Advanced NiCr/NiSi Thin-Film Thermocouples for Precise Temperature Sensing in Lithium-Ion Battery Systems
Abstract
1. Introduction
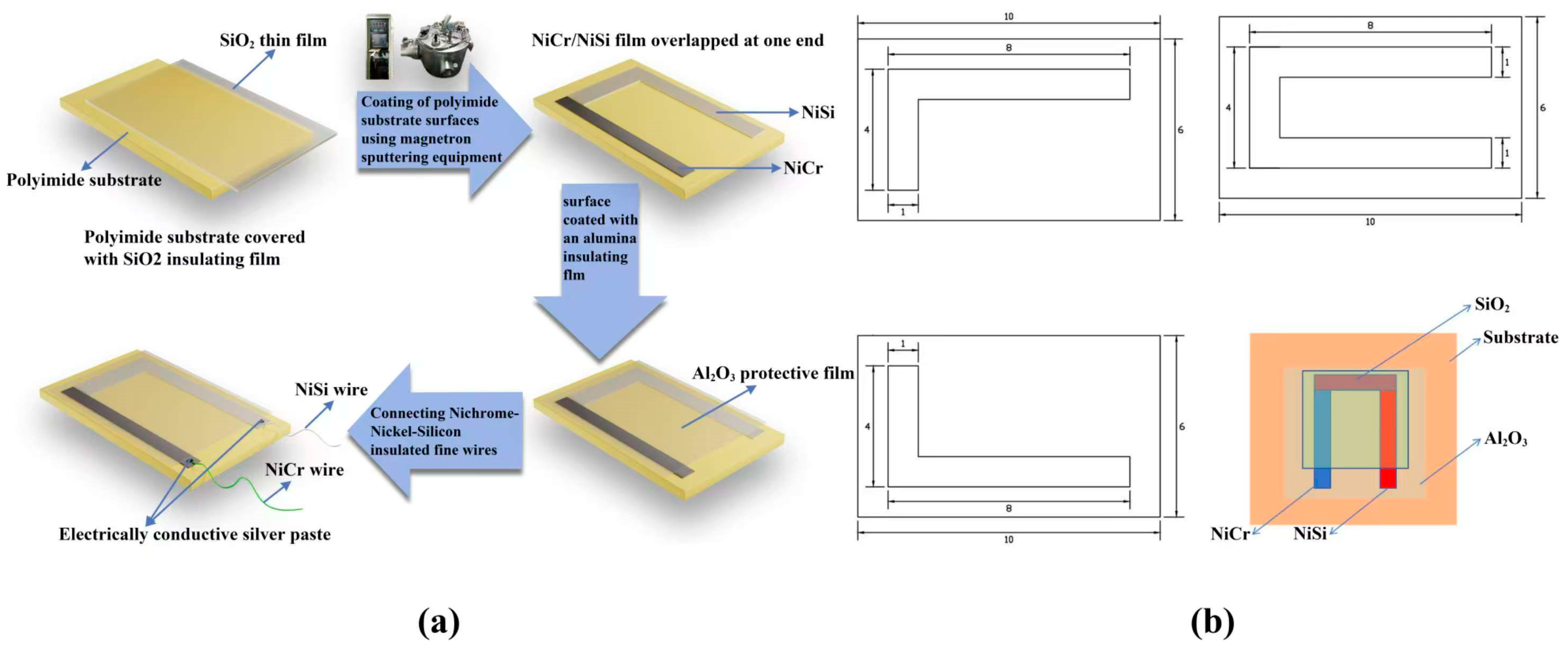
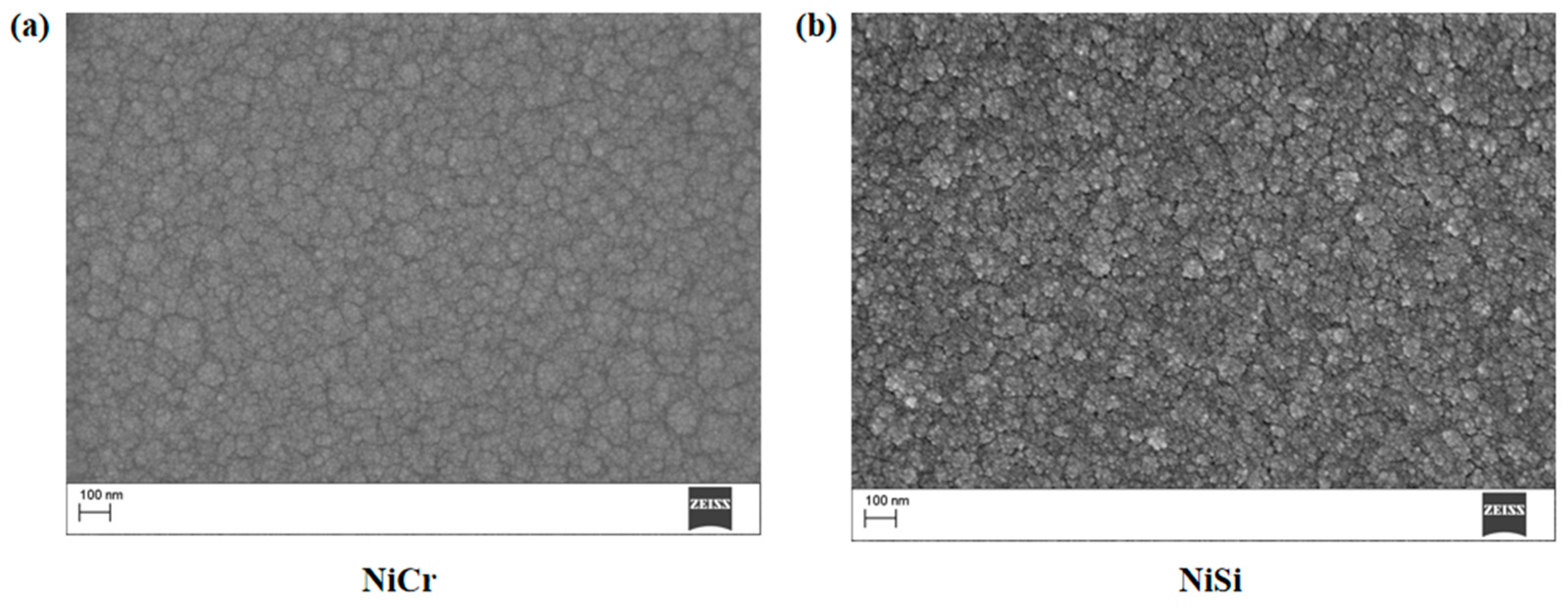
2. Thin-Film Thermocouple Design and Fabrication
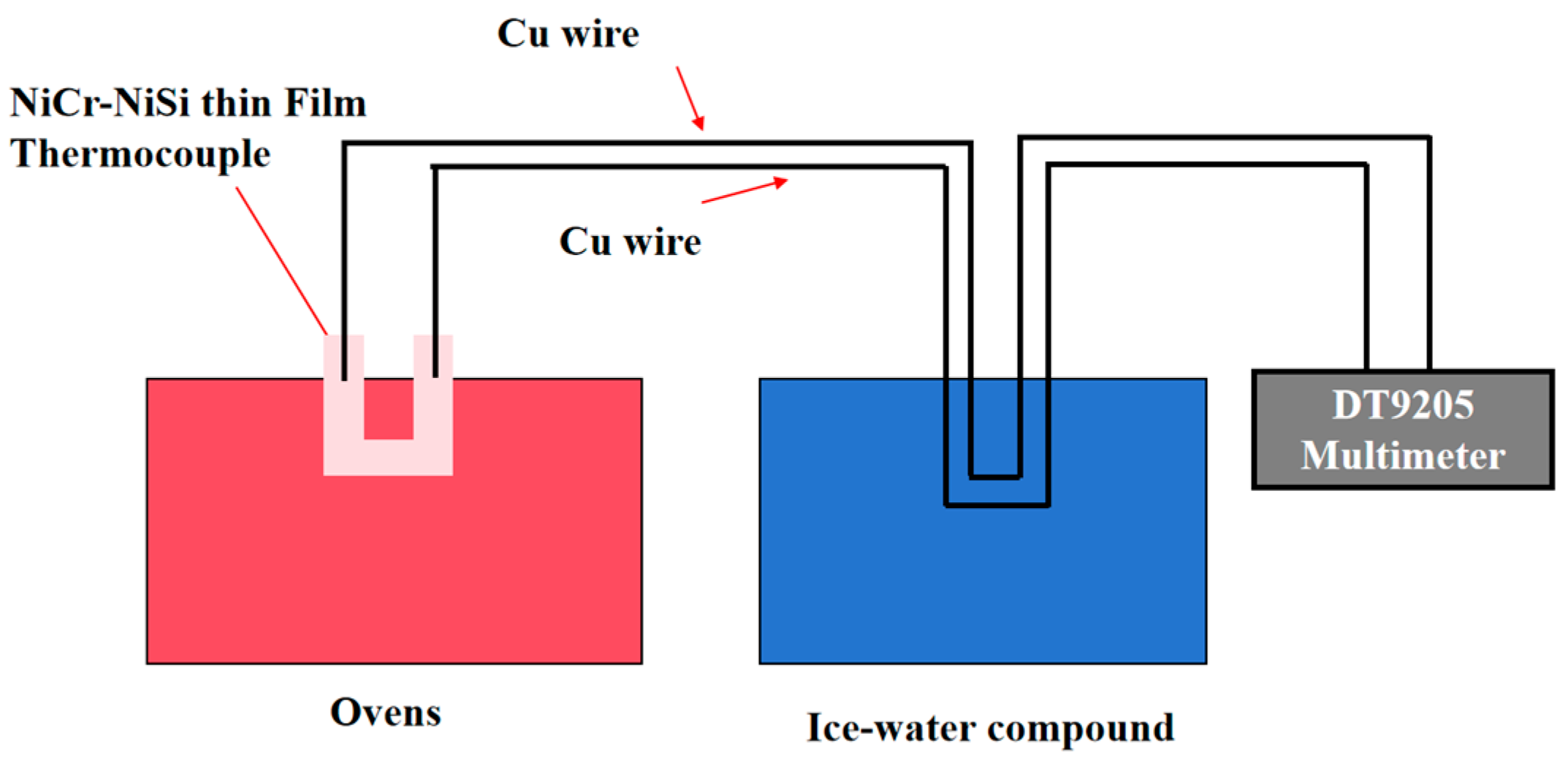
3. Static Calibration of Thin Film Thermocouples
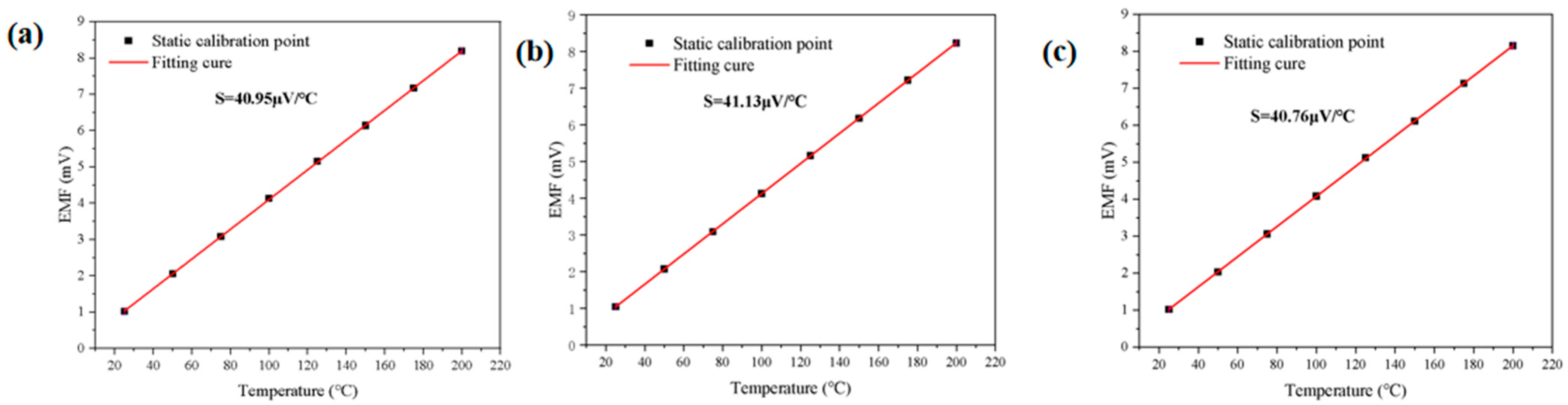
3.1. Thin Film Thermocouple Characterization
3.2. Build an Experimental Platform
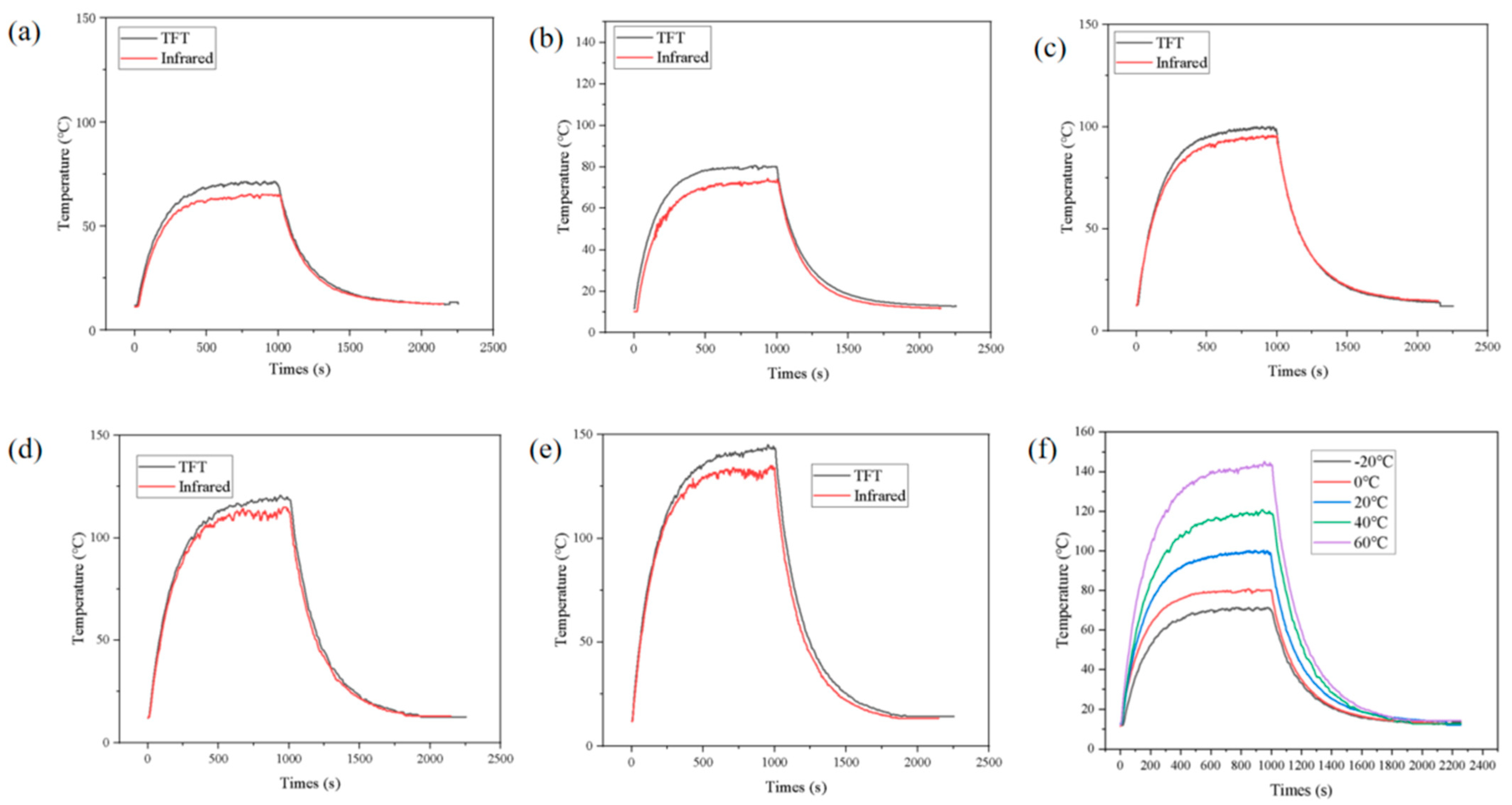
3.3. Repeatability and Consistency
3.3.1. Consistency Test
3.3.2. Repeatable Experiments
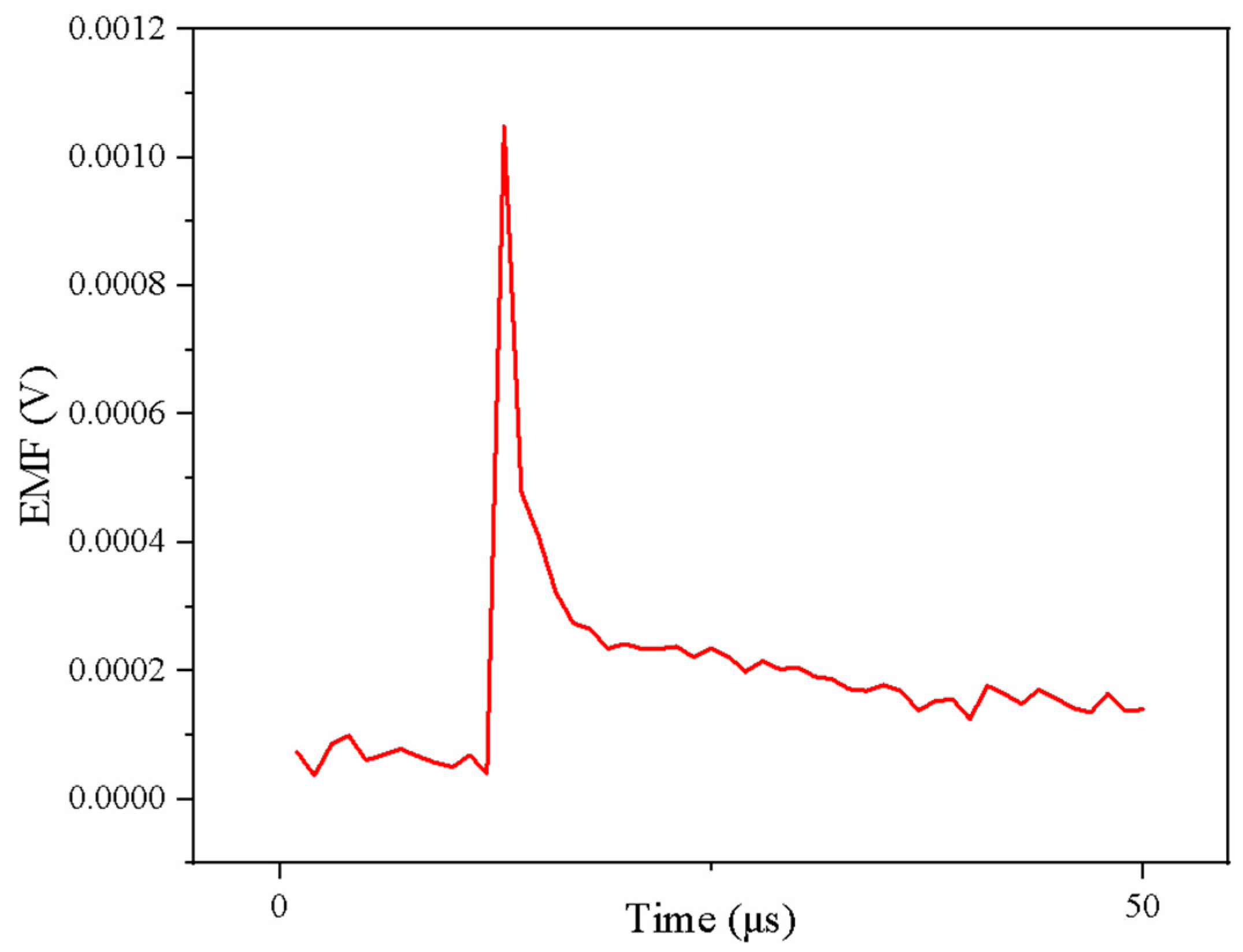
3.4. Dynamic Calibration
4. Temperature Measurement
4.1. Emulate
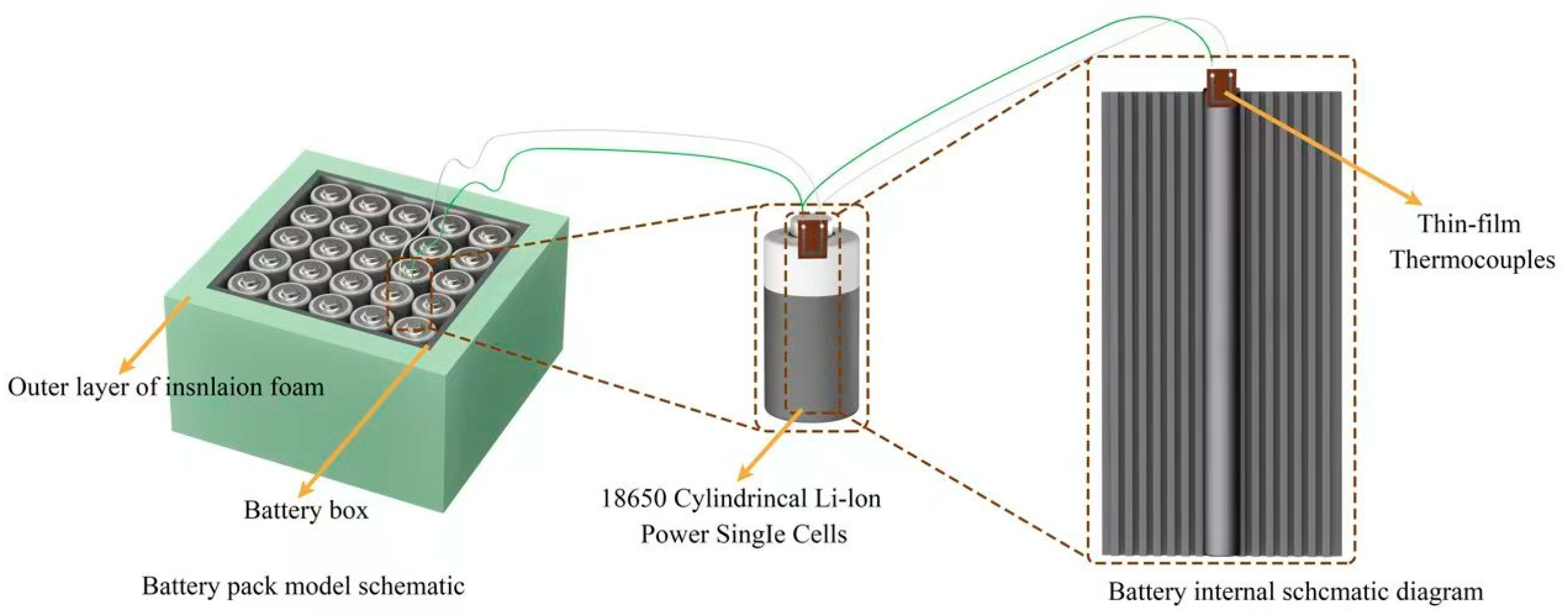
4.1.1. Determining the Topology of the Lithium-Ion Battery Box for the Experiments in This Paper Using Simulation
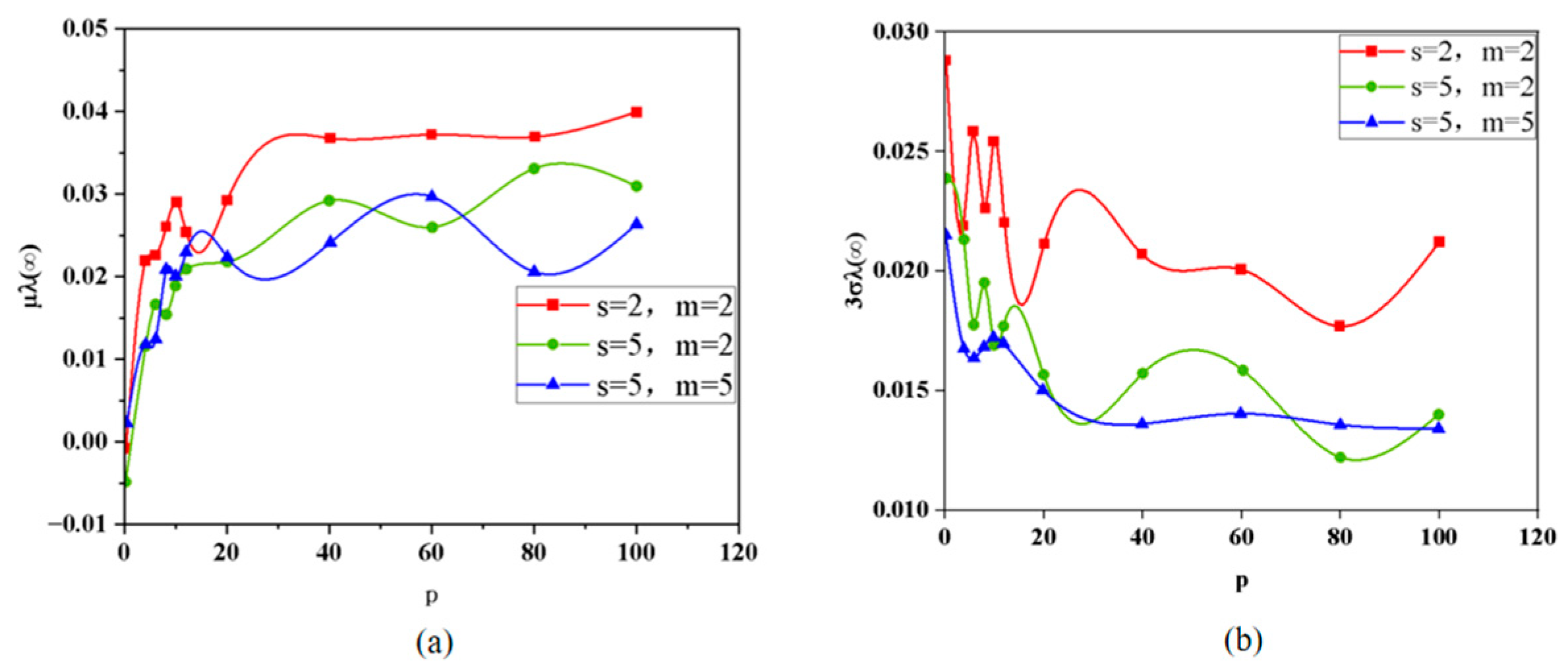
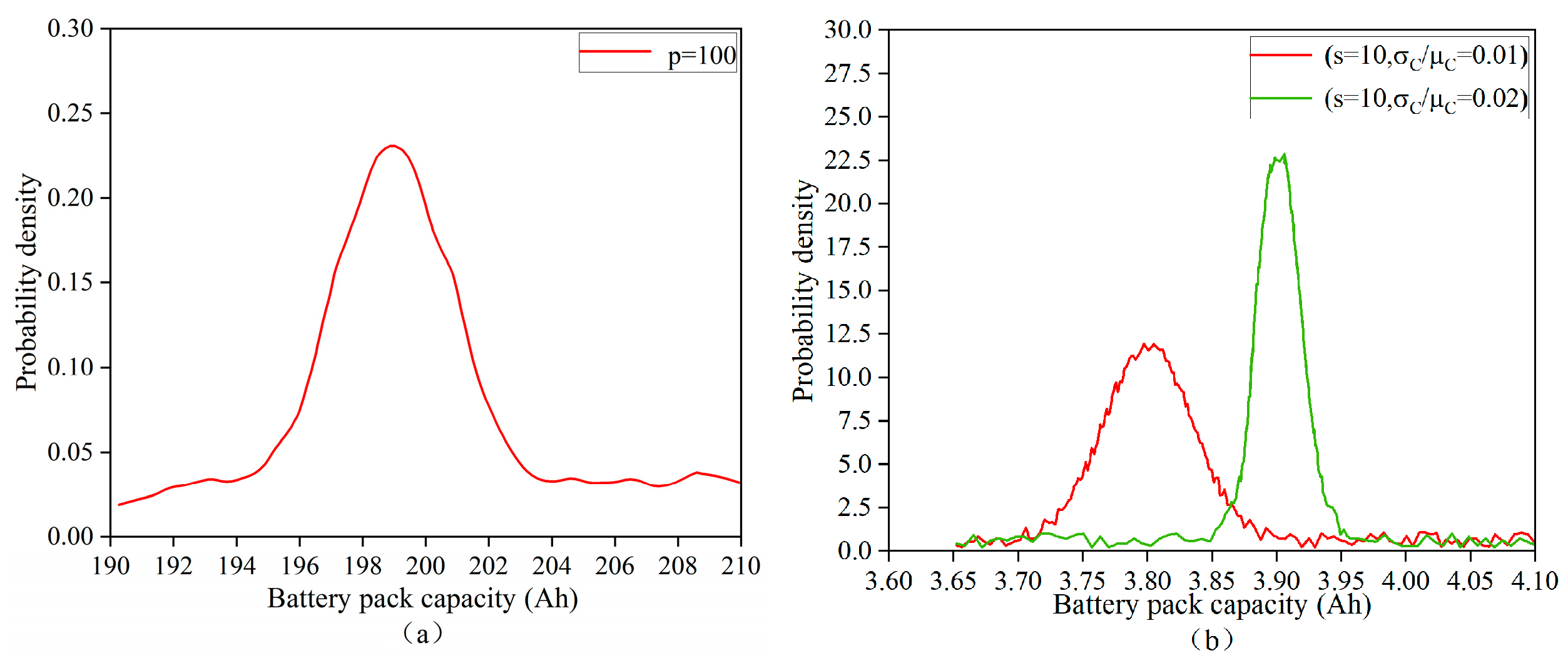
4.1.2. Probability Density Profile of Parallel Battery Pack Capacity
4.1.3. Determine the Optimal Solution for the NMC Battery Pack C
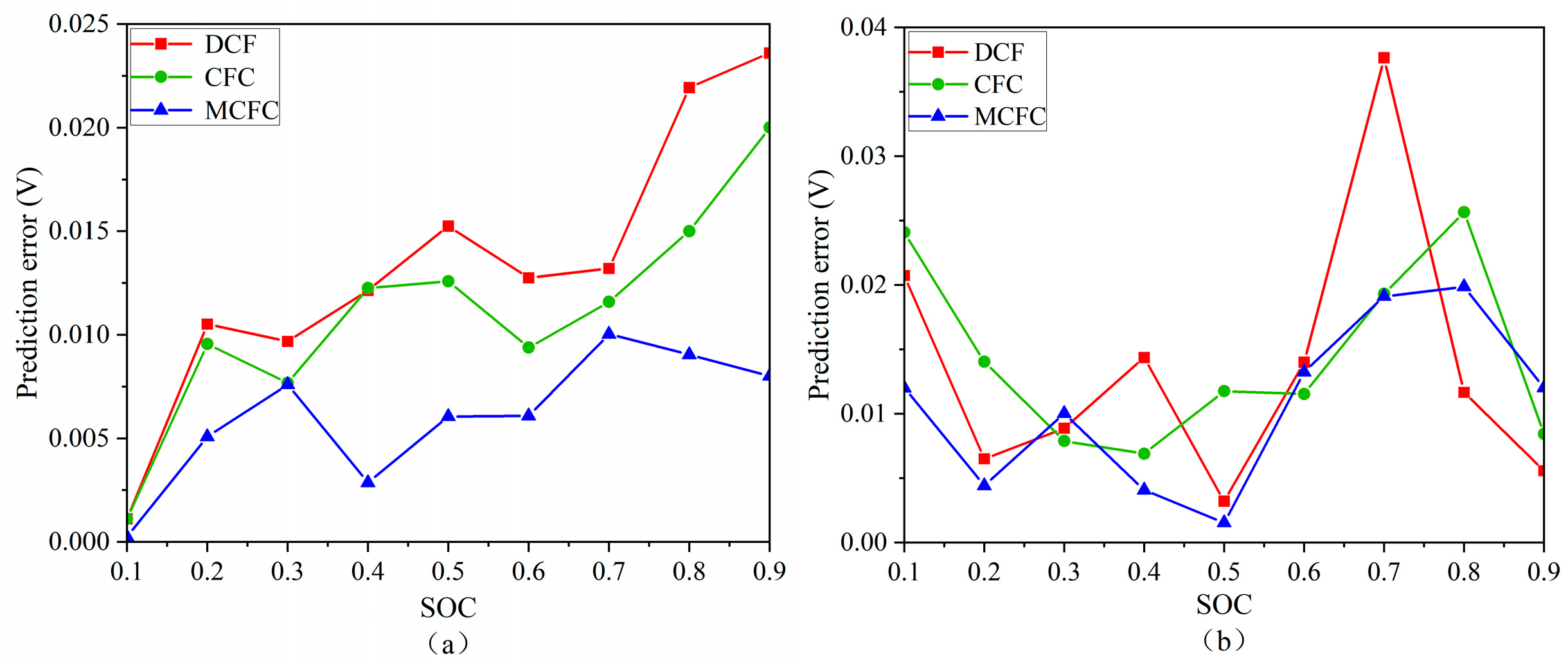
4.1.4. Absolute Errors in Predicting Open-Circuit Voltage of Batteries During Charging and Discharging by Three Methods
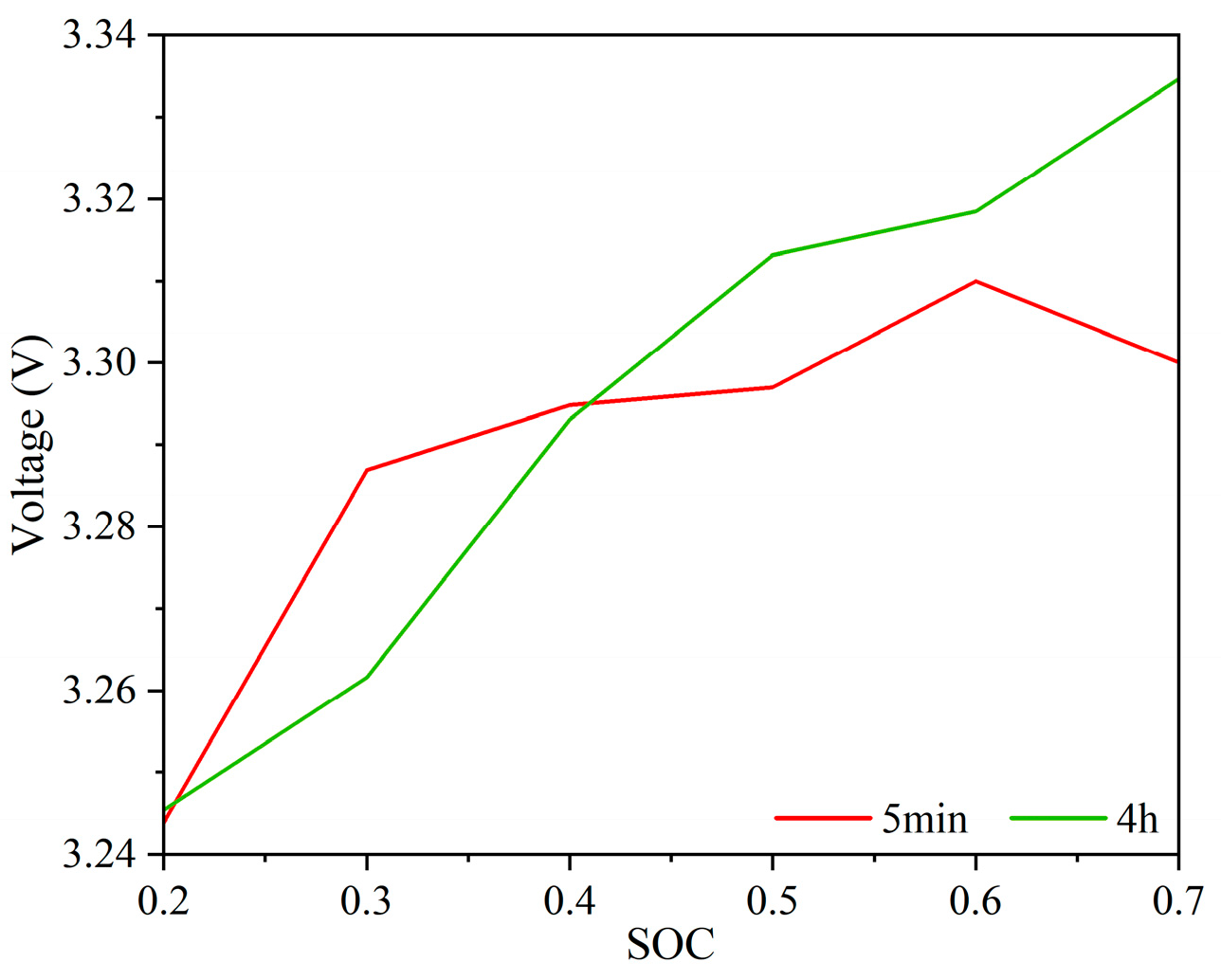
4.1.5. Exploring the Feasibility of Reducing Voltage Test Time with Simulation
4.1.6. Influence of Battery Cell Capacity on Battery Pack Capacity
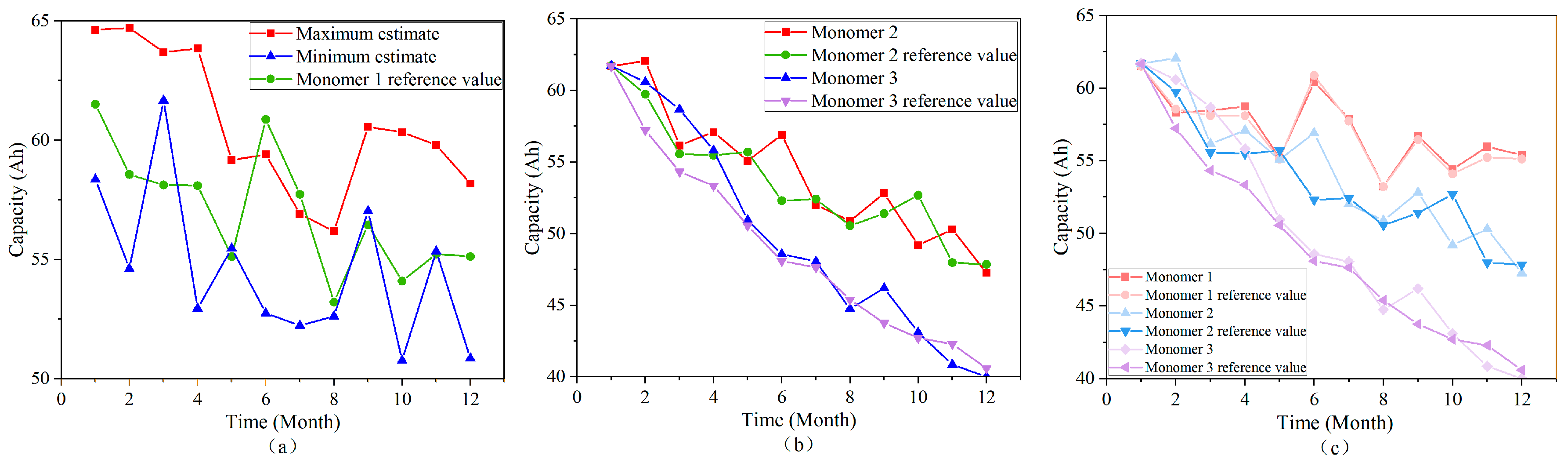
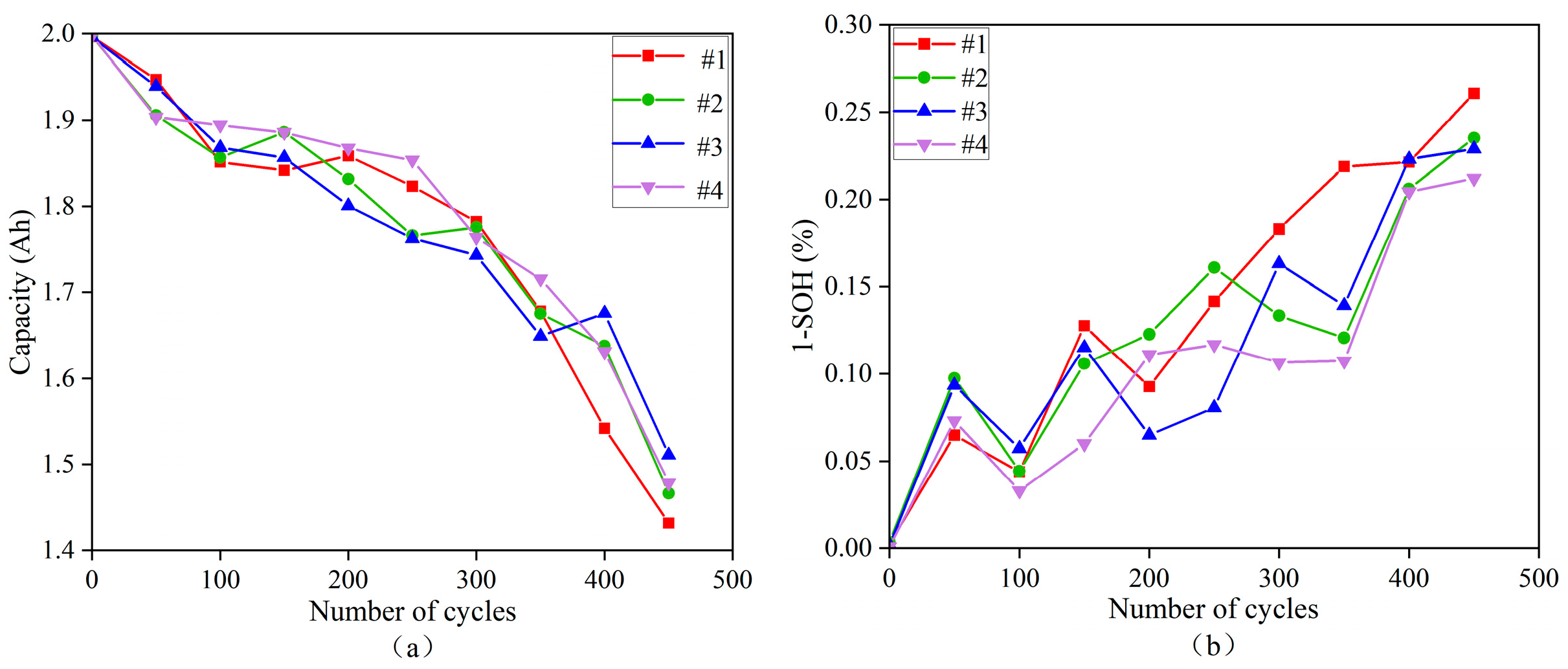
4.1.7. Simulating the Capacity Degradation Process of a Battery Pack
