Development of an SPRi Immune Method for the Quantitative Detection of Osteopontin
Abstract
Highlights
- SPRi biosensors for osteopontin determination have been developed.
- The bioreceptors are mouse or rabbit monoclonal anti-osteopontin antibodies attached to the gold surface of a chip via a cysteamine linker.
- The biosensors have sufficient sensitivity to determine osteopontin at levels characteristic of this protein in blood plasma without any signal enhancement.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. SPRi Measurement Methodology
2.3. Sensor Biofunctionalization
2.4. Sensor Regeneration
2.5. ELISA Measurements
3. Results and Discussion
3.1. Formation of Layers on the Biosensor Surface
3.2. Optimization of Experimental Parameters
3.2.1. Selecting the Bioreceptor Concentration
3.2.2. Optimization of Time Interaction
3.3. Estimating the Calibration Range
3.4. Precision, Accuracy, and Recovery of the SPRi Method for the Determination of Osteopontin
3.5. Interference Studies
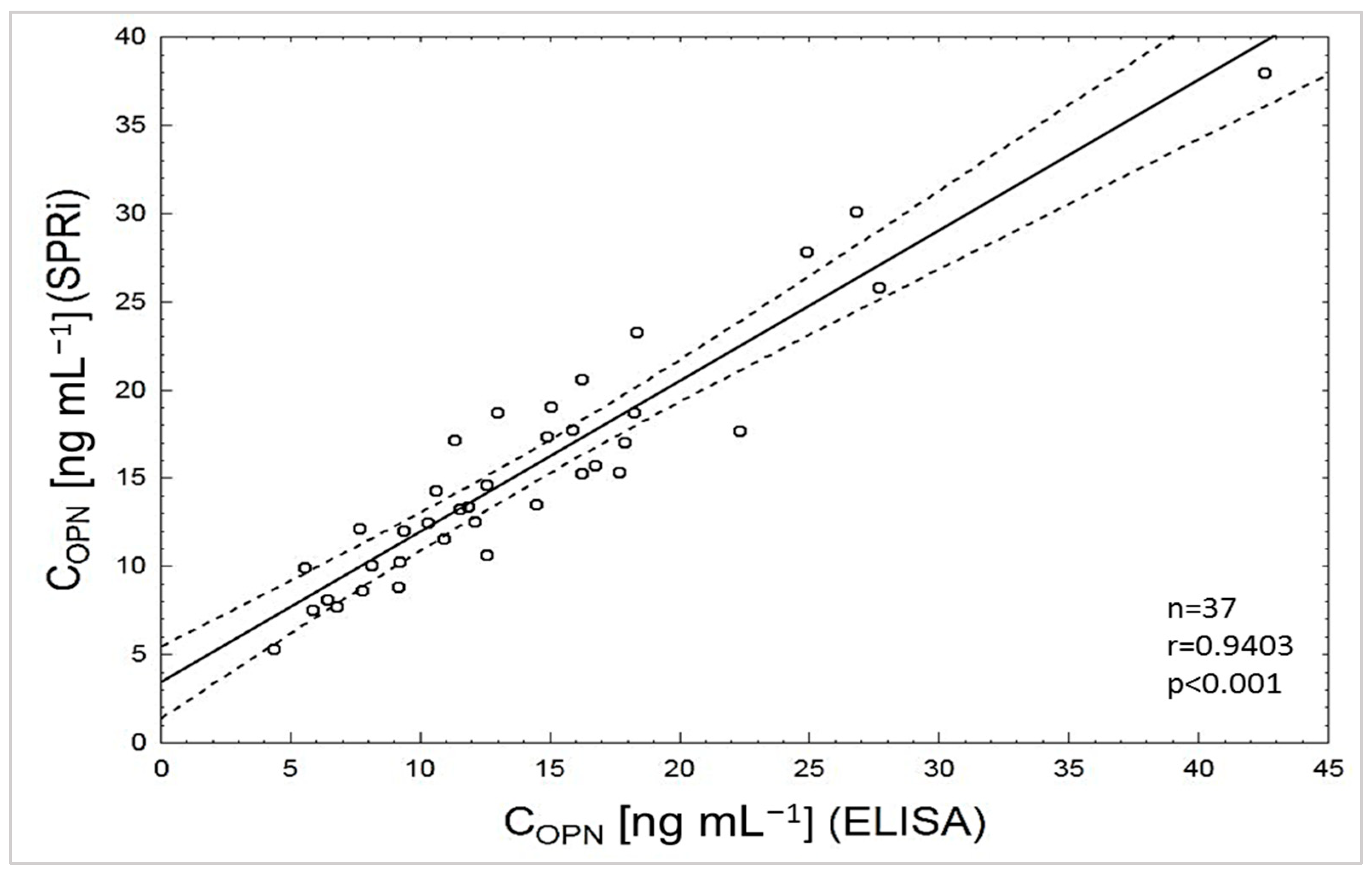
