Effects of Sampling Frequency on Human Activity Recognition with Machine Learning Aiming at Clinical Applications
Abstract
1. Introduction
2. Related Work
| Study | Target Activities | Sampling Frequencies | Classifier | Main Findings | Relevance to Clinical HAR |
|---|---|---|---|---|---|
| Khan et al. [12] | General daily activities (5 benchmark datasets + 4 subjects) | 4–250 Hz | SVM a | 12–63 Hz sufficient | Relevance to physical activity monitoring |
| Allik et al. [13] | Static, low intensity, moderate intensity, rhythmical intensity, walking, running, outdoor cycling | 13–50 Hz | Decision tree | 13 Hz sufficient for most activities except for outdoor cycling | Relevance to physical activity monitoring |
| Bieber et al. [14] | Sleeping, walking, running, cycling, office work, resting, being active | 0.0166 Hz (1/60 Hz) | Decision tree | Classified at 0.0166 Hz (1/60 Hz) | Extremely low sampling rate possible for HAR |
| Zhang et al. [15] | Sedentary, household, walking, running | 5–80 Hz | Logistic regression, decision tree, SVM | 10 Hz maintain high accuracy | Relevance to physical activity monitoring |
| Brophy et al. [16] | Walking, running, high resistance exercise bike, low resistance exercise bike | 1–256 Hz | CNNs b | 5–10 Hz maintain high accuracy | Relevance to physical activity monitoring |
| Fan et al. [17] | Walking, running, high-speed cyclic movements | 10–1600 Hz | 4 SFAs c: FSM d, ECF e, VQF f, SEL g | 100 Hz sufficient for walking, 200 Hz running, 400 Hz high-speed cyclic movements | Orientation estimation |
| Antonio Santoyo-Ramón et al. [18] | Activities of daily living (ADL), fall (15 public datasets) | 1–238 Hz | CNNs | 20 Hz sufficient for fall detection | Fall detection |
3. Materials and Methods
3.1. Participants
3.2. Experimental Setup
3.3. Activity Selection
3.4. Data Processing and Feature Extraction
3.5. Sampling Frequency
- (i)
- At 100 Hz: Original sampling frequency during data collection.
- (ii)
- At 50 Hz: Every second data point in the 100 Hz dataset.
- (iii)
- At 25 Hz: Every second data point in the 50 Hz dataset.
- (iv)
