]
- Verdini, F.; Mengarelli, A.; Chemello, G.; Salvatori, B.; Morettini, M.; Göbl, C.; Tura, A. Sensors and Devices Based on Electrochemical Skin Conductance and Bioimpedance Measurements for the Screening of Diabetic Foot Syndrome: Review and Meta-Analysis. Biosensors 2025, 15, 73. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Cho, P.; Kim, J.; Bent, B.; Dunn, J. BIG IDEAs Lab Glycemic Variability and Wearable Device Data, version 1.1.2; PhysioNet: London, UK, 2023.
- Constantinou, A.; Oikonomou, S.; Konstantinou, C.; Makris, K.C. A Randomized Cross-over Trial Investigating Differences in 24-h Personal Air and Skin Temperatures Using Wearable Sensors between Two Climatologically Contrasting Settings. Sci. Rep. 2021, 11, 22020. [Google Scholar] [CrossRef]
- Park, C.; Kim, H.; Wang, M. Investigation of Finite-Sample Properties of Robust Location and Scale Estimators. Commun Stat Simul. Comput. 2022, 51, 2619–2645. [Google Scholar] [CrossRef]
- Broll, S.; Urbanek, J.; Buchanan, D.; Chun, E.; Muschelli, J.; Punjabi, N.M.; Gaynanova, I. Interpreting Blood GLUcose Data with R Package Iglu. PLoS ONE 2021, 16, e0248560. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, J. “J”-Index. A New Proposition of the Assessment of Current Glucose Control in Diabetic Patients. Horm. Metab. Res. 1995, 27, 41–42. [Google Scholar] [CrossRef]
- Schlichtkrull, J.; Munck, O.; Jersild, M. The M-Value, an Index of Blood-Sugar Control in Diabetics. Acta Med. Scand. 2009, 177, 95–102. [Google Scholar] [CrossRef]
- Manikandan, S. Data Transformation. J. Pharmacol. Pharmacother. 2010, 1, 126–127. [Google Scholar] [CrossRef]
- Vellei, M.; Chinazzo, G.; Zitting, K.-M.; Hubbard, J. Human Thermal Perception and Time of Day: A Review. Temperature 2021, 8, 320–341. [Google Scholar] [CrossRef]
- Howsmon, D.; Bequette, B.W. Hypo- and Hyperglycemic Alarms. J. Diabetes Sci. Technol. 2015, 9, 1126–1137. [Google Scholar] [CrossRef]
- D’Antoni, F.; Petrosino, L.; Marchetti, A.; Bacco, L.; Pieralice, S.; Vollero, L.; Pozzilli, P.; Piemonte, V.; Merone, M. Layered Meta-Learning Algorithm for Predicting Adverse Events in Type 1 Diabetes. IEEE Access 2023, 11, 9074–9094. [Google Scholar] [CrossRef]
- Sivanandam, S.; Anburajan, M.; Venkatraman, B.; Menaka, M.; Sharath, D. Estimation of Blood Glucose by Non-Invasive Infrared Thermography for Diagnosis of Type 2 Diabetes: An Alternative for Blood Sample Extraction. Mol. Cell. Endocrinol. 2013, 367, 57–63. [Google Scholar] [CrossRef]