3.6. Determination of Osteopontin Concentration in Biological Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Foster, C.R.; Dalal, S.; Singh, K. Osteopontin: Role in Extracellular Matrix Deposition and Myocardial Remodeling Post-MI. J. Mol. Cell. Cardiol. 2010, 48, 538–543. [Google Scholar] [CrossRef]
- Du, Y.; Mao, L.; Wang, Z.; Yan, K.; Zhang, L.; Zou, J. Osteopontin—The Stirring Multifunctional Regulatory Factor in Multisystem Aging. Front. Endocrinol. 2022, 13, 1014853. [Google Scholar] [CrossRef] [PubMed]
- Merritt, K. Immune Response. In Handbook of Biomaterial Properties, 2nd ed.; Springer: New York, NY, USA, 2016; pp. 593–606. [Google Scholar] [CrossRef]
- Wai, P.Y.; Kuo, P.C. The Role of Osteopontin in Tumor Metastasis. J. Surg. Res. 2004, 121, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Gezmen-Karadag, M. The Multiple Functions and Mechanisms of Osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Park, M.G.; Oh, M.M.; Yoon, J.H.; Park, J.Y.; Park, H.S.; Moon, D.G.; Yoon, D.K. The value of plasma osteopontin levels as a predictive factor of disease stage and recurrence in patients with bladder urothelial carcinoma: A prospective study. Kaohsiung J. Med. Sci. 2012, 28, 526–530. [Google Scholar] [CrossRef]
- Rangaswami, H.; Bulbule, A.; Kundu, G.C. Osteopontin: Role in Cell Signaling and Cancer Progression. Trends Cell Biol. 2006, 16, 79–87. [Google Scholar] [CrossRef]
