Endogenous γ-Secretase Is Linked to Phagocytic Activity in Microglial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid DNA, Antibodies, and Reagents
2.2. Cell Culture and Transfection
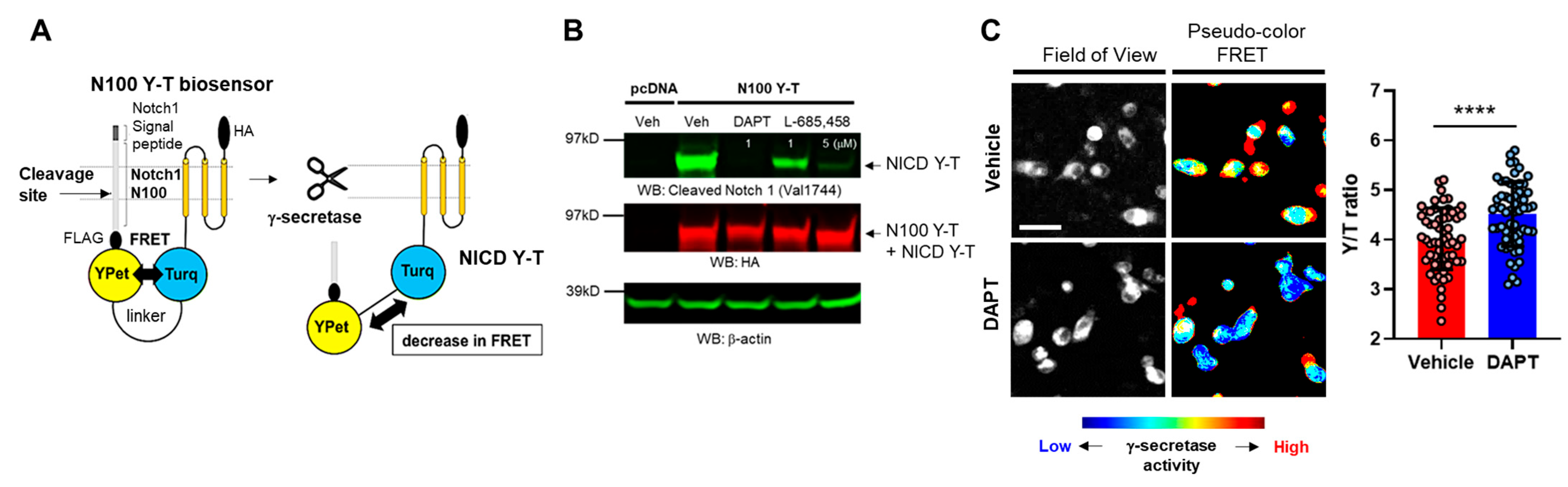
2.3. Confocal Microscopy and FRET
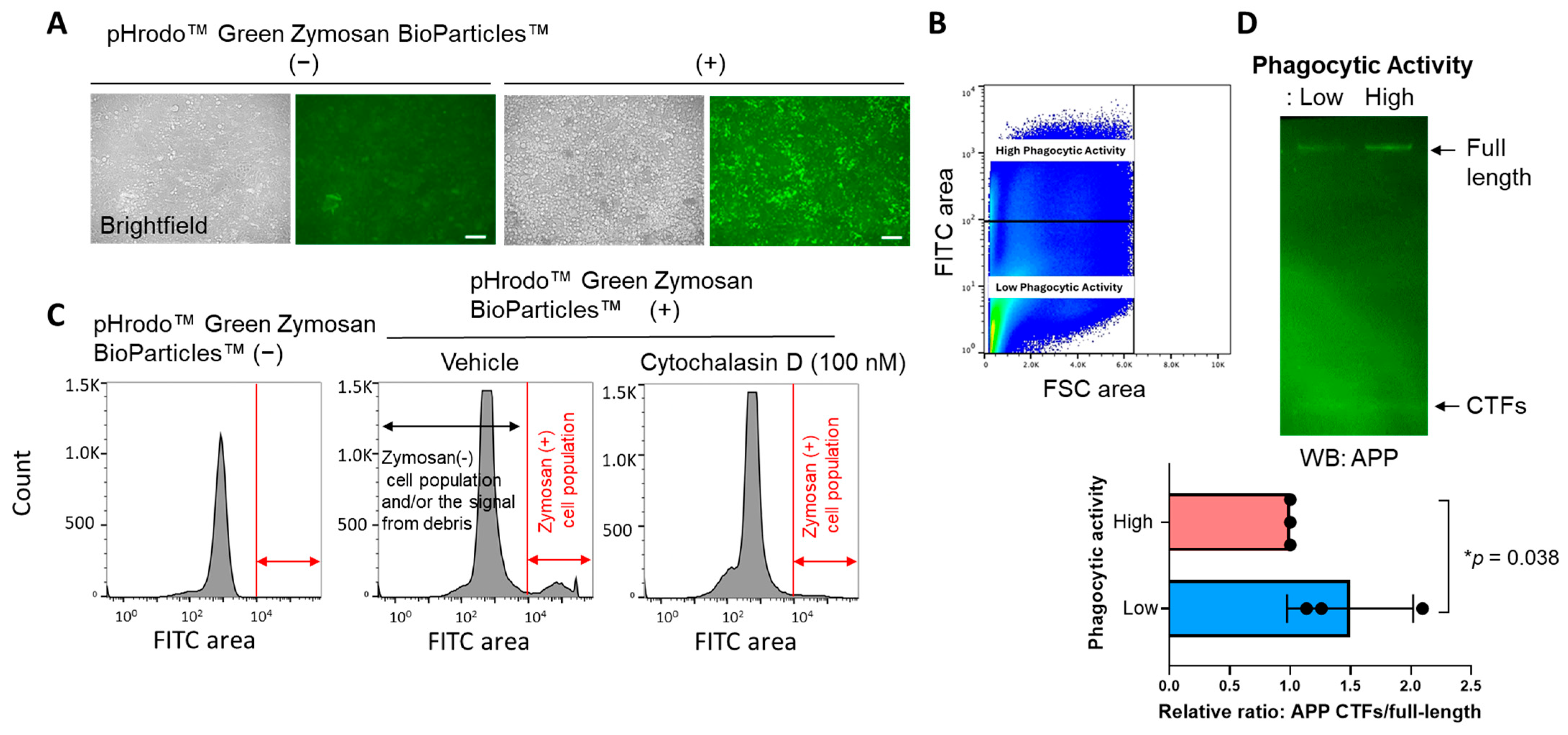
2.4. Fluorescence-Activated Cell Sorting (FACS) and Western Blot
2.5. Statistical Analysis
3. Results
3.1. Recording Endogenous γ-Secretase Activity in Individual BV-2 Microglial Cells Using the N100 Y-T Biosensor
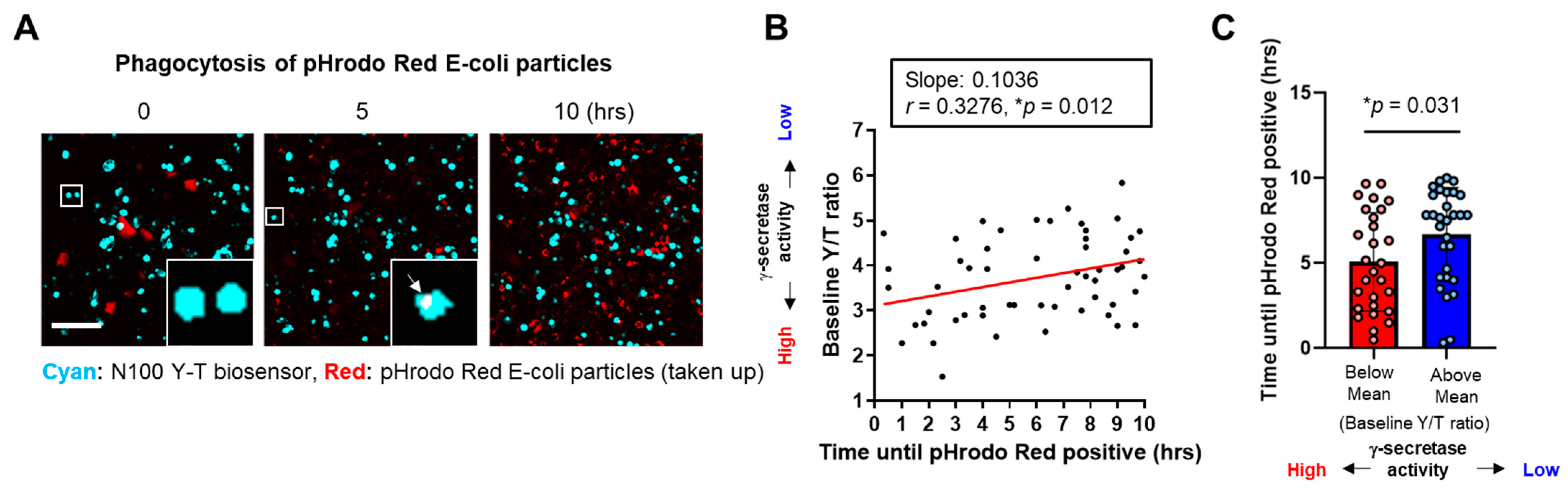
3.2. Phagocytosis Is Impaired in the BV-2 Cells with Lower γ-Secretase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | amyloid precursor protein |
| FACS | fluorescence-activated cell sorting |
| FRET | Förster resonance energy transfer |
| Y-T | YPet-mTurquoise-GL |
References
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Pallarés-Moratalla, C.; Bergers, G. The ins and outs of microglial cells in brain health and disease. Front. Immunol. 2024, 15, 1305087. [Google Scholar] [CrossRef]
- De Strooper, B.; Annaert, W.; Cupers, P.; Saftig, P.; Craessaerts, K.; Mumm, J.S.; Schroeter, E.H.; Schrijvers, V.; Wolfe, M.S.; Ray, W.J.; et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 1999, 398, 518–522. [Google Scholar] [CrossRef]
- Costa, R.M.; Honjo, T.; Silva, A.J. Learning and memory deficits in Notch mutant mice. Curr. Biol. 2003, 13, 1348–1354. [Google Scholar] [CrossRef]
- Alberi, L.; Liu, S.; Wang, Y.; Badie, R.; Smith-Hicks, C.; Wu, J.; Pierfelice, T.J.; Abazyan, B.; Mattson, M.P.; Kuhl, D.; et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 2011, 69, 437–444. [Google Scholar] [CrossRef]
- De Strooper, B.; Saftig, P.; Craessaerts, K.; Vanderstichele, H.; Guhde, G.; Annaert, W.; Von Figura, K.; Van Leuven, F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 1998, 391, 387–390. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef]
