1. Introduction
Digital health offers substantial benefits to patients and the medical field, yet it also introduces several vulnerabilities that could compromise these gains. For example, it facilitates the diagnosis, treatment, and monitoring of health conditions [
1], optimizes healthcare operations, reduces administrative burdens, and ultimately shifts focus toward patient care [
2]. However, it can also give rise to a range of undesirable environmental effects throughout the life cycle of enabling technology, including global warming [
3], resource depletion [
4] and electronic waste [
5]. How can the advantages and disadvantages of technology be effectively differentiated to enable a comprehensive and pragmatic assessment and ecological design of digital health innovations?
In the context of smart technologies and the Internet of Medical Things (IoMT), addressing this research question is of utmost importance. Recent findings raise concerns that the anticipated benefits of commercially available IoMT solutions (e.g., reduction in hospital service use [
6] or personalized medical assistance and monitor health [
7]) may be deeply undermined by the negative implications of the excessive production of sensor devices [
8,
9], their uncontrolled disposal [
4,
10,
11] and their electricity consumption [
4,
12,
13].
In general, Health Technology Assessment (HTA) is the most widely adopted and endorsed approach for evaluating the balance between the returns and tradeoffs of innovations in the medical field. In fact, according to the World Health Organization (WHO), HTA represents a systematic evaluation of the properties, effects, and/or impacts of health technologies, intended to inform decision-making [
14].
Recently, certain digital health technologies have been integrated into HTA frameworks to support not only decision-makers but also policymakers, particularly through cost–benefit, cost-effectiveness, and cost–utility analyses (CBA, CEA, CUA). Within the literature, the interest in telemedicine is considerable and, although most studies address common dimensions such as outcomes, ethical considerations, efficiency, and efficacy, their specific emphases vary significantly.
In terms of clinical and patient outcomes, for instance, some authors [
15,
16,
17,
18] focus on assessing the influence of telemedicine on mortality, morbidity, adherence, and quality of life, while others [
19] investigate patient satisfaction and technology usability. Additionally, there are researchers that delve into emotional factors that affect patients’ quality of life [
20].
With respect to ethical aspects, Grigsby et al. [
17] examined the impact of telemedicine on the cost of care, whereas Khoja et al. [
21] explored issues related to technology access and its social implications. Furthermore, Ekeland et al. [
15] and Kidholm et al. [
16] addressed socio-cultural and legal dimensions, while other authors [
22,
23,
24] focused on cost, acceptability, and affordability.
In the context of efficiency of telemedicine, Brear M. [
25] evaluated technical performance, other researchers [
15,
16,
18,
21] investigated the use and allocation of resources, and Ohinmaa et al. [
19] examined diagnostic quality. Regarding efficacy, Ohinmaa et al. [
19] addressed service quality and reliability, Khoja et al. [
21] considered the appropriateness of technology, Ekeland et al. [
15] and Kidholm et al. [
16] explored technological maturity, Dechant et al. [
23] assessed accuracy, and Brown et al. [
26] and Shaw N.T. [
27] focused on training in the use of digital health technologies.
On the other hand, the integration of environmental aspects into HTA and the examination of eHealth technologies through the lens of sustainability have drawn increasing scholarly attention in recent years. Regarding the green transition of HTA, Toolan et al. [
3] identified four strategies to support HTA agencies in incorporating ecological criteria and conducting environmental impact assessments of healthcare innovations. These include reusing existing environmental data, analyzing environmental data separately from established economic evaluations, adopting or developing new methodologies, and focusing solely on environmental benefits (regardless of clinical outcomes).
A recent review by Iandolo et al. [
28] underscored the lack of a holistic perspective in current impact estimation methodologies, especially in capturing the direct and indirect effects of innovation in healthcare. Williams et al. [
29] show that some emerging tools address this gap by incorporating incremental carbon footprint cost ratios, or by integrating environmental impact into multi-criteria decision-making frameworks. However, as they point out, no existing method, framework, or tool is yet suitable for widespread use. Therefore, the further tailoring and adaptation of assessment methodologies appear to be inevitable [
30].
