Determination of the Precision of Glucometers Used in Saudi Arabia
Abstract
Highlights
- The prevalence of self-monitoring blood glucose among individuals diagnosed with diabetes in Saudi Arabia is 95%, using commercially available glucometers.
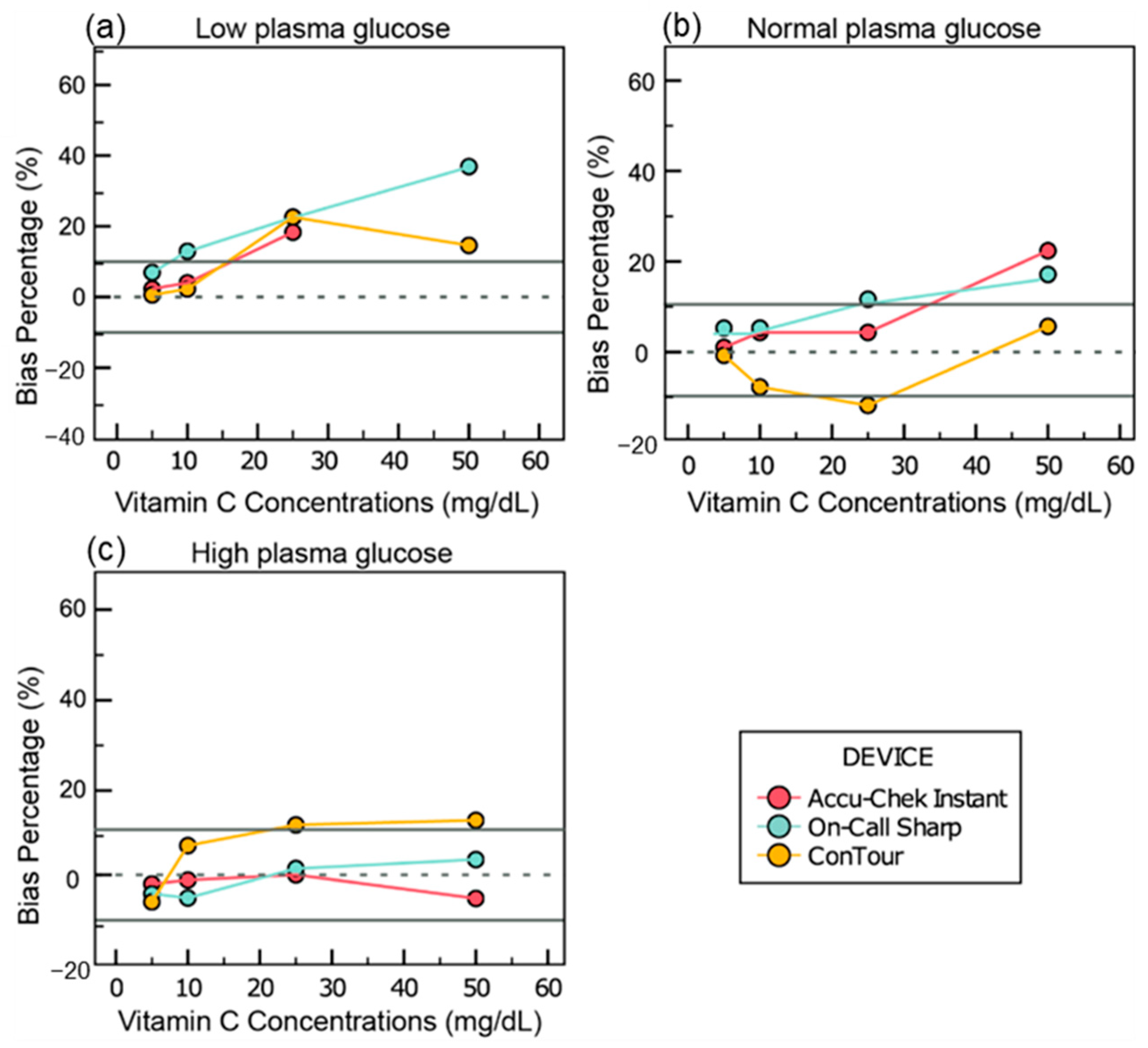
- Among the three glucometers tested, only Accu-Chek Instant met precision standards, while ConTour showed significant positive interference from vitamin C, exceeding acceptable bias limits.