4.1.8. Investigation of Electrochemical Stability of Battery Packs
4.2. Experiments
4.2.1. Construction of the Overall Experimental Platform
4.2.2. Abuse Behavior of Lithium Batteries with Different States of Charge During External Short Circuit
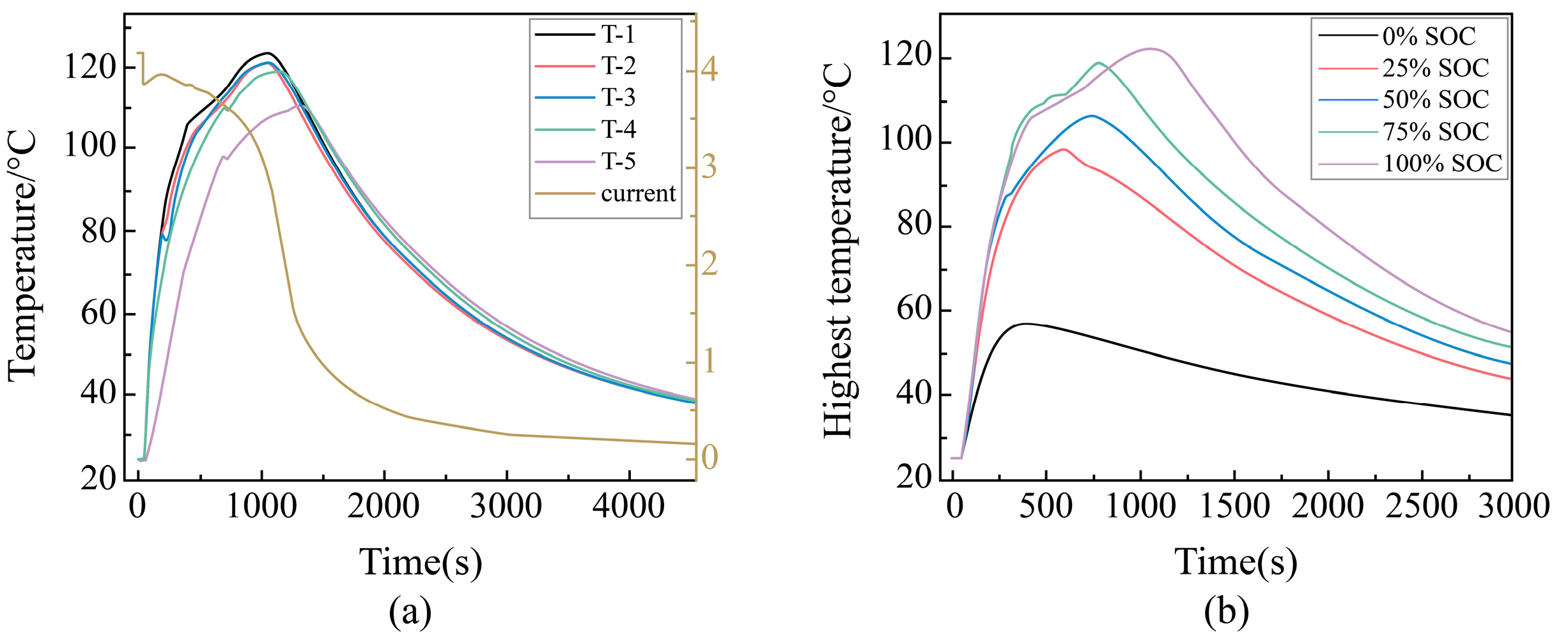
Temperature Change Rule During External Short Circuit of Different SOC Batteries
Maximum Temperature During External Short-Circuit for Different SOC Cells
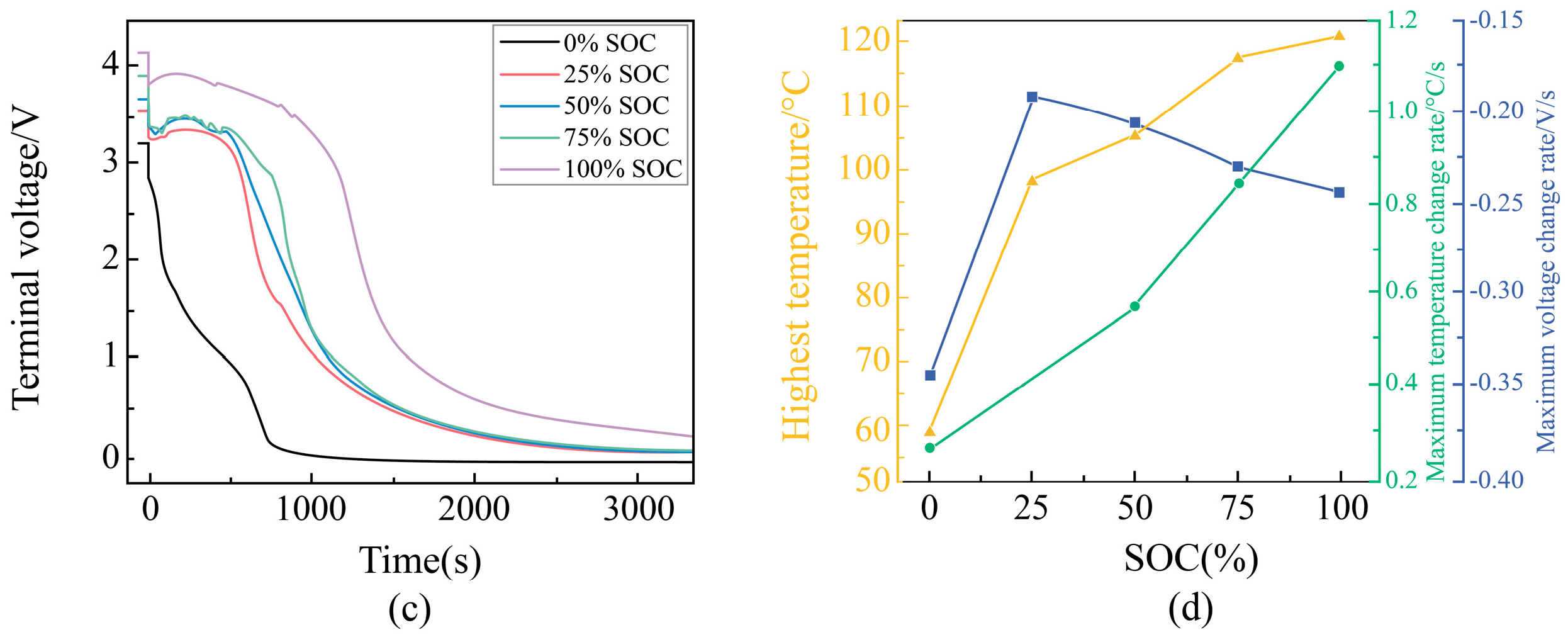
Voltage Change Rule During External Short Circuit of Different SOC Batteries
Change Rule of Characteristic Parameters During External Short Circuit of Different SOC Batteries
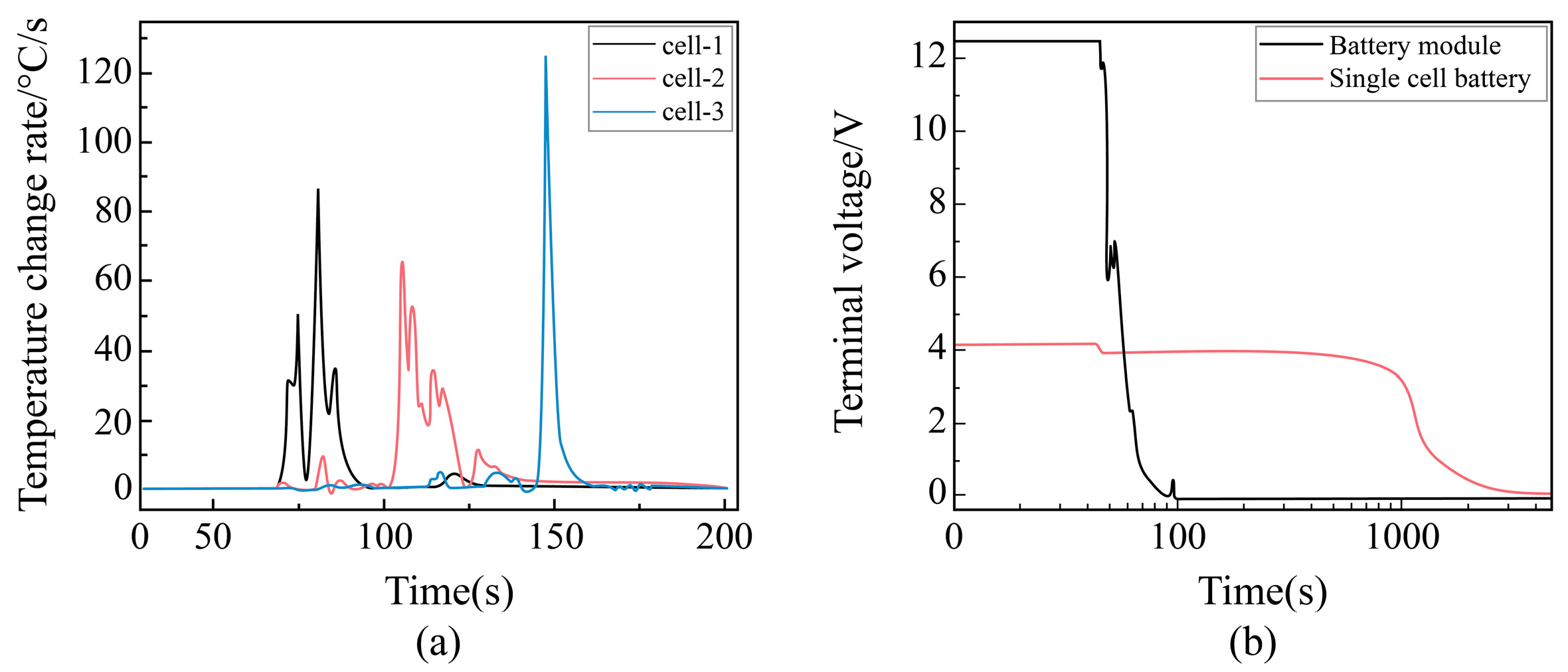
Temperature Change Rule of Each Point with Time During External Short Circuit of Battery Module
4.2.3. Abuse Performance of Lithium Batteries with Different States of Charge During Needling
Abuse Behavior of Lithium Batteries in the Same State of Charge at Various Points During Pinning
Changes of Maximum Temperature During Needling Outside Different SOC Cells
Voltage Change Pattern During External Pinning of Different SOC Cells
Changes of Characteristic Parameters During Needling of Different SOC Cells
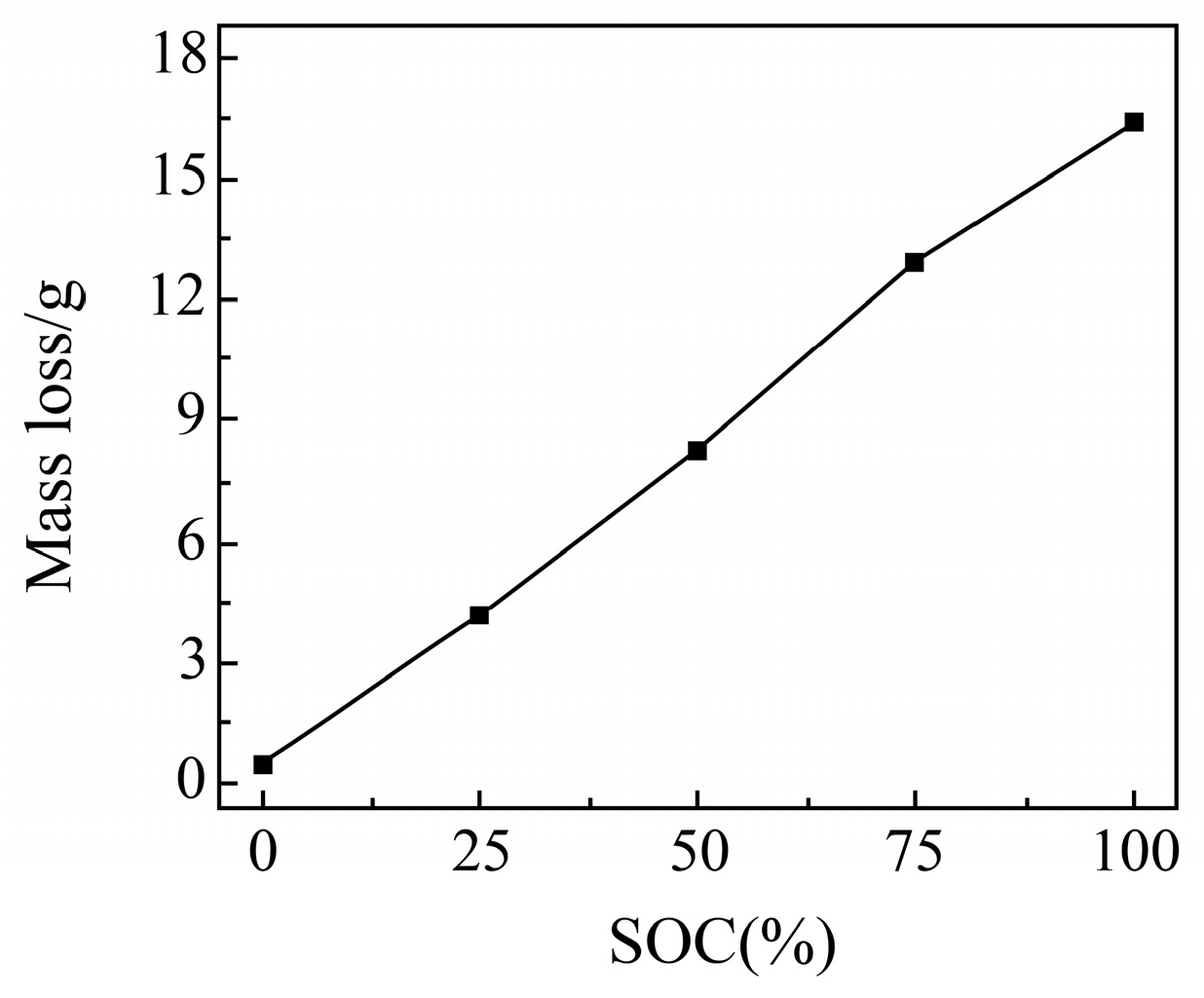
Mass Change Before and After Cell Needling
Change Rule of Temperature Change Rate with Time at Each Point During Pinning of Battery Module
Voltage Change Law with Time at Each Point During Needling
