Hydrogen Sensors Based on Pd-Based Materials: A Review
Abstract
1. Introduction
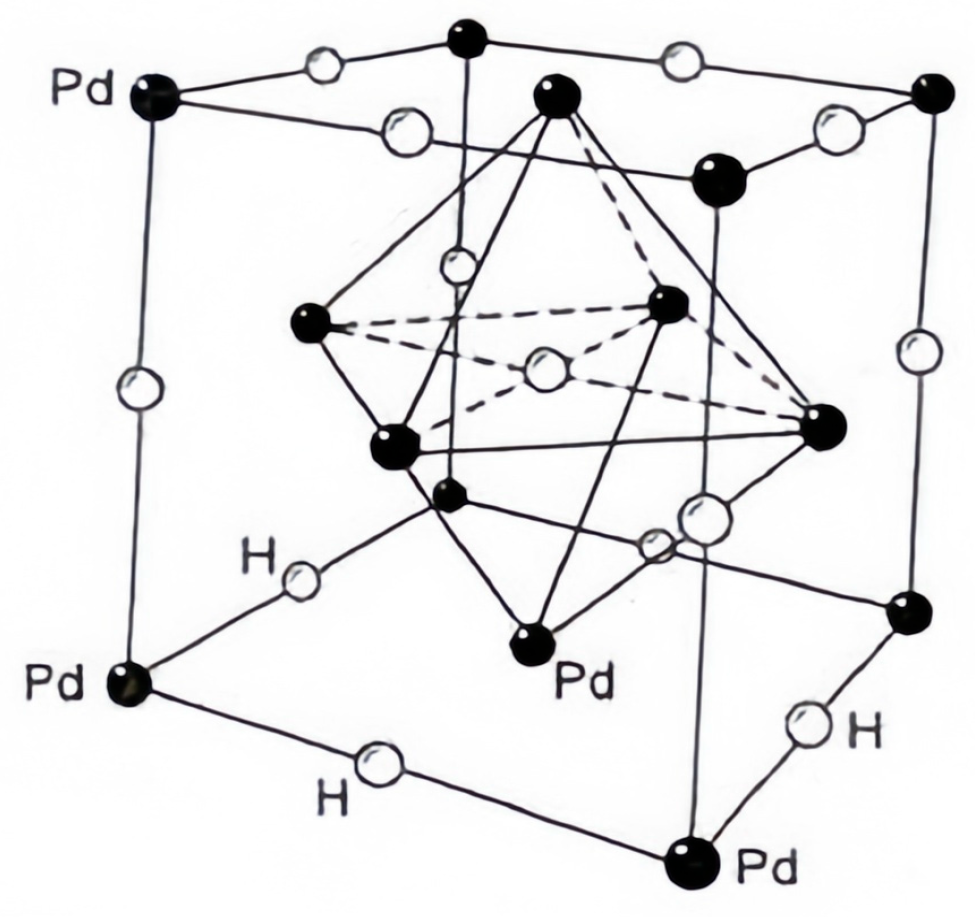
2. Principle of Detection
3. Palladium-Based Hydrogen Sensitive Materials Hydrogen Sensors
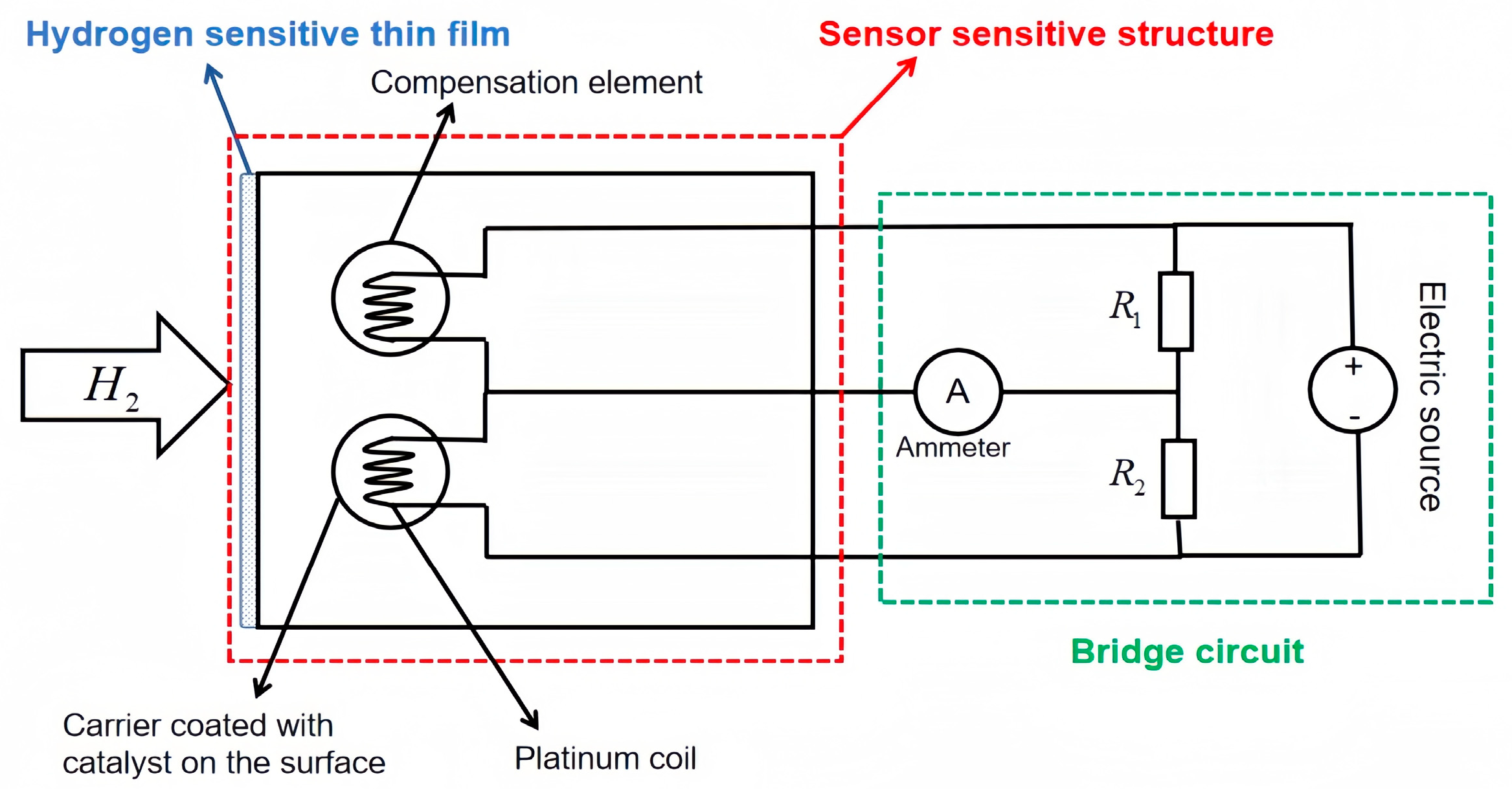
3.1. Catalytic Hydrogen Sensor
3.2. Current Hydrogen Sensors
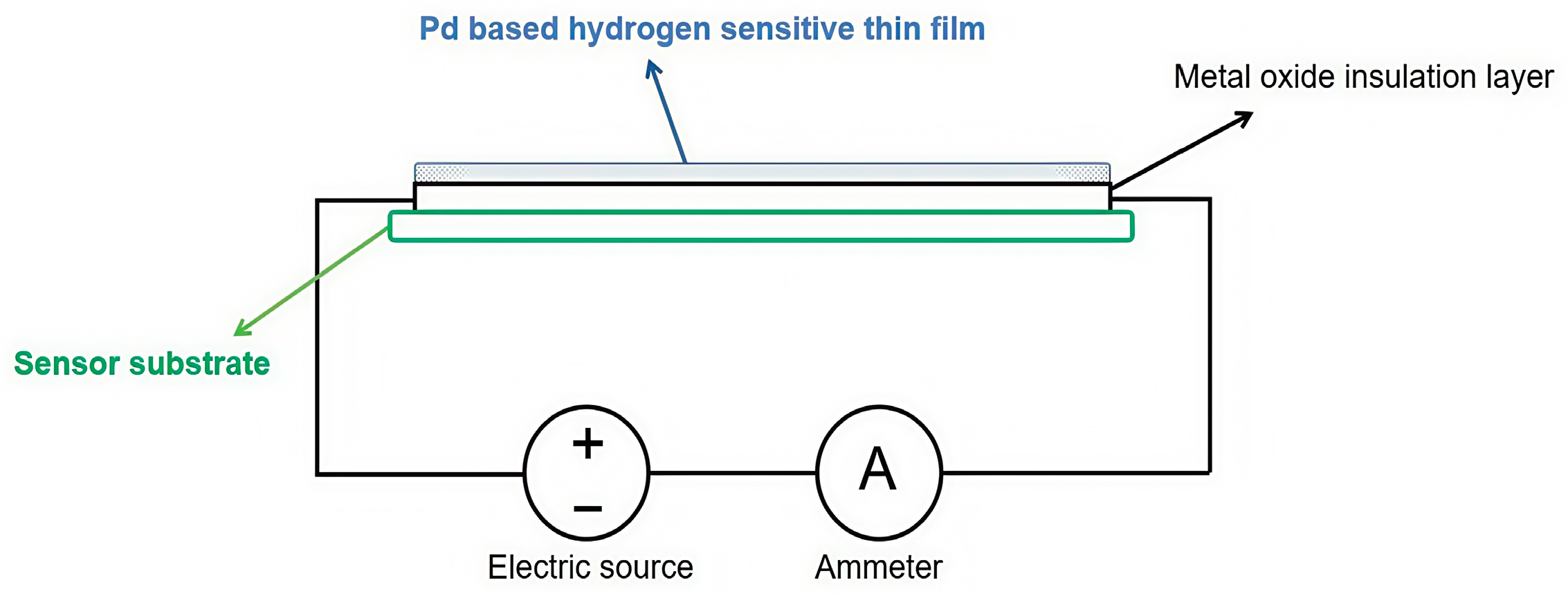
3.3. Resistive Hydrogen Sensors
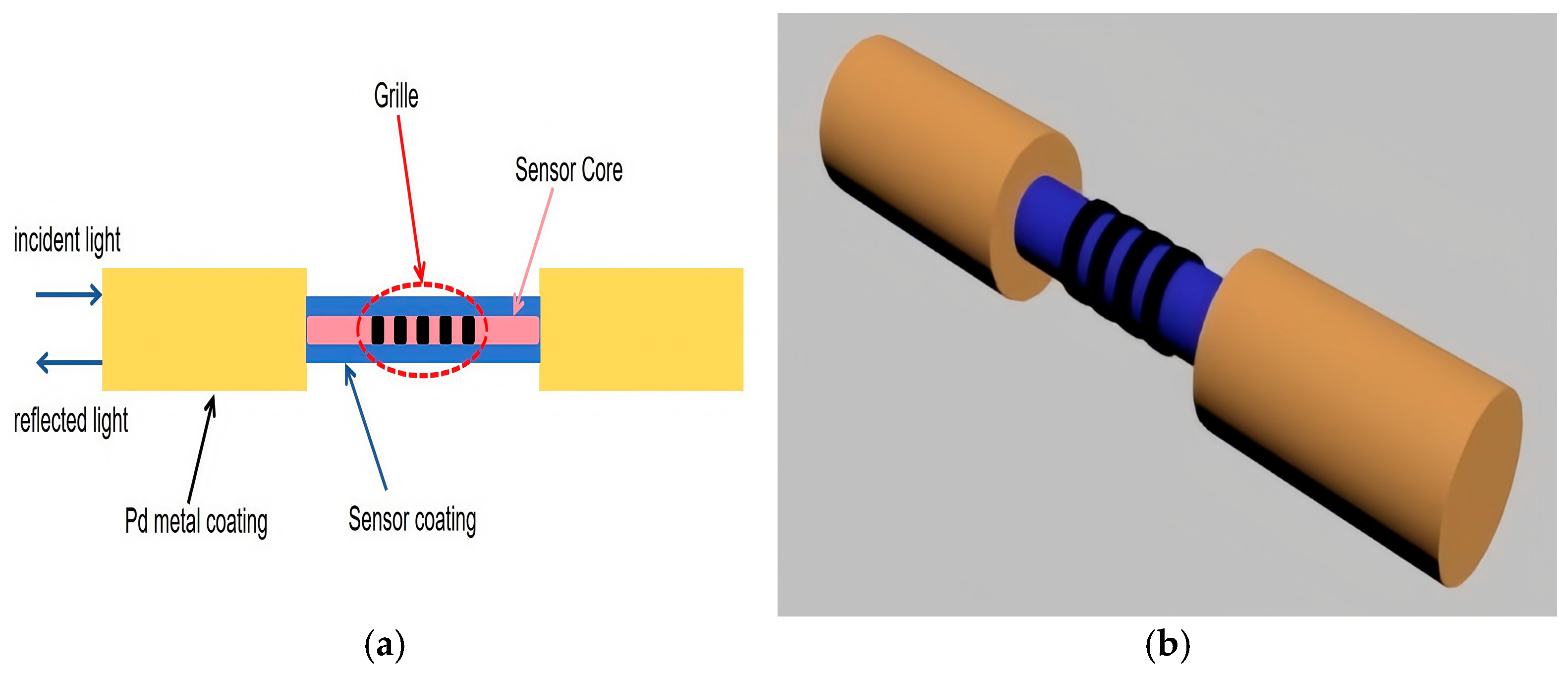
3.4. Fiber Optic Hydrogen Sensors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Pan, A.; Liu, J.; Liu, Z.; Yang, Y.; Yang, X.; Zhang, M. Application of Hydrogen Energy and Review of Current Conditions. IOP Conf. Ser. Earth Environ. Sci. 2020, 526, 012124. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, Z. Functional industrial policy mechanism under natural resource conflict: A case study on the Chinese new energy vehicle industry. Resour. Policy 2023, 81, 103417. [Google Scholar] [CrossRef]
- Yeung, G. ‘Made in China 2025’: The development of a new energy vehicle industry in China. Area Dev. Policy 2019, 4, 39–59. [Google Scholar] [CrossRef]
- Shatnawi, M.; Al Qaydi, N.; Aljaberi, N.; Aljaberi, M. Hydrogen-based energy storage systems: A review. In Proceedings of the 2018 7th International Conference on Renewable Energy Research and Applications (ICRERA), Paris, France, 14–17 October 2018; pp. 697–700. [Google Scholar]
- Onorati, A.; Payri, R.; Vaglieco, B.M.; Agarwal, A.K.; Bae, C.; Bruneaux, G.; Canakci, M.; Gavaises, M.; Günthner, M.; Hasse, C.; et al. The role of hydrogen for future internal combustion engines. Int. J. Engine Res. 2022, 23, 529–540. [Google Scholar] [CrossRef]
- Marchenko, O.V.; Solomin, S.V. The future energy: Hydrogen versus electricity. Int. J. Hydrogen Energy 2015, 40, 3801–3805. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Pour, G.B.; Aval, L.F. Highly sensitive work function hydrogen gas sensor based on PdNPs/SiO2/Si structure at room temperature. Results Phys. 2017, 7, 1993–1999. [Google Scholar] [CrossRef]
- Schönauer, A.L.; Glanz, S. Hydrogen in future energy systems: Social acceptance of the technology and its large-scale infrastructure. Int. J. Hydrogen Energy 2022, 47, 12251–12263. [Google Scholar] [CrossRef]
- Behzadi Pour, G.; Fekri Aval, L. Monitoring of hydrogen concentration using capacitive nanosensor in a 1% H2–N2 mixture. Micro. Nano Lett. 2018, 13, 149–153. [Google Scholar] [CrossRef]
- Tabib-Azar, M.; Sutapun, B.; Petrick, R.; Kazemi, A. Highly sensitive hydrogen sensors using palladium coated fiber optics with exposed cores and evanescent field interactions. Sens. Actuators B Chem. 1999, 56, 158–163. [Google Scholar] [CrossRef]