- Saura, C.A.; Chen, G.; Malkani, S.; Choi, S.Y.; Takahashi, R.H.; Zhang, D.; Gouras, G.K.; Kirkwood, A.; Morris, R.G.; Shen, J. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J. Neurosci. 2005, 25, 6755–6764. [Google Scholar] [CrossRef]
- Walter, J.; Kemmerling, N.; Wunderlich, P.; Glebov, K. γ-Secretase in microglia–implications for neurodegeneration and neuroinflammation. J. Neurochem. 2017, 143, 445–454. [Google Scholar] [CrossRef]
- Hou, P.; Zielonka, M.; Serneels, L.; Martinez-Muriana, A.; Fattorelli, N.; Wolfs, L.; Poovathingal, S.; T’Syen, D.; Balusu, S.; Theys, T.; et al. The γ-secretase substrate proteome and its role in cell signaling regulation. Mol. Cell 2023, 83, 4106–4122.e10. [Google Scholar] [CrossRef] [PubMed]
- Farfara, D.; Trudler, D.; Segev-Amzaleg, N.; Galron, R.; Stein, R.; Frenkel, D. γ-Secretase component presenilin is important for microglia β-amyloid clearance. Ann. Neurol. 2011, 69, 170–180. [Google Scholar] [CrossRef]
- Kakuda, N.; Funamoto, S.; Yagishita, S.; Takami, M.; Osawa, S.; Dohmae, N.; Ihara, Y. Equimolar production of amyloid beta-protein and amyloid precursor protein intracellular domain from beta-carboxyl-terminal fragment by gamma-secretase. J. Biol. Chem. 2006, 281, 14776–14786. [Google Scholar] [CrossRef]
- Cao, X.; Südhof, T.C. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 2001, 293, 115–120. [Google Scholar] [CrossRef]
- Maesako, M.; Sekula, N.M.; Aristarkhova, A.; Feschenko, P.; Anderson, L.C.; Berezovska, O. Visualization of PS/γ-Secretase Activity in Living Cells. iScience 2020, 23, 101139. [Google Scholar] [CrossRef]
- Houser, M.C.Q.; Hou, S.S.; Perrin, F.; Turchyna, Y.; Bacskai, B.J.; Berezovska, O.; Maesako, M. A Novel NIR-FRET Biosensor for Reporting PS/γ-Secretase Activity in Live Cells. Sensors 2020, 20, 5980. [Google Scholar] [CrossRef]
- Axline, S.G.; Reaven, E.P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J. Cell Biol. 1974, 62, 647–659. [Google Scholar] [CrossRef]
