Review on Multispectral Photoacoustic Imaging Using Stimulated Raman Scattering Light Sources
Abstract
1. Introduction
2. Principles
2.1. Principles of Photoacoustic Imaging
2.2. Principles of Stimulated Raman Scattering
3. SRS Light Source in the Visible Spectral Region
4. SRS Light Source at the Near-Infrared Spectral Region
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef]
- Beard, P. Biomedical Photoacoustic Imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Kim, H.-H.; Kim, C.-S.; Kim, J. Review on Optical Imaging Techniques for Multispectral Analysis of Nanomaterials. Nanotheranostics 2022, 6, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.E. Basic Physics of Ultrasound Imaging. Crit. Care. Med. 2007, 35, S131–S137. [Google Scholar] [CrossRef]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional Photoacoustic Imaging: From Nano-and Micro-to Macro-Scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic Tomography of Blood Oxygenation: A Mini Review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Knieling, F.; Clingman, B.; Bohndiek, S.; Wang, L.V.; Kim, C. Clinical Translation of Photoacoustic Imaging. Nat. Rev. Bioeng. 2025, 3, 193–212. [Google Scholar] [CrossRef]
- Lee, C.; Cho, S.; Lee, D.; Lee, J.; Park, J.-I.; Kim, H.-J.; Park, S.H.; Choi, W.; Kim, U.; Kim, C. Panoramic Volumetric Clinical Handheld Photoacoustic and Ultrasound Imaging. Photoacoustics 2023, 31, 100512. [Google Scholar] [CrossRef]
- Dima, A.; Ntziachristos, V. In-Vivo Handheld Optoacoustic Tomography of the Human Thyroid. Photoacoustics 2016, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; Timmermans, A.C.; Wouters, P.C.; Kacprowicz, M.; van Dam, G.M.; Ntziachristos, V. Optoacoustic Imaging of Human Vasculature: Feasibility by using a Handheld Probe. Radiology 2016, 281, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A Review of Clinical Photoacoustic Imaging: Current and Future Trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Kim, C. Clinical Photoacoustic Imaging Platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, E.-Y.; Park, B.; Choi, W.; Lee, K.J.; Kim, C. Towards Clinical Photoacoustic and Ultrasound Imaging: Probe Improvement and Real-Time Graphical User Interface. Exp. Biol. Med. 2020, 245, 321–329. [Google Scholar] [CrossRef]
- Ivankovic, I.; Merčep, E.; Schmedt, C.-G.; Deán-Ben, X.L.; Razansky, D. Real-Time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 45–50. [Google Scholar] [CrossRef]
- Paul, S.; Mulani, S.; Daimary, N.; Singh, M.S. Simplified-Delay-Multiply-and-Sum-based Promising Beamformer for Real-Time Photoacoustic Imaging. IEEE Trans. Instrum. Meas. 2022, 71, 4006509. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S.; et al. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef]