- Li, Z.; Guo, P.; Han, R.; Sun, H. Current status and development trend of wind power generation-based hydrogen production technology. Energy Explor. Exploit. 2019, 37, 5–25. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Moretti, I.; Françolin, J.; Pacheco, C.; D’Agostino, A.; Werly, J.; Rupin, F. Natural Hydrogen Continuous Emission from Sedimentary Basins: The Example of a Brazilian H2-Emitting Structure. Int. J. Hydrogen Energy 2019, 44, 5676–5685. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A critical review of renewable hydrogen production methods: Factors affecting their scale-up and its role in future energy generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.-H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.-B.; Hong, S.-H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuators B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrogen Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef]
- Kowalska, E.; Czerwosz, E.; Kamińska, A.; Kozłowski, M. Investigation of Pd content in C–Pd films for hydrogen sensor applications. J. Therm. Anal. Calorim. 2012, 108, 1017–1023. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. A novel Pd nanocube–graphene hybrid for hydrogen detection. Sens. Actuators B Chem. 2014, 199, 354–360. [Google Scholar] [CrossRef]
- Sun, Y.; Du, B.; Wang, Y.; Zhang, M.; Zhang, S. Hydrogen spillover-accelerated selective hydrogenation on WO3 with ppm-level Pd. ACS Appl. Mater. Interfaces 2023, 15, 20474–20482. [Google Scholar] [CrossRef]
- Chen, H.-I.; Cheng, Y.-C.; Chang, C.-H.; Chen, W.-C.; Liu, I.-P.; Lin, K.-W.; Liu, W.-C. Hydrogen sensing performance of a Pd nanoparticle/Pd film/GaN-based diode. Sens. Actuators B Chem. 2017, 247, 514–519. [Google Scholar] [CrossRef]
- Foucher, A.C.; Ngan, H.T.; Shirman, T.; Filie, A.; Duanmu, K.; Aizenberg, M.; Madix, R.J.; Friend, C.M.; Aizenberg, J.; Sautet, P.; et al. Influence of Pd Concentration in Au–Pd Nanoparticles for the Hydrogenation of Alkynes. ACS Appl. Nano Mater. 2023, 6, 22927–22938. [Google Scholar] [CrossRef]
- Wang, J.W.; Song, M.; He, Y.H.; Gong, H.R. Stability, adsorption, and diffusion of hydrogen in Pd3Ag phases. J. Membr. Sci. 2016, 503, 124–131. [Google Scholar] [CrossRef]
- Nishijima, Y.; Shimizu, S.; Kurihara, K.; Hashimoto, Y.; Takahashi, H.; Balčytis, A.; Seniutinas, G.; Okazaki, S.; Juodkazytė, J.; Iwasa, T.; et al. Optical readout of hydrogen storage in films of Au and Pd. Opt. Express 2017, 25, 24081–24092. [Google Scholar] [CrossRef]
- Porwal, G.; Gupta, S.; Sreedhala, S.; Elizabeth, J.; Khan, T.S.; Haider, M.A.; Vinod, C.P. Mechanistic insights into the pathways of phenol hydrogenation on Pd nanostructures. ACS Sustain. Chem. Eng. 2019, 7, 17126–17136. [Google Scholar] [CrossRef]
- Jiraskova, Y.; Bursik, J.; Zemanova, A.; Cizek, J.; Hruska, P.; Zivotsky, O. Effect of hydrogen on Fe and Pd alloying and physical properties. Int. J. Hydrogen Energy 2017, 42, 6885–6901. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrogen Energy 2019, 44, 20552–20571. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. Characteristics of resistivity-type hydrogen sensing based on palladium-graphene nanocomposites. Int. J. Hydrogen Energy 2014, 39, 620–629. [Google Scholar] [CrossRef]