- At 20 Hz: Every fifth data point in the 100 Hz dataset.
- (v)
- At 10 Hz: Every second data point in the 20 Hz dataset.
- (vi)
- At 1 Hz: Every hundredth data point in the 100 Hz dataset.
3.6. Model Training and Testing
3.7. Performance Evaluation
- Precision: The proportion of predicted positive cases that were correctly identified, calculated as follows:
- Recall: The proportion of actual positive cases correctly identified, calculated as follows:
- F-value: The harmonic mean of precision and recall, calculated as follows:
3.8. Sensor Placement
4. Results
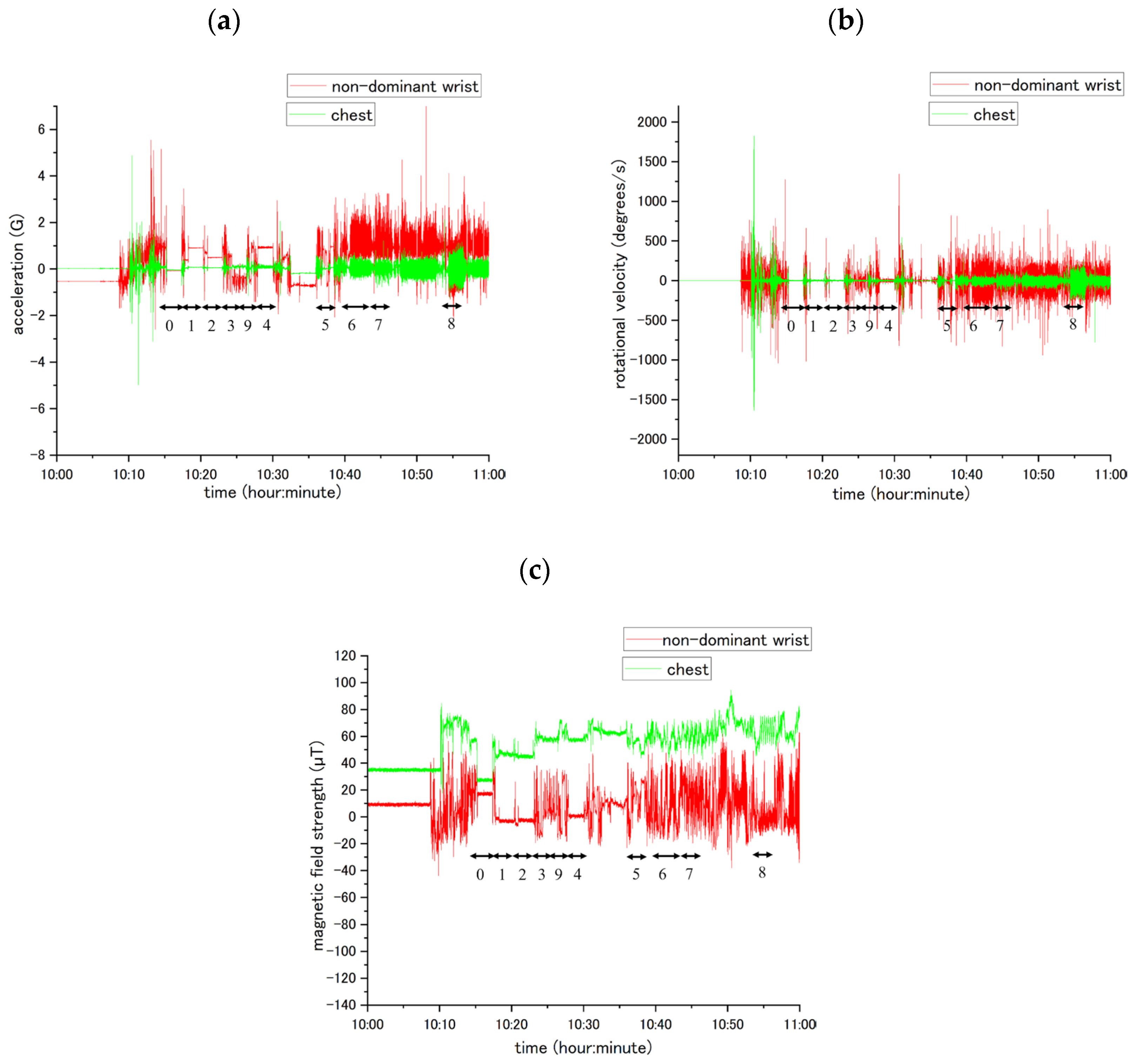
4.1. Waveform Data
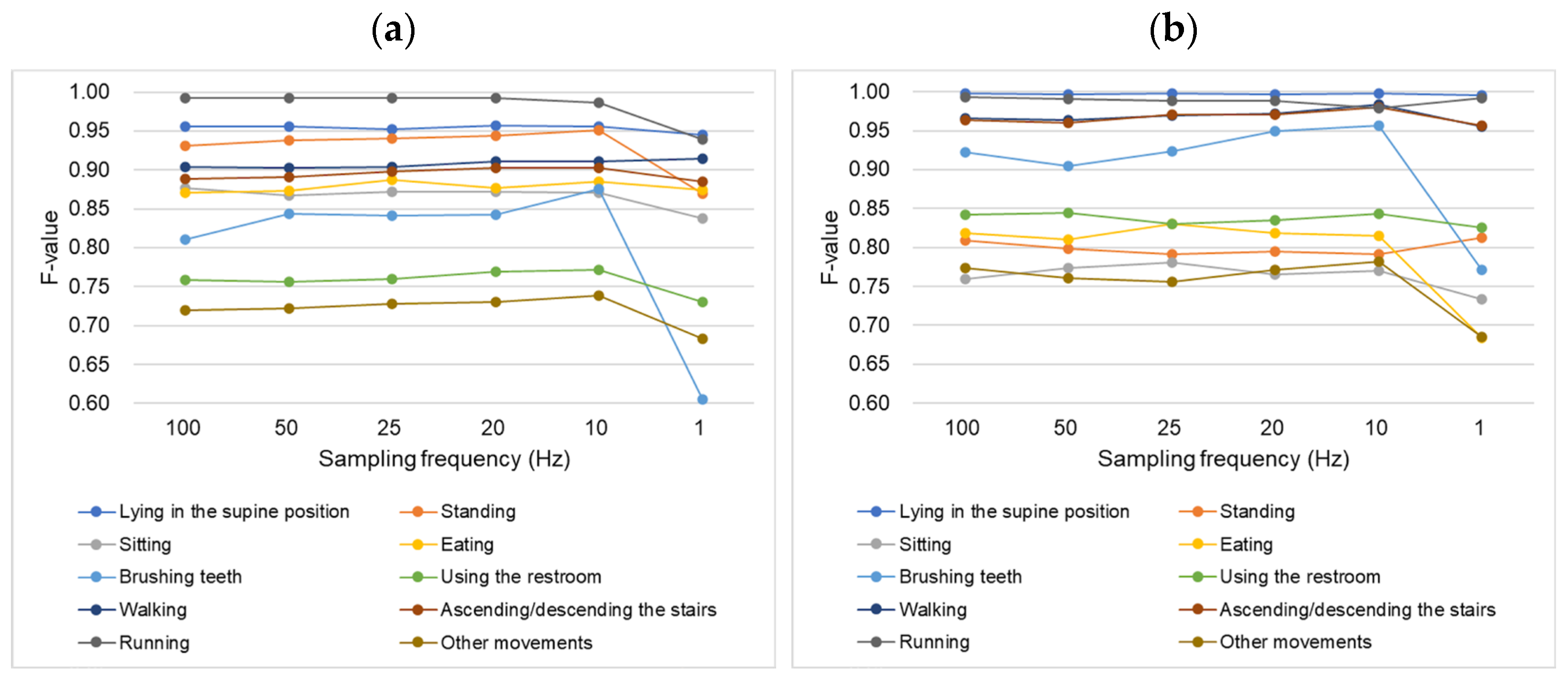
4.2. Sampling Frequency and Activity Recognition Results
4.2.1. Non-Dominant Wrist Classifier
4.2.2. Chest Classifier
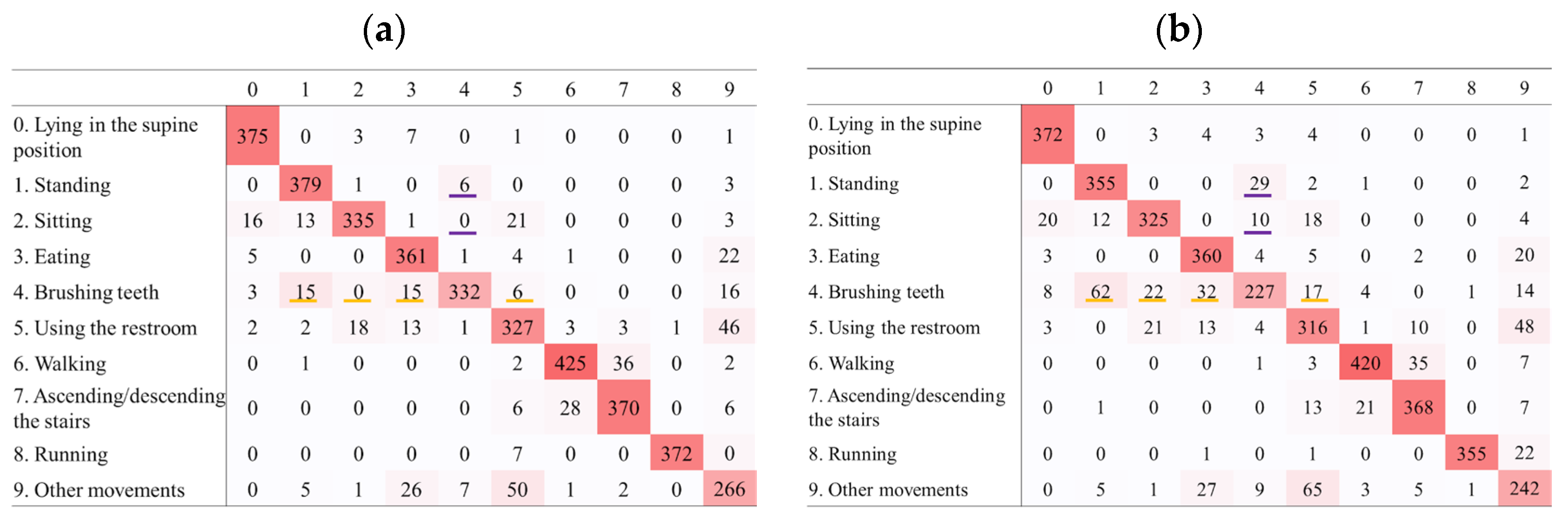
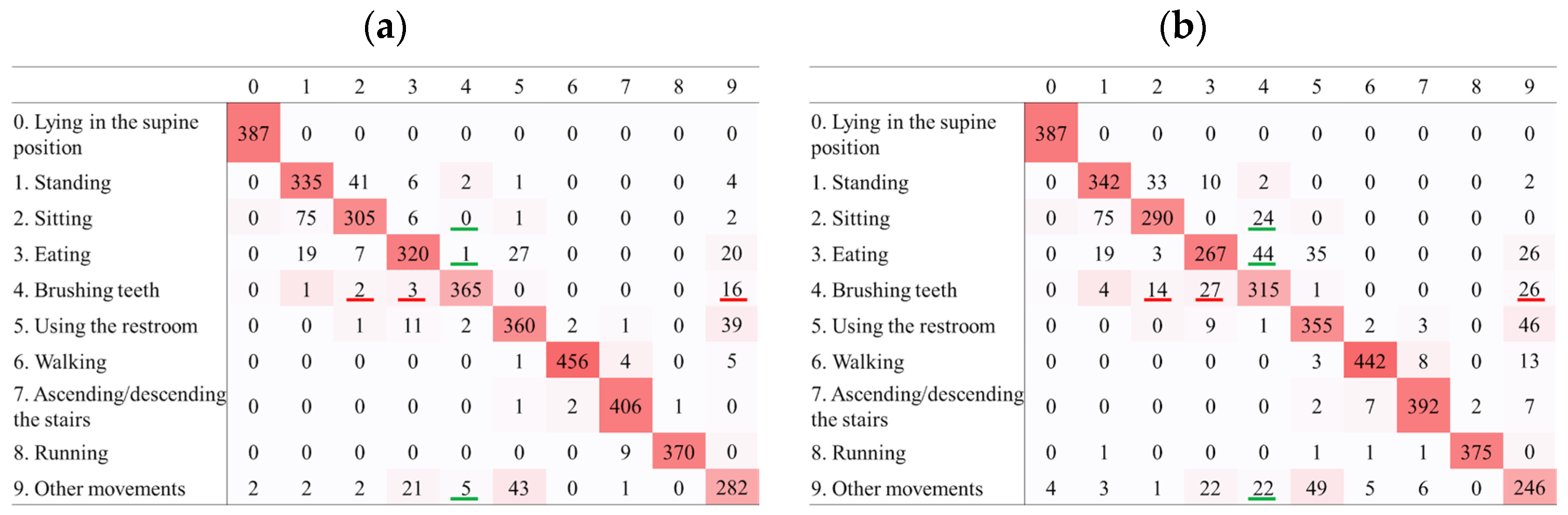
4.3. Confusion Matrices
5. Discussion
- ∙
- A sampling frequency of 10 Hz is sufficient to maintain reliable recognition accuracy when using data from wearable accelerometers, even in long-term monitoring scenarios.