- Thirunavukkarasu, U.; Umapathy, S.; Janardhanan, K.; Thirunavukkarasu, R. A Computer Aided Diagnostic Method for the Evaluation of Type II Diabetes Mellitus in Facial Thermograms. Phys. Eng. Sci. Med. 2020, 43, 871–888. [Google Scholar] [CrossRef]
- Chiacchiaretta, P.; Tumini, S.; Mascitelli, A.; Sacrini, L.; Saltarelli, M.A.; Carabotta, M.; Osmelli, J.; Di Carlo, P.; Aruffo, E. The Impact of Atmospheric Temperature Variations on Glycaemic Patterns in Children and Young Adults with Type 1 Diabetes. Climate 2024, 12, 121. [Google Scholar] [CrossRef]
- Böttcher, S.; Vieluf, S.; Bruno, E.; Joseph, B.; Epitashvili, N.; Biondi, A.; Zabler, N.; Glasstetter, M.; Dümpelmann, M.; Van Laerhoven, K.; et al. Data Quality Evaluation in Wearable Monitoring. Sci. Rep. 2022, 12, 21412. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.; Prendin, F.; Facchinetti, A.; Sparacino, G.; Favero, S. Del Individualized Models for Glucose Prediction in Type 1 Diabetes: Comparing Black-Box Approaches to a Physiological White-Box One. IEEE Trans. Biomed. Eng. 2023, 70, 3105–3115. [Google Scholar] [CrossRef]
- Prendin, F.; Del Favero, S.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Forecasting of Glucose Levels and Hypoglycemic Events: Head-to-Head Comparison of Linear and Nonlinear Data-Driven Algorithms Based on Continuous Glucose Monitoring Data Only. Sensors 2021, 21, 1647. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous Glucose Monitoring and Metrics for Clinical Trials: An International Consensus Statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef]
- Dubosson, F.; Ranvier, J.-E.; Bromuri, S.; Calbimonte, J.-P.; Ruiz, J.; Schumacher, M. The Open D1NAMO Dataset: A Multi-Modal Dataset for Research on Non-Invasive Type 1 Diabetes Management. Inform. Med. Unlocked 2018, 13, 92–100. [Google Scholar] [CrossRef]
- Marling, C.; Bunescu, R. The OhioT1DM Dataset for Blood Glucose Level Prediction: Update 2020. In Proceedings of the CEUR Workshop Proceedings, Honolulu, HI, USA, 26 April 2020; pp. 71–74. [Google Scholar]
- Zhu, Y.; Aimandi, N.B.; Ul Alam, M.A. Enhancing Wearable Based Real-Time Glucose Monitoring via Phasic Image Representation Learning Based Deep Learning. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; pp. 1–4. [Google Scholar]
- Chowdhury, M.H.; Chowdhury, M.E.H.; Alqahtani, A. MMG-Net: Multi Modal Approach to Estimate Blood Glucose Using Multi-Stream and Cross Modality Attention. Biomed. Signal Process. Control 2024, 92, 105975. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Samra, J.K.; Mullainathan, S. Individual Differences in Normal Body Temperature: Longitudinal Big Data Analysis of Patient Records. BMJ 2017, 359, j5468. [Google Scholar] [CrossRef]