- Roll, W.; Markwardt, N.A.; Masthoff, M.; Helfen, A.; Claussen, J.; Eisenblätter, M.; Hasenbach, A.; Hermann, S.; Karlas, A.; Wildgruber, M. Multispectral Optoacoustic Tomography of Benign and Malignant Thyroid Disorders—A Pilot Study. J. Nucl. Med. 2019, 60, 1461–1466. [Google Scholar] [CrossRef]
- Noltes, M.E.; Bader, M.; Metman, M.J.; Vonk, J.; Steinkamp, P.J.; Kukačka, J.; Westerlaan, H.E.; Dierckx, R.A.; van Hemel, B.M.; Brouwers, A.H. Towards In Vivo Characterization of Thyroid Nodules Suspicious for Malignancy using Multispectral Optoacoustic Tomography. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2736–2750. [Google Scholar] [CrossRef] [PubMed]
- Schoustra, S.M.; Piras, D.; Huijink, R.; Op’t Root, T.J.; Alink, L.; Kobold, W.M.F.; Steenbergen, W.; Manohar, S. Twente Photoacoustic Mammoscope 2: System Overview and Three-Dimensional Vascular Network Images in Healthy Breasts. J. Biomed. Opt. 2019, 24, 121909. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.-R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous Transrectal Ultrasound and Photoacoustic Human Prostate Imaging. Sci. Transl. Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef]
- Park, B.; Bang, C.H.; Lee, C.; Han, J.H.; Choi, W.; Kim, J.; Park, G.S.; Rhie, J.W.; Lee, J.H.; Kim, C. 3D Wide-Field Multispectral Photoacoustic Imaging of Human Melanomas In Vivo: A Pilot Study. J. Eur. Acad. Dermatol. 2020, 35, 669–676. [Google Scholar] [CrossRef]
- Choi, W.; Park, B.; Choi, S.; Oh, D.; Kim, J.; Kim, C. Recent Advances in Contrast-Enhanced Photoacoustic Imaging: Overcoming the Physical and Practical Challenges. Chem. Rev. 2023, 123, 7379–7419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Swartchick, C.B.; Chan, J. Targeted Contrast Agents and Activatable Probes for Photoacoustic Imaging of Cancer. Chem. Soc. Rev. 2022, 51, 829–868. [Google Scholar] [CrossRef]
- Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Singh, S.; Giammanco, G.; Hu, C.-H.; Bush, J.; Cordova, L.S.; Lawrence, D.J.; Moran, J.L.; Chitnis, P.V.; Veneziano, R. Size-Tunable ICG-based Contrast Agent Platform for Targeted Near-Infrared Photoacoustic Imaging. Photoacoustics 2022, 29, 100437. [Google Scholar] [CrossRef]
- Kilian, H.I.; Ma, C.; Zhang, H.; Chen, M.; Nilam, A.; Quinn, B.; Tang, Y.; Xia, J.; Yao, J.; Lovell, J.F. Intraperitoneal Administration for Sustained Photoacoustic Contrast Agent Imaging. Photoacoustics 2022, 28, 100406. [Google Scholar] [CrossRef]
- Jiang, Z.; Ding, Y.; Lovell, J.F.; Zhang, Y. Design and Application of Organic Contrast Agents for Molecular Imaging in the Second Near Infrared (NIR-II) Window. Photoacoustics 2022, 28, 100426. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to Drug Delivery and Responses via Photoacoustic Imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Kim, G.; Lee, S.; Lee, H.-J.; Kim, Y.B.; Byun, Y.; Oh, Y.-K. Image-Guided Synergistic Photothermal Therapy using Photoresponsive Imaging Agent-Loaded Graphene-Based Nanosheets. J. Control. Release 2015, 211, 28–36. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging From Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yang, J.; Lee, S.Y.; Kim, J.; Lee, J.; Kim, W.J.; Lee, S.; Kim, C. Deep Learning Enhances Multiparametric Dynamic Volumetric Photoacoustic Computed Tomography In Vivo (DL-PACT). Adv. Sci. 2023, 10, 2202089. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Tong, X.; Na, S.; Cao, R.; Yuan, X.; Garrett, D.C.; Shi, J.; Maslov, K.; Wang, L.V. High-Speed Three-Dimensional Photoacoustic Computed Tomography for Preclinical Research and Clinical Translation. Nat. Commun. 2021, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Huda, K.; Wu, C.; Sider, J.G.; Bayer, C.L. Spherical-View Photoacoustic Tomography for Monitoring in vivo Placental Function. Photoacoustics 2020, 20, 100209. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Kim, T.; Jung, S.; Choi, W.; Ahn, J.; Yoon, D.; Kim, J.; Jeon, S.; Lee, D. Quadruple Ultrasound, Photoacoustic, Optical Coherence, and Fluorescence Fusion Imaging with a Transparent Ultrasound Transducer. Proc. Natl. Acad. Sci. USA 2021, 118, e1920879118. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic Microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Nasiriavanaki, M.; Xia, J.; Wan, H.; Bauer, A.Q.; Culver, J.P.; Wang, L.V. High-Resolution Photoacoustic Tomography of Resting-State Functional Connectivity in the Mouse Brain. Proc. Natl. Acad. Sci. USA 2014, 111, 21–26. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Park, S.; Cho, S.; Yoo, J.; Kim, C.; Kim, J. Ultrasound-Guided Breath-Compensation in Single-Element Photoacoustic Imaging for Three-Dimensional Whole-Body Images of Mice. Front. Phys. 2022, 10, 457. [Google Scholar] [CrossRef]
- Park, E.-Y.; Park, S.; Lee, H.; Kang, M.; Kim, C.; Kim, J. Simultaneous Dual-Modal Multispectral Photoacoustic and Ultrasound Macroscopy for Three-Dimensional Whole-Body Imaging of Small Animals. Photonics 2021, 8, 13. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, J.; Kim, C. Multiplane Spectroscopic Whole-Body Photoacoustic Imaging of Small Animals In Vivo. Med. Biol. Eng. Comput. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, N.; Li, T.; Zhang, J.; Lin, R.; Gong, X.; Song, L.; Liu, Z.; Liu, C. Motion Correction in Optical Resolution Photoacoustic Microscopy. IEEE T. Med. Imaging 2019, 38, 2139–2150. [Google Scholar] [CrossRef]
- Qin, W.; Jin, T.; Guo, H.; Xi, L. Large-Field-of-View Optical Resolution Photoacoustic Microscopy. Opt. Express 2018, 26, 4271–4278. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast Optical-Resolution Photoacoustic Microscopy using a 2-Axis Water-Proofing MEMS Scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef]