- ∙
- Reducing the sampling rate from 100 Hz to 10 Hz significantly decreases data volume and power consumption, enabling longer battery life and extended monitoring, which is particularly advantageous for clinical use.
- ∙
- For specific high-frequency, short-duration movements such as brushing teeth, higher sampling rates may improve recognition. However, in the context of Holter ECG monitoring, where brushing teeth can be identified based on ECG waveform patterns, a sampling rate as low as 1 Hz may be acceptable.
- ∙
- Therefore, we suggest 10 Hz as a general lower limit for HAR in clinical applications, with the possibility of further reduction (e.g., to 1 Hz).
6. Conclusions
- (1)
- Reducing the sampling frequency to 10 Hz did not considerably affect the recognition accuracy for most activities when using data from either the non-dominant wrist or chest sensors.
- (2)
- Further reduction to 1 Hz resulted in decreased accuracy for many activities, with a decline in the recognition of brushing teeth.
- (3)
- The ability to maintain recognition accuracy at a sampling frequency of 10 Hz has important implications for clinical applications, allowing for a substantial reduction in data volume, enabling long-term patient monitoring. A reduced data volume can lead to extended battery life, faster data processing and transmission, and potential device miniaturization. These improvements have made wearable devices suitable for continuous long-term monitoring in clinical settings.
- (4)
- The study demonstrated the feasibility of using lower sampling frequencies for HAR in clinical applications, particularly for conditions where the relationship between symptom onset and patient activity is crucial, such as COPD and arrhythmia. This study provides valuable insights for the development of more efficient and patient-friendly wearable devices for clinical monitoring. Optimizing the sampling frequency will pave the way for improved long-term patient monitoring, and potentially more objective and simplified diagnostic approaches for various medical conditions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dBM | Digital biomarker |
| HAR | Human activity recognition |
| COPD | Chronic obstructive pulmonary disease |
| ECG | Electrocardiography |
| LOSO | Leave-one-subject-out |
| RF | Random forest |
| AUC | Area under the curve |
| PAF | Paroxysmal atrial fibrillation |
References
- Yousaf, R.; Arif, M.; Ullah, Q.; Rafique, S.; Hanif, A.; Ali, M. Daily Activity Related Quality of Life in Chronic Obstructive Pulmonary Disease in Adults. Int. J. Front. Sci. 2019, 3, 10–23. [Google Scholar] [CrossRef][Green Version]
- Miao, F.; Cheng, Y.; He, Y.; He, Q.; Li, Y. A Wearable Context-Aware ECG Monitoring System Integrated with Built-in Kinematic Sensors of the Smartphone. Sensors 2015, 15, 11465–11484. [Google Scholar] [CrossRef]
- Gacto-Sánchez, M.; Lozano-Meca, J.A.; Lozano-Guadalajara, J.V.; Montilla-Herrador, J. Concurrent validity of the 2-and 6-min walk test in knee osteoarthritis. Knee 2023, 43, 34–41. [Google Scholar] [CrossRef]
- Weir, N.A.; Brown, A.W.; Shlobin, O.A.; Smith, M.A.; Reffett, T.; Battle, E.; Ahmad, S.; Nathan, S.D. The influence of alternative instruction on 6-min walk test distance. Chest 2013, 144, 1900–1905. [Google Scholar] [CrossRef]
- Yamane, T.; Kimura, M.; Morita, M. Application of Nine-Axis Accelerometer-Based Recognition of Daily Activities in Clinical Examination. Phys. Act. Health 2024, 8, 29–46. [Google Scholar] [CrossRef]
- Babrak, L.M.; Menetski, J.; Rebhan, M.; Nisato, G.; Zinggeler, M.; Brasier, N.; Baerenfaller, K.; Brenzikofer, T.; Baltzer, L.; Vogler, C.; et al. Traditional and Digital Biomarkers: Two Worlds Apart? Digit. Biomark. 2019, 3, 92–102. [Google Scholar] [CrossRef]
