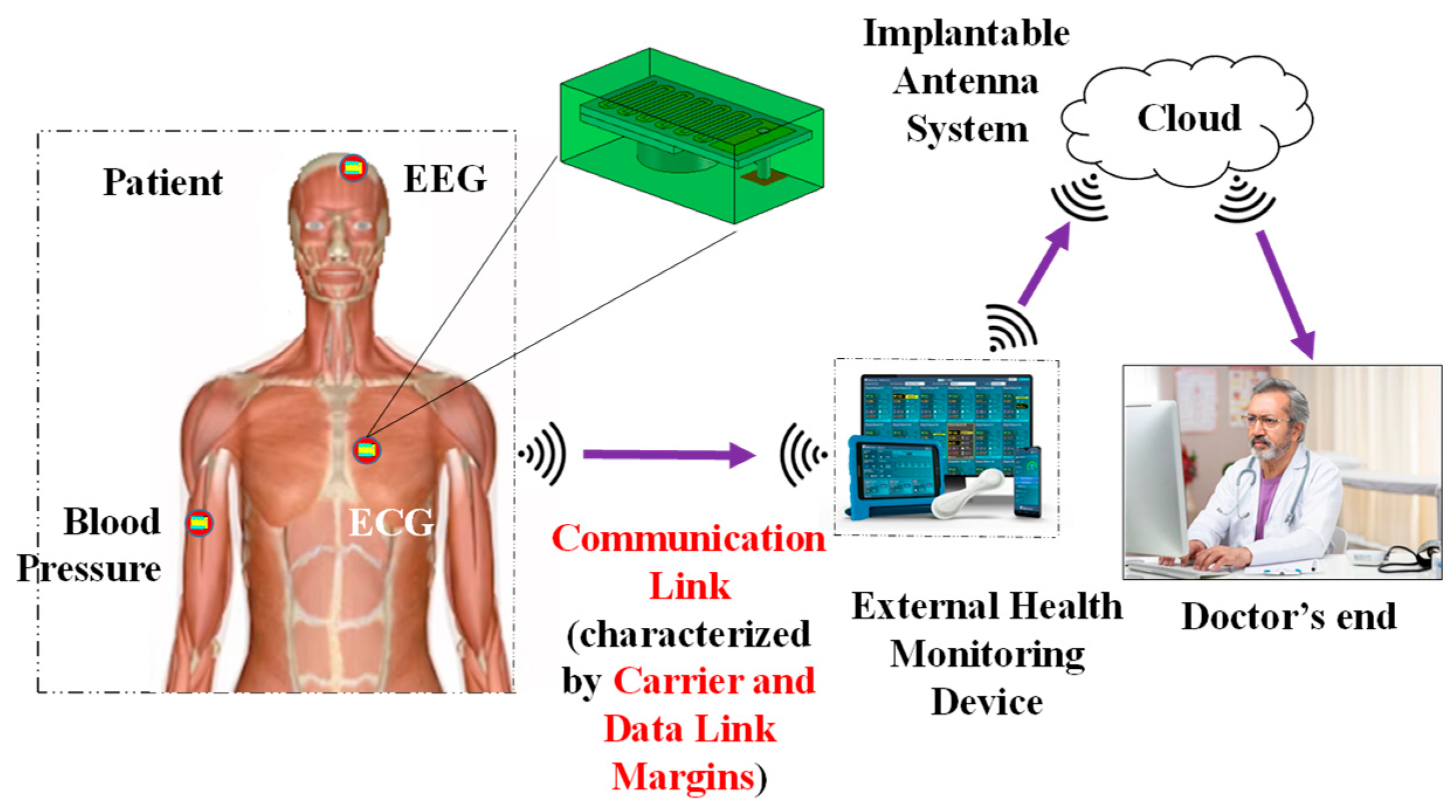
Effects of Dielectric Properties of Human Body on Communication Link Margins and Specific Absorption Rate of Implanted Antenna System
Abstract
Highlights
- The carrier and data links margins of a biocompatible gelatin-encapsulated implantable medical device have been demonstrated.
- The performance matrices [including signal radiation (gain) and reflection (S11)] within the body model are comparable with the leading implantable antennas.
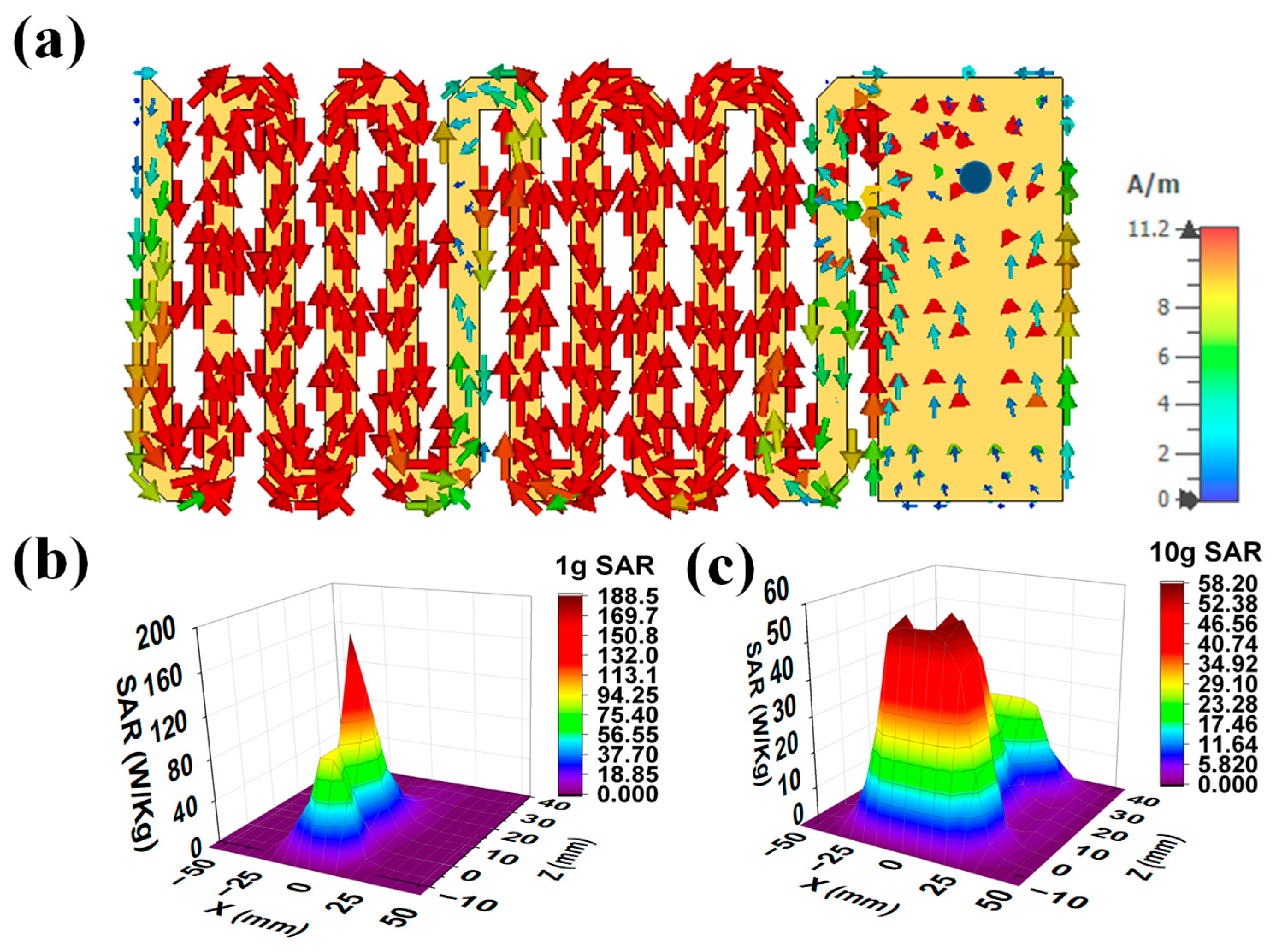
- The SAR value (220.26 W/kg) for a 1 g tissue at 1 W input power ensures the patient’s safety after implantation compared with the existing works.
- ANN modelling was performed to analyze the accuracy of the dependence. The innovative use of ANN in this field further underlines the pioneering nature of this research.
- The reported research findings will aid the innovative development of 5G/6G IoMT devices for existing and/or emerging wearable technology use cases and/or applications.