- Frolov, S.M.; Medvedev, S.N.; Basevich, V.Y.; Frolov, F.S. Self-ignition of hydrocarbon–hydrogen–air mixtures. Int. J. Hydrogen Energy 2013, 38, 4177–4184. [Google Scholar] [CrossRef]
- Kumar, M.K.; Rao, M.R.; Ramaprabhu, S. Structural, morphological and hydrogen sensing studies on pulsed laser deposited nanostructured palladium thin films. J. Phys. D Appl. Phys. 2006, 39, 2791. [Google Scholar] [CrossRef]
- Athayde, A.L.; Baker, R.W.; Nguyen, P. Metal composite membranes for hydrogen separation. J. Membr. Sci. 1994, 94, 299–311. [Google Scholar] [CrossRef]
- Pugachev, V.A.; Busol, F.I.; Nikolaev, E.I.; Nam, B.P. Penetration and diffusion of hydrogen in palladium–silver alloys. Russ. J. Phys. Chem 1975, 49. [Google Scholar]
- Axelrod, S.D.; Makrides, A.C. X-ray Studies of Hydrogen—Silver—Palladium Electrodes. J. Phys. Chem. 1964, 68, 2154–2159. [Google Scholar] [CrossRef]
- Kansara, S.; Gupta, S.K.; Sonvane, Y.; Gajjar, P.N. Ultrathin Pd and Pt nanowires for potential applications as hydrogen economy. Mater. Today Commun. 2021, 26, 101761. [Google Scholar] [CrossRef]
- Dai, Y.; Jiang, H.; Zhao, X.; Tian, J.; Deng, X.; Zhang, W. A temperature-stable Pd nanofilm hydrogen sensor with a Wheatstone bridge structure. J. Mater. Sci. Mater. Electron. 2023, 34, 833. [Google Scholar] [CrossRef]
- Niu, J.S.; Huang, C.H.; Shao, W.C.; Tsai, J.H.; Liu, W.C. Pd Nanoparticle/Pd/Al2O3 Resistive Sensor for Hydrogen Detection in a High-Temperature Environment. ECS J. Solid State Sci. Technol. 2022, 11, 067003. [Google Scholar] [CrossRef]
- Jen, S.U.; Chen, T.P.; Chang, S.A. Electrical resistivity of Co-Ni-Pd and Co-Pd alloys. J. Appl. Phys. 1991, 70, 5831–5833. [Google Scholar] [CrossRef]
- Kato, R.; Yoshida, T.; Iimori, R.; Zizhou, T.; Shiga, M.; Inagaki, Y.; Kimura, T.; Kawae, T. Resistivity Measurements in Palladium Hydride Film Prepared by Low-Temperature Hydrogen Absorption Method. J. Phys. Soc. Jpn. 2024, 93, 024703. [Google Scholar] [CrossRef]
- Tóth, J.; Péter, L.; Bakonyi, I.; Tompa, K. Peculiarities of the electrolytic hydrogenation of Pd as revealed by resistivity measurements. J. Alloys Compd. 2005, 387, 172–178. [Google Scholar] [CrossRef]
- Houlet, L.F.; Shin, W.; Tajima, K.; Nishibori, M.; Izu, N.; Itoh, T.; Matsubara, I. Thermopile sensor-devices for the catalytic detection of hydrogen gas. Sens. Actuators B Chem. 2008, 130, 200–206. [Google Scholar] [CrossRef]
- Kalinin, I.A.; Roslyakov, I.V.; Bograchev, D.; Kushnir, S.E.; Ivanov, I.I.; Dyakov, A.V.; Napolskii, K.S. High performance microheater-based catalytic hydrogen sensors fabricated on porous anodic alumina substrates. Sens. Actuators B Chem. 2024, 404, 135270. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Baranov, A.M.; Talipov, V.A.; Mironov, S.M.; Akbari, S.; Kolesnik, I.V.; Orlova, E.D.; Napolskii, K.S. Investigation of catalytic hydrogen sensors with platinum group catalysts. Sens. Actuators B Chem. 2021, 346, 130515. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of electrochemical hydrogen sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, I.H.; Hassan, H.A.; Abdullah, Q.N. Hydrogen gas sensor based on nanocrystalline SnO 2 thin film grown on bare Si substrates. Nano-Micro Lett. 2016, 8, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, J.; Noh, J.-S.; Kim, W.; Lee, T.; Maeng, S.; Lee, W. Pd–Ni hydrogen sponge for highly sensitive nanogap-based hydrogen sensors. Int. J. Hydrogen Energy 2012, 37, 14702–14706. [Google Scholar] [CrossRef]