- Morita, M.; Honjoh, M.; Yamane, Y. Trend of Digital Biomarkers (dBM) as Endpoints in Clinical Trials: Secondary Analysis of Open Data. In Proceedings of the MEDINFO 2025, Taipei, Taiwan, 9–13 August 2025. [Google Scholar]
- Vijayan, V.; Connolly, J.P.; Condell, J.; McKelvey, N.; Gardiner, P. Review of Wearable Devices and Data Collection Considerations for Connected Health. Sensors 2021, 21, 5589. [Google Scholar] [CrossRef]
- Karoulla, E.; Matsangidou, M.; Frangoudes, F.; Paspalides, P.; Neokleous, K.; Pattichis, C.S. Tracking Upper Limb Motion via Wearable Solutions: Systematic Review of Research From 2011 to 2023. J. Med. Internet Res. 2024, 26, e51994. [Google Scholar] [CrossRef]
- Vavasour, G.; Giggins, O.M.; Doyle, J.; Kelly, D. How wearable sensors have been utilised to evaluate frailty in older adults: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 112. [Google Scholar] [CrossRef]
- Zhou, L.; Fischer, E.; Tunca, C.; Brahms, C.M.; Ersoy, C.; Granacher, U.; Arnrich, B. How We Found Our IMU: Guidelines to IMU Selection and a Comparison of Seven IMUs for Pervasive Healthcare Applications. Sensors 2020, 20, 4090. [Google Scholar] [CrossRef]
- Khan, A.; Hammerla, N.; Mellor, S.; Plötz, T. Optimising sampling rates for accelerometer-based human activity recognition. Pattern Recognit. Lett. 2016, 73, 33–40. [Google Scholar] [CrossRef]
- Allik, A.; Pilt, K.; Karai, D.; Fridolin, I.; Leier, M.; Jervan, G. Optimization of Physical Activity Recognition for Real-Time Wearable Systems: Effect of Window Length, Sampling Frequency and Number of Features. Appl. Sci. 2019, 9, 4833. [Google Scholar] [CrossRef]
- Bieber, G.; Kirste, T.; Gaede, M. Low sampling rate for physical activity recognition. In Proceedings of the 7th International Conference on PErvasive Technologies Related to Assistive Environments (PETRA’14), Rhodes, Greece, 27–30 May 2014; Volume 15, pp. 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Murray, P.; Zillmer, R.; Eston, R.G.; Catt, M.; Rowlands, A.V. Activity classification using the GENEA: Optimum sampling frequency and number of axes. Med. Sci. Sports Exerc. 2012, 44, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Brophy, E.; Muehlhausen, W.; Smeaton, A.F.; Ward, T.E. CNNs for Heart Rate Estimation and Human Activity Recognition in Wrist Worn Sensing Applications. In Proceedings of the 2020 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops), Austin, TX, USA, 23–27 March 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, L.; Cai, S.; Du, M.; Liu, T.; Li, Q.; Shull, P. Influence of Sampling Rate on Wearable IMU Orientation Estimation Accuracy for Human Movement Analysis. Sensors 2025, 25, 1976. [Google Scholar] [CrossRef]
- Santoyo-Ramón, J.; Casilari, E.; Cano-Garcia, J.M. A Study of the Influence of the Sensor Sampling Frequency on the Performance of Wearable Fall Detectors. Measurement 2022, 193, 110945. [Google Scholar] [CrossRef]
- Hounslow, J.; Brewster, L.; Lear, K.; Guttridge, T.; Daly, R.; Whitney, N. Assessing the effects of sampling frequency on behavioural classification of accelerometer data. J. Exp. Mar. Biol. Ecol. 2019, 512, 22–30. [Google Scholar] [CrossRef]
- Trost, S.G.; Zheng, Y.; Wong, W.K. Machine learning for activity recognition: Hip versus wrist data. Physiol. Meas. 2014, 35, 2183–2189. [Google Scholar] [CrossRef]
- Leotta, M.; Fasciglione, A.; Verri, A. Daily Living Activity Recognition Using Wearable Devices: A Features-Rich Dataset and a Novel Approach. In Pattern Recognition. ICPR International Workshops and Challenges; Springer International Publishing: Cham, Switzerland, 2021; pp. 171–187. [Google Scholar] [CrossRef]