4.3. Influence of Ambient Temperature on the Temperature Variation of the Battery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; He, Y.-Q.; Wang, H.-G.; Wu, R.-F. Low-carbon promotion of new energy vehicles: A quadrilateral evolutionary game. Renew. Sustain. Energy Rev. 2023, 188, 113795. [Google Scholar] [CrossRef]
- Paneerselvam, P.; Narendranathan, S.K.; Suyamburajan, V.; Murugaiyan, T.; Shekhawat, K.S.; Rengasamy, G. A review on recent progress in battery thermal management system in electric vehicle application. Mater. Today Proc. 2024, in press. [Google Scholar] [CrossRef]
- Zhu, X.; Ren, M.; Wu, G.; Pei, J.; Pardalos, P.M. Promoting new energy vehicles consumption: The effect of implementing carbon regulation on automobile industry in China. Comput. Ind. Eng. 2019, 135, 211–226. [Google Scholar] [CrossRef]
- He, M.; Zhou, J.; Liu, L. A study of supporting legal policies for improving China’s new energy automobile industry based on environmental benefits equilibrium-enlightenment from the environmental subsidies of Germany legal system. Int. J. Hydrogen Energy 2017, 42, 18699–18708. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Lee, S.-Y.; Zhang, F.; Xie, J.; Ye, B.; Geng, W.; Xiao, K.; Lee, J.-H.; Park, S.-J.; et al. Dual-strategy-encapsulated phase change materials with thermal immune functions for efficient energy storage and all-climate battery thermal management. Compos. Sci. Technol. 2023, 243, 110256. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, W.; Wei, X. Lithium-ion battery thermal safety evolution during high-temperature nonlinear aging. Fuel 2024, 362, 130845. [Google Scholar] [CrossRef]