- Goddette, D.W.; Frieden, C. Actin polymerization. The mechanism of action of cytochalasin D. J. Biol. Chem. 1986, 261, 15974–15980. [Google Scholar] [CrossRef]
- Houser, M.C.Q.; Turchyna, Y.; Perrin, F.; Chibnik, L.; Berezovska, O.; Maesako, M. Limited Substrate Specificity of PS/γ-Secretase Is Supported by Novel Multiplexed FRET Analysis in Live Cells. Biosensors 2021, 11, 169. [Google Scholar] [CrossRef]
- Maesako, M.; Houser, M.C.Q.; Turchyna, Y.; Wolfe, M.S.; Berezovska, O. Presenilin/γ-Secretase Activity Is Located in Acidic Compartments of Live Neurons. J. Neurosci. 2022, 42, 145–154. [Google Scholar] [CrossRef]
- McKendell, A.K.; Houser, M.C.Q.; Mitchell, S.P.C.; Wolfe, M.S.; Berezovska, O.; Maesako, M. In-Depth Characterization of Endo-Lysosomal Aβ in Intact Neurons. Biosensors 2022, 12, 663. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.S.; Ikegawa, Y.; Kwon, Y.; Wieckiewicz, N.; Houser, M.C.Q.; Lundin, B.; Bacskai, B.J.; Berezovska, O.; Maesako, M. Recording γ-secretase activity in living mouse brains. Elife 2024, 13, RP96848. [Google Scholar] [CrossRef]
- Güner, G.; Lichtenthaler, S.F. The substrate repertoire of γ-secretase/presenilin. Semin. Cell Dev. Biol. 2020, 105, 27–42. [Google Scholar] [CrossRef]
- Wong, P.C.; Zheng, H.; Chen, H.; Becher, M.W.; Sirinathsinghji, D.J.; Trumbauer, M.E.; Chen, H.Y.; Price, D.L.; Van der Ploeg, L.H.; Sisodia, S.S. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 1997, 387, 288–292. [Google Scholar] [CrossRef]
- Coric, V.; van Dyck, C.H.; Salloway, S.; Andreasen, N.; Brody, M.; Richter, R.W.; Soininen, H.; Thein, S.; Shiovitz, T.; Pilcher, G.; et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch. Neurol. 2012, 69, 1430–1440. [Google Scholar] [CrossRef]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef]
- Bateman, R.J.; Siemers, E.R.; Mawuenyega, K.G.; Wen, G.; Browning, K.R.; Sigurdson, W.C.; Yarasheski, K.E.; Friedrich, S.W.; Demattos, R.B.; May, P.C.; et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann. Neurol. 2009, 66, 48–54. [Google Scholar] [CrossRef]