- Ayesh, A.I. Linear hydrogen gas sensors based on bimetallic nanoclusters. J. Alloys Compd. 2016, 689, 1–5. [Google Scholar] [CrossRef]
- Xiang, C.; She, Z.; Zou, Y.; Cheng, J.; Chu, H.; Qiu, S.; Zhang, H.; Sun, L.; Xu, F. A room-temperature hydrogen sensor based on Pd nanoparticles doped TiO2 nanotubes. Ceram. Int. 2014, 40, 16343–16348. [Google Scholar] [CrossRef]
- Alenezy, E.K.; Sabri, Y.M.; Kandjani, A.E.; Korcoban, D.; Rashid, S.S.A.A.H.; Ippolito, S.J.; Bhargava, S.K. Low-temperature hydrogen sensor: Enhanced performance enabled through photoactive Pd-decorated TiO2 colloidal crystals. ACS Sens. 2020, 5, 3902–3914. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Srivastava, S.; Solanki, P.R.; Puri, N.K. Electrochemical hydrogen gas sensing employing palladium oxide/reduced graphene oxide (PdO-rGO) nanocomposites. IEEE Sens. J. 2019, 19, 8262–8271. [Google Scholar] [CrossRef]
- Lee, B.; Cho, S.; Jeong, B.J.; Lee, S.H.; Kim, D.; Kim, S.H.; Park, J.H.; Yu, H.K.; Choi, J.Y. Highly responsive hydrogen sensor based on Pd nanoparticle-decorated transfer-free 3D graphene. Sens. Actuators B Chem. 2024, 401, 134913. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Zhao, S.; Jin, T.; Zhong, M.; Cen, Z.; Gao, P.; Yan, W.; Ling, M. Room Temperature Resistive Hydrogen Sensor for Early Safety Warning of Li-Ion Batteries. Chemosensors 2023, 11, 344. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.; Zhang, J.; Huang, Y.; Tian, J.; Deng, X.; Zhao, X.; Zhang, W. Flexible nanofiber sensor for low-concentration hydrogen detection. Nanotechnology 2019, 31, 015504. [Google Scholar] [CrossRef]
- Hu, Y.; Lei, J.; Wang, Z.; Yang, S.; Luo, X.; Zhang, G.; Chen, W.; Gu, H. Rapid response hydrogen sensor based on nanoporous Pd thin films. Int. J. Hydrogen Energy 2016, 41, 10986–10990. [Google Scholar] [CrossRef]
- Yu, S.; Welp, U.; Hua, L.Z.; Rydh, A.; Kwok, W.K.; Wang, H.H. Fabrication of palladium nanotubes and their application in hydrogen sensing. Chem. Mater. 2005, 17, 3445–3450. [Google Scholar] [CrossRef]
- RaviPrakash, J.; McDaniel, A.H.; Horn, M.; Pilione, L.; Sunal, P.; Messier, R.; McGrath, R.T.; Schweighardt, F.K. Hydrogen sensors: Role of palladium thin film morphology. Sens. Actuators B Chem. 2007, 120, 439–446. [Google Scholar] [CrossRef]
- Lundström, K.I.; Shivaraman, M.S.; Svensson, C.M. A hydrogen-sensitive Pd-gate MOS transistor. J. Appl. Phys. 1975, 46, 3876–3881. [Google Scholar] [CrossRef]
- Stiblert, L.; Svensson, C. Hydrogen leak detector using a Pd-gate MOS transistor. Rev. Sci. Instrum. 1975, 46, 1206–1208. [Google Scholar] [CrossRef]