Concerning the sustainability of eHealth technologies, Alajlan et al. [
31] emphasized the importance of first establishing a clear definition of sustainability in healthcare before developing evaluation approaches. Their work highlights critical enabling factors—such as ICT infrastructure, hardware, and data/system quality—and calls for impact assessment methods that consider these alongside organizational, economic, social, and resource-related dimensions. In this context, Bobini et al. [
32] analyzed the pivotal role of Life Cycle Assessment (LCA) in green HTA for telemedicine, concluding that accounting for both the environmental costs of equipment production and the environmental benefits of its use is essential. Along these lines, Brown, C. et al. [
33] advocated for frugal innovation as a path to affordable, low-ecological-impact healthcare solutions.
While all this literature is undoubtedly valuable and enlightening, the specificities of smart technology remain largely unexplored, and the nuances of modern IoMT equipment have yet to be systematically integrated from both Health Technology Assessment and design perspectives.
Bridging this gap in knowledge is critically urgent, especially considering that, according to the WHO’s global strategy, the medical sector should already be improving physical and psychological well-being for all, everywhere, by accelerating the development and adoption of appropriate, accessible, affordable, scalable and sustainable person-centered digital solutions [
34]. Clearly, they did not anticipate that integrating the sustainability assessment and eco design of smart technologies with a cost-effectiveness and comprehensive mindset is challenging, particularly in the case of IoMT systems and prototypes, which are conditioned to numerous issues such as the rapid evolution of hardware [
35,
36,
37] or effectiveness–efficacy tradeoffs [
38].
In this work, we strive to address this challenge, arguing that the integral Health Technology Assessment and eco design of sustainable smart technology and IoMT solutions should embrace a pragmatic approach primarily grounded in their capacities to sense, size and transform information.
1.1. Hypotheses
Sensing, sizing and transforming data are the most distinctive features of smart technology [