- Gebri, E.; Kiss, A.; Tóth, F.; Hortobágyi, T. Salivary Osteopontin as a Potential Biomarker for Oral Mucositis. Metabolites 2021, 11, 208. [Google Scholar] [CrossRef]
- Zou, C.; Pei, S.; Yan, W.; Lu, Q.; Zhong, X.; Chen, Q.; Pan, S.; Wang, Z.; Wang, H.; Zheng, D. Cerebrospinal Fluid Osteopontin and Inflammation-Associated Cytokines in Patients with Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Front. Neurol. 2020, 11, 519692. [Google Scholar] [CrossRef]
- Swami, S.; Kayenat, F.; Wajid, S. SPR Biosensing: Cancer Diagnosis and Biomarkers Quantification. Microchem. J. 2024, 197, 109792. [Google Scholar] [CrossRef]
- Sina, A.A.I.; Vaidyanathan, R.; Wuethrich, A.; Carrascosa, L.G.; Trau, M. Label-Free Detection of Exosomes Using a Surface Plasmon Resonance Biosensor. Anal. Bioanal. Chem. 2019, 411, 1311–1318. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Kim, K.H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in Surface Plasmon Resonance–Based Biosensor Technologies for Cancer Biomarker Detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Sankiewicz, A.; Hermanowicz, A.; Grycz, A.; Łukaszewski, Z.; Gorodkiewicz, E. An SPR Imaging Immunosensor for Leptin Determination in Blood Plasma. Anal. Methods 2021, 13, 642–646. [Google Scholar] [CrossRef]