- Eakin, E.G.; Resnikoff, P.M.; Prewitt, L.M.; Ries, A.L.; Kaplan, R.M. Validation of a new dyspnea measure: The UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998, 113, 619–624. [Google Scholar] [CrossRef]
- Liu, S.; Gao, R.X.; Freedson, P.S. Computational methods for estimating energy expenditure in human physical activities. Med. Sci. Sports Exerc. 2012, 44, 2138–2146. [Google Scholar] [CrossRef]
- Gholamiangonabadi, D.; Kiselov, N.; Grolinger, K. Deep Neural Networks for Human Activity Recognition With Wearable Sensors: Leave-One-Subject-Out Cross-Validation for Model Selection. IEEE Access 2020, 8, 133982–133994. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Loef, B.; Wong, A.; Janssen, N.A.H.; Strak, M.; Hoekstra, J.; Picavet, H.S.J.; Boshuizen, H.C.H.; Verschuren, W.M.M.; Herber, G.M. Using random forest to identify longitudinal predictors of health in a 30-year cohort study. Sci. Rep. 2022, 12, 10372. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.B.u.d.; Dogar, A.B.; Fatima, R.; Yasin, A.; Shafiq, M.; Khan, J.A.; Assam, M.; Mohamed, A.; Attia, E.-A. Stochastic Recognition of Human Physical Activities via Augmented Feature Descriptors and Random Forest Model. Sensors 2022, 22, 6632. [Google Scholar] [CrossRef]
- Taiwo, S.A.; Iyaomolere, B.A. Hyperparameter Optimization of Random Forest Classifiers for Enhanced Performance in Sensor-Based Human Activity Recognition. Adv. J. Sci. Eng. Technol. 2025, 10, 103–119. [Google Scholar]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A readers’ guide to the interpretation of diagnostic test properties: Clinical example of sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Carrington, A.M.; Manuel, D.G.; Fieguth, P.W.; Ramsay, T.; Osmani, V.; Wernly, B.; Bennett, C.; Hawken, S.; Magwood, O.; Sheikh, Y.; et al. Deep ROC Analysis and AUC as Balanced Average Accuracy, for Improved Classifier Selection, Audit and Explanation. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 329–341. [Google Scholar] [CrossRef]
- Niazi, A.; Yazdansepas, D.; Gay, J.; Maier, F.; Ramaswamy, L.; Rasheed, K.; Buman, M. Statistical Analysis of Window Sizes and Sampling Rates in Human Activity Recognition. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies—Volume 5: BIOSTEC, Porto, Portugal, 21–23 February 2017; pp. 319–325. [Google Scholar] [CrossRef]
- Small, S.; Khalid, S.; Dhiman, P.; Chan, S.; Jackson, D.; Doherty, A.; Price, A. Impact of Reduced Sampling Rate on Accelerometer-Based Physical Activity Monitoring and Machine Learning Activity Classification. J. Meas. Phys. Behav. 2021, 4, 298–310. [Google Scholar] [CrossRef]
- Koyama, T.; Hirai, S. Measure of Vibrations by Everyday Life Activities in Front of a Washstand on a Floor and Its Monitoring System. In Proceedings of the IPSJ Interaction 2016, Tokyo, Japan, 2–4 March 2016; Presentation Number 2B36. Available online: https://www.interaction-ipsj.org/proceedings/2016/data/pdf/2B36.pdf (accessed on 16 June 2025). (only the abstract In English).
- Studd, E.K.; Landry-Cuerrier, M.; Menzies, A.K.; Boutin, S.; McAdam, A.G.; Lane, J.E.; Humphries, M.M. Behavioral classification of low-frequency acceleration and temperature data from a free-ranging small mammal. Ecol. Evol. 2018, 9, 619–630. [Google Scholar] [CrossRef]
- User Guide ActiGraph GT9X Link + ActiLife. Available online: https://s3.amazonaws.com/actigraphcorp.com/wp-content/uploads/2020/03/05155628/ActiGraph_Link_UserGuide_E.200.6001_Revision6_FINAL.pdf (accessed on 19 March 2025).
- Lotus Heart. Available online: https://lotus-heart.jp/#top (accessed on 19 March 2025). (In Japanese).
- Philips. Available online: https://www.philips.co.jp/healthcare/resources/landing/epatch (accessed on 19 March 2025). (In Japanese).