39] and represent the primary functions of the Internet of Things, all designed to provide information to human and/or machines autonomously [
40]. In the medical field, this suggests that “IoMT devices continuously sense and collect data, thereby generating
information that can be subsequently analyzed and contextualized to implement medical treatments efficiently and effectively, and allocate healthcare resources optimally” (Hypothesis 1).
Information, on the other hand, can be defined as “the message from which a sustainable digital-based health service or paradigm can exist, and with which specific barriers for integral assessment and eco design can be surpassed” (Hypothesis 2).
1.2. Proposition
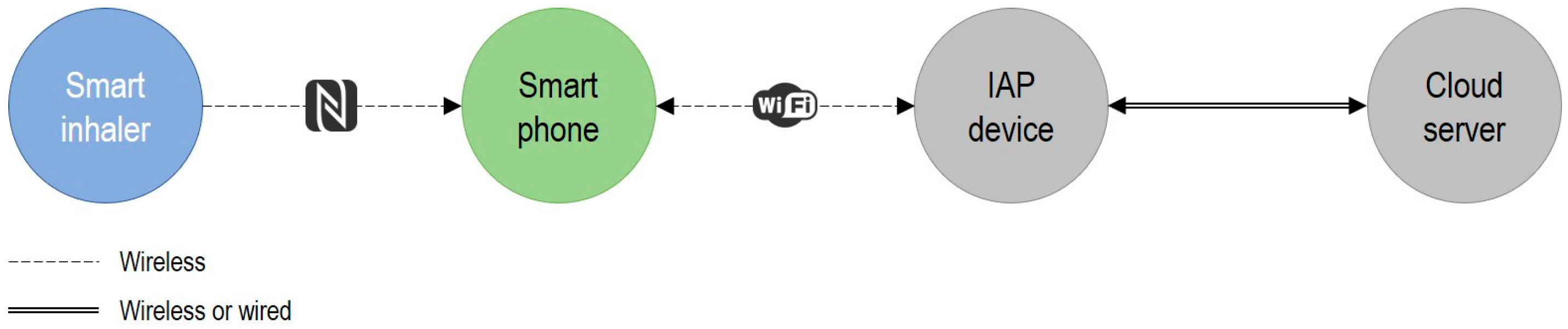
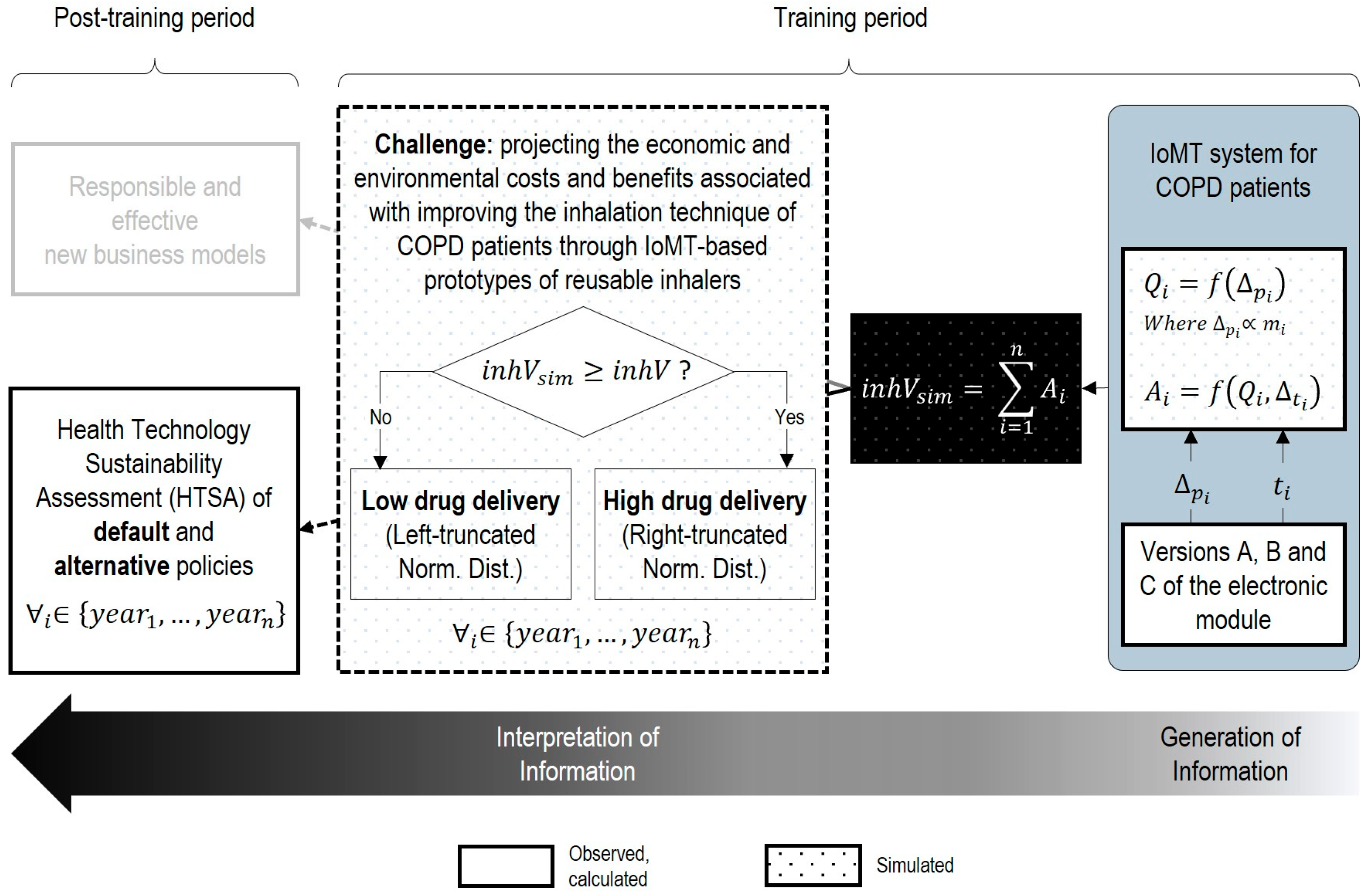
Building on our hypotheses and recognizing that smart technologies integrate advanced computing into smart systems (comprising local equipment and shared infrastructure [
41]), we propose in
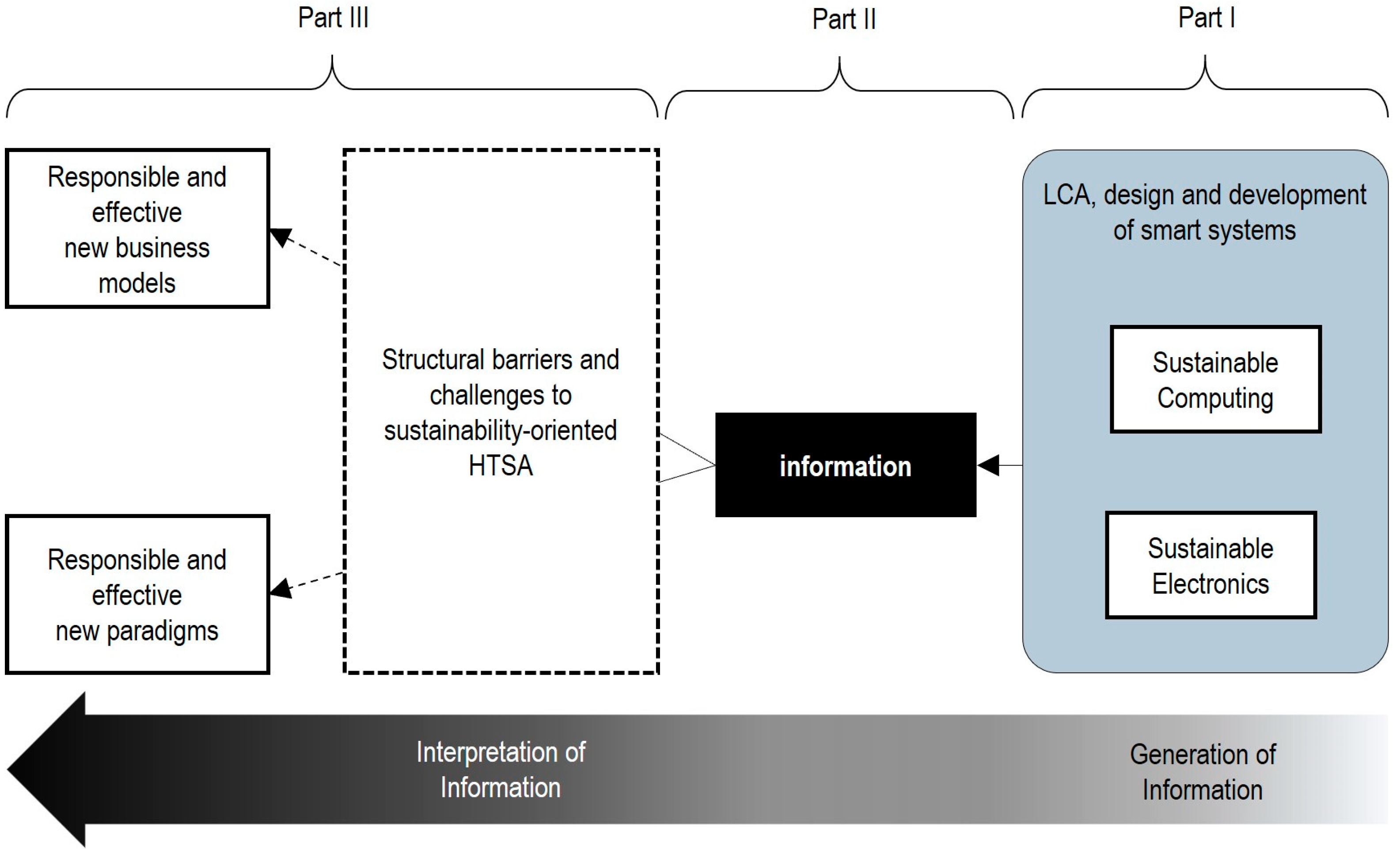
Figure 1 a novel framework to pragmatically integrate sustainability and eco design into the Health Technology Assessment of smart technologies and IoMT systems.