- Kumar, A.; Thundat, T.; Swihart, M.T. Ultrathin palladium nanowires for fast and hysteresis-free H2 sensing. ACS Appl. Nano Mater. 2022, 5, 5895–5905. [Google Scholar] [CrossRef]
- Thokala, N.; Vankayala, K.; Basavaiah, K.; Kalidindi, S.B. Spontaneously decorated palladium nanoparticles on redox active covalent organic framework for chemiresistive hydrogen gas sensing. Int. J. Hydrogen Energy 2024, 81, 270–279. [Google Scholar] [CrossRef]
- Rashid, T.R.; Phan, D.T.; Chung, G.S. A flexible hydrogen sensor based on Pd nanoparticles decorated ZnO nanorods grown on polyimide tape. Sens. Actuators B Chem. 2013, 185, 777–784. [Google Scholar] [CrossRef]
- Nguyen, K.; Hung, C.M.; Ngoc, T.M.; Le, D.T.T.; Nguyen, D.H.; Van, D.N.; Van, H.N. Low-temperature prototype hydrogen sensors using Pd-decorated SnO2 nanowires for exhaled breath applications. Sens. Actuators B Chem. 2017, 253, 156–163. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Mahmoud, S.T.; Ahmad, S.J.; Haik, Y. Novel hydrogen gas sensor based on Pd and SnO2 nanoclusters. Mater. Lett. 2014, 128, 354–357. [Google Scholar] [CrossRef]
- Sokovykh, E.V.; Oleksenko, L.P.; Maksymovych, N.P.; Matushko, I.P. Influence of conditions of Pd/SnO2 nanomaterial formation on properties of hydrogen sensors. Nanoscale Res. Lett. 2017, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Deivasegamani, R.; Karunanidhi, G.; Santhosh, C.; Gopal, T.; Saravana achari, S.; Neogi, A.; Nivetha, R.; Pradeep, N.; Venkatraman, U.; Bhatnagar, A.; et al. Chemoresistive sensor for hydrogen using thin films of tin dioxide doped with cerium and palladium. Microchim. Acta 2017, 184, 4765–4773. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, K.; Kohli, N.; Kaur, A.; Singh, R.C. Temperature dependent selective detection of hydrogen and acetone using Pd doped WO3/reduced graphene oxide nanocomposite. Chem. Phys. Lett. 2018, 701, 115–125. [Google Scholar] [CrossRef]
- Boudiba, A.; Zhang, C.; Navio, C.; Bittencourt, C.; Snyders, R.; Debliquy, M. Preparation of highly selective, sensitive and stable hydrogen sensors based on Pd-doped tungsten trioxide. Procedia Eng. 2010, 5, 180–183. [Google Scholar] [CrossRef]
- Boudiba, A.; Zhang, C.; Umek, P.; Bittencourt, C.; Snyders, R.; Olivier, M.G.; Debliquy, M. Sensitive and rapid hydrogen sensors based on Pd–WO3 thick films with different morphologies. Int. J. Hydrogen Energy 2013, 38, 2565–2577. [Google Scholar] [CrossRef]
- Duan, P.; Duan, Q.; Peng, Q.; Jin, K.; Sun, J. Design of ultrasensitive gas sensor based on self-assembled Pd-SnO2/rGO porous ternary nanocomposites for ppb-level hydrogen. Sens. Actuators B Chem. 2022, 369, 132280. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, S.; Men, G.; Han, D.; Gu, F. Sensitization of Pd loading for remarkably enhanced hydrogen sensing performance of 3DOM WO3. Sens. Actuators B Chem. 2018, 262, 577–587. [Google Scholar] [CrossRef]
- Woo, J.A.; Phan, D.T.; Jung, Y.W.; Jeon, K.J. Fast response of hydrogen sensor using palladium nanocube-TiO2 nanofiber composites. Int. J. Hydrogon Energy 2017, 42, 18754–18761. [Google Scholar] [CrossRef]