- Yousef, H.; Ramezanpour Ahangar, E.; Varacallo, M. Physiology, Thermal Regulation; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Laganà, F.; Bibbò, L.; Calcagno, S.; De Carlo, D.; Pullano, S.A.; Pratticò, D.; Angiulli, G. Smart Electronic Device-Based Monitoring of SAR and Temperature Variations in Indoor Human Tissue Interaction. Appl. Sci. 2025, 15, 2439. [Google Scholar] [CrossRef]
- Cuesta, M.; Boudreau, P.; Cermakian, N.; Boivin, D.B. Skin Temperature Rhythms in Humans Respond to Changes in the Timing of Sleep and Light. J. Biol. Rhythms 2017, 32, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Obayashi, K.; Yamagami, Y.; Saeki, K. Association between Circadian Skin Temperature Rhythms and Actigraphic Sleep Measures in Real-Life Settings. J. Clin. Sleep Med. 2023, 19, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Palotti, J.; Mall, R.; Aupetit, M.; Rueschman, M.; Singh, M.; Sathyanarayana, A.; Taheri, S.; Fernandez-Luque, L. Benchmark on a Large Cohort for Sleep-Wake Classification with Machine Learning Techniques. NPJ Digit. Med. 2019, 2, 50. [Google Scholar] [CrossRef]
- Piersanti, A.; Giurato, F.; Göbl, C.; Burattini, L.; Tura, A.; Morettini, M. Software Packages and Tools for the Analysis of Continuous Glucose Monitoring Data. Diabetes Technol. Ther. 2023, 25, 69–85. [Google Scholar] [CrossRef]

| Participants | |
|---|---|
| P | 16 |
| Sex (M/F) | 7M/9F |
| Age (years) | NR (inclusion criteria 35–65 years) |
| Hba1c (%) | 5.7 ± 0.3 |
| Monitoring period | 8–10 days |
| CGM data | |
| Total number of samples | 36,872 |
| Number of samples across subjects | 2318 ± 302 |
| Percent of data completeness across subjects (%) | 97 ± 6 |
| Skin temperature data | |
| Total number of samples | 37,543,280 |
| Number of samples across subjects | 2,346,455 ± 400,741 |
| Percent of data completeness across subjects (%) | 79 ± 19 |
| Subject | Daytime | Nighttime | ||||
|---|---|---|---|---|---|---|
| IR | AR | BR | IR | AR | BR | |
| S1 | 1.07 [0.21; 4.04] | 3.52 [0.93; 5.01] * | 0.88 [0.53; 3.82] | 12.86 [8.88; 15.11] | 6.35 [5.17; 13.12] * | 11.59 [11.01; 12.17] |
| S2 | 3.30 [1.62; 5.03] | 3.72 [1.90; 5.99] * | - | 3.52 [1.82; 5.12] | 3.29 [1.75; 4.89] | - |
| S3 | 1.11 [0.16; 4.02] | 6.60 [1.88; 9.79] * | 0.04 [0.00; 0.11] * | 3.73 [1.25; 7.57] | 8.88 [1.45; 13.18] * | - |