This framework is grounded in information science theory and the Data–Information–Knowledge (DIK) hierarchy [
42,
43]. It consists of three parts, operating as follows: In part I, the Life Cycle Assessment, eco design and development of sustainable computing and electronic prototypes are addressed. In part II, only the sufficient data are collected or defined to generate the information for both the primary function and the evaluation of medical applications addressed by the system. Finally, Part III involves interpretation, which helps in addressing specific difficulties, barriers, challenges and dilemmas that hinder the integral cost-effectiveness analysis and development of new medical devices, business models and paradigms.
The aim of the next sections is to illustrate the use of this framework and test our hypotheses presented in
Section 1.1.
With respect to the first hypothesis, this work will gather evidence to determine whether, based on specific information, a particular IoMT-based treatment can be made more effective while simultaneously reducing its implementation costs.
With respect to the second hypothesis, this work will gather evidence to determine whether, based on specific information, the potential cost–benefit balance of using IoMT devices in a medical treatment can be quantified in a practical and objective manner.
To this end, we project and contrast, through a systematic implementation of parts I, II and III of our proposed methodology, the economic and ecological cost–benefit sides of transforming a medical device into a “smart” medical device.
4. Discussion
The literature somewhat related to the focus of this article does not reconcile HTA and the design of the enabling technology, and much less from a sustainability perspective. For example, the methodology proposed by Caulfield et al. [
58] enables the identification and evaluation of suitable sensor devices for healthcare applications based on specific requirements (such as timeframe, objectives, and data needs) and certain criteria (such as physical dimensions, sensing capabilities, and connectivity), but it does not address the ecological design aspects of the selected devices. Similarly, the guidelines presented by Baumel et al. [