- Barcellona, S.; Codecasa, L.; Colnago, S.; Laurano, C.; Piegari, L.; Toscani, S. A technique for separating the impact of cycle aging and temperature on Li-ion battery capacity. J. Energy Storage 2024, 91, 112087. [Google Scholar] [CrossRef]
- Fathoni, A.M.; Putra, N.; Mahlia, T.M.I. A systematic review of battery thermal management systems based on heat pipes. J. Energy Storage 2023, 73, 109081. [Google Scholar] [CrossRef]
- An, Z.; Jia, L.; Ding, Y.; Dang, C.; Li, X. A review on lithium-ion power battery thermal management technologies and thermal safety. J. Therm. Sci. 2017, 26, 391–412. [Google Scholar] [CrossRef]
- Xie, J.; Yang, R.; Gooi, H.B.; Nguyen, H.D. PID-based CNN-LSTM for accuracy-boosted virtual sensor in battery thermal management system. Appl. Energy 2023, 331, 120424. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, R.; Lu, J.; Li, X. Temperature rise prediction of lithium-ion battery suffering external short circuit for all-climate electric vehicles application. Appl. Energy 2018, 213, 375–383. [Google Scholar] [CrossRef]
- Lian, Y.; Chen, X.; Zhang, T.; Liu, C.; Lin, L.; Lin, F.; Li, Y.; Chen, Y.; Zhang, M.; Zhou, W. Temperature measurement performance of thin-film thermocouple cutting tool in turning titanium alloy. Ceram. Int. 2023, 49, 2250–2261. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, L.; Yuan, J. Oxidation behaviour of NiSi–NiCr thin film thermocouples and antioxidation effect of SiNxOy film. Ceram. Int. 2024, 50, 25810–25821. [Google Scholar] [CrossRef]