| S4 | 2.96 [0.57; 6.27] | 1.55 [0.10; 4.53] * | - | 6.85 [3.55; 8.93] | 6.72 [2.87; 9.29] | - |
| S5 | 0.36 [0.06; 1.38] | 0.48 [0.00; 1.69] | - | 1.68 [0.56; 3.76] | 10.08 [9.25; 10.92] * | - |
| S6 | 1.27 [0.20; 3.64] | 0.45 [0.13; 1.84] * | - | 1.84 [0.56; 3.47] | 0.51 [0.12; 1.72] * | - |
| S7 | 0.57 [0.05; 3.88] | 2.49 [0.35; 7.90] * | 0.26 [0.03; 1.40] * | 4.58 [1.13; 6.85] | 0.10 [0.00; 0.57] * | 0.01 [0.00; 0.08] * |
| S8 | 1.38 [0.26; 4.42] | 0.81 [0.10; 2.08] * | - | 4.12 [0.91; 6.77] | 0.28 [0.22; 0.33] | 3.39 [1.66; 4.53] |
| S9 | 1.31 [0.14; 3.69] | 0.48 [0.04; 3.09] * | - | 5.59 [3.26; 7.79] | 3.17 [1.51; 5.49] * | - |
| S10 | 0.84 [0.21; 2.41] | 0.42 [0.07; 1.64] * | 0.62 [0.12; 2.76] | 6.86 [4.43; 8.32] | 0.04 [0.01; 0.40] * | - |
| S11 | 0.47 [0.05; 1.96] | 0.53 [0.07; 2.71] | - | 7.59 [5.07; 9.03] | 6.93 [5.29; 8.82] | - |
| S12 | 0.74 [0.09; 3.01] | 1.94 [0.43; 4.19] * | - | 3.93 [1.14; 6.00] | 2.15 [0.84; 5.47] * | - |
| S13 | 0.73 [0.12; 2.48] | 0.14 [0.02; 0.94] * | - | 1.61 [0.25; 3.67] | 0.21 [0.03; 0.94] * | - |
| S14 | 0.43 [0.05; 2.90] | 1.13 [0.33; 5.26] * | - | 5.99 [3.60; 8.19] | 4.53 [1.72; 8.29] | - |
| S16 | 1.52 [0.25; 5.20] | 1.56 [0.21; 4.29] | - | 4.17 [1.46; 6.49] | 7.39 [4.56; 8.21] | 2.02 [1.64; 2.60] |
| Subject | Daytime | Nighttime | ||||
|---|---|---|---|---|---|---|
| IR | AR | BR | IR | AR | BR | |
| S1 | 0.35 [0.34; 0.36] | 0.35 [0.35; 0.35] | 0.39 [0.37; 0.40] * | 0.20 [0.20; 0.21] | 0.22 [0.20; 0.22] * | 0.20 [0.20; 0.20] |
| S2 | 0.26 [0.25; 0.28] | 0.25 [0.25; 0.27] * | - | 0.16 [0.16; 0.16] | 0.16 [0.15; 0.16] * | - |
| S3 | 0.57 [0.54; 0.60] | 0.51 [0.50; 0.55] * | 0.50 [0.50; 0.50] * | 0.34 [0.33; 0.35] | 0.32 [0.31; 0.33] * | - |
| S4 | 0.38 [0.38; 0.40] | 0.38 [0.38; 0.41] | - | 0.19 [0.17; 0.23] | 0.23 [0.17; 0.23] * | - |
| S5 | 0.34 [0.33; 0.34] | 0.32 [0.30; 0.33] * | - | 0.26 [0.26; 0.27] | 0.28 [0.27; 0.28] * | - |
| S6 | 0.36 [0.34; 0.38] | 0.35 [0.35; 0.36] | - | 0.26 [0.24; 0.27] | 0.26 [0.24; 0.27] | - |
| S7 | 0.41 [0.39; 0.43] | 0.43 [0.42; 0.44] * | 0.44 [0.41; 0.45] * | 0.29 [0.28; 0.31] | 0.31 [0.30; 0.31] | 0.29 [0.28; 0.29] |
| S8 | 0.39 [0.39; 0.40] | 0.39 [0.39; 0.39] * | - | 0.28 [0.26; 0.30] | 0.30 [0.30; 0.30] | 0.41 [0.36; 0.43] * |
| S9 | 0.43 [0.40; 0.47] | 0.42 [0.40; 0.46] | - | 0.27 [0.23; 0.30] | 0.31 [0.27; 0.41] * | - |
| S10 | 0.47 [0.47; 0.48] | 0.47 [0.46; 0.48] * | 0.46 [0.46; 0.47] * | 0.17 [0.16; 0.18] | 0.17 [0.17; 0.17] | - |
| S11 | 0.41 [0.35; 0.42] | 0.42 [0.36; 0.42] * | - | 0.12 [0.11; 0.12] | 0.12 [0.11; 0.14] * | - |