Abstract
1. Introduction
- (i)
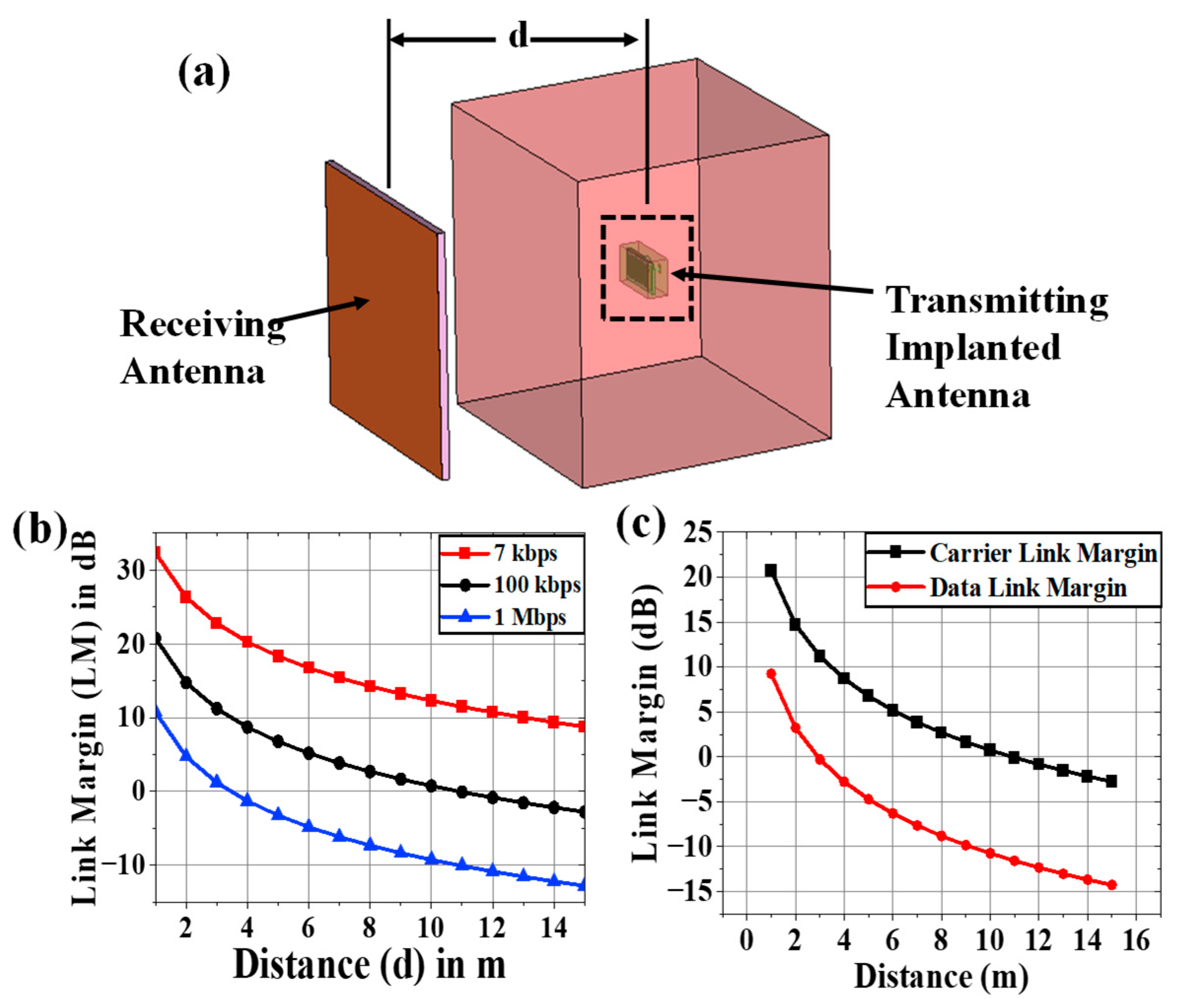
- According to Table 1, this is the first study to examine the carrier link margin and data link margin of an external monitoring device on a muscle-implanted antenna.
- (ii)
- In comparison to previous research, the SAR value (220.26 W/kg) for a 1 g tissue at 1 W input power guarantees the patient’s safety following implantation.
- (iii)
- The body model’s performance matrices, which include signal radiation (gain) and reflection (S11), are on par with the top implantable antennas.
- (iv)
- An implanted antenna with a communication range of up to 13 m was created by the authors in [36]. The impact of phantom size on the spiral-implanted antenna’s efficiency and gain was examined in [39]. Based on just six samples of permittivity values, it was possible that fluctuations in the relative permittivity of the body may cause variations in the S11 and frequency of an implanted antenna [36]. The impact of both human body electrical characteristics (such as effective relative permittivity and conductivity) on the link margins (CLM and DLM) is examined and validated in our work using a thorough methodology. The prevailing performance matrices for potential IoMT applications include operating frequency, realized gain, data rate, channel loss, the range of communication, bandwidth, and the SAR signature of the implanted antenna.
- (v)
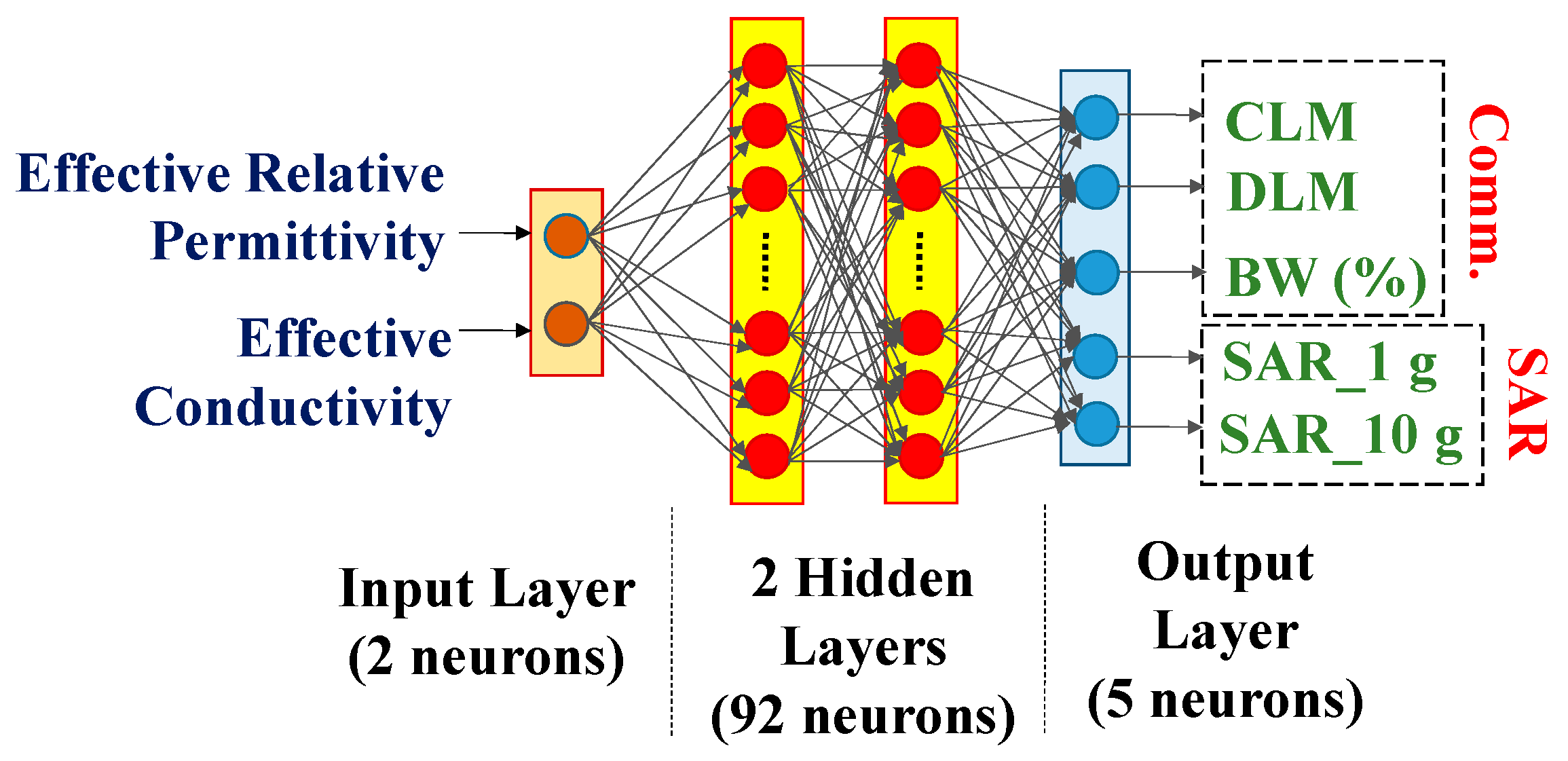
- To examine the correctness of the dependence, ANN modeling was conducted on 2500 samples of effective body models. The creative application of ANN in this area emphasizes the groundbreaking character of this study even more.
- (vi)
- This study offers the first comprehensive examination of the electrical characteristics of the human body that depends on the SAR profile and implantable antenna communication link performance.
- (vii)
- The presented study results will support the creation of 5G/6G IoMT devices for current and/or future wearable technology applications and use cases.