- Regular use of glucometers is widespread, underscoring the importance of evaluating device accuracy and reliability.
- Certain glucometers may produce misleading glucose readings in the presence of interfering substances like vitamin C, highlighting the need for greater awareness among healthcare providers and patients.
Abstract
1. Introduction
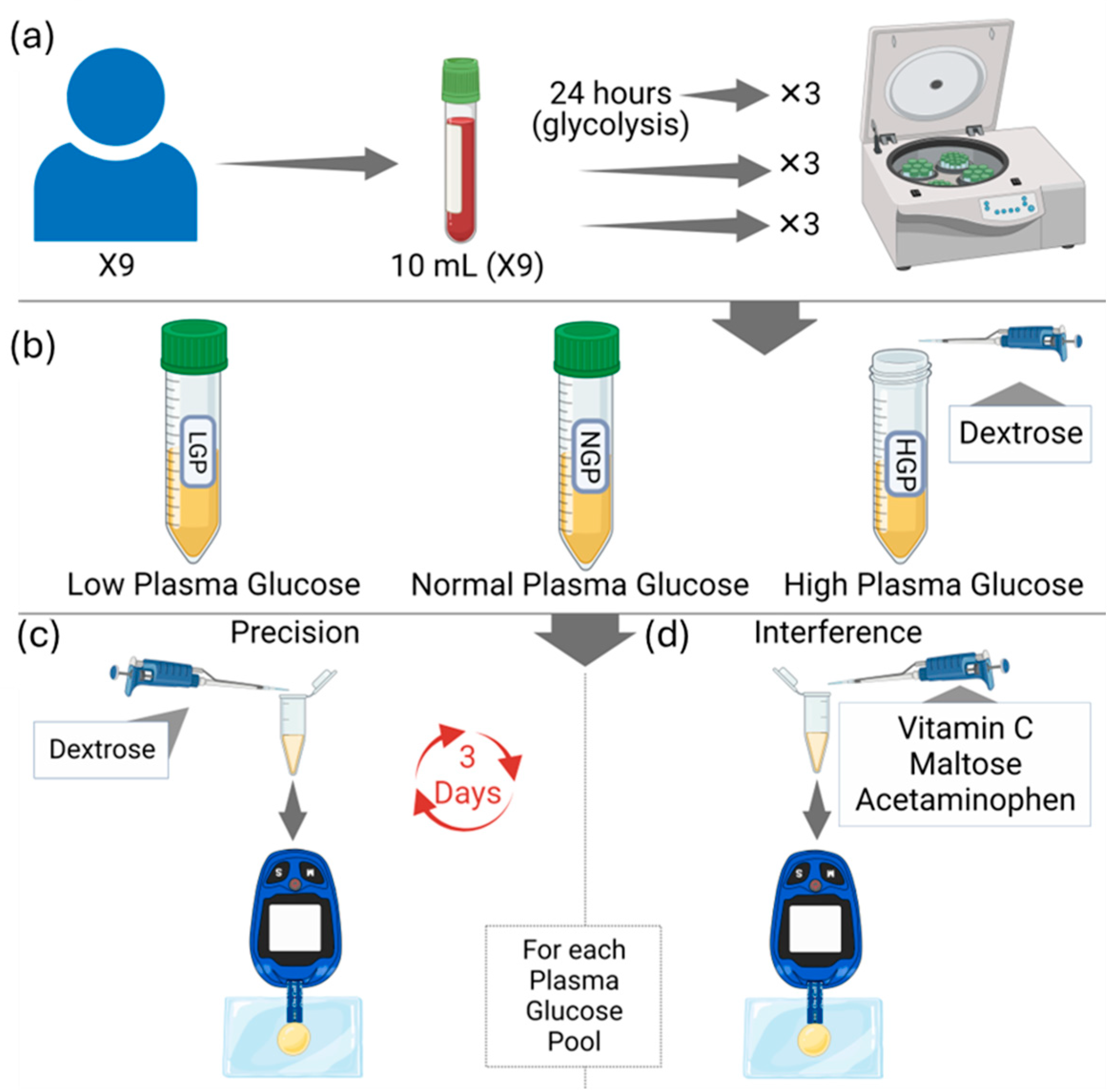
2. Materials and Methods
- 0–0.5 = poor reliability;
- 0.5–0.75 = moderate reliability;
- 0.75–0.9 = good reliability;
- 0.9–1 = excellent reliability.