59] allow for estimating the success of digital health interventions by evaluating digital products as a whole (considering usability and patients’ needs, engagement, stability, and continuous improvement), but do not estimate the economic or environmental costs of the enabling technologies.
In contrast to these works, our contribution addresses central limitations that hinder timely HTA analysis and efficient design within the sustainability domain.
Indeed, our framework emphasizes the core functionalities of smart systems and prototypes, aiming to facilitate not only their cost-effectiveness assessment, but also their sustainability evaluation and design in a pragmatic and integrated manner.
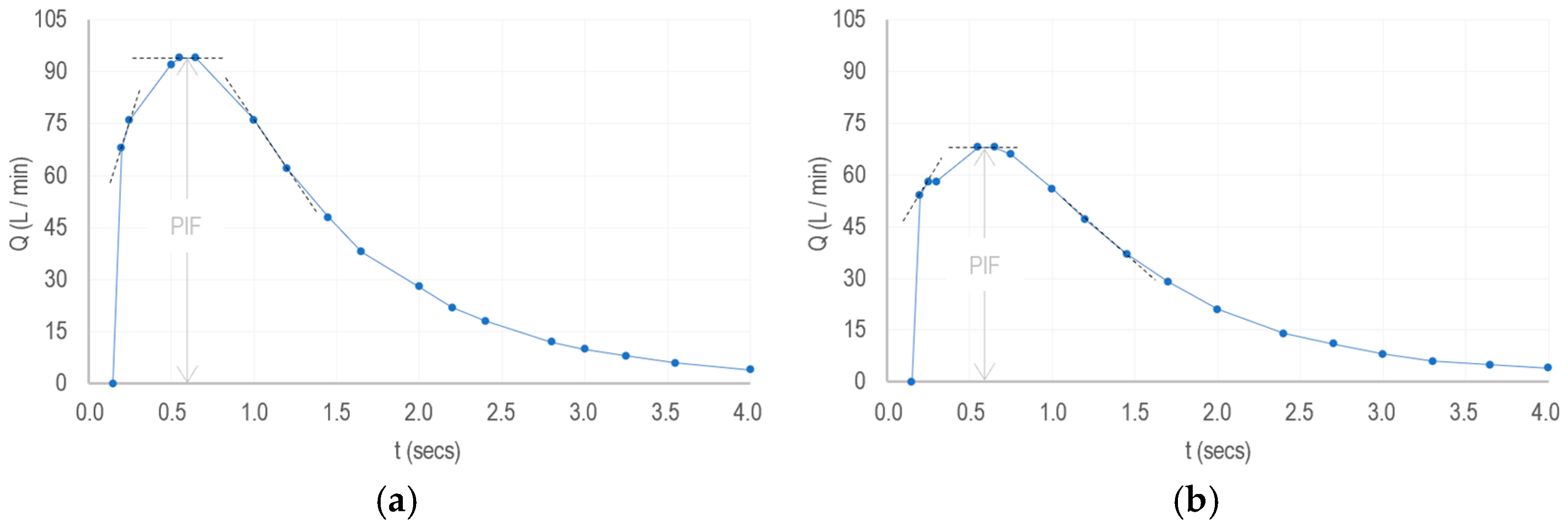
To demonstrate this, we applied our framework to analyze the core functions of smart inhalers designed for COPD patients, resulting in the design of three distinct pilot devices. In the economic context, for the same benefits obtained over a five-year period, we found that smart inhaler prototypes with a high-performance design are less costly than those with a feature-rich design (refer to
Table 6). Conversely, in the environmental context, for the same savings obtained over the same period, smart inhaler prototypes with a high-performance design are slightly more costly than those with a lightweight design (refer
Table 11).
As such, our framework provides a structured basis not only for identifying critical aspects that guide decision-making in the ecological design of final IoMT devices and systems, but also for shaping sustainable policy interventions. Consider, for example,
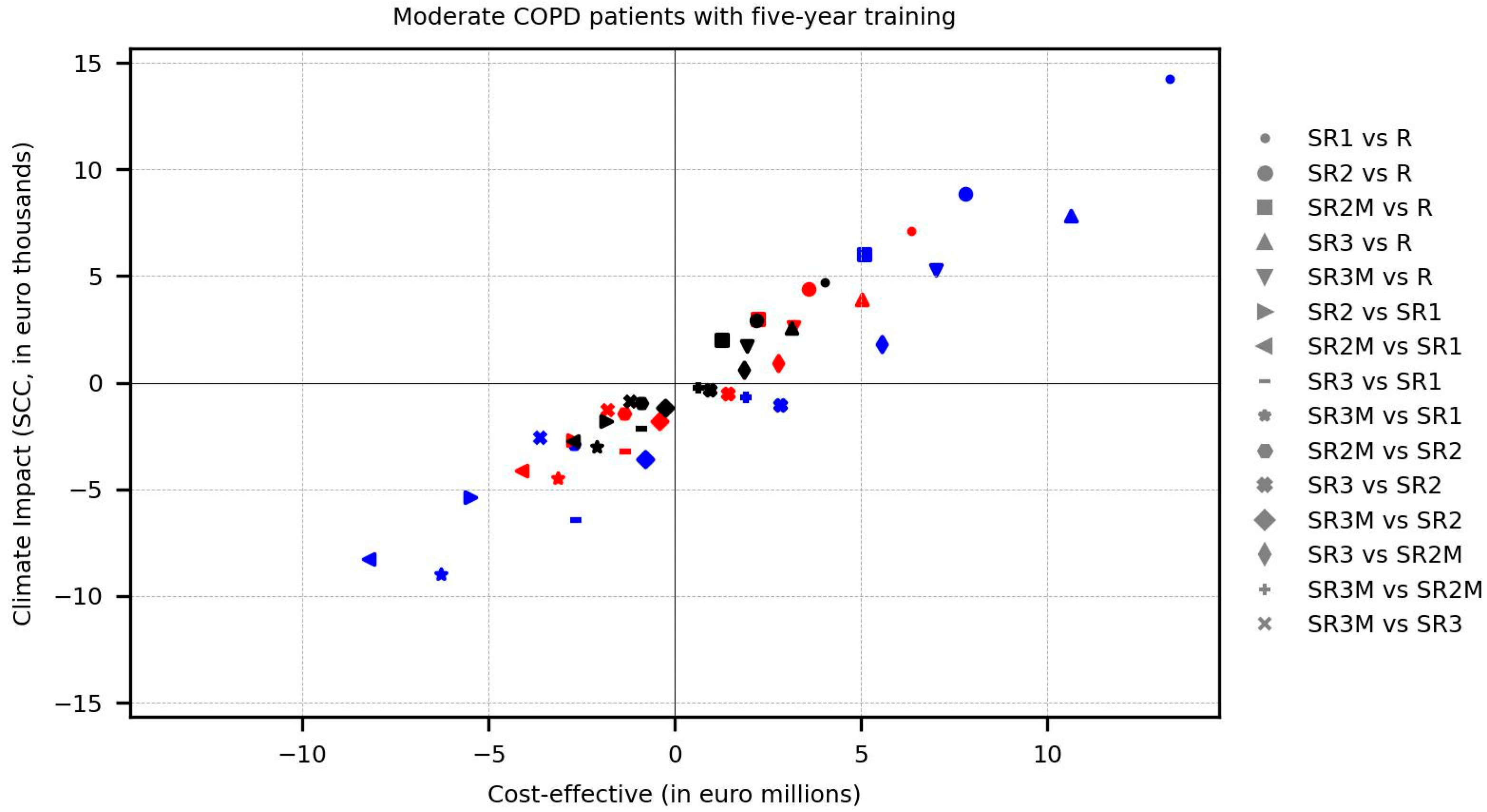
Figure 5 below, which adapts the sustainable net, cost-effective balance framework proposed by Raymakers, A. J. N., et al. [
60] for comparing the economic and ecologic Incremental Cost-Effectiveness Ratio (ICER) of policies studied in this article.