2. Models and Methods
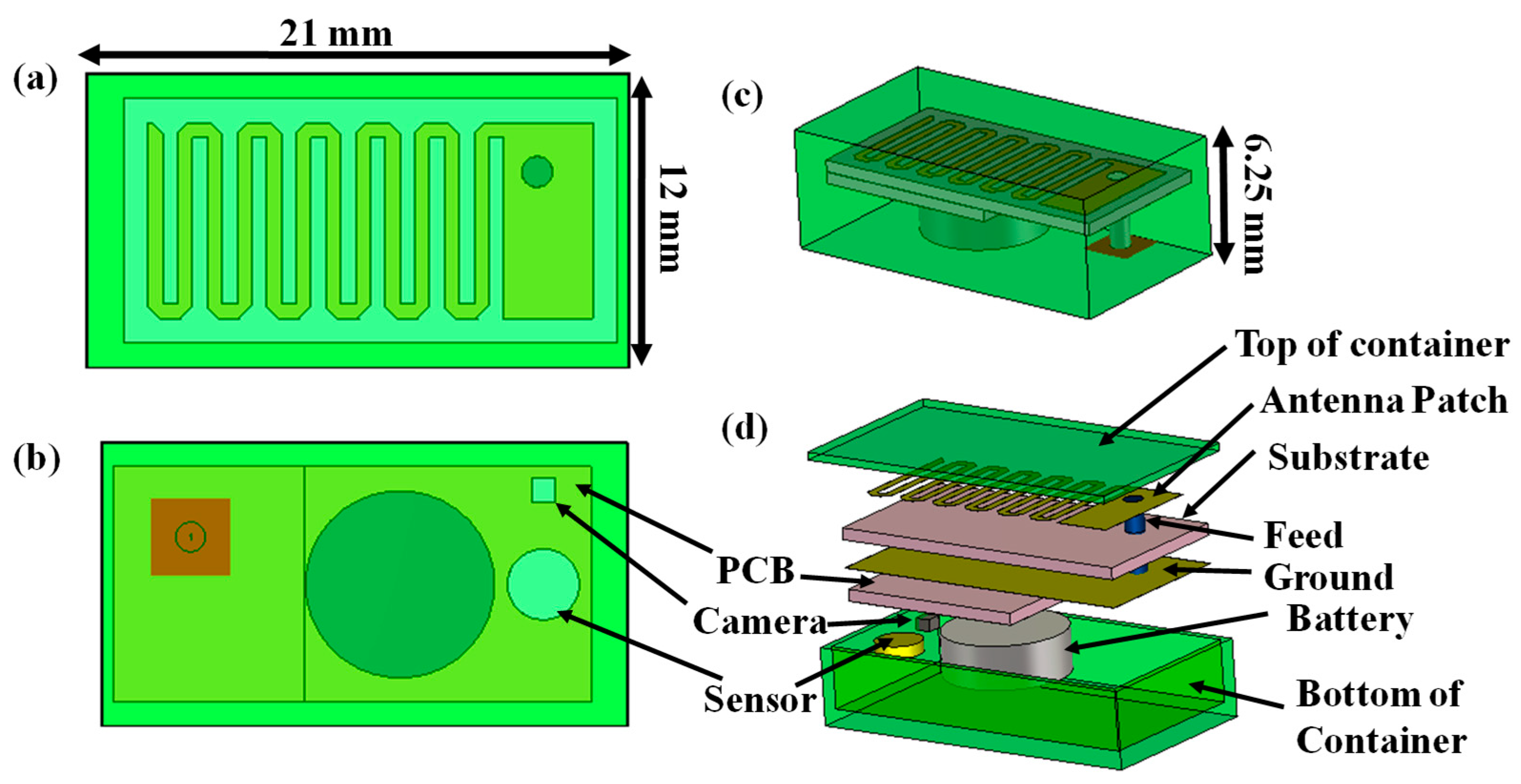
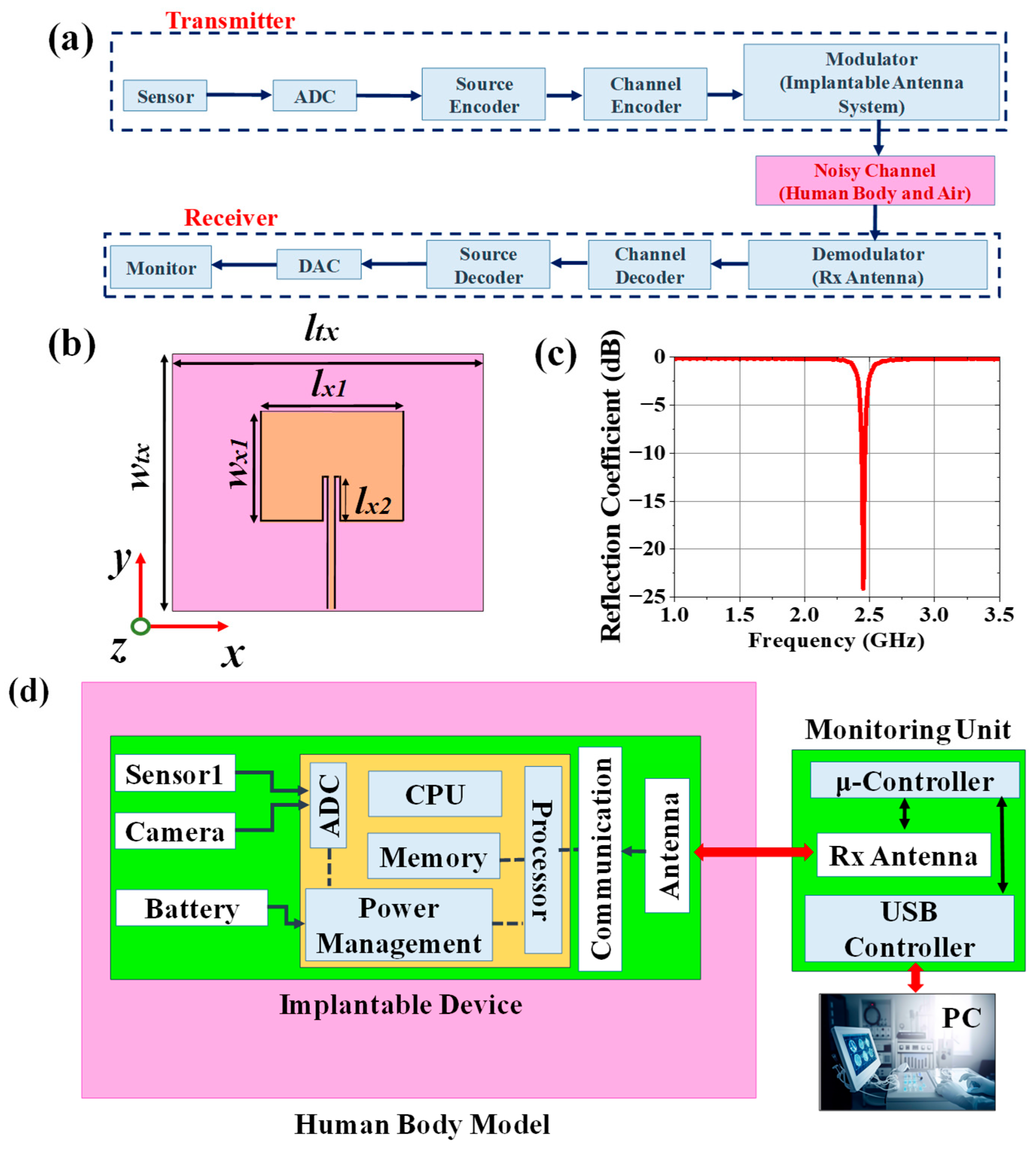
2.1. Implantable Transmitting Antenna System Design
2.1.1. Design Procedure
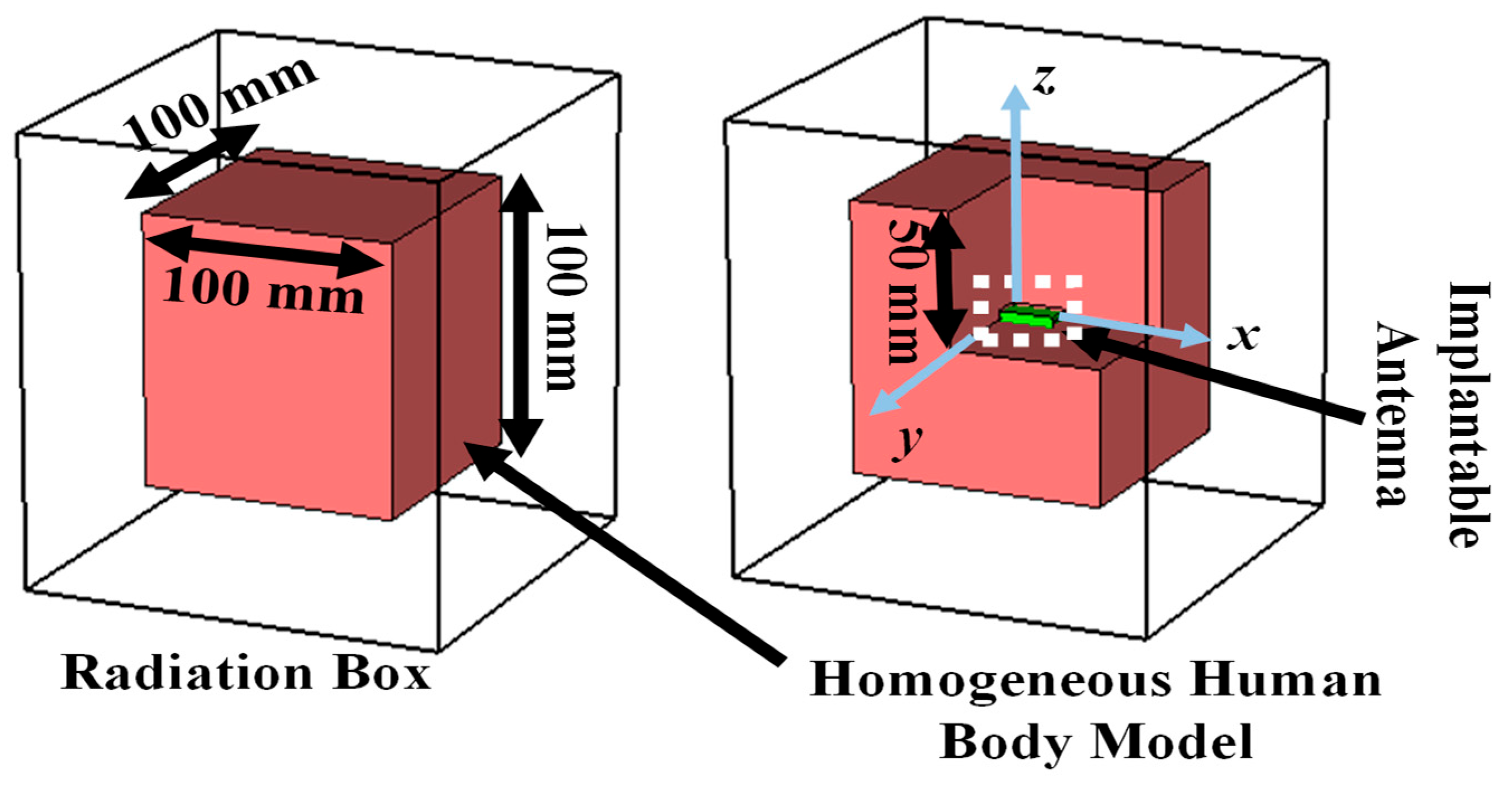
2.1.2. Simulation Setup
2.1.3. Design Evolution
2.1.4. Current Distribution and SAR Profile
2.2. Antenna Design for Monitoring Device
2.3. Communication Performance Characterization
2.4. Carrier Link Margin and Data Link Margin Calculation
3. Dependence Analysis and Discussion
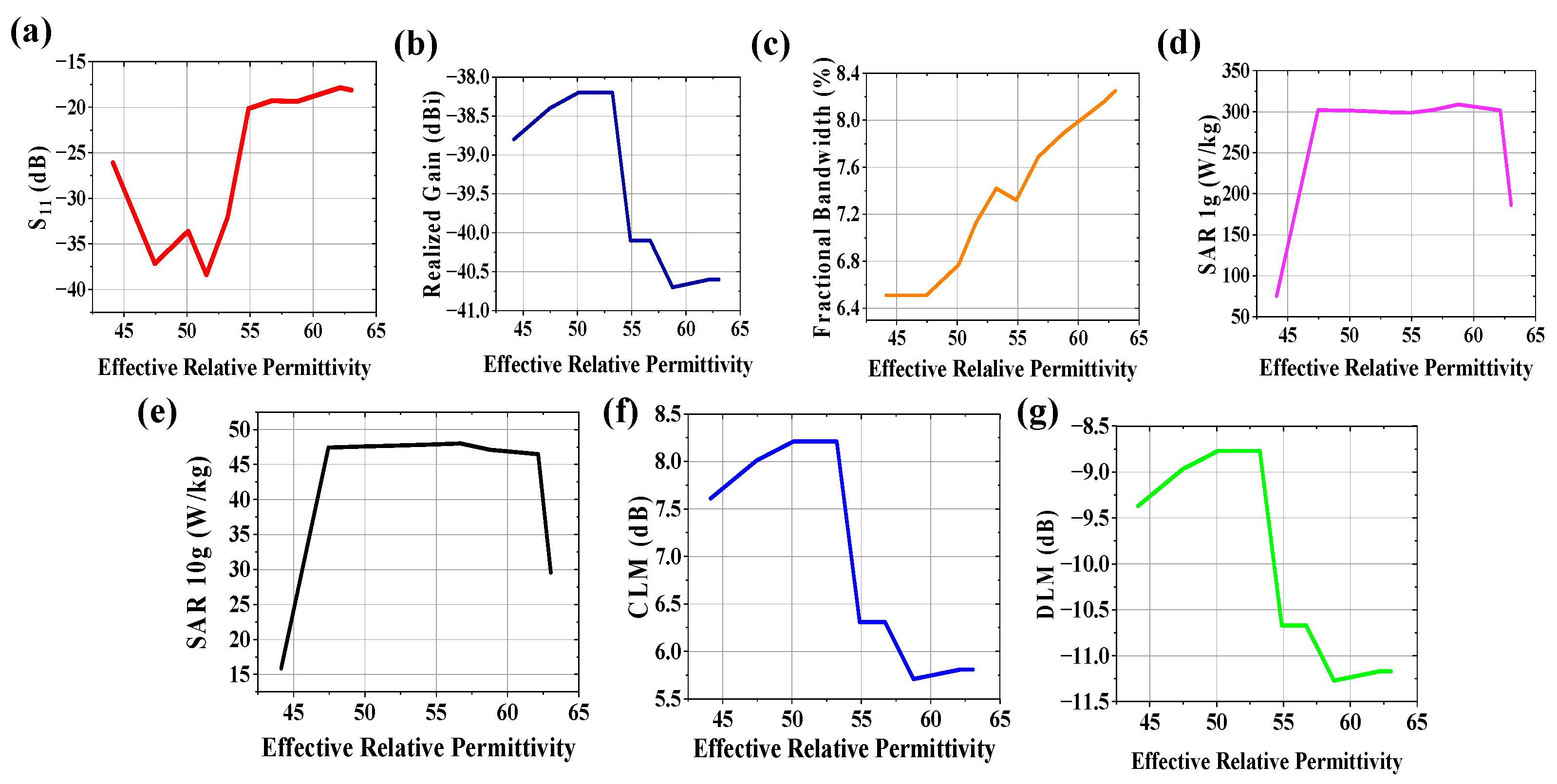
3.1. Variation in Effective Relative Permittivity
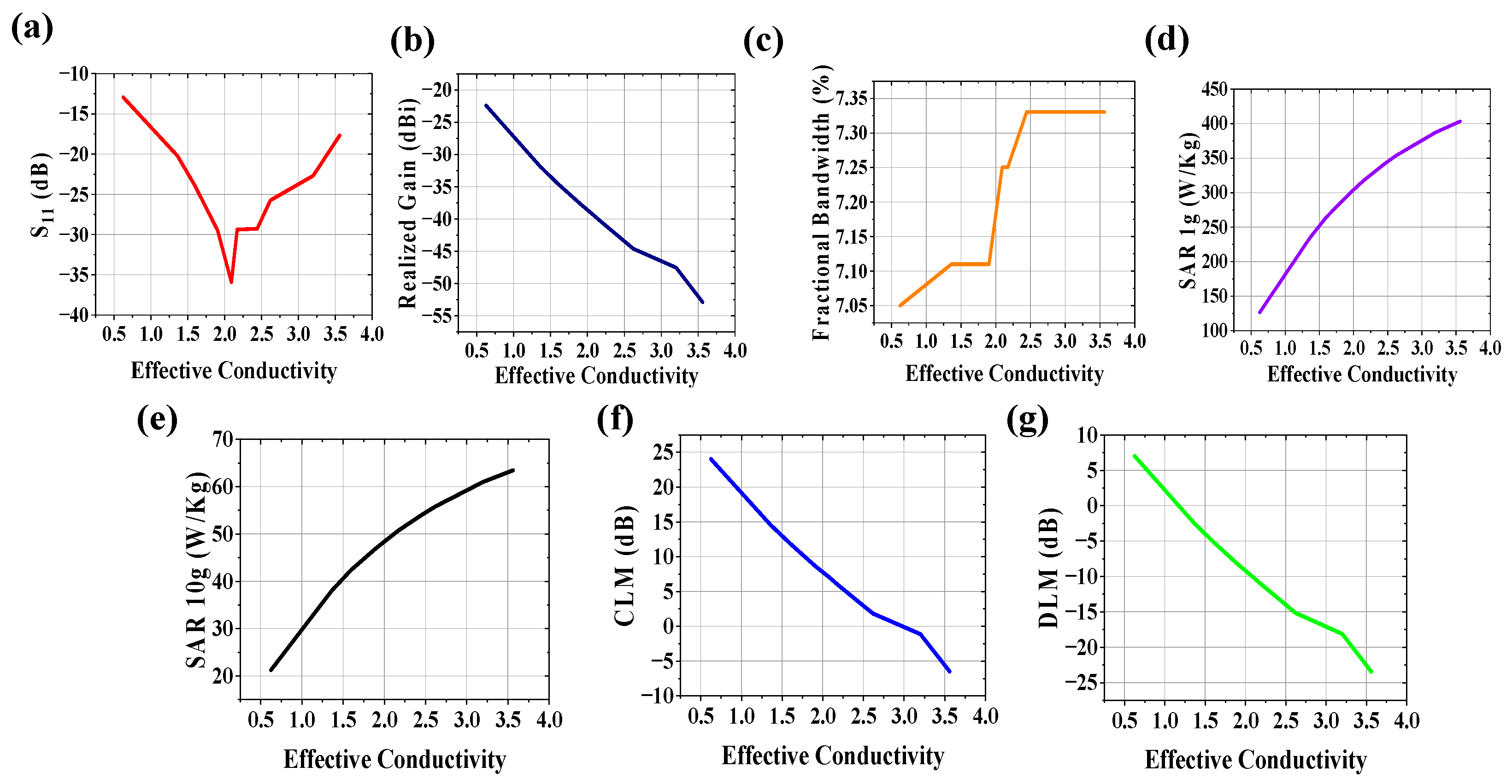
3.2. Variation in Effective Conductivity
3.3. Variations in Both Effective Relative Permittivity and Conductivity
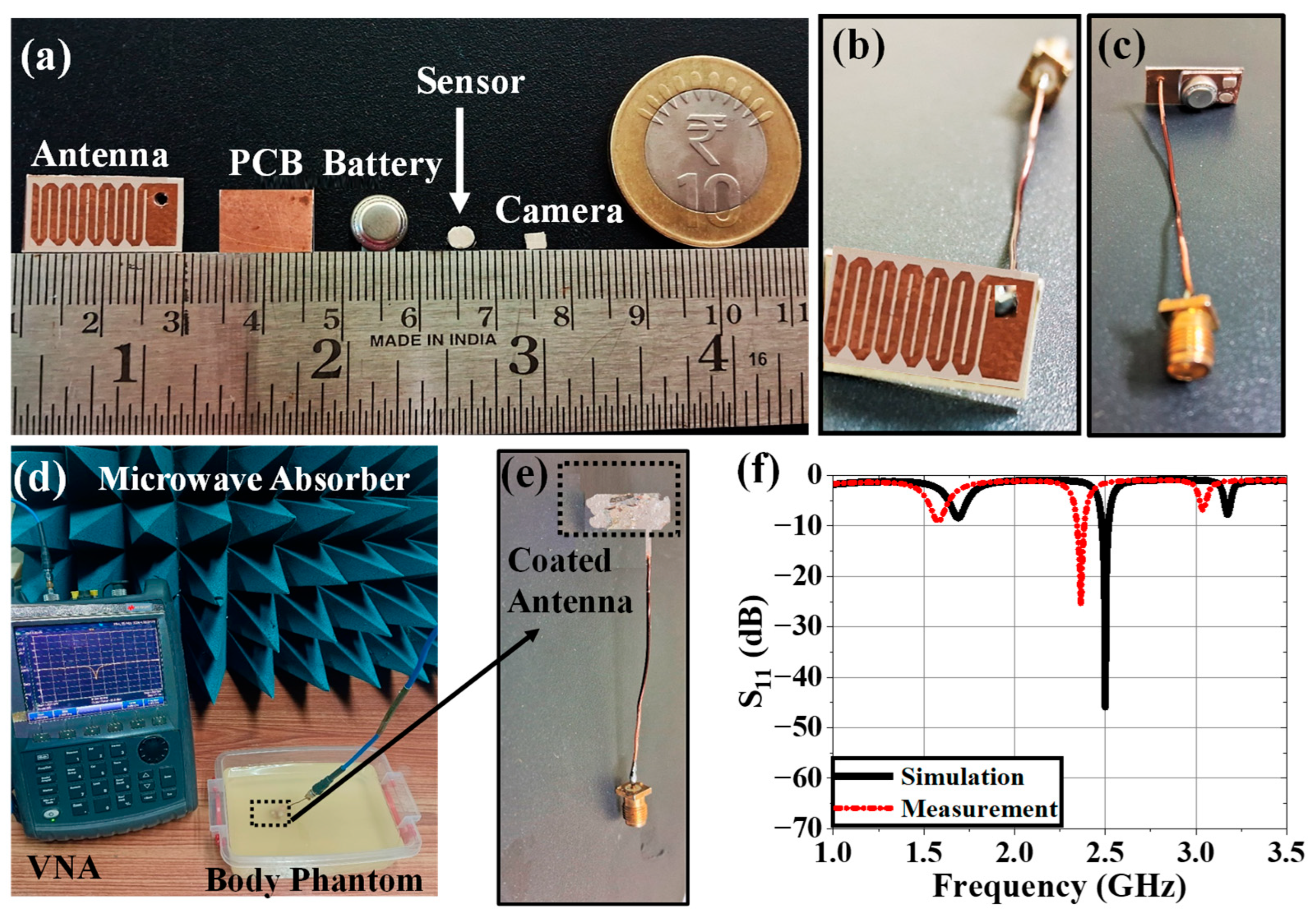
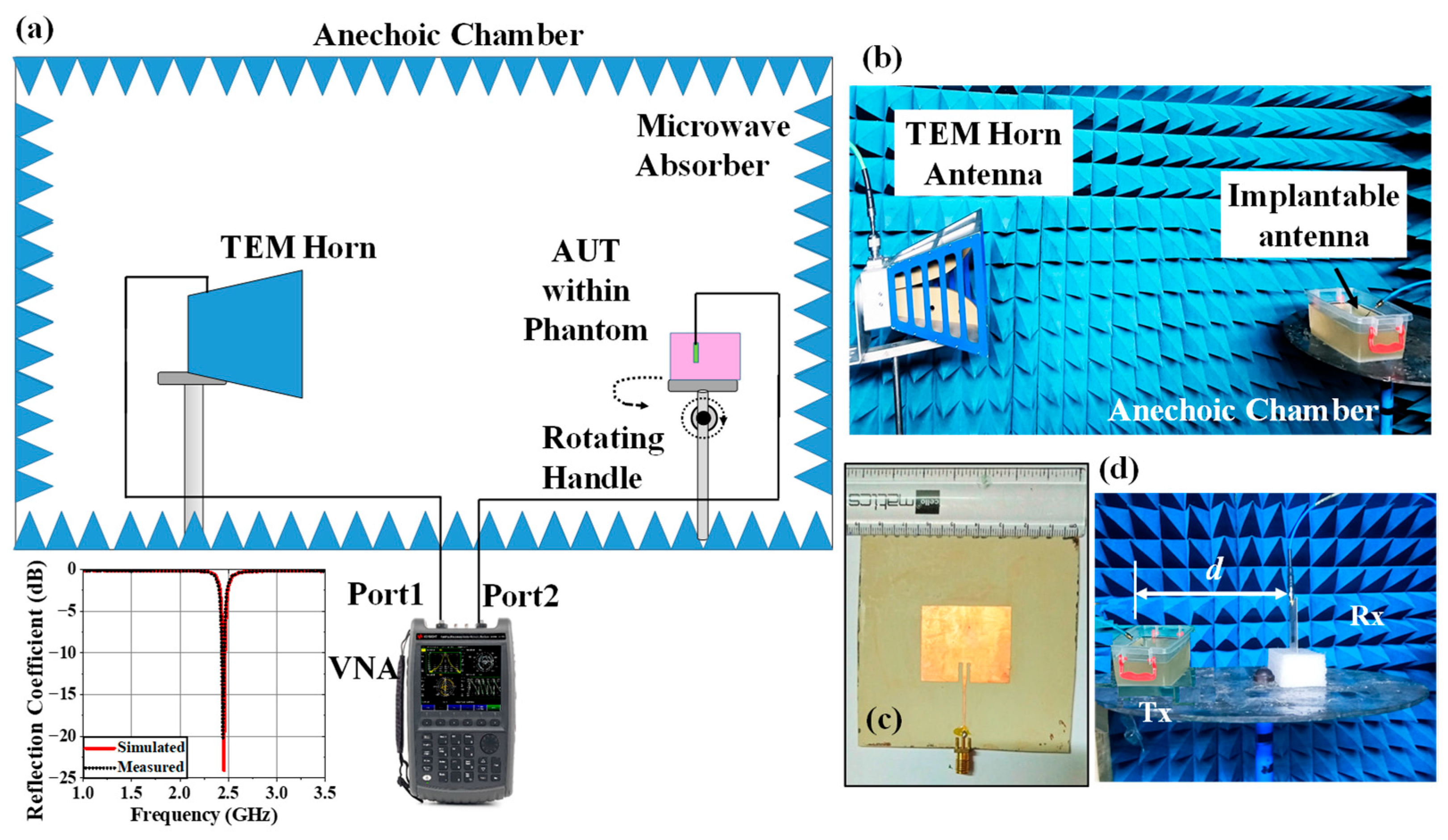
4. Experimental Setup and Measurement
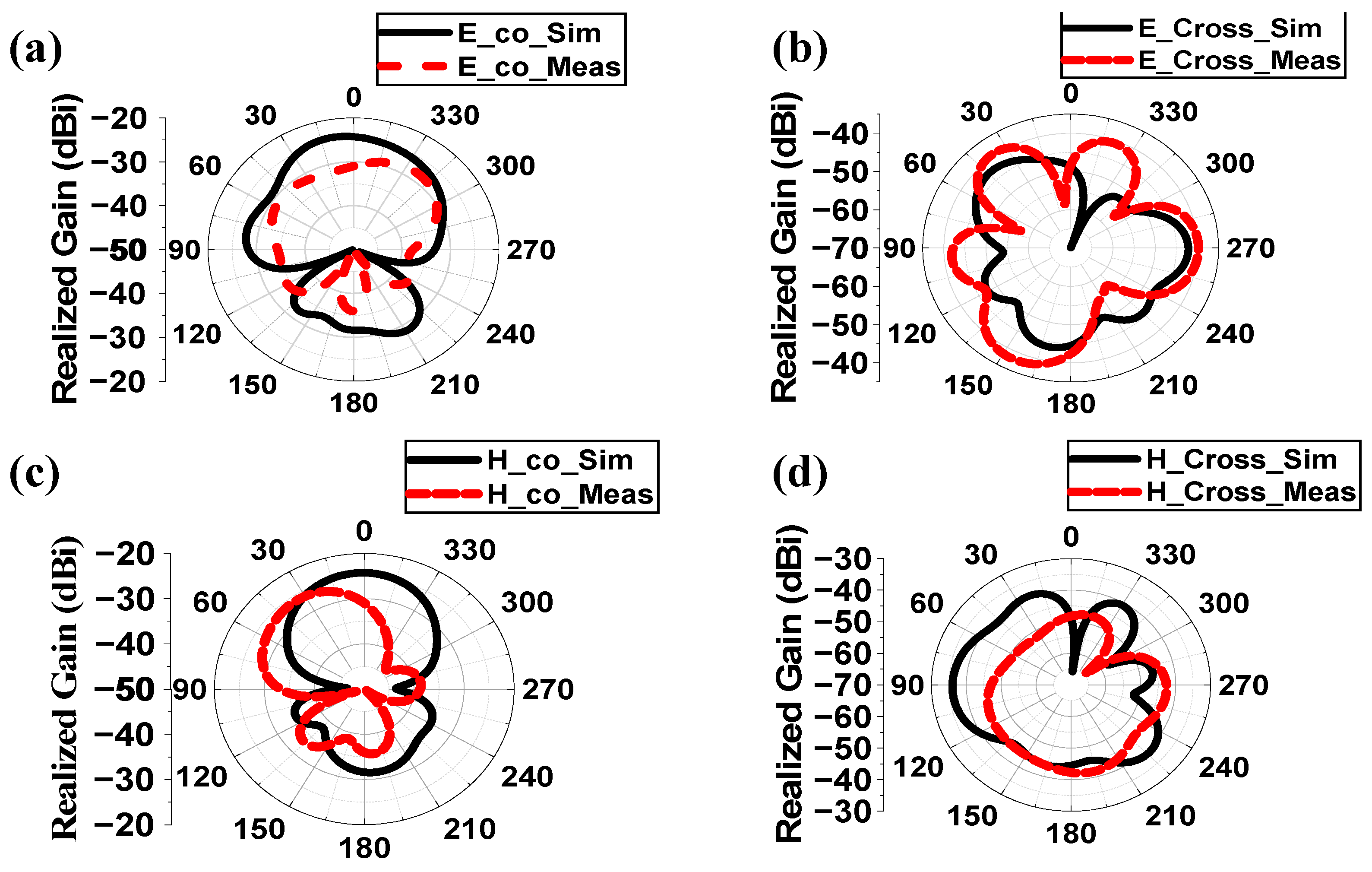
4.1. Implantable Antenna System
4.2. Monitoring Antenna