- Moon, J.; Hedman, H.-P.; Kemell, M.; Tuominen, A.; Punkkinen, R. Hydrogen sensor of Pd-decorated tubular TiO2 layer prepared by anodization with patterned electrodes on SiO2/Si substrate. Sens. Actuators B Chem. 2016, 222, 190–197. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Labat, F.; Ciofini, I.; Pauporté, T.; Adelung, R. Ultra-sensitive and selective hydrogen nanosensor with fast response at room temperature based on a single Pd/ZnO nanowire. Sens. Actuators B Chem. 2018, 254, 1259–1270. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Renitta, A.; Monamary, A. Substantial effect of Pd incorporation on the room temperature hydrogen sensing performance of ZnO/ITO nanowires prepared by spray pyrolysis method. J. Mater. Sci. Mater. Electron. 2018, 29, 21023–21032. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, K.; Kim, S.W.; Park, C.W.; Kim, S.I.; Mirzaei, A.; Lee, J.H.; Jeong, D.Y. Enhancement of H2 Gas Sensing Using Pd Decoration on ZnO Nanoparticles. Chemosensors 2024, 12, 90. [Google Scholar] [CrossRef]
- Phan, D.T.; Uddin, A.S.M.I.; Chung, G.S. A large detectable-range, high-response and fast-response resistivity hydrogen sensor based on Pt/Pd core–shell hybrid with graphene. Sens. Actuators B Chem. 2015, 220, 962–967. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.S. Pd/Ag alloy as an application for hydrogen sensing. Int. J. Hydrogen Energy 2017, 42, 25446–25452. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.S. Graphene decorated Pd-Ag nanoparticles for H2 sensing. Int. J. Hydrogen Energy 2018, 43, 11397–11402. [Google Scholar] [CrossRef]
- Hassan, K.; Uddin, A.S.M.I.; Chung, G.S. Mesh of ultrasmall Pd/Mg bimetallic nanowires as fast response wearable hydrogen sensors formed on filtration membrane. Sens. Actuators B Chem. 2017, 252, 1035–1044. [Google Scholar] [CrossRef]
- Gautam, Y.K.; Sanger, A.; Kumar, A.; Chandra, R. A room temperature hydrogen sensor based on Pd–Mg alloy and multilayers prepared by magnetron sputtering. Int. J. Hydrogen Energy 2015, 40, 15549–15555. [Google Scholar] [CrossRef]
- Yoshimura, K.; Nakano, S.; Uchinashi, S.; Yamaura, S.; Kimura, H.; Inoue, A. A hydrogen sensor based on Mg–Pd alloy thin film. Meas. Sci. Technol. 2007, 18, 3335. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.M.; Lee, E.; Noh, J.S.; Joe, J.H.; Jung, B.; Lee, W. Hydrogen gas sensing performance of Pd–Ni alloy thin films. Thin Solid Film. 2010, 519, 880–884. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, M.; Yu, Y.; Tian, X.; Zhao, X.; Zhang, W.; Zhang, J.; Huang, Y.; Yu, K. Integrated temperature and hydrogen sensors with MEMS technology. Sensors 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tian, X.; Deng, X.; Zhao, X.; Zhang, L.; Zhang, W.; Zhang, J.; Huang, Y. Low concentration response hydrogen sensors based on wheatstone bridge. Sensors 2019, 19, 1096. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gong, H.; Peng, D.; Meng, G. Pd–Ni thin films grown on porous Al2O3 substrates by metalorganic chemical vapor deposition for hydrogen sensing. Thin Solid Film. 1999, 345, 217–221. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Feng, X.; Zhao, F.; Wang, H. Wheatstone Bridge MEMS Hydrogen Sensor with ppb-Level Detection Limit Based on the Palladium–Gold Alloy. ACS Sens. 2024, 9, 6082–6091. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Chen, P.; Lu, S.; Zhang, Q.; Chen, Y.; Du, B.; Tang, J.; He, J.; Liao, C.; et al. Fiber optic hydrogen sensor based on a Fabry–Perot interferometer with a fiber Bragg grating and a nanofilm. Lab A Chip 2021, 21, 1752–1758. [Google Scholar] [CrossRef]
- Hu, T.Y.; Wang, D.N.; Wang, M.; Li, Z.; Yang, M. Miniature hydrogen sensor based on fiber inner cavity and Pt-doped WO 3 coating. IEEE Photonics Technol. Lett. 2014, 26, 1458–1461. [Google Scholar] [CrossRef]
- Jia, C.; Zhao, L.; Huang, G.; Liu, L.; Wang, W.; Yang, Y.; Miao, Y. A Review of Hydrogen Sensors for ECLSS: Fundamentals, Recent Advances, and Challenges. Appl. Sci. 2023, 13, 6869. [Google Scholar] [CrossRef]
- Yang, M.; Dai, J. Fiber optic hydrogen sensors: A review. Photonic Sens. 2014, 4, 300–324. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Hunze, A. Improving the sensitivity of palladium-based fiber optic hydrogen sensors. J. Light. Technol. 2018, 36, 2166–2174. [Google Scholar] [CrossRef]
- Zou, M.; Dai, Y.; Zhou, X.; Dong, K.; Yang, M. Femtosecond laser ablated FBG with composite microstructure for hydrogen sensor application. Sensors 2016, 16, 2040. [Google Scholar] [CrossRef] [PubMed]
- Buric, M.; Chen, T.; Maklad, M.; Swinehart, P.R.; Chen, K.P. Multiplexable low-temperature fiber Bragg grating hydrogen sensors. IEEE Photonics Technol. Lett. 2009, 21, 1594–1596. [Google Scholar] [CrossRef]
- Buric, M.; Chen, K.P.; Bhattarai, M.; Swinehart, P.R.; Maklad, M. Active fiber Bragg grating hydrogen sensors for all-temperature operation. IEEE Photonics Technol. Lett. 2007, 19, 255–257. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, Y.; Zou, M.; Karanja, J.M.; Yang, M. FBG hydrogen sensor based on spiral microstructure ablated by femtosecond laser. Sens. Actuators B Chem. 2016, 236, 392–398. [Google Scholar] [CrossRef]
- Zhou, X.; Karanja, J.M.; Yang, M.; Zhou, F.; Liu, K.; Ming, X.; Dai, Y. FBG Hydrogen Sensor Based on Pd87–Ni13/Pd4–Ag1 Thin Film and Femtosecond Laser Ablation. Integr. Ferroelectr. 2021, 221, 1–11. [Google Scholar] [CrossRef]
- Coelho, L.; Almeida, J.M.M.M.d.; Santos, J.L.; Viegas, D. Fiber optic hydrogen sensor based on an etched Bragg grating coated with palladium. Appl. Opt. 2015, 54, 10342–10348. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yang, Z.; Li, Z.; Wang, Y.; Wang, G.; Zhang, Y.; Zhuang, Z. Enhanced sensitivity of fiber Bragg grating hydrogen sensor using flexible substrate. Sens. Actuators B Chem. 2014, 196, 604–609. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Janssens, S.; Hunze, A. Palladium-based hydrogen sensors using fiber Bragg gratings. J. Light. Technol. 2017, 36, 850–856. [Google Scholar] [CrossRef]