3. Results
3.1. Baseline
3.2. %CV
3.3. Intraclass Correlation (ICC)
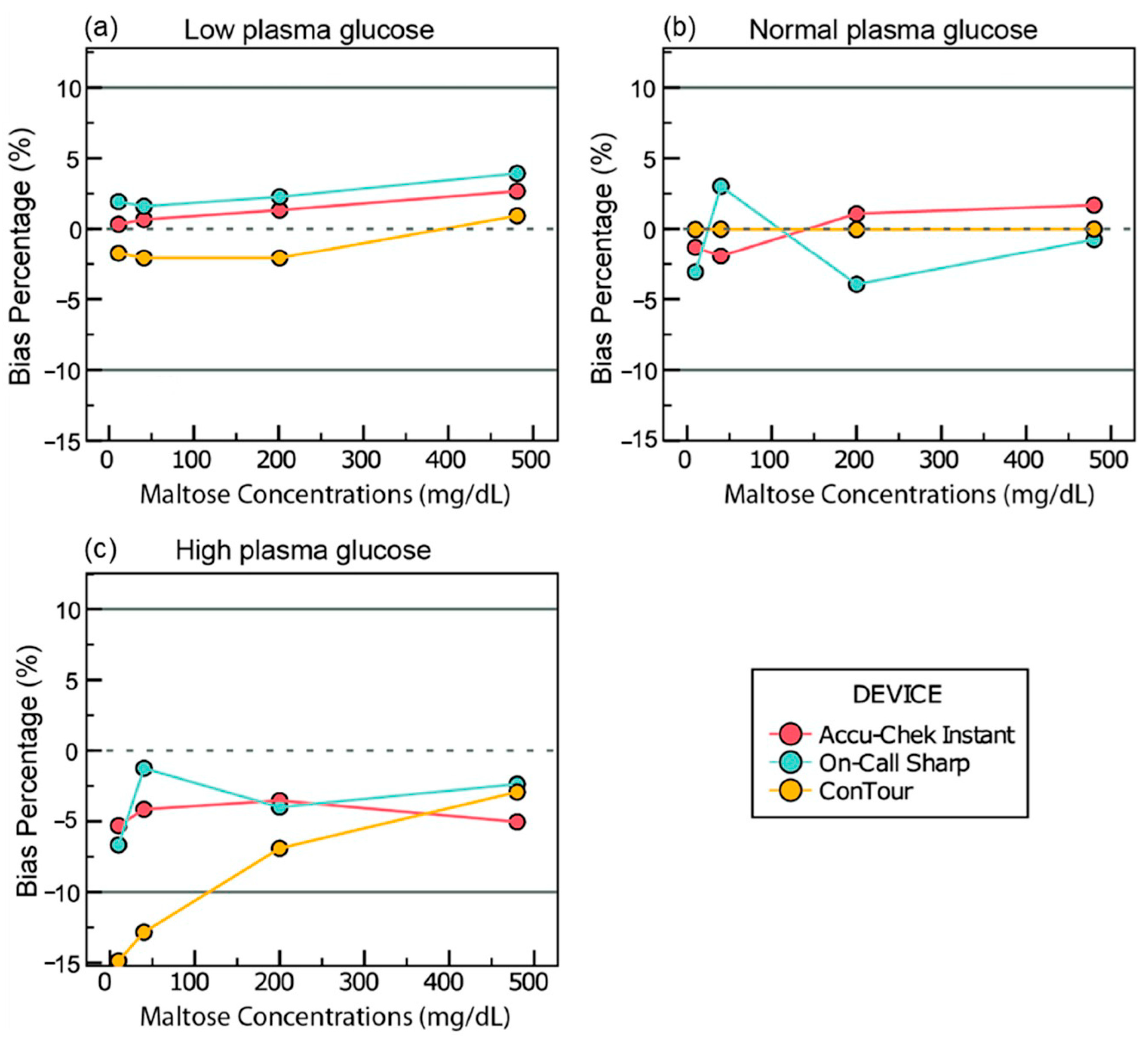
3.4. Interference Data
3.4.1. Vitamin C
3.4.2. Maltose
3.4.3. Acetaminophen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dakhale, G.N.; Chaudhari, H.V.; Shrivastava, M. Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: A randomized, double-blind study. Adv. Pharmacol. Pharm. Sci. 2011, 2011, 195271. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef]
- Abdulaziz Al Dawish, M.; Alwin Robert, A.; Braham, R.; Abdallah Al Hayek, A.; Al Saeed, A.; Ahmed Ahmed, R.; Sulaiman Al Sabaan, F. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr. Diabetes Rev. 2016, 12, 359–368. [Google Scholar] [CrossRef]
- Meng, L. Tailoring Conducting Polymer Interface for Sensing and Biosensing; Linköping University Electronic Press: Linköping, Sweden, 2020; Volume 2094. [Google Scholar]
- Puttananjegowda, K.; Thomas, S. A low-power low-noise multi-stage transimpedance amplifier for amperometric based blood glucose monitoring systems. Analog Integr. Circuits Signal Process. 2020, 102, 659–666. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose meters: A review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Loew, N.; Tsugawa, W.; Nagae, D.; Kojima, K.; Sode, K. Mediator preference of two different FAD-dependent glucose dehydrogenases employed in disposable enzyme glucose sensors. Sensors 2017, 17, 2636. [Google Scholar] [CrossRef]
- Cho, J.; Ahn, S.; Yim, J.; Cheon, Y.; Jeong, S.H.; Lee, S.-G.; Kim, J.-H. Influence of vitamin C and maltose on the accuracy of three models of glucose meters. Ann. Lab. Med. 2016, 36, 271–274. [Google Scholar] [CrossRef]
- Erbach, M.; Freckmann, G.; Hinzmann, R.; Kulzer, B.; Ziegler, R.; Heinemann, L.; Schnell, O. Interferences and limitations in blood glucose self-testing: An overview of the current knowledge. J. Diabetes Sci. Technol. 2016, 10, 1161–1168. [Google Scholar] [CrossRef]
- Girard, P.; Sourdet, S.; Cantet, C.; de Souto Barreto, P.; Rolland, Y. Acetaminophen safety: Risk of mortality and cardiovascular events in nursing home residents, a prospective study. J. Am. Geriatr. Soc. 2019, 67, 1240–1247. [Google Scholar] [CrossRef]
- Maahs, D.M.; DeSalvo, D.; Pyle, L.; Ly, T.; Messer, L.; Clinton, P.; Westfall, E.; Wadwa, R.P.; Buckingham, B. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015, 38, e158–e159. [Google Scholar] [CrossRef]
- Tang, Z.; Du, X.; Louie, R.F.; Kost, G.J. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am. J. Clin. Pathol. 2000, 113, 75–86. [Google Scholar] [CrossRef]