- Silverman, R.H.; Kong, F.; Chen, Y.; Lloyd, H.O.; Kim, H.H.; Cannata, J.M.; Shung, K.K.; Coleman, D.J. High-Resolution Photoacoustic Imaging of Ocular Tissues. Ultrasound Med. Biol. 2010, 36, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.; Lim, H.; Yang, H.; Kim, J.; Kim, J.; Kim, Y.; Kim, H.H.; Kim, C. Deep Learning Alignment of Bidirectional Raster Scanning in High Speed Photoacoustic Microscopy. Sci. Rep. 2022, 12, 16238. [Google Scholar] [CrossRef]
- Ly, C.D.; Vo, T.H.; Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Kim, C.-S.; Oh, J. Full-View In Vivo Skin and Blood Vessels Profile Segmentation in Photoacoustic Imaging based on Deep Learning. Photoacoustics 2022, 25, 100310. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, J.Y.; Choi, W.; Kim, C. High-Resolution Functional Photoacoustic Monitoring of Vascular Dynamics in Human Fingers. Photoacoustics 2021, 23, 100282. [Google Scholar] [CrossRef]
- Ning, B.; Kennedy, M.J.; Dixon, A.J.; Sun, N.; Cao, R.; Soetikno, B.T.; Chen, R.; Zhou, Q.; Shung, K.K.; Hossack, J.A. Simultaneous Photoacoustic Microscopy of Microvascular Anatomy, Oxygen Saturation, and Blood Flow. Opt. Lett. 2015, 40, 910–913. [Google Scholar] [CrossRef]
- Agarwal, A.; Huang, S.; O’Donnell, M.; Day, K.; Day, M.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007, 102, 064701. [Google Scholar] [CrossRef]
- Cao, R.; Kilroy, J.P.; Ning, B.; Wang, T.; Hossack, J.A.; Hu, S. Multispectral Photoacoustic Microscopy based on an Optical–Acoustic Objective. Photoacoustics 2015, 3, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Ma, T.; Li, J.; Wiedmann, M.T.; Huang, S.; Yu, M.; Kirk Shung, K.; Zhou, Q.; Kim, C.-S.; Chen, Z. High Speed Intravascular Photoacoustic Imaging with Fast Optical Parametric Oscillator Laser at 1. 7 μm. Appl. Phys. Lett. 2015, 107, 083701. [Google Scholar] [CrossRef]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emelianov, S. Multiwavelength Photoacoustic Imaging and Plasmon Resonance Coupling of Gold Nanoparticles for Selective Detection of Cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.-J.; Jeong, E.J.; Jung, M.Y.; Lee, S.; Kim, B.K.; Song, D.H. Gain-Switched Ti: Sapphire Laser-based Photoacoustic Imaging. Appl. Opt. 2016, 55, 5419–5422. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jeong, E.-J.; Song, H.-W.; Ahn, C.-G.; Noh, H.W.; Sim, J.Y.; Song, D.H.; Jeon, M.Y.; Lee, S.; Kim, H. Photoacoustic Imaging Probe for Detecting Lymph Nodes and Spreading of Cancer at Various Depths. J. Biomed. Opt. 2017, 22, 091513. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, C.; Yu, H.-B.; Wang, Z.-M.; Zuo, J.-W.; Xia, Y.-Q.; Bian, Q.; Bo, Y.; Gao, H.-W.; Guo, Y.-D. High Pulse Energy, High Beam Quality Microsecond-Pulse Ti: Sapphire Laser at 819. 7 nm. Appl. Phys. B 2017, 123, 94. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Jeon, M.Y.; Kim, J.; Kim, C. In Vitro Photoacoustic Measurement of Hemoglobin Oxygen Saturation using a Single Pulsed Broadband Supercontinuum Laser Source. Appl. Opt. 2014, 53, 3884–3889. [Google Scholar] [CrossRef]