- Cao, Q.; Li, P.; Lu, J.; Dheen, S.T.; Kaur, C.; Ling, E.A. Nuclear factor-κB/p65 responds to changes in the Notch signaling pathway in murine BV-2 cells and in amoeboid microglia in postnatal rats treated with the γ-secretase complex blocker DAPT. J. Neurosci. Res. 2010, 88, 2701–2714. [Google Scholar] [CrossRef]
- Jayadev, S.; Case, A.; Eastman, A.J.; Nguyen, H.; Pollak, J.; Wiley, J.C.; Möller, T.; Morrison, R.S.; Garden, G.A. Presenilin 2 is the predominant γ-secretase in microglia and modulates cytokine release. PLoS ONE 2010, 5, e15743. [Google Scholar] [CrossRef]
- Kemmerling, N.; Wunderlich, P.; Theil, S.; Linnartz-Gerlach, B.; Hersch, N.; Hoffmann, B.; Heneka, M.T.; de Strooper, B.; Neumann, H.; Walter, J. Intramembranous processing by γ-secretase regulates reverse signaling of ephrin-B2 in migration of microglia. Glia 2017, 65, 1103–1118. [Google Scholar] [CrossRef]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 2003, 3, 445–453. [Google Scholar] [CrossRef]
- Kawabori, M.; Kacimi, R.; Kauppinen, T.; Calosing, C.; Kim, J.Y.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015, 35, 3384–3396. [Google Scholar] [CrossRef]
- Xiang, X.; Werner, G.; Bohrmann, B.; Liesz, A.; Mazaheri, F.; Capell, A.; Feederle, R.; Knuesel, I.; Kleinberger, G.; Haass, C. TREM2 deficiency reduces the efficacy of immunotherapeutic amyloid clearance. EMBO Mol. Med. 2016, 8, 992–1004. [Google Scholar] [CrossRef]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef]
- Wunderlich, P.; Glebov, K.; Kemmerling, N.; Tien, N.T.; Neumann, H.; Walter, J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J. Biol. Chem. 2013, 288, 33027–33036. [Google Scholar] [CrossRef]
- Schlepckow, K.; Kleinberger, G.; Fukumori, A.; Feederle, R.; Lichtenthaler, S.F.; Steiner, H.; Haass, C. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol. Med. 2017, 9, 1356–1365. [Google Scholar] [CrossRef]