4.3. Variation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, M.S.; Ghosh, J.; Ghosh, S.; Sarkhel, A. Miniaturized Dual-Antenna System for Implantable Biotelemetry Application. IEEE Antennas Wirel. Propag. Lett. 2021, 20, 1394–1398. [Google Scholar] [CrossRef]
- Dixon, A.M.R.; Allstot, E.G.; Gangopadhyay, D.; Allstot, D.J. Compressed Sensing System Considerations for ECG and EMG Wireless Biosensors. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Kiourti, A.; Nikita, K.S. Numerical Assessment of the Performance of a Scalp-Implantable Antenna: Effects of Head Anatomy and Dielectric Parameters. Bioelectromagnetics 2012, 34, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rahmat-Samii, Y. Implanted antennas inside a human body: Simulations, designs, and characterizations. IEEE Trans. Microw. Theory Tech. 2004, 52, 1934–1943. [Google Scholar] [CrossRef]
- Hayat, S.; Shah, S.A.A.; Yoo, H. Miniaturized Dual-Band Circularly Polarized Implantable Antenna for Capsule Endoscopic System. IEEE Trans. Antennas Propag. 2021, 69, 1885–1895. [Google Scholar] [CrossRef]
- Crumley, G.C.; Evans, N.E.; Burns, J.B.; Trouton, T.G. On the design and assessment of a 2.45 GHz radio telecomm and system for remote patient monitoring. Med. Eng. Phys. 1999, 20, 750–755. [Google Scholar] [CrossRef]
- McIntosh, R.L.; Iskra, S.; McKenzie, R.J.; Chambers, J.; Metzenthen, B.; Anderson, V. Assessment of SAR and thermal changes near a cochlear implant system for mobile phone type exposures. Bioelectromagnetics 2007, 29, 71–80. [Google Scholar] [CrossRef]
- Ghosh, S.; Kundu, A.; Gupta, B. Slot based Miniaturized Human Body Implantable Antenna Design at 2.45 GHz ISM Band. In Proceedings of the 2022 IEEE Wireless Antenna and Microwave Symposium, Rourkela, India, 5–8 June 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Yeap, K.; Voon, C.; Hiraguri, T.; Nisar, H. A compact dual-band implantable antenna for medical telemetry. Microw. Opt. Technol. Lett. 2019, 61, 2105–2109. [Google Scholar] [CrossRef]
- Karacolak, T.; Hood, A.Z.; Topsakal, E. Design of a Dual—Band Implantable Antenna and Development of Skin Mimicking Gels for Continuous Glucose Monitoring. IEEE Trans. Microw. Theory Tech. 2008, 56, 1001–1008. [Google Scholar] [CrossRef]
- Yilmaz, T.; Karacolak, T.; Topsakal, E. Characterization and Testing of a Skin Mimicking Material for Implantable Antennas Operating at ISM Band (2.4 GHz–2.48 GHz). IEEE Antennas Wirel. Propag. Lett. 2008, 7, 418–420. [Google Scholar] [CrossRef]
- Zada, M.; Yoo, H. A Miniaturized Triple—Band Implantable Antenna System for Bio -Telemetry Applications. IEEE Trans. Antennas Propag. 2018, 66, 7378–7382. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 KHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, R.F., Jr.; Sylvar, D.M.; Ulcek, J.L. Evaluating compliance with FCC guidelines for human exposure to radiofrequency electromagnetic fields. FCC OET Bull. 1997, 65, 1–57. [Google Scholar]
- Department of Telecommunication (DoT). A Journey for EMF. 2012. Available online: https://dot.gov.in/sar-mobile-phones (accessed on 14 April 2025).