- Dasa, M.K.; Nteroli, G.; Bowen, P.; Messa, G.; Feng, Y.; Petersen, C.R.; Koutsikou, S.; Bondu, M.; Moselund, P.M.; Podoleanu, A. All-Fibre Supercontinuum Laser for in vivo Multispectral Photoacoustic Microscopy of Lipids in the Extended Near-Infrared Region. Photoacoustics 2020, 18, 100163. [Google Scholar] [CrossRef]
- Shu, X.; Bondu, M.; Dong, B.; Podoleanu, A.; Leick, L.; Zhang, H.F. Single All-Fiber-based Nanosecond-Pulsed Supercontinuum Source for Multispectral Photoacoustic Microscopy and Optical Coherence Tomography. Opt. Lett. 2016, 41, 2743–2746. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Chen, R.; Shi, J. Recent Advances in High-Speed Photoacoustic Microscopy. Photoacoustics 2021, 24, 100294. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.M.; Park, J.; Cho, S.-W.; Han, S.; Ahn, J.; Cho, S.; Kim, C.; Kim, C.-S.; Kim, J. Transportable Multispectral Optical-Resolution Photoacoustic Microscopy using Stimulated Raman Scattering Spectrum. IEEE Trans. Instrum. Meas. 2024, 73, 4502309. [Google Scholar] [CrossRef]
- Wang, L.; Maslov, K.; Yao, J.; Rao, B.; Wang, L.V. Fast Voice-Coil Scanning Optical-Resolution Photoacoustic Microscopy. Opt. Lett. 2011, 36, 139–141. [Google Scholar] [CrossRef]
- Hu, S.; Maslov, K.; Wang, L.V. Second-Generation Optical-Resolution Photoacoustic Microscopy with Improved Sensitivity and Speed. Opt. Lett. 2011, 36, 1134–1136. [Google Scholar] [CrossRef]
- Wang, L.V.; Wu, H.-i. Biomedical Optics: Principles and Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sehgal, C.M.; Greenleaf, J.F. Scattering of Ultrasound by Tissues. Ultrason. Imaging 1984, 6, 60–80. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
- Keshava, N. A Survey of Spectral Unmixing Algorithms. Linc. Lab. J. 2003, 14, 55–78. [Google Scholar]
- Baksalary, O.M.; Trenkler, G. The Moore–Penrose Inverse: A Hundred Years on a Frontline of Physics Research. Eur. Phys. J. H 2021, 46, 9. [Google Scholar] [CrossRef]
- Arabul, M.; Rutten, M.; Bruneval, P.; van Sambeek, M.; van de Vosse, F.; Lopata, R. Unmixing Multi-Spectral Photoacoustic Sources in Human Carotid Plaques using Non-Negative Independent Component Analysis. Photoacoustics 2019, 15, 100140. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, F.; Hu, S. Temporal and Spectral Unmixing of Photoacoustic Signals by Deep Learning. Opt. Lett. 2021, 46, 2690–2693. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; You, H.; Wu, H.; Zhao, Q.; Dong, X.; Bai, S.; He, H.; Dong, J. Dual-Wavelength, Nanosecond, Miniature Raman Laser Enables Efficient Photoacoustic Differentiation of Water and Lipid. APL Photonics 2024, 9, 096104. [Google Scholar] [CrossRef]
- Agrawal, G.P. Nonlinear Fiber Optics: Its History and Recent Progress. J. Opt. Soc. Am. B 2011, 28, A1–A10. [Google Scholar] [CrossRef]
- Supradeepa, V.; Feng, Y.; Nicholson, J.W. Raman Fiber Lasers. J. Opt. 2017, 19, 023001. [Google Scholar] [CrossRef]