- Guszcz, T.; Sankiewicz, A.; Gorodkiewicz, E. Application of Surface Plasmon Resonance Imaging Biosensors for Determination of Fibronectin, Laminin-5 and Type IV Collagen in Serum of Transitional Bladder Cancer Patients. J. Pharm. Biomed. Anal. 2023, 222, 115103. [Google Scholar] [CrossRef]
- Mukama, O.; Wu, W.; Wu, J.; Lu, X.; Liu, Y.; Liu, Y.; Liu, J.; Zeng, L. A Highly Sensitive and Specific Lateral Flow Aptasensor for the Detection of Human Osteopontin. Talanta 2020, 210, 120624. [Google Scholar] [CrossRef]
- Pereira, A.C.; Moreira, F.T.C.; Rodrigues, L.R.; Sales, M.G.F. Paper-Based Aptasensor for Colorimetric Detection of Osteopontin. Anal. Chim. Acta 2022, 1198, 339557. [Google Scholar] [CrossRef]
- Li, Z.; Gu, Y.; Ge, S.; Mao, Y.; Gu, Y.; Cao, X.; Lu, D. An Aptamer-Based SERS–LFA Biosensor with Multiple Channels for the Ultrasensitive Simultaneous Detection of Serum VEGF and Osteopontin in Cervical Cancer Patients. New J. Chem. 2022, 46, 20629–20642. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Electrochemical Aptasensor for Human Osteopontin Detection Using a DNA Aptamer Selected by SELEX. Anal. Chim. Acta 2017, 987, 25–37. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface Plasmon Resonance (SPR) Sensors: Approaching Their Limits? Opt. Express 2009, 17, 16505. [Google Scholar] [CrossRef]
- Falkowski, P.; Mrozek, P.; Lukaszewski, Z.; Oldak, L.; Gorodkiewicz, E. An Immunosensor for the Determination of Cathepsin S in Blood Plasma by Array SPRi-A Comparison of Analytical Properties of Silver-Gold and Pure Gold Chips. Biosensors 2021, 11, 298. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Tokarzewicz, A.; Gorodkiewicz, E. Regeneration of Surface Plasmone Resonance Chips for Multiple Use Regeneration of Surface Plasmone Resonance Chips for Multiple Use. Bulgar. Chem. Commun. 2015, 47, 477–482. [Google Scholar]
- Igawa, T.; Mimoto, F.; Hattori, K. PH-Dependent Antigen-Binding Antibodies as a Novel Therapeutic Modality. Biochim. Biophys. Acta—Proteins Proteom. 2014, 1844, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, P.; Lombardi, G.; Colombini, A.; Grasso, D.; Banfi, G. Stability of Osteopontin in Plasma and Serum. Clin. Chem. Lab. Med. 2012, 50, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Przepiórka-Kosińska, J.M.; Bartosińska, J.; Raczkiewicz, D.; Bojar, I.; Kosiński, J.; Krasowska, D.; Chodorowska, G. Serum Concentration of Osteopontin and Interleukin 17 in Psoriatic Patients. Adv. Clin. Exp. Med. 2020, 29, 203–208. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, C.H.; Hsu, B.G.; Tsai, J.P. Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis. Life 2022, 12, 397. [Google Scholar] [CrossRef]
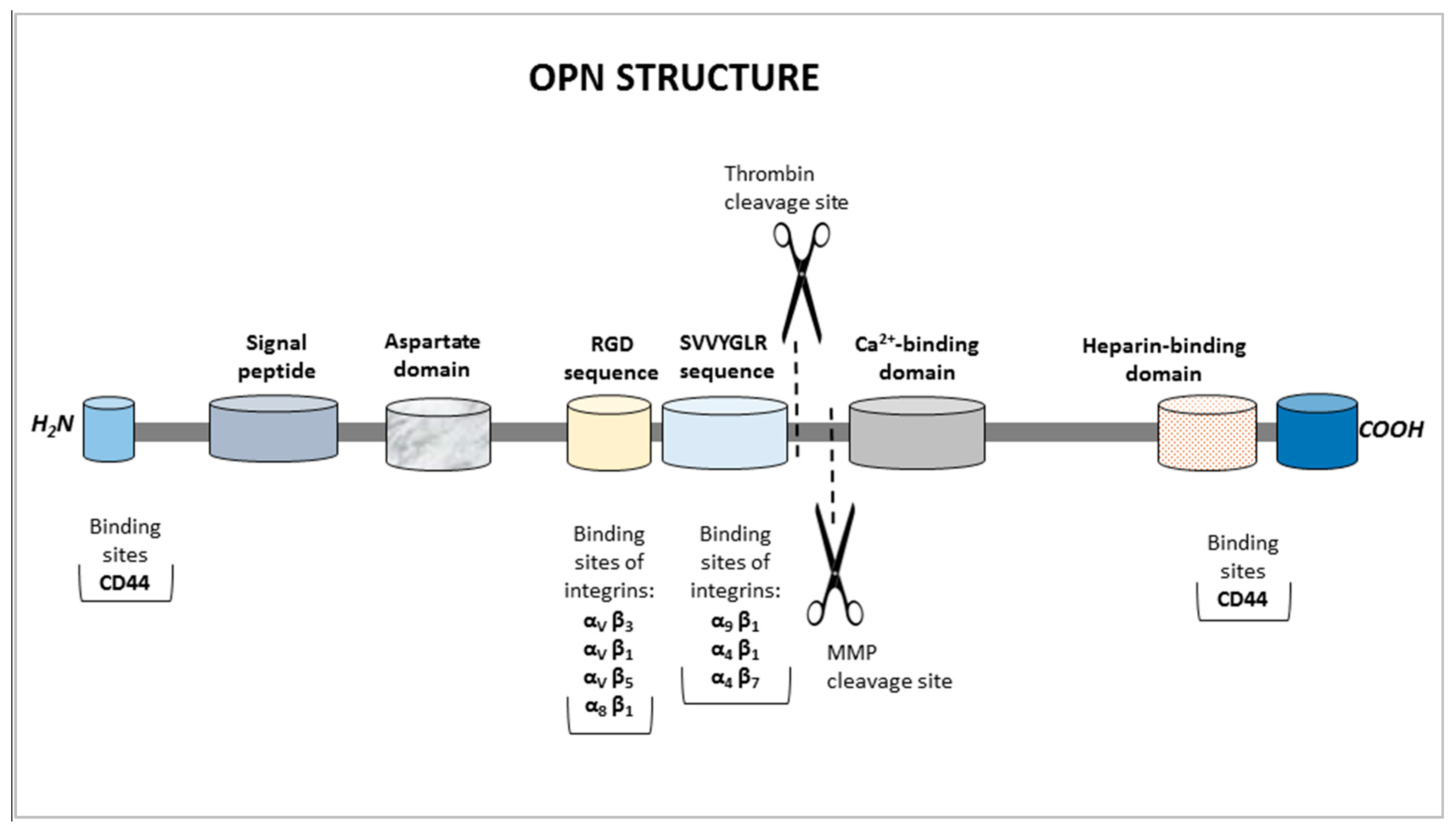
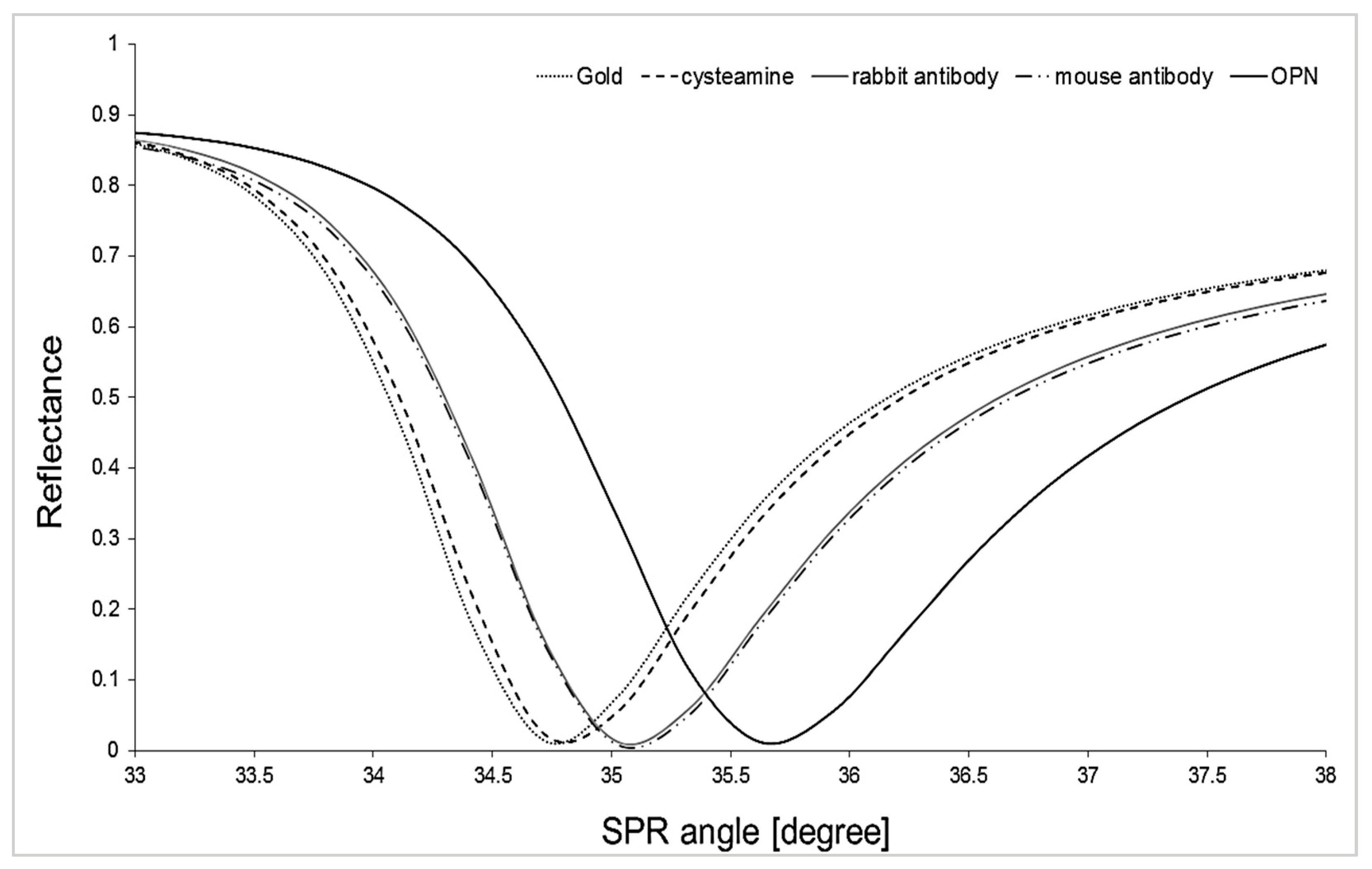
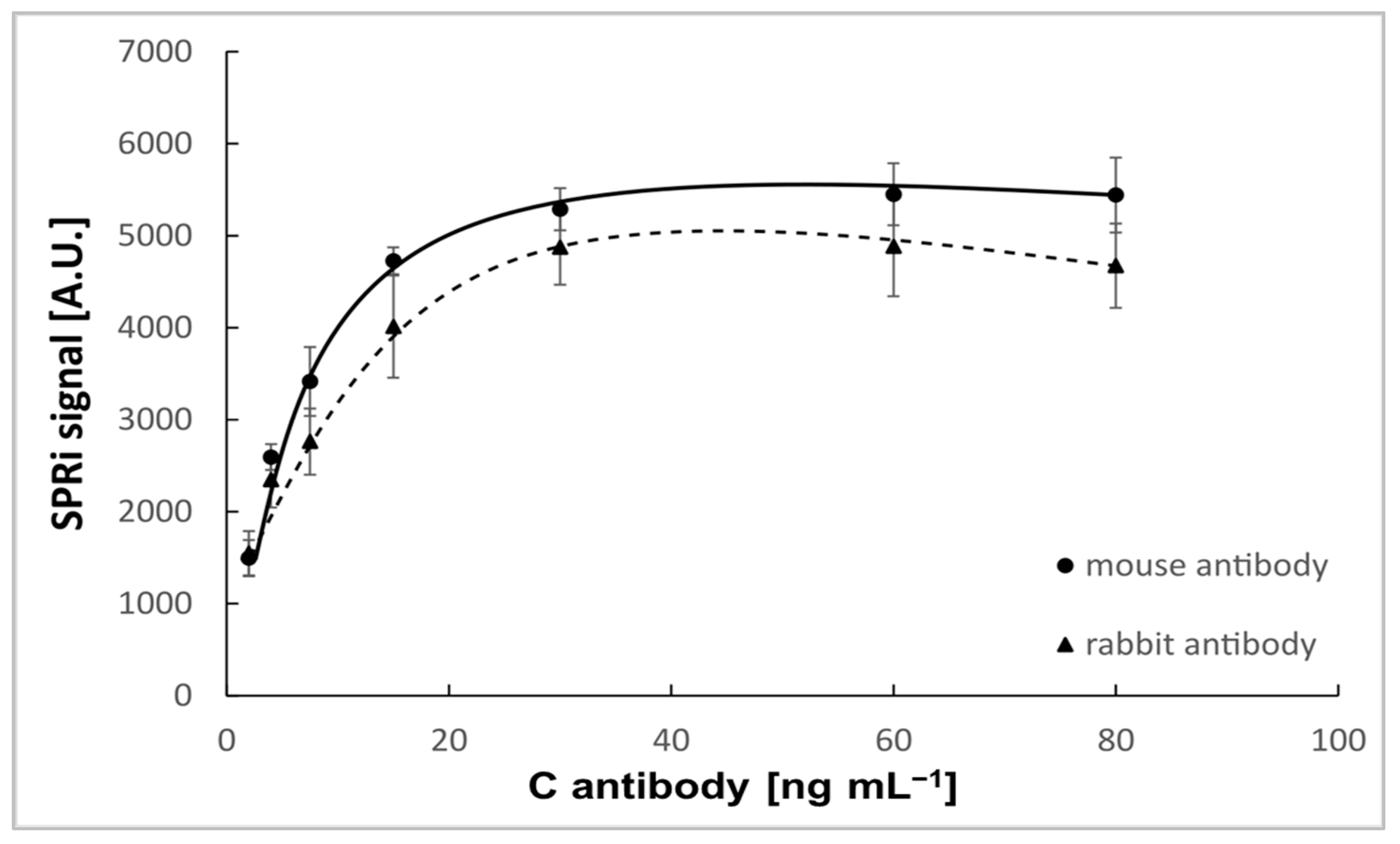
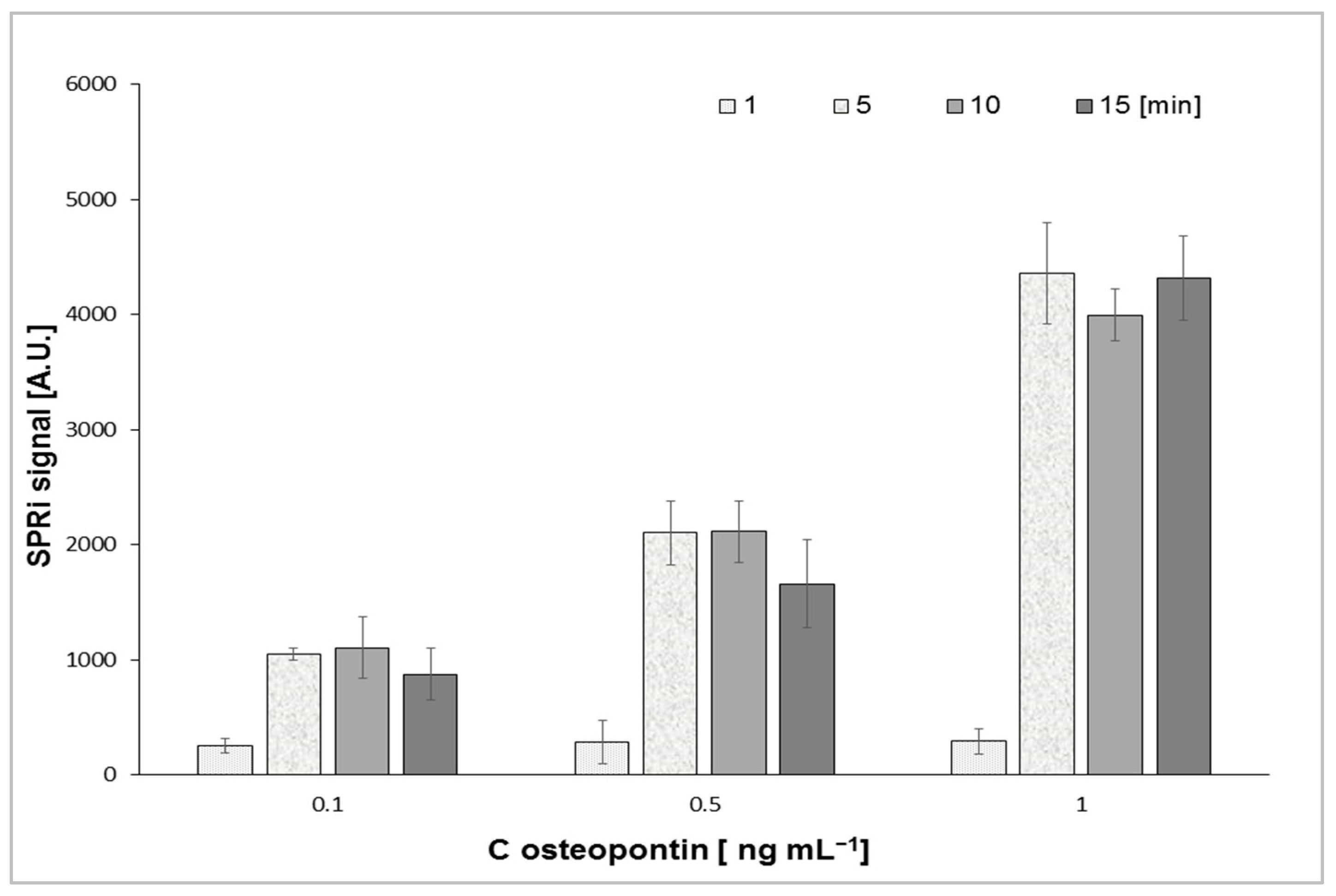
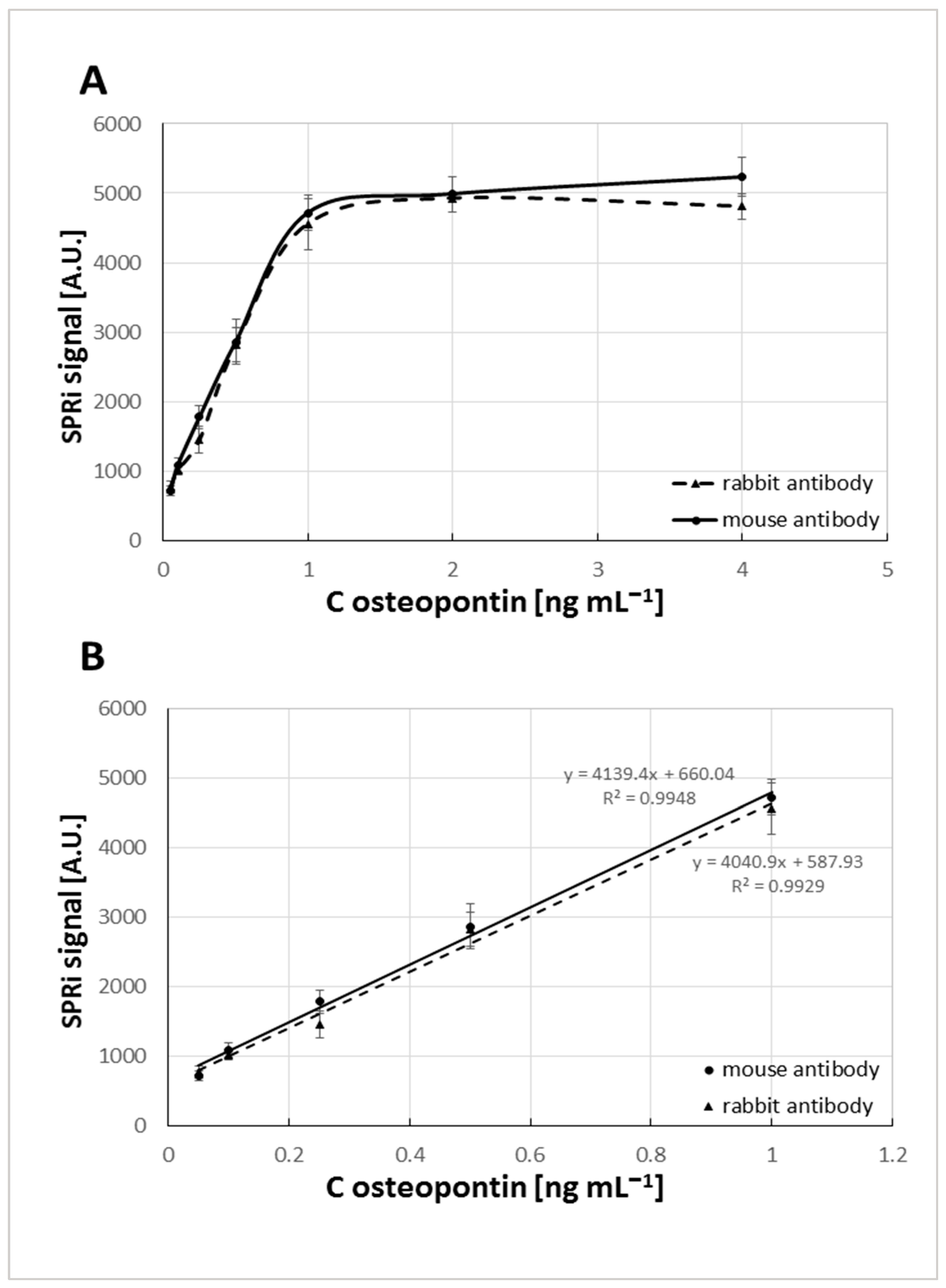
| Biosensor with Mouse Antibody | Biosensor with Rabbit Antibody | |||||||
|---|---|---|---|---|---|---|---|---|
| C Added [ng mL−1] | C Found Mean [ng mL−1] | SD | Recovery [%] | CV [%] | C Found Mean [ng mL−1] | SD | Recovery [%] | CV [%] |
| 0.050 | 0.046 | 0.004 | 93 | 7.6 | 0.049 | 0.004 | 99 | 7.3 |
| 0.250 | 0.248 | 0.002 | 99 | 0.7 | 0.258 | 0.010 | 103 | 3.9 |
| 0.500 | 0.521 | 0.008 | 104 | 1.4 | 0.509 | 0.014 | 102 | 2.8 |
| 1.000 | 0.981 | 0.017 | 99 | 1.7 | 1.016 | 0.035 | 102 | 3.4 |