- Absar, S.; Ruszkiewicz, B.J.; Skovron, J.D.; Mears, L.; Abke, T.; Zhao, X.; Choi, H. Temperature measurement in friction element welding process with micro thin film thermocouples. Procedia Manuf. 2018, 26, 485–494. [Google Scholar] [CrossRef]
- Yang, K. Research on the preparation process of metal-based Pt/ITO thin film thermocouples//Chinese Vacuum Society. In Abstracts of the 2014 Annual Meeting of the Chinese Vacuum Society; State Key Laboratory of Electronic Thin Film and Integrated Devices, University of Electronic Science and Technology: Chengdu, China, 2014; Volume 2. [Google Scholar]
- Liu, Y. Research on Probe Type Metal-Semiconductor Type Thin Film Thermocouple Temperature Sensor Based on MEMS Technology. Master’s Thesis, Xi’an Jiaotong University, Xi’an, China, 2021. [Google Scholar]
- Xie, H. Design and Fabrication of Ceramic-Based Thick-Film Heat Flow Sensor and Performance Research. Master’s Thesis, North Central University, Minneapolis, MN, USA, 2023. [Google Scholar]
- Werschmoeller, D.; Li, X. Measurement of tool internal temperatures in the tool–chip contact region by embedded micro thin film thermocouples. J. Manuf. Process. 2011, 13, 147–152. [Google Scholar] [CrossRef]
- Radajewski, M.; Decker, S.; Krüger, L. Direct temperature measurement via thermocouples within an SPS/FAST graphite tool. Measurement 2019, 147, 106863. [Google Scholar] [CrossRef]
- Kim, S.; Lim, S.; Jeong, M.H.; Kim, W.; Baik, S.; Suk, J.W. Flexible thermocouple using a thermoelectric graphene fiber with a seamless junction. J. Mater. Sci. Technol. 2024, 172, 15–22. [Google Scholar] [CrossRef]