- Turakhia, M.P.; Hoang, D.D.; Zimetbaum, P.; Miller, J.D.; Froelicher, V.F.; Kumar, U.N.; Xu, X.; Yang, F.; Heidenreich, P.A. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am. J. Cardiol. 2013, 112, 520–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Clark, W.W.; Tillman, B.; Chun, Y.J.; Liu, S.; Cho, S.K. A System to Track Stent Location in the Human Body by Fusing Magnetometer and Accelerometer Measurements. Sensors 2023, 23, 4887. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Park, J.; Oh, B.; Kim, S.C. Recognition of Gait Patterns in Older Adults Using Wearable Smartwatch Devices: Observational Study. J. Med. Internet Res. 2022, 24, e39190. [Google Scholar] [CrossRef]
- Dingeto, H.; Kim, J. Exploring Cutout and Mixup for Robust Human Activity Recognition on Sensor and Skeleton Data. Appl. Sci. 2024, 14, 10286. [Google Scholar] [CrossRef]
- Gao, X.; Luo, H.; Wang, Q.; Zhao, F.; Ye, L.; Zhang, Y. A Human Activity Recognition Algorithm Based on Stacking Denoising Autoencoder and LightGBM. Sensors 2019, 19, 947. [Google Scholar] [CrossRef]

| (a) | ||||
|---|---|---|---|---|
| Sampling Frequency (Hz) | Precision | Recall | F-Value | |
| Lying in the supine position | 100 | 0.9446 | 0.9737 | 0.9552 |
| 50 | 0.9452 | 0.9763 | 0.9563 | |
| 25 | 0.9454 | 0.9686 | 0.9527 | |
| 20 | 0.9480 | 0.9737 | 0.9567 | |
| 10 | 0.9509 | 0.9686 | 0.9559 | |
| 1 | 0.9411 | 0.9611 | 0.9454 | |
| Standing | 100 | 0.9350 | 0.9459 | 0.9306 |
| 50 | 0.9351 | 0.9588 | 0.9383 | |
| 25 | 0.9372 | 0.9588 | 0.9406 | |
| 20 | 0.9422 | 0.9615 | 0.9438 | |
| 10 | 0.9413 | 0.9744 | 0.9511 | |
| 1 | 0.8622 | 0.9126 | 0.8694 | |
| Sitting | 100 | 0.9171 | 0.8690 | 0.8769 |
| 50 | 0.9150 | 0.8613 | 0.8669 | |
| 25 | 0.9185 | 0.8641 | 0.8724 | |
| 20 | 0.9189 | 0.8641 | 0.8720 | |
| 10 | 0.9233 | 0.8615 | 0.8707 | |
| 1 | 0.8866 | 0.8353 | 0.8382 | |
| Eating | 100 | 0.8650 | 0.9068 | 0.8712 |
| 50 | 0.8695 | 0.9042 | 0.8737 | |
| 25 | 0.8751 | 0.9196 | 0.8867 | |
| 20 | 0.8691 | 0.9093 | 0.8765 | |
| 10 | 0.8728 | 0.9196 | 0.8852 | |
| 1 | 0.8584 | 0.9160 | 0.8746 | |
| Brushing teeth | 100 | 0.8433 | 0.7979 | 0.8104 |
| 50 | 0.8889 | 0.8209 | 0.8433 | |
| 25 | 0.8810 | 0.8207 | 0.8418 | |
| 20 | 0.8816 | 0.8233 | 0.8420 | |
| 10 | 0.9275 | 0.8541 | 0.8756 | |
| 1 | 0.6851 | 0.5829 | 0.6053 | |
| Using the restroom | 100 | 0.7904 | 0.7634 | 0.7588 |
| 50 | 0.7955 | 0.7582 | 0.7560 | |
| 25 | 0.7956 | 0.7601 | 0.7597 | |
| 20 | 0.8057 | 0.7733 | 0.7694 | |
| 10 | 0.8007 | 0.7841 | 0.7720 | |
| 1 | 0.7300 | 0.7555 | 0.7307 | |
| Walking | 100 | 0.9295 | 0.9010 | 0.9039 |
| 50 | 0.9312 | 0.8999 | 0.9024 | |
| 25 | 0.9259 | 0.9071 | 0.9041 | |
| 20 | 0.9295 | 0.9126 | 0.9113 | |
| 10 | 0.9355 | 0.9137 | 0.9110 | |
| 1 | 0.9412 | 0.9049 | 0.9150 | |
| Ascending/descending the stairs | 100 | 0.8979 | 0.8920 | 0.8890 |
| 50 | 0.8979 | 0.8949 | 0.8905 | |
| 25 | 0.9087 | 0.8970 | 0.8982 | |
| 20 | 0.9151 | 0.8994 | 0.9028 | |
| 10 | 0.9142 | 0.9017 | 0.9023 | |
| 1 | 0.8812 | 0.8991 | 0.8847 | |
| Running | 100 | 0.9974 | 0.9897 | 0.9926 |
| 50 | 0.9974 | 0.9897 | 0.9926 | |
| 25 | 0.9974 | 0.9897 | 0.9926 | |
| 20 | 0.9974 | 0.9897 | 0.9926 | |
| 10 | 0.9974 | 0.9821 | 0.9864 | |
| 1 | 0.9952 | 0.9361 | 0.9394 | |
| Other movements | 100 | 0.7316 | 0.7423 | 0.7192 |
| 50 | 0.7315 | 0.7484 | 0.7226 | |
| 25 | 0.7329 | 0.7587 | 0.7281 | |
| 20 | 0.7380 | 0.7523 | 0.7300 | |