| Bioreceptor | Nr Samples | OPN Concentration [ng mL−1] | |||
|---|---|---|---|---|---|
| Plasma Not Spiked with Osteopontin | Expected | Observed | Recovery [%] | ||
| Mouse antibody | 1 | 12.0 | 16.0 | 15.9 | 99 |
| 2 | 12.6 | 16.6 | 17.1 | 103 | |
| 3 | 11.6 | 15.6 | 16.7 | 107 | |
| 4 | 10.3 | 14.3 | 13.5 | 94 | |
| 5 | 13.5 | 17.5 | 17.3 | 99 | |
| 6 | 18.7 | 22.7 | 21.1 | 93 | |
| 7 | 10.1 | 14.1 | 13.9 | 99 | |
| 8 | 15.3 | 19.3 | 20.2 | 105 | |
| 9 | 7.8 | 11.8 | 12.2 | 104 | |
| Rabbit antibody | 1 | 12.8 | 16.8 | 15.6 | 93 |
| 2 | 13.1 | 17.4 | 17.9 | 105 | |
| 3 | 12.8 | 16.8 | 15.6 | 93 | |
| 4 | 11.5 | 15.5 | 14.4 | 93 | |
| 5 | 12.3 | 16.3 | 16.4 | 101 | |
| 6 | 17.9 | 21.9 | 20.7 | 95 | |
| 7 | 9.7 | 13.7 | 15.2 | 111 | |
| 8 | 15.4 | 19.4 | 17.9 | 92 | |
| 9 | 8.6 | 12.6 | 12.7 | 101 | |
| Potential Interferent | OPN Spike [ng mL−1] | Excess Over OPN | Found OPN (Mouse Antibody as Bioreceptor [ng mL−1] | Recovery [%] | Found OPN (Rabbit Antibody as Bioreceptor [ng mL−1] | Recovery [%] |
|---|---|---|---|---|---|---|
| Fibronectin | 0.50 | 1:10 | 0.514 ± 0.022 | 103 | 0.518 ± 0.011 | 102 |
| 0.50 | 1:100 | 0.501 ± 0.012 | 100 | 0.514 ± 0.018 | 103 | |
| Collagen IV | 0.50 | 1:10 | 0.515 ± 0.023 | 103 | 0.519 ± 0.022 | 104 |