As observed, our estimations show that accounting for the environmental impacts and economic costs of integrated circuits (IC) in electronic modules is of paramount importance. From the environmental perspective, the difference in impact between the use of smart reusable inhalers with a feature-rich design (included in the policy configuration SR1) and the use of reusable inhalers (benchmark policy R) is substantial across all treatment patterns (small circle marks). However, it can be significantly reduced by using simpler microcontrollers in a high-performance design version of smart inhalers (included in policy configuration SR2, big circle marks) and marginally reduced by opting for simpler memory and sensors in the lightweight design version of smart inhalers (included in policy configuration SR3, upward-pointing triangle marks). Moreover, using both design versions exclusively with patients who did not correct their inhalation technique (refer to policy configuration SR2M and SR3M) moderately decreases the gap from reusable inhalers (square and downward-pointing triangle marks), while combined with a policy based exclusively on treatment pattern 3 (black marks), it achieves a markedly greater reduction. However, the above applies only to the policy configuration SR2M within the economic context, as the smart inhalers with a lightweight design used in the policy configuration SR3M unfortunately employ more expensive sensors (albeit slightly more environmentally friendly).
Our framework also enables a first-order cost-effectiveness appreciation, providing rapid insights into whether the introduction of specific IoMT devices into health systems is worthwhile by means of multiple strategies for assessing the associated benefits. Indeed, throughout our analysis, we found that the use of smart inhalers presents more drawbacks than advantages—at least in the context of our assumptions and our tactics for modeling the adjacent benefit. This is critical for simplifying the decision to either adjust our evaluation strategy early or simply abandon the idea of replacing reusable inhalers with smart reusable inhalers in the mid-term (even gradually), as our estimates indicate that the environmental and economic investments required to progressively improve drug delivery through smart inhalers exceed the environmental and economic investments of treating COPD patients with non-smart reusable inhalers.