- Schleis, T.G. Interference of maltose, icodextrin, galactose, or xylose with some blood glucose monitoring systems. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1313–1321. [Google Scholar] [CrossRef]
- Reno, C.M.; Daphna-Iken, D.; Chen, Y.S.; VanderWeele, J.; Jethi, K.; Fisher, S.J. Severe hypoglycemia–induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013, 62, 3570–3581. [Google Scholar] [CrossRef]
- Albloui, F.; John, J.; Alghamdi, O.; Alseraye, F.; Alqahtani, A.; Tamimi, W.; Albloshi, A.; Aldakheel, F.M.; Mateen, A.; Syed, R. Effect of hematocrit, galactose and ascorbic acid on the blood glucose readings of three point-of-care glucometers. Scand. J. Clin. Lab. Investig. 2022, 82, 563–570. [Google Scholar] [CrossRef]
- Jendrike, N.; Baumstark, A.; Kamecke, U.; Haug, C.; Freckmann, G. ISO 15197: 2013 evaluation of a blood glucose monitoring system’s measurement accuracy. J. Diabetes Sci. technology 2017, 11, 1275–1276. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Salacinski, A.J.; Alford, M.; Drevets, K.; Hart, S.; Hunt, B.E. Validity and reliability of a glucometer against industry reference standards. J. Diabetes Sci. Technol. 2014, 8, 95–99. [Google Scholar] [CrossRef]
- Kermani, S.K.; Khatony, A.; Jalali, R.; Rezaei, M.; Abdi, A. Accuracy and precision of measured blood sugar values by three glucometers compared to the standard technique. J. Clin. Diagn. Res. JCDR 2017, 11, OC05. [Google Scholar] [CrossRef]
- Vanavanan, S.; Santanirand, P.; Chaichanajarernkul, U.; Chittamma, A.; DuBois, J.A.; Shirey, T.; Heinz, M. Performance of a new interference-resistant glucose meter. Clin. Biochem. 2010, 43, 186–192. [Google Scholar] [CrossRef]
- Lyon, M.E.; DuBois, J.A.; Fick, G.H.; Lyon, A.W. Estimates of total analytical error in consumer and hospital glucose meters contributed by hematocrit, maltose, and ascorbate. J. Diabetes Sci. Technol. 2010, 4, 1479–1494. [Google Scholar] [CrossRef]
- Katzman, B.M.; Kelley, B.R.; Deobald, G.R.; Myhre, N.K.; Agger, S.A.; Karon, B.S. Unintended consequence of high-dose vitamin c therapy for an oncology patient: Evaluation of ascorbic acid interference with three hospital-use glucose meters. J. Diabetes Sci. Technol. 2021, 15, 897–900. [Google Scholar] [CrossRef]