| 0.50 | 1:100 | 0.506 ± 0.034 | 101 | 0.506 ± 0.026 | 101 | |
| Albumin | 0.50 | 1:100 | 0.531 ± 0.029 | 106 | 0.528 ± 0.017 | 106 |
| 0.50 | 1:1000 | 0.516 ± 0.038 | 103 | 0.518 ± 0.022 | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankiewicz, A.; Żelazowska-Rutkowska, B.; Guszcz, T.; Gorodkiewicz, E. Development of an SPRi Immune Method for the Quantitative Detection of Osteopontin. Sensors 2025, 25, 3628. https://doi.org/10.3390/s25123628
Sankiewicz A, Żelazowska-Rutkowska B, Guszcz T, Gorodkiewicz E. Development of an SPRi Immune Method for the Quantitative Detection of Osteopontin. Sensors. 2025; 25(12):3628. https://doi.org/10.3390/s25123628
Chicago/Turabian StyleSankiewicz, Anna, Beata Żelazowska-Rutkowska, Tomasz Guszcz, and Ewa Gorodkiewicz. 2025. "Development of an SPRi Immune Method for the Quantitative Detection of Osteopontin" Sensors 25, no. 12: 3628. https://doi.org/10.3390/s25123628
APA StyleSankiewicz, A., Żelazowska-Rutkowska, B., Guszcz, T., & Gorodkiewicz, E. (2025). Development of an SPRi Immune Method for the Quantitative Detection of Osteopontin. Sensors, 25(12), 3628. https://doi.org/10.3390/s25123628