- El-Muraikhi, M.D.; Ayesh, A.I.; Mirzaei, A. Resistive gas sensors based on inorganic nanotubes: A review. J. Alloys Compd. 2025, 1020, 179585. [Google Scholar] [CrossRef]
- Lobaev, M.; Radishev, D.; Vikharev, A.; Gorbachev, A.; Bogdanov, S.; Isaev, V.; Demidov, E.; Arkhipova, E.; Korolev, S.; Okhapkin, A.; et al. Real-time temperature sensor based on integrated diamond Schottky diode. Mater. Sci. Semicond. Process. 2025, 188, 109267. [Google Scholar] [CrossRef]
- Wang, L. Development of Leaded Self-Connecting Temperature Measurement Tool for Multi-Point Measurement of Transient Milling Temperature. Master’s Thesis, Dalian Jiaotong University, Dalian, China, 2023. [Google Scholar]
- Fang, C. Design and Test of a Thin Film Thermocouple Calibration and Alignment Device for Aviation. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar]
- Tai, H. Research on the Design and Preparation Technology of Flexible Thin-Film Thermocouple for Transient Temperature Measurement on Warhead Surface. Master’s Thesis, University of Electronic Science and Technology, Chengdu, China, 2021. [Google Scholar]
- Liu, Z.; Wang, Q.; Guo, S.; Wang, H.; Jiang, W.; Liu, S.; Liu, C.; Wang, N.; Cui, Y.; Ding, W. The preliminary exploration on change mechanism of Seebeck coefficient for NiCr/NiSi thin film thermocouple with different thickness. J. Alloys Compd. 2023, 931, 167573. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, C.; Wang, Y.; Kang, Z.; Gao, X.; Guo, Y. Enhanced high-temperature stability of indium tin oxide—Indium oxide thermocouples by two-step annealing. Thin Solid Film. 2023, 773, 139780. [Google Scholar] [CrossRef]