- Le, T.T.; Kim, Y.-D.; Yun, T.-Y. A Triple-Band Dual-Open-Ring High-Gain High-Efficiency Antenna for Wearable Applications. IEEE Access 2021, 9, 118435–118442. [Google Scholar] [CrossRef]
- Fear, E.C.; Meaney, P.M.; Stuchly, M.A. Microwaves for breast cancer detection? IEEE Potentials 2003, 22, 12–18. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kopera, D. Impact of Testosterone on Hair and Skin. Endocrinol. Metab. Syndr. 2015, 4, 1–4. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Li, Y. Adipose Tissue Aging and Metabolic Disorder, and the impact of Nutritional Inventions. Nutrients 2022, 14, 3134. [Google Scholar] [CrossRef]
- Walia, N.S.; Raj, R.; Tak, C.S. Gottron’s syndrome. Indian J. Dermatol. Venereol. Lepr. 2001, 67, 211. [Google Scholar]
- Szewc, M.; Sitarz, R.; Moroz, N.; Maciejewski, R.; Wierzbicki, R. Madelung’s disease—Progressive, excessive, and symmetrical deposition of adipose tissue in the subcutaneous layer: Case report and literature review. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 819–825. [Google Scholar] [CrossRef]
- Ghodgaonkar, D.K.; Gandhi, O.P.; Iskander, M.F. Complex permittivity of human skin in vivo in the frequency band 26.5–60 GHz. In Proceedings of the IEEE Antennas and Propagation Society International Symposium. Transmitting Waves of Progress to the Next Millennium. 2000 Digest. Held in Conjunction with: USNC/URSI National Radio Science Meeting (C), Salt Lake City, UT, USA, 16–21 July 2000; Volume 2, pp. 1100–1103. [Google Scholar]
- Hwang, H.; Yim, J.; Cho, J.-W.; Cheon, C.; Kwon, Y. 110 GHz broadband measurement of permittivity on human epidermis using 1 mm coaxial probe. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, 2003, Philadelphia, PA, USA, 8–13 June 2003; Volume 1, pp. 399–402. [Google Scholar]
- Boric-Lubecke, O.; Nikawa, Y.; Snyder, W.; Lin, J.; Mizuno, K. Novel microwave and millimeter-wave biomedical applications. In Proceedings of the 4th International Conference on Telecommunications in Modern Satellite, Cable and Broadcasting Services. TELSIKS’99 (Cat. No.99EX365), Nis, Yugoslavia, 13–15 October 1999; pp. 186–193. [Google Scholar]
- Peyman, A.; Kos, B.; Djokic, M.; Trotovsek, B.; Limbaeck-Stokin, C.; Sersa, G.; Miklavcic, D. Variation in Dielectric Properties Due to Pathological Changes in Human Liver. Bioelectromagnetics 2015, 36, 603–612. [Google Scholar] [CrossRef]
- Johnson, J. Statistical Analysis of Detuning Effects for Implantable Microstrip Antennas. Ph.D. Thesis, University of Utah, Salt Lake City, UT, USA, 2007. [Google Scholar]
- Vidal, N.; Curto, S.; Lopez Villegas, J.M.; Sieiro, J.; Ramos, F.M. Detuning study of implantable antennas inside the human body. Prog. Electromagn. Res. 2012, 124, 265–283. [Google Scholar] [CrossRef]
- Ekpo, S.C.; George, D. Impact of Noise Figure on a Satellite Link Performance. IEEE Commun. Lett. 2011, 15, 977–979. [Google Scholar] [CrossRef]
- Ekpo, S.C. Parametric System Engineering Analysis of Capability-Based Small Satellite Missions. IEEE Syst. J. 2019, 13, 3546–3555. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Tiwari, R.-N.; Choi, H.C.; Kim, K.W. On body and off body communication using a compact wideband and high gain wearable textile antenna. Sci. Rep. 2024, 14, 14493. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Li, H.; Ding, X.; Shao, W.; Xiao, S. Compact and Broadband Circularly Polarized Implantable Antenna for Wireless Implantable Medical Devices. IEEE Antennas Wirel. Propag. Lett. 2023, 22, 1236–1240. [Google Scholar] [CrossRef]
- Fang, X.; Du, X.; Bärhold, M.; Wang, Q.; Plettemeier, D. MICS-Band Helical Dipole Antenna for Biomedical Implants. IEEE Antennas Wirel. Propag. Lett. 2022, 21, 2502–2506. [Google Scholar] [CrossRef]
- Ali, R.; Cho, Y.; Shah, I.A.; Hayat, S.; Basir, A.; Yoo, H. Compact Dual-Band MIMO Implantable Antenna System for High-Data-Rate Cortical Visual Prostheses Applications. IEEE Trans. Antennas Propag. 2024, 72, 6374–6386. [Google Scholar] [CrossRef]
- Ashvanth, B.; Partibane, B. Miniaturized dual wideband MIMO antenna for implantable biomedical applications. Microw. Opt. Technol. Lett. 2023, 65, 3296–3302. [Google Scholar] [CrossRef]
- Perez, M.D.; Jeong, S.H.; Raman, S.; Nowinski, D.; Wu, Z.; Redzwan, S.; Velander, J.; Peng, Z.; Hjort, K.; Augustine, R. Head-compliant microstrip split ring resonator for noninvasive healing monitoring after craniosynostosis-based surgery. Healthc. Technol. Lett. 2020, 7, 29–34. [Google Scholar] [CrossRef]
- Iqbal, A.; Al-Hasan, M.; Mabrouk, I.B.; Denidni, T.A. Deep-Implanted MIMO Antenna Sensor for Implantable Medical Devices. IEEE Sens. J. 2023, 23, 2105–2112. [Google Scholar] [CrossRef]
- Iqbal, A.; Sura, P.R.; Smida, A.; Al-Hasan, M.; Ben Mabrouk, I.; Denidni, T.A. Dual-Band 3-D Implantable MIMO Antenna for IoT-Enabled Wireless Capsule Endoscopy. IEEE Internet Things J. 2024, 11, 31385–31393. [Google Scholar] [CrossRef]
- Vidal, N.; Garcia-Miquel, A.; Lopez-Villegas, J.M.; Sieiro, J.J.; Ramos, F.M. Influence of phantom models on implantable antenna performance for biomedical applications. In Proceedings of the 2015 9th European Conference on Antennas and Propagation (EuCAP), Lisbon, Portugal, 13–17 April 2015; pp. 1–4. [Google Scholar]
- Patra, K. Analytical Modelling of Microstrip Travelling Wave Antennas. Ph.D. Dissertation, Electronics and Tele-Communication Engineering, Jadavpur University, Kolkata, India, 2018. [Google Scholar]
- IEEE Std C95.1-2019; IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz—Redline. IEEE Standards Association: Piscataway, NJ, USA, 2019.
- Hasgall, P.A.; Gennaro, F.-D.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenbock, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Version 4.1. 2022. Available online: https://itis.swiss/virtual-population/tissue-properties/database/dielectric-properties (accessed on 14 April 2025). [CrossRef]
- Liu, C.; Guo, Y.-X.; Xiao, S. Capacitively Loaded Circularly Polarized Implantable Patch Antenna for ISM Band Biomedical Applications. IEEE Trans. Antennas Propag. 2014, 62, 2407–2417. [Google Scholar] [CrossRef]