- Agrawal, G.P. Nonlinear Fiber Optics, in Nonlinear Science at the Dawn of the 21st Century; Springer: Berlin/Heidelberg, Germany, 2000; pp. 195–211. [Google Scholar]
- Zijlstra, W.; Buursma, A.; Meeuwsen-Van der Roest, W. Absorption Spectra of Human Fetal and Adult Oxyhemoglobin, De-oxyhemoglobin, Carboxyhemoglobin, and Methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Cho, S.-W.; Kang, H.; Park, S.M.; Lim, G.; Piao, Z.; Lee, S.-W.; Kim, C.-S.; Lee, T.G. Optimal Generation of Ten Individual Green-to-Red Raman Source for Wavelength-Dependent Real-Time OR-PAM Images. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 1400109. [Google Scholar] [CrossRef]
- Koeplinger, D.; Liu, M.; Buma, T. Photoacoustic Microscopy with a Pulsed Multi-Color Source based on Stimulated Raman Scattering. In Proceedings of the IEEE International Ultrasonics Symposium 2011, Orlando, FL, USA, 18–21 October 2011. [Google Scholar]
- Loya, A.K.; Dumas, J.; Buma, T. Photoacoustic Microscopy with a Tunable Source based on Cascaded Stimulated Raman Scattering in a Large-Mode Area Photonic Crystal Fiber. In Proceedings of the IEEE International Ultrasonics Symposium 2012, Dresden, Germany, 7–10 October 2012. [Google Scholar]
- Strohm, E.M.; Moore, M.J.; Kolios, M.C. High Resolution Ultrasound and Photoacoustic Imaging of Single Cells. Photoacoustics 2016, 4, 36–42. [Google Scholar] [CrossRef]
- Bui, N.Q.; Cho, S.-W.; Moorthy, M.S.; Park, S.M.; Piao, Z.; Nam, S.Y.; Kang, H.W.; Kim, C.-S.; Oh, J. In Vivo Photoacoustic Monitoring using 700-nm Region Raman Source for Targeting Prussian Blue Nanoparticles in Mouse Tumor Model. Sci. Rep. 2018, 8, 2000. [Google Scholar] [CrossRef]
- Hajireza, P.; Forbrich, A.; Zemp, R. In-Vivo Functional Optical-Resolution Photoacoustic Microscopy with Stimulated Raman Scattering Fiber-Laser Source. Biomed. Opt. Express 2014, 5, 539–546. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Q.; DiSpirito, A.; Vu, T.; Rong, Q.; Peng, X.; Sheng, H.; Shen, X.; Zhou, Q.; Jiang, L. Real-Time Whole-Brain Imaging of Hemodynamics and Oxygenation at Micro-Vessel Resolution with Ultrafast Wide-Field Photoacoustic Microscopy. Light-Sci. Appl. 2022, 11, 138. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Li, X.; Zhu, J.; Li, D.; Li, S.; Lee, C.-S.; Wang, L. Confocal Visible/NIR Photoacoustic Microscopy of Tumors with Structural, Functional, and Nanoprobe Contrasts. Photonics Res. 2020, 8, 1875–1880. [Google Scholar] [CrossRef]
- Liu, C.; Liang, Y.; Wang, L. Single-Shot Photoacoustic Microscopy of Hemoglobin Concentration, Oxygen Saturation, and Blood Flow in Sub-Microseconds. Photoacoustics 2020, 17, 100156. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qu, Z.; Amjadian, M.; Tang, X.; Chen, J.; Wang, L. All-Fiber Three-Wavelength Laser for Functional Photoacoustic Microscopy. Photoacoustics 2025, 42, 100703. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; He, L.; Liang, Y.; Wang, L. Wide-Field Polygon-Scanning Photoacoustic Microscopy of Oxygen Saturation at 1-MHz A-Line Rate. Photoacoustics 2020, 20, 100195. [Google Scholar] [CrossRef]