| S12 | 0.71 [0.70; 0.76] | 0.71 [0.70; 0.75] | - | 0.36 [0.35; 0.39] | 0.39 [0.36; 0.40] * | - |
| S13 | 0.35 [0.34; 0.36] | 0.35 [0.34; 0.35] | - | 0.25 [0.24; 0.26] | 0.25 [0.24; 0.26] | - |
| S14 | 0.40 [0.34; 0.41] | 0.41 [0.40; 0.41]* | - | 0.24 [0.23; 0.27] | 0.24 [0.23; 0.24] | - |
| S16 | 0.47 [0.46; 0.51] | 0.47 [0.45; 0.51] | - | 0.28 [0.28; 0.29] | 0.28 [0.27; 0.28] | 0.28 [0.28; 0.28] |
| Subject | Daytime | Nighttime | ||||
|---|---|---|---|---|---|---|
| IR | AR | BR | IR | AR | BR | |
| S1 | 0.49 [0.47; 0.54] | 0.44 [0.44; 0.47] * | 0.19 [0.17; 0.22] * | 0.47 [0.28; 0.51] | 0.50 [0.49; 0.51] * | 0.28 [0.28; 0.29] |
| S2 | 0.23 [0.21; 0.26] | 0.22 [0.20; 0.24] * | - | 0.16 [0.15; 0.18] | 0.15 [0.10; 0.18] * | - |
| S3 | 0.43 [0.40; 0.45] | 0.37 [0.36; 0.42] * | 0.36 [0.36; 0.36] * | 0.34 [0.29; 0.35] | 0.27 [0.27; 0.28] * | - |
| S4 | 0.25 [0.25; 0.28] | 0.25 [0.25; 0.29] | - | 0.26 [0.19; 0.28] | 0.28 [0.20; 0.28] * | - |
| S5 | 0.22 [0.22; 0.23] | 0.22 [0.21; 0.22] * | - | 0.20 [0.20; 0.21] | 0.23 [0.23; 0.23] * | - |
| S6 | 0.29 [0.27; 0.34] | 0.32 [0.32; 0.33] * | - | 0.23 [0.20; 0.29] | 0.23 [0.20; 0.27] | - |
| S7 | 0.38 [0.34; 0.39] | 0.39 [0.39; 0.40] * | 0.40 [0.38; 0.40] * | 0.31 [0.29; 0.34] | 0.32 [0.32; 0.32] | 0.29 [0.29; 0.29] |
| S8 | 0.32 [0.32; 0.33] | 0.32 [0.31; 0.32] * | - | 0.24 [0.24; 0.27] | 0.26 [0.26; 0.26] | 0.34 [0.32; 0.36] * |
| S9 | 0.31 [0.29; 0.33] | 0.30 [0.29; 0.32] | - | 0.29 [0.26; 0.33] | 0.34 [0.30; 0.40] * | - |
| S10 | 0.40 [0.36; 0.42] | 0.40 [0.37; 0.41] | 0.36 [0.36; 0.39] | 0.24 [0.23; 0.27] | 0.24 [0.23; 0.28] | - |
| S11 | 0.33 [0.26; 0.36] | 0.36 [0.27; 0.36] * | - | 0.13 [0.13; 0.14] | 0.14 [0.12; 0.16] * | - |
| S12 | 0.53 [0.51; 0.55] | 0.53 [0.50; 0.55] | - | 0.42 [0.41; 0.44] | 0.45 [0.41; 0.46] * | - |
| S13 | 0.28 [0.26; 0.28] | 0.28 [0.26; 0.29] * | - | 0.23 [0.20; 0.24] | 0.23 [0.20; 0.23] | - |
| S14 | 0.30 [0.25; 0.31] | 0.30 [0.29; 0.31] | - | 0.25 [0.25; 0.26] | 0.26 [0.24; 0.27] | - |
| S16 | 0.40 [0.39; 0.43] | 0.40 [0.39; 0.44] | - | 0.30 [0.26; 0.30] | 0.27 [0.27; 0.27] | 0.32 [0.32; 0.32] * |
| Subject | Daytime | Nighttime | ||||
|---|---|---|---|---|---|---|
| IR | AR | BR | IR | AR | BR | |
| S1 | 1.79 [1.72; 1.86] | 1.76 [1.75; 1.80] | 0.28 [0.22; 0.50] * | 0.77 [0.66; 0.84] | 0.75 [0.66; 0.82] | 0.78 [0.78; 0.78] |
| S2 | 1.21 [1.09; 1.31] | 1.15 [1.08; 1.24] * | - | 0.65 [0.63; 0.67] | 0.64 [0.49; 0.66] * | - |
| S3 | 1.37 [1.23; 1.39] | 1.38 [1.30; 1.44] * | 1.40 [1.40; 1.40] * | 0.98 [0.86; 1.58] | 0.80 [0.79; 0.81] * | - |