| 10 | 0.7520 | 0.7623 | 0.7386 | |
| 1 | 0.7124 | 0.6930 | 0.6826 | |
| (b) | ||||
| Sampling Frequency (Hz) | Precision | Recall | F-Value | |
| Lying in the supine position | 100 | 0.9956 | 1.0000 | 0.9976 |
| 50 | 0.9938 | 1.0000 | 0.9966 | |
| 25 | 0.9956 | 1.0000 | 0.9976 | |
| 20 | 0.9938 | 1.0000 | 0.9966 | |
| 10 | 0.9956 | 1.0000 | 0.9976 | |
| 1 | 0.9922 | 1.0000 | 0.9956 | |
| Standing | 100 | 0.8528 | 0.8675 | 0.8094 |
| 50 | 0.8571 | 0.8466 | 0.7984 | |
| 25 | 0.8526 | 0.8438 | 0.7913 | |
| 20 | 0.8189 | 0.8590 | 0.7942 | |
| 10 | 0.8137 | 0.8590 | 0.7917 | |
| 1 | 0.8303 | 0.8769 | 0.8123 | |
| Sitting | 100 | 0.8119 | 0.7667 | 0.7591 |
| 50 | 0.8346 | 0.7846 | 0.7737 | |
| 25 | 0.8265 | 0.7923 | 0.7804 | |
| 20 | 0.8472 | 0.7795 | 0.7651 | |
| 10 | 0.8192 | 0.7846 | 0.7704 | |
| 1 | 0.7993 | 0.7462 | 0.7330 | |
| Eating | 100 | 0.8442 | 0.8161 | 0.8187 |
| 50 | 0.8488 | 0.8008 | 0.8100 | |
| 25 | 0.8714 | 0.8195 | 0.8302 | |
| 20 | 0.8551 | 0.8144 | 0.8185 | |
| 10 | 0.8525 | 0.8118 | 0.8151 | |
| 1 | 0.7625 | 0.6743 | 0.6843 | |
| Brushing teeth | 100 | 0.9386 | 0.9171 | 0.9224 |
| 50 | 0.9294 | 0.8989 | 0.9048 | |
| 25 | 0.9366 | 0.9220 | 0.9229 | |
| 20 | 0.9712 | 0.9353 | 0.9496 | |
| 10 | 0.9762 | 0.9429 | 0.9564 | |
| 1 | 0.8086 | 0.8122 | 0.7715 | |
| Using the restroom | 100 | 0.8567 | 0.8569 | 0.8420 |
| 50 | 0.8569 | 0.8656 | 0.8449 | |
| 25 | 0.8515 | 0.8543 | 0.8306 | |
| 20 | 0.8491 | 0.8615 | 0.8346 | |
| 10 | 0.8588 | 0.8675 | 0.8433 | |
| 1 | 0.8421 | 0.8546 | 0.8258 | |
| Walking | 100 | 0.9761 | 0.9593 | 0.9661 |
| 50 | 0.9730 | 0.9568 | 0.9632 | |
| 25 | 0.9778 | 0.9637 | 0.9694 | |
| 20 | 0.9752 | 0.9710 | 0.9717 | |
| 10 | 0.9912 | 0.9776 | 0.9839 | |
| 1 | 0.9681 | 0.9466 | 0.9549 | |
| Ascending/descending the stairs | 100 | 0.9532 | 0.9785 | 0.9641 |
| 50 | 0.9501 | 0.9737 | 0.9598 | |
| 25 | 0.9619 | 0.9833 | 0.9712 | |
| 20 | 0.9657 | 0.9761 | 0.9704 | |
| 10 | 0.9734 | 0.9901 | 0.9798 | |
| 1 | 0.9592 | 0.9563 | 0.9563 | |
| Running | 100 | 1.0000 | 0.9889 | 0.9933 |
| 50 | 1.0000 | 0.9861 | 0.9912 | |
| 25 | 1.0000 | 0.9833 | 0.9889 | |
| 20 | 1.0000 | 0.9833 | 0.9889 | |
| 10 | 0.9976 | 0.9750 | 0.9788 | |
| 1 | 0.9951 | 0.9897 | 0.9921 | |
| Other movements | 100 | 0.7917 | 0.7777 | 0.7739 |
| 50 | 0.7781 | 0.7660 | 0.7606 | |
| 25 | 0.7779 | 0.7621 | 0.7556 | |
| 20 | 0.7977 | 0.7722 | 0.7713 | |
| 10 | 0.7997 | 0.7890 | 0.7817 | |
| 1 | 0.7189 | 0.6893 | 0.6849 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamane, T.; Kimura, M.; Morita, M. Effects of Sampling Frequency on Human Activity Recognition with Machine Learning Aiming at Clinical Applications. Sensors 2025, 25, 3780. https://doi.org/10.3390/s25123780
Yamane T, Kimura M, Morita M. Effects of Sampling Frequency on Human Activity Recognition with Machine Learning Aiming at Clinical Applications. Sensors. 2025; 25(12):3780. https://doi.org/10.3390/s25123780
Chicago/Turabian StyleYamane, Takahiro, Moeka Kimura, and Mizuki Morita. 2025. "Effects of Sampling Frequency on Human Activity Recognition with Machine Learning Aiming at Clinical Applications" Sensors 25, no. 12: 3780. https://doi.org/10.3390/s25123780
APA StyleYamane, T., Kimura, M., & Morita, M. (2025). Effects of Sampling Frequency on Human Activity Recognition with Machine Learning Aiming at Clinical Applications. Sensors, 25(12), 3780. https://doi.org/10.3390/s25123780