- Park, S.M.; Cho, S.-W.; Kim, B.-M.; Lee, T.G.; Kim, C.-S.; Lee, S.-W. Quickly Alternating Green and Red Laser Source for Real-Time Multispectral Photoacoustic Microscopy. Photoacoustics 2020, 20, 100204. [Google Scholar] [CrossRef]
- Buma, T.; Wilkinson, B.C.; Sheehan, T.C. Near-Infrared Spectroscopic Photoacoustic Microscopy using a Multi-Color Fiber Laser Source. Biomed. Opt. Express 2015, 6, 2819–2829. [Google Scholar] [CrossRef]
- Wilkinson, B.C.; Sheehan, T.C.; Buma, T. Spectroscopic Photoacoustic Microscopy in the 1064–1300 nm Range using a Pulsed Multi-Color Source based on Stimulated Raman Scattering. In Proceedings of the IEEE International Ultrasonics Symposium 2014, Chicago, IL, USA, 3–6 September 2014. [Google Scholar]
- Choi, S.W.; Buma, T. Injection-Seeded Raman Fiber Amplifier for Photoacoustic Microscopy of Lipids. In Proceedings of the IEEE International Ultrasonics Symposium 2016, Tours, France, 18–21 September 2016. [Google Scholar]
- Lee, H.; Seeger, M.R.; Lippok, N.; Nadkarni, S.K.; van Soest, G.; Bouma, B.E. Nanosecond SRS Fiber Amplifier for Label-Free Near-Infrared Photoacoustic Microscopy of Lipids. Photoacoustics 2022, 25, 100331. [Google Scholar] [CrossRef]
- Lee, H.; Seeger, M.R.; Bouma, B.E. Electronically Controlled Dual-Wavelength Switchable SRS Fiber Amplifier in the NIR-II Region for Multispectral Photoacoustic Microscopy. Laser Photonics Rev. 2024, 18, 2400144. [Google Scholar] [CrossRef]
- Park, S.M.; Bak, S.; Kim, G.H.; Kim, C.S.; Cho, S.W.; Bouma, B.E.; Lee, H. Wavelength-Switchable Synchronously Pumped Raman Fiber Laser Near 1. 7 µm for Multispectral Photoacoustic Microscopy. Laser Photonics Rev. 2025, 19, 2401080. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.; Yang, J.-M.; Gao, L.S.; Maslov, K.I.; Wang, L.V.; Huang, C.-H.; Zou, J. Wide-Field Fast-Scanning Photoacoustic Microscopy based on a Water-Immersible MEMS Scanning Mirror. J. Biomed. Opt. 2012, 17, 080505. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Zhang, Y.; Zhu, J.; Wang, L. Five-Wavelength Optical-Resolution Photoacoustic Microscopy of Blood and Lymphatic Vessels. Adv. Photonics 2021, 3, 016002. [Google Scholar] [CrossRef]
- Wang, T.; Sun, N.; Cao, R.; Ning, B.; Chen, R.; Zhou, Q.; Hu, S. Multiparametric Photoacoustic Microscopy of the Mouse Brain with 300-kHz A-Line Rate. Neurophotonics 2016, 3, 045006. [Google Scholar] [CrossRef] [PubMed]