| S4 | 1.14 [1.12; 1.17] | 1.14 [1.12; 1.16] | - | 0.78 [0.76; 0.80] | 0.80 [0.80; 0.77] * | - |
| S5 | 0.92 [0.88; 0.93] | 0.88 [0.71; 0.93] * | - | 0.86 [0.83; 0.89] | 0.77 [0.76; 0.96] * | - |
| S6 | 1.55 [1.47; 1.62] | 1.54 [1.49; 1.55] | - | 0.92 [0.89; 0.95] | 0.90 [0.88; 0.27] * | - |
| S7 | 2.05 [1.98; 2.22] | 1.97 [1.96; 2.04] * | 1.99 [1.96; 2.07] * | 1.20 [1.17; 1.24] | 1.29 [1.29; 1.30] * | 1.18 [1.17; 1.18] |
| S8 | 2.01 [1.95; 2.06] | 1.96 [1.94; 2.01] * | - | 1.53 [1.47; 1.62] | 1.73 [1.73; 1.74] | 1.25 [1.05; 1.25] * |
| S9 | 1.46 [1.43; 1.50] | 1.46 [1.45; 1.49] | - | 1.16 [1.04; 1.22] | 1.27 [1.18; 1.50] * | - |
| S10 | 1.10 [1.06; 1.12] | 1.10 [1.08; 1.14] * | 1.12 [1.09; 1.12] | 0.83 [0.75; 0.87] | 0.85 [0.81; 0.88] | - |
| S11 | 1.45 [1.14; 1.48] | 1.45 [1.27; 1.49] * | - | 0.41 [0.34; 0.43] | 0.42 [0.32; 0.45] * | - |
| S12 | 1.68 [1.67; 1.71] | 1.71 [1.67; 1.73] | - | 1.30 [1.22; 1.35] | 1.31 [1.22; 1.32] * | - |
| S13 | 1.23 [0.92; 1.26] | 1.23 [1.19; 1.26] | - | 1.24 [1.05; 1.25] | 1.25 [1.11; 1.29] * | - |
| S14 | 1.54 [1.46; 1.61] | 1.50 [1.45; 1.59] * | - | 0.89 [0.85; 0.92] | 0.91 [0.90; 0.92] | - |
| S16 | 2.03 [1.96; 2.03] | 2.04 [1.94; 2.06] | - | 2.12 [2.02; 2.44] | 2.52 [2.50; 2.53] * | 2.20 [2.19; 2.20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piersanti, A.; Littero, M.; Del Giudice, L.L.; Marcantoni, I.; Burattini, L.; Tura, A.; Morettini, M. Variability of the Skin Temperature from Wrist-Worn Device for Definition of Novel Digital Biomarkers of Glycemia. Sensors 2025, 25, 4038. https://doi.org/10.3390/s25134038
Piersanti A, Littero M, Del Giudice LL, Marcantoni I, Burattini L, Tura A, Morettini M. Variability of the Skin Temperature from Wrist-Worn Device for Definition of Novel Digital Biomarkers of Glycemia. Sensors. 2025; 25(13):4038. https://doi.org/10.3390/s25134038
Chicago/Turabian StylePiersanti, Agnese, Martina Littero, Libera Lucia Del Giudice, Ilaria Marcantoni, Laura Burattini, Andrea Tura, and Micaela Morettini. 2025. "Variability of the Skin Temperature from Wrist-Worn Device for Definition of Novel Digital Biomarkers of Glycemia" Sensors 25, no. 13: 4038. https://doi.org/10.3390/s25134038
APA StylePiersanti, A., Littero, M., Del Giudice, L. L., Marcantoni, I., Burattini, L., Tura, A., & Morettini, M. (2025). Variability of the Skin Temperature from Wrist-Worn Device for Definition of Novel Digital Biomarkers of Glycemia. Sensors, 25(13), 4038. https://doi.org/10.3390/s25134038