- Bahrami, H.; Mirbozorgi, S.A.; Rusch, L.A.; Gosselin, B. Biological Channel Modeling and Implantable UWB Antenna Design for Neural Recording Systems. IEEE Trans. Biomed. Eng. 2015, 62, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.A.; Basir, A.; Lim, Y.-H.; Yoo, H. A Novel Efficient Wirelessly Powered Biotelemetric Endovascular Aortic Stent Antenna System. IEEE Trans. Antennas Propag. 2023, 71, 7132–7145. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, S.; Ghosh, S.; Gupta, B. Impact of Electrical Properties Variations of Human-Body Phantom on Implantable Antenna Performance During In-Vitro Measurement. IETE J. Res. 2025, 1–11. [Google Scholar] [CrossRef]

| Ref. | Freq. (GHz) | S11 (dB) | Gain (dBi) | SAR_1 g (1 W) (W/kg) | CLM (dB) | DLM (dB) | Comm. Range (m) | Uncertain Parameters | Samples | Variation Analysis Techniques | Variation in Parameters Tested |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [32] | 2.4 | −25 | −24.7 | 697.5 | No | No | 17 (7 kbps), 4.3 (100 kbps) and 1.4 (1 Mbps) | No | No | No | No |
| [33] | 0.4 | −20 | −18.9 | No | No | No | No | No | No | No | No |
| [34] | 0.915, 2.45 | −25, −40 | −30.47, −24.71 | 658, 589 | No | No | 2 | No | No | No | No |
| [35] | 0.915, 2.45 | −20, −37 | −36, −30.1 | 333 | No | No | No | No | No | No | No |
| [37] | 0.915 | −20 | −23.23 | 270.3 | No | No | 13 | Effective permittivity | 6 | Cartesian plot | Sensing Performance |
| [38] | 0.915, 2.45 | −19, −15 | −26.30, −20.9 | 306.19, 252.36 | No | No | 8 | No | No | No | No |
| [39] | 0.402, 2.45 | No | −37, −24.5 | No | No | No | No | Phantom Size | 2 | Cartesian plot | Gain, efficiency |
| [36] | 2.45 | −11 | No | No | No | No | No | Relative Permittivity | 6 | Cartesian plot | S11 and frequency |
| This Work | 2.5 | −45.9 | −38.42 | 220.26 | 20.73 (d = 1 m, Ts = 13 K) (First) | 9.28 (d = 1 m, Ts = 13 K) (First) | 15 (7 kbps), 10 (100 kbps) and 3.5 (1 Mbps) | Effective permittivity and Conductivity | 2500 | ANN modeling (First) | CLM, DLM, bandwidth and SAR performance (First) |
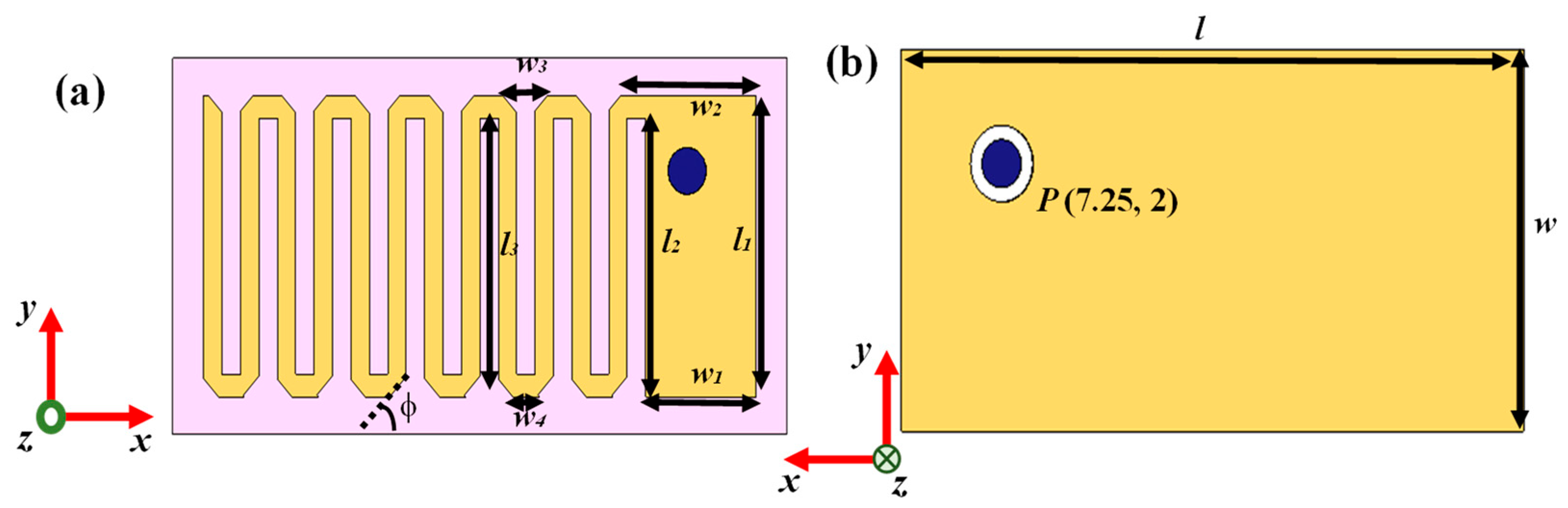
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| l | 20 mm | w2 | 4.397 mm |
| w | 10 mm | w3 | 1.46 mm |
| l1 | 8 mm | w4 | 0.86 mm |
| w1 | 3.6 mm | φ | 45° |
| l2 | 7.4 mm | P | (7.25 mm, 2 mm) |
| Layer | Relative Permittivity | Conductivity (S/m) |
|---|---|---|
| Skin | 3.8 | 1.46 |
| Fat | 5.28 | 0.1 |
| Muscle | 54.8 | 2.26 |
| Cortical Bone | 11.4 | 0.39 |
| Cancellous Bone | 36.2 | 1.21 |
| Parameters | Variable | Values | |
|---|---|---|---|
| Transmitter | Frequency | fr | 2.5 GHz |
| Transmitted Power | PTX | 8.45 dBm | |
| Tx Antenna Gain | GTX | −38.42 dBi | |
| Receiver | Receiving Antenna Gain | GRX | 4.95 dBi |
| Polarization | P | LP | |
| Temperature | To | 293 K | |
| Boltzmann Constant | K | 1.38 × 10−23 | |
| Noise Power Density | No | 199.95 dB/Hz | |
| Signal Quality | Distance | d | 1–15 m |
| Ideal-BPSK | Eb/No | 9.6 dB | |
| Coding Gain | GC | 0 | |
| Fixing Deterioration | GD | 2.5 dB |
| Effective Properties | Simulation | ANN | Measurement | % Error of Prediction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ɛeff | σeff | BW (%) | CLM (dB) | DLM (dB) | BW (%) | CLM (dB) | DLM (dB) | BW (%) | CLM (dB) | DLM (dB) | BW | CLM | DLM | |||
| Sim | Meas | Sim | Meas | Sim | Meas | |||||||||||
| 44.13 | 2.10 | 6.18 | 13.86 | 6.12 | 6.26 | 13.84 | 6.08 | 5.27 | 13.48 | 6.21 | 0.01 | 0.16 | 0.08 | 4.47 | 0.66 | 2.14 |
| 46.02 | 0.92 | 5.58 | 26.06 | 16.98 | 5.62 | 26.02 | 17.57 | 4.96 | 25.97 | 17.24 | 0.01 | 0.12 | 0.14 | 0.27 | 3.36 | 1.88 |
| 46.02 | 3.56 | 7.07 | 0.71 | −4.22 | 7.13 | 0.67 | −4.25 | 6.94 | 0.24 | −4.38 | 0.01 | 0.03 | 0.14 | 5.22 | 0.71 | 3.06 |
| 49.80 | 1.80 | 6.79 | 17.11 | 8.94 | 6.85 | 17.02 | 8.97 | 6.48 | 16.85 | 8.85 | 0.01 | 0.05 | 1.52 | 2.12 | 1.79 | 3.36 |
| 51.69 | 1.51 | 6.88 | 19.06 | 9.58 | 6.91 | 18.99 | 9.77 | 6.72 | 18.78 | 9.48 | 0.00 | 0.03 | 0.64 | 1.92 | 1.94 | 2.97 |
| 53.59 | 1.80 | 7.37 | 15.98 | 8.66 | 7.43 | 15.95 | 8.64 | 7.24 | 15.76 | 8.12 | 0.01 | 0.03 | 0.11 | 2.48 | 0.23 | 6.02 |
| 55.48 | 2.10 | 7.37 | 13.46 | 6.54 | 7.43 | 13.44 | 6.47 | 7.19 | 13.37 | 6.78 | 0.01 | 0.03 | 0.08 | 0.64 | 1.08 | 4.79 |
| 57.37 | 0.63 | 7.88 | 28.36 | 20.75 | 7.75 | 28.25 | 20.65 | 7.52 | 28.12 | 20.45 | 0.02 | 0.03 | 1.67 | 1.73 | 0.48 | 0.97 |
| 59.26 | 0.92 | 7.81 | 23.11 | 17.02 | 7.69 | 22.95 | 16.89 | 7.72 | 23.04 | 16.95 | 0.02 | 0.00 | 2.02 | 1.52 | 0.77 | 0.36 |
| 63.04 | 2.97 | 6.72 | 4.26 | 0.65 | 6.89 | 4.21 | 0.67 | 6.65 | 3.97 | 0.64 | 0.02 | 0.03 | 0.27 | 3.34 | 2.99 | 4.48 |
| Sample | Effective Properties | Simulation | ANN | % Error of Prediction w.r.t Simulation | ||||
|---|---|---|---|---|---|---|---|---|
| ɛeff | σeff | 1 g | 10 g | 1 g | 10 g | 1 g | 10 g | |
| 1 | 46.02 | 0.63 | 250.15 | 65.09 | 247.60 | 64.98 | 1.03 | 0.17 |
| 2 | 46.02 | 0.92 | 147.78 | 43.57 | 145.98 | 42.65 | 1.23 | 2.16 |
| 3 | 46.02 | 3.56 | 302.63 | 74.26 | 300.98 | 75.95 | 0.55 | 2.23 |
| 4 | 49.80 | 1.80 | 227.38 | 61.01 | 227.22 | 61.42 | 0.07 | 0.67 |
| 5 | 51.69 | 1.51 | 203.46 | 56.43 | 202.65 | 57.23 | 0.40 | 1.40 |
| 6 | 53.59 | 1.80 | 225.20 | 60.72 | 226.18 | 60.55 | 0.43 | 0.28 |
| 7 | 55.48 | 2.10 | 348.30 | 64.03 | 345.45 | 65.22 | 0.83 | 1.82 |
| 8 | 57.37 | 0.63 | 109.15 | 33.91 | 108.45 | 33.22 | 0.65 | 2.08 |
| 9 | 59.26 | 0.92 | 127.85 | 42.47 | 125.65 | 42.58 | 1.75 | 0.26 |
| 10 | 63.04 | 2.97 | 231.16 | 68.81 | 235.22 | 69.18 | 1.73 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Ekpo, S.C.; Elias, F.; Alabi, S.; Gupta, B. Effects of Dielectric Properties of Human Body on Communication Link Margins and Specific Absorption Rate of Implanted Antenna System. Sensors 2025, 25, 3498. https://doi.org/10.3390/s25113498
Ghosh S, Ekpo SC, Elias F, Alabi S, Gupta B. Effects of Dielectric Properties of Human Body on Communication Link Margins and Specific Absorption Rate of Implanted Antenna System. Sensors. 2025; 25(11):3498. https://doi.org/10.3390/s25113498
Chicago/Turabian StyleGhosh, Soham, Sunday C. Ekpo, Fanuel Elias, Stephen Alabi, and Bhaskar Gupta. 2025. "Effects of Dielectric Properties of Human Body on Communication Link Margins and Specific Absorption Rate of Implanted Antenna System" Sensors 25, no. 11: 3498. https://doi.org/10.3390/s25113498
APA StyleGhosh, S., Ekpo, S. C., Elias, F., Alabi, S., & Gupta, B. (2025). Effects of Dielectric Properties of Human Body on Communication Link Margins and Specific Absorption Rate of Implanted Antenna System. Sensors, 25(11), 3498. https://doi.org/10.3390/s25113498