On the other hand, although our methodological framework was applied to a case study involving a specific disease, it is designed to highlight the fundamental role of information interpretation in a general context, thereby facilitating its systematic use in estimating the benefits of enabling technology in digital health. For example, in the context of cardiovascular diseases, beyond leveraging atypical data sources to monitor and detect cardiac dysfunctions through IoT technology embedded in electrocardiogram equipment [
62], our framework would also help emphasize the importance of the frequency of such anomalies as an interpretation of the improvement or deterioration in the patient’s quality of life. Similarly, in the context of neurodegenerative diseases, in addition to passively collecting smartphone sensor data (e.g., accelerometer data) to track the progression of amyotrophic lateral sclerosis [
63], our framework would further underscore the relevance of these data at specific time points as a mode of interpretation of the effectiveness of complementary therapies, such as physiotherapy aimed at maintaining mobility and preventing contractures.
Finally, as with any back-of-the-envelope estimation, this research is based on certain assumptions and presents limitations in its results, to which the reader should pay particular attention. Regarding assumptions, it is presumed that clinical outcomes derived from using smart inhalers and from using regular inhalers are, at least, the same. Also, it is assumed that the clinical outcomes when passing from using smart inhalers to using regular ones (policy configuration SR2M and SR3M) are unchanged. On the other hand, the Monte Carlo models assume the normal distribution of drug delivery rates, with a linear improvement of inhalation technique over the years for simplicity. In addition, it is acknowledged that the analysis of aspects related to patients (e.g., acceptance and preference around use or cessation of use of smart inhalers, behavioral regression, etc.) is absent in this study.
Regarding the results, it is recognized that the estimations of environmental costs are based exclusively on global warming in relation to producing smart reusable inhalers, to be coherent with the reference study. Moreover, economic and environmental uncertainties arising throughout the life cycle of smart inhalers (e.g., industrial scalability or waste flow of electronic modules) are not taken into account.
5. Conclusions
Smart technologies offer both advantages and drawbacks for digital health. The multitude of contributing factors on either side make the development and implementation of efficient and responsible innovations in the medical field a formidable challenge—particularly in the absence of specialized tools capable of catalyzing the agile and effective deployment of HTA to support early ecological design. In this article, we introduce a novel three-stage HTSA framework designed to address this gap, specifically within the domain of smart technologies and the context of IoMT systems. The framework is grounded in information science theory and two hypotheses, themselves derived from an abstraction of the fundamental purpose of such technologies.
Through the application of our approach to a specific case study involving the deployment of smart inhalers in the treatment of COPD, we found evidence supporting our second hypothesis; specifically, that even a limited set of information can enable the adoption of pragmatic strategies to quantify the costs and intrinsic benefits of smart assisted inhalation medication, while simultaneously facilitating the economic and environmental projection of policies implementing the partial or full use of three types of smart inhaler prototypes in the medium term. Regarding our first hypothesis, we found that the effectiveness of smart inhalers in increasing drug delivery for COPD treatment may be quite limited, while the implementation costs are relatively high (at least based on the specific information interpreted here). However, we also acknowledge the potential of smart inhaler prototypes based on pressure drop sensing with high-performance and lightweight designs, especially when combined with mixed policies.
In this manner, our framework positions itself as a preliminary tool for assessing the potential benefits and risks of smart technologies—potentially even prior to large-scale industrial production. However, it is not intended to replace the empirical rigor of clinical studies, but rather to complement and accelerate them.
Importantly, the case study analyzed in this work focuses solely on the economic and ecological dimensions of HTSA, and was developed through a simulation to estimate the cost and potential benefits of using three types of smart devices before the design of interventional studies. In future research, we aim to address these limitations and explore the transferability of our framework to other medical devices used in the treatment of different conditions, particularly neurodegenerative diseases. Through this process, we also expect to gather clinical evidence that may reinforce or reshape our main hypotheses, and also help address the research question raised in this article.