- Thornton, P.; Sevalle, J.; Deery, M.J.; Fraser, G.; Zhou, Y.; Ståhl, S.; Franssen, E.H.; Dodd, R.B.; Qamar, S.; Gomez Perez-Nievas, B.; et al. TREM2 shedding by cleavage at the H157-S158 bond is accelerated for the Alzheimer’s disease-associated H157Y variant. EMBO Mol. Med. 2017, 9, 1366–1378. [Google Scholar] [CrossRef]
- Steiner, A.; Schlepckow, K.; Brunner, B.; Steiner, H.; Haass, C.; Hagn, F. γ-Secretase cleavage of the Alzheimer risk factor TREM2 is determined by its intrinsic structural dynamics. EMBO J. 2020, 39, e104247. [Google Scholar] [CrossRef]
- Glebov, K.; Wunderlich, P.; Karaca, I.; Walter, J. Functional involvement of γ-secretase in signaling of the triggering receptor expressed on myeloid cells-2 (TREM2). J. Neuroinflamm. 2016, 13, 17. [Google Scholar] [CrossRef]
- Miot, H.A. Correlation analysis in clinical and experimental studies. J. Vasc. Bras. 2018, 17, 275–279. [Google Scholar] [CrossRef]
- Rusakov, D.A. A misadventure of the correlation coefficient. Trends Neurosci. 2023, 46, 94–96. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.; Houser, M.C.Q.; Torres, S.; Wieckiewicz, N.; Sadek, M.; Yokomizo, M.; Maesako, M. Endogenous γ-Secretase Is Linked to Phagocytic Activity in Microglial Cells. Sensors 2025, 25, 3298. https://doi.org/10.3390/s25113298
Williams E, Houser MCQ, Torres S, Wieckiewicz N, Sadek M, Yokomizo M, Maesako M. Endogenous γ-Secretase Is Linked to Phagocytic Activity in Microglial Cells. Sensors. 2025; 25(11):3298. https://doi.org/10.3390/s25113298
Chicago/Turabian StyleWilliams, Emily, Mei C. Q. Houser, Sebastian Torres, Natalia Wieckiewicz, Michael Sadek, Midori Yokomizo, and Masato Maesako. 2025. "Endogenous γ-Secretase Is Linked to Phagocytic Activity in Microglial Cells" Sensors 25, no. 11: 3298. https://doi.org/10.3390/s25113298
APA StyleWilliams, E., Houser, M. C. Q., Torres, S., Wieckiewicz, N., Sadek, M., Yokomizo, M., & Maesako, M. (2025). Endogenous γ-Secretase Is Linked to Phagocytic Activity in Microglial Cells. Sensors, 25(11), 3298. https://doi.org/10.3390/s25113298