- Hu, X.; Hu, W.; Dai, J.; Ye, H.; Zhang, F.; Yang, M.; Buchfellner, F.; Bian, Q.; Hopf, B.; Roths, J. Performance of Fiber-Optic Hydrogen Sensor Based on Locally Coated π-Shifted FBG. IEEE Sens. J. 2022, 22, 23982–23989. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, G.M.; Li, C.R.; Song, H.T.; Luo, Y.T.; Wang, H.B. Highly sensitive dissolved hydrogen sensor based on side-polished fiber Bragg grating. IEEE Photonics Technol. Lett. 2015, 27, 1453–1456. [Google Scholar] [CrossRef]
- Silva, S.; Coelho, L.; Almeida, J.M.; Frazao, O.; Santos, J.L.; Malcata, F.X.; Becker, M.; Rothhardt, M.; Bartelt, H. H 2 sensing based on a Pd-coated tapered-FBG fabricated by DUV femtosecond laser technique. IEEE Photonics Technol. Lett. 2013, 25, 401–403. [Google Scholar] [CrossRef]
- Okazaki, S.; Kawada, H.; Koshiba, Y.; Kasai, N.; Maru, Y.; Mizutani, T.; Takesaki, Y.; Shimano, S. Catalytic combustion type optical fiber Bragg grating hydrogen gas sensor using platinum-loaded fumed silica powder. Int. J. Hydrogen Energy 2023, 48, 9512–9527. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, X.; Peng, B.; Li, Z.; Xia, X.; Lu, C.; You, D.; Li, K.; Guo, T. Ultrafast and reproducible fiber-optic hydrogen sensor via a tilted fiber grating with pd/wo3 nanocoating. Photonic Sens. 2025, 15, 1–10. [Google Scholar] [CrossRef]
- Dai, J.; Yin, K.; Chen, Z.; Hu, W.; Yang, M.; Fu, J.; Sun, X.; Chen, X. Improved performance of a fiber-optic hydrogen sensor based on a controllable optical heating technology. Opt. Lett. 2024, 49, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Hu, Y.; Chen, H.; Zhang, S.; Li, J.; Liang, H.; Wu, C.; Guan, B.O. Hydrogen and temperature measurement using a functionalized superstructure of Bragg gratings in a helical-core fiber. Opt. Laser Technol. 2024, 174, 110551. [Google Scholar] [CrossRef]
- Abdalwareth, A.; Flachenecker, G.; Angelmahr, M.; Schade, W. Optical fiber evanescent hydrogen sensor based on palladium nanoparticles coated Bragg gratings. Sens. Actuators A Phys. 2023, 361, 114594. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Cao, Y.; Su, Y.; Huang, B.; Chen, C.; Yu, X.; Xu, A.; Wu, T. Hydrogen Sensors Based on Pd-Based Materials: A Review. Sensors 2025, 25, 3402. https://doi.org/10.3390/s25113402
Yan S, Cao Y, Su Y, Huang B, Chen C, Yu X, Xu A, Wu T. Hydrogen Sensors Based on Pd-Based Materials: A Review. Sensors. 2025; 25(11):3402. https://doi.org/10.3390/s25113402
Chicago/Turabian StyleYan, Shubin, Yuhao Cao, Yiru Su, Biyi Huang, Changxin Chen, Xianfeng Yu, Aiwei Xu, and Taiquan Wu. 2025. "Hydrogen Sensors Based on Pd-Based Materials: A Review" Sensors 25, no. 11: 3402. https://doi.org/10.3390/s25113402
APA StyleYan, S., Cao, Y., Su, Y., Huang, B., Chen, C., Yu, X., Xu, A., & Wu, T. (2025). Hydrogen Sensors Based on Pd-Based Materials: A Review. Sensors, 25(11), 3402. https://doi.org/10.3390/s25113402