- Ding, J.; Chen, R.; Wang, J.; Yang, S.; Ye, S. Static characteristics calibration of Pt/Au thin-film thermocouple by means of laser heating and temperature extrapolation method. Sens. Actuators A Phys. 2018, 279, 178–190. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. The voltage–current curve of a direct methanol fuel cell: “exact” and fitting equations. Electrochem. Commun. 2002, 4, 939–946. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Liu, H.; Wang, Z. Non-unit protection scheme for flexible DC transmission lines based on fitting the rate of change of line-mode voltage reverse traveling waves. Int. J. Electr. Power Energy Syst. 2025, 164, 110415. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Fang, W.-Z.; Lin, H.; Tao, W.-Q. Thin film thermocouple fabrication and its application for real-time temperature measurement inside PEMFC. Int. J. Heat Mass Transf. 2019, 141, 1152–1158. [Google Scholar] [CrossRef]
- Kesriklioglu, S.; Pfefferkorn, F.E. Real time temperature measurement with embedded thin-film thermocouples in milling. Procedia CIRP 2018, 77, 618–621. [Google Scholar] [CrossRef]
- Choi, H.; Li, X. Fabrication and application of micro thin film thermocouples for transient temperature measurement in nanosecond pulsed laser micromachining of nickel. Sens. Actuators A Phys. 2007, 136, 118–124. [Google Scholar] [CrossRef]
- Kennedy, F.E.; Frusescu, D.; Li, J. Thin film thermocouple arrays for sliding surface temperature measurement. Wear 1997, 207, 46–54. [Google Scholar] [CrossRef]