- Hosseinaee, Z.; Ecclestone, B.; Pellegrino, N.; Khalili, L.; Mukhangaliyeva, L.; Fieguth, P.; Reza, P.H. Functional Photoacoustic Remote Sensing Microscopy using a Stabilized Temperature-Regulated Stimulated Raman Scattering Light Source. Opt. Express 2021, 29, 29745–29754. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-D.; Kim, Y.H.; Park, J.-H.; Yim, S.-Y.; Jeong, M.S. Note: Automatic Laser-to-Optical-Fiber Coupling System based on Monitoring of Raman Scattering Signal. Rev. Sci. Instrum. 2012, 83, 096104. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kye, H.; Kim, C.-S.; Kim, T.-K.; Yoo, J.; Kim, J. Automated Laser-Fiber Coupling Module for Optical-Resolution Photoacoustic Microscopy. Sensors 2023, 23, 6643. [Google Scholar] [CrossRef]
- Yang, J.; Choi, S.; Kim, J.; Lee, J.; Kim, W.J.; Kim, C. Multiplane Spectroscopic Whole-Body Photoacoustic Computed Tomography of Small Animals In Vivo. Laser Photonics Rev. 2024, 19, 2400672. [Google Scholar] [CrossRef]




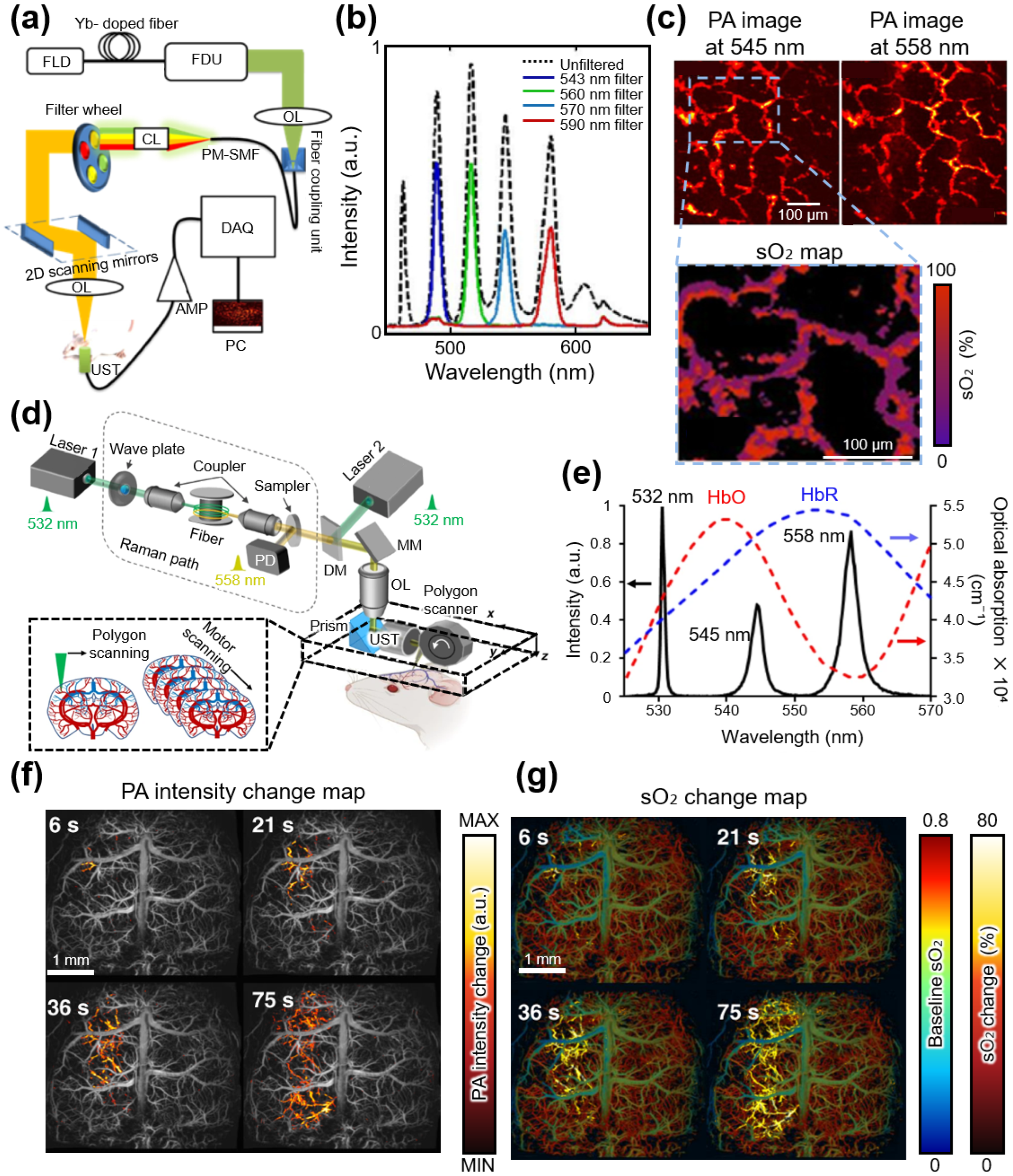
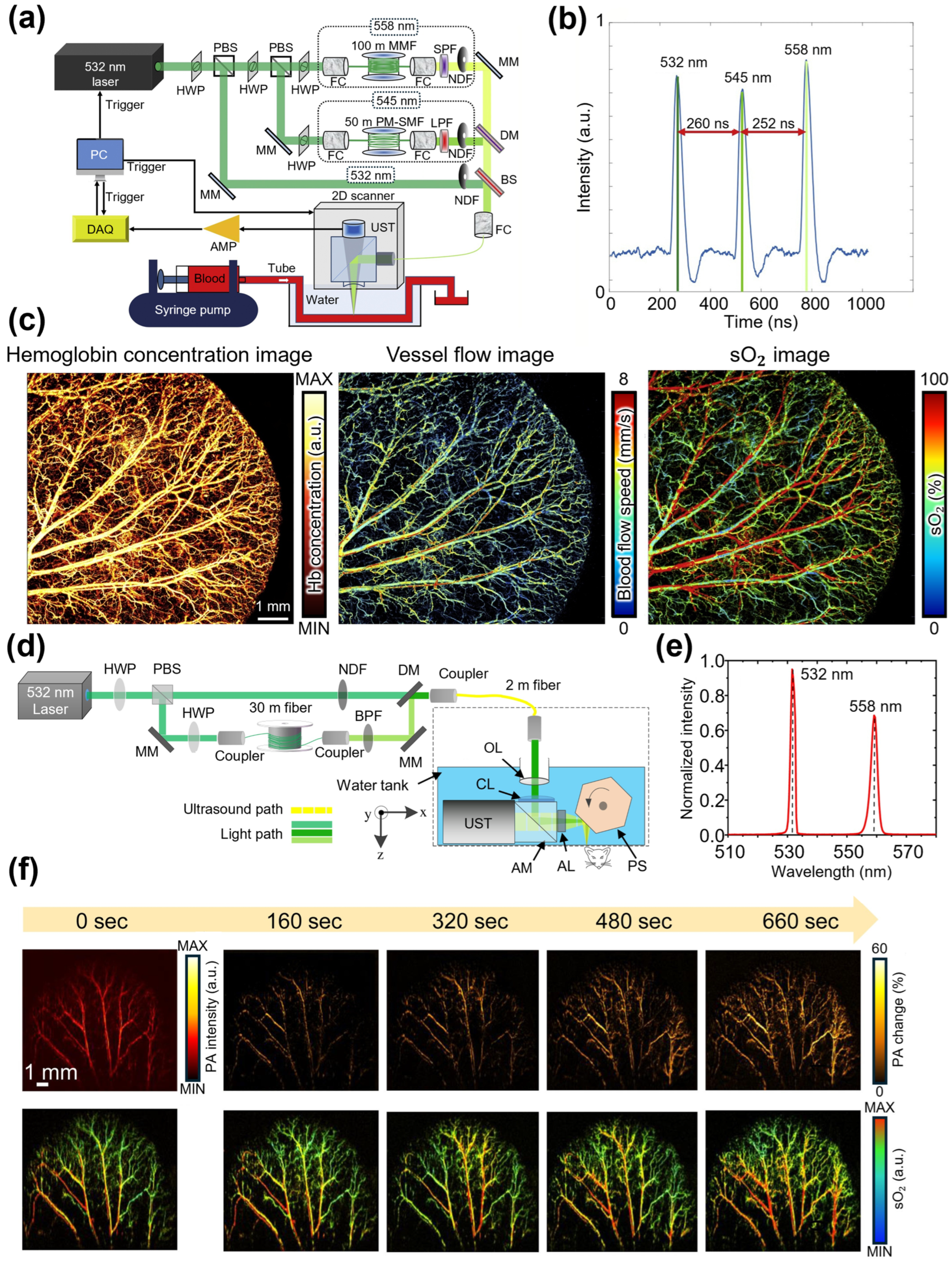
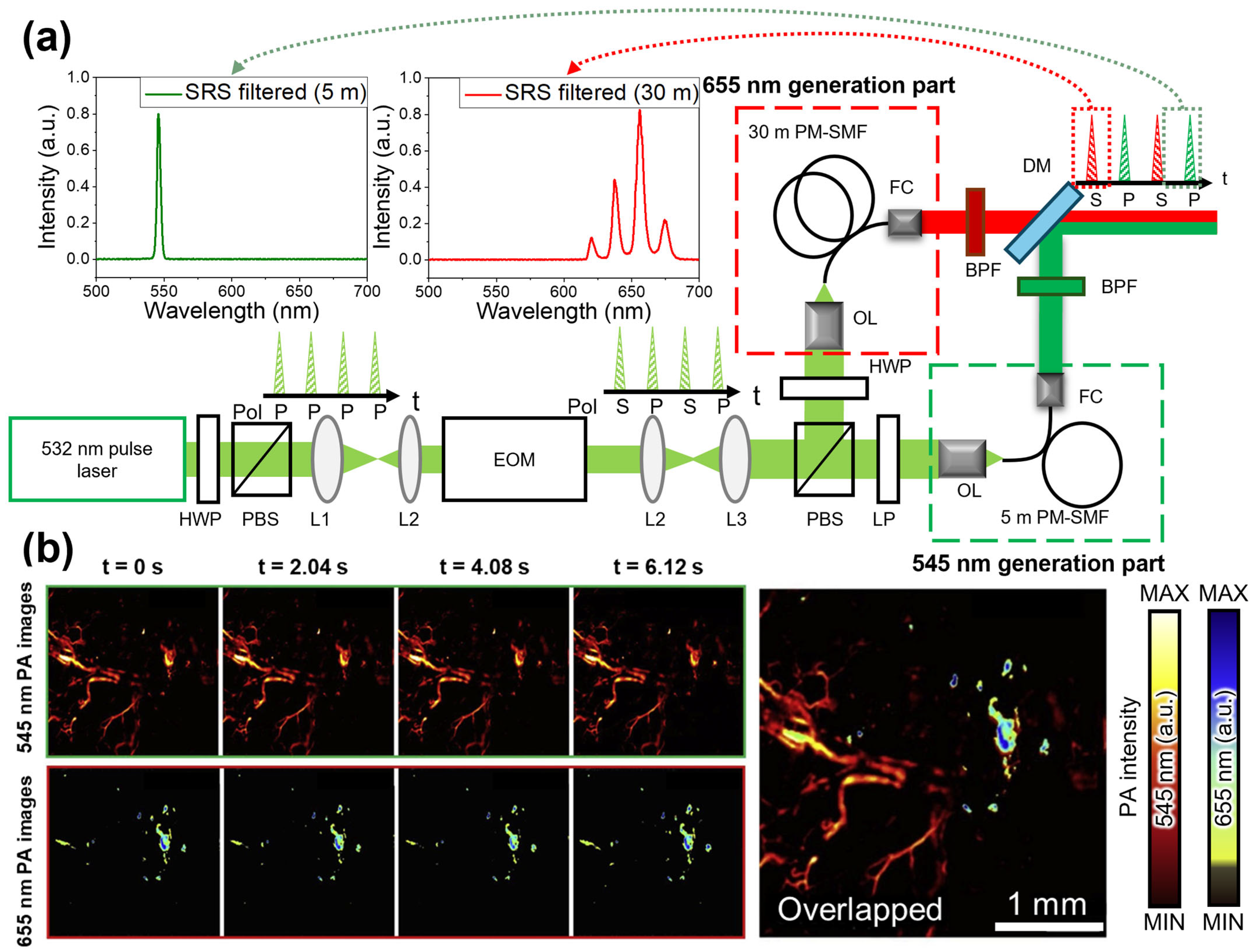


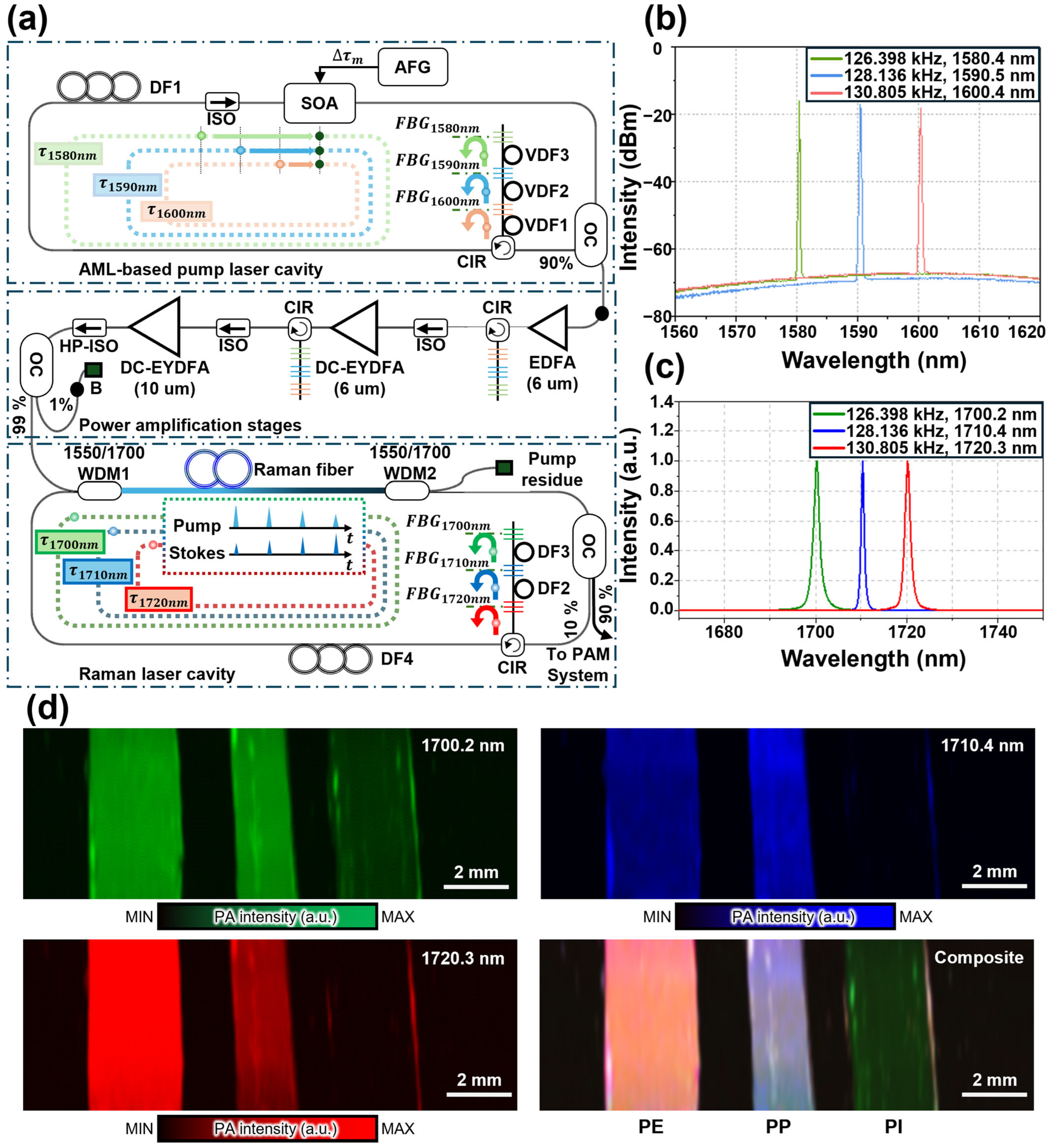
| Spectral Region | Type | [nm] | [kW] | PRR [kHz] | [nm] | [nJ] | Target | Ref. |
|---|---|---|---|---|---|---|---|---|
| Visible | SRS | 532 | - | - | 532, 600 | 1–5 | Wright–Giemsa stain | [80] |
| 532 | 8.89 | 5 | 532, 700 | 300 | Hemoglobin, PB NPs | [81] | ||
| 532 | 1.875 | 40 | 532, 545, 560, 590 | 300–500 | Hemoglobin | [82] | ||
| 532 | - | 800 | 532, 558 | 200 | Hemoglobin | [83] | ||
| 532 | - | 4 | 532, 545, 558 | 100 | Hemoglobin | [85] | ||
| 532 | - | 1000 | 532, 558 | 64–85 | Hemoglobin | [87] | ||
| Polarization- modulated SRS | 532 | 1.5 | 300 | 532, 655 | 200 | Hemoglobin, AuNRs | [88] | |
| NIR | SRS | 1064 | 13.7 | 7.4 | 1064, 1100, 1175, 1225, 1275, 1325 | - | Lipid phantom | [90] |
| Injection-seeded SRS | 1047 | 10 | 2.5 | 1048, 1098, 1153, 1206, 1275 | - | Drosophila larva | [91] | |
| 1068 | - | 200 | 1192 | 467 | White adipocytes | [92] | ||
| 1030 | - | 200 | 1168, 1202 | 271 | PE, PI, PP | [93] | ||
| Synchronously pumped SRS | 1580, 1590, 1600 | ~0.264 | ~130 | 1700, 1710, 1720 | 400 | PE, PI, PP | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Park, S.M.; Jeong, Y.; Kim, J.; Lee, H. Review on Multispectral Photoacoustic Imaging Using Stimulated Raman Scattering Light Sources. Sensors 2025, 25, 3325. https://doi.org/10.3390/s25113325
Song Y, Park SM, Jeong Y, Kim J, Lee H. Review on Multispectral Photoacoustic Imaging Using Stimulated Raman Scattering Light Sources. Sensors. 2025; 25(11):3325. https://doi.org/10.3390/s25113325
Chicago/Turabian StyleSong, Yuon, Sang Min Park, Yongjae Jeong, Jeesu Kim, and Hwidon Lee. 2025. "Review on Multispectral Photoacoustic Imaging Using Stimulated Raman Scattering Light Sources" Sensors 25, no. 11: 3325. https://doi.org/10.3390/s25113325
APA StyleSong, Y., Park, S. M., Jeong, Y., Kim, J., & Lee, H. (2025). Review on Multispectral Photoacoustic Imaging Using Stimulated Raman Scattering Light Sources. Sensors, 25(11), 3325. https://doi.org/10.3390/s25113325