- Karon, B.S.; Griesmann, L.; Scott, R.; Bryant, S.C.; Dubois, J.A.; Shirey, T.L.; Presti, S.; Santrach, P.J. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol. Ther. 2008, 10, 111–120. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, G.; Kang, X.; Yuan, H.; Lv, Y.; Wang, W.; Randall, R. Factors interfering with the accuracy of five blood glucose meters used in Chinese hospitals. J. Clin. Lab. Anal. 2013, 27, 354–366. [Google Scholar] [CrossRef]
- Lockyer, M.; Fu, K.; Edwards, R.; Collymore, L.; Thomas, J.; Hill, T.; Devaraj, S. Evaluation of the Nova StatStrip glucometer in a pediatric hospital setting. Clin. Biochem. 2014, 47, 840–843. [Google Scholar] [CrossRef]
- Floré, K.M.; Delanghe, J.R. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: An overview. Perit. Dial. Int. 2009, 29, 377–383. [Google Scholar] [CrossRef]
- Iwasa, H.; Ozawa, K.; Sasaki, N.; Kinoshita, N.; Yokoyama, K.; Hiratsuka, A. Fungal FAD-dependent glucose dehydrogenases concerning high activity, affinity, and thermostability for maltose-insensitive blood glucose sensor. Biochem. Eng. J. 2018, 140, 115–122. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Chenoweth, J.; Dang, T.; Gao, G.; Tran, N. Acetaminophen interference with Nova StatStrip® Glucose Meter: Case report with bench top confirmation. Clin. Toxicol. 2020, 58, 1067–1070. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/self-monitoring-blood-glucose-test-systems-over-counter-use (accessed on 1 April 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Othman, S.A.; Al-Zaidany, Z.H.; Al-Ghannam, S.H.; Al-Turki, A.M.; Al-Abdulazeem, A.A.; Vatte, C.; Habara, A.; Al-Ali, A.K.; Al-Awami, M.F. Determination of the Precision of Glucometers Used in Saudi Arabia. Sensors 2025, 25, 3561. https://doi.org/10.3390/s25113561
Al-Othman SA, Al-Zaidany ZH, Al-Ghannam SH, Al-Turki AM, Al-Abdulazeem AA, Vatte C, Habara A, Al-Ali AK, Al-Awami MF. Determination of the Precision of Glucometers Used in Saudi Arabia. Sensors. 2025; 25(11):3561. https://doi.org/10.3390/s25113561
Chicago/Turabian StyleAl-Othman, Shoug A., Zahra H. Al-Zaidany, Shahad H. Al-Ghannam, Ahmed M. Al-Turki, Abdulrahman A. Al-Abdulazeem, Chittibabu Vatte, Alawi Habara, Amein K. Al-Ali, and Mohammed F. Al-Awami. 2025. "Determination of the Precision of Glucometers Used in Saudi Arabia" Sensors 25, no. 11: 3561. https://doi.org/10.3390/s25113561
APA StyleAl-Othman, S. A., Al-Zaidany, Z. H., Al-Ghannam, S. H., Al-Turki, A. M., Al-Abdulazeem, A. A., Vatte, C., Habara, A., Al-Ali, A. K., & Al-Awami, M. F. (2025). Determination of the Precision of Glucometers Used in Saudi Arabia. Sensors, 25(11), 3561. https://doi.org/10.3390/s25113561