- Li, J.; Tao, B.; Huang, S.; Yin, Z. Cutting tools embedded with thin film thermocouples vertically to the rake face for temperature measurement. Sens. Actuators A Phys. 2019, 296, 392–399. [Google Scholar] [CrossRef]
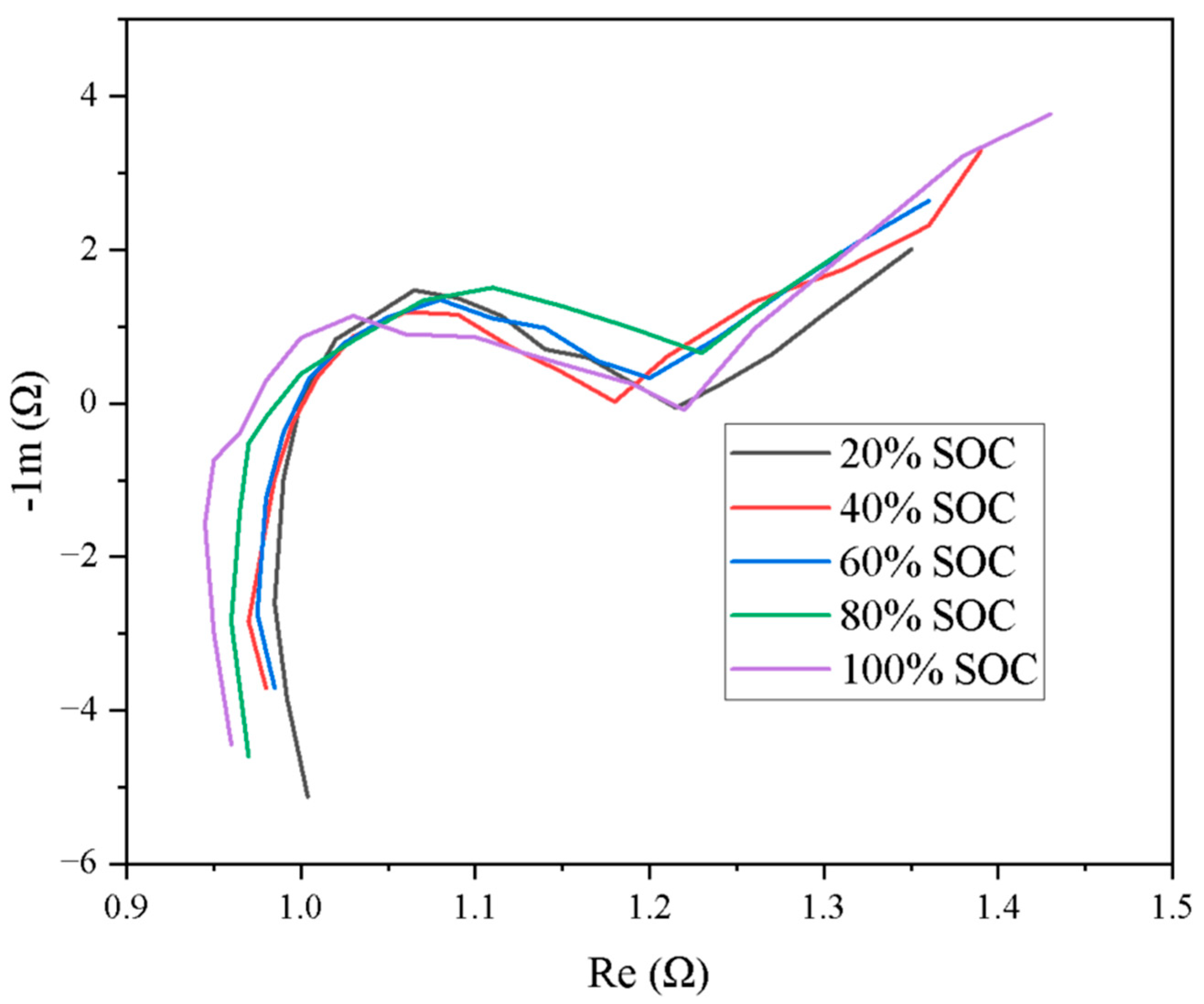
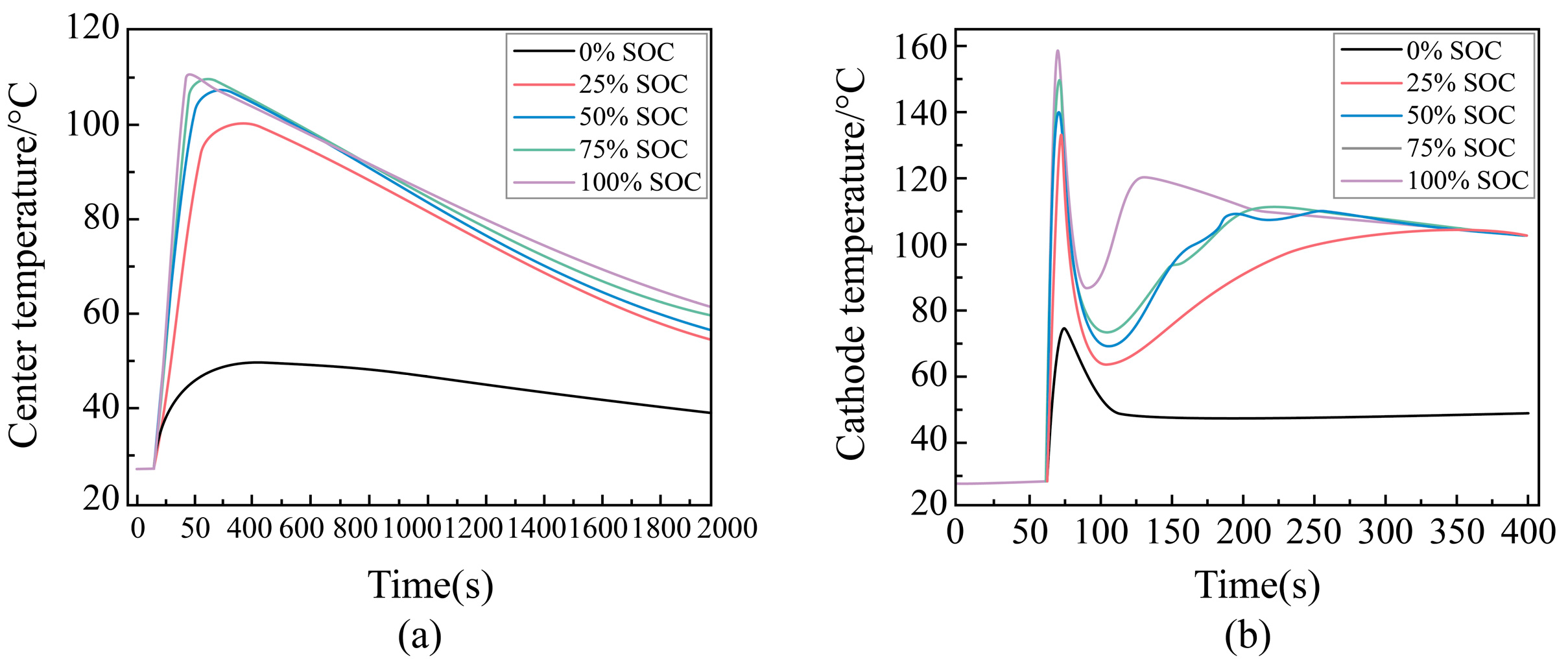
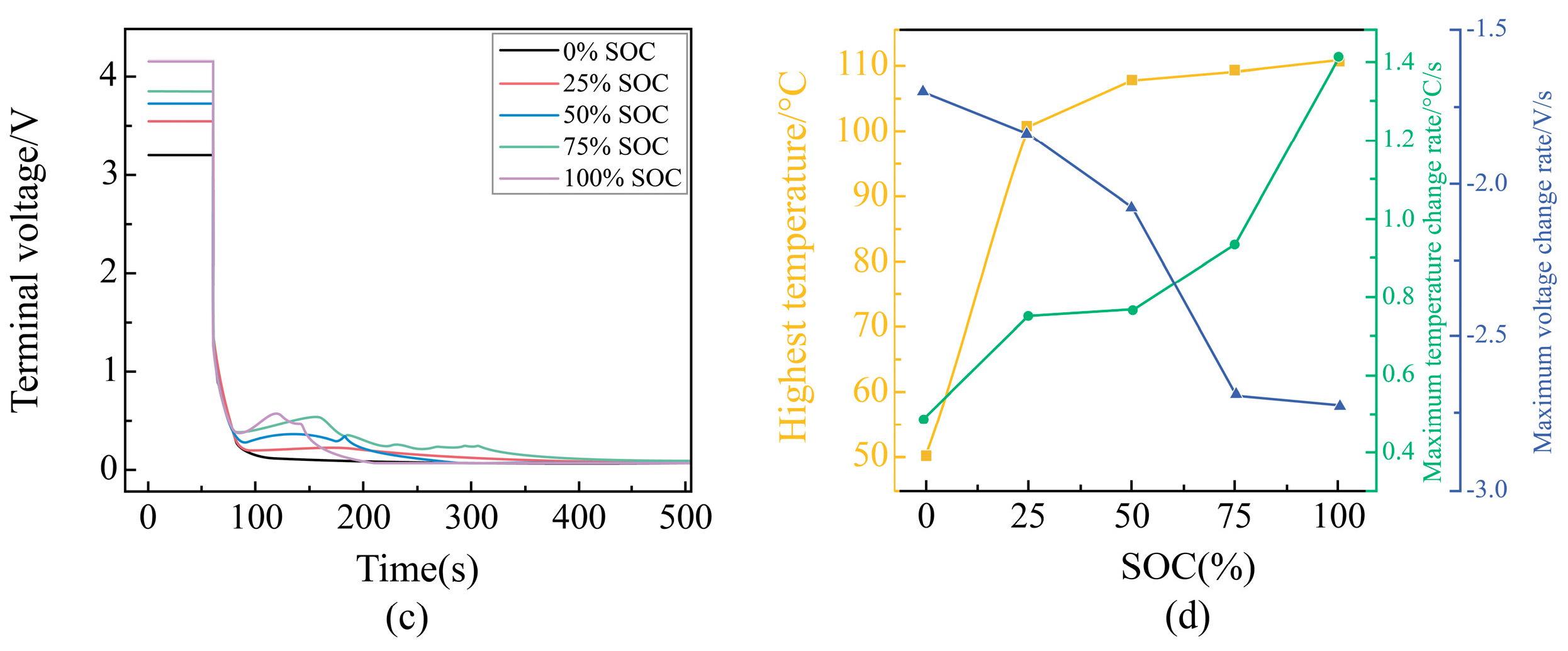
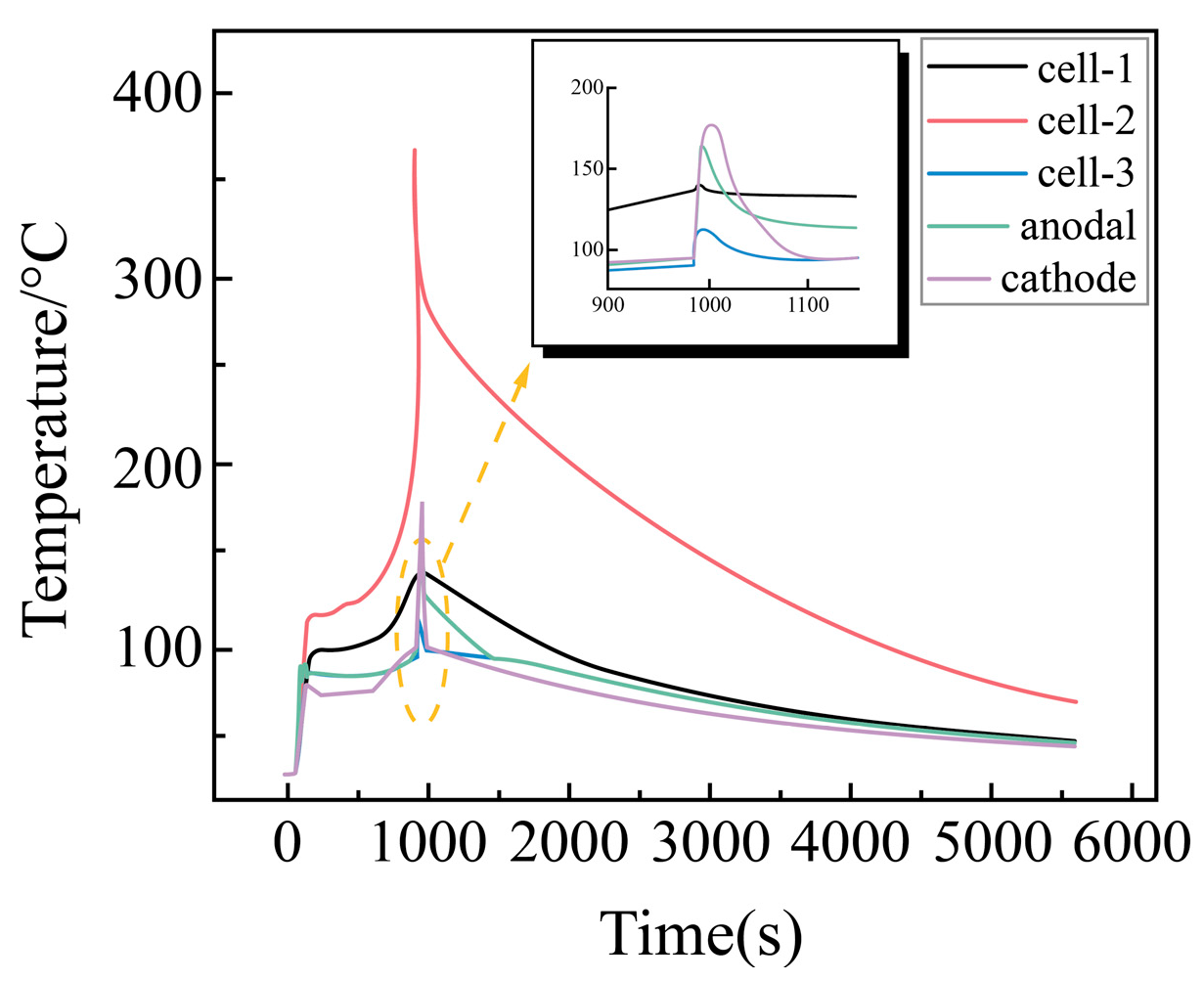
| Battery Model | |||||
|---|---|---|---|---|---|
| #1 | 0.0257 | 1.142 | 0.012 | 0.1543 | 1.3050 × 10−4 |
| #2 | 0.0324 | 1.125 | 0.016 | 0.1286 | 1.2054 × 10−4 |
| #3 | 0.0278 | 1.159 | 0.013 | 0.1409 | 1.3124 × 10−4 |
| #4 | 0.0294 | 1.203 | 0.012 | 0.1539 | 1.3498 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Mao, Y. Advanced NiCr/NiSi Thin-Film Thermocouples for Precise Temperature Sensing in Lithium-Ion Battery Systems. Sensors 2025, 25, 3438. https://doi.org/10.3390/s25113438
Liu X, Mao Y. Advanced NiCr/NiSi Thin-Film Thermocouples for Precise Temperature Sensing in Lithium-Ion Battery Systems. Sensors. 2025; 25(11):3438. https://doi.org/10.3390/s25113438
Chicago/Turabian StyleLiu, Xiyao, and Yanpeng Mao. 2025. "Advanced NiCr/NiSi Thin-Film Thermocouples for Precise Temperature Sensing in Lithium-Ion Battery Systems" Sensors 25, no. 11: 3438. https://doi.org/10.3390/s25113438
APA StyleLiu, X., & Mao, Y. (2025). Advanced NiCr/NiSi Thin-Film Thermocouples for Precise Temperature Sensing in Lithium-Ion Battery Systems. Sensors, 25(11), 3438. https://doi.org/10.3390/s25113438